Abstract
Background
Heterochronic parabiosis has identified growth differentiation factor (GDF)-11 as a potential means of cardiac rejuvenation, but findings have been inconsistent. A major barrier has been lack of assay specificity for GDF-11 and its homolog GDF-8.
Methods
We tested the hypothesis that GDF-11 and GDF-8, and their major antagonists follistatin and follistatin-like (FSTL)-3, are associated with incident heart failure (HF) and its subtypes in elders. Based on validation experiments, we used liquid chromatography–tandem mass spectrometry to measure total serum GDF-11 and GDF-8, along with follistatin and FSTL-3 by immunoassay, in 2 longitudinal cohorts of older adults.
Results
In 2 599 participants (age 75.2 ± 4.3) followed for 10.8 ± 5.6 years, 721 HF events occurred. After adjustment, neither GDF-11 (HR per doubling: 0.93 [0.67, 1.30]) nor GDF-8 (HR: 1.02 per doubling [0.83, 1.27]) was associated with incident HF or its subtypes. Positive associations with HF were detected for follistatin (HR: 1.15 [1.00, 1.32]) and FLST-3 (HR: 1.38 [1.03, 1.85]), and with HF with preserved ejection fraction for FSTL-3 (HR: 1.77 [1.03, 3.02]). (All HRs per doubling of biomarker.) FSTL-3 associations with HF appeared stronger at higher follistatin levels and vice versa, and also for men, Blacks, and lower kidney function.
Conclusions
Among older adults, serum follistatin and FSTL-3, but not GDF-11 or GDF-8, were associated with incident HF. These findings do not support the concept that low serum levels of total GDF-11 or GDF-8 contribute to HF late in life, but do implicate transforming growth factor-β superfamily pathways as potential therapeutic targets.
Keywords: Follistatin, Follistatin-like 3, Growth and differentiation factor 8, Growth and differentiation factor 11, Heart failure
Heart failure (HF) is a foremost cardiovascular disorder of aging (1). Its 2 major subtypes, HF with reduced (HFrEF) and preserved ejection fraction (HFpEF), portend similarly poor survival (2). The need for effective therapies remains pressing, especially for HFpEF (2). Thus, an improved understanding of HF’s molecular underpinnings is of major public health importance.
Heterochronic parabiosis raised prospects in this regard, showing the reversal of cardiac hypertrophy in older mice by humoral factors from younger mice (3). The molecule responsible was a member of the transforming growth factor (TGF)-β superfamily, growth differentiation factor (GDF)-11, whose levels were deficient in older rodents. Subsequent studies, however, failed to replicate these results. In one report, GDF-11 levels increased with age (4). In another, recombinant GDF-11 produced regression of cardiac hypertrophy (5), but also caused cachexia (6).
It later became clear that the aptamer technique used in the original experiments couldn’t distinguish GDF-11 from its homolog, GDF-8 (7). Unlike GDF-11, which is synthesized widely, GDF-8 is chiefly produced by skeletal muscle, of which it is a principal negative regulator (7). Nor could immunoassays differentiate between the homologs (8) or avoid cross-reactivity (9). A longitudinal study associated low aptamer-determined GDF-8/11 levels with cardiovascular disease (CVD), HF, and death (10). Another study using liquid chromatography–tandem mass spectrometry (LC-MS/MS), however, showed that GDF-8, but not GDF-11, decreased with age (11), and related higher GDF-11 to adverse outcomes in aortic stenosis (11).
Further complicating GDF-11 and GDF-8 assessment is that each circulates as a latent complex, wherein the mature ligand is inactivated by the noncovalent binding of its prodomain, and is only capable of receptor binding upon cleavage by proteases (7). Moreover, both GDF-11 and GDF-8 are further inactivated by various peptides that bind to their latent complexes, including follistatin, follistatin-like (FSTL)-3, and GDF-associated serum proteins (GASP)-1 and -2 (7). Another LC-MS/MS study demonstrated a positive correlation of the GDF-11 prodomain and its mature ligand with age, showing independent (negative) correlations with knee strength for follistatin and GASP-2, but not GDF-11 or GDF-8 (12). Among these GDF-11 and GDF-8 inhibitors, FSTL-3 seems of particular relevance to the heart. Produced by cardiomyocytes, among other cells, FSTL-3 has shown adverse effects in rodent models of cardiac injury (13–15), although benefits from its inhibition of TGF-β-superfamily ligands have also been documented (16).
To circumvent the limitations of aptamer and immunoassay techniques, we validated an LC-MS/MS method to measure GDF-11 and GDF-8 with high specificity. Using this method, together with immunoassay-based measurement of follistatin and FSTL-3, we leveraged 2 prospective older cohorts to test the hypothesis that these TGF-β-superfamily members, and their 2 main circulating antagonists, are associated with incident HF and its subtypes late in life.
Method
Study Population
Procedures for the Cardiovascular Health Study (CHS) and Health Aging and Body Composition (Health ABC) study have been described (17–19). CHS is a longitudinal study of CVD in community-living adults ≥65 years old. Participants were identified from a random sample of Medicare-eligible individuals from 4 U.S. communities (17,18). An original cohort of 5 201 participants was recruited in 1989–1990, followed in 1992–1993 by a supplemental cohort of 687 African American participants. In-person examinations were conducted annually from 1989–1990 to 1998–1999, and again in 2005–2006. Health ABC was a prospective cohort study of risk factors for physical decline in older adults (19). The study enrolled 3 075 functionally independent participants ages 70–79 years in 1997–1998 selected from Medicare-eligible White or Black residents near 2 urban centers. Repeat in-person evaluation was conducted annually for 5 years and biannually thereafter. Clinic examinations in both CHS and Health ABC included medical history, physical examination, diagnostic testing, and blood collection. Institutional Review Boards approved the study protocols for both studies, as well as for our assay validation activities. All subjects provided written informed consent.
Selection of our epidemiologic sample is detailed in Supplementary Figure 1. There were 4 842 participants who attended the 1994–1995 CHS examination. A random list of 1 600 participants from the original cohort was created, of whom 1 051 had available serum. A second random list of 650 participants was then generated, of whom 455 had available specimens, yielding a total of 1 506 participants for biomarker measurement. Of the 3 075 participants in the 1997–1998 Health ABC examination, a random list of 1 300 individuals was generated. Among these, 1 237 had available serum. After the exclusion of prevalent HF, 1 413 participants from CHS and 1 186 participants from Health ABC were included in our analytic sample.
Biochemical Profiling
We performed double-blind experiments in native (“neat”) and spiked specimens from healthy volunteers to identify an assay with maximal recovery and no cross-reactivity for serum GDF-11 and GDF-8. The LC-MS/MS method selected is described in Supplementary Material. Serum follistatin and FSTL-3 were measured by enzyme-linked immunoassay (ELISA), also described in Supplementary Material. For epidemiologic analyses, all candidate biomarkers were measured in specimens from 1994–1995 in CHS and 1997–1998 in Health ABC.
Ascertainment of HF
Participants in both CHS and Health ABC were followed semiannually through in-person or telephone contacts (20). The primary endpoint was incident HF, with HFpEF and HFrEF as secondary endpoints. Identification of HF events in CHS and Health ABC involved a common protocol (20). All potential cases were adjudicated by study investigators (18,21). Ascertainment of HF rested on physician diagnosis, symptoms and signs, pharmacotherapy, or diagnostic imaging evidence (18,21). Assessment of HF subtypes was based on documented left ventricular ejection fraction (LVEF) (20). HFrEF was assigned for LVEF < 50%, and HFpEF for LVEF ≥ 50%. Event adjudication extended to 2014 (CHS) and 2012 (Health ABC).
Cardiac Phenotyping
Methods for the 1994–1995 CHS echocardiographic evaluation and subsequent speckle-tracking strain analyses are described in Supplementary Material. In CHS, N-terminal pro-B-type natriuretic peptide (NT-proBNP) was measured by immunoassay in 1992–1993.
Covariates
Assessment of covariates was similar in CHS and Health ABC (20). In CHS, covariates came from the 1994–1995 or 1992–1993 examination, except for urine albumin/creatinine ratio, available in 1996–1997. In Health ABC, covariates from the 1997–1998 visit were used. Diabetes was defined as fasting glucose ≥126 mg/dL, nonfasting glucose ≥200 mg/dL, or use of antihyperglycemic medication. Health ABC modeled CHS approaches for the determination of coronary heart disease (CHD), defined as myocardial infarction or percutaneous/surgical revascularization; stroke, defined as ischemic and hemorrhagic cerebrovascular events; and peripheral arterial disease, defined as claudication (18,22). In Health ABC, prevalent cases were from study baseline (1997–1998), whereas in CHS, they included baseline (1989–1990) through the 1994–1995 examination (18). In both cohorts, atrial fibrillation (AF) was determined by a 12-lead electrocardiogram or ICD-9 code. Forced expiratory volume in 1 second (FEV1) was obtained by spirometry. Glomerular filtration rate was estimated from cystatin C (eGFRcys). C-reactive protein was measured by ELISA.
Statistical Analysis
Epidemiologic analyses combined individual-level data from CHS and Health ABC. Biochemical markers underwent log2 transformation to improve normality. Correlations were determined by Pearson coefficients, and geometric means compared with the Student t test. Cox models were fit to evaluate associations of biochemical exposures with HF events. All biochemical markers were modeled linearly after inspection of generalized additive model plots. Risk estimates were expressed per doubling of each biomarker in 4 sequential models. Model 1 was unadjusted. Model 2 adjusted for age, sex, race, and cohort. Model 3 additionally adjusted for body mass index (BMI), systolic blood pressure, antihypertensive medication, diabetes, current smoking, heavy alcohol use, FEV1, and prevalent CHD, stroke, claudication, and AF. Model 4 was further adjusted for eGFRcys. Sensitivity analyses additionally adjusted for estrogen replacement therapy, urine albumin/creatinine ratio, or C-reactive protein. In addition, pairwise interaction terms of biomarkers with one another, as well as age, sex, race, cohort, or eGFRcys, were included in Model 4. For secondary cross-sectional analyses of biochemical exposures with continuous cardiac phenotypes (echocardiographic measures or NT-proBNP), associations were examined with sequential linear regression models including the same covariates as above. All analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC). Given previous evidence implicating the 4 candidate biomarkers of interest in relation to the primary endpoint, we did not correct for multiple comparisons, defining significance as p < .05.
Results
Validation of GDF-11 and GDF-8 Assays
Concentrations of GDF-11 and GDF-8 in native and spiked serum determined by each of the measurement techniques are shown in Supplementary Table 1. Serum concentrations were ~1.5–8-fold higher for GDF-8 than for GDF-11. The highest protein concentrations were for the LC-MS/MS method from the Bhasin Laboratory (Boston, MA). The latter method also showed maximum recovery at both spiked concentrations, exceeding 98.1%.
Correlations of GDF-11 and GDF-8 levels recorded in native serum by each of the measurement techniques are presented in Supplementary Table 2. Correlation coefficients ranged from modest (r = 0.29) for the Bhasin LC-MS/MS method to strong (r = 0.88) for the updated Somamer GDF-11 and GDF-8 assays. As shown in Supplementary Figure 2A, pairwise comparison of GDF-11 assays revealed generally weak correlations, with the exception of the Bhasin and LeBrasseur LC-MS/MS techniques with each other (r2 = 0.76), and each with the updated Somascan assay (r2 = 0.78 and r2 = 0.65, respectively). The corresponding pairwise correlations across GDF-8 assays (Supplementary Figure 2B) were generally mild to moderate, except for that between the Bhasin LC-MS/MS method and the immunoassay, which was strong (r2 = 0.91). Comparison of the updated Somamer assays for GDF-11 and GDF-8 in both native serum and serum spiked with GDF-8 and GDF-11 yielded a similarly high correlation (r2 = 0.92; Supplementary Figure 3A) as seen in the unspiked specimens only. In turn, the original GDF-8/11 somamer assay showed values approximately twofold higher than the updated GDF-11 and GDF-8 somamers, along with moderate correlations with each (r2 = 0.53 and r2 = 0.47, respectively; Supplementary Figure 3B). Based on its high recovery and lack of meaningful cross-reactivity, the Bhasin LC-MS/MS method was selected for use in the epidemiologic cohorts.
Baseline Characteristics of Epidemiologic Sample
Participants from CHS and Health ABC included in our study sample were more often White, and exhibited less hypertension, diabetes, prevalent AF, and lung or kidney disease than their excluded counterparts (Supplementary Table 3). Their baseline characteristics are presented in Table 1. Compared with Health ABC, CHS participants were older and less often male or Black; had lower BMI, blood pressure or treatment, current smoking, measures of kidney and lung function, prevalent stroke, and prevalent claudication; and showed higher LDL cholesterol, along with more frequent prevalent CHD and AF. CHS participants had higher GDF-8, GDF-11, and FSTL-3 levels than Health ABC.
Table 1.
Baseline Characteristics of Study Sample
| Characteristics | Health ABC, n = 1 186 | CHS, n = 1 413 | Overall, n = 2 599 |
|---|---|---|---|
| Age, y | 73.4 ± 2.9 | 76.7 ± 4.7 | 75.2 ± 4.3 |
| Male, n (%) | 596 (50.3) | 567 (40.1) | 1 163 (44.8) |
| White, n (%) | 727 (61.3) | 1 351 (95.6) | 2 078 (80.0) |
| BMI, kg/m2 | 27.1 ± 4.6 | 26.4 ± 4.3 | 26.7 ± 4.4 |
| Systolic BP, mm Hg | 135 ± 19 | 132 ± 20 | 134 ± 20 |
| Antihypertensive medication, n (%) | 619 (52.5) | 678 (48.0) | 1 297 (50.0) |
| Diabetes, n (%) | 197 (16.6) | 200 (15.2) | 397 (15.9) |
| LDL cholesterol, mg/dL | 122 ± 35 | 129 ± 32 | 126 ± 34 |
| HDL cholesterol, mg/dL | 54 ± 17 | 53 ± 14 | 53 ± 15 |
| Triglycerides, mg/dL | 139 ± 80 | 145 ± 85 | 143 ± 83 |
| Statin use, n (%) | 159 (13.5) | 107 (7.6) | 266 (10.3) |
| Current smoker, n (%) | 118 (10.0) | 106 (7.6) | 224 (8.7) |
| Heavy alcohol use, n (%) | 41 (3.5) | 142 (10.1) | 183 (7.1) |
| Estrogen use (women), n (%) | 142 (12.0) | 151 (10.7) | 293 (11.3) |
| Prevalent CHD, n (%) | 212 (17.9) | 297 (21.0) | 509 (19.6) |
| Prevalent stroke, n (%) | 105 (8.9) | 70 (5.0) | 175 (6.3) |
| Prevalent AF, n (%) | 31 (2.6) | 108 (7.6) | 139 (5.4) |
| Prevalent claudication, n (%) | 56 (4.8) | 40 (2.8) | 96 (3.7) |
| eGFRcys, mL/min/1.73 m2 | 74 ± 18 | 67 ± 16 | 70 ± 18 |
| Urine albumin/creatinine ratio, mg/g | 33 ± 111 | 45 ± 237 | 39 ± 184 |
| FEV1, L | 2.22 ± 0.64 | 2.04 ± 0.63 | 2.12 ± 0.64 |
| C-reactive protein, mg/L | 2.5 ± 3.2 | 4.4 ± 8.5 | 3.6 ± 6.6 |
| GDF-11, ng/mL | 3.28 ± 0.82 | 3.39 ± 0.84 | 3.34 ± 0.83 |
| GDF-8, ng/mL | 6.73 ± 1.97 | 7.55 ± 2.20 | 7.18 ± 2.14 |
| Follistatin, ng/mL | 1.87 ± 0.88 | 1.81 ± 1.00 | 1.83 ± 0.96 |
| FSTL-3, ng/mL | 8.70 ± 2.29 | 9.62 ± 2.74 | 9.21 ± 2.59 |
| Percent predicted LV mass | NA | 112.7 ± 29.9 | NA |
| LV longitudinal strain*, % | NA | 13.1 ± 3.5 | NA |
| LV early-diastolic strain rate*, s−1 | NA | 0.58 ± 0.21 | NA |
| LA longitudinal strain*, % | NA | 37.3 ± 16.6 | NA |
| Mitral e’, cm/s | NA | 2.5 ± 0.9 | NA |
| Mitral E/e’ | NA | 32.6 ± 15.9 | NA |
| NT-proBNP, pg/mL | NA | 229 ± 360 | NA |
Notes: Bold type: p < .05. *All strain parameters reported as absolute values. AF = atrial fibrillation; BMI = body mass index; BP = blood pressure; CHD = coronary heart disease; GDF = growth differentiation factor; eGFRcys = estimated glomerular filtration rate based on cystatin C; FEV1 = forced expiratory volume in 1 s; FSTL-3 = follistatin-like 3; HDL = high density lipoprotein; LA = left atrial; LDL = low density lipoprotein; LV = left ventricular; NT-proBNP = N-terminal pro-B-type natriuretic peptide.
Biomarkers and Covariates
Cross-sectional associations are shown in Table 2. Serum GDF-11, GDF-8, follistatin, and FSTL-3 showed negligible or no correlations with one another. There were no or minimal associations of GDF-11 and GDF-8 with baseline covariates, except for GDF-8’s inverse relationship with estrogen replacement therapy and FEV1. Follistatin was positively associated with female sex, diabetes, and estrogen replacement therapy. FSTL-3 showed positive relationships with age, White race, BMI, blood pressure measures, diabetes, prevalent CVD, and inverse associations with current smoking, estrogen replacement therapy, and kidney and lung function.
Table 2.
Associations of Biomakers with Baseline Characteristics
| Characteristic | Log2 GDF-11 | Log2 GDF-8 | Log2 Follistatin | Log2 FSTL-3 | ||||
|---|---|---|---|---|---|---|---|---|
| Pearson Rho or Geometric Mean | p | Pearson Rho or Geometric Mean | p | Pearson Rho or Geometric Mean | p | Pearson Rho or Geometric Mean | p | |
| Age | 0.04 | .024 | 0.08 | <.001 | 0 | .883 | 0.3 | <.001 |
| Sex | .494 | <.001 | <.001 | .643 | ||||
| Male | 1.18 (1.17, 1.19) | 1.94 (1.93, 1.95) | 7.35 (7.33, 7.38) | 9.10 (9.08, 9.11) | ||||
| Female | 1.18 (1.17, 1.19) | 1.90 (1.88, 1.91) | 7.44 (7.42, 7.47) | 9.10 (9.09, 9.11) | ||||
| Race/ethnicity | .787 | .143 | .203 | <.001 | ||||
| White | 1.18 (1.17, 1.19) | 1.91 (1.90, 1.92) | 7.41 (7.39, 7.43) | 9.12 (9.11, 9.13) | ||||
| Black or other | 1.18 (1.17, 1.19) | 1.93 (1.91, 1.95) | 7.38 (7.34, 7.42) | 8.99 (8.97, 9.02) | ||||
| BMI | −0.01 | .775 | 0.04 | .032 | 0 | .861 | 0.16 | <.001 |
| Systolic BP | 0 | .850 | 0.03 | .122 | 0.01 | .524 | 0.10 | <.001 |
| Antihypertensive medication | .382 | .015 | .955 | <.001 | ||||
| Yes | 1.18 (1.17, 1.19) | 1.90 (1.89, 1.92) | 7.40 (7.38, 7.43) | 9.14 (9.12, 9.15) | ||||
| No | 1.18 (1.17, 1.19) | 1.93 (1.92, 1.94) | 7.40 (7.38, 7.43) | 9.06 (9.04, 9.07) | ||||
| Diabetes | .553 | .046 | .007 | .044 | ||||
| Yes | 1.18 (1.17, 1.20) | 1.94 (1.91, 1.96) | 7.45 (7.41, 7.50) | 9.12 (9.09, 9.15) | ||||
| No | 1.18 (1.17, 1.18) | 1.91 (1.90, 1.92) | 7.39 (7.37, 7.41) | 9.09 (9.08, 9.10) | ||||
| LDL cholesterol | 0.01 | .535 | 0.05 | .015 | −0.02 | .340 | −0.02 | .252 |
| HDL cholesterol | −0.03 | .122 | −0.05 | .010 | 0.15 | <.001 | −0.12 | <.001 |
| Triglycerides | −0.02 | .382 | -0.07 | <.001 | 0.08 | <.001 | 0.14 | <.001 |
| Statin use | .340 | .029 | .651 | .062 | ||||
| Yes | 1.17 (1.15, 1.19) | 1.88 (1.85, 1.91) | 7.42 (7.36, 7.47) | 9.07 (9.04, 9.10) | ||||
| No | 1.18 (1.17, 1.19) | 1.92 (1.91, 1.93) | 7.40 (7.38, 7.42) | 9.10 (9.09, 9.11) | ||||
| Current smoking | .422 | .047 | .839 | .001 | ||||
| Yes | 1.19 (1.17, 1.21) | 1.88 (1.85, 1.92) | 7.40 (7.34, 7.45) | 9.04 (9.00, 9.08) | ||||
| No | 1.18 (1.17, 1.18) | 1.92 (1.91. 1.93) | 7.40 (7.38, 7.42) | 9.10 (9.09, 9.11) | ||||
| Heavy alcohol use | .786 | .456 | .773 | .256 | ||||
| Yes | 1.18 (1.16, 1.20) | 1.93 (1.89, 1.97) | 7.41 (7.34, 7.48) | 9.12 (9.08, 9.16) | ||||
| No | 1.18 (1.17, 1.19) | 1.92 (1.90, 1.93) | 7.40 (7.38, 7.42) | 9.10 (9.09, 9.11) | ||||
| Estrogen replacement (women) | <.001 | <.001 | <.001 | <.001 | ||||
| Yes | 1.15 (1.3, 1.16) | 1.82 (1.79, 1.85) | 7.75 (7.69, 7.82) | 9.04 (9.02, 9.07) | ||||
| No | 1.18 (1.18, 1.19) | 1.93 (1.92, 1.94) | 7.36 (7.35, 7.38) | 9.11 (9.09, 9.12) | ||||
| Prevalent CHD | .114 | .942 | .093 | <.001 | ||||
| Yes | 1.19 (1.8, 1.20) | 1.92 (1.90, 1.94) | 7.38 (7.34, 7.41) | 9.16 (9.13, 9.18) | ||||
| No | 1.18 (1.17, 1.18) | 1.92 (1.90, 1.93) | 7.41 (7.39. 7.43) | 9.08 (9.07, 9.09) | ||||
| Prevalent stroke | .857 | .043 | .089 | .001 | ||||
| Yes | 1.18 (1.16, 1.20) | 1.88 (1.94, 1.92) | 7.46 (7.39, 7.52) | 9.17 (9.12, 9.21) | ||||
| No | 1.18 (1.17, 1.19) | 1.92 (1.91, 1.93) | 7.40 (7.38, 7.42) | 9.09 (9.08, 9.10) | ||||
| Prevalent AF | .488 | .746 | .800 | <.001 | ||||
| Yes | 1.19 (1.17, 1.21) | 1.92 (1.89, 1.96) | 7.41 (7.34, 7.48) | 9.22 (9.18, 9.26) | ||||
| No | 1.18 (1.17, 1.19) | 1.92 (1.91, 1.93) | 7.40 (7.38, 7.42) | 9.09 (9.08, 9.10) | ||||
| Prevalent claudication | .461 | .919 | .578 | .050 | ||||
| Yes | 1.19 (1.16, 1.22) | 1.92 (1.86, 1.98) | 7.43 (7.34, 7.52) | 9.16 (9.10, 9.23) | ||||
| No | 1.18 (1.7, 1.19) | 1.92 (1.91, 1.93) | 7.40 (7.38, 7.42) | 9.10 (9.09, 9.11) | ||||
| eGFRcys | -0.03 | .082 | -0.02 | 0.248 | -0.04 | 0.072 | -0.64 | <.001 |
| Urine albumin/creatinine ratio | 0.04 | .054 | 0.02 | .425 | 0.03 | .280 | 0.18 | <0.001 |
| FEV1 | −0.08 | <.001 | −0.20 | <.001 | 0.05 | .029 | −0.18 | <.001 |
| C-reactive protein | −0.01 | .745 | −0.03 | .111 | 0.06 | .007 | 0.11 | <.001 |
| Log2 GDF-8 | 0.10 | <.001 | — | −0.09 | <.001 | 0.04 | .027 | |
| Log2 GDF-11 | — | 0.10 | <.001 | -0.04 | .094 | 0.04 | .045 | |
| Log2 Follistatin | −0.04 | .094 | −0.09 | <.001 | — | 0.05 | .014 | |
| Log2 FSTL-3 | 0.04 | .045 | 0.04 | .027 | 0.05 | .014 | — | |
Notes: AF = atrial fibrillation; BMI = body mass index; BP = blood pressure; CHD = coronary heart disease; GDF = growth and differentiation factor; eGFRcys = estimated glomerular filtration rate based on cystatin C; FEV1 = forced expiratory volume in 1 s; FSTL-3 = follistatin-like 3; HDL = high density lipoprotein; LDL = low density lipoprotein.
Biomarkers and Incident HF
During the mean follow-up of 10.5 ± 6.2 years in CHS, there were 483 HF events (169 [35.0%] HFpEF, 128 [26.5%] HFrEF, 186 [31.5%] unclassified). Over the mean follow-up of 11.3 ± 4.7 years in Health ABC, 238 HF events occurred (81 [34.0%] HFpEF, 91 [38.2%] HFrEF, 66 [27.4%] unclassified). Overall, this resulted in 721 incident HF events (250 HFpEF and 219 HFrEF) over 10.8 ± 5.6 years. Age-adjusted incidence rates of HF were 29.5 (95% confidence interval [CI]: 26.6, 32.3) and 17.8 (95% CI: 15.3, 20.3) per 1 000 person-years in CHS and Health ABC, respectively.
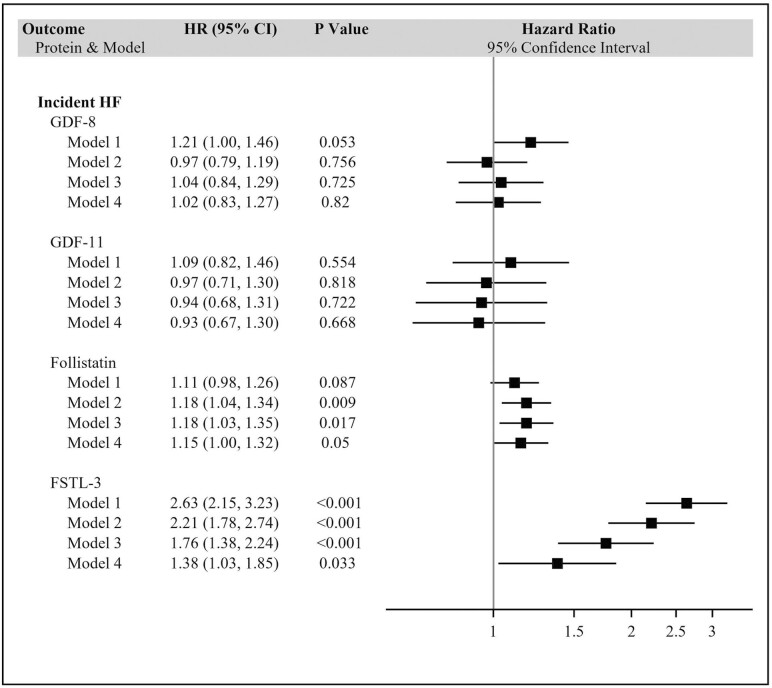
The associations of biomarkers with HF and HF subtypes are given in Figure 1 and Supplementary Figure 4, respectively. Serum levels of GDF-11 or GDF-8 showed no significant associations with incident HF or its subtypes in any of the models. By contrast, follistatin showed a significant association with incident HF in the minimally adjusted model (Model 2), which became marginally significant after full adjustment. Specifically, each doubling in follistatin level was associated with a 15% (95% CI, 0%–32%) higher risk of HF. There was no significant association, however, with HFpEF or HFrEF. FSTL-3 also showed a significant association with incident HF, one that was present in both unadjusted and minimally adjusted models, and was attenuated but remained significant after serial adjustment. After full adjustment (Model 4), each doubling of FSTL-3 was associated with a 38% (95% CI: 3%–85%) increase in risk of HF. This significant association extended to HFpEF—but not HFrEF—for which there was a 77% (95% CI: 3%–202%) higher incidence per doubling of FSTL-3 in the fully adjusted model. Associations were not meaningfully altered by additional adjustment for estrogen replacement therapy, urine albumin/creatinine ratio, or C-reactive protein. Concurrent inclusion of all 4 exposure biomarkers in Model 4 did not alter the relationship of FSTL-3 with incident HF, but that for follistatin was attenuated and no longer significant (p = .058).
Figure 1.
Associations of GDF-11, GDF-8, follistatin, and FSTL-3 with incident heart failure. *Per doubling. Model 1: unadjusted. Model 2: adjusted for age, sex, race and cohort. Model 3: additionally adjusted for BMI, systolic blood pressure, antihypertensive medication, diabetes, current smoking, heavy alcohol use, FEV1, CHD, stroke, claudication, and atrial fibrillation. Model 4: additionally adjusted for eGFRcys. BMI = body mass index CHD = coronary heart disease; GDF = growth and differentiation factor; eGFRcys = estimated glomerular filtration rate based on cystatin C; FEV1 = forced expiratory volume in 1 s; FSTL-3 = follistatin-like 3.
There was no evidence for the interaction of GDF-11, GDF-8, or follistatin with one another or with age, sex, race, cohort, or eGFRcys in relation to HF (all p ≥ .065). FSTL-3 did show evidence of interaction with follistatin (and vice versa), as well as sex, race, and eGFRcys. As illustrated in Supplementary Table 4, the effect modification of FSTL-3 was such that its association with HF was stronger for men, Black participants, lower eGFRcys, and higher levels of follistatin. The associations for follistatin were also stronger at higher levels of FSTL-3.
Cardiac Phenotypes
Cross-sectional relationships of biomarkers with intermediate phenotypes in CHS are shown in Table 3. GDF-11 and GDF-8 were largely unrelated to echocardiographic measures, with the exception of a positive association between GDF-8 and medial e’ after full adjustment. In the demographically adjusted models (Model 2), FSTL-3 showed a positive association with E/e’, together with inverse associations with LV longitudinal strain, LV early-diastolic strain rate, and medial e’—as did follistatin for medial e’. These associations were attenuated and ceased to be significant after full adjustment. In turn, neither GDF-11 nor GDF-8 was associated with NT-proBNP concentration at any level of adjustment, but follistatin and FSTL-3 did exhibit positive associations with this marker of cardiac stretch. The latter associations showed attenuation with adjustment, particularly in the case of FSTL-3, but both remained significant in Model 4, such that each doubling of follistatin and FSTL-3 was associated with 9.4% (1.0%–18.5%) and 28.4% (7.3%–53.7%) increases in plasma NT-proBNP level.
Table 3.
Cross-Sectional Associations of GDF-11, GDF-8, FST, and FSTL-3 With Cardiac Phenotypes (CHS Only)
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| β* (95% CI) | p | β* (95% CI) | p | β* (95% CI) | p | β* (95% CI) | p | |
| LVLS | ||||||||
| GDF-11 | 0.58 (−0.77, 1.38) | .436 | 0.68 (−0.67, 1.45) | .32 | 0.49 (−0.91, 1.38) | .57 | 0.59 (−0.85, 1.46) | .47 |
| GDF-8 | 0.43 (−0.67, 1.11) | .494 | 0.58 (−0.52, 1.21) | .29 | 0.52 (−0.63, 1.19) | .4 | 0.52 (−0.62, 1.2) | .39 |
| FST | 0.39 (−0.34, 0.81) | .283 | 0.16 (−0.53, 0.66) | .76 | 0.41 (−0.36, 0.84) | .29 | 0.36 (−0.41, 0.81) | .38 |
| FSTL-3 | −1.37 (−1.81, −0.85) | <.001 | −1.32 (−1.78, −0.76) | <.001 | −0.77 (−1.38, 0.35) | .14 | −0.31 (−1.19, 0.95) | .75 |
| LVDSR | ||||||||
| GDF-11 | 0.06 (−0.13, 0.18) | .631 | 0.08 (−0.12, 0.19) | .479 | 0.1 (−0.11, 0.22) | .38 | 0.12 (−0.09, 0.24) | .25 |
| GDF-8 | 0.04 (−0.11, 0.15) | .704 | 0.06 (−0.09, 0.16) | .465 | 0.05 (−0.11, 0.15) | .66 | 0.05 (−0.11, 0.16) | .64 |
| FST | 0.05 (−0.06, 0.11) | .363 | 0.02 (−0.08, 0.09) | .788 | 0.04 (−0.07, 0.11) | .54 | 0.04 (−0.07, 0.11) | .61 |
| FSTL-3 | −0.17 (−0.24, −0.09) | .001 | −0.14 (−0.21, −0.03) | .024 | −0.05 (−0.16, 0.11) | .61 | 0.05 (−0.14, 0.17) | .74 |
| LARS | ||||||||
| GDF-11 | −2.03 (−4.34, 2.03) | .327 | −1.99 (−4.31, 2.06) | .339 | −2.31 (−4.72, 2) | .28 | −2.01 (−4.57, 2.37) | .39 |
| GDF-8 | 0.91 (−2.28, 3.07) | .681 | 0.91 (−2.29, 3.07) | .682 | 0.51 (−2.62, 2.96) | .87 | 0.65 (−2.57, 3.04) | .81 |
| FST | 0.9 (−1.3, 2.22) | .469 | 1.1 (−1.13, 2.38) | .334 | 0.72 (−1.54, 2.19) | .63 | 0.55 (−1.67, 2.12) | .74 |
| FSTL-3 | −2.17 (−3.8, 0.81) | .115 | −2.11 (−3.83, 1.04) | .15 | 0.99 (−2.39, 3.26) | .67 | 1.76 (−2.26, 4.14) | .42 |
| Medial e’ | ||||||||
| GDF-11 | −0.05 (−0.45, 0.42) | .929 | −0.07 (−0.45, 0.41) | .892 | −0.07 (−0.48, 0.44) | .9 | 0.05 (−0.45, 0.48) | .94 |
| GDF-8 | 0.37 (0.05, 0.58) | 0.037 | 0.34 (−0.05, 0.56) | 0.064 | 0.37 (0.02, 0.59) | 0.05 | 0.38 (0.05, 0.6) | 0.04 |
| FST | −0.24 (−0.38, −0.01) | 0.047 | −0.18 (−0.34, 0.11) | 0.018 | −0.17 (−0.34, 0.13) | 0.24 | −0.17 (−0.34, 0.14) | 0.26 |
| FSTL-3 | −0.46 (−0.64, −0.23) | 0.002 | −0.44 (−0.63, −0.19) | 0.005 | −0.31 (−0.55, 0.12) | 0.12 | 0.04 (−0.41, 0.43) | 0.95 |
| E/e’ | ||||||||
| GDF-11 | −0.23 (−3.26, 3.16) | .965 | 0.07 (−3.16, 3.18) | .994 | −0.68 (−3.58, 3.11) | .85 | −1.11 (−3.85, 2.92) | .7 |
| GDF-8 | −1.28 (−3.21, 1.9) | .48 | −0.84 (−2.91, 2.2) | .698 | −1.93 (−3.7, 1.3) | .21 | −2 (−3.77, 1.22) | .19 |
| FST | 1.6 (−0.37, 2.69) | .081 | 1.07 (−1.07, 2.29) | .327 | 1.26 (−0.91, 2.47) | .23 | 1.13 (−1.08, 2.39) | .31 |
| FSTL-3 | 4.16 (2.71, 5.41) | <.001 | 3.58 (1.91, 4.94) | .001 | 2.95 (0.73, 4.54) | .02 | 1.84 (−1.95, 4.02) | .35 |
| ppLVM | ||||||||
| GDF-11 | 3.09 (−3.18, 6.69) | 0.337 | 3.17 (−3.07, 6.72) | 0.317 | 3.19 (−3.33, 6.93) | 0.34 | 2.91 (−3.62, 6.75) | 0.41 |
| GDF-8 | −0.41 (−4.28, 4.08) | 0.946 | −1.28 (−4.69, 3.64) | 0.723 | 0.58 (−4.24, 4.57) | 0.92 | 0.76 (−4.18, 4.66) | 0.88 |
| FST | −2.64 (−4.27, −0.06) | 0.049 | −2.24 (−3.96, 0.93) | 0.127 | −2.67 (−4.43, 0.48) | 0.07 | −2.59 (−4.39, 0.66) | 0.09 |
| FSTL-3 | 3.69 (−0.79, 6.19) | 0.077 | 2.19 (−2.87, 5.2) | 0.428 | 2.21 (−3.26, 5.53) | 0.48 | 2.03 (−4.04, 5.93) | 0.6 |
| Ln NT-proBNP† | ||||||||
| GDF-11 | −0.02 (−0.23, 0.19) | 0.827 | −0.04 (−0.24, 0.16) | 0.683 | −0.14 (−0.34, 0.07) | 0.193 | −0.14 (−0.34, 0.05) | 0.153 |
| GDF-8 | −0.02 (−0.18, 0.14) | .804 | 0.02 (−0.13, 0.17) | .822 | 0.06 (−0.09, 0.21) | .446 | 0.07 (−0.08, 0.22) | .349 |
| FST | 0.13 (0.04, 0.21) | .004 | 0.09 (0.01, 0.17) | .027 | 0.09 (0.01, 0.17) | .036 | 0.09 (0.01, 0.17) | .031 |
| FSTL-3 | 0.71 (0.57, 0.85) | <.001 | 0.49 (0.35, 0.63) | <.001 | 0.52 (0.36, 0.67) | <.001 | 0.25 (0.07, 0.43) | .007 |
Notes: Model 1: unadjusted. Model 2: adjusted for age, sex, and race. Model 3: adjusted for Model 2 covariates plus body mass index, systolic blood pressure, antihypertensive medication, diabetes, current smoking, heavy alcohol use, FEV1, prevalent CHD, prevalent stroke, prevalent PAD, prevalent AF. Model 4: adjusted for Model 3 covariates plus eGFRcys. FST = follistatin; FSTL-3 = follistatin-like 3; LARS = left atrial reservoir strain; Ln = natural logarithm; LVDSR = left ventricular early-diastolic strain rate; LVLS = left ventricular longitudinal strain; NT-proBNP = N-terminal pro-B-type natriuretic peptide; ppLVM = percent predicted left ventricular mass.
*Per doubling.
†To interpret β coefficients for natural-log transformed NT-proBNP, exponentiating of the coefficient gives the ratio of the geometric means of NT-proBNP associated with each doubling of the biomarker in question. For example, for the FST effect estimate in Model 4, e0.09 = 1.094, representing a 9.4% increase in NT-proBNP per doubling in FST. Similarly, for the FSTL-3 risk estimate in Model 4, e0.25 = 1.284, or a 28.4% increase in NT-proBNP, per doubling in FSTL-3.
Discussion
Main Findings
Based on comparison of different laboratory techniques for the measurement of GDF-11 and GDF-8, we selected an LC-MS/MS method demonstrating superior accuracy and specificity for the evaluation of these biomarkers in the epidemiologic setting. In analyses involving 2 prospective studies of older adults, we did not find significant associations for serum GDF-11 or GDF-8 measured by this LC-MS/MS technique with incident HF or its subtypes. Nor did either biomarker exhibit consistent cross-sectional associations with cardiac phenotypes available in one of the cohorts. Instead, there were near-significant or significant associations between serum follistatin and FSTL-3, assessed using standard immunoassays, and incident HF. In the case of FSTL-3, this was also seen in relation to HFpEF, but not HFrEF, and cross-sectionally with circulating NT-proBNP. There was also a suggestion of effect modification, such that associations for FSTL-3 and HF were stronger at higher follistatin levels (and vice versa), as well as in men, Black participants, and at lower eGFRcys.
Findings in Context
Prior investigations of GDF-11 and GDF-8 in relation to HF, whether jointly with follistatin and FSTL-3 or not, have relied on aptamer-based methods (10,23–25). This technique fails to differentiate GDF-11 from GDF-8 (10) and predominantly reflects GDF-8, whose blood concentrations are considerably higher than GDF-11—findings replicated here for the original GDF-8/11 assay, as well as for second-generation GDF-8 and GDF-11 aptamers. Hence, the findings documented in an initial study (10) that GDF-8/11 levels decrease with age, and show inverse associations with CVD outcomes, could mostly relate to the biology of GDF-8 levels. Accumulating evidence from studies with a broad age span, unlike our own, supports the concept that GDF-8 levels decline with age in response to, and not as a cause of, decreasing skeletal muscle mass (11). Accordingly, because skeletal muscle decline is a risk factor for adverse events, and particularly HF (26), such findings could relate to GDF-8’s serving as a marker of such decline, rather than to its, or GDF-11’s, direct contributions to HF or CVD pathogenesis.
In aptamer-based proteomic analyses in 2 different prospective middle-aged to older cohorts (24,25), GDF-8/11 emerged as a significant hit for overall HF in the first study of White participants (24), with an inverse association consistent with the original findings (10). No association of GDF-8/11 with overall HF was seen in the second Black population (25), but secondary analyses of HF subtypes revealed divergent associations—inverse for HFpEF but positive for HFrEF—that require replication. In a subsequent study (23) that evaluated co-regulated networks in the aptamer-measured blood proteome in relation to cardiometabolic outcomes in elders, a protein module containing GDF-8/11 showed no association with HF. Neither did another module containing follistatin, but a separate module in which FSTL-3 was identified as a hub protein did exhibit a significant relationship with prevalent and incident HF. Individually, FSTL-3 showed a positive association with prevalent HF and mortality (though not incident HF), but models were not adjusted for clinical risk factors. Another study (27) identified a positive relationship between follistatin and incident HF in elders after extensive adjustment for covariates, including eGFR.
To our knowledge, this is the first prospective study to evaluate serum GDF-11 and GDF-8 in relation to HF and its subtypes using a gold-standard technique of LC-MS/MS, and to do so alongside immunoassay measurements of their major antagonists, follistatin and FSTL-3. The observation that neither GDF-11 nor GDF-8 was associated with incident HF is consistent with findings from a cross-sectional study of skeletal muscle strength in elders (12). Using selective reaction monitoring LC-MS/MS, the latter study failed to detect independent relationships of GDF-11 and GDF-8 with quadriceps strength.
By contrast, another study employing immunoplexed LC-MS/MS found that in patients with severe aortic stenosis, GDF-11 levels were associated with greater frailty, diabetes, and adverse outcomes (11). The latter technique differs from the LC-MS/MS method (28) selected for our epidemiologic investigations in its use of antibodies for immunofixation of TGF-β-superfamily members and antagonists, whose recognition of exposed epitopes on the circulating complex of GDF-11 and its antagonists is a key determinant of ligand measurement. Another distinction is that the immunoplexed LC-MS/MS method used acid, and not urea-based, denaturation to achieve dissociations of noncovalent binding antagonists, including the prodomain. Thus, detected levels in the prior study would have been confined to captured forms of GDF-11 and GDF-8 complexes, and not total mature protein with and without its prodomain. Indeed, the higher recovery demonstrated for the immunofixation-free method, together with higher protein concentrations observed in native serum, supports the capture of total protein levels instead, since recombinant protein was allowed to equilibrate in serum to generate all GDF-11 (and GDF-8) forms (inactive pro-complex, inactive latent complex, active ligand) before measurement.
Notably, native serum concentrations and recovery of spiked recombinant protein were also higher for our LC-MS/MS technique than for the somamer assays. This suggests that, apart from their lack of specificity, these somamer assays do not capture total mature protein for GDF-11 or GDF-8. Such technical differences between assays likely account in large measure for differences in results based on the LC-MS/MS method used here (28), and those involving immunoplexed LC-MS/MS (11) or somamer-based (10,24,25) measurements reported previously. Yet earlier studies included distinct populations and, in the case of the immunoplexed LC-MS/MS method, outcomes, which may have contributed to the different findings. As relates to the inverse associations with HF of aptamer-based GDF-8/11 (10,24), these were observed in younger populations with a broader age range than ours, which may have provided a greater dynamic range of protein concentrations for their pathobiological consequences to become manifest. Because the aptamer GDF-8/11 assay in question was not measured in our epidemiologic cohorts, we are not able to test the latter premise here.
Potential Mechanisms
Unlike GDF-11 and GDF-8, follistatin and FSTL-3 did show positive associations with HF, and NT-proBNP, a marker of cardiac stretch. The 2 TGF-β-superfamily antagonists share structural and functional homology, but have notable differences. FSTL-3 lacks a heparin-binding site, and therefore functions to inhibit TGF-β-superfamily ligands in an endocrine/paracrine fashion, whereas follistatin can also do this in an autocrine fashion (29). Follistatin and FSTL-3 inhibit GDF-11, GDF-8, and activin A, though follistatin also inhibits certain bone morphogenetic proteins. Their tissue distributions are wide ranging but do differ, with follistatin expressed especially in ovary, pituitary, and kidney, while FSTL-3 is expressed particularly in cardiac muscle and testis (30). These differences in mode of action, protein ligands, and tissue distribution could explain the interaction observed between the 2 factors to amplify HF risk.
As potential explanations for their associations with HF, follistatin and FSTL-3 have each been implicated in the regulation of glucose metabolism and inflammatory responses. Knockout of FSLT-3 in mice leads to greater pancreatic islet number and size, improved glucose tolerance, and increased liver steatosis (31), whereas inactivation of follistatin in mouse liver alleviates hyperglycemia (32). In humans, both follistatin and FSTL-3 are associated with age and adiposity (33), an association seen only for FSTL-3 here. They are also associated with inflammation, which upregulates follistatin (34) and FSTL-3 (35) levels to counter the pro-inflammatory actions of activin. In the case of follistatin, bariatric surgery produces a decline in circulating levels, which are associated with subsequent improvement in insulin sensitivity (36). In our analyses, cross-sectional associations with diabetes and C-reactive protein were seen for FSTL-3, but not follistatin. Only diabetes attenuated FSTL-3’s association with HF, suggesting a contribution from the latter disorder.
The relationship documented for these molecules, and especially FSTL-3, likely also reflects their prominent roles in the heart (37). A number of animal models have shown deleterious effects of FSTL-3 on cardiac phenotypes (13–15), although such studies have not involved detailed assessments of cardiac function. More recently, mouse experiments confirmed that cardiac FSTL-3 expression is upregulated by engagement of the activin type II receptor (ActIIR) by TGF-β-superfamily ligands, particularly activin A (16). Both activin A and GDF-11 stimulation of ActIIR was shown to decrease cardiac muscle mass, but, contrary to prior reports, GDF-11 also adversely affected cardiac function and failed to rescue HF phenotypes in different models. Moreover, activin A, GDF-11, and GDF-8 binding of ActIIR led to transcriptional activation of a range of pathways characteristic of the HF phenotype (16). The same study also reported higher activin A and FSTL-3 levels, but lower aptamer-based GDF-8/11 levels, with age in a human cohort, and linked higher activin A and FSTL-3 to HF severity and frailty in patients with aortic stenosis. Together, these findings led to the propostion that activation of ActIIR signaling is the operative deleterious pathway in the heart, whether for activin A or GDF-11 (16). According to this premise, FSLT-3 increases are secondary, and not primary, drivers of cardiac dysfunction. This is supported by some (13,14), though not all (15), prior experimental results. Given the lack of corresponding human tissue, an additional study will be needed to define the mechanisms responsible in the clinical setting.
Another consideration is the role of kidney function. Although both follistatin and FSTL-3 are filtered at the glomerulus, the former’s binding of cell surface glycosaminoglycans affords it protection from renal excretion. By contrast, FSTL-3 is known to be strongly inversely related to eGFR, as seen here (38). Indeed, eGFR substantially attenuated the association for FSTL-3, suggesting that the relationship relates considerably to reduced elimination by the kidney. There was also evidence of effect modification, such that the association was more pronounced at lower eGFR, though this was only of nominal statistical significance. It is nonetheless possible that the FSTL-3 relation to HF and HFpEF may not solely be a marker of diminished elimination with CKD, but that higher levels could themselves have deleterious effects. Rodent models have shown that in the setting of kidney disease, there is upregulation of FSTL-3 transcription in the liver (38), which could further contribute to the rising concentration and have adverse consequences. The pathobiology of FSTL-3 in CKD merits further investigation.
The explanation for observed effect modification of FSTL-3 by sex and race is uncertain. p values for interaction were only nominally significant in the context of multiple testing, and similar effect modification was not observed for follistatin. The stronger associations suggested for men and Black participants could relate to differences in various risk factors with which FSTL-3 was associated, other features, or the play of chance. Also uncertain is the basis for FSTL-3’s association with the HFpEF subtype. The links between FSTL-3 and metabolic dysregulation, inflammation, and kidney disease make it more likely to reflect or contribute to the pathobiology of this subphenotype, which bears an especially close relationship with multiple aging-related comorbidities (2). This secondary finding will require additional study.
Implications
Our results involving specific measures of GDF-11 and GDF-8, assessed by a newly developed LC-MS/MS technique that achieves superior GDF-11 and GDF-8 recovery and specificity than tested LC-MS/MS, aptamer, and immunoassay methods, contribute novel and important information. Contrary to earlier findings from heterochronic parabiosis (3), they show that total circulating levels of neither GDF-11 nor GDF-8 exhibit meaningful age-related declines in older adults, nor are these levels associated with incident HF. Our LC-MS/MS technique cannot directly measure the mature proteins or their local activity, an assessment that requires direct analysis of target tissues. However, the present findings provide solid population-level evidence against the concept that circulating GDF-11 deficiency, as defined by the aggregate of circulating GDF-11 forms, is a risk factor for HF incidence in older human populations.
By contrast, follistatin and, especially, FSTL-3, major circulating antagonists of TGF-β-superfamily ligands, did show positive associations with HF incidence. The finding for follistatin is consistent with the previously reported association with HF, while that for FSTL-3 newly shows that this extends to incident HF, is independent of potential confounders or putative mediators, and applies particularly to HFpEF. Because the functions of follistatin and FSTL-3 do not depend on a tissue-level activation step, and because they integrate inhibition against a number of TGF-β-superfamily members, their circulating levels likely represent a better measure of TGF-β superfamily-related pathway activity. We are unable to determine here whether these homologs are markers or drivers of adverse effects. As noted, however, animal studies have shown that elevated FSTL-3 levels in large measure reflect upregulation by activin A, suggesting that FSTL-3 (and, likewise, follistatin) primarily indicate activation of TGF-β-superfamily pathways. This is consistent with lack of correlation of FSTL-3 and follistatin with total GDF-11 or GDF-8 levels here, although confirmation of this concept in humans will require further studies of relevant tissues.
Limitations
The target biomarkers were measured in subsets from CHS and Health ABC that were more commonly White and generally healthier than the remainder of participants in these cohorts. As such, our findings are not necessarily generalizable to all older adults, nor to younger or race/ethnically distinct populations. Because of compelling prior probabilities, we did not correct for multiple comparisons, justifying our tradeoff of a potentially higher false-positive rate for a lower false-negative rate; our findings should be interpreted in this context. Specimen collection in the CHS 1994–1995 exam was nonfasting, which may have led to misclassification specifically for follistatin levels, which are known to be suppressed by (post-prandial) insulin. There was no evidence, however, that this affected the associations of interest relative to Health ABC, where fasting specimens were obtained. There was a substantial proportion of HF cases that could not be subphenotyped, limiting our secondary analysis. Last, we did not measure levels of the GDF-11 and GDF-8 antagonists GASP-1 or GASP-2, nor of activin A, and cannot assess their roles here.
Conclusion
Among older adults, total serum levels of GDF-11 and GDF-8 showed no association with incident HF, but their circulating antagonists, follistatin and FSTL-3, were associated with increased risk of this outcome—and, in the case of FSTL-3, HFpEF. Based on enhanced measurements by LC-MS/MS, the present findings do not support a role for a decline in total GDF-11 levels as an important risk factor for HF in elders. The positive associations for follistatin and FSTL-3 implicate TGF-β-superfamily pathways in the development of HF late in life. Whether these are markers or mediators of new-onset HF, and the mechanisms involved, will require further study.
Supplementary Material
Contributor Information
Jorge R Kizer, Cardiology Section, San Francisco Veterans Affairs Health Care System, San Francisco, California, USA; Department of Medicine, University of California San Francisco, San Francisco, California, USA; Department of Epidemiology and Biostatistics, University of California San Francisco, San Francisco, California, USA.
Sheena Patel, Research Institute, California Pacific Medical Center, San Francisco, California, USA.
Peter Ganz, Department of Medicine, University of California San Francisco, San Francisco, California, USA; Cardiology Division, Zuckerberg San Francisco General Hospital, San Francisco, California, USA.
Anne B Newman, Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Shalender Bhasin, Research Program in Men’s Health: Aging and Metabolism, Boston Claude D. Pepper Older Americans Independence Center, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Se-Jin Lee, The Jackson Laboratory and University of Connecticut School of Medicine, Farmington, Connecticut, USA.
Peggy M Cawthon, Department of Epidemiology and Biostatistics, University of California San Francisco, San Francisco, California, USA; Research Institute, California Pacific Medical Center, San Francisco, California, USA.
Nathan K LeBrasseur, Robert and Arlene Kogod Center on Aging, and Department of Physical Medicine and Rehabilitation, Mayo Clinic, Rochester, Minnesota, USA.
Sanjiv J Shah, Division of Cardiology, Department of Medicine, Northwestern University School of Medicine, Chicago, Illinois, USA.
Bruce M Psaty, Cardiovascular Health Research Unit, Departments of Medicine, Epidemiology, and Health Systems and Population Health, University of Washington, Seattle, Washington, USA.
Russell P Tracy, Department of Pathology and Laboratory Medicine, University of Vermont Larner College of Medicine, Burlington, Vermont, USA.
Steven R Cummings, Department of Epidemiology and Biostatistics, University of California San Francisco, San Francisco, California, USA; Research Institute, California Pacific Medical Center, San Francisco, California, USA.
Roger Fielding, (Medical Sciences Section).
Funding
The present research was supported by R01 AG052964 from the National Institute on Aging (NIA) to J.R.K., P.G., A.B.N., R.P.T., and S.R.C. The Cardiovascular Health Study (C.H.S.) was supported by R01 AG053325 from the NIA; and by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, 75N92021D00006, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the NIA. A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. Health ABC was supported by NIA Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, and National Institute of Nursing Research grant R01-NR012459. The research was funded in part by the Intramural Research Program of the NIH, NIA.
Conflict of Interest
J.R.K. discloses stock ownership in AbbVie, Abbott, Bristol-Myers Squibb, Johnson & Johnson, Medtronic, Merck and Pfizer. S.B. reports receiving research grants from AbbVie, Metro International Technology, Transition Therapeutics, and Function Promoting Therapies, LLC; equity interest in Function Promoting Therapies, LLC; and personal consulting fees from OPKO and Aditum. B.M.P. serves on the Steering Committee of the Yale Open Data Access Project funded by Johnson & Johnson. P.M.C. and S.R.C. receive consulting fees from BioAge. S.J.S. has received research grants from Actelion, AstraZeneca, Corvia, Novartis, and Pfizer and has received consulting fees from Abbott, Actelion, AstraZeneca, Amgen, Aria CV, Axon Therapies, Bayer, Boehringer-Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardiora, Coridea, CVRx, Cyclerion, Cytokinetics, Edwards Lifesciences, Eidos, Eisai, Imara, Impulse Dynamics, Intellia, Ionis, Ironwood, Lilly, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Prothena, Regeneron, Rivus, Sanofi, Shifamed, Tenax, Tenaya, and United Therapeutics. The other authors declare no conflict of interest.
References
- 1. Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation. 2022;145:e153–e639. 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 2. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:CIR0000000000001063. 10.1161/CIR.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 3. Loffredo FS, Steinhauser ML, Jay SM, et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. 10.1016/j.cell.2013.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Egerman MA, Cadena SM, Gilbert JA, et al. GDF11 increases with age and inhibits skeletal muscle regeneration. Cell Metab. 2015;22:164–174. 10.1016/j.cmet.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith SC, Zhang X, Zhang X, et al. GDF11 does not rescue aging-related pathological hypertrophy. Circ Res. 2015;117:926–932. 10.1161/CIRCRESAHA.115.307527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harper SC, Johnson J, Borghetti G, et al. GDF11 decreases pressure overload-induced hypertrophy, but can cause severe cachexia and premature death. Circ Res. 2018;123:1220–1231. 10.1161/CIRCRESAHA.118.312955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walker RG, Poggioli T, Katsimpardi L, et al. Biochemistry and biology of GDF11 and myostatin: similarities, differences, and questions for future investigation. Circ Res. 2016;118:1125–1141; discussion 1142. 10.1161/CIRCRESAHA.116.308391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harper SC, Brack A, MacDonnell S, et al. Is growth differentiation factor 11 a realistic therapeutic for aging-dependent muscle defects? Circ Res. 2016;118:1143–1150; discussion 1150. 10.1161/CIRCRESAHA.116.307962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Poggioli T, Vujic A, Yang P, et al. Circulating growth differentiation factor 11/8 levels decline with age. Circ Res. 2016;118:29–37. 10.1161/CIRCRESAHA.115.307521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Olson KA, Beatty AL, Heidecker B, et al. Association of growth differentiation factor 11/8, putative anti-ageing factor, with cardiovascular outcomes and overall mortality in humans: analysis of the Heart and Soul and HUNT3 cohorts. Eur Heart J. 2015;36:3426–3434. 10.1093/eurheartj/ehv385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schafer MJ, Atkinson EJ, Vanderboom PM, et al. Quantification of GDF11 and myostatin in human aging and cardiovascular disease. Cell Metab. 2016;23:1207–1215. 10.1016/j.cmet.2016.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Semba RD, Zhang P, Zhu M, et al. Relationship of circulating growth and differentiation factors 8 and 11 and their antagonists as measured using liquid chromatography-tandem mass spectrometry with age and skeletal muscle strength in healthy adults. J Gerontol A Biol Sci Med Sci. 2019;74:129–136. 10.1093/gerona/gly255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oshima Y, Ouchi N, Shimano M, et al. Activin A and follistatin-like 3 determine the susceptibility of heart to ischemic injury. Circulation. 2009;120:1606–1615. 10.1161/CIRCULATIONAHA.109.872200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shimano M, Ouchi N, Nakamura K, et al. Cardiac myocyte-specific ablation of follistatin-like 3 attenuates stress-induced myocardial hypertrophy. J Biol Chem. 2011;286:9840–9848. 10.1074/jbc.M110.197079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Panse KD, Felkin LE, Lopez-Olaneta MM, et al. Follistatin-like 3 mediates paracrine fibroblast activation by cardiomyocytes. J Cardiovasc Transl Res. 2012;5:814–826. 10.1007/s12265-012-9400-9 [DOI] [PubMed] [Google Scholar]
- 16. Roh JD, Hobson R, Chaudhari V, et al. Activin type II receptor signaling in cardiac aging and heart failure. Sci Transl Med. 2019;11:eaau8680. 10.1126/scitranslmed.aau8680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. 10.1016/1047-2797(91)90005-w [DOI] [PubMed] [Google Scholar]
- 18. Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. 10.1016/1047-2797(94)00093-9 [DOI] [PubMed] [Google Scholar]
- 19. Simonsick EM, Newman AB, Nevitt MC, et al. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001;56:M644–M649. 10.1093/gerona/56.10.m644 [DOI] [PubMed] [Google Scholar]
- 20. Zhang L, Bartz TM, Santanasto A, et al. Body composition and incident heart failure in older adults: results from 2 prospective cohorts. J Am Heart Assoc. 2022;11:e023707. 10.1161/JAHA.121.023707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Butler J, Kalogeropoulos A, Georgiopoulou V, et al. ; Health ABC Study. Incident heart failure prediction in the elderly: the Health ABC heart failure score. Circ Heart Fail. 2008;1:125–133. 10.1161/CIRCHEARTFAILURE.108.768457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cesari M, Penninx BW, Newman AB, et al. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108:2317–2322. 10.1161/01.CIR.0000097109.90783.FC [DOI] [PubMed] [Google Scholar]
- 23. Emilsson V, Ilkov M, Lamb JR, et al. Co-regulatory networks of human serum proteins link genetics to disease. Science. 2018;361:769–773. 10.1126/science.aaq1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nayor M, Short MI, Rasheed H, et al. ; CHARGE-Heart Failure Working Group. Aptamer-based proteomic platform identifies novel protein predictors of incident heart failure and echocardiographic traits. Circ Heart Fail. 2020;13:e006749. 10.1161/CIRCHEARTFAILURE.119.006749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Katz DH, Tahir UA, Ngo D, et al. Multiomic profiling in black and white populations reveals novel candidate pathways in left ventricular hypertrophy and incident heart failure specific to Black adults. Circ Genom Precis Med. 2021;14:e003191. 10.1161/CIRCGEN.120.003191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haykowsky MJ, Tomczak CR, Scott JM, Paterson DI, Kitzman DW.. Determinants of exercise intolerance in patients with heart failure and reduced or preserved ejection fraction. J Appl Physiol (1985). 2015;119:739–744. 10.1152/japplphysiol.00049.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stenemo M, Nowak C, Byberg L, et al. Circulating proteins as predictors of incident heart failure in the elderly. Eur J Heart Fail. 2018;20:55–62. 10.1002/ejhf.980 [DOI] [PubMed] [Google Scholar]
- 28. Peng L, Gagliano-Jucá T, Pencina KM, et al. Age trends in growth and differentiation factor-11 and myostatin levels in healthy men, and differential response to testosterone, measured using liquid chromatography-tandem mass spectrometry. J Gerontol A Biol Sci Med Sci. 2022;77:763–769. 10.1093/gerona/glab146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sidis Y, Mukherjee A, Keutmann H, Delbaere A, Sadatsuki M, Schneyer A.. Biological activity of follistatin isoforms and follistatin-like-3 is dependent on differential cell surface binding and specificity for activin, myostatin, and bone morphogenetic proteins. Endocrinology. 2006;147:3586–3597. 10.1210/en.2006-0089 [DOI] [PubMed] [Google Scholar]
- 30. Tortoriello DV, Sidis Y, Holtzman DA, Holmes WE, Schneyer AL.. Human follistatin-related protein: a structural homologue of follistatin with nuclear localization. Endocrinology. 2001;142:3426–3434. 10.1210/endo.142.8.8319 [DOI] [PubMed] [Google Scholar]
- 31. Mukherjee A, Sidis Y, Mahan A, et al. FSTL3 deletion reveals roles for TGF-beta family ligands in glucose and fat homeostasis in adults. Proc Natl Acad Sci U S A. 2007;104:1348–1353. 10.1073/pnas.0607966104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tao R, Wang C, Stohr O, et al. Inactivating hepatic follistatin alleviates hyperglycemia. Nat Med. 2018;24:1058–1069. 10.1038/s41591-018-0048-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perakakis N, Mougios V, Fatouros I, et al. Physiology of activins/follistatins: associations with metabolic and anthropometric variables and response to exercise. J Clin Endocrinol Metab. 2018;103:3890–3899. 10.1210/jc.2018-01056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jones KL, Mansell A, Patella S, et al. Activin A is a critical component of the inflammatory response, and its binding protein, follistatin, reduces mortality in endotoxemia. Proc Natl Acad Sci U S A. 2007;104:16239–16244. 10.1073/pnas.0705971104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brandt C, Pedersen M, Rinnov A, et al. Obesity and low-grade inflammation increase plasma follistatin-like 3 in humans. Mediators Inflamm. 2014;2014:364209. 10.1155/2014/364209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perakakis N, Kokkinos A, Peradze N, et al. Follistatins in glucose regulation in healthy and obese individuals. Diabetes Obes Metab. 2019;21:683–690. 10.1111/dom.13572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lara-Pezzi E, Felkin LE, Birks EJ, et al. Expression of follistatin-related genes is altered in heart failure. Endocrinology. 2008;149:5822–5827. 10.1210/en.2008-0151 [DOI] [PubMed] [Google Scholar]
- 38. Kralisch S, Hoffmann A, Kloting N, et al. FSTL3 is increased in renal dysfunction. Nephrol Dial Transplant. 2017;32:1637–1644. 10.1093/ndt/gfw472 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.