Abstract
Cellular senescence is a biological aging process that is exacerbated by obesity and leads to inflammation and age- and obesogenic-driven chronic diseases including type 2 diabetes. Caloric restriction (CR) may improve metabolic function in part by reducing cellular senescence and the pro-inflammatory senescence-associated phenotype (SASP). We conducted an ancillary investigation of an 18-week randomized controlled trial (RCT) of CR (n = 31) or Control (n = 27) in 58 middle-aged/older adults (57.6 ± 5.8 years; 75% Women) with obesity and prediabetes. We measured mRNA expression of select senescence and apoptosis genes in blood CD3 + T cells (qRT-PCR) and a panel of 25 plasma SASP proteins (Luminex/multiplex; ELISA). Participants randomized to CR lost −10.8 ± 0.9 kg (−11.3% ± 5.4%) over 18 weeks compared with +0.5 ± 0.9 kg (+0.03% ± 3.5%) in Control group. T-cell expression of senescence biomarkers, p16INK4a and p21CIP1/WAF1, and apoptosis markers, BCL2L1 and BAK1, was not different between CR and Control groups in age, race, and sex-adjusted mixed models (p > .05, all). Iterative principal axis factor analysis was used to develop composite SASP Factors, and the Factors comprising TNFRI, TNFRII, uPAR, MMP1, GDF15, OPN, Fas, and MPO were significantly altered with CR intervention (age, sex, race-adjusted mixed model time × treatment F = 4.17, p ≤ .05) and associated with the degree of weight loss (R2 = 0.12, p ≤ .05). Our study provides evidence from an RCT that specific circulating biomarkers of senescent cell burden are changed by CR in middle-aged and older adults with obesity and prediabetes. Future studies compare tissue and circulating levels of p16INK4a and pro-inflammatory SASP biomarkers in other populations, and interventions.
Keywords: Aging, Biomarkers, Cell senescence, Dietary restriction, Inflammation
Cellular senescence is a fundamental biological aging process that is induced or exacerbated by obesity (1,2). Several studies have shown a positive correlation between obesity and elevated senescent cell burden in organs such as adipose tissue, liver, and pancreatic β cells (2–5). These senescent cells can secrete several pro-inflammatory and profibrotic proteins, growth factors, and matrix metalloproteinases that affect other cells and tissues both locally and at a distance resulting in further aging- and obesogenic-driven dysfunction and may accelerate aging-associated pathologies such as type 2 diabetes (T2D), cardiovascular diseases (CVD), and other chronic diseases (1,6–12). Pharmacologic and nonpharmacologic interventions that mitigate senescent cell burden and the pro-inflammatory senescence-associated secretome (SASP) mitigate obesity-induced metabolic dysfunction in mice (8,13,14). For example, in rodents, elevated adipose senescent cell burden induced by aging or “fast-food” Westernized diet is accompanied by systemic inflammation, but lower senescent cell abundance and SASP cytokine expression were linked to attenuated fat mass accumulation, and was accompanied by robust improvements in glucose tolerance, fasting insulin, and beta cell mass (8). Furthermore, transplanting adipose tissue from obese humans into lean younger mice caused insulin resistance, but this insulin resistance was mitigated by eliminating the senescent cells from the transplanted human fat using senolytics (14). In sum, cell senescence and SASP signaling appear to play a causative role in aging- and obesity-related metabolic dysfunction in rodents.
Translating these findings to human health requires accessible and informative biomarkers of senescent cell burden evaluated with randomized controlled trials (RCTs) capable of affecting aging biology and obesity. Promising blood-based cellular senescence biomarker candidates include cell expression of cyclin-dependent kinase inhibitors and apoptotic pathways and circulating proteins that are mediators and components of the SASP (15–17). However, these have rarely been evaluated in RCTs that target aging and obesity. Caloric restriction (CR) attenuates the effects of excess adiposity and obesity, and is one of the most robust interventions that alter biological aging, health, and life span across animal models (18). Moreover, CR reduces senescence markers in various mouse organs (19,20). Even short-term CR-initiated mid- to late life in mice reduces senescent cell burden in adipose tissue, small intestine, and liver leading to a healthier aging phenotype (21,22). Evaluating the effects of CR in an RCT using easy-to-access circulating biomarkers of senescent cells and the SASP is a key step for clinical translation.
In this analysis, we leveraged an 18-week RCT designed to achieve 10% weight loss in the CR group compared with a health education control group (Control) in 58 middle-aged and older adults with obesity and prediabetes. We prospectively collected blood samples and analyzed mRNA expression of select senescence and apoptosis gene transcripts (p16INK4a, p21CIP1/WAF1, BCL2L1, BAK1) in peripheral blood CD3 + T cells and a panel of 25 plasma SASP proteins in biospecimen collected before and after the 18-week intervention. We hypothesized that CR will reduce circulating biomarkers of cellular senescence and SASP proteins compared with Control, be associated with the degree of weight loss, and may correlate with improvements in metabolic health and function.
Method
Study Design
We performed a prospective ancillary investigation to an RCT of 10% CR-based weight loss relative to Control conducted at Wake Forest University School of Medicine from 2017 through 2020 (NCT02869659). The primary results from the parent trial are pending publication; for a preliminary report, see (23). Our ancillary study was conducted prospectively, with a biospecimen collection of randomized participants and biomarker analyses performed under masked conditions.
Study Design: Participants, Randomization, Intervention
In brief, inclusion criteria were age of 40–70 years, but the cellular senescence biomarkers ancillary study reported here was restricted to participants aged 50–70 years, as it was. Participants had a body mass index (BMI) of 30–50 or 27–30 kg/m2, if waist circumference was >100 cm (men) or>89 cm (women); prediabetes (fasting blood glucose 100–125 mg/dL OR HbA1c 5.7–6.4%); and sedentary (exercise <20 minutes; 2 d/wk). Participants did not have diabetes or use diabetes medications or insulin; did not have high triglycerides (>400 mg/dL) or uncontrolled hypertension (blood pressure >160/90 mmHg); were not regular smokers or heavy alcohol drinkers; had not had bariatric surgery; and did not have current or past major chronic diseases that would limit their ability to complete the study. All participants provided written informed consent. The trial was approved by the Wake Forest School of Medicine Institutional Review Board and registered at clinicaltrials.gov (NCT02869659). The RCT used the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines (24); the CONSORT flow diagram of the cellular senescence biomarker ancillary study is presented in Supplementary Figure 1.
The 58 participants eligible for the study after screening were randomized 1:1 to CR (n = 31) or Control (n = 27) using a web-based randomization scheme with blocking stratified by gender and race. Participants randomized to the CR program underwent a high-protein dietary CR intervention, targeting 10% baseline weight. The Medifast Achieve 4 & 2 & 1 Plan was used to achieve a caloric deficit by providing 1 100–1 300 calories per day through 4 meal replacement products (90–110 kcals, 11–15 g protein), 2 Lean & Green meals (each containing 5–7 oz. lean protein, 3 servings of nonstarchy vegetable, and up to 2 healthy fat servings), and 1 healthy snack (a serving of fruit, dairy, or grain). A Registered Dietitian led biweekly behavioral group counseling sessions to monitor CR, encourage consumption of only CR plan-approved items, and discuss topics pertinent to weight control. Participants were encouraged to maintain baseline physical activity levels (all sedentary) and complete daily food logs, which were reviewed biweekly to verify diet compliance. Participants randomized to Control condition attended biweekly group behavioral education sessions and were monitored by study staff to ensure weight stability (within ±5% of baseline) throughout the study.
Cellular Measures of Senescence and Apoptosis
Fasted blood samples were collected in the early morning before and after the intervention for biomarker analyses of gene expression in CD3± T cells (primary) and plasma SASP proteins (secondary). Blood sample handling was performed using established standardized protocols by trained professional technicians under consistent conditions across all collections. Most cell isolations were started within 2 hours of collection, and all cell isolations were completed in under 4–6 hours. Cell isolation protocols were performed under the Biosafety Cabinet and in an isolated area with little traffic and no other samples or potential contaminants. CD3 ± T cells were isolated from K2 EDTA tubes by negative selection using magnetic bead separation, plasma was aliquoted, and both plasma and T-cell pellets were cryopreserved at −80°C at Wake Forest University School of Medicine’s Claude D. Pepper Center Integrative Biology Core’s biobank until CD3 + T-cell pellets were batch shipped to Sapere Bio for processing using previously validated methods (15,25). Briefly, total RNA was isolated, cDNA prepared, and expression of p16INK4a, p21CIP1/WAF1, BCL2L1, and BAK1 was measured by real-time TaqMan quantitative reverse-transcription polymerase chain reaction (qRT-qPCR) and normalized to housekeeping genes. Extensive external and internal controls were included per the standard operating protocols of Sapere Bio.
We examined plasma concentrations of 25 prototypical SASP components, including cytokines, chemokines, matrix remodeling proteins, and growth factors using a multiplex assay were measured as previously described (16,17). In brief, the investigators induced senescence in endothelial cells, fibroblasts, preadipocytes, epithelial cells, and myoblasts to profile secreted proteins. This list of proteins was refined by evaluating associations between plasma senescent cell-secreted proteins and clinical data across the life span and in older adults undergoing surgery for prevalent age-related diseases or clinical events (16). All biomarker analyses were performed under masked conditions; technicians and investigators were blinded to treatment group assignment during assays and subsequent biomarker analyses.
Cardiometabolic Health
Body weight was measured on a digital scale, height (cm) without shoes; height and weight were used to calculate BMI (kg/m2). Waist and hip circumference were assessed by tape measure (cm). Fasting lipoprotein lipids, metabolic panel, glucose, insulin, and HbA1c were assessed from fasting blood draw. An estimate of insulin resistance by the homeostasis model assessment (HOMA) score was calculated using fasting insulin and glucose levels (insulin × glucose ÷ 22.5) (26,27). Blood pressure (3 trials) was measured in the seated position after a 5-minute rest and averaged.
Statistical Analyses
All statistical analyses were performed with SAS software (SAS Studio 3.8 Enterprise Edition). All hypothesis tests were 2 sided at a nominal 0.05 of significance. Biomarker levels (cell expression and SASP protein markers) were inspected visually; most deviated from normality and were log transformed. We used Spearman correlation coefficients to evaluate associations between individual biomarker analyte levels. We analyzed the change in individual biomarkers between CR and Control groups using a linear mixed-effect model by examining the time × treatment effect. Linear regression analyses were used to examine the associations between changes in biomarkers and response to intervention (degree of weight lost). All models were adjusted for age, sex, and race. Hypotheses related to intervention effects were considered separately for these 2 classes of circulating biomarkers.
To reduce the dimension of the 25 multiplexed SASP proteins, we conducted an exploratory iterative principal factor analysis with a Varimax rotation on assays performed at baseline, prior to randomization (see Supplementary Figure 2 for overview). In brief, circulating SASP data was grouped using well-recognized criteria: (a) data were screened and correlation matrix evaluated; notably the minimum amount of data for factor analysis was not satisfied, with a final sample size of 56 (using listwise deletion for SASP factors), providing fewer than 10 cases per variable; (b) initial extraction of factors was based on estimation of communality threshold of 0.3; (c) determined the number of factors to be retained based on scree plot of the preliminary eigenvalues and proportion of variance; (d) we evaluated initial factor analysis results after Varimax rotation; (e) chose a conservative factor loading cut point of 0.4 for SASP variables to remain in a factor, and confirmed factor analyses and SASP constituents within each factor by replicating analysis using least squares method rather than iterative principal factor analysis; (f) initial results were interpreted in the context of existing scientific literature; and (g) estimated SASP factor-based scores were calculated as the mean of the items with primary loadings on each factor (denoted by “SASP Factor”). These SASP Factor scores were calculated at baseline and postintervention. Linear mixed-effect models and linear regression were used to examine the intervention effect on the 3 combined SASP Factors and individual proteins.
We explored associations between blood-based biomarkers and biomarkers with measures of cardiometabolic health. We used Spearman correlations between measures at baseline and change versus change scores, with change as log-fold difference (postintervention–baseline) in biomarkers and percent change with intervention for functional measures.
Results
VEGGIE Trial Patient Characteristics and Clinical Effects of Intervention
Participant characteristics at baseline are shown in Table 1. The CR intervention resulted in a change of body weight by −10.8 ± 0.9 kg (−11.3% ± 5.4%) whereas weight did not change in the Control group (+0.5 ± 0.9 kg; 0.03% ± 3.5%; adjusted for age, sex, and race, p < .001, Supplementary Table 1). HbA1c (p < .001), waist circumference (p < .001), fasting insulin (p = .03), HOMA-IR (p = .03), and diastolic blood pressure (p = .03) were also reduced in the CR, but there were no differences between groups in fasting glucose or other health measures (Supplementary Table 1).
Table 1.
Participant Characteristics in the Cell Senescence/SASP Biomarker Ancillary to VEGGIE
| Baseline | |||
|---|---|---|---|
| Overall | CR | Control | |
| Characteristics (N) | 58 | 31 | 27 |
| Women | 43 | 23 | 20 |
| Men | 15 | 8 | 7 |
| Race | |||
| Black/African American | 20 | 14 | 6 |
| White | 35 | 15 | 20 |
| Other | 3 | 2 | 1 |
| Mean ± SD | Mean ± SD | Mean ± SD | |
|---|---|---|---|
| Age (yr) | 57.6 ± 5.8 | 57.4 ± 6.2 | 57.7 ± 5.3 |
| BMI (kg/m2) | 37.0 ± 5.2 | 36.1 ± 5.3 | 37.9 ± 5.9 |
| Body weight (kg) | 103 ± 14 | 100.9 ± 15.4 | 105.6 ± 11.2 |
| Waist circumference (cm) | 111.9 ± 10.5 | 110.4 ± 12.1 | 113.5 ± 7.9 |
| Fasting insulin (mIU/L) | 18.1 ± 11 | 14.6 ± 7.0 | 21.9 ± 13.3 |
| Hemoglobin A1c (HbA1c, %) | 5.75 ± 0.35 | 5.74 ± 0.41 | 5.75 ± 0.26 |
| Insulin Resistance Index (HOMA-IR) | 4.91 ± 3.06 | 4.02 ± 2.17 | 5.88 ± 3.59 |
| Fasting glucose (mg/dL) | 103.4 ± 8.3 | 102.7 ± 7.9 | 104.0 ± 7.9 |
| Creatinine (mg/dL) | 0.86 ± 0.18 | 0.86 ± 0.19 | 0.86 ± 0.16 |
| LDL cholesterol (mg/dL) | 113.2 ± 29.2 | 114.7 ± 28.4 | 111.4 ± 30.6 |
| Total cholesterol (mg/dL) | 187.8 ± 35.8 | 189.7 ± 37.1 | 185.8 ± 34.9 |
| Systolic blood pressure (mmHg) | 129.9 ± 18.5 | 131.9 ± 18.9 | 127.7 ± 18.0 |
| Diastolic blood pressure (mmHg) | 73.8 ± 8.5 | 75.4 ± 7.5 | 72.0 ± 9.3 |
Notes: BMI = body mass index; CR = Caloric restriction; HbA1c = hemoglobin A1c; HOMA = Homeostatic Model Assessment; SASP = senescence-associated secretory phenotype; SD = standard deviation.
Effect of CR on Peripheral Blood T-Cell Expression of Cell Senescence and Apoptosis Markers
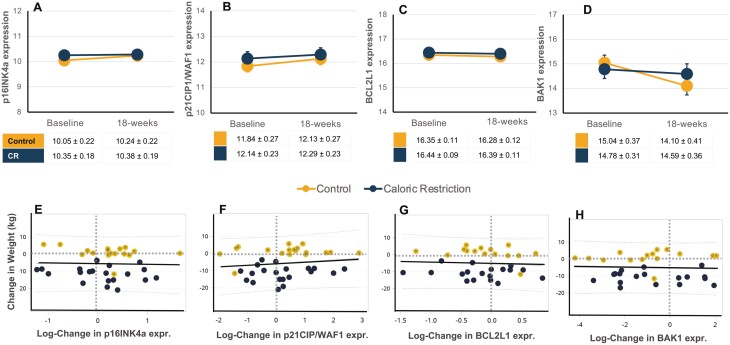
Overall, no changes were observed in peripheral blood CD3 + T-cell expression of cell senescence and apoptosis markers with CR versus Control dietary intervention (Figure 1). In mixed models adjusted for age, sex, and race, no significant changes were observed in T-cell expression of p16INK4a, p21CIP1/WAF1, BCL2L1, or BAK1 expression (Supplementary Table 2). Simple linear regression was used to test if a change in T-cell gene expression significantly correlated with a change in weight, but no associations were observed (Figure 1, Supplementary Table 2).
Figure 1.
Intervention effect on circulating T-cell expression of biomarkers of cellular senescence and apoptosis. Individual data points and mean ± SEM data are shown at baseline and 18-week postintervention of caloric restriction (CR, blue) or health education control (Control, orange) for CD3 + T-cell gene expression of log-transformed markers of cellular senescence p16INK4a (A), and p21CIP1/WAF1 (B), apoptosis marker BCL2L1 (C), and anti-apoptosis marker BAK1 (D). Estimated means and SEM are shown in tables; mixed models adjusted for age, sex, and race were performed. Simple linear regression model with R2-values for log-fold change in gene expression with change in body with intervention in CR (blue) or Control (orange) groups for p16INK4a (E), p21CIP1/WAF1 (F), BCL2L1 (G), BAK1 (H). No significant changes or between group effects were observed. CR = Caloric restriction; SEM = standard error of the mean.
Effect of CR on Plasma SASP: Individual Markers and Composite SASP Factors
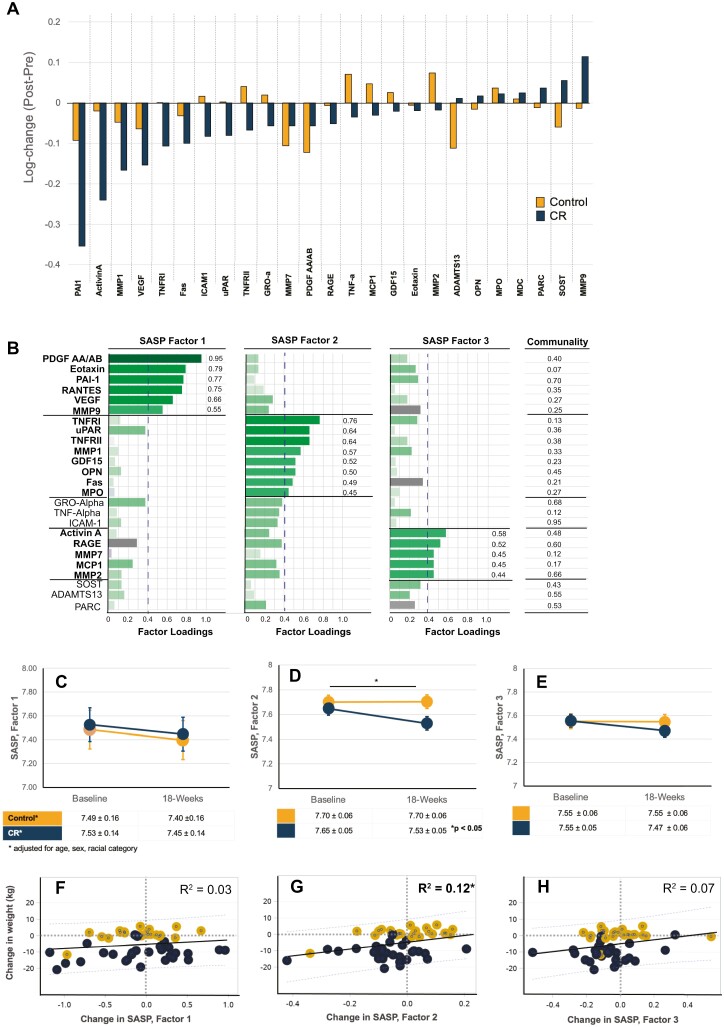
Log-change showed that levels of plasma SASP biomarkers were generally reduced over time with CR versus Control (Figure 2A). Of the 25 SASP biomarkers, 9 were significantly reduced in the CR group (Activin A, Fas, sICAM-1, MMP1, PAI-1, TNFRI, TNFRII, TNF-α, uPAR, VEGF) whereas 1 factor (ADAMTS13) was lower in the Control group (Supplementary Table 3); only Activin A, sICAM-1, and TNFR1 were statistically significant at Bonferroni corrected p ≤ .002. Eight SASP biomarkers were significantly reduced with CR versus Control after adjustment for age, sex, and race (Supplementary Table 3).
Figure 2.
Change in senescence-associated secretory phenotype (SASP) proteins with 18-week intervention of Caloric restriction (CR) or Control. (A) All data are as log-fold change in biomarker levels (post–pre) for CR (blue) or Control (orange) groups, displayed as waterfall chart with largest reduction in biomarker level with CR on the left, and largest increase with CR on the right. (B) An exploratory factor analysis using iterative principal axis factor with a Varimax (orthogonal) rotation was performed on assays prior to randomization to reduce dimensionality. The results of an orthogonal rotation of the solution are shown with three SASP Factors (note, capitalized “Factor” hereafter refers to the composite score from each of three Factors identified by iterative principal factor analysis). A conservative cut point of 0.4 for variables contributing to Factors to remain in factor analysis was used. A composite score for each Factor was calculated by averaging the values of each contributing factor determined in Panel B, for example, SASP Factor 1 score = average (PDGF AA + Eotaxin + PAI-1 + RANTES + VEGF + MMP9). Mixed models adjusted for age, sex, and race were performed, and Estimated means and SEM are shown and time × treatment interactions indicated as *p ≤ .05 for: SASP Factor 1 score (C), SASP Factor 2 score (D), and SASP Factor 3 score (E). Lower panels show simple regression models with R2 values for log-fold change in composite SASP Factors 1 (F), Factor 2 (G), and Factor 3 (H) with change in body weight in CR (blue) or Control (orange) groups.
The results of the exploratory factor analysis are shown in Figure 2B; a simplified overview and links between procedures and results are shown in Supplementary Figure 2. First, the factorability of the 25 SASP proteins was examined and all plasma SASP biomarkers were correlated at least r = 0.25 with at least 1 other biomarker (Supplementary Table 4); PDGF AA and PDGF-AB were highly correlated (r > 0.9) and averaged to create a combined PDGF AA/AB item. Second, the initial extraction of factors revealed that MDC did not meet estimation of communality threshold of 0.3 and was excluded from further analysis. Third, we determined that three factors would be retained based on (a) the scree plot of the preliminary eigenvalues (>1.0) and proportion of variance (>0.1) (Supplementary Figure 3), and (b) the eigenvalues of the reduced correlation matrix (>1.0) and proportion of the common variance explained >10% (Supplementary Table 5; Total = 9.70, Average = 0.39). Fourth, Varimax rotation was used to simplify the results. Fifth, we evaluated initial factor analysis results after rotation; the results and components of the 3 identified SASP factors are shown in Figure 2B. A total of 6 SASP proteins (GRO-α, TNF-α, sICAM-1, SOST, ADAMTS13, and PARC) were eliminated from factor scores because they did not contribute to a simple factor structure and failed to meet the minimum criteria of having a primary factor loading of 0.4 or above. The factor analysis was confirmed using least squares method; factor numbers, and variables that comprise the 3 factors were the same (results not shown). Sixth, for interpretation of initial results, we provide a description of each SASP protein’s class and role in Supplementary Table 6. We opted against providing factor labels to limit overinterpretation of factor results given the small sample size and limitation that minimum amount of data for factor analysis was not satisfied, but finally, estimated SASP factor-based scores were created based on the mean of the items with primary loadings on each factor. For example, SASP factor 1 for participant 1 is the average of PDGF AA/AB, Eotaxin, PAI-1, RANTES, VEGF, and MMP9, calculated at baseline and at post 18-week intervention. In general, higher scores indicated elevated circulating SASP Factors.
Intervention effect on composite SASP Factor scores at baseline and 18-week postintervention of CR or Control is shown in Figure 2C–E and data in Supplementary Table 7. SASP Factor 2, which was comprised of TNFRI, TNFRII, uPAR, MMP1, GDF15, OPN, Fas, and MPO, was reduced with CR versus Control (p = .046). Simple regression analyses indicated that change in composite SASP Factor 2 associated with the degree of weight loss (R2 = 0.13 unadjusted; p = .01), but not SASP Factor 1 or SASP Factor 3.
Associations Between Biomarkers and Metabolic Health
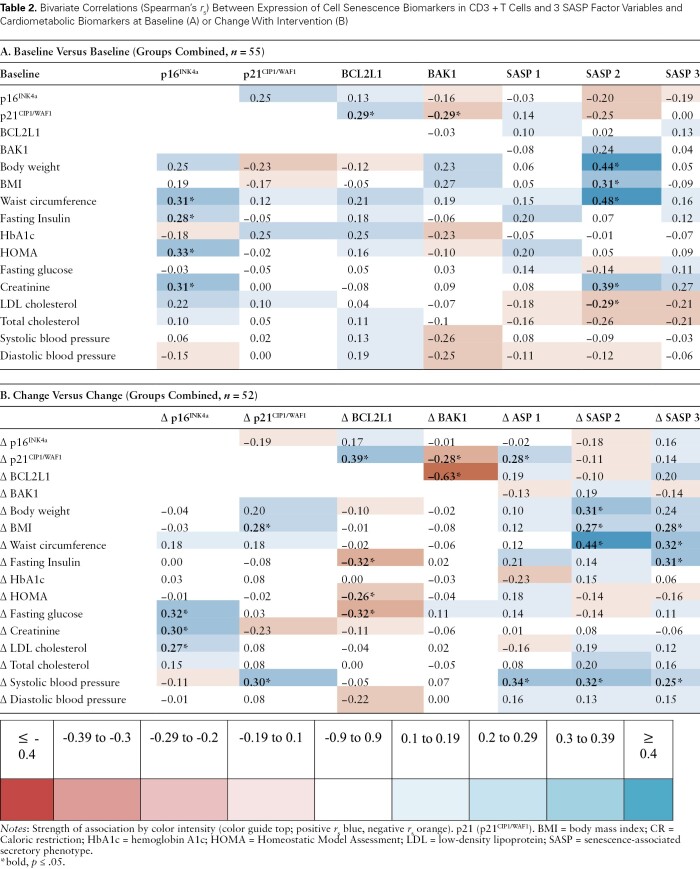
We explored bivariate correlations between biomarkers and metabolic variables at baseline (Table 2) and change in biomarkers with change in cardiometabolic health parameters (Table 2). At baseline, T-cell expression of p16INK4a was positively correlated with baseline fasting insulin, HOMA-IR, and creatinine. SASP Factor 2 was positively correlated with body weight, BMI, waist circumference, and creatinine levels, but negatively correlated with low-density lipoprotein (LDL) cholesterol levels. Change in p16INK4a expression was also positively associated with change in fasting glucose, creatinine, LDL cholesterol, and change in p21CIP1/WAF1 expression with change in BMI and systolic blood pressure (p ≤ .05). In contrast, change in BCL2L1 expression was negatively correlated with insulin, glucose, and HOMA-IR (p ≤ .05 all). Changes in all 3 composite SASP Factors were positively associated with change in blood pressure, and SASP Factors 2 and 3 with change in BMI and waist circumference, and Factor 2 with change in fasting insulin (p ≤ .05, all).
Table 2.
Bivariate Correlations (Spearman’s rs) Between Expression of Cell Senescence Biomarkers in CD3 + T Cells and 3 SASP Factor Variables and Cardiometabolic Biomarkers at Baseline (A) or Change With Intervention (B)
Discussion
Plasma pro-inflammatory and apoptosis-related SASP markers were lowered in middle-aged and older adults with obesity and prediabetes randomized to an 18-week CR-based weight-loss intervention. In contrast, circulating T-cell expression of key cell senescence biomarkers p16INK4a and p21CIP1/WAF1 was unchanged with CR and weakly correlated with 3 plasma composite SASP Factors. However, both p16INK4a expression and composite SASP Factors were independently associated with measures of obesity and metabolic health at baseline, and changes in these during the intervention correlated with changes in metabolic parameters. The results of this study suggest a nuanced relationship between circulating T-cell markers of cellular senescence and its secretome in persons undergoing a short-term CR intervention.
Functional Consequences of Cellular Senescence in Aging and Obesity
Whether or not cell senescence is at the nexus of inflammatory SASP proteins in persons with obesity and prediabetes cannot be inferred based on T-cell gene expression in this study. The tumor suppressor gene p16INK4a is a mediator of cellular senescence and may be a biomarker of molecular or cellular age in tissues including T cells (15,28,29). Expression of p16INK4a is not thought to be an epiphenomenon of aging, but causally linked to both aging of the immune system and cellular senescence and immune aging can drive aging in solid organs (30). Cell senescence can be established and maintained by at least two major pathways—the p53/p21CIP1/WAF1 and p16INK4a/pRB pathways. When induced, expression of p16INK4a may lead to permanent cell-cycle arrest and cellular senescence. Senescent cells can secrete SASP proteins, which includes a variety of soluble signaling factors, such as pro-inflammatory cytokines, chemokines, growth modulators, angiogenic factors, proteases, bioactive lipids, extracellular matrix components, and matrix metalloproteinases (MMPs), many of which were assayed in this study (31–34). Though there is disagreement in the field regarding definitive gene expression signatures of senescence, some investigators posit that neither the expression of either p16INK4a or the cell-cycle inhibitor p21CIP1/WAF1 alone is both necessary and sufficient to reflect that a cell is, in fact, senescent. For example, the activation of p21CIP1/WAF1 in senescent cells might be only transient and decreases after the establishment of growth arrest (35).
Nonetheless, T-cell expression of p16INK4a is regarded as a potential biomarker of cellular aging and cell senescence for clinical trials (17,36,37). We did not reveal changes in p16INK4a expression with CR-based weight-loss intervention or association with body weight. However, we did find direct associations with cardiometabolic health parameters at baseline and change with intervention in persons who are obese with prediabetes. This suggests that T-cell p16INK4a expression may reflect health and functional status. This is consistent with previous studies that promote p16INK4a expression as a biomarker of aging associated with chronologic age and health (15,17,28). Studies find that p16INK4a mRNA expression in T cells differs by age, race, breast cancer risk, family history of cancer, marital status, annual income, and smoking status (38). Moreover, cellular senescence and p16INK4a expression have been shown to be important in the function of pancreatic β cells (39), but our observed association between T-cell p16INK4a expression and indices of insulin resistance is novel.
We did not find an association between T-cell p16INK4a expression and pro-inflammatory SASP Factors, which could be explained by the clinical characteristics of this patient population. Adipose tissue is a potent endocrine tissue. Adipose accumulation and dysfunction in obesity drive chronic pro-inflammatory status, including many of the cytokines and chemokines assayed in our SASP panel. In this study, SASP Factors were associated with body weight, BMI, and waist circumference at baseline and changes in SASP Factors were associated with changes in these anthropomorphic estimates of obesity. Thus, it is possible that changes in adipose tissue mediated the change in cytokine signaling in our study, but this was not reflected in the change in T-cell expression. Future work should directly explore the expression of p16INK4a and p21CIP1/WAF1 in adipose tissues and circulating cells and evaluate their associations with circulating SASP proteins in RCTs of CR and senolytics. Another plausible explanation is that CR might have a senomorphic effect on senescent cells, reducing inflammation and possibly preventing new senescent cell generation without changing the pre-existing senescent cell burden or altering T-cell p16INK4a expression (40).
Caloric Restriction and Weight-Loss Intervention in Older Adults
CR with adequate nutrient intake is the most powerful intervention for extending health span and life span in multiple animal models (20,22,41). A reduction of dietary calories by 20% up to 50% results in a substantial extension of both average and maximal life span in most strains of mice. In this study, CR was administered to achieve 10% weight loss for the treatment of obesity and prediabetes. Calories were reduced to 1 100–1 300 calories per day, a reduction of ~50% of caloric intake prior to randomization in some participants. The mechanisms mediating the health benefits of CR for weight loss in the context of obesity versus CR interventions designed to extend life span in the absence of obesity are not fully understood.
CR does reduce senescence markers in various mouse organs and in the human colon mucosa (3,20,21,42). It is speculated that CR reduces the generation of senescent cells by preventing cellular damage that may induce cells to senescence (20,43). Accumulating evidence also supports obesity as a condition that accelerates or exacerbates biological aging and targeting cell senescence can enhance metabolic function in old animals (44). Thus, either through CR-specific biological pathways or indirectly through mechanisms mediated by reducing obesity, we hypothesized that CR should have a meaningful effect on senescence biomarkers and the senescent secretome. The broad effects of CR versus Control on circulating pro-inflammatory SASP markers certainly suggest a strong intervention effect that could lead to meaningful change in biological aging, disease risk factors, and health. Moreover, as speculated above, CR could have a senomorphic effect by reducing inflammatory secretome by existing senescent cells without leading to apoptosis or senescent cell clearance in the timeframe studied in this RCT. However, this hypothesis would need to be tested in model systems.
Estimated SASP Factor Scores
Given our limited sample size, we prefer not to overinterpret effects on individual components of the estimated SASP Factors, but we did observe a statistically significant effect of CR on SASP Factor 2 only. SASP Factor 2 consists of pro-inflammatory cytokines, and factors related to immune cell regulation, extracellular matrix remodeling, and apoptosis. In obesity, adipose tissue macrophages are polarized into pro-inflammatory M1 macrophages which secrete pro-inflammatory cytokines, and in aging and obesity, senescence macrophages in adipose tissue are a source of inflammation including TNF family cytokines (for review, see (45,46)). Our data suggest that short-term CR weight loss may alter adipose tissue secretion of these cytokines, but whether this is mediated by change in senescent cell burden in adipose tissue or M1 versus M2 macrophage polarization cannot be determined by circulating cytokine levels alone. It will be important for future studies to explore tissue-specific effects that may lead to altered circulating biomarker levels, and the cell types these circulating SASP proteins originate from.
Potential effects of CR on SASP Factor 3, SASP Factor 1, or their constituents are less clear. SASP Factor 2 is also comprised of senescence-associated proteins that drive extracellular matrix remodeling and inflammation. Obesity drives excess deposition of extracellular matrix in adipose tissue, and both diet and exercise have been shown to induce adipose tissue extracellular matrix remodeling (for review, see (47)). It is possible that larger future trials may reveal statistically significant changes in adipose tissue ECM remodeling linked to SASP signaling in older adults that we were not powered to detect. Despite common factorability at baseline many of the constituent proteins within SASP Factor 1 responded to CR weight-loss intervention in strikingly different ways. For example, Activin A and PAI-1 demonstrated the largest log-fold decreases observed in the CR group, and MMP9 showed the largest log-fold increase. The combined SASP Factor 1 may have effectively canceled out the change in individual components. Larger intervention studies are needed to re-evaluate SASP grouping and their combined effects.
Circulating T-Cell and SASP Biomarker Validation
The purpose of this study was to evaluate intervention-induced change in circulating biomarkers of cellular senescence and SASP proteins, which is a small but important step in biomarker assessment. The circulating biomarkers were purposely selected given use in existing cohort studies. For example, a 7-protein SASP panel consisting of GDF15, FAS ligand, OPN, TNFR1, activin A, macrophage inflammatory protein-1 α, (MIP-1α), and interleukin-15 (IL-15) predicted adverse events within 12 months of surgery for severe aortic stenosis; the receiver-operating curve (ROC) area under the curve was 0.84, respectively, compared with predictive ability of frailty index of 0.66, and age plus sex of 0.56 (16). The same SASP biomarker panel was used in a post hoc analysis of baseline data and biospecimens obtained from older females and males with mobility limitations who participated in the Lifestyle Interventions in Elders (LIFE) study, a large, single-blind, randomized clinical trial conducted at 8 centers across the United States. In this study, the 10-protein SASP panel was more closely associated with physical performance (ROC = 0.86) than models comprised of traditional factors such as chronologic age, sex, race, and BMI (ROC = 0.59) (48). Moreover, the same SASP panel and CD3 + T-cell expression were evaluated in a small single-arm open 12-week structured exercise program, which found that the intervention lowered the expression of key markers of the senescence program, including p16INK4a, and p21CIP1/WAF1 in CD3 + T cells, and the circulating concentrations of multiple SASP proteins (17). The current study is the first randomized clinical trial to evaluate circulating cell senescence and SASP biomarker assays. Leveraging similar assays permits a common currency across studies, which could permit future meta-analyses and biomarker validation studies.
Limitations and Future Directions
This is a preliminary investigation performed as an ancillary to an existing clinical trial, so we acknowledge several limitations. There was a slightly higher number of missing data for CD3 + T cells given the technical challenge of cell isolation and expression levels; this may have a minor impact on analyses by limiting sample size, but technical losses were balanced between treatment groups and did not bias the analyses. Important next steps should confirm our findings by evaluating the T-cell expression of p16INK4a and pro-inflammatory SASP biomarkers in other populations that include an older age range than the 50–70-year-old adults included herein, and using other types of interventions, such as pharmacologic intervention with rapamycin, metformin, or senolytics. Our 18-week clinical trial duration was relatively brief; it is unknown if weight-loss maintenance versus immediate effects of CR intervention may alter the circulating expression of senescence markers. Effects of CR and other pharmacologic interventions of longer duration are needed to inform factor analyses of SASP cytokines and chemokines, as are larger cohorts with longer longitudinal follow-up. Finally, senescent cell abundance is tissue, cell, and inducer dependent; extensive tissue mapping for these rare cells across tissues is now underway. It is of high importance to evaluate tissue level and circulating cell expression of markers of cellular senescence longitudinally and in populations with varying risk factors and exposures. Such information, together with biomarker analyses in clinical trials as performed in this study, will provide a better understanding of the interaction between tissues, biomarkers of senescence, and the responsiveness of biomarkers to interventions.
Conclusion
This study provides evidence that a CR-based intentional weight-loss intervention in middle-aged and older adults with obesity and prediabetes is associated with change in circulating SASP proteins but not T-cell expression of the biomarkers of senescence, p16INK4a or p21CIP1/WAF1, and therefore may be senomorphic but not senolytic. However, there were associations between both p16INK4a and SASP biomarkers with cardiometabolic health risk factors. Although promising, these results are not definitive. Larger, prospective trials with a comparison of tissue and circulating levels of select biomarkers are needed to validate and build upon our findings and to guide clinical trials designed to test interventions that target cellular senescence.
Supplementary Material
Acknowledgments
We thank the men and women who volunteered for this study as well as the research staff who conducted recruitment and assessments and the WFUSM Clinical Research Unit nursing and phlebotomy personnel. We also give special thanks to John Stone and Heather Gregory at WFUSM Biogerontology Lab for assistance with data and biospecimen retrieval. We would like to thank WFUSM Center for Precision Medicine summer internship students Victoria Uchman and Margaret Templeton for assistance with organizing data and preliminary analyses in CD3 + T-cell expression. We would like to thank Dr. Mike Miller for consultation on use of factor analyses and data reduction techniques in randomized clinical trials.
Contributor Information
Jamie N Justice, Sticht Center for Healthy Aging and Alzheimer’s Prevention, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA; Department of Internal Medicine, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA.
Xiaoyan I Leng, Sticht Center for Healthy Aging and Alzheimer’s Prevention, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA; Department of Biostatistics and Data Science, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA.
Nathan K LeBrasseur, Robert and Arlene Kogod Center on Aging, Mayo Clinic, Rochester, Minnesota, USA; Department of Physical Medicine and Rehabilitation, Mayo Clinic, Rochester, Minnesota, USA.
Tamara Tchkonia, Robert and Arlene Kogod Center on Aging, Mayo Clinic, Rochester, Minnesota, USA; Department of Physiology and Biomedical Engineering, Mayo Clinic College of Medicine, Rochester, Minnesota, USA.
James L Kirkland, Department of Physiology and Biomedical Engineering, Mayo Clinic College of Medicine, Rochester, Minnesota, USA; Division of General Internal Medicine, Department of Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Natalia Mitin, Sapere Bio, Triangle Research Park, North Carolina, USA.
Yongmei Liu, Department of Medicine, Duke University School of Medicine, Durham, North Carolina, USA.
Stephen B Kritchevsky, Sticht Center for Healthy Aging and Alzheimer’s Prevention, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA; Department of Internal Medicine, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA.
Barbara J Nicklas, Sticht Center for Healthy Aging and Alzheimer’s Prevention, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA; Department of Internal Medicine, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA.
Jingzhong Ding, Sticht Center for Healthy Aging and Alzheimer’s Prevention, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA; Department of Internal Medicine, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA.
Funding
This work was supported by the National Institutes of Health program grant to Wake Forest University School of Medicine (WFUSM) Claude D. Pepper Older Americans Independence Center P30 AG21332 (PI: S.B.K; Co-I’s J.N.J., B.J.N., I.L., J.D.), career development grant: K01 AG059837 (PI: J.N.J.), and research grants: U01 AG060897 (PI: J.D.), R37 AG13925 (PI: J.L.K.), and R33 AG61456 (PI: J.L.K.). The VEGGIE primary clinical trial was supported by R01 DK103531 (PI: J.D.; Co-Is: I.L. and B.J.N.). SASP biomarker analyses were supported by R01AG55529 (PI: N.K.L.). Jason Pharmaceuticals, Inc., a wholly owned subsidiary of Medifast Inc., provided an in-kind donation of the meal replacements used in the study by participants in the CR group. The project was supported in part by the Jarrahi Research Scholars Fund in Geroscience Innovation (J.N.J.). Research was conducted at WFUSM Clinical Research Unit, which is supported by National Center for Advancing Translational Sciences UL1 TR001420. J.N.J. is dual-affiliated with 501(c)(3) nonprofit XPRIZE Foundation and WFUSM, but all works related to this manuscript were performed at WFUSM and pre-date secondary affiliation.
Conflict of Interest
N.M. is a cofounder of Sapere Bio, holds equity in the company, and is an inventor of intellectual property applications. The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
The study concept and design: J.N.J., J.D., S.B.K., B.J.N., J.L.K.. Patient recruitment, data collection, research, analysis: J.D., I.L., B.J.N. Cellular senescence assays and biomarker analyses: J.N.J., N.K.L., N.M. Statistical analysis: J.N.J., I.L. Interpretation: J.N.J., S.B.K., B.J.N., N.M., N.K.L., J.L.K., J.D. Drafted manuscript: J.N.J. Critically revised manuscript: J.D., S.B.K., B.J.N., N.K.L., T.T., J.L.K. Responsible for the final content of manuscript: J.N.J. Read and approved the final manuscript: all authors.
Permission to Reuse and Copyright
Authors confirm all figures and images are original to this publication.
References
- 1. Narasimhan A, Flores RR, Camell CD, Bernlohr DA, Robbins PD, Niedernhofer LJ.. Cellular senescence in obesity and associated complications: a new therapeutic target. Curr Diab Rep. 2022;22:537–548. 10.1007/s11892-022-01493-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ogrodnik M, Zhu Y, Langhi LGP, et al. Obesity-induced cellular senescence drives anxiety and impairs neurogenesis. Cell Metab. 2019;29:1061–1077.e8. 10.1016/j.cmet.2018.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ogrodnik M, Miwa S, Tchkonia T, et al. Cellular senescence drives age-dependent hepatic steatosis. Nat Commun. 2017;8:15691. 10.1038/ncomms15691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tchkonia T, Morbeck DE, Von Zglinicki T, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–684. 10.1111/j.1474-9726.2010.00608.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Justice JN, Gregory H, Tchkonia T, et al. Cellular senescence biomarker p16INK4a+ cell burden in thigh adipose is associated with poor physical function in older women. J Gerontol A Biol Sci Med Sci. 2017;73:939–945. 10.1093/gerona/glx134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moreno-Fernandez ME, Sharma V, Stankiewicz TE, et al. Aging mitigates the severity of obesity-associated metabolic sequelae in a gender independent manner. Nutr Diabetes. 2021;11:15. 10.1038/s41387-021-00157-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palmer AK, Gustafson B, Kirkland JL, Smith U.. Cellular senescence: at the nexus between ageing and diabetes. Diabetologia. 2019;62:1835–1841. 10.1007/s00125-019-4934-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schafer MJ, White TA, Evans G, et al. Exercise prevents diet-induced cellular senescence in adipose tissue. Diabetes. 2016;65:1606–1615. 10.2337/db15-0291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A.. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14:576–590. 10.1038/s41574-018-0059-4 [DOI] [PubMed] [Google Scholar]
- 10. Schafer MJ, Miller JD, LeBrasseur NK.. Cellular senescence: implications for metabolic disease. Mol Cell Endocrinol. 2017;455:93–102. 10.1016/j.mce.2016.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu M, Pirtskhalava T, Farr JN, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24:1246–1256. 10.1038/s41591-018-0092-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yousefzadeh MJ, Flores RR, Zhu Y, et al. An aged immune system drives senescence and ageing of solid organs. Nature. 2021;594:100–105. 10.1038/s41586-021-03547-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Palmer AK, Xu M, Zhu Y, et al. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell. 2019;18:e12950. 10.1111/acel.12950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang L, Wang B, Gasek NS, et al. Targeting p21(Cip1) highly expressing cells in adipose tissue alleviates insulin resistance in obesity. Cell Metab. 2022;34:75–89.e8. 10.1016/j.cmet.2021.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu Y, Sanoff HK, Cho H, et al. Expression of p16(INK4a) in peripheral blood T-cells is a biomarker of human aging. Aging Cell. 2009;8:439–448. 10.1111/j.1474-9726.2009.00489.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schafer MJ, Zhang X, Kumar A, et al. The senescence-associated secretome as an indicator of age and medical risk. JCI Insight. 2020;5:e133668. 10.1172/jci.insight.133668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Englund DA, Sakamoto AE, Fritsche CM, et al. Exercise reduces circulating biomarkers of cellular senescence in humans. Aging Cell. 2021;20:e13415. 10.1111/acel.13415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Broadbent HM, Peden JF, Lorkowski S, et al. ; PROCARDIS consortium. Susceptibility to coronary artery disease and diabetes is encoded by distinct, tightly linked SNPs in the ANRIL locus on chromosome 9p. Hum Mol Genet. 2008;17:806–814. 10.1093/hmg/ddm352 [DOI] [PubMed] [Google Scholar]
- 19. Fontana L, Mitchell SE, Wang B, et al. The effects of graded caloric restriction: XII. Comparison of mouse to human impact on cellular senescence in the colon. Aging Cell. 2018;17:e12746. 10.1111/acel.12746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fontana L, Nehme J, Demaria M.. Caloric restriction and cellular senescence. Mech Ageing Dev. 2018;176:19–23. 10.1016/j.mad.2018.10.005 [DOI] [PubMed] [Google Scholar]
- 21. Wang C, Maddick M, Miwa S, et al. Adult-onset, short-term dietary restriction reduces cell senescence in mice. Aging (Albany NY). 2010;2:555–566. 10.18632/aging.100196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Green CL, Lamming DW, Fontana L.. Molecular mechanisms of dietary restriction promoting health and longevity. Nat Rev Mol Cell Biol. 2022;23:56–73. 10.1038/s41580-021-00411-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ding J, Lohman K, Kritchevsky SB, et al. Monocyte cholesterol metabolism during weight loss and glycemic improvement. Diabetes. 2022;71:1428-P. 10.2337/db22-1428-P [DOI] [Google Scholar]
- 24. Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Trials. 2010;11:32. 10.1186/1745-6215-11-3220334632 [DOI] [Google Scholar]
- 25. Mitin N, Nyrop KA, Strum SL, et al. A biomarker of aging, p16, predicts peripheral neuropathy in women receiving adjuvant taxanes for breast cancer. npj Breast Cancer. 2022;8:103. 10.1038/s41523-022-00473-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bloomgarden ZT. Measures of insulin sensitivity. Clin Lab Med. 2006;26:611–633, vi. 10.1016/j.cll.2006.06.007 [DOI] [PubMed] [Google Scholar]
- 27. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC.. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 28. Liu Y, Sharpless NE.. Tumor suppressor mechanisms in immune aging. Curr Opin Immunol. 2009;21:431–439. 10.1016/j.coi.2009.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shachar SS, Deal AM, Reeder-Hayes KE, et al. Effects of breast cancer adjuvant chemotherapy regimens on expression of the aging biomarker, p16(INK4a). JNCI Cancer Spectr. 2020;4:pkaa082. 10.1093/jncics/pkaa082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nelson JA, Krishnamurthy J, Menezes P, et al. Expression of p16(INK4a) as a biomarker of T-cell aging in HIV-infected patients prior to and during antiretroviral therapy. Aging Cell. 2012;11:916–918. 10.1111/j.1474-9726.2012.00856.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Coppe JP, Desprez PY, Krtolica A, Campisi J.. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. 10.1146/annurev-pathol-121808-102144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lopes-Paciencia S, Saint-Germain E, Rowell MC, Ruiz AF, Kalegari P, Ferbeyre G.. The senescence-associated secretory phenotype and its regulation. Cytokine. 2019;117:15–22. 10.1016/j.cyto.2019.01.013 [DOI] [PubMed] [Google Scholar]
- 33. Kumari R, Jat P.. Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Front Cell Dev Biol. 2021;9:645593. 10.3389/fcell.2021.645593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. 10.1146/annurev-physiol-030212-183653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stein GH, Drullinger LF, Soulard A, Dulic V.. Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol Cell Biol. 1999;19:2109–2117. 10.1128/MCB.19.3.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu JY, Souroullas GP, Diekman BO, et al. Cells exhibiting strong p16(INK4a) promoter activation in vivo display features of senescence. Proc Natl Acad Sci U S A. 2019;116:2603–2611. 10.1073/pnas.1818313116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kirkland JL, Tchkonia T.. Senolytic drugs: from discovery to translation. J Intern Med. 2020;288:518–536. 10.1111/joim.13141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shen J, Song R, Fuemmeler BF, McGuire KP, Chow WH, Zhao H.. Biological aging marker p16(INK4a) in T cells and breast cancer risk. Cancers (Basel). 2020;12:3122. 10.3390/cancers12113122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Helman A, Klochendler A, Azazmeh N, et al. p16(Ink4a)-induced senescence of pancreatic beta cells enhances insulin secretion. Nat Med. 2016;22:412–420. 10.1038/nm.4054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Niedernhofer LJ, Robbins PD.. Senotherapeutics for healthy ageing. Nat Rev Drug Discov. 2018;17:377. 10.1038/nrd.2018.44 [DOI] [PubMed] [Google Scholar]
- 41. Fontana L, Partridge L, Longo VD.. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. 10.1126/science.1172539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Krishnamurthy J, Torrice C, Ramsey MR, et al. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114:1299–1307. 10.1172/JCI22475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Maduro AT, Luis C, Soares R.. Ageing, cellular senescence and the impact of diet: an overview. Porto Biomed J. 2021;6:e120. 10.1097/j.pbj.0000000000000120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu M, Palmer AK, Ding H, et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife. 2015;4:e12997. 10.7554/eLife.12997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zatterale F, Longo M, Naderi J, et al. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front Physiol. 2019;10:1607. 10.3389/fphys.2019.01607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stout MB, Justice JN, Nicklas BJ, Kirkland JL.. Physiological aging: links among adipose tissue dysfunction, diabetes, and frailty. Physiology (Bethesda). 2017;32:9–19. 10.1152/physiol.00012.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ruiz-Ojeda FJ, Mendez-Gutierrez A, Aguilera CM, Plaza-Diaz J.. Extracellular matrix remodeling of adipose tissue in obesity and metabolic diseases. Int J Mol Sci. 2019;20:4888. 10.3390/ijms20194888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fielding RA, Atkinson EJ, Aversa Z, et al. Associations between biomarkers of cellular senescence and physical function in humans: observations from the Lifestyle Interventions for Elders (LIFE) study. Geroscience. 2022;44:2757–2770. 10.1007/s11357-022-00685-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.