Abstract
Deer antlers are a bony organ solely able to acquired distinct unique attributes during evolution and all these attributes are against thus far known natural rules. One of them is as the fastest animal growing tissue (2 cm/day), they are remarkably cancer-free, despite high cell division rate. Although tumor-like nodules on the long-lived castrate antlers in some deer species do occur, but they are truly benign in nature. In this review, we tried to find the answer to this seemingly contradictory phenomenon based on the currently available information and give insights into possible clinic application. The antler growth center is located in its tip; the most intensive dividing cells are resident in the inner layer of reserve mesenchyme (RM), and these cells are more adopted to osteosarcoma rather than to normal bone tissues in gene expression profiles but acquire their energy mainly through aerobic oxidative phosphorylation pathway. To counteract propensity of neoplastic transformation, antlers evolved highly efficient apoptosis exactly in the RM, unparalleled by any known tissues; and annual wholesale cast to jettison the corps. Besides, some strong cancer suppressive genes including p53 cofactor genes and p53 regulator genes are highly positively selected by deer, which would have certainly contributed to curb tumorigenesis. Thus far, antler extracts and RM cells/exosomes have been tried on different cancer models either in vitro or in vivo, and all achieved positive results. These positive experimental results together with the anecdotal folklore that regular consumption of velvet antler is living with cancer-free would encourage us to test antlers in clinic settings.
Subject terms: Cell biology, Cancer
Facts
The antler is a secondary sexual character of male deer; these boney organs are the only complex mammalian organ that, once lost, can fully regenerate.
During the growth phase (around 70 days), the antlers in males of the larger species can elongate at a rate of around 2 cm/day.
The antler growth center is located in the antler tip; based on gene expression profiling, the cells of the antler growth center are more like an osteosarcoma than normal developing bone tissue.
The antler cycle is under the control of androgen hormones, which are in turn regulated by changes in day-length; the newly regenerated antler grows in spring following casting of the previous hard (boney) antler; when the androgens rise in late summer, and under androgen stimulation, blood supply is shut down, the velvet skin of the antler dies, the antler stops growing and matures to bone.
Tumor-like antlers are known in some species of the deer family; castration in such species can induce tumor-like antlers or antleromas to occur.
Evidence gathered to date indicates the abnormal antlers are truly benign in nature and can respond to androgen hormones.
Preparations of the tissue of antlers have been shown to suppress cancer cell propagation in animal models of certain types of cancer, such as prostate cancer and glioblastoma.
Open questions
Antlers are an extremely fast growing mammalian tissue but remain cancer free. Ironically, antler extracts can effectively retard tumor growth in certain types of cancer. What are the underlying mechanisms?
How has the deer evolved mechanisms for highly efficient apoptosis in the antler growth center to solve the problem of becoming cancerous during the transient antler growing phase?
Is it possible that the annual cycle of ossification and casting of the calcified antlers removes any residual cells that might possess a tendency towards neoplastic transformation?
Have the genes that have been positively selected during deer evolution and that are highly expressed in the antler growth center, such as p53 cofactor genes and p53 regulator genes, helped to prevent antlers from becoming cancerous?
How do antler preparations (through which factors and pathways) effectively suppress cancer cell propagation?
Introduction
The hypotheses on the cause and on the origin of tumors are a predominant part of the history of medicine. In 2015, the provoking work of Tomasetti and Vogelstein’s hypothesized the ‘bad-luck’ intrinsic origin of cancer; accordingly, the lifetime risk of cancers of many different types is directly correlated with the total number of divisions of the normal self-renewing stem cells maintaining tissues’ homeostasis [1], see also [2]. Conversely, shortly after, the group of Hannun raised the question that the overwhelming majority of cancers develop following environmental external insults, with only ~10–30% of the risk attributable to intrinsic factors, thus supporting the “toxic insults” theory [3]. Whilst these hypotheses report correlations, without detailing the causal underlying molecular mechanisms, the message of these papers could not be in more strident contradiction, with the effect of igniting a public debate on this very hot topic [2].
A trade-off model system recently established by Boddy et al. [4] convincingly predicted that sexually selected traits such as exaggerated appendages or large body size require high levels of cell proliferation and appear to be associated with greater cancer susceptibility. Trade-offs between reproductive competitiveness and cancer defenses may be instantiated by various mechanisms, including higher levels of growth factors and hormones, less efficient cell-cycle control and less DNA repair, or simply a larger number of cell divisions. For example, after the artificially selected for daily ovulation of jungle fowl hen, the occurrence of their ovarian cancer advanced significantly to as young as 2 years of age, and the incidence of ovarian cancer increased sharply to 30–35% of the population (highly malignant disease by 3.5 years of age) [5, 6]. In women, the reduction of ovulation associated with the number of pregnancies is loosely related to less ovarian cancers [7]. Boddy et al. [4] believe that selection for (1) rapid cell proliferation could simultaneously enhance extreme trait expression as well as cancer formation, which may involve mutations or epigenetic silencing of cancer suppression genes; and (2) increased allocation of energy to reproductive traits rather than to somatic maintenance could elevate cancer risk by increasing somatic mutation rates, which could be due to altered allocation of a finite energy pool towards reproduction at the expense of somatic maintenance, such as DNA repair or immune defenses.
An exception to this aforementioned trade-off model, the deer antlers, arguably the fastest growing mammalian tissue, have not been reported thus far to grow malignant tumors. Therefore, Goss [8] has called antlers improbable mammalian appendages, solely because they have evolved a few unique attributes that are in defiance of all known general rules in life science, such as annually full regeneration from their pedicles, the permanent bony protuberances [9, 10]. In this account, we will focus on reviewing one of the antler’s unique attributes, unprecedented growing rate but barely subject to neoplastic transformation, based on the currently available information [11].
The fastest growing tissue
The annual cycle of antler regeneration starts from the top of the pedicle immediately after casting of the previous hard bony antler in spring (Fig. 1Aa). The fastest growing period of antlers occurs in late spring and early summer (Fig. 1Ab), with the elongation rate reaching more than 2 cm/day. Fully grown antlers (around 90 days of growth) in large species can be over 1.5 m in length with annual repeating species-specific branch patterns; and undergo total calcification and velvet shedding in autumn (Fig. 1Ac). Dead exposed antlers are ready for fighting during the rutting season in late autumn. During the post-rut period over the winter, the dead bony antlers remain firmly attached to their living pedicles (Fig. 1Ad) until casting again next spring (over half a year), then triggers another cycle of antler regeneration [12].
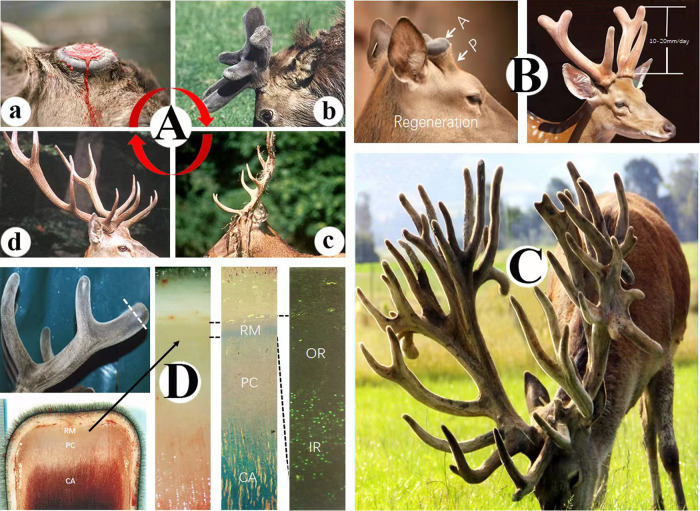
Fig. 1. Fastest growing tissue.
A Antler growth cycle in red deer. a casting surface of a pedicle immediately after the hard antler drops off in spring, note that the surface was still with fresh bleeding; (b) early-mid fast growing stage antlers in early summer, note antlers acquired three-branches and enveloped in special pelage, the velvet; (c). fully calcified antlers in autumn, note that velvet shedding was nearly completed; (d) exposed dead antlers firmly attached to their living pedicles in winter. B Antler regeneration in sika deer. Left, wound healing has completed over the top of the pedicle (P), note that healing skin is the typical velvet and early regenerating antler bud (A) has formed; Right, mid-late growing stage antlers with 3-branches, note that at this stage antler elongation rate can reach 10–20 mm/day. C Farmed red deer with huge multi-branched antlers. The weight of the antlers can be estimated around 30 kg. D Antler growth center localization in red deer. Left panel: upper photo, mid growth stage antlers with 3-branches, note that tip of the antler was removed at the level of dotted line for the investigation of histological makeup of antler growth center; lower photo, longitudinally cut surface of the antler tip from the upper photo, note that three layers can be morphologically distinguished, i.e., reserve mesenchyme (RM), precartilage (PC) and cartilage (CA) (for detailed classification, refer to Li et al, 2002); Mid 1: Higher magnification of the antler center from the left panel; Mid 2: Same tissue of Mid 1 but with histological staining to show the differentiation of three layers, note that RM layer can be further divided into two sublayers based on the staining: outer (no staining) and inner (blue staining) sublayers, PC is essentially no staining, and CA has heavily blue staining; Right panel: BrdU labeled RM layer, note that RM outer layer is essentially devoid of BrdU labeled cells, whereas inner layer cells are intensively labeled, as BrdU only labels dividing cells, thus antler elongation is mainly driven by the proliferation of RM inner sublayer cells.
Unprecedented elongation rate in the animal kingdom
Wound healing over the pedicle stump is a rapid regeneration in nature although the healing skin is atypical called velvet skin, and normally is complete within a week in red deer or sika deer [13]. The early antler bud or blastema [14] starts to establish as soon as the wound healing is complete (Fig. 1B left). Once the antler blastema is properly formed, antler elongation starts to accelerate to a phenomenal rate, and this is clearly evident by day 15 after casting [15]. Even in medium-sized deer, such as sika deer, the elongation rate may reach 13 mm/day (Fig. 1B right) [16], and in some larger species, like North American wapiti, can reach 27.5 mm/day [17]. For some extreme examples, wapiti or red deer (Fig. 1C) can produce 30 kg or so antlers in weight around 70–80 days of growth, with over 400 g of fresh tissue being added to the growing antlers per day. It is a formidable challenge for a body weight of 180–200 kg animal to produce over 1/6 tissue mass of the whole-body each year within such a limited period.
Histological make-up of the antler growth center
Since antler growth is so fast, one would inevitably ask where the growth center is located in the antler? Interestingly, the antler growth center was initially identified in a very primitive but effective way cited by Chapman [18]: a metal screw was inserted into an early growing antler at the level 3.5 cm from the base and 1.5 cm from the tip; 11 days later, the distance of the screw to the base was unchanged, but to the tip became to 5.5 cm. Thus, the center for antler elongation was determined to be at its tip (Fig. 1D Left upper).
The antler growth center histologically comprises three layers [19] distoproximally: reserve mesenchyme (RM), precartilage (PC), and cartilage (CA; Fig. 1D Left lower, Mid 1 and Mid 2). By means of the BrdU labeling approach to pinpoint proliferation cells in the antler growth center in vivo, Li et al. [20] found that the RM layer can be further divided into outer (OR) and inner (IR) two sublayers. The OR is essentially devoid of dividing cells, whereas the dividing cells are very densely populated in the IR (Fig. 1D Right). Therefore, it is the extensive cell proliferation in the IR that constitutes the main force driving antler elongation to that astonishing speed. Interestingly, the OR may constitute a stem cell reservoir to replenish the gradually exhausting proliferating cells in the IR [20].
The rapid proliferation of RM cells is stimulated by insulin-like growth factors (IGFs), and the RM cells react to IGF1 in vitro in a dose-dependent manner [21]. This stimulation may be achieved through direct binding to the RM cells, as an abundance of both type I and type II IGF receptors have been detected in the antler growth center [22, 23]. Expectedly, fastest antler growth always coincides with the highest peak period of circulating IGF1 [24, 25].
Underpinning genes
With the ability to achieve such an astonishing elongation rate, antler growth must be underpinned at the gene level. Indeed, Wang et al. [26] reported that gene expression profiles of fast-growing antlers are more similar to osteosarcoma (r = 0.67–0.78) than to normal bone tissues (r = 0.33–0.47), showing similar patterns of developmental programs in antler growth and oncogenesis. In the study, Wang et al. [26] found that 3 proto-oncogenes (FOS, FAM83A, and REL) were under positive selection and 5 growth factor and receptor genes (FGF19, FGF21, FGFBP3, PDGFD, and PDGFRL) were highly and specifically expressed in growing antlers. In addition, a deer-specific highly conserved element (HCE) was located in the 3ʹUTR of NOVA1, a gene that is believed to activate telomerase and promote tumor growth in vivo [27]. Analysis of differentially expressed genes between antler and osteosarcoma revealed the enrichment for cancer- and metabolism-related pathways. According to the findings of Wang et al. [26], it seems possible that in order to successfully complete the growth phase of such huge appendages (up to 30 kg), proportional to their body size within a limited timeframe (around 90 days), deer have adopted molecular machinery that cancer cells use to drive their development.
The least cancer organ
Although the velocity at which antlers elongate far exceeds that of most cancers, antlers are remarkably free from neoplasia. This apparent absence of cancer in antlers may simply reflect the fact that most tumors require more than a few months to develop. During their limited growth period, antler cells form the bone tissue that is identical histologically to their somatic counterpart with proper Haversian systems [28, 29]. Revealing the mechanism as to how can such extremely rapidly proliferating mammalian cells stabilize their genome and avoid becoming cancerous would undoubtedly help our understanding how some of the kinds of cancer develop.
There would seem to be no reason why velvet antlers ought not to become cancerous, for they are composed of many of the same tissues which can develop tumors elsewhere [8]. A question, thus, arises as to whether there might be a wider perspective on whether deer themselves are protected from neoplasia. Interestingly, the cancer incidence rates are ~5 times lower in deer than in other mammals according to the records from both the Philadelphia (0.4–0.8%) and San Diego (2.1–4.6%) zoos [30, 31].
Contrast of energy metabolism: TCA cycles vs glycolysis
Most malignant cells utilize anaerobic glycolysis rather than aerobic oxidative phosphorylation as the major metabolic pathway to generate ATP [32]. The shift of energy metabolism from oxidative phosphorylation (TCA cycle) to glycolysis is known as the Warburg effect [33]. Glycolysis has now been known as one of the hallmarks of malignant tumors [34]. Therefore, we sought to define the energy pathway in the RM cells of fast-growing antlers compared with mouse mesenchymal cells (non-cancerous) and Hela cells (cancerous) using Seahorse XF Analyzer. The results demonstrated unambiguously that the energy source of the RM cells was almost entirely obtained from the TCA cycles (Fig. 2A), suggesting that the antler is a type of normal tissue.
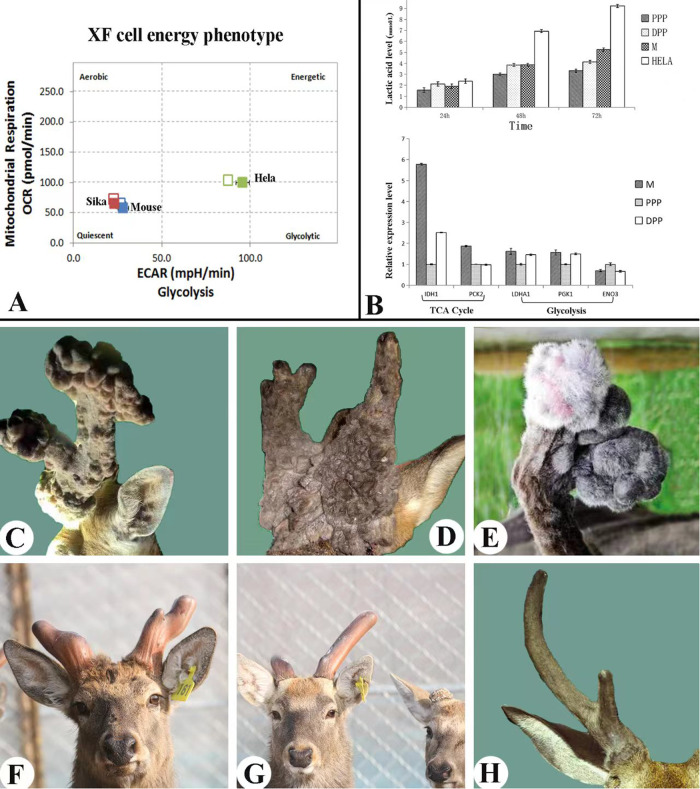
Fig. 2. Least cancer organ.
A, B Energy metabolism pathway of the RM cells in the antler growth center, note that RM cells obtain their energy were mainly through TCA cycles. OCR oxygen consumption rate, ECAR extracellular acidification rate, MO mouse osteoblasts, RM antler reserve mesenchymal cells, HeLa cancer cell line. A Tested using Agilent Seahorse XF Analyzer, note that both RM and MO cells fell in the quadrant of aerobic and quiescent; whereas, HeLa cells were within the area of the glycolysis quadrant (Sun et al., unpublished). B upper. Production of lactic acids, note that lactic acid level generated by HeLa cells was highly significantly higher than those of M, PPP and DPP cells. M, RM cells; PPP, potentiated pedicle periosteal cells; DPP, dormant pedicle periosteal cells. RM cells are initially differentiated from the PP cells. B lower. Expression levels of the genes involved in either Aerobic respiration or Anaerobic metabolism, note that RM cells highly significantly expressed genes related to TCA cycles compared to the quiescent pedicle cells; whereas, no general conclusion can be drawn from the expression levels of the genes related to glycolysis among three deer cell lines (Guo et al, unpublished). C Tumor-like nodules on the castrate antlers of fellow deer, known as “antleromas”. D Wig-like antlers grown by a castrated roe deer, known as “peruke”. E Tumor-like nodules on the castrate antlers of reindeer. F, G Antlers grown by castrated sika deer, note that although these antlers were deviated from their species-specific shape, they did not form tumor-like structures. F Three years after castration. G Four years of castration. H Antlers grown by a castrated red deer, note that the specie-specific antler shape was pretty much retained.
Tumors are heavily dependent on glucose for energy through glycolysis, but are often in a glucose-deprived state. Surprisingly, solid tumors are apparently resistant to glucose deprivation [35]. Wu et al. [36] subsequently discovered that lactic acidosis constitutes a potent tumor microenvironmental factor that allows cancer cells to strongly resist cell death induced by glucose deprivation, thus significantly extending the survival time of cancer cells under such conditions. Lactic acidosis arises as the tumor exhibits abnormally high glycolysis and converts a considerable amount of glucose to lactate [37]. Therefore, to further examine glucose metabolism in the antler, we compared lactic acid levels in the culture medium of RM cells of fast-growing antlers and mitotically-quiescent pedicle periosteal cells (PP cells), from which RM cells were derived; (Li et al. [9])with Hela cells (cancer cells) as the control. After 48 and 72 h in culture, lactic acid levels of both antler and pedicle cells achieved about half of that of Hela cells, with no significant difference between the deer cells (Fig. 2B upper) either in the state of mitotically active or quiescent. Relevant TCA cycle genes were highly significantly expressed in the RM cells than in the PP cells (Fig. 2B lower), indicating that fast proliferating cells (RM) require more energy than the mitotically quiescent cells (PP cells; Guo et al., unpublished). Overall, all these results demonstrate that antler cells obtain their energies through TCA cycles rather than glycolysis, therefore they are from normal tissue. Interestingly, the differences in expression levels of the glycolysis-related genes between the antler cells were negligible.
Tumor-like antlers are truly benign tissue
Cancers often require long periods of development before becoming overt, but antlers are transient structures and normally remain viable for only 3 months until their elongation slows down and the velvet skin is shed from the fully calcified and dead bone [38]. Given this constrained lifespan, one wonders what would possibly happen if growing antlers are allowed to grow longer or indefinitely, through suppression of circulating androgens by means of castration, for example.
Castration has the interesting effect of prolonging the life of the antler indefinitely. If carried out at the hard bone stage (in autumn-winter), the hard antlers are cast within two weeks (owing to the abrupt decline in circulating androgens) and new soft antlers start to grow. These castrate antlers remain immature and fail to peel off their velvet skin. If deer are castrated while their antlers are at growing phase (spring-summer), such antlers remain viable for deer’s life [39]. Although antlers of castrated deer are a perennially living tissue, Goss [8] reported that these antlers do not grow incessantly but stop elongating when their normal dimensions have been attained (despite the continued absence of androgens). However, they resume growth in the following spring as the day length increases, but this growth mainly causing increase in thickness rather than length, and eventually produces tumorlike nodules in some species, such as fellow deer (Fig. 2C). Again, growth is not continuous, but ceases until the following spring when the nodules may mushroom into amorphous tumors known as “antleromas” [40]. In castrated roe deer, antlers are referred to as perukes because of the way they grow down over the head like a wig (Fig. 2D). The castrated white-tailed deer, mule deer and reindeer (Fig. 2E) have also been observed to produce amorphous growths on the surfaces of their antlers. It would be interesting to see if the soft tumors on the castrate antler can survive the surge of circulating androgen hormones, which naturally cause the demise of the entire antler via full calcification, when dissociated from the antler maturation phase, such as being ectopically transplanted elsewhere on deer body
The nature of antler tumors grown by castrated deer is not well understood. They may not even be the same in different species. Perukes of roe deer have been reported to contain bone although overwhelmed by the skin component [38]. The antleromas of the fallow deer were believed to be made up of quantities of disorganized collagen fibrils with interspersed fibroblasts and blood vessels [40]; but later on, antleromas were found also contain bone tissue [41]. Thus far, members of the genus Cervus, such as sika deer (Fig. 2F, G), red deer (Fig. 2H) or wapiti, are not known to grow tumor-like nodules following castration [40]. Why some species form tumor-like tissues on their castrate antlers while others do not is not known and deserves further investigation. Goss [8] stated that antleromas in fellow deer, perukes in roe deer, and their counterparts in Odocoileus deer may well represent abortive attempts to produce antlers in the absence of the morphogenetic factors normally responsible for prescribing shape, rather than the true neoplastic tissue.
The fact that such overgrowths in antlers of castrated deer in some species exhibit annually recurring activation and cessation suggests not only that control of growth is still effectively operational in these tumor-like nodules, but also that this control is exerted by some non-gonadal hormones (circulating steroids are barely detectable in spring), such as IGF1 [24]. The even stronger evidence for claiming that antler tumors are benign is the response of these structures to treatment with high doses of exogenous testosterone where they become totally calcified and dead tissue [42]. Consequently, these so-called antler tumors or antleromas are likely to be the true benign tissue, which may only represent the example where growth is devoiced from morphogenesis. To substantiate their trade-off model, Boddy et al. [4] used antleromas as an example. However, we do not believe this is appropriate because (1) antleromas are truly benign as they are still under the control of endogenous signals and do not go metastasis; (2) antleromas are only confined to certain species such as fellow deer and roe deer, but not to others, such as Cervus genera, and antlers from some species of the Cervus genera (wapiti and red deer etc.) are much bigger than fellow and roe deer but do not undergo such neoplastic-like “transformation”.
The possible strategy for preventing neoplastic transformation
To find the answer to the question of why the fastest-growing antler tissue does not go cancerous is as challenging as it is intriguing. Based on the currently available insights, deer have evolved at least the three possible strategies that contribute to this phenomenon.
Highly efficient cell apoptosis mechanism
Tissue growth and remodeling, especially at the extremes of growth rate, require very rapid cell proliferation, differentiation, and programmed cell death [43]. The unprecedented rate of antler elongation is achieved mainly through intensive cell proliferation in the IR sublayer of a growing antler tip [20]. Interestingly, Colitti et al. [44] found that the percentage of apoptotic cells was the highest in the IR (up to 64%) of the growing antler, and this was higher than that recorded in any other adult tissue. Likewise, the ratio of proliferation to apoptosis was also highest in the IR (up to 22%). Furthermore, these authors detected high-level expression of both Bcl-2 and Bax in the mesenchymal tissue, likely in the cells with a precancerous state, which can be ascertained via single-cell sequencing. It is known that one of the major intracellular apoptosis cascades is the mitochondrial pathway [45] and that this pathway is regulated by members of the BCL-2 protein family including Bax for pro-apoptotic activity, providing further support for the localization of vastly apoptotic cells in the IR. Colitti et al. [44] postulated that this extensive cell death phenomenon probably reflects the formidable rate of morphogenesis and tissue remodeling that takes place in a growing antler. However, this high rate of apoptosis would also be expected to prevent the aberrant cells, being resulted from the fastest proliferating process, to undergo neoplastic transformation.
In conclusion, the very high proportion of apoptotic cells must be one key to the maintenance of a well-organized antler tissue, in that a highly efficient apoptotic mechanism coupled with a short period of active growth has resolved the problem of aberrant cells surviving and going cancerous, at least as temporary expediency. In this respect, understanding how apoptosis is triggered in the antlers of castrated deer could be revealing, albeit that there could well be variation between species.
Total calcification and an annual wholesale cast of the antler
An apoptotic strategy, especially in those potentially transformed cells, during the rapid antler growth phase, is very powerful in preventing neoplastic transformation but may not be “once and for all”, so deer invented another effective means, arguably the most powerful one, to completely prevent this to happen by annually killing the tissue through total calcification [46]. Each year in late summer/autumn, growing antlers (Fig. 3A) become totally calcified and all cells died (Fig. 3B) due to a sharp increase in circulating androgen hormones just prior to the starting of rutting season [39, 47, 48]. Initially dead antlers are firmly attached to their living pedicles (Fig. 3C) but in spring, these hard antlers are naturally cast due to robust activities of osteoclasts along the interface between the dead antler and living pedicle (Fig. 3D, E). It may be totally another question how deer have evolved the mechanism of jettisoning the corpse in the first place, but the phenomenon of seasonal demise and wholesale casting of the antler may have served the purpose of the second and the most thorough checkpoint for the neoplastic transformation during the deer’s life.
Fig. 3. Possible strategy for preventing neoplastic transformation.
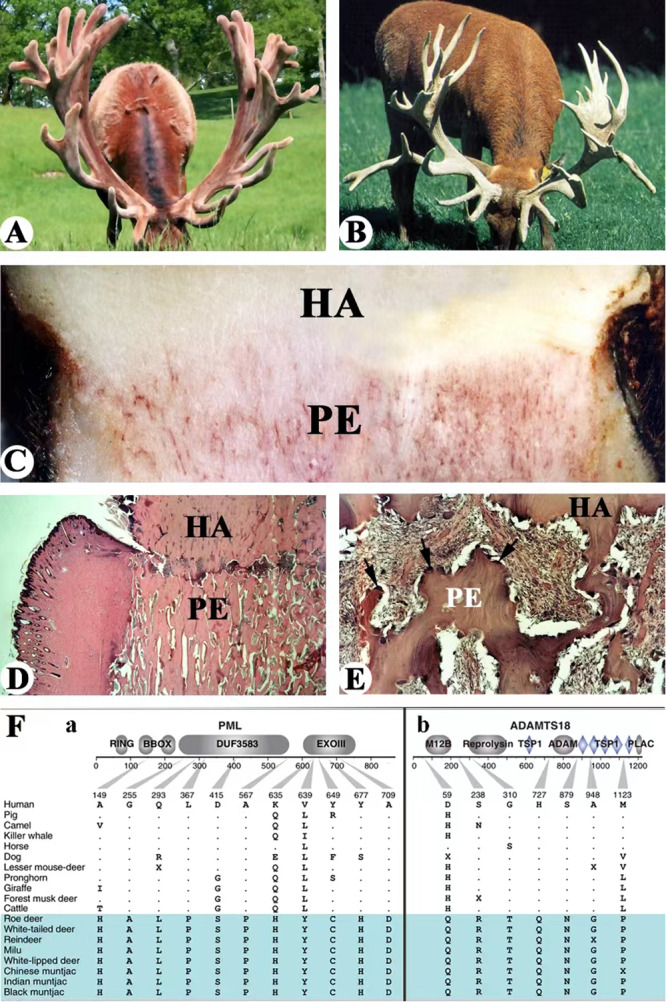
A Huge size of velvet antlers grown by a red deer stag in summer. B Huge size of hard antlers grown by a red deer stag in autumn, note that the antlers were fully calcified and dead. C Interface between dead hard antler (HA) and live pedicle (PE), note that texture of hard antlers are dense and white, and live pedicles are densely populated with blood vessels (red thread-like). D, E Process of hard antler (HA) casting from its living pedicle (PE). D At lower magnification, note that abscission line had developed between the antler and pedicle. E Higher magnification to show the abscission line, note that osteoclasts were densely distributed along the abscission line (arrows). F Examples of positively selected tumor suppressor genes in deer. A Gene models showing deer-specific mutations of two positively selected tumor suppressor genes, PML (a) and ADAMTS18 (b), note that PML genes has the strongest selection signals detected in deer. (modified from Fig. 4A, Wang et al. [26]; courtesy of Prof. Qiu Qiang).
Positive selection of cancer suppressive genes specific to deer
Besides efficient apoptosis [49–51] and annual wholesale casting, positive selection of some strong cancer suppressive genes to deer during evolution have been discovered recently through transcriptomic and comparative genomic analyses [26]. The positive selection of cancer suppressive genes may have constituted the third strategy of cancer prevention in deer. These genes include three p53 cofactor genes (PML, NMT2, and CD2AP) and five p53 regulator genes (ELOVL6, S100A8, ISG15, CNOT3, and CCDC69), all of which are strongly expressed in the growing antler (Fig. 3Fa). Given these genes and cofactors have functions in the p53 pathway [52–54], it suggests that deer may have evolved an enhanced TP53 signaling pathway to constrain tumor growth.
Among these, the tumor suppressor gene PML is of particular interest. It has 11 deer-specific non-synonymous changes and carries the strongest signal of positive selection detected in deer. PML is a transcriptional coactivator of p53, and its overexpression enhances p53 transcriptional activation and leads to arrest of cell growth [55]. The TP53 signaling pathway plays a central role in the regulation of cell division and prevention of tumor formation [56–58]. TP53 itself has also been identified as a rapidly evolving gene in the deer lineage from the evolutionary analysis of 51 ruminant genomes [59]. Several other tumor suppressor genes which appear to be under positive selection in deer are also expressed in antlers; these include ADAMTS18 (Fig. 3Fb), a member of the ADAMTS family. Several genes (TP73, TP53I13, SLF1, RHNO1, and DDB2) involved in DNA damage response pathways are also evident in deer-specific evolution. Of these, TP73 and TP53I13 which suppress tumors through their roles in the p53-mediated DNA damage response pathway [60] are specifically expressed in antlers [26]. Recently, the team, who discovered those tumor suppressor genes, led by Prof. Wang is actively investigating the roles of these genes in human tumor cells through the approach of gain-of-function analysis. The preliminary results have been encouraging, particularly with the overexpression of PML genes (personal communication). Overall, the deer-specific expression and genetic changes in these tumor suppressor and DNA repair genes may play important roles in fine-tuning of the regulatory network of rapid antler regeneration, and at the same time helping prevent the onset of cancers.
Now one wonders how such a thing can be possible for an organ that has adopted the way of cancer metabolism in order to form the tissue mass proportional to the body size of its carrier in a very limited timeframe; on the other hand, the carrier evolved potent ability to activate anticancer genes to counteract the tendency of neoplastic transformation of rapidly proliferating cells in the organ. One possible explanation would be that tumor suppressor genes only selectively express in some cells, such as pre-cancerous cells; but not in others in order for keeping these cells in fast proliferation mode, which is evidenced by the differential proliferation rate among these cells (personal observation).
Revealing the mechanism underlying the fine balance between the unparalleled growth rate and maintenance of genome stability in the antler model would undoubtedly help further understanding of the initial occurrence of cancer and devise more targeted drugs for cancer treatment.
Applications in the clinic?
Although not being recorded and formally published in the literature (but normally stated in the textbooks, such as Ma et al. [61]), anti-cancer activities of velvet antlers have been frequently talked about by folks. For instance, to deer themselves, people who have long, intimate experience with deer, such as deer farmers and hunters, would notice that deer are almost disease-free, certainly including tumor-free, during the period of velvet growing season, but not before (till early antler growing stage) or after (artificially removed during the growth period for velvet production or reach to the final calcification stage naturally). To humans, people who live in deer hometowns in Northeast China believe that the consumption of velvet antlers not only boosts the immune system, anti-fatigue and enhances sexuality, but also suppresses tumor formation. Thus, regular consumers of velvet antlers are rare dead of cancer. Therefore, it would be very fruitful to carry out a formal survey to register whether the inverse relationship between antler and tumor is purely coincidental or causal.
From the foregoing review of the relationship between the fastest-growing speed and lowest cancer incidence in antlers has inspired researchers to consider the antler model in addressing some aspects of cancer treatment. One approach has been taken, i.e., to treat cancer-bearing animals by directly applying different types of antler preparations (such as extracts, powder, and slices). The second, still speculative though, is to induce total calcification of developing tumors. The latter is inspired by the phenomenon of the annual demise of deer antlers through intensive mineralization.
Effects of velvet antler preparations on different types of cancers
Deer antlers have been used in traditional Chinese medicine (TCM) for over two thousand years as tonics and for treating different diseases [62]. To retain the maximum medicinal value, antlers are harvested during their rapid growing phase; and as these antlers are still enveloped in the velvet skin, so-called velvet antlers. After removal, velvet antlers are either dried in a traditional way (boiling and air-drying) or by freeze-drying. Dried antlers are further processed to final form as slices, powder, or extracts.
Given the evidence for the lack of neoplasia in the growing antler and the positive selection for cancer suppressive genes during deer evolution [26], the hypothesis is that some ‘gene products’ (including downstream derivatives) of these tumor suppressor genes would show anticancer activities. Therefore, given some survival of active forms of these ‘gene products’ through processing, the interest is in the evaluation of processed velvet antler extracts (particularly through freeze-drying) for their anti-neoplastic properties.
The first relevant experiment relating to the anti-cancer effects of velvet antler extracts (VAE) was reported by Fan et al. [63] in mice inoculated with sarcoma180 cells. They showed that administration of VAE via intraperitoneal injection significantly prolonged the life of the cancer-bearing mice from 15 to 20 days. Xiong et al. [64] achieved even more dramatic results by applying VAE of the antler tip in the same animal model (sarcoma 180 cells) via intraperitoneal injection. They found that VAE treatment significantly reduced tumor weight (0.52 g vs 3.11 g in control), and prolonged the life of tumor-bearing mice (42.2 d vs 19.2 d in control). Fraser et al. [65] reported treatment of colon cancer in a mouse model where oral administration of VAE effectively decreased the severity of colon cancer, evidenced by a prolonged the survival period and reduced the tumor weight of mice injected abdominally with sarcoma 180 cells. Hu et al. [66] found a polypeptide extract effectively inhibited the proliferation and telomerase activity of the rat breast cancer cell line MA782 in vitro.
AVE has also been evaluated in prostate cancer (PC) by Tang et al. [67] and Yang et al. [68]. They reported that VAE significantly inhibited proliferation and formation of colony units of PC cells (LNCap and PC3M cell lines, respectively); whereas, no adverse side-effects were detected in the non-cancer cell lines; and even with strong mitogenic effects on some of these cell lines (such as HEK 293). Subsequently, Tang et al. [69] carried out a study on mice and found that VAE was administered via gavage and effectively inhibited tumor growth (Fig. 4A), evidenced by significantly reduced tumor size (Fig. 4B) and weight (Fig. 4C) as effectively as cisplatin for the PC, but did not show adverse side-effects. Given cisplatin is one of the important chemotherapeutic agents for the treatment of various cancers, but has severe side effects [70]. Therefore, if the VAE is used to treat PC patients, it would be predictably safe and effective.
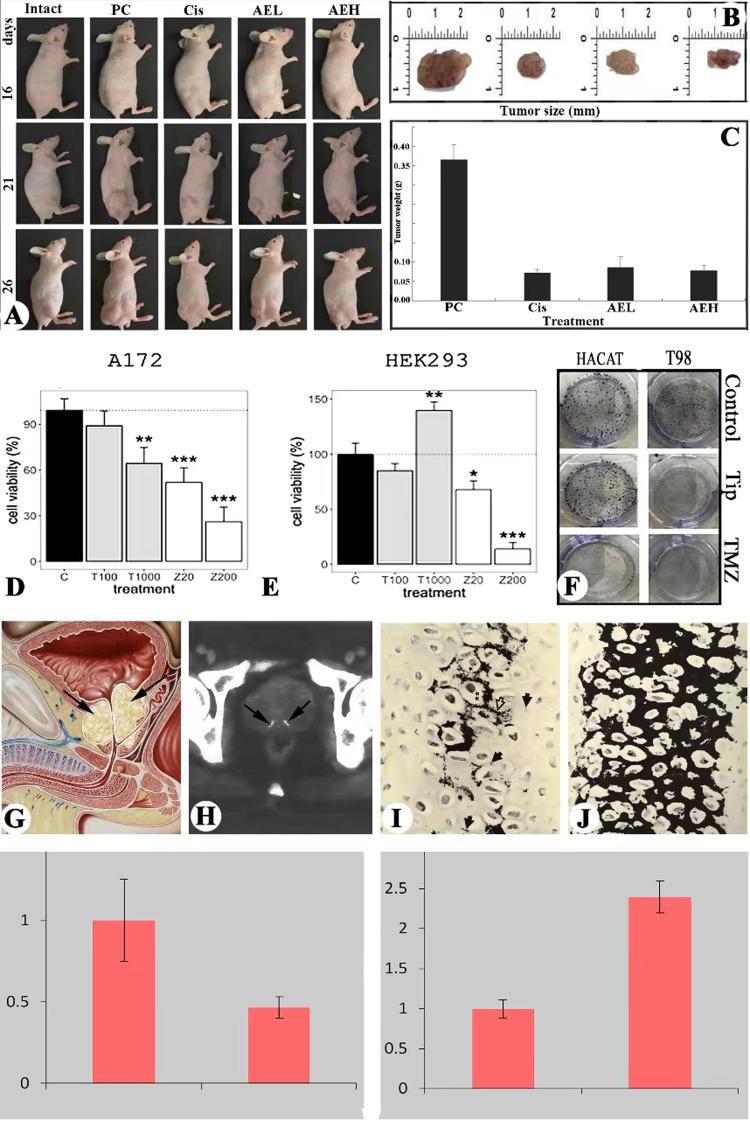
Fig. 4. Insights into clinic use.
A Nude mice bearing human prostate cancer; note that VAE treatment significantly inhibited tumor growth compared to the positive control (no treatment). VAE, velvet antler extracts; Cis, cisplatin; AEL, low concentration of VAE; AEH, high concentration of VAE. B Tumor size from the different treatment groups; note that the size of tumors from the AVE treated groups are comparable to that from the Cis treated groups (positive control). C Tumor weight from the different treatment groups; note that the weight of tumors from the VAE treated groups are comparable to that from the Cis treated groups (positive control). (Figures A–C: Modified from Fig. 2. Tang et al, 2018. Courtesy of Dr Yujiao Tang). D, F Effects of AVE treatment on glioblastoma cell line T98G and non-cancer cell line HEK293. D VAE significantly inhibited T98G cell proliferation compared to the control, and in a dose-dependent manner; (E) VAE significantly stimulated T98G cell proliferation compared to the control; and (F). VAE significantly reduced toxicity of TMZ to HEK 293 cells. TMZ, temozolomide; 519L1, extract of a growing antler tip of #519 red deer. (Figures D–F: Courtesy of Dr Louis Chonco). G Schematic drawing of prostate glands (arrows), note that the glands are located between the bladder (asterisk) and the urogenital diaphragm and surround the root of the urethra. H A computed tomography (CT) scan of prostate gland, arrows point to the localized foci of calcification. I, J Cartilaginous columns in the cartilage zone of the antler growth center, note that these columns were subjecting intensive calcification (black color) around the lacunae of chondrocytes. I At the more distal end of the zone, note that the column was still forming and mature chondrocytes located in the center that were initializing mineralization. J At the center region of cartilage zone, note that cartilaginous cells of the entire column had matured and subjecting intensive mineralization. (Modified from Fig. 13 and 14, respectively, after Banks and Newbrey, 1982). K, L Cell proliferation rate either singular or co-cultured with antler RM cells via CCK8. K Human osteosarcoma cell line U2OS (cancer cell line); (L) Fibroblast cell line 3T3 (normal cell line). Note that when co-cultured with antler RM cells, proliferation rate of U2OS was significantly decreased; whereas, that of 3T3 was significantly increase (Hu et al., unpublished).
In the last few years, we participated a project led by Prof. Landete-Castillejos using VAE from growing antler tips to treat glioblastoma cell lines T98G and A172 in vitro [71]. Glioblastoma is a type of neuronal cancer that has a rather low survival rate. The results convincingly demonstrated that VAE significantly inhibited A172 proliferation (Fig. 4D); whereas, had strong positive mitogenic effects on HEK 293 (Fig. 4E), indicating no adverse side-effects of VAE on non-cancerous cells/tissues. Furthermore, VAE significantly stimulated the formation of colony units of non-cancerous cell line HACAT, but inhibited cancerous cell line T98G doing so (Fig. 4F). Overall, VAE from the antler tip has the similar anti-glioblastoma effect to TMZ, but no adverse side effects are detected. Therefore, VAE contains bioactive compounds with tumor suppressor properties and might be developed as a valuable therapeutic drug for the treatment of glioblastoma in the future.
Recently, our group investigated the effects of antler RM cells (antler growth center cells) on the proliferation of human osteosarcoma cell line U2OS via a co-culture approach. The preliminary results showed that RM cells significantly inhibited U2OS cell proliferation; whereas, significantly increased 3T3 cell proliferation (Hu et al, unpublished). Suppression effects of the RM cells/exosomes on human osteosarcomas in vivo are currently under investigation. These results not only demonstrate that antler growth center cells have suppression effects on a certain type of cancers but also have a reasonable wide spectrum of anti-tumor activities.
Antler full calcification gives insights into the treatment of prostate cancer
The prostate is an unpaired (two halves) accessory (parenchymal) gland of the male reproductive system; located between the bladder and the urogenital diaphragm and surrounding the root of the urethra (Fig. 4G). The occurrence of PC is generally related to genetic factors. Although PC can be treated successfully at an early stage by radical prostatectomy or radiation therapy, most patients later experience local recurrence and distant metastases [72]. Not uncommonly, after short-term remission (18-24 months), surviving tumor cells recur, causing castrate-resistant PC (CRPC) with inevitable progression and death within 2–3 years in most men [73, 74]. In CRPC progression, tumor cells acquire the ability to both survive in the absence of androgens and proliferate using non-androgenic stimuli for mitogenesis [75].
Calcification of prostate gland tissue in humans has been reported particularly in aged men, but only in a very localized manner (foci) under normal levels of circulating sex hormones (Fig. 4H). Calcification is normally caused by chronic inflammation due to the deposition of the calcium salts in the acini of the prostate gland. If the mechanism of the total calcification of antler tissue under high levels of circulating sex hormones can be defined, PC tissue may be efficiently killed through targeted administration of high levels of androgens, possibly preferentially fully calcifying the interstitial tissue from which collagens are secreted. In so doing, the life of the patients may be effectively prolonged.
Acknowledgements
We authors thank Dr. Peter Fennessy for critically reading through the paper and helpful and constructive criticisms.
Author contributions
CL, YL, WW, GM, RD and YS conceived the project and wrote the manuscript. All of the Authors have written, corrected and approved the submitted version.
Funding
This study was supported by grants from the National Natural Science Foundation of China (No. U20A20403).
Data availability
Data are available from the corresponding authors upon reasonable request.
Competing interests
GM and YS are members of the Editorial Board of Cell Death & Differentiation and of Cell Death & Disease. The authors declare no other conflict of interests.
Ethics
All the procedures carried out in the research with participation of humans were in compliance with the ethical standards of the institutional and/or national ethics committee and with the Helsinki Declaration of 1964 and its subsequent changes or with comparable ethics standards. Informed voluntary consent was obtained from every participant of the study. Animal work was according to Ministry of Health regulation, respecting ethics and safety for the mice. All authorizations were obtained for specific strains and detailed experiments.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gerry Melino, Email: melino@uniroma2.it.
Rui Du, Email: durui197107@sina.com.
Yufang Shi, Email: shiyufang2@gmail.com.
References
- 1.Tomasetti C, Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015;347:78–81. doi: 10.1126/science.1260825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carbone M, Amelio I, Affar EB, Brugarolas J, Cannon-Albright LA, Cantley LC, et al. Consensus report of the 8 and 9th Weinman Symposia on Gene x Environment Interaction in carcinogenesis: novel opportunities for precision medicine. Cell Death Differ. 2018;25:1885–904. doi: 10.1038/s41418-018-0213-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu S, Powers S, Zhu W, Hannun YA. Substantial contribution of extrinsic risk factors to cancer development. Nature. 2016;529:43–47. doi: 10.1038/nature16166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boddy AM, Kokko H, Breden F, Wilkinson GS, Aktipis CA. Cancer susceptibility and reproductive trade-offs: a model of the evolution of cancer defences. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140220. [DOI] [PMC free article] [PubMed]
- 5.Fredrickson TN. Ovarian tumors of the hen. Environ Health Perspect. 1987;73:35–51. doi: 10.1289/ehp.877335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson PA, Giles JR. The hen as a model of ovarian cancer. Nat Rev Cancer. 2013;13:432–6. doi: 10.1038/nrc3535. [DOI] [PubMed] [Google Scholar]
- 7.Kotsopoulos J, Lubinski J, Gronwald J, Cybulski C, Demsky R, Neuhausen SL, et al. Factors influencing ovulation and the risk of ovarian cancer in BRCA1 and BRCA2 mutation carriers. Int J Cancer. 2015;137:1136–46. doi: 10.1002/ijc.29386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goss RJ. Future directions in antler research. Anat Rec. 1995;241:291–302. doi: 10.1002/ar.1092410302. [DOI] [PubMed] [Google Scholar]
- 9.Li C, Suttie JM, Clark DE. Morphological observation of antler regeneration in red deer (Cervus elaphus) J Morphol. 2004;262:731–40. doi: 10.1002/jmor.10273. [DOI] [PubMed] [Google Scholar]
- 10.Landete-Castillejos T, Kierdorf H, Gomez S, Luna S, Garcia AJ, Cappelli J, et al. Antlers - Evolution, development, structure, composition, and biomechanics of an outstanding type of bone. Bone. 2019;128:115046. doi: 10.1016/j.bone.2019.115046. [DOI] [PubMed] [Google Scholar]
- 11.Li C, Fennessy P. The periosteum: a simple tissue with many faces, with special reference to the antler-lineage periostea. Biol Direct. 2021;16:17. doi: 10.1186/s13062-021-00310-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.>Li C Annual antler renewal: a unique case of stem cell-based mammalian organ regeneration. 19th Annual Queenstown Molecular Biology Meeting. 2009; Queenstown. p. 38.
- 13.Guo Q, Liu Z, Zheng J, Zhao H, Li C. Substances for regenerative wound healing during antler renewal stimulated scar-less restoration of rat cutaneous wounds. Cell Tissue Res. 2021;386:99–116. doi: 10.1007/s00441-021-03505-9. [DOI] [PubMed] [Google Scholar]
- 14.Li C, Chu W. The regenerating antler blastema: the derivative of stem cells resident in a pedicle stump. Front Biosci (Landmark Ed) 2016;21:455–67. doi: 10.2741/4401. [DOI] [PubMed] [Google Scholar]
- 15.Fennessy P, Corson I, Suttie J, Littlejohn R Antler growth patterns in young red deer stags. The Biology of Deer. 1992; Mississippi State University: Springer-Verlag: pp. 487-92.
- 16.Gao Z, Li C. The study on the relationship between antler’s growth rate, relative bone mass and circulation testosterone, estradiol, AKP in sika deer. Acta Vet Zootech Sin. 1988;19:224–31. [Google Scholar]
- 17.Goss RJ. Problems of antlerogenesis. Clin Orthop Relat Res. 1970;69:227–38. [PubMed] [Google Scholar]
- 18.Chapman DI. Antlers-bones of contention. Mammal Rev. 1975;5:121–72. [Google Scholar]
- 19.Banks W, Newbrey J. Antler Development in Cervidae. Kingsville: Caesar Kleberg Wildl. Res. Inst.; 1982. Light microscopic studies of the ossification process in developing antlers; pp. 231–60. [Google Scholar]
- 20.Li C, Clark DE, Lord EA, Stanton JA, Suttie JM. Sampling technique to discriminate the different tissue layers of growing antler tips for gene discovery. Anat Rec. 2002;268:125–30. doi: 10.1002/ar.10120. [DOI] [PubMed] [Google Scholar]
- 21.Sadighi M, Haines SR, Skottner A, Harris AJ, Suttie JM. Effects of insulin-like growth factor-I (IGF-I) and IGF-II on the growth of antler cells in vitro. J Endocrinol. 1994;143:461–9. doi: 10.1677/joe.0.1430461. [DOI] [PubMed] [Google Scholar]
- 22.Elliott JL, Oldham JM, Ambler GR, Bass JJ, Spencer GS, Hodgkinson SC, et al. Presence of insulin-like growth factor-I receptors and absence of growth hormone receptors in the antler tip. Endocrinology. 1992;130:2513–20. doi: 10.1210/endo.130.5.1315246. [DOI] [PubMed] [Google Scholar]
- 23.Elliott JL, Oldham JM, Ambler GR, Molan PC, Spencer GS, Hodgkinson SC, et al. Receptors for insulin-like growth factor-II in the growing tip of the deer antler. J Endocrinol. 1993;138:233–42. doi: 10.1677/joe.0.1380233. [DOI] [PubMed] [Google Scholar]
- 24.Suttie JM, Gluckman PD, Butler JH, Fennessy PF, Corson ID, Laas FJ. Insulin-like growth factor 1 (IGF-1) antler-stimulating hormone? Endocrinology. 1985;116:846–8. doi: 10.1210/endo-116-2-846. [DOI] [PubMed] [Google Scholar]
- 25.Suttie JM, Fennessy PF Growth promoting hormones and antler development. 18th Congress, IUGB. 1987; Krakow. pp. 194-5.
- 26.Wang Y, Zhang C, Wang N, Li Z, Heller R, Liu R, et al. Genetic basis of ruminant headgear and rapid antler regeneration. Science. 2019;364:eaav6335. [DOI] [PubMed]
- 27.Ludlow AT, Wong MS, Robin JD, Batten K, Yuan L, Lai TP, et al. NOVA1 regulates hTERT splicing and cell growth in non-small cell lung cancer. Nat Commun. 2018;9:3112. doi: 10.1038/s41467-018-05582-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li C, Suttie JM. Light microscopic studies of pedicle and early first antler development in red deer (Cervus elaphus) Anat Rec. 1994;239:198–215. doi: 10.1002/ar.1092390211. [DOI] [PubMed] [Google Scholar]
- 29.Li C, Suttie JM, Clark DE. Histological examination of antler regeneration in red deer (Cervus elaphus) Anat Rec A Discov Mol Cell Evol Biol. 2005;282:163–74. doi: 10.1002/ar.a.20148. [DOI] [PubMed] [Google Scholar]
- 30.Lombard LS, Witte EJ. Frequency and types of tumors in mammals and birds of the Philadelphia Zoological Garden. Cancer Res. 1959;19:127–41. [PubMed] [Google Scholar]
- 31.Griner LA. A review of necropsies conducted over a fourteen-year period at the San Diego Zoo and San Diego Wild Animal Park. Pathology of Zoo Animals. 1983.
- 32.Annibaldi A, Widmann C. Glucose metabolism in cancer cells. Curr Opin Clin Nutr Metab Care. 2010;13:466–70. doi: 10.1097/MCO.0b013e32833a5577. [DOI] [PubMed] [Google Scholar]
- 33.Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 34.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–10. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 36.Wu H, Ding Z, Hu D, Sun F, Dai C, Xie J, et al. Central role of lactic acidosis in cancer cell resistance to glucose deprivation-induced cell death. J Pathol. 2012;227:189–99. doi: 10.1002/path.3978. [DOI] [PubMed] [Google Scholar]
- 37.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goss RJ. Regeneration, Function and Evolution. New York: Academic Press; 1983. Deer Antlers. [Google Scholar]
- 39.Bubenik GA Endocrine regulation of the antler cycle. Antler Development in Cervidae. Kingsville: Caesar Kleberg Wildl. Res. Inst.; 1982. pp. 73-107.
- 40.Goss RJ. Tumor-like growth of antlers in castrated fallow deer: an electron microscopic study. Scanning Microsc. 1990;4:715–20. [PubMed] [Google Scholar]
- 41.Kierdorf U, Kierdorf H, Schultz M, Rolf HJ. Histological structure of antlers in castrated male fallow deer. Discoveries Mol Cell Evolut Biol. 2004;281:1352–62. doi: 10.1002/ar.a.20127. [DOI] [PubMed] [Google Scholar]
- 42.Goss RJ. Inhibition of growth and shedding of antlers by sex hormones. Nature. 1968;220:83–85. doi: 10.1038/220083a0. [DOI] [PubMed] [Google Scholar]
- 43.Guo M, Hay BA. Cell proliferation and apoptosis. Curr Opin Cell Biol. 1999;11:745–52. doi: 10.1016/s0955-0674(99)00046-0. [DOI] [PubMed] [Google Scholar]
- 44.Colitti M, Allen SP, Price JS. Programmed cell death in the regenerating deer antler. J Anat. 2005;207:339–51. doi: 10.1111/j.1469-7580.2005.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Antonsson B, Martinou JC. The Bcl-2 protein family. Exp Cell Res. 2000;256:50–57. doi: 10.1006/excr.2000.4839. [DOI] [PubMed] [Google Scholar]
- 46.Suttie J, Fennessy P Recent advances in the physiological control of velvet antler growth. The Biology of Deer. 1992 Springer-Verlag. pp. 471-86.
- 47.Fennessy PF, Suttie JM, Crosbie SF, Corson ID, Elgar HJ, Lapwood KR. Plasma LH and testosterone responses to gonadotrophin-releasing hormone in adult red deer (Cervus elaphus) stags during the annual antler cycle. J Endocrinol. 1988;117:35–41. doi: 10.1677/joe.0.1170035. [DOI] [PubMed] [Google Scholar]
- 48.Suttie JM, Fennessy PF, Lapwood KR, Corson ID. Role of steroids in antler growth of red deer stags. J Exp Zool. 1995;271:120–30. doi: 10.1002/jez.1402710207. [DOI] [PubMed] [Google Scholar]
- 49.Moujalled D, Strasser A, Liddell JR. Molecular mechanisms of cell death in neurological diseases. Cell Death Differ. 2021;28:2029–44. doi: 10.1038/s41418-021-00814-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dasgupta S, Ghosh T, Dhar J, Bhuniya A, Nandi P, Das A, et al. RGS5-TGFbeta-Smad2/3 axis switches pro- to anti-apoptotic signaling in tumor-residing pericytes, assisting tumor growth. Cell Death Differ. 2021;28:3052–76. doi: 10.1038/s41418-021-00801-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martens S, Bridelance J, Roelandt R, Vandenabeele P, Takahashi N. MLKL in cancer: more than a necroptosis regulator. Cell Death Differ. 2021;28:1757–72. doi: 10.1038/s41418-021-00785-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yin K, Lee J, Liu Z, Kim H, Martin DR, Wu D, et al. Mitophagy protein PINK1 suppresses colon tumor growth by metabolic reprogramming via p53 activation and reducing acetyl-CoA production. Cell Death Differ. 2021;28:2421–35. doi: 10.1038/s41418-021-00760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Y, Cui K, Zhang Q, Li X, Lin X, Tang Y, et al. FBXL6 degrades phosphorylated p53 to promote tumor growth. Cell Death Differ. 2021;28:2112–25. doi: 10.1038/s41418-021-00739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jin JO, Lee GD, Nam SH, Lee TH, Kang DH, Yun JK, et al. Sequential ubiquitination of p53 by TRIM28, RLIM, and MDM2 in lung tumorigenesis. Cell Death Differ. 2021;28:1790–803. doi: 10.1038/s41418-020-00701-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pearson M, Pelicci PG. PML interaction with p53 and its role in apoptosis and replicative senescence. Oncogene. 2001;20:7250–6. doi: 10.1038/sj.onc.1204856. [DOI] [PubMed] [Google Scholar]
- 56.Kastenhuber ER, Lowe SW. Putting p53 in Context. Cell. 2017;170:1062–78. doi: 10.1016/j.cell.2017.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amelio I, Melino G. The p53 family and the hypoxia-inducible factors (HIFs): determinants of cancer progression. Trends Biochem Sci. 2015;40:425–34. doi: 10.1016/j.tibs.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 58.Candi E, Cipollone R, Rivetti di Val Cervo P, Gonfloni S, Melino G, Knight R. p63 in epithelial development. Cell Mol Life Sci. 2008;65:3126–33. doi: 10.1007/s00018-008-8119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen L, Qiu Q, Jiang Y, Wang K, Lin Z, Li Z, et al. Large-scale ruminant genome sequencing provides insights into their evolution and distinct traits. Science. 2019;364:eaav6202. [DOI] [PubMed]
- 60.Meek DW. Tumour suppression by p53: a role for the DNA damage response? Nat Rev Cancer. 2009;9:714–23. doi: 10.1038/nrc2716. [DOI] [PubMed] [Google Scholar]
- 61.Ma. Deer Production and Disease. Jilin Press of Science and Technology, 1998.
- 62.Kong YC, But PPH Deer: The ultimate medicinal animal (antler and deer parts in medicine). Biology of deer production. 1985 Wellington. pp. 311-24.
- 63.Fan YL, Xing Z, Wei Q. A study on the extraction separation and anticancer activity of velvet antler protein. J Economic Anim. 1998;3:27–31. [Google Scholar]
- 64.Xiong HL Extraction and isolation of activity component from velvet antler and research of its anti-tumor effect. Northwest A&F University. 2007.
- 65.Fraser A, Haines SR, Stuart EC, Scandlyn MJ, Alexander A, Somers-Edgar TJ, et al. Deer velvet supplementation decreases the grade and metastasis of azoxymethane-induced colon cancer in the male rat. Food Chem Toxicol. 2010;48:1288–92. doi: 10.1016/j.fct.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 66.Hu W, Qi L, Tian YH, Hu R, Wu L, Meng XY. Studies on the purification of polypeptide from sika antler plate and activities of antitumor. BMC Complement Alter Med. 2015;15:328. doi: 10.1186/s12906-015-0845-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang Y, Jeon BT, Wang Y, Choi EJ, Kim YS, Hwang JW, et al. First Evidence that Sika Deer (Cervus nippon) Velvet Antler Extract Suppresses Migration of Human Prostate Cancer Cells. Korean J Food Sci Anim Resour. 2015;35:507–14. doi: 10.5851/kosfa.2015.35.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang H, Wang L, Sun H, He X, Zhang J, Liu F. Anticancer activity in vitro and biological safety evaluation in vivo of Sika deer antler protein. J Food Biochem. 2017;41:e12421.
- 69.Tang Y, Fan M, Choi YJ, Yu Y, Yao G, Deng Y, et al. Sika deer (Cervus nippon) velvet antler extract attenuates prostate cancer in xenograft model. Biosci Biotechnol Biochem. 2019;83:348–56. doi: 10.1080/09168451.2018.1537775. [DOI] [PubMed] [Google Scholar]
- 70.Palipoch S, Punsawad C. Biochemical and histological study of rat liver and kidney injury induced by Cisplatin. J Toxicol Pathol. 2013;26:293–9. doi: 10.1293/tox.26.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chonco L, Landete-Castillejos T, Serrano-Heras G, Serrano MP, Perez-Barberia FJ, Gonzalez-Armesto C, et al. Anti-tumour activity of deer growing antlers and its potential applications in the treatment of malignant gliomas. Sci Rep. 2021;11:42. doi: 10.1038/s41598-020-79779-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fleshner N. Defining high-risk prostate cancer: current status. Can J Urol. 2005;12:14–17. [PubMed] [Google Scholar]
- 73.Gleave ME, Bruchovsky N, Moore MJ, Venner P. Prostate cancer: 9. Treatment of advanced disease. CMAJ. 1999;160:225–32. [PMC free article] [PubMed] [Google Scholar]
- 74.Moreau JP, Delavault P, Blumberg J. Luteinizing hormone-releasing hormone agonists in the treatment of prostate cancer: a review of their discovery, development, and place in therapy. Clin Ther. 2006;28:1485–508. doi: 10.1016/j.clinthera.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 75.Lamoureux F, Thomas C, Yin MJ, Kuruma H, Fazli L, Gleave ME, et al. A novel HSP90 inhibitor delays castrate-resistant prostate cancer without altering serum PSA levels and inhibits osteoclastogenesis. Clin Cancer Res. 2011;17:2301–13. doi: 10.1158/1078-0432.CCR-10-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the corresponding authors upon reasonable request.