Abstract
The denitrifying bacterium Azoarcus anaerobius LuFRes1 grows anaerobically with resorcinol (1,3-dihydroxybenzene) as the sole source of carbon and energy. The anaerobic degradation of this compound was investigated in cell extracts. Resorcinol reductase, the key enzyme for resorcinol catabolism in fermenting bacteria, was not present in this organism. Instead, resorcinol was hydroxylated to hydroxyhydroquinone (HHQ; 1,2,4-trihydroxybenzene) with nitrate or K3Fe(CN)6 as the electron acceptor. HHQ was further oxidized with nitrate to 2-hydroxy-1,4-benzoquinone as identified by high-pressure liquid chromatography, UV/visible light spectroscopy, and mass spectroscopy. Average specific activities were 60 mU mg of protein−1 for resorcinol hydroxylation and 150 mU mg of protein−1 for HHQ dehydrogenation. Both activities were found nearly exclusively in the membrane fraction and were only barely detectable in extracts of cells grown with benzoate, indicating that both reactions were specific for resorcinol degradation. These findings suggest a new strategy of anaerobic degradation of aromatic compounds involving oxidative steps for destabilization of the aromatic ring, different from the reductive dearomatization mechanisms described so far.
The biochemistry of anaerobic degradation of aromatic compounds has received considerable attention because it comprises rather unusual reactions and mechanisms. In contrast to the aerobic degradation pathways, which are quite uniform and widely understood, in the anaerobic world a larger variety of pathways is known, and more are expected to be found in the future (for reviews, see references 13, 14, and 25). Three central intermediates in the anaerobic metabolism of aromatic compounds are known to be substrates for dearomatization as a preparation for ring cleavage: benzoyl coenzyme A (benzoyl-CoA), phloroglucinol (1,3,5-trihydroxybenzene), and resorcinol (1,3-dihydroxybenzene). In all of these cases, a reduction was shown to be the key reaction for dearomatization, and the corresponding reductases were purified (2, 11, 25a, 28). Recently, a further reducing activity with hydroxyhydroquinone (1,2,4-trihydroxybenzene [HHQ]) as the substrate was found in the sulfate-reducing bacterium Desulfovibrio inopinatus (24).
An exception to these reductive strategies for dearomatization appeared to be manifested in two strains of denitrifying bacteria growing with resorcinol as the sole source of carbon and energy, since neither resorcinol reductase nor any of the key enzymes of the other anaerobic pathways could be detected (10, 16). Strain LuFRes1, which was isolated from sewage sludge and described as an obligately nitrate-reducing bacterium, converts resorcinol with nitrate completely to CO2 and N2 (10). This strain was recently described as a new species of the genus Azoarcus, A. anaerobius (26). In resorcinol degradation studies with dense suspensions of resting cells, a new compound which was identified by mass spectroscopy as 5-oxo-2-hexenoic acid was detected. This product would result from direct hydrolysis of resorcinol, but no resorcinol-hydrolyzing activity was detectable in cell extracts (10).
In this work, we reexamined resorcinol degradation by A. anaerobius and found in cell extracts two new reactions which most probably initiate the degradation pathway.
MATERIALS AND METHODS
Medium and growth conditions.
A. anaerobius LuFRes1 (DSM 12081) was grown in nonreduced bicarbonate-buffered mineral medium (30) containing 8 mM NaNO3–1 mM Na2SO4 as the sulfur source, vitamin solution, selenite-tungstate solution (29), and the trace element solution SL10 (31). The medium was dispensed anoxically into infusion bottles which were sealed with butyl rubber septa. Substrates were added from sterile anoxic stock solutions. Growth was followed by measuring the turbidity at 578 nm in a Hitachi 100-40 spectrophotometer.
Preparation of dense cell suspensions.
Cells were grown in 1-liter infusion bottles starting with 2 mM resorcinol. After substrate depletion, another 1 mM resorcinol was added. Final optical densities of 0.5 to 1.0 were reached. Cells were harvested in the late logarithmic phase under anoxic conditions by centrifugation at 6,000 × g for 25 min at 4°C in a Sorvall centrifuge. The pellets were washed once with anoxic potassium phosphate buffer (50 mM, pH 7.0). Dense cell suspensions were prepared by resuspending the pellets in small amounts of the same buffer (mostly 3 ml of buffer for a pellet resulting from 1 liter of culture). For degradation experiments, cells were added to 2 ml of 50 mM anoxic potassium phosphate buffer (pH 7.0) containing 4 mM nitrate to a final optical density of 2.0 (equivalent to ca. 0.43 mg [dry weight] per 2 ml). Experiments were performed under nitrogen gas in butyl rubber sealed Hungate tubes, and reactions were started by addition of resorcinol.
Preparation of cell extracts.
Dense cell suspensions were passed anoxically two times through a French press at 138 MPa. The crude extract was separated from cell debris by centrifugation at 20,000 × g for 20 min at 4°C. Fractionation of the crude extract was obtained by centrifugation at 100,000 × g and 4°C for 1 h in a TL ultracentrifuge (Beckman Instruments, Munich, Germany). The membrane fraction was resuspended in the original volume of the cytosol (mostly 1 to 3 ml) with anoxic potassium phosphate buffer (50 mM, pH 7.0) containing 150 mM KCl. For solubilization experiments, membranes were resuspended in the same buffer containing 5% (wt/vol) glycerol. Membrane proteins were solubilized by adding detergents {1% (vol/vol) Triton X-100 or 1% (wt/vol) 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS)} and incubating the mixtures for 1 h at 4°C under gentle stirring. Solubilized proteins were separated from the membranes by a further ultracentrifugation run.
Protein was quantified by the method of Bradford (3), with bovine serum albumin as the standard.
Measurement of enzyme activities.
All measurements of enzyme activities were performed under strictly anoxic conditions at 30°C in 5-ml Hungate tubes or 1.5-ml cuvettes, using anoxic buffers and solutions. The tubes and cuvettes were flushed with N2 and closed with butyl septa. All additions and samplings were done with gastight Unimetrix microliter syringes (Macherey-Nagel, Düren, Germany). Linear correlations between protein amount and product formation or substrate consumption rates were determined for all assays.
Resorcinol-hydroxylating activity was measured either continuously by a photometric assay or discontinuously by high-performance liquid chromatography (HPLC) analysis. A standard reaction mixture contained Tris HCl (50 mM, pH 7.1) with 2.5 mM CaCl2, 75 μl of crude extract (0.2 to 0.3 mg of protein), and 1 mM K3Fe(CN)6, and the reaction was started by addition of 1 mM resorcinol. In the photometric assay, the reduction of K3Fe(CN)6 was monitored in a Hitachi 100-40 spectrophotometer at 420 nm [ɛ420 of K3Fe(CN)6 = 0.9 mM−1 cm−1]. Discontinuous assays were performed by monitoring resorcinol degradation in samples taken at regular intervals from the reaction mixture. Samples (100 μl) were injected immediately into 400 μl of H3PO4 (100 mM) to stop all enzymatic reactions. After acidification, the samples were stable and could be stored at −20°C before HPLC analysis.
HHQ-oxidizing activity was measured discontinuously by monitoring HHQ degradation. A standard reaction mixture contained Tris HCl (50 mM, pH 8.0) or potassium phosphate buffer (50 mM, pH 7.0), 75 μl of crude extract (0.2 to 0.3 mg protein), and 1 mM HHQ, and the reaction was started by addition of 1 mM NaNO3. Samples (100 μl) were taken at regular intervals. Due to the high reactivity of the formed nitrite in acidic solutions, the test could not be stopped in H3PO4 but samples were diluted in ice-cold potassium phosphate buffer (400 μl, 50 mM, pH 7.0). These samples had to be injected immediately into the HPLC apparatus because HHQ and the reaction products rapidly autoxidized when exposed to air.
Maleylacetate reductase (15) and β-ketoadipate:succinyl-CoA transferase (21) were measured as described previously.
Analytical methods.
Aromatic compounds were analyzed with a high-performance liquid chromatograph (System Gold, Beckman Instruments) equipped with a C18 reversed-phase column (Grom, Herrenberg, Germany), a UV detector (Beckman 166 or 167), and an autosampler (Beckman 502). The eluent was composed of ammonium phosphate buffer (100 mM, pH 2.6) and methanol. Routine analysis was performed isocratically (15 or 20% methanol) with a detection wavelength of 206 nm. 2-Hydroxy-1,4-benzoquinone was analyzed with an ammonium acetate buffer (10 mM, pH adjusted to 4.79 by addition of acetic acid) and detection wavelength of 280 nm. Concentrations of aromatic compounds were calculated from external standards. Compounds were identified by comparison of retention times and by coelution with reference substances as well as by UV/visible light (VIS) spectroscopy using a Uvikon 930 spectrophotometer (Kontron Instruments, Zurich, Switzerland) or on-line scanning with a Beckman 168 diode array detector. Nitrite was determined chemically (22).
HPLC-mass spectroscopy.
Mass spectra were acquired on a Platform LC (Micromass, Manchester, United Kingdom), using negative ion electrospray. The needle voltage was set to 3.75 kV and the cone voltage was set to 26 V, giving soft ionization conditions. Nitrogen gas was used as the nebulizer and drying gas (150°C). The mass spectrometer was scanned from 50 to 600 Da at one scan per second. HPLC analysis was performed under the conditions described above for separation of 2-hydroxy-1,4-benzoquinone, using a Hewlett-Packard 1100 system.
Thermodynamic and electrochemical calculations.
ΔG°′ values were calculated from published data (27); the value for HHQ (−358 kJ mol−1) was taken from reference 24). The E° value for the redox pair 2-hydroxy-1,4-benzoquinone–HHQ (600 mV [8]) was corrected for pH 7.0 to E°′ = 180 mV.
Chemicals.
All chemicals were of analytical grade and highest purity available. Maleylacetate was prepared by alkaline hydrolysis of its cis-dienelactone, which was kindly provided by W. Reineke (Wuppertal, Germany) and H.-J. Seitz (Hohenheim, Germany).
RESULTS
Resorcinol degradation in dense cell suspensions.
Initial investigations on the resorcinol degradation pathway in A. anaerobius were performed with suspensions of resting cells. Previous studies with dense cell suspensions had shown that resorcinol degradation depended on the integrity of cytoplasmic membranes and on the presence of nitrate (10). Nitrate could be replaced by nitrite, N2O, and oxygen as electron acceptors (not shown). In experiments with nitrite, resorcinol reacted chemically with nitrite when samples were transferred to phosphoric acid to stop biological reactions. The chromatographic and UV/VIS spectroscopic properties of this chemical reaction product were identical to those of the previously described hydrolysis product 5-oxo-2-hexenoic acid. Therefore, formation of this compound was most probably an artifact of the analytical process.
Initial reaction of resorcinol degradation in cell extracts.
Various efforts were made to measure resorcinol degradation in cell extracts. The addition of ATP, phosphoenolpyruvate, acetyl-CoA, free CoA plus ATP, or a mixture of trace elements, as well as changes of pH and salt concentrations or application of reducing conditions, did not result in detectable degradation of resorcinol. A decrease in resorcinol concentration was observed only if electron acceptors with a positive redox potential, such as K3Fe(CN)6, dichlorophenol indophenol, NO3−, or O2, were used, suggesting that the initiating reaction in resorcinol catabolism was oxidative. An assay for resorcinol oxidation with K3Fe(CN)6 (E°′ = 360 mV) as the electron acceptor was developed for continuous and discontinuous tests. The reaction was catalyzed nearly exclusively by the membrane fraction (Table 1). Boiled extracts (incubation at 95°C for 10 min) had no activity. No activity could be detected at pH 4.5 and 5.8. At pH values higher than 7.5, resorcinol started to react chemically with K3Fe(CN)6, and at pH 8.4 this chemical reaction could not be distinguished from the biological one any more.
TABLE 1.
Initial specific activities of resorcinol-hydroxylating and HHQ-dehydrogenating enzymes after fractionation of the cell extract by ultracentrifugation (100,000 × g, 1 h)a
| Prepn | Protein (mg ml−1) | Activity
|
|||
|---|---|---|---|---|---|
| Resorcinol hydroxylating
|
HHQ dehydrogenating
|
||||
| Sp act (mU mg−1) | Vol act (mU ml−1) | Sp act (mU mg−1) | Vol act (mU ml−1) | ||
| Cell extract | 16.6 | 23.0 | 375.1 (100%) | 45.0 | 742.6 (100%) |
| Cytosolic fraction | 12.2 | <0.1 | <0.1 | 10.1 | 122.0 (16%) |
| Membrane fraction | 3.2 | 65.2 (2.8) | 206.2 (55%) | 175.1 (3.9) | 554.2 (75%) |
| Membrane fraction of benzoate-grown cells | 1.2 | 9.3 | 20.8 | ||
HHQ dehydrogenation was measured at pH 8.0. Protein concentrations of membrane fractions of resorcinol- and benzoate-grown cells applied in the assays were equal. Percentages in parentheses indicate yields; other values in parentheses represent enrichment factors.
Identification of the reaction product.
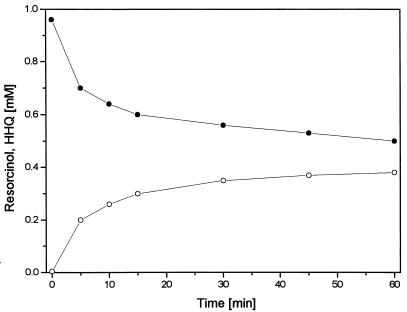
During resorcinol oxidation, HPLC chromatograms displayed a prominent new peak which increased proportionally with resorcinol consumption. The retention time of this reaction product was shorter than that of resorcinol, indicating a more hydrophilic character. By comparison of the chromatographic and UV spectroscopic properties of the product with those of the three trihydroxybenzene isomers (HHQ, 1,2,4-trihydroxybenzene; pyrogallol, 1,2,3-trihydroxybenzene; and phloroglucinol, 1,3,5-trihydroxybenzene), the unknown product was identified as HHQ. As shown in Fig. 1, 0.5 mM resorcinol was degraded but only up to 0.35 mM HHQ accumulated. This nonstoichiometric conversion was due to chemical oxidation of HHQ by K3Fe(CN)6 (see below). The reaction showed a relatively high initial rate which decreased within the first 10 min. In the initial phase, the specific activity reached values of 40 to 150 mU mg of protein−1.
FIG. 1.
Conversion of resorcinol (•) to HHQ (○) by the membrane fraction of A. anaerobius with 1 mM K3Fe(CN)6 as the electron acceptor.
Degradation of HHQ.
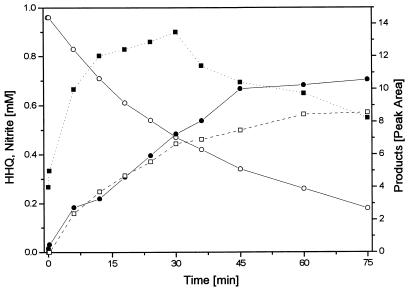
Cell extracts were examined for known reactions involved in HHQ metabolism by anaerobic bacteria. A transhydroxylating activity converting HHQ to phloroglucinol as observed in Pelobacter massiliensis (4) could not be detected. Phloroglucinol reductase was not present, nor could we detect reduction of HHQ to an unknown product which was recently described for a sulfate-reducing bacterium growing with HHQ (24). Under strictly anoxic conditions, HHQ was completely stable in cell extracts without addition of exogenous compounds, indicating that there was no direct hydrolysis. Addition of ATP plus either free CoA or acetyl-CoA had no effect. No HHQ oxidation was observed with electron acceptors of redox potentials lower than +100 mV (NAD, NADP, methylene blue, phenazine methosulfate, and naphthoquinone), while acceptors of higher redox potentials [K3Fe(CN)6 and dichlorophenol indophenol] reacted chemically with HHQ in the absence of cell extract. Nitrate was the only electron acceptor tested which did not react chemically with HHQ and caused HHQ degradation only in the presence of cell extract. After optimization of the analytical procedure (see Materials and Methods), a strictly nitrate-dependent oxidation of HHQ with concomitant nitrite formation was observed to be catalyzed by the membrane fraction (Fig. 2). The initial specific activities of this reaction were 70 to 120 mU mg of protein−1 at pH 7.0, 150 to 230 mU mg of protein−1 at pH 8.0, and 20 mU mg of protein−1 at pH 4.5. Heat-inactivated extracts showed no activity. At the beginning of the reaction, nitrite accumulated stoichiometrically. With increasing nitrite concentration, a small deviation from a 1:1 stoichiometry was observed, indicating that nitrite might react further with components of the assay mixture. However, if 1 mM nitrite instead of nitrate was used as the electron acceptor, HHQ was degraded only slowly (12 mU mg of protein−1).
FIG. 2.
Oxidation of HHQ (○) with 1 mM nitrate as the electron acceptor by the membrane fraction of A. anaerobius at pH 7.0 and formation of nitrite (•), 2-hydroxy-1,4-benzoquinone (▪), and a hypothetical dimer of 2-hydroxy-1,4-benzoquinone (□) as products. A typical example for this reaction is shown; data are based on two experiments with membrane fractions of the same preparation.
Identification of reaction products.
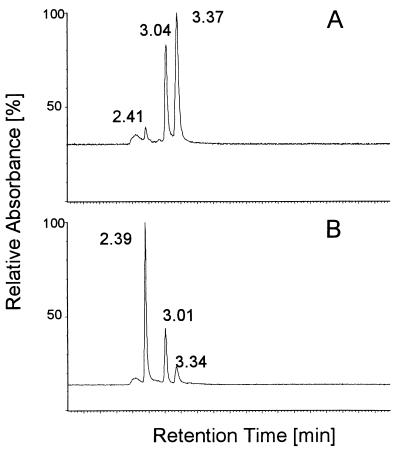
HPLC analysis of products formed during HHQ oxidation revealed two prominent product peaks (Fig. 3). One product accumulated at the beginning of the reaction and thereafter was replaced continuously by a second peak of shorter retention time (Fig. 2 and 3B).
FIG. 3.
HPLC chromatograms of samples taken from an HHQ oxidation assay 1 min (A) and 10 min (B) after the start of the reaction. The peaks (detected at 280 nm) represent the following compounds (retention times in chromatograms A and B in parentheses): HHQ (3.37 and 3.34 min), 2-hydroxy-1,4-benzoquinone (3.04 and 3.01 min), and the hypothetical dimer of 2-hydroxy-1,4-benzoquinone (2.41 and 2.39 min).
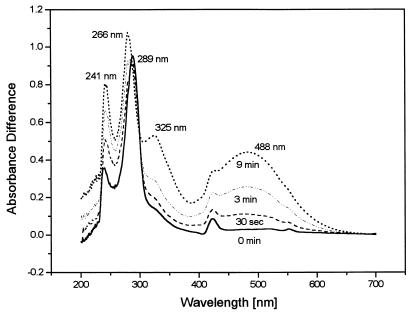
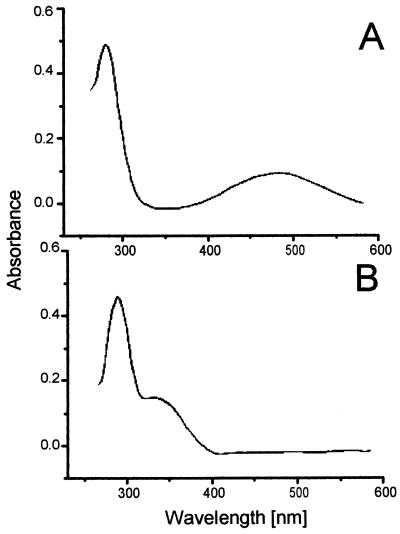
During the reaction, the assay mixture turned from colorless to brick red. When the reaction was monitored in a double-beam photometer against a reference cuvette from which HHQ was omitted, absorption maxima developed at wavelengths of 241, 266, and 488 nm while the maximum at 289 nm attributed to HHQ disappeared (Fig. 4). As the reaction proceeded, a shoulder at 325 nm appeared. A further small maximum at 423 nm was caused by reduced c-type cytochromes present in the membrane fraction.
FIG. 4.
Absorption difference spectra of a reaction mixture oxidizing 0.1 mM HHQ with 1 mM nitrate monitored against a reference containing all components of the assay except HHQ. The reaction was performed at pH 8.0; scans were repeated at the intervals indicated in the graph.
The first prominent product appearing in the HPLC chromatogram had an absorption spectrum very similar to that of the whole assay mixture (Fig. 5A). Thus, the absorption maxima at wavelengths of 488 and 266 nm could be ascribed to the first product of HHQ oxidation. Furthermore, the same product was formed if HHQ was allowed to autoxidize with oxygen (not shown).
FIG. 5.
On-line spectra of peak fractions after separation by HPLC. The spectra represent two reaction products of HHQ oxidation, 2-hydroxy-1,4-benzoquinone (A) and hypothetical dimer of 2-hydroxy-1,4-benzoquinone (B).
These results provide strong evidence that 2-hydroxy-1,4-benzoquinone was formed in this reaction since such UV/VIS spectroscopic properties are expected for this compound, based on studies of the chemical oxidation of HHQ with oxygen (6, 7) and its biological oxidation (18). As this compound is not commercially available, further analytical evidence was provided by HPLC-mass spectroscopy.
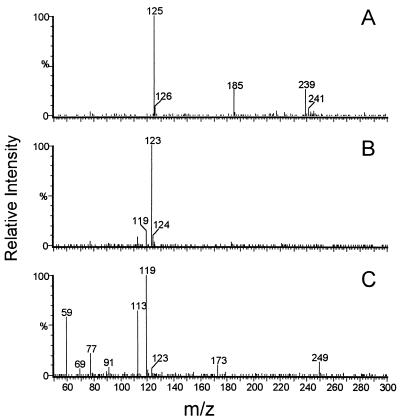
Figure 6 shows the negative ion mass spectra for the fractions detected by HPLC analysis. The peak at 3.37 min had a mass of 125 Da, which corresponds to HHQ after loss of one proton. A mass of 123 Da was attributed to the peak at 3.04 min, equivalent to hydroxybenzoquinone with a dissociated hydroxyl group. Combined with the UV/VIS spectra, these results prove that HHQ is converted to the corresponding hydroxybenzoquinone. After a 10-min reaction time, a second peak accumulated at 2.39 min (Fig. 3B) while the 2-hydroxy-1,4-benzoquinone peak decreased. UV/VIS on-line scans of this peak (Fig. 5B) indicated that this compound was responsible for the appearance of a shoulder at 325 nm in the later phase of the reaction (Fig. 4). Mass spectroscopy revealed a mass of 249 Da (Fig. 6C) for this compound, which would be equivalent to a dimer of 2-hydroxy-1,4-benzoquinone connected, e.g., by a peroxo linkage between the hydroxyl group of one molecule and the oxo group of the other one. This dimerization was markedly favored at pH values higher than 7.0.
FIG. 6.
Corresponding negative ion mass spectra of the substances separated by HPLC as shown in Fig. 3. (A) Deprotonated HHQ with a mass of 125 Da; (B) deprotonated 2-hydroxy-1,4-benzoquinone with a mass of 123 Da; (C) hypothetical dimer of 2-hydroxy-1,4-benzoquinone with a mass of 249 Da. Other masses appearing in the spectra are due to low amounts of contaminating compounds.
Localization of the reactions.
As mentioned above, both oxidative reactions were found in the membrane fraction; the cytosolic fraction contained no resorcinol-oxidizing activity and very low HHQ-dehydrogenating activity (Table 1). Neither activity could be resolved from the membranes by washing with phosphate buffer containing high salt concentrations, but both were enriched in the membrane fraction by this procedure. After addition of detergents (1% CHAPS and 1% Triton X-100), both activities were found in the supernatant after ultracentrifugation, indicating that they were due to membrane-bound enzymes. The resorcinol-hydroxylating activity could be restored more effectively if glycerol was added to the solubilized fraction (Table 2).
TABLE 2.
Specific activities of resorcinol-hydroxylating and HHQ-dehydrogenating activities after washing with 50 mM potassium phosphate buffer (pH 7.0) containing 150 mM KCl and after solubilization with CHAPSa
| Prepn | Sp act (U mg−1)
|
|
|---|---|---|
| Resorcinol-hydroxylating activity | HHQ-dehydrogenating activity | |
| Washed membrane fraction | 88.9 | 89.2 |
| Supernatant after washing of membrane fraction | <0.1 | <0.1 |
| Membrane fraction after treatment, with 1% (wt/vol) CHAPS | <0.1 | 38.8 |
| Supernatant after treatment with 1% (wt/vol) CHAPS | 47.6 (after addition of 50% glycerol) | 23.9 |
The HHQ dehydrogenase assay was performed at pH 7.0, and activity was calculated from HHQ consumption after 30 min.
Specificity of the reactions.
Dense suspensions of resting cells of A. anaerobius grown with benzoate as the sole source of carbon and energy did not degrade resorcinol. Slow degradation was observed after several hours but did not occur in the presence of chloramphenicol. In addition, benzoate-grown cells showed long lag phases of up to several days when transferred to a resorcinol-containing medium, and vice versa (not shown). Cell extracts of benzoate-grown cells exhibited only low resorcinol-hydroxylating and HHQ-oxidizing activities (Table 1). Patterns of protein bands in a sodium dodecyl sulfate-polyacrylamide gel from membrane fractions of cells grown with either substrate showed numerous differences in major bands (not shown).
Catechol (1,2-dihydroxybenzene), hydroquinone (1,4-dihydroxybenzene), and phloroglucinol (1,3,5-trihydroxybenzene) were not oxidized by the membrane fraction of resorcinol-grown cells with nitrate as the electron acceptor. A slow reaction was found only with pyrogallol (1,2,3-trihydroxybenzene).
Further reactions of the degradation pathway.
To investigate later steps in the degradation pathway, we determined whether two enzymes involved in aerobic HHQ degradation, maleylacetate reductase and 3-oxoadipate-succinyl-CoA transferase, were present in cell extracts. A low maleylacetate reductase activity (15 mU mg of protein−1) was measured but lasted only for a short time when recorded in a continuous assay. CoA transferase activity was not detected.
DISCUSSION
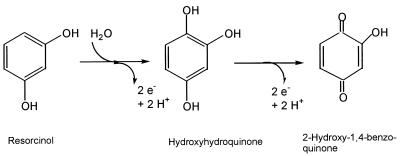
Since anaerobic nitrate-dependent degradation of resorcinol by A. anaerobius obviously did not involve any of the known pathways of anaerobic degradation of aromatic compounds, we checked for alternative routes and found two reactions which most probably initiate a new pathway. The first reaction was a hydroxylation of resorcinol to the trihydroxybenzene HHQ. This intermediate was oxidized to the corresponding hydroxybenzoquinone in a second step (Fig. 7).
FIG. 7.
Initial reactions in anaerobic resorcinol degradation by A. anaerobius.
The characteristics of the two reactions presented in this study convey evidence that they are of physiological relevance in resorcinol metabolism by A. anaerobius. Both activities were the only metabolic transformations of the substrates detectable in cell extracts in a broad screening by different assays. No evidence of direct hydrolysis or any other conversion of resorcinol or HHQ could be obtained. Average specific activities of about 60 mU mg of protein−1 for the resorcinol-hydroxylating system and about 150 mU mg of protein−1 for the HHQ dehydrogenation were in the range of the in vivo activity calculated from growth parameters (43 to 67 mU mg of protein−1; calculated from data in reference 10). Resorcinol degradation by cell suspensions of A. anaerobius depended on the presence of electron acceptors and on intact membranes. These observations agree with the finding that both oxidations were due to membrane-bound enzymes. In cell extracts of benzoate-grown cells, both activities were only weakly detectable, and benzoate-grown cells were not induced for resorcinol catabolism. Thus, it can be concluded that both activities are specific for resorcinol degradation and that the corresponding pathway is different from the benzoyl-CoA route, the only degradation pathway of aromatic compounds found thus far in denitrifying bacteria. We reported recently that HHQ was formed also as an intermediate in the anaerobic degradation of α-resorcylate (3,5-dihydroxybenzoate) by Thauera aromatica AR-1 (9). These findings suggest that HHQ may be a new central intermediate in anaerobic degradation of aromatic compounds, at least in denitrifying bacteria.
No pathways analogous to the pathway proposed in this work have been found in anaerobic metabolism of aromatic compounds, although some channeling reactions involving oxidative steps have been described. Hydroxylations of alkyl chains attached to the ring were recently identified as initial reactions in the anaerobic degradation of ethylbenzene (1) and of meta-cresol (17), but these reactions transform those compounds finally to benzoyl-CoA and are not involved in the breakdown of the aromatic nucleus itself. All anaerobic pathways studied so far imply a reductive step for dearomatization of the ring system to prepare its cleavage (14). In the present study, an oxidative destabilization of the ring is suggested to form HHQ as an appropriate substrate possessing hydroxyl groups in ortho position to each other. Electrons from the oxidation of the aromatic compounds could be fed directly into the denitrification process (equations 1 and 2). The physiological electron acceptors of these reactions are unknown, but with E°′ values of −33 mV for the hydroxylating activity and +180 mV for the dehydrogenation of HHQ, electrons could enter the electron transport chain at the levels of ubiquinone and cytochrome b, respectively:
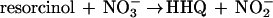 |
1 |
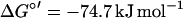 |
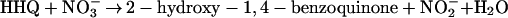 |
2 |
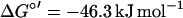 |
Such a strategy for preparing the aromatic ring for cleavage would be of advantage for nitrate-reducing bacteria since it requires no expenditure of energy, in contrast to the costly benzoyl-CoA pathway. An appropriate topology of the enzymatic systems in the cytoplasmic membrane would even allow energy conservation by proton translocation.
The oxidative strategy of ring destabilization presented in this study shows analogies to the aerobic degradation of aromatic compounds. In Pseudomonas putida, orcinol (5-methyl-1,3-dihydroxybenzene) and resorcinol are converted to HHQ, which is cleaved by a dioxygenase to yield maleylacetate (5, 6). This HHQ variant of the common aerobic pathways leading to catechol or protocatechuate is found also in fungi (19). In the denitrifying bacterium described here, the function of oxygenases present in aerobic bacteria would be partly fulfilled by membrane-bound hydroxylases and dehydrogenases.
2-Hydroxy-1,4-benzoquinone as the first nonaromatic intermediate of the degradation pathway should be prone to ring fission, but the cleavage reaction is unknown. The β-ketoadipate pathway characteristic of aerobic bacteria growing on resorcinylic compounds appears not to be present in A. anaerobius. 2-Hydroxy-1,4-benzoquinone is very reactive. Apart from its tendency to form dimers, it reacts very fast with thiols to form addition products (5, 23). Such products with an absorbance maximum at 345 nm appeared when CoA was added to an HHQ-dehydrogenating assay mixture. These competing chemical reactions were also observed with components of the crude extract and render investigations on the further metabolism of this substance difficult. On the other hand, the high reactivity of this compound should also facilitate ring cleavage. Fission between carbon atoms 1 and 2 seems feasible since this would resemble an intradiol cleavage, similar to the case for anaerobic acetoin oxidation (20). A similar mechanism was recently suggested for degradation of cyclohexane-1,2-diol by a recently identified Azoarcus species (12). However, no indication of such reactions has been detected in A. anaerobius. Studies with 2-hydroxy-1,4-benzoquinone as the substrate are under way in our laboratory to elucidate the further degradation pathway.
ACKNOWLEDGMENTS
We thank Marc J.-F. Suter (EAWAG, Dübendorf, Switzerland) for performing the HPLC-mass spectrometry analysis.
This study was supported by the Deutsche Forschungsgemeinschaft (Germany) through its special research program, Biochemistry of Anaerobic Bacteria.
REFERENCES
- 1.Ball H A, Johnson H A, Reinhard M, Spormann A M. Initial reactions in anaerobic ethylbenzene oxidation by a denitrifying bacterium, strain EB1. J Bacteriol. 1996;178:5755–5761. doi: 10.1128/jb.178.19.5755-5761.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boll M, Fuchs G. Benzoyl-CoA reductase (dearomatizing), a key enzyme of anaerobic aromatic metabolism. ATP dependence of the reaction, purification and some properties of the enzyme from Thauera aromatica strain K172. Eur J Biochem. 1995;234:921–933. doi: 10.1111/j.1432-1033.1995.921_a.x. [DOI] [PubMed] [Google Scholar]
- 3.Bradford M M. A rapid sensitive method for the quantification of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Brune A, Schnell S, Schink B. Sequential transhydroxylations converting hydroxyhydroquinone to phloroglucinol in the strictly anaerobic, fermentative bacterium Pelobacter massiliensis. Appl Environ Microbiol. 1992;58:1861–1868. doi: 10.1128/aem.58.6.1861-1868.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman P J, Ribbons D W. Metabolism of resorcinylic compounds by bacteria: orcinol pathway in Pseudomonas putida. J Bacteriol. 1976;125:975–984. doi: 10.1128/jb.125.3.975-984.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman P J, Ribbons D W. Metabolism of resorcinylic compounds by bacteria: alternative pathways for resorcinol catabolism in Pseudomonas putida. J Bacteriol. 1975;125:985–998. doi: 10.1128/jb.125.3.985-998.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corbett J F. The chemistry of hydroxy-quinones. Part VI. Formation of 2-hydroxy-semiquinones during autoxidation of the benzene-1,2,4-triols in alkaline solution. J Chem Soc C. 1970;1970:2101–2106. [Google Scholar]
- 8.Flaig W, Beutelspacher H, Riemer H, Kälke E. Einfluß von Substituenten auf das Redoxpotential substituierter Benzochinone-(1,4) Liebigs Ann Chem. 1968;719:96–111. doi: 10.1002/jlac.19687190112. [DOI] [PubMed] [Google Scholar]
- 9.Gallus C, Schink B. Anaerobic degradation of α-resorcylate by Thauera aromatica strain AR-1 proceeds via oxidation and decarboxylation to hydroxyhydroquinone. Arch Microbiol. 1998;169:333–338. doi: 10.1007/s002030050579. [DOI] [PubMed] [Google Scholar]
- 10.Gorny N, Wahl G, Brune A, Schink B. A Strictly anaerobic nitrate-reducing bacterium growing with resorcinol and other aromatic compounds. Arch Microbiol. 1992;158:48–53. doi: 10.1007/BF00249065. [DOI] [PubMed] [Google Scholar]
- 11.Haddock J D, Ferry J G. Purification and properties of phloroglucinol reductase from Eubacterium oxidoreducens. J Biol Chem. 1989;264:4423–4427. [PubMed] [Google Scholar]
- 12.Harder J. Anaerobic degradation of cyclohexane-1,2-diol by a new Azoarcus species. Arch Microbiol. 1997;168:199–204. [Google Scholar]
- 13.Harwood C S, Gibson J. Shedding light on anaerobic benzene ring degradation: a process unique to prokaryotes? J Bacteriol. 1997;179:301–309. doi: 10.1128/jb.179.2.301-309.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heider J, Fuchs G. Anaerobic metabolism of aromatic compounds. Eur J Biochem. 1997;243:577–596. doi: 10.1111/j.1432-1033.1997.00577.x. [DOI] [PubMed] [Google Scholar]
- 15.Kasberg T, Seibert V, Schlömann M, Reineke W. Cloning, characterization, and sequence analysis of the clcE gene encoding the maleylacetate reductase of Pseudomonas sp. strain B13. J Bacteriol. 1997;179:3801–3803. doi: 10.1128/jb.179.11.3801-3803.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kluge C, Tschech A, Fuchs G. Anaerobic metabolism of resorcyclic acids (m-dihydroxybenzoic acids) and resorcinol (1,3-benzenediol) in a fermenting and in a denitrifying bacterium. Arch Microbiol. 1990;155:68–74. [Google Scholar]
- 17.Londry K L, Fedorak P M, Suflita J M. Anaerobic degradation of m-cresol by a sulfate-reducing bacterium. Appl Environ Microbiol. 1997;63:3170–3175. doi: 10.1128/aem.63.8.3170-3175.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mason H S. The chemistry of melanin. VI. Mechanism of the oxidation of catechol by tyrosinase. J Biol Chem. 1949;181:803–812. [PubMed] [Google Scholar]
- 19.Middlehoven W J. Catabolism of benzene compounds by ascomycetous and basidomycetous yeasts and yeast-like fungi. A literature review and an experimental approach. Antonie Leeuwenhoek. 1993;63:125–144. doi: 10.1007/BF00872388. [DOI] [PubMed] [Google Scholar]
- 20.Oppermann F B, Schmidt B, Steinbüchel A. Purification and characterization of acetoin:2,6-dichloroindophenol oxidoreductase and dihydrolipoamide acetyltransferase of the Pelobacter carbinolicus acetoin dehydrogenase enzyme system. J Bacteriol. 1991;173:757–767. doi: 10.1128/jb.173.2.757-767.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parales R E, Harwood C S. Characterization of the genes encoding β-ketoadipate:succinyl-coenzyme A transferase in Pseudomonas putida. J Bacteriol. 1992;174:4657–4666. doi: 10.1128/jb.174.14.4657-4666.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prochazkova L. Bestimmung der Nitrate im Wasser. Z Anal Chem. 1959;167:254–260. [Google Scholar]
- 23.Redfearn E R. Plastoquinone. In: Morton A, editor. Biochemistry of quinones. New York, N.Y: Academic Press Inc.; 1965. pp. 149–181. [Google Scholar]
- 24.Reichenbecher W, Schink B. Desulfovibrio inopinatus, sp. nov., a new sulfate-reducing bacterium that degrades hydroxyhydroquinone (1,2,4-trihydroxybenzene) Arch Microbiol. 1997;168:338–344. doi: 10.1007/s002030050507. [DOI] [PubMed] [Google Scholar]
- 25.Schink B, Brune A, Schnell S. Anaerobic degradation of aromatic compounds. In: Winkelmann G, editor. Microbial degradation of organic compounds. Weinheim, Germany: VCH; 1992. pp. 220–242. [Google Scholar]
- 25a.Schueler, K. H., and B. Schink. Unpublished data.
- 26.Springer, N., W. Ludwig, B. Philipp, and B. Schink. Azoarcus anaerobius sp. nov., a resorcinol-degrading strictly anaerobic denitrifying bacterium. Int. J. Syst. Bacteriol., in press. [DOI] [PubMed]
- 27.Thauer R K, Jungermann K, Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977;41:100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tschech A, Schink B. Fermentative degradation of resorcinol and resorcylic acids. Arch Microbiol. 1985;143:52–59. [Google Scholar]
- 29.Tschech A, Pfennig N. Growth yield increase linked to caffeate reduction in Acetobacterium woodii. Arch Microbiol. 1984;134:163–167. [Google Scholar]
- 30.Widdel F, Pfennig N. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. I. Isolation of new sulfate-reducing bacteria enriched with acetate from saline environments. Description of Desulfobacter postgatei gen. nov., sp. nov. Arch Microbiol. 1981;129:395–400. doi: 10.1007/BF00406470. [DOI] [PubMed] [Google Scholar]
- 31.Widdel F, Kohring G W, Mayer F. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. III. Characterization of the filamentous gliding Desulfonema limicola gen. nov. sp. nov., and Desulfonema magnum sp. nov. Arch Microbiol. 1983;134:286–294. [Google Scholar]