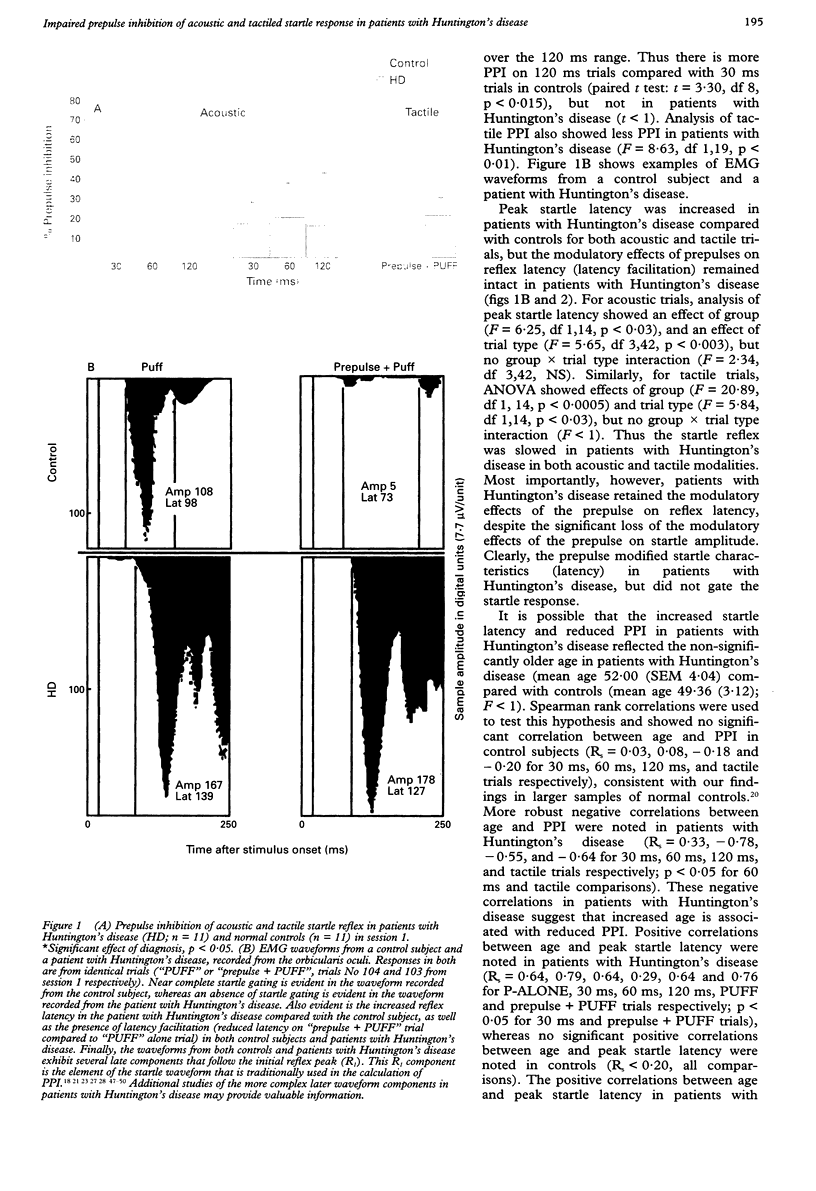
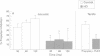Abstract
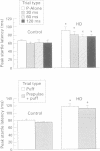
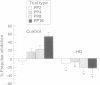
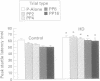
The corpus striatum serves a critical function in inhibiting involuntary, intrusive movements. Striatal degeneration in Huntington's disease results in a loss of motor inhibition, manifested by abnormal involuntary choreiform movements. Sensorimotor inhibition, or "gating", can be measured in humans using the startle reflex: the startle reflex is normally inhibited when the startling stimulus is preceded 30-500 ms earlier by a weak prepulse. In the present study, prepulse inhibition (PPI) was measured in patients with Huntington's disease to quantify and characterise sensorimotor gating. Compared with age matched controls, patients with Huntington's disease exhibit less PPI. Startle gating deficits are evident in patients with Huntington's disease when startle is elicited by either acoustic or tactile stimuli. Even with stimuli that elicit maximal PPI in normal subjects, patients with Huntington's disease exhibit little or no PPI, and their pattern of startle gating does not show the normal modulatory effects usually elicited by changing the prepulse interval or intensity. Startle amplitude and habituation and latency facilitation are largely intact in these patients, although reflex latency is significantly slowed. In patients with Huntington's disease, startle reflex slowing correlates with cognitive impairment measured by the dementia rating scale, and with the performance disruptive effects of interference measured by the Stroop test. These findings document a profound disruption of sensorimotor gating in patients with Huntington's disease and are consistent with preclinical findings that identify the striatum and striatopallidal GABAergic efferent circuitry as critical substrates for sensorimotor gating of the startle reflex.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenthal T. D., Gescheider G. A. Modification of the acoustic startle reflex by a tactile prepulse: the effects of stimulus onset asynchrony and prepulse intensity. Psychophysiology. 1987 May;24(3):320–327. doi: 10.1111/j.1469-8986.1987.tb00302.x. [DOI] [PubMed] [Google Scholar]
- Blumenthal T. D., Levey B. J. Prepulse rise time and startle reflex modification: different effects for discrete and continuous prepulses. Psychophysiology. 1989 Mar;26(2):158–165. doi: 10.1111/j.1469-8986.1989.tb03148.x. [DOI] [PubMed] [Google Scholar]
- Braff D. L., Geyer M. A. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiatry. 1990 Feb;47(2):181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Braff D. L., Grillon C., Geyer M. A. Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry. 1992 Mar;49(3):206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Braff D., Stone C., Callaway E., Geyer M., Glick I., Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978 Jul;15(4):339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Butler R. W., Jenkins M. A., Braff D. L. The abnormality of normal comparison groups: the identification of psychosis proneness and substance abuse in putatively normal research subjects. Am J Psychiatry. 1993 Sep;150(9):1386–1391. doi: 10.1176/ajp.150.9.1386. [DOI] [PubMed] [Google Scholar]
- Butters N., Sax D., Montgomery K., Tarlow S. Comparison of the neuropsychological deficits associated with early and advanced Huntington's disease. Arch Neurol. 1978 Sep;35(9):585–589. doi: 10.1001/archneur.1978.00500330033006. [DOI] [PubMed] [Google Scholar]
- Caine S. B., Geyer M. A., Swerdlow N. R. Carbachol infusion into the dentate gyrus disrupts sensorimotor gating of startle in the rat. Psychopharmacology (Berl) 1991;105(3):347–354. doi: 10.1007/BF02244429. [DOI] [PubMed] [Google Scholar]
- Caine S. B., Geyer M. A., Swerdlow N. R. Hippocampal modulation of acoustic startle and prepulse inhibition in the rat. Pharmacol Biochem Behav. 1992 Dec;43(4):1201–1208. doi: 10.1016/0091-3057(92)90503-8. [DOI] [PubMed] [Google Scholar]
- Coon H., Plaetke R., Holik J., Hoff M., Myles-Worsley M., Waldo M., Freedman R., Byerley W. Use of a neurophysiological trait in linkage analysis of schizophrenia. Biol Psychiatry. 1993 Sep 1;34(5):277–289. doi: 10.1016/0006-3223(93)90085-r. [DOI] [PubMed] [Google Scholar]
- Davis M., Gendelman D. S., Tischler M. D., Gendelman P. M. A primary acoustic startle circuit: lesion and stimulation studies. J Neurosci. 1982 Jun;2(6):791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson I. T., Lenman J. A., Johnston B. B. Habituation of the orbicularis oculi reflex in dementia and dyskinetic states. J Neurol Neurosurg Psychiatry. 1978 Sep;41(9):824–828. doi: 10.1136/jnnp.41.9.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein S. E., Jensen B., Leigh R. J., Folstein M. F. The measurement of abnormal movement: methods developed for Huntington's disease. Neurobehav Toxicol Teratol. 1983 Nov-Dec;5(6):605–609. [PubMed] [Google Scholar]
- Graham F. K. Presidential Address, 1974. The more or less startling effects of weak prestimulation. Psychophysiology. 1975 May;12(3):238–248. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Groves P. M. A theory of the functional organization of the neostriatum and the neostriatal control of voluntary movement. Brain Res. 1983 Mar;286(2):109–132. doi: 10.1016/0165-0173(83)90011-5. [DOI] [PubMed] [Google Scholar]
- Groves P. M., Boyle R. D., Welker R. L., Miller S. W. On the mechanism of prepulse inhibition. Physiol Behav. 1974 Mar;12(3):367–375. doi: 10.1016/0031-9384(74)90111-5. [DOI] [PubMed] [Google Scholar]
- Hackley S. A., Graham F. K. Effects of attending selectively to the spatial position of reflex-eliciting and reflex-modulating stimuli. J Exp Psychol Hum Percept Perform. 1987 Aug;13(3):411–424. doi: 10.1037//0096-1523.13.3.411. [DOI] [PubMed] [Google Scholar]
- Ison J. R., McAdam D. W., Hammond G. R. Latency and amplitude changes in the acoustic startle reflex of the rat produced by variation in auditory prestimulation. Physiol Behav. 1973 Jun;10(6):1035–1039. doi: 10.1016/0031-9384(73)90185-6. [DOI] [PubMed] [Google Scholar]
- Kemble E. D., Ison J. R. Limbic lesions and the inhibition of startle reactions in the rat by conditions of preliminary stimulation. Physiol Behav. 1971 Dec;7(6):925–928. doi: 10.1016/0031-9384(71)90068-0. [DOI] [PubMed] [Google Scholar]
- Kuwert T., Lange H. W., Langen K. J., Herzog H., Aulich A., Feinendegen L. E. Cortical and subcortical glucose consumption measured by PET in patients with Huntington's disease. Brain. 1990 Oct;113(Pt 5):1405–1423. doi: 10.1093/brain/113.5.1405. [DOI] [PubMed] [Google Scholar]
- Penney J. B., Jr, Young A. B. Speculations on the functional anatomy of basal ganglia disorders. Annu Rev Neurosci. 1983;6:73–94. doi: 10.1146/annurev.ne.06.030183.000445. [DOI] [PubMed] [Google Scholar]
- Schwarcz R., Tamminga C. A., Kurlan R., Shoulson I. Cerebrospinal fluid levels of quinolinic acid in Huntington's disease and schizophrenia. Ann Neurol. 1988 Oct;24(4):580–582. doi: 10.1002/ana.410240417. [DOI] [PubMed] [Google Scholar]
- Schwarcz R., Whetsell W. O., Jr, Mangano R. M. Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science. 1983 Jan 21;219(4582):316–318. doi: 10.1126/science.6849138. [DOI] [PubMed] [Google Scholar]
- Shoulson I., Fahn S. Huntington disease: clinical care and evaluation. Neurology. 1979 Jan;29(1):1–3. doi: 10.1212/wnl.29.1.1. [DOI] [PubMed] [Google Scholar]
- Smith S. J., Lees A. J. Abnormalities of the blink reflex in Gilles de la Tourette syndrome. J Neurol Neurosurg Psychiatry. 1989 Jul;52(7):895–898. doi: 10.1136/jnnp.52.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow N. R., Auerbach P., Monroe S. M., Hartston H., Geyer M. A., Braff D. L. Men are more inhibited than women by weak prepulses. Biol Psychiatry. 1993 Aug 15;34(4):253–260. doi: 10.1016/0006-3223(93)90079-s. [DOI] [PubMed] [Google Scholar]
- Swerdlow N. R., Benbow C. H., Zisook S., Geyer M. A., Braff D. L. A preliminary assessment of sensorimotor gating in patients with obsessive compulsive disorder. Biol Psychiatry. 1993 Feb 15;33(4):298–301. doi: 10.1016/0006-3223(93)90300-3. [DOI] [PubMed] [Google Scholar]
- Swerdlow N. R., Braff D. L., Geyer M. A. GABAergic projection from nucleus accumbens to ventral pallidum mediates dopamine-induced sensorimotor gating deficits of acoustic startle in rats. Brain Res. 1990 Nov 5;532(1-2):146–150. doi: 10.1016/0006-8993(90)91754-5. [DOI] [PubMed] [Google Scholar]
- Swerdlow N. R., Braff D. L., Masten V. L., Geyer M. A. Schizophrenic-like sensorimotor gating abnormalities in rats following dopamine infusion into the nucleus accumbens. Psychopharmacology (Berl) 1990;101(3):414–420. doi: 10.1007/BF02244063. [DOI] [PubMed] [Google Scholar]
- Swerdlow N. R., Geyer M. A. Clozapine and haloperidol in an animal model of sensorimotor gating deficits in schizophrenia. Pharmacol Biochem Behav. 1993 Mar;44(3):741–744. doi: 10.1016/0091-3057(93)90193-w. [DOI] [PubMed] [Google Scholar]
- Swerdlow N. R., Geyer M. A. Prepulse inhibition of acoustic startle in rats after lesions of the pedunculopontine tegmental nucleus. Behav Neurosci. 1993 Feb;107(1):104–117. doi: 10.1037//0735-7044.107.1.104. [DOI] [PubMed] [Google Scholar]
- Wan F. J., Swerdlow N. R. Intra-accumbens infusion of quinpirole impairs sensorimotor gating of acoustic startle in rats. Psychopharmacology (Berl) 1993;113(1):103–109. doi: 10.1007/BF02244341. [DOI] [PubMed] [Google Scholar]