Abstract
RpoS (sigma-S or sigma-38) controls a large array of genes that are expressed during stationary phase and under various stress conditions in Escherichia coli and other bacteria. We document here that plant pathogenic and epiphytic Erwinia species, such as E. amylovora; E. carotovora subsp. atroseptica, betavasculorum, and carotovora; E. chrysanthemi; E. herbicola; E. rhapontici; and E. stewartii, possess rpoS genes and produce the alternate sigma factor. We show that rpoS transcription in E. carotovora subsp. carotovora is driven from a major promoter which resides within the nlpD gene located upstream of rpoS as in E. coli. RpoS− E. carotovora subsp. carotovoa strain AC5061, constructed by marker exchange, is more sensitive to hydrogen peroxide, carbon starvation, and acidic pH than its RpoS+ parent strain, AC5006. The basal levels of extracellular pectate lyase, polygalacturonase, and cellulase as well as those of transcripts of E. carotovora subsp. carotovora hrpN (hrpNEcc), the gene for the elicitor of the hypersensitive reaction, are higher in the RpoS− strain than in the RpoS+ parent. Likewise, compared to AC5006, AC5061 causes more extensive maceration of celery petioles. Our findings with the RpoS− mutant and strains carrying multiple copies rpoS+ DNA reveal that rpoS positively controls rsmA expression. We also present evidence that supports the hypothesis that the RpoS effect on extracellular enzyme levels, hrpNEcc expression, and virulence manifests itself by the modulation of rsmA expression.
Bacteria have the remarkable capacity to quickly adapt to various stresses including nutrient limitations, temperature extremes, acidic conditions, osmolarity extremes, and toxic chemicals. Under such conditions bacteria generally deploy sigma factors to activate specific sets of genes. In Escherichia coli and various other bacteria, several alternate sigma factors such as sigma-24, sigma-28, sigma-32, sigma-38, and sigma-54 have been identified (reference 14 and references cited therein). Of these, sigma-38 (also called KatF, RpoS, and sigma-S) is involved in the expression of a number of genes that are activated during postexponential growth or upon nutrient limitation or exposure to acidic pH (12, 36). In addition, sigma-S contributes to bacterial resistance to the toxic effects of hydrogen peroxide (1, 6), to the production of certain secondary metabolites (31), and in some instances to the virulence of animal pathogens, for example in Salmonella typhimurium (8, 9). Several studies with soft-rotting Erwinia spp. have disclosed that virulence factors are expressed during postexponential growth and are subject to global regulation (2, 4, 5, 11, 13, 15, 25, 28, 34, 35). These features prompted the notion that such virulence factors may also be controlled by sigma-S. To test this idea we have undertaken studies with rpoS genes of Erwinia species. Here we (i) document the occurrence of rpoS homologs and the production of the alternate sigma factor; (ii) show that transcription of rpoS is mainly driven by a promoter located within the nlpD gene upstream of rpoS; (iii) report that RpoS of Erwinia carotovora is required for the bacterium to cope with carbon starvation, hydrogen peroxide toxicity, and acidic pH; and (iv) document that RpoS controls the expression of rsmA, a global negative regulator gene. We also present data that demonstrate that RpoS affects extracellular enzyme production, E. carotovora subsp. carotovora hrpN (hrpNEcc) expression, and virulence by modulating rsmA expression.
MATERIALS AND METHODS
Bacterial strains, plasmids and media.
Bacterial strains and plasmids are described in Table 1. The strains carrying antibiotic markers were maintained on Luria-Bertani (LB) agar containing appropriate antibiotics. The wild-type Erwinia and other enterobacterial strains, described in our previous report (5), were maintained on LB agar.
TABLE 1.
Erwinia carotovora subsp. carotovora strains and plasmids
| Bacterial strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| Strains | ||
| 71 | Wild type | 35 |
| AC5006 | Lac− mutant of 71 | 25 |
| AC5047 | Nalr derivative of AC5006 | 4 |
| AC5061 | RpoS− derivative of AC5006 by marker exchange with pAKC941; Spcr | This study |
| AC5070 | RsmA− mini-Tn5-Km mutant of AC5047; Kmr | 4 |
| AC5072 | RpoS− derivative of AC5070 by marker exchange with pAKC941; Kmr Spcr | This study |
| Plasmids | ||
| pRK415 | Tcr | 16 |
| pTB2 | RpoS+; 4.3-kb EcoRI-PstI fragment in pGem3Z; Apr | 3 |
| pAKC940 | RpoS+; 4.3-kb EcoRI-PstI fragment from pTB2 in pRK415; Tcr | This study |
| pAKC941 | RpoS::Ωspc; pAKC940 inactivated with Ω-Spcr; Spcr Tcr | This study |
| pAKC943 | rpoS-lacZ; 0.76-kb SspI-HpaI fragment of pTB2 in pMP220; Tcr | This study |
| pAKC944 | rpoS-lacZ; 0.3-kb SspI-PvuII fragment of pTB2 in pMP220; Tcr | This study |
| pAKC945 | rpoS-lacZ; 0.45-kb PvuII-HpaI fragment of pTB2 in pMP220; Tcr | This study |
The compositions of King’s B (KB) medium, LB medium, minimal salts medium, nutrient gelatin agar, polygalacturonate-yeast extract agar, and M9 medium have been described previously (23, 27, 30). To test the effects of osmolarity on rpoS expression, bacteria were grown in TY medium (1% [wt/vol] tryptone, 0.5% [wt/vol] yeast extract) either without NaCl or with 0.1 or 0.25 M NaCl. When required, antibiotics and drugs were supplemented as follows (concentrations are in micrograms per milliliter): ampicillin, 100; kanamycin, 50; nalidixic acid, 50; spectinomycin, 50; and tetracycline (TC), 10. Media were solidified by the addition of 1.5% agar.
The compositions of agarose media for semiquantitative assays of enzymatic activities were described previously by Chatterjee et al. (4).
Enzyme assays.
The preparation of enzyme samples for pectate lyase (Pel), polygalacturonase (Peh), cellulase (Cel), and protease (Prt) assays and the assay procedures were described previously (4, 27).
DNA techniques.
Standard procedures were used in the isolation of plasmid and chromosomal DNAs, transformation, restriction endonuclease digests, gel electrophoresis, and DNA ligation (30). Southern hybridizations were carried out as described by Cui et al. (5). Restriction and modifying enzymes were obtained from Promega Biotec (Madison, Wis.). The Prime-a-Gene DNA labeling system of Promega Biotec was used for labeling DNA.
RNA isolation and Northern blot analyses.
Bacterial cultures were grown at 28°C in different media and harvested for total RNA extraction at the Klett values indicated in the figure legends. The procedures for RNA isolation and Northern blot analysis have been described previously (21). The 875-bp HpaI-MluI fragment from pTB2 (Table 1) was used as the rpoS probe, the 779-bp EcoRV-SmaI fragment of pAKC924 was used as the hrpNEcc probe (25), and the 183-bp NdeI-SalI fragment of pAKC882 was used as the rsmA probe (24).
S1 nuclease protection assay.
Ten picomoles of primer rpoS1 (5′-TGCCGACAGCAGCTGTATTGCTG-3′; complementary to the base positions −417 to −440 from the translational start codon) was end labeled by polynucleotide kinase (Promega Biotec) and [γ-32P]ATP (New England Nuclear Life Science Products, Boston, Mass.). The end-labeled probe was amplified by PCR using end-labeled primer rpoS1, the opposing unlabeled primer rpoS2 (5′-TGGCAACAACCGATGCCACACGCG-3′; corresponding to the base positions −633 to −610 from the translational start codon), and pAKC942 as the template DNA. The conditions of PCR, hybridization, S1 nuclease digestion, and analysis of products were as described previously (19).
Western blot analysis.
Bacterial strains were grown at 28°C in LB medium to a Klett value of ca. 180. Cells were collected by centrifugation (4,000 × g, 10 min, 4°C), resuspended in TESP buffer (50 mM Tris-HCl [pH 8.0], 1 mM EDTA, 100 mM NaCl, 0.1 mM phenylmethylsulfonyl fluoride), and sonicated with a Braunsonic 1510 sonicator (B. Braun Biotech Inc., Allentown, Pa.) at a 100-W output. The samples were centrifuged at 15,000 × g for 15 min at 4°C, and the supernatants were collected. The protein concentration of the cell lysates was determined by using the bicinchoninic acid (Pierce Corp., Rockford, Ill.) method with bovine serum albumin as a standard. Double-strength sodium dodecyl sulfate (SDS) loading buffer (100 mM Tris-HCl [pH 6.8], 200 mM dithiothreitol, 4% SDS, 0.2% bromophenol blue, 2% glycerol) was added, and the samples were boiled for 5 min. Proteins were fractionated by 0.1% SDS–12% polyacrylamide gel electrophoresis and transferred to a NitroBind nitrocellulose membrane (Micron Separations, Inc., Westboro, Mass.). The blots were probed with antibodies raised against E. coli sigma-38 (14) and visualized with goat anti-rabbit antibody conjugated with alkaline phosphatase (Promega Biotec).
Construction of RpoS− strains by marker exchange.
The plasmid pAKC941 (Table 1), wherein rpoS was inactivated by inserting an Ω-Spc cassette (29) at the HpaI site (Fig. 1), was transferred into AC5006 and AC5070 by using helper plasmid pRK2013 (10). Transconjugants were selected on minimal salts agar containing sucrose and supplemented with spectinomycin. Isolates that were Spcr and Tcs were selected for further studies. The marker exchange was confirmed by Southern blot hybridization as well as Northern and Western blot analyses.
FIG. 1.
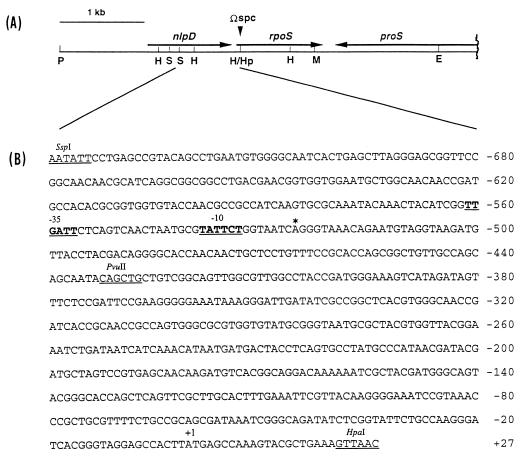
(A) Restriction map of the 4.5-kb DNA segment of E. carotovora subsp. carotovora 71 containing rpoS, nlpD, and proS genes. This genetic organization is deduced from the nucleotide sequence data of Calcutt et al. (3). The locations and directions of the genes are indicated by arrows. The site of Ω-Spc insertion in the HpaI site, resulting in the inactivation of rpoS, is indicated. The 875-bp HpaI-MluI fragment was used as the probe in Northern and Southern blot hybridizations. E, EcoRI; H, HincII; Hp, HpaI; M, MluI; P, PstI; S, SspI. (B) Nucleotide sequence of the SspI-HpaI fragment containing parts of nlpD and rpoS genes of strain 71. The asterisk and +1 indicate transcriptional and putative translational start sites, respectively. The putative −10 and −35 regions and some restriction enzyme sites are shown. The numbers on the right refer to the positions of the nucleotides.
Construction of rpoS-lacZ fusions and β-galactosidase assay.
A series of rpoS-lacZ transcriptional fusions were constructed by cloning sequences from nucleotides (nt) −736 to +24, from nt −736 to −429, and from nt −429 to +24 into promoter probe vector pMP220 (32) to produce pAKC943, pAKC944, and pAKC945, respectively. The orientation of the cloned fragments in these constructs was determined by restriction mapping. β-Galactosidase assays were carried out as described by Miller (23), and the units of activity are expressed as A420 units per min per A600 unit.
Sensitivity to environmental stress.
The RpoS− strain and its parent were grown overnight in M9 medium at 28°C in a shaker incubator. Stationary-phase cells were collected by centrifugation and resuspended in the desired media (A600 of 1.0) and exposed to the indicated stress. For H2O2 treatment cells were washed and resuspended in 0.9% NaCl to an A600 of 1.0. H2O2 was added to a final concentration of 5 mM, and the mixture was incubated for 10 min (1, 18). To find out the effects of starvation, cells were washed and resuspended in M9 medium containing 0.025% glucose and incubated for 100 h. For acid tolerance determination cells were grown overnight in LB broth and resuspended in LB medium buffered with MES (morpholineethanesulfonic acid; final concentration, 5 mM; pH 3.0) and incubated for 3 min (1). After exposure to the respective stresses in the incubation media at 28°C in a shaker incubator, the bacterial suspensions were diluted and appropriate dilutions were plated on LB agar.
Plant tissue maceration.
The celery petiole assay has been previously described (28). The extent of tissue maceration was visually estimated.
RESULTS AND DISCUSSION
Evidence for the occurrence of rpoS homologs.
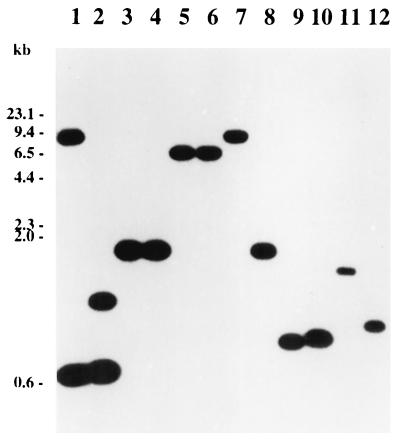
Calcutt et al. (3) determined the nucleotide sequence of rpoS and DNA flanking this gene for E. carotovora subsp. carotovora strain 71 (hereafter strain 71). Their data showed that (i) the nucleotide sequence of the rpoS structural gene is 81% identical to that of the corresponding E. coli gene; (ii) the Erwinia gene could encode a protein of 331 amino acid residues that is 89 to 91% identical to RpoS of E. coli; and (iii) the putative nlpD gene is located upstream of rpoS, as in E. coli. To ascertain if homologs of strain 71 rpoS occur in other Erwinia species, we conducted Southern blot hybridizations using an internal fragment of strain 71 rpoS (the 875-bp HpaI-MluI fragment; Fig. 1) as the probe. The data in Fig. 2 show that strain 71 rpoS DNA hybridized with genomic fragments of all tested strains of Erwinia species as well as E. coli, Yersinia enterocolitica, and S. typhimurium.
FIG. 2.
Southern blot hybridization of HincII-digested chromosomal DNAs of wild-type strains of Erwinia, Salmonella, Yersinia, and E. coli with rpoS of E. carotovora subsp. carotovora 71. Lane 1, E. carotovora subsp. carotovora 71; lane 2, E. carotovora subsp. atroseptica Eca12; lane 3, E. carotovora subsp. betavasculorum Ecb11129; lane 4, Erwinia chrysanthemi EC16; lanes 5 and 6, Erwinia amylovora E9 and Ea321; lane 7, Erwinia rhapontici Er1; lane 8, Erwinia herbicola EH105; lane 9, Erwinia stewartii Es1; lane 10, S. typhimurium LT2; lane 11, Y. enterocolitica 8081v; and lane 12, E. coli AE908.
To detect if rpoS genes are expressed in these Erwinia species, cell lysates of bacteria were subjected to Western blot analysis. All the tested Erwinia strains produced protein species of about 38 kDa that cross-reacted with polyclonal antibodies raised against E. coli RpoS (data not shown). Since this cross-reacting material was absent in the extract of an RpoS− E. carotovora subsp. carotovora strain, we concluded that the 38-kDa protein represents the RpoS species. These data, taken along with the results of Southern blot analysis (Fig. 2), establish the occurrence of active rpoS alleles in these plant-pathogenic and plant-associated bacteria.
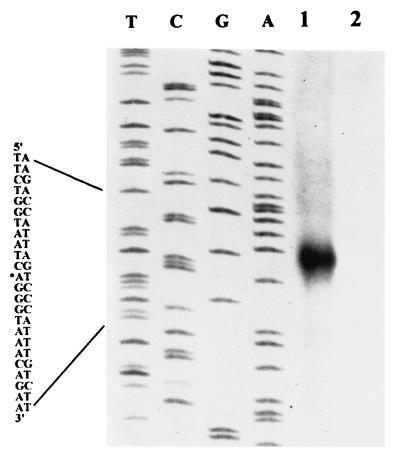
The 5′ end of the rpoS transcript of strain 71 was localized by an S1 nuclease protection assay at the A residue at base −525 relative to the translational start site (Fig. 3; also see Fig. 1). The calculated sizes of the rpoS transcripts presumed to be initiated from this start site matched well to the 1,600-base size determined by Northern blot assays (Fig. 4A). Upstream of the putative rpoS transcriptional start site, there are a −10 (TATTCT) element and a −35 (TTGATT) element, which are highly similar to the consensus E. coli sigma-70 promoter. The results of the S1 nuclease protection assay also indicated that this putative promoter is located within the coding region of nlpD in strain 71, as is the major rpoSp1 promoter of E. coli (17). To confirm that this promoter is actually functional in E. carotovora subsp. carotovora, we made the following transcriptional rpoS-lacZ fusions: an SspI-HpaI fragment corresponding to nt −736 to +24 in pAKC943; the upstream region, i.e., the SspI-PvuII fragment from nt −736 to −429 in pAKC944; and the downstream region, i.e., the PvuII-HpaI fragment from nt −429 to +24 in pAKC945 (Fig. 1 and Table 1). E. carotovora subsp. carotovora AC5006 carrying each of these plasmids or the promoter probe vector, pMP220, was grown in LB broth to a Klett value of ca. 180, and β-galactosidase activity was assayed. AC5006 carrying pAKC943 produced 3,694 Miller units of β-galactosidase activity, and AC5006 carrying pAKC944 produced 5,568 Miller units. By contrast, AC5006 carrying vector pMP220 produced 155 Miller units of β-galactosidase activity. The high expression levels of the rpoS-lacZ fusions in these constructs and the location of the consensus sigma-70 promoter strongly suggest that the major rpoS promoter (rpoSpM) is present within nt −561 and −525 (Fig. 1). The transcription of rpoS from this promoter was stimulated during postexponential growth and by medium osmolarity (data not shown), as in E. coli (12, 22).
FIG. 3.
S1 nuclease mapping of the putative transcriptional start site of rpoS. Strain 71 was grown in LB medium to a Klett value of ca. 200 for RNA isolation. Lane 1, 100,000 cpm of end-labeled DNA probe with 20 μg of total RNA; lane 2, 100,000 cpm of end-labeled DNA probe without RNA. The nucleotides on the left refer to the nucleotide sequence beyond the 5′ end. The asterisk denotes the A residue at which transcription was presumed to be initiated.
FIG. 4.
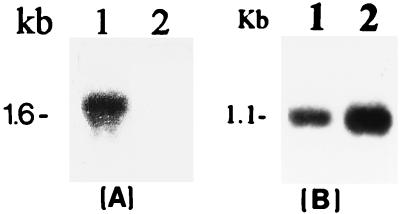
Northern blot analysis of rpoS (A) and hrpNEcc (B) mRNA in E. carotovora subsp. carotovora AC5006 (lane 1) and its RpoS− derivative, AC5061 (lane 2). Total RNA was extracted from bacteria grown in minimal salts medium supplemented with sucrose (0.5% [wt/vol]) to a Klett value of 200. Each lane contained 10 μg of total RNA.
AC5006 carrying pAKC945 produced 887 Miller units of β-galactosidase activity, which is about sixfold higher than the levels produced by bacteria carrying promoter probe vector pMP220 but much lower than the levels produced by bacteria carrying either pAKC943 or pAKC944. These data suggest that there may be another weak promoter (rpoSpW) behind rpoSpM. This conclusion is supported by the S1 nuclease protection assay, which revealed the presence of weakly protected bands within nt −166 and +19 (data not shown). It is noteworthy that in E. coli, rpoS transcription is mainly driven by a single major promoter, rpoSp1, which is homologous to the strain 71 rpoSpM promoter. Although there is a second putative promoter about 251 bp downstream of rpoSp1 (17), this promoter apparently does not play a significant role in the expression of rpoS in E. coli. A very similar situation probably occurs in strain 71 since by Northern blot analysis we did not detect an rpoS transcript smaller than 1,600 bases (Fig. 4A).
Effects of RpoS on the production of extracellular enzymes and expression of hrpNEcc.
To analyze the regulatory role of RpoS in E. carotovora subsp. carotovora, we constructed an RpoS-deficient mutant, AC5061, by a marker exchange procedure. The exchange of the wild-type rpoS gene by inactivated rpoS::Ω fragment was confirmed by Northern blot hybridizations (Fig. 4A) as well as Western blot assay and Southern blot hybridization (data not shown). The RpoS− mutant strain was also tested for various characteristics. The inactivation of rpoS resulted in enhanced sensitivity to carbon starvation, acidic pH, and hydrogen peroxide (Table 2). These characteristics are typical of RpoS− bacteria (22).
TABLE 2.
Survival of E. carotovora subsp. carotovora AC5006 and its RpoS− mutant (AC5061) upon exposure to environmental stresses
| Stress factor | Strain | % Survivala |
|---|---|---|
| Hydrogen peroxide | AC5006 | 35 |
| AC5061 | 2.7 | |
| Low pH | AC5006 | 29 |
| AC5061 | 2.5 | |
| Carbon starvation | AC5006 | 150 |
| AC5061 | 36 |
These values were obtained by dividing bacterial CFU after exposure to stresses by CFU at zero time, i.e., immediately prior to exposure to stresses, and multiplying by 100.
In the course of characterization of RpoS− strain AC5006, we noted that the levels of extracellular Pel, Peh, and Cel were higher in RpoS− bacteria than in the RpoS+ parent strain. The results of quantitative assays (Table 3) show that Pel-specific activity was about twofold higher in AC5061 than in the RpoS+ parent, AC5006. Similarly, the levels of hrpNEcc transcripts were higher in RpoS− bacteria than in the RpoS+ parent strain (Fig. 4B). The RpoS− strain was more virulent (Fig. 5) than the RpoS+ parent as would be expected from the production of higher levels of extracellular enzymes, specially the pectinases. These observations were unexpected since RpoS is generally known to activate gene expression in the stationary-growth phase (12). This pleiotropic effect of RpoS deficiency was somewhat reminiscent of the phenotype of the RsmA− strains of E. carotovora subsp. carotovora (4, 5). We therefore argued that RpoS positively regulates rsmA expression and that the reduced pool of RsmA in RpoS− bacteria accounts for the elevated levels of extracellular enzymes, the higher level of expression of hrpNEcc, and the greater plant tissue maceration. The data presented below substantiate this hypothesis.
TABLE 3.
The effect of rpoS on Pel production in E. carotovora subsp. carotovora
| Bacterial constructa | Relevant phenotype | Pel activityb |
|---|---|---|
| AC5006* | RsmA+ RpoS+ | 0.35 ± 0.005 |
| AC5061* | RsmA+ RpoS− | 0.58 ± 0.004 |
| AC5070* | RsmA− RpoS+ | 9.6 ± 0.22 |
| AC5072* | RsmA− RpoS− | 10.1 ± 0.35 |
| AC5006/pRK415† | RsmA+ RpoS+/RpoS− | 0.059 ± 0.006 |
| AC5061/pRK415† | RsmA+ RpoS−/RpoS− | 0.24 ± 0.016 |
| AC5061/pAKC940† | RsmA+ RpoS−/RpoS+ | 0.015 ± 0.0016 |
| AC5070/pRK415† | RsmA− RpoS+/RpoS− | 2.0 ± 0.036 |
| AC5072/pRK415† | RsmA− RpoS−/RpoS− | 2.1 ± 0.097 |
| AC5072/pAKC940† | RsmA− RpoS−/RpoS+ | 2.0 ± 0.063 |
Bacteria were grown in minimal salts plus sucrose (*) or minimal salts plus sucrose plus TC (†) to a Klett value of ca. 200. Cultural supernatants were assayed for Pel activity.
Expressed as units per milliliter per A600 unit.
FIG. 5.
Plant tissue maceration induced by E. carotovora subsp. carotovora AC5006 and its RpoS− mutant, AC5061. About 2 × 108 cells were injected into the celery petiole at each inoculation site. The inoculated celery petiole was incubated in a moist chamber at 25°C for 24 h. (A) water injection; (B) AC5006 injection; (C) AC5061 injection.
RpoS causes accumulation of rsmA transcripts.
The data shown in Fig. 6 demonstrate that the levels of rsmA transcripts were much lower in RpoS− strain AC5061 than in RpoS+ strain AC5006. To assess the effect of rpoS gene dosage, the levels of rpoS and rsmA transcripts in the RpoS− strain, the parent strain carrying a chromosomal copy of rpoS+, and the RpoS− strain carrying low-copy-number RpoS+ plasmid pAKC940 were determined. Bacteria were grown in KB medium containing TC. Total RNA was extracted from cells and subjected to Northern blot analysis. The results were as follows. (i) The level of rpoS mRNA was higher in the strain carrying multiple copies of rpoS+ than in the strain carrying a single copy of rpoS+ (data not shown). As expected, no rpoS mRNA was detected in RpoS− mutant AC5061. (ii) The highest level of rsmA transcripts was observed in bacteria producing the most rpoS mRNA (i.e., the strain carrying rpoS+ plasmid pAKC940) (Table 4).
FIG. 6.
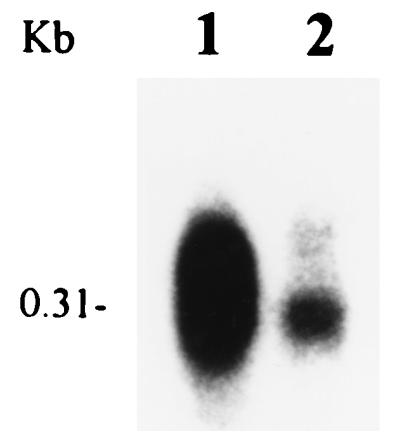
Northern blot analysis of rsmA mRNA in E. carotovora subsp. carotovora AC5006 (lane 1) and its RpoS− derivative, AC5061 (lane 2). Each lane contained 20 μg of total RNA isolated from bacteria grown in LB medium to a Klett value of ca. 200.
TABLE 4.
Effect of rpoS+ copies on the levels of rsmA transcriptsa
| Bacterial construct | Relative value of contour (OD × mm2)b |
|---|---|
| E. carotovora subsp. carotovora | |
| AC5006/pRK415* | 100 |
| AC5061/pRK415* | ND |
| AC5061/pAKC940* | 249 |
| 71/pRK415† | 100 |
| 71/pAKC940† | 336 |
| SCRI193/pRK415† | 100 |
| SCRI193/pAKC940† | 258 |
| E. carotovora subsp. atroseptica | |
| Eca12/pRK415† | 100 |
| Eca12/pAKC940† | 207 |
| E. carotovora subsp. betavasculorum | |
| Ecb11129/pRK415† | 100 |
| Ecb11129/pAKC940† | 294 |
Total RNA was isolated from bacteria grown in KB plus TC (*) to an A600 of 4.0 or in LB plus TC (†) to a Klett value of ca. 100. The RNA samples (10 μg of total RNA for each strain) were subjected to Northern blot analysis. The densities of the hybridization bands were quantified by using the QS30 optically enhanced densitometry system (Fisher Scientific, Pittsburgh, Pa.).
Data are presented as relative values by defining the value for the wild-type strains carrying cloning vector pRK415 as 100. pAKC940 carries the Erwinia rpoS DNA cloned into pRK415 (see Table 1). ND, a band corresponding to rsmA mRNA was not detected; the relative value of the contour is <20. OD, optical density.
To determine if there was a correlation between the dosage of rpoS+ DNA and the levels of extracellular enzymes, those bacterial constructs were grown in minimal salts medium containing sucrose and TC, and culture supernatants were assayed for enzymatic activities. The level of Pel activity in AC5061 carrying multiple copies of rpoS+ DNA was 6% of the activity found in AC5061 carrying the vector (Table 3). A similar effect of rpoS+ copies occurred with Peh, Cel, and Prt activities (data not shown). We attribute this effect to overexpression of rsmA by rpoS+ copies, followed by RsmA-promoted decay of the cognate transcripts of the extracellular enzyme genes.
We performed the following experiments to establish that the RpoS effect was mediated via RsmA. We constructed an RpoS− RsmA− double mutant, and determined that the levels of Pel were similar in the mutant, AC5072, and its RpoS+ counterpart, AC5070 (Table 3). We then transferred RpoS+ plasmid pAKC940 or cloning vector pRK415 into the RpoS− RsmA− double mutant, AC5072. The RpoS+ RsmA− strain, AC5070, carrying pRK415 was used as a control. These constructs were grown in minimal salts medium containing sucrose and TC, and culture supernatants were assayed for enzymatic activities. The levels of Pel activity were very similar in these constructs (Table 3). Similar results were obtained with Peh, Cel, and Prt activities (data not shown). These observations demonstrate that in the absence of a functional rsmA allele RpoS does not have a significant effect on extracellular enzyme production. This clearly contrasts with the suppression of enzyme levels by RpoS in the RsmA+ strain (see above). A straightforward interpretation of these observations is that the RpoS effect manifests itself primarily by regulating rsmA expression.
To determine if multiple copies of the strain 71 rpoS+ gene have a generalized effect on rsmA expression in different bacteria, we introduced pAKC940 into E. carotovora subsp. carotovora SCRI193, E. carotovora subsp. atroseptica Eca12, and E. carotovora subsp. betavasculorum Ecb11129. Strain 71 carrying pAKC940 served as the positive control. The plasmid-carrying strains were grown in LB containing TC, and total RNA was extracted from cells grown to a Klett value of 100. The data in Table 4 show that rsmA transcripts were consistently higher in strains carrying the strain 71 rpoS allele than in cells carrying cloning vector pRK415.
Since high levels of rsmA transcripts are only detected in the presence of RpoS, we do not consider it merely coincidental that the rsmA promoter consists of a −10 region comprising CTAAACT and no consensus −35 region (5). This −10 sequence is typical of sigma-S-dependent promoters (see, for example, reference 7). These observations and the finding that the level of rsmA expression is higher in the postexponential than in the exponential growth stage (20) provide strong support for the hypothesis that rsmA expression is positively affected by RpoS in E. carotovora subsp. carotovora. In fact, we have found that the levels of rsmA (csrA) transcripts in S. typhimurium are reduced by RpoS deficiency (data not shown), raising the possibility that expression of rsmA may be under the control of this alternate sigma factor in other enterobacteria as well. We have initiated studies in collaboration with Tony Romeo to determine if E. coli csrA, the rsmA homolog, is also controlled by this alternate sigma factor. We should note that expression of rsmA occurs to some extent in RpoS-deficient E. carotovora subsp. carotovora strains. Thus, the gene is controlled by sigma-70 as well as sigma-S. In fact, this prediction is consistent with the observation that many sigma-S-controlled genes in E. coli are also activated by sigma-70 (33).
In summary, we have shown that some of the structural characteristics of E. carotovora rpoS and its functions are generally similar to those in E. coli. These similarities notwithstanding, our data reveal several novel features as well. For example, the levels of extracellular enzymes and hrpNEcc transcripts and the degree of plant virulence are higher in RpoS-deficient bacteria than in the RpoS+ parent. We have established that this effect manifests itself through the reduction in rsmA expression. The rationale for a dual control of rsmA expression by sigma-70 and sigma-S can perhaps be appreciated by invoking an important housekeeping role of RsmA as well as its function as a regulator of secondary metabolites.
ACKNOWLEDGMENTS
This work was supported by the Food for the 21st Century Program of the University of Missouri and by the National Science Foundation (grant DMB-94-19403).
We thank Mick Calcutt for RpoS plasmids.
Footnotes
Journal series 12,671 of the Missouri Agricultural Experiment Station.
REFERENCES
- 1.Badger J L, Miller V L. Role of RpoS in survival of Yersinia enterocolitica to a variety of environmental stresses. J Bacteriol. 1995;177:5370–5373. doi: 10.1128/jb.177.18.5370-5373.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barras F, van Gijsegem F, Chatterjee A K. Extracellular enzymes and pathogenesis of soft-rot Erwinia. Annu Rev Phytopathol. 1994;32:201–234. [Google Scholar]
- 3.Calcutt M J, Becker-Hapak M, Gaut M, Hoerter J, Eisenstark A. The rpoS gene of Erwinia carotovora: gene organization and functional expression in E. coli. FEMS Microbiol Lett. 1998;159:275–281. doi: 10.1111/j.1574-6968.1998.tb12872.x. [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee A, Cui Y, Liu Y, Dumenyo C K, Chatterjee A K. Inactivation of rsmA leads to overproduction of extracellular pectinases, cellulases, and proteases in Erwinia carotovora subsp. carotovora in the absence of the starvation/cell density sensing signal, N-(3-oxohexanoyl)-l-homoserine lactone. Appl Environ Microbiol. 1995;61:1959–1967. doi: 10.1128/aem.61.5.1959-1967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui Y, Chatterjee A, Liu Y, Dumenyo C K, Chatterjee A K. Identification of a global repressor gene, rsmA, of Erwinia carotovora subsp. carotovora that controls extracellular enzymes, N-(3-oxohexanoyl)-l-homoserine lactone, and pathogenicity in soft-rotting Erwinia spp. J Bacteriol. 1995;177:5108–5115. doi: 10.1128/jb.177.17.5108-5115.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisenstark A, Calcutt M J, Becker-Hapak M, Ivanova A. Role of Escherichia coli rpoS and associated genes in defense against oxidative damage. Free Radic Biol Med. 1996;21:975–993. doi: 10.1016/s0891-5849(96)00154-2. [DOI] [PubMed] [Google Scholar]
- 7.Espinosa-Urgel M, Chamizo C, Tormo A. A consensus structure for ςs-dependent promoters. Mol Microbiol. 1996;21:657–659. doi: 10.1111/j.1365-2958.1996.tb02573.x. [DOI] [PubMed] [Google Scholar]
- 8.Fang F C, Chen C-Y, Guiney D G, Xu Y. Identification of ςs-regulated genes in Salmonella typhimurium: complementary regulatory interactions between ςs and cyclic AMP receptor protein. J Bacteriol. 1996;178:5112–5120. doi: 10.1128/jb.178.17.5112-5120.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang F C, Libby S J, Buchmeier N A, Loewen P C, Switala J, Harwood J, Guiney D G. The alternative sigma factor KatF (RpoS) regulates Salmonella virulence. Proc Natl Acad Sci USA. 1992;89:11978–11982. doi: 10.1073/pnas.89.24.11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figurski D H, Helinski D R. Replication of an origin-containing derivative of a plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Handa A K, Chiu J, Rozycki H, Bennetzen L. Evidence for global regulation of the expression of pathogenicity genes in soft-rot Erwinia. In: Klement Z, editor. Plant pathogenic bacteria. Budapest, Hungary: Akademiai Kiado; 1990. pp. 707–712. [Google Scholar]
- 12.Hengge-Aronis R. Regulation of gene expression during entry into stationary phase. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: ASM Press; 1996. pp. 1497–1512. [Google Scholar]
- 13.Hugouvieux-Cotte-Pattat N, Condemine G, Nasser W, Reverchon S. Regulation of pectinolysis in Erwinia chrysanthemi. Annu Rev Microbiol. 1996;50:213–257. doi: 10.1146/annurev.micro.50.1.213. [DOI] [PubMed] [Google Scholar]
- 14.Jishage M, Iwata A, Ueda S, Ishihama A. Regulation of RNA polymerase sigma subunit synthesis in Escherichia coli: intracellular levels of four species of sigma subunit under various growth conditions. J Bacteriol. 1996;178:5447–5451. doi: 10.1128/jb.178.18.5447-5451.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones S, Yu B, Bainton N J, Birdsall M, Bycroft B W, Chhabra S R, Cox A J R, Golby P, Reeves P J, Stephens S, Winson M K, Salmond G P C, Stewart G S A B, Williams P. The lux autoinducer regulates the production of exoenzyme virulence determinants in Erwinia carotovora and Pseudomonas aeruginosa. EMBO J. 1993;12:2477–2482. doi: 10.1002/j.1460-2075.1993.tb05902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keen N T, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 17.Lange R, Fischer D, Hengge-Aronis R. Identification of transcriptional start sites and the role of ppGpp in the expression of rpoS, the structural gene for the ςs subunit of RNA polymerase in Escherichia coli. J Bacteriol. 1995;177:4676–4680. doi: 10.1128/jb.177.16.4676-4680.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lange R, Hengge-Aronis R. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol Microbiol. 1991;5:49–59. doi: 10.1111/j.1365-2958.1991.tb01825.x. [DOI] [PubMed] [Google Scholar]
- 19.Liu, Y., Y. Cui, A. Mukherjee, and A. K. Chatterjee. Characterization of a novel RNA regulator of Erwinia carotovora subsp. carotovora that controls production of extracellular enzymes and secondary metabolites. Mol. Microbiol., in press. [DOI] [PubMed]
- 20.Liu, Y., and A. Mukherjee. Unpublished data.
- 21.Liu Y, Murata H, Chatterjee A, Chatterjee A K. Characterization of a novel regulatory gene aepA that controls extracellular enzyme production in the phytopathogenic bacterium Erwinia carotovora subsp. carotovora. Mol Plant-Microbe Interact. 1993;6:299–308. doi: 10.1094/mpmi-6-299. [DOI] [PubMed] [Google Scholar]
- 22.Loewen P C, Hengge-Aronis R. The role of the sigma factor ςs (KatF) in bacterial global regulation. Annu Rev Microbiol. 1994;48:53–80. doi: 10.1146/annurev.mi.48.100194.000413. [DOI] [PubMed] [Google Scholar]
- 23.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 24.Mukherjee A, Cui Y, Liu Y, Dumenyo C K, Chatterjee A K. Global regulation in Erwinia species by Erwinia carotovora rsmA, a homologue of Escherichia coli csrA: repression of secondary metabolites, pathogenicity and hypersensitive reaction. Microbiology. 1996;142:427–434. doi: 10.1099/13500872-142-2-427. [DOI] [PubMed] [Google Scholar]
- 25.Mukherjee A, Cui Y, Liu Y, Chatterjee A K. Molecular characterization and expression of the Erwinia carotovora hrpNEcc gene, which encodes an elicitor of the hypersensitive reaction. Mol Plant-Microbe Interact. 1997;10:462–471. doi: 10.1094/MPMI.1997.10.4.462. [DOI] [PubMed] [Google Scholar]
- 26.Murata H, Fons M, Chatterjee A, Collmer A, Chatterjee A K. Characterization of transposon insertion Out− mutants of Erwinia carotovora subsp. carotovora defective in enzyme export and of a DNA segment that complements out mutations in E. carotovora subsp. carotovora, E. carotovora subsp. atroseptica, and Erwinia chrysanthemi. J Bacteriol. 1990;172:2970–2978. doi: 10.1128/jb.172.6.2970-2978.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murata H, McEvoy J L, Chatterjee A, Collmer A, Chatterjee A K. Molecular cloning of an aepA gene that activates production of extracellular pectolytic, cellulolytic, and proteolytic enzymes in Erwinia carotovora. Mol Plant-Microbe Interact. 1991;4:239–246. [Google Scholar]
- 28.Pirhonen M, Flego D, Heikinheimo R, Palva E T. A small diffusible signal molecule is responsible for the global control of virulence and exoenzyme production in the plant pathogen Erwinia carotovora. EMBO J. 1993;12:2467–2476. doi: 10.1002/j.1460-2075.1993.tb05901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prentki P, Krisch H M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 31.Sarniguet A, Kraus J, Henkels M D, Muehlchen A M, Loper J E. The sigma factor ςs affects antibiotic production and biological control activity of Pseudomonas fluorescens Pf-5. Proc Natl Acad Sci USA. 1995;92:12255–12259. doi: 10.1073/pnas.92.26.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spaink H P, Okker R J H, Wijffelman C A, Pees E, Lugtenberg B J J. Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL 1JI. Plant Mol Biol. 1987;9:27–39. doi: 10.1007/BF00017984. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka K, Takayanagi Y, Fujita N, Ishihama A, Takahashi H. Heterogeneity of the principal ς factor in Escherichia coli: the rpoS gene product, ς38, is a second principal ς factor of RNA polymerase in stationary-phase Escherichia coli. Proc Natl Acad Sci USA. 1993;90:3511–3515. doi: 10.1073/pnas.90.8.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomson N R, Cox A, Bycroft B W, Stewart G S A B, Williams P, Salmond G P C. The Rap and Hor proteins of Erwinia, Serratia and Yersinia: a novel subgroup in a growing superfamily of proteins regulating diverse physiological processes in bacterial pathogens. Mol Microbiol. 1997;26:531–544. doi: 10.1046/j.1365-2958.1997.5981976.x. [DOI] [PubMed] [Google Scholar]
- 35.Wharam S D, Mulholland V, Salmond G P C. Conserved virulence factor regulation and secretion systems in bacterial pathogens of plants and animals. Eur J Plant Pathol. 1995;101:1–13. [Google Scholar]
- 36.Zambrano M M, Kolter R. Gasping for life in stationary phase. Cell. 1996;86:181–184. doi: 10.1016/s0092-8674(00)80089-6. [DOI] [PubMed] [Google Scholar]
- 37.Zink R T, Kemble R J, Chatterjee A K. Transposon Tn5 mutagenesis in Erwinia carotovora subsp. carotovora and E. carotovora subsp. atroseptica. J Bacteriol. 1984;157:809–814. doi: 10.1128/jb.157.3.809-814.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]