Extract
Peripheral pulmonary artery stenosis (PPS) is defined as the obstruction of the pulmonary artery from the pulmonary artery trunk to the peripheral arteries. PPS is considered a paediatric-onset disease associated with systemic congenital diseases (congenital rubella syndrome, Williams syndrome, Alagille syndrome, Ehlers–Danlos syndrome and Noonan syndrome) [1–4]. Apart from PPS associated with congenital syndromes, adult-onset idiopathic PPS has been reported [5]; however, it is rare and has not been reported in more than 10 cases. Transcatheter pulmonary angioplasty for chronic thromboembolic pulmonary hypertension (CTEPH) has been widely performed in Japan during the last decade, which has led to more frequent pulmonary angiographies (PAGs), resulting in a marked increase in the opportunity to detect PPS in adults compared with previous years.
Graphical abstract
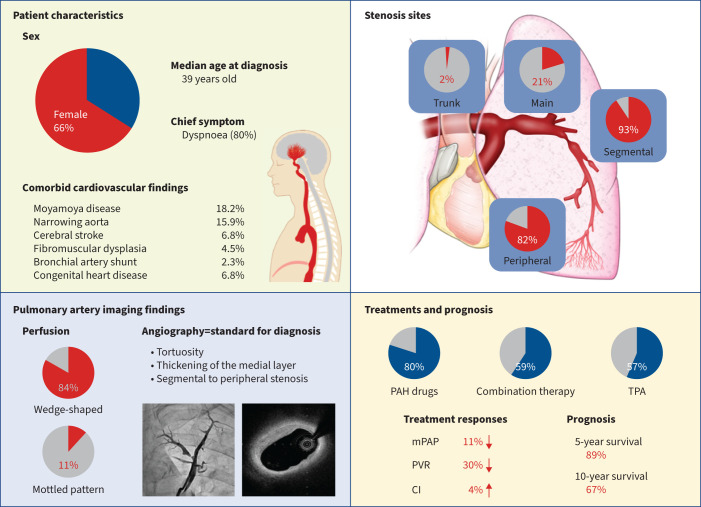
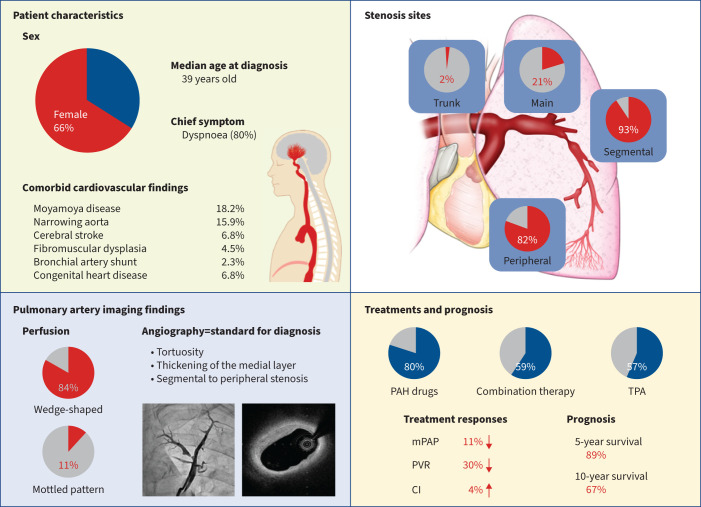
Clinical characteristics of adult-onset idiopathic peripheral pulmonary artery stenosis (PPS). Overview of patient backgrounds, imaging findings, site of stenosis in pulmonary angiography, treatment profiles, treatment response and prognosis in this cohort study of patients with adult-onset idiopathic PPS. PAH: pulmonary arterial hypertension; TPA: transcatheter pulmonary angioplasty; mPAP: mean pulmonary arterial pressure; PVR: pulmonary vascular resistance; CI: cardiac index.
Abstract
Background
Peripheral pulmonary artery stenosis (PPS) refers to stenosis of the pulmonary artery from the trunk to the peripheral arteries. Although paediatric PPS is well described, the clinical characteristics of adult-onset idiopathic PPS have not been established. Our objectives in this study were to characterise the disease profile of adult-onset PPS.
Methods
We collected data in Japanese centres. This cohort included patients who underwent pulmonary angiography (PAG) and excluded patients with chronic thromboembolic pulmonary hypertension or Takayasu arteritis. Patient backgrounds, right heart catheterisation (RHC) findings, imaging findings and treatment profiles were collected.
Results
44 patients (median (interquartile range) age 39 (29–57) years; 29 females (65.9%)) with PPS were enrolled from 20 centres. In PAG, stenosis of segmental and peripheral pulmonary arteries was observed in 41 (93.2%) and 36 patients (81.8%), respectively. 35 patients (79.5%) received medications approved for pulmonary arterial hypertension (PAH) and 22 patients (50.0%) received combination therapy. 25 patients (56.8%) underwent transcatheter pulmonary angioplasty. RHC data showed improvements in both mean pulmonary arterial pressure (44 versus 40 mmHg; p<0.001) and pulmonary vascular resistance (760 versus 514 dyn·s·cm−5; p<0.001) from baseline to final follow-up. The 3-, 5- and 10-year survival rates of patients with PPS were 97.5% (95% CI 83.5–99.6%), 89.0% (95% CI 68.9–96.4%) and 67.0% (95% CI 41.4–83.3%), respectively.
Conclusions
In this study, patients with adult-onset idiopathic PPS presented with segmental and peripheral pulmonary artery stenosis. Although patients had severe pulmonary hypertension at baseline, they showed a favourable treatment response to PAH drugs combined with transcatheter pulmonary angioplasty.
Tweetable abstract
This investigation illuminated patient attributes and imaging manifestations in individuals afflicted with adult-onset idiopathic PPS, signifying that the majority experienced stenosis in segmental and peripheral pulmonary arteries. https://bit.ly/40isWao
Introduction
Peripheral pulmonary artery stenosis (PPS) is defined as the obstruction of the pulmonary artery from the pulmonary artery trunk to the peripheral arteries. PPS is considered a paediatric-onset disease associated with systemic congenital diseases (congenital rubella syndrome, Williams syndrome, Alagille syndrome, Ehlers–Danlos syndrome and Noonan syndrome) [1–4]. Apart from PPS associated with congenital syndromes, adult-onset idiopathic PPS has been reported [5]; however, it is rare and has not been reported in more than 10 cases. Transcatheter pulmonary angioplasty for chronic thromboembolic pulmonary hypertension (CTEPH) has been widely performed in Japan during the last decade, which has led to more frequent pulmonary angiographies (PAGs), resulting in a marked increase in the opportunity to detect PPS in adults compared with previous years.
Due to the rarity of reports, there is no established diagnostic approach or treatment strategy for patients with adult-onset idiopathic PPS. Although PPS is a cause of pulmonary hypertension (PH), it is often misdiagnosed as pulmonary arterial hypertension (PAH) or CTEPH [5]. Therefore, it is important to evaluate the clinical, laboratory and imaging findings of idiopathic PPS to make an early and accurate diagnosis. In terms of treatment, it remains unclear whether pulmonary vasodilators (medications approved for PAH) are effective in idiopathic PPS. The efficacy of transcatheter pulmonary angioplasty and surgical treatment has been reported [6–13].
Given this background, we collected clinical data on idiopathic PPS from multiple centres in Japan with the aim of characterising patient backgrounds, laboratory and imaging findings, treatment efficacy, and prognosis of patients with idiopathic PPS, which leads to establishment of the disease profile.
Methods
Ethics approval
This study conformed to the ethical guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of the International University of Health and Welfare, Mita Hospital, Tokyo, Japan (approval 5-21-108). The Institutional Review Boards of all participating centres approved the study design. The requirement for informed consent was waived owing to the retrospective nature of the study.
Study participants
This retrospective cohort study included patients diagnosed with PPS at hospitals providing specialised treatment for PH in Japan. A national survey was conducted by a government-based research group, with participation from centres that agreed to participate in the study. Patients diagnosed as PH at age ≥15 years were included. For a confirmed diagnosis of PPS, PAG was performed in all patients and a definitive diagnosis was made by the expert upon observation of the presence of diffuse and whole area stenosis and/or tortuosity lesions in the vessels from the pulmonary artery trunk to the peripheral pulmonary arteries. Takayasu arteritis and CTEPH were differentiated by PAG images. PPS was differentiated from CTEPH based on the PAG findings by specialists at each PH centre. Typical findings are from previous reports [14–16], which are described in the Results section.
Evaluation and definition of clinical variables
We collected basic characteristic data, including age, sex, date of diagnosis and final follow-up, coexisting disease, World Health Organization (WHO) Functional Class, 6-min walk distance, haemodynamics, laboratory data and medication for PAH. Baseline data were collected during the first right heart catheterisation (RHC) at each centre. We evaluated two clinical outcomes: 1) all-cause death and/or 2) hospital admission due to right heart failure (RHF) after the first contact at each PH centre.
PAH was defined as mean pulmonary arterial pressure (mPAP) >20 mmHg at rest, pulmonary arterial wedge pressure (PAWP) ≤15 mmHg and pulmonary vascular resistance (PVR) >3 WU measured by RHC [17]. Combination therapy was defined as treatment with two or more PAH drugs administered simultaneously during the follow-up period. According to the PAG findings, the pulmonary arteries were classified into the pulmonary trunk, main pulmonary artery (right or left), segmental pulmonary artery and peripheral pulmonary artery to identify the site of stenosis [14]. Lung perfusion scintigraphy was used to evaluate the presence of wedge-shaped defects, diffuse defects and mottled patterns [18–21]. Composite outcomes were all-cause death and hospitalisation due to RHF.
Statistical analysis
Continuous variables are presented as mean with standard deviation for those with a normal distribution and as median (interquartile range (IQR)) for those with a non-normal distribution. Categorical variables are presented as number (percentage). The Mann–Whitney U-test or t-test was used to compare continuous variables between groups. The Wilcoxon signed-rank test was used to compare continuous variables at first contact in PH centres and at final follow-up. Fisher's exact test was used to compare the proportions of categorical variables between groups. We analysed Kaplan–Meier curves generated for all-cause death and hospitalisation-free survival. Time to event was defined as the time from PPS diagnosis to death or hospitalisation for RHF. The maximum observation period was 10 years after PPS diagnosis. A two-sided p-value <0.05 was considered significant for all statistical tests. Statistical analyses were performed using R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
We enrolled 48 patients diagnosed with PPS from 20 Japanese PH centres. Patients diagnosed with PH <15 years of age were excluded. As a result, 44 patients (median (IQR) age at diagnosis 39 (29–57) years; 29 females (65.9%)) were included in this study (table 1).
TABLE 1.
Baseline characteristics of study participants (n=44)
| Age at diagnosis for PPS (years) | 39 (29–57) |
| Age at diagnosis for PH (years) | 34 (25–53) |
| Age at symptom awareness (years) | 38 (22–55) |
| Female | 29 (65.9) |
| BMI (kg·m−2) | 20.8 (18.8–23.4) |
| Symptom at diagnosis for PPS | |
| Dyspnoea on exertion | 35 (79.5) |
| Chest pain | 3 (6.8) |
| Cough | 2 (4.5) |
| Syncope | 2 (4.5) |
| None (ECG or chest radiography abnormality) | 6 (13.6) |
| Comorbid disease | |
| Moyamoya disease | 8 (18.2) |
| Cerebral stroke | 3 (6.8) |
| Congenital heart disease | 3 (6.8) |
| Fibromuscular dysplasia | 2 (4.5) |
| Cardiac findings at first contact | |
| 6MWD (m) | 360 (200–445) |
| BNP (pg·mL−1) | 56.7 (24.2–252.5) |
| CRP (mg·dL−1) | 0.10 (0.03–0.39) |
| WHO Functional Class | |
| I | 6 (13.6) |
| II | 21 (47.7) |
| III | 16 (36.4) |
| IV | 1 (2.3) |
| Right heart catheterisation | |
| Mean PAP (mmHg) | 44 (39–62) |
| Systolic PAP (mmHg) | 81 (67–116) |
| Diastolic PAP (mmHg) | 25 (20–31) |
| Systolic RVP (mmHg) | 82 (67–106) |
| Diastolic RVP (mmHg) | 11 (8–14) |
| PAWP (mmHg) | 8 (6–11) |
| Mean RAP (mmHg) | 6 (3–9) |
| PVR (dyn·s·cm−5) | 760 (551–1226) |
| Cardiac index (L·min−1·m−2) | 2.44 (2.09–2.88) |
| SvO2 (%) | 69 (63–73) |
Data are presented as median (interquartile range) or n (%). PPS: peripheral pulmonary artery stenosis; PH: pulmonary hypertension; BMI: body mass index; 6MWD: 6-min walk distance; BNP: brain natriuretic peptide; CRP: C-reactive protein; WHO: World Health Organization; PAP: pulmonary arterial pressure; RVP: right ventricular pressure; PAWP: pulmonary arterial wedge pressure; RAP: right arterial pressure; PVR: pulmonary vascular resistance; SvO2: mixed venous oxygen saturation.
The baseline patient characteristics are shown in table 1. Dyspnoea on exertion was the most common trigger for the diagnosis. Complications included moyamoya disease in eight patients (18.2%) and fibromuscular dysplasia in two patients (4.5%). There was no evidence of a severe inflammatory response suggestive of Takayasu disease. All patients had mPAP >20 mmHg. The median mPAP and PVR at the first contact in the PH centres were 44 mmHg and 760 dyn·s·cm−5, respectively. The median (IQR) time from symptom onset to diagnosis was 15 (5–48) months. 16 patients (36.4%) were treated with home-based oxygen therapy.
Imaging findings in patients with PPS
The findings of computed tomography (CT), PAG, lung perfusion scintigraphy and optical coherence tomography in a few cases of adult-onset PPS are presented in figure 1. The imaging findings of the participants are presented in table 2 and supplementary table S1. The most common finding of lung perfusion scintigraphy was a wedge-shaped defect in 37 patients (84.1%). PAG identified stenosis in the pulmonary artery trunk and main pulmonary artery in one and nine patients, respectively. Optical coherence tomography evaluation of the luminal structure of the pulmonary artery revealed the presence of a narrowing of the vessel with thickening of the vascular media, completely different from the arterial phenotypes observed in patients with CTEPH, where a web of thrombi was present. This clearly shows that vascular degeneration of the pulmonary artery was the predominant lesion in idiopathic PPS. Figure 2 shows the number of patients according to the location of the stenosis in the pulmonary vessels. A pattern of stenosis in the segmental and peripheral pulmonary arteries was observed in two-thirds of patients with PPS.
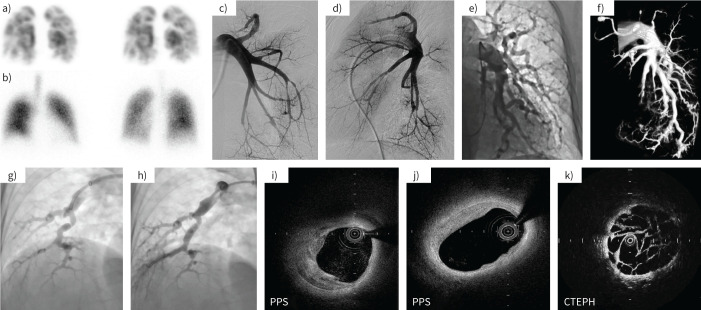
FIGURE 1.
Comparative representative images of peripheral pulmonary artery stenosis (PPS) and chronic thromboembolic pulmonary hypertension (CTEPH). a, b) In a patient with PPS, a) lung perfusion scintigraphy showed multiple wedge-shaped defects and b) lung ventilation scintigraphy showed normal findings. c–e) Pulmonary angiography (PAG) from typical PPS cases presenting with c, d) multiple stenosis and e) tortuosity in segmental pulmonary arteries. f) Three-dimensional image constructed from PAG showed pulmonary arterial stenosis. g, h) PAG images g) before and h) after percutaneous pulmonary angioplasty. i–k) Optical coherence tomography showed thickening of the medial layer of the pulmonary artery and the vascular characteristics were different from those of CTEPH. These images were obtained in a few cases.
TABLE 2.
Imaging findings of study participants (n=44)
| Computed tomography findings | |
| Bronchial artery–pulmonary artery shunt | 1 (2.3) |
| Narrowing aorta | 7 (15.9) |
| Arterial abnormalities other than pulmonary artery | 11 (25.0) |
| Pulmonary angiography findings | |
| Stenosis site | |
| Pulmonary trunk | 1 (2.3) |
| Main pulmonary artery (right or left) | 9 (20.5) |
| Segmental pulmonary artery | 41 (93.2) |
| Peripheral pulmonary artery | 36 (81.8) |
| Tortuosity | 19 (43.2) |
| Lung perfusion scintigraphy | |
| Wedge-shaped defect | 37 (84.1) |
| Diffuse defect | 13 (29.5) |
| Mottled pattern | 5 (11.4) |
Data are presented as n (%).
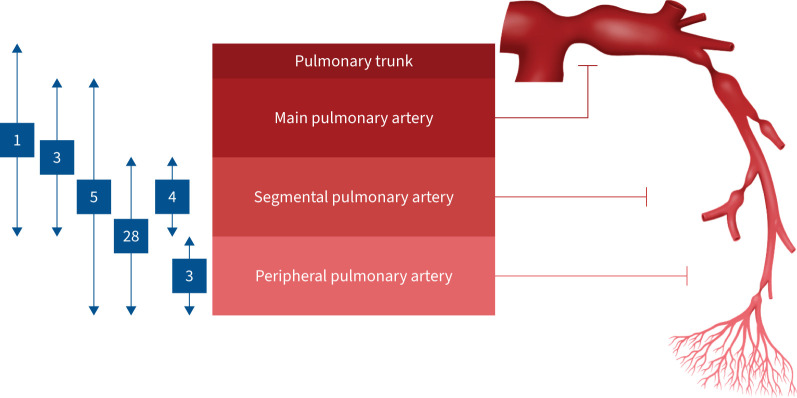
FIGURE 2.
Location of pulmonary arterial stenosis and the numbers of patients within the spectrum of stenosis. We divided the pulmonary artery into four segments (pulmonary trunk, main pulmonary artery, segmental pulmonary artery and peripheral pulmonary artery) on pulmonary angiography and evaluated which site had stenosis. Patients with stenosis in the segmental and peripheral pulmonary arteries were the most common.
Treatment details and clinical outcomes
The median (IQR) observation period from PPS diagnosis to final follow-up was 60 (24–114) months. Among the 44 patients, 35 were treated with PAH drugs during the clinical course, and 28 underwent RHC before and after the use of PAH drugs. The treatment details at the time of final follow-up or RHC are shown in table 3. In the total cohort, 26 patients received combination therapy, among whom 13 received triple combination therapy. Drugs targeting the nitric oxide pathway are the most commonly used PAH drugs. Three patients had their medications discontinued or reduced due to side-effects from PAH drugs. Of these, only one was due to systemic hypotension. Furthermore, 25 patients (56.8%) were treated with percutaneous pulmonary angioplasty, 20 of whom were treated with PAH drugs. Patients undergoing percutaneous pulmonary angioplasty had a median (IQR) of 6 (4–8) sessions. Seven patients underwent stenting and no patients were treated with a drug-coated balloon. Restenosis after percutaneous pulmonary angioplasty was observed in 10 patients. Complications from percutaneous pulmonary angioplasty included pulmonary haemorrhage, pulmonary oedema, and perforation and rupture of the pulmonary arteries, which were seen in 14 patients. The frequency of complications per number of sessions was 23.2%.
TABLE 3.
Therapeutic interventions at the follow-ups
| At final follow-up in total cohort (n=44) | At follow-up RHC in patients with PAH drugs (n=28) | |
| PAH drugs | 35 (79.5) | 28 (100) |
| Monotherapy | 9 (20.5) | 7 (25.0) |
| Double combination therapy | 13 (29.5) | 12 (42.9) |
| Triple combination therapy | 13 (29.5) | 9 (32.1) |
| Type of PAH drugs | ||
| Endothelin receptor antagonists | 26 (59.1) | 18 (64.3) |
| Macitentan | 18 (40.9) | 12 (42.9) |
| Ambrisentan | 3 (6.8) | 2 (7.1) |
| Bosentan | 5 (11.4) | 4 (14.3) |
| Drugs targeting the NO pathway# | 33 (75.0) | 28 (100) |
| Tadalafil | 10 (22.7) | 11 (39.3) |
| Sildenafil | 6 (13.6) | 5 (17.9) |
| Riociguat | 19 (43.2) | 14 (50.0) |
| Oral prostacyclin analogues | 16 (36.4) | 13 (46.4) |
| Selexipag | 9 (20.5) | 5 (17.9) |
| Beraprost | 7 (15.9) | 8 (28.6) |
| Parenteral prostacyclin therapy | 1 (2.3) | 0 (0.0) |
| Epoprostenol | 1 (2.3) | 0 (0.0) |
| Home oxygen therapy | 16 (36.4) | 13 (46.4) |
| Percutaneous pulmonary angioplasty | 25 (56.8) | 18 (64.3) |
Data are presented as n (%). PAH: pulmonary arterial hypertension; NO: nitric oxide. #: two patients were treated with two drugs targeting the NO pathway (tadalafil+riociguat and tadalafil+sildenafil).
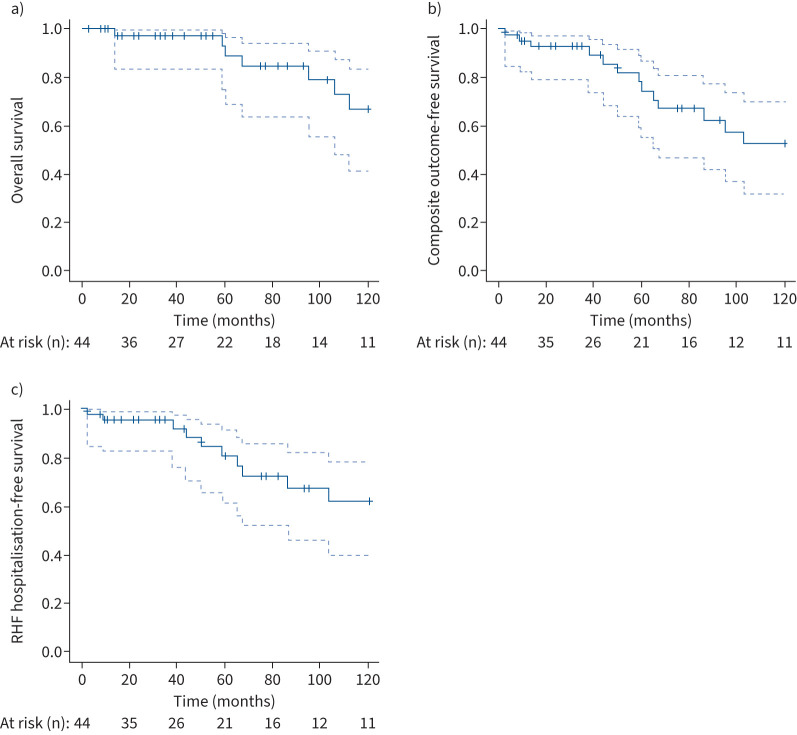
Table 4 shows the haemodynamics and clinical parameters of the patients with follow-up RHC and PAH drugs at the time of first contact in PH centres and at final follow-up (median (IQR) 44 (18–72) months). During follow-up, haemodynamic improvements were observed in this cohort. Both mPAP and PVR decreased significantly. Haemodynamics at baseline and at final follow-up were compared by patients according to whether or not percutaneous pulmonary angioplasty was performed (supplementary table S2). Haemodynamics improved significantly in both groups, but tended to improve more in patients who underwent percutaneous pulmonary angioplasty. During the follow-up period, death and hospitalisation for RHF occurred in 10 (22.7%) and 11 (25.0%) patients, respectively. Of the 10 patients who died, six had cardiovascular deaths, including sudden death. Within 10 years from the diagnosis of PPS, there were seven deaths and 10 hospitalisations due to RHF. Kaplan–Meier curves for survival in patients with PPS at 3, 5 and 10 years were 97.5% (95% CI 83.5–99.6), 89.0% (95% CI 68.9–96.4) and 67.0% (95% CI 41.4–83.3), respectively (figure 3a). In Kaplan–Meier curves, the composite outcome-free rates at 1, 3, 5 and 10 years were 95.3% (95% CI 82.6–98.8%), 92.8% (95% CI 79.4–97.6%), 74.8% (95% CI 55.1–86.8%) and 53.1% (95% CI 32.2–70.3%), respectively (figure 3b).
TABLE 4.
Changes in pulmonary haemodynamics and clinical parameters of patients with follow-up right heart catheterisation and pulmonary arterial hypertension drugs (n=28)
| First evaluation | Final follow-up | Difference | p-value | |
| Mean PAP (mmHg) | 46 (41–61) | 41 (35–51) | −7 (−14– −1) | <0.001 |
| Systolic PAP (mmHg) | 81 (71–116) | 71 (63–86) | −10 (−32–2) | <0.001 |
| Diastolic PAP (mmHg) | 24 (20–31) | 23 (15–28) | −4 (−11–1) | 0.084 |
| Systolic RVP (mmHg) | 82 (72–110) | 71 (59–80) | −14 (−28–0) | <0.01 |
| Diastolic RVP (mmHg) | 12 (7–14) | 9 (5–13) | −1 (−4–2) | <0.01 |
| PAWP (mmHg) | 8 (6–11) | 9 (7–11) | 2 (−2–3) | 0.30 |
| Mean RAP (mmHg) | 6 (4–9) | 7 (4–9) | 1 (−2–3) | 0.75 |
| PVR (dyn·s·cm−5) | 759 (566–1302) | 536 (408–902) | −178 (−362– −28) | <0.001 |
| Cardiac index (L·min−1·m−2) | 2.29 (1.95–2.84) | 2.55 (2.18–3.04) | 0.21 (−0.11–0.68) | 0.084 |
| SvO2 (%) | 69 (64–73) | 66 (62–72) | −2 (−3–5) | 0.62 |
| 6MWD | 375 (235–453) | 425 (332–467) | 15 (−1–70) | 0.57 |
| WHO Functional Class | 0.57 | |||
| I | 5 (17.9) | 4 (14.3) | ||
| II | 13 (46.4) | 15 (53.6) | ||
| III | 10 (35.7) | 7 (25.0) | ||
| IV | 0 (0.0) | 2 (7.1) |
Values are presented as median (interquartile range) or n (%), unless otherwise stated. PAP: pulmonary arterial pressure; RVP: right ventricular pressure; PAWP: pulmonary arterial wedge pressure; RAP: right arterial pressure; PVR: pulmonary vascular resistance; SvO2: mixed venous oxygen saturation; 6MWD: 6-min walk distance; WHO: World Health Organization.
FIGURE 3.
Kaplan–Meier curves of survival in patients with peripheral pulmonary artery stenosis (PPS): estimates of a) overall survival and b, c) outcome-free survival (b) all-cause death and hospitalisation due to right heart failure (RHF) and c) hospitalisation due to RHF). PPS was diagnosed on day 0. The dashed lines indicate 95% confidence intervals.
Discussion
In this study, we characterised the clinical backgrounds, laboratory and imaging findings, treatment patterns and prognosis of patients with adult-onset idiopathic PPS by Japanese multicentre collaborations (figure 4). To the best of our knowledge, this is the first report that constitutes a significant number of cases of this disease. The median age at diagnosis was 39 years and the diagnosis was made 15 months after the onset of symptoms. PAG was performed in all patients for a definite diagnosis; most patients had stenosis in the segmental to more peripheral pulmonary arteries. While the baseline pulmonary arterial pressures were severely high, haemodynamic improvements were observed with treatment.
FIGURE 4.
Clinical characteristics of adult-onset idiopathic peripheral pulmonary artery stenosis (PPS). Overview of patient backgrounds, imaging findings, site of stenosis in pulmonary angiography, treatment profiles, treatment response and prognosis in this cohort study of patients with adult-onset idiopathic PPS. PAH: pulmonary arterial hypertension; TPA: transcatheter pulmonary angioplasty; mPAP: mean pulmonary arterial pressure; PVR: pulmonary vascular resistance; CI: cardiac index.
Adult-onset idiopathic PPS is diagnosed late because of its infrequency and there is a need for careful differentiation from other diseases [16]. Therefore, appropriate imaging evaluation is important for early diagnosis (table 5). In this study, there was a median difference of 15 months between symptom presentation and PPS diagnosis. PPS has long been considered a paediatric disease and the fact that idiopathic PPS is not yet well known may be one reason for its delayed diagnosis. Another reason is the difficulty in differentiating PPS from CTEPH. In our cohort, >80% of the patients had a wedge-shaped defect on lung perfusion scintigraphy, which is a common finding in CTEPH [23]. PAG is the gold standard for the diagnosis of PPS [16] and was performed in all patients in this study. In PAG, stenosis of the segmental to peripheral pulmonary arteries was the most common pattern of stenosis. Tortuosity of the pulmonary artery was also a unique finding observed in >40% of the patients in the present study. These findings in PAG help to confirm the diagnosis of idiopathic PPS. As for how rare this disease is, the Japanese multicentre registry participating in this study enrolled over 1000 cases of both PAH and CTEPH, indicating that the incidence of idiopathic PPS is <5% compared with those diseases.
TABLE 5.
Differential diagnosis of adult-onset peripheral pulmonary artery stenosis (PPS)
| Disease (Nice classification [22]) | ||||||
|
Adult-onset idiopathic PPS
(Group 4) |
CTEPH
(Group 4) |
Takayasu arteritis
(Group 4) |
Congenital PPS
(Group 4) |
Fibrosing mediastinitis
(Group 5) |
Pulmonary artery sarcoma
(Group 4) |
|
| Pathophysiology | Symptomatic in adulthood due to multiple pulmonary artery stenoses without obvious congenital disease | Organised thromboembolic material leads to stenoses and occlusions | Primary granulomatous large vessel vasculitis affecting mainly the aorta and pulmonary arteries | Result of congenital disease characterised by multiple pulmonary artery stenoses | Benign fibro-proliferative disease affecting mediastinal structures | Sarcoma originating from pulmonary arteries |
| Risk factor or aetiologies | Associated with moyamoya disease (18.2%) or fibromuscular dysplasia (4.5%); younger age at diagnosis (median 39 years) | History of acute pulmonary embolism and several other factors including chronic inflammatory diseases and genetic factors | Causes of vasa vasorum-specific inflammation remain unknown | Associated with congenital rubella syndrome, Williams syndrome, Alagille syndrome, Ehlers–Danlos syndrome and Noonan syndrome; mutations in elastin genes | Abnormal immune response triggered by various factors including infections and autoimmune diseases | Unknown |
| V/Q scans | Mismatched segmental perfusion defects and wedge-shaped defects (84.1%) | Mismatched segmental defects and blurred edges | Large mismatched defects | Mismatched large defects | Unilateral large defects; severe cases present matched ventilation and perfusion defects | Unilateral large defects |
| Other imaging findings (CT, MRI, etc.) | CT shows multiple and focal (ring-shaped) stenoses from the trunk to the peripheral pulmonary arteries; narrowing aorta (15.9%) | CT indicates bilateral thrombus presence, mosaic perfusions and obstruction pattern; 3D CT angiography reveals chronic embolic findings consistent with PAG | CT reveals irregular unilateral or bilateral stenoses with vascular wall thickness centred on a main trunk of pulmonary arteries, sometimes with thrombus; inflammation in arteries is sometimes observed | CT shows multiple and focal (ring-shaped) stenoses from the trunk to peripheral pulmonary arteries | CT indicates fibrous tissue hyperplasia affecting lung structures | CT shows pulmonary thromboembolic-like characteristics; MRI and PET further provide additional diagnostic details as sarcoma |
| PAG | Multiple and focal narrowing stenoses are likely located in segmental to peripheral pulmonary arteries; tortuosity (43.2%); thickening medial layer in the peripheral pulmonary arteries | Intimal irregularities (mural thrombus or eccentric emboli), webs/bands, abrupt narrowing of the major pulmonary arteries, chronic total occlusions (pouch lesions or tapered lesions), vessel retractions or atrophies and post-stenotic dilatation | Lobar or segmental arterial occlusion, stenoses, aneurysm and luminal irregularity in pulmonary trunk, or in unilateral or bilateral pulmonary arteries | Multiple and focal (ring-shaped) stenoses of pulmonary arteries from the trunk to the peripheral arteries | Localised unilateral stenosis proximal to segmental pulmonary arteries | Localised unilateral stenosis in a pulmonary artery at the tumour site |
CTEPH: chronic thromboembolic pulmonary hypertension; V/Q: ventilation/perfusion; CT: computed tomography; MRI: magnetic resonance imaging; 3D: three-dimensional; PAG: pulmonary angiography; PET: positron emission tomography.
The presence of systemic diseases also aids in the diagnosis of idiopathic PPS. In the present study, 22.7% of patients had moyamoya disease or fibromuscular dysplasia associated with other extrapulmonary vascular lesions [24], suggesting the possibility of systemic vascular involvement. In addition, CT revealed narrowing of the aorta in 15.9% of patients and arterial abnormalities except in the pulmonary artery in 25.0% of patients. Thus, the presence of idiopathic PPS should be suspected in PH cases with complications of other extrapulmonary vascular lesions (e.g. moyamoya disease or fibromuscular dysplasia) or abnormalities of other extrapulmonary vessels on imaging studies.
PAH drugs and transcatheter pulmonary angioplasty may be effective for treating idiopathic PPS. In the present cohort, PAH drugs were administered to 80% of the patients with idiopathic PPS. Combination therapy was given in more than half of the cases and triple combination therapy was given in 29.5% of the cases. Percutaneous pulmonary angioplasty was performed in approximately half of the patients. As a result, haemodynamic improvements, such as PVR and mPAP improvements, were observed in patients with follow-up RHC. In this study, the 3-, 5- and 10-year survival rates for idiopathic PPS were 97.5%, 89.0% and 67.0%, respectively, which were comparable to the favourable survival rate for PAH (3-year survival rate of 95.7%) reported in a Japanese registry [25]. These results suggest that idiopathic PPS is a disease with a good prognosis for the severity of PH if appropriately diagnosed and treated using a combined treatment approach with transcatheter pulmonary angioplasty and PAH drugs. While the baseline haemodynamics were as severe as patients with PAH from the Japanese registry (mPAP 48 versus 44 mmHg; PVR 1036 versus 760 dyn·s·cm−5), only one patient required parenteral prostacyclin. As for percutaneous pulmonary angioplasty, several reports have indicated its efficacy [6–10], and our study also emphasised the importance of combining appropriate catheter interventions and oral medical therapies.
This study has several limitations. First, this was a retrospective study and the number of patients was limited. However, to the best of our knowledge, this is the largest report of idiopathic PPS. Second, because there are no established diagnostic criteria, diagnostic procedures other than PAG might differ among centres. Third, the patients in this study were presumed to be severe for the actual patients with PPS, given their symptoms and PH. Fourth, while approximately half of the patients underwent transcatheter pulmonary angioplasty, the study could not illuminate the specific protocols pertaining to percutaneous pulmonary angioplasty or the effectiveness of drugs that specifically target PAH. After establishing the disease definition in this study, further studies are needed to evaluate the efficacy of single therapy using either transcatheter pulmonary angioplasty or PAH drugs.
In adult-onset idiopathic PPS, PAG is required and stenosis in the segmental to peripheral pulmonary arteries is common. The prognosis of patients with idiopathic PPS was relatively good, suggesting the efficacy of PAH drugs combined with transcatheter pulmonary angioplasty.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary tables ERJ-00763-2023.SUPPLEMENT (151KB, pdf)
Shareable PDF
Acknowledgements
We would like to express our gratitude to all the participants and staff involved in this study, especially Rika Takeyasu and Yui Shiga (International University of Health and Welfare, Mita Hospital, Tokyo, Japan), for data management.
Footnotes
Ethics statement: This study conformed to the ethical guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of the International University of Health and Welfare, Mita Hospital, Tokyo, Japan (approval 5-21-108).
This article has an editorial commentary: https://doi.org/10.1183/13993003.02085-2023
Conflict of interest: All authors have nothing to disclose.
Support statement: Grant-in-Aid for Scientific Research (Health and Labor Sciences Research Grants: 20FC1027, 23FC1031) from the Ministry of Health, Labour and Welfare of Japan. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Ko S, Komuro J, Katsumata Y, et al. . Peripheral pulmonary stenosis with Noonan syndrome treated by balloon pulmonary angioplasty. Pulm Circ 2020; 10: 2045894020954310. doi: 10.1177/2045894020954310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reesink HJ, Henneman OD, van Delden OM, et al. . Pulmonary arterial stent implantation in an adult with Williams syndrome. Cardiovasc Intervent Radiol 2007; 30: 782–785. doi: 10.1007/s00270-007-9009-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saidi AS, Kovalchin JP, Fisher DJ, et al. . Balloon pulmonary valvuloplasty and stent implantation. For peripheral pulmonary artery stenosis in Alagille syndrome. Tex Heart Inst J 1998; 25: 79–82. [PMC free article] [PubMed] [Google Scholar]
- 4.Geggel RL, Gauvreau K, Lock JE. Balloon dilation angioplasty of peripheral pulmonary stenosis associated with Williams syndrome. Circulation 2001; 103: 2165–2170. doi: 10.1161/01.CIR.103.17.2165 [DOI] [PubMed] [Google Scholar]
- 5.Kreutzer J, Landzberg MJ, Preminger TJ, et al. . Isolated peripheral pulmonary artery stenoses in the adult. Circulation 1996; 93: 1417–1423. doi: 10.1161/01.CIR.93.7.1417 [DOI] [PubMed] [Google Scholar]
- 6.Kenny D, Amin Z, Slyder S, et al. . Medium-term outcomes for peripheral pulmonary artery stenting in adults with congenital heart disease. J Interv Cardiol 2011; 24: 373–377. doi: 10.1111/j.1540-8183.2011.00638.x [DOI] [PubMed] [Google Scholar]
- 7.Kan JS, Marvin WJ Jr, Bass JL, et al. . Balloon angioplasty – branch pulmonary artery stenosis: results from the Valvuloplasty and Angioplasty of Congenital Anomalies Registry. Am J Cardiol 1990; 65: 798–801. doi: 10.1016/0002-9149(90)91391-I [DOI] [PubMed] [Google Scholar]
- 8.Gentles TL, Lock JE, Perry SB. High pressure balloon angioplasty for branch pulmonary artery stenosis: early experience. J Am Coll Cardiol 1993; 22: 867–872. doi: 10.1016/0735-1097(93)90205-F [DOI] [PubMed] [Google Scholar]
- 9.Zeevi B, Berant M, Blieden LC. Midterm clinical impact versus procedural success of balloon angioplasty for pulmonary artery stenosis. Pediatr Cardiol 1997; 18: 101–106. doi: 10.1007/s002469900125 [DOI] [PubMed] [Google Scholar]
- 10.Ing FF, Khan A, Kobayashi D, et al. . Pulmonary artery stents in the recent era: immediate and intermediate follow-up. Catheter Cardiovasc Interv 2014; 84: 1123–1130. doi: 10.1002/ccd.25567 [DOI] [PubMed] [Google Scholar]
- 11.Al-Khaldi A, Tamimi O. Surgical reconstruction of peripheral pulmonary arteries: strategies, outcomes, and new classification. Ann Thorac Surg 2015; 100: 623–630. doi: 10.1016/j.athoracsur.2015.04.097 [DOI] [PubMed] [Google Scholar]
- 12.Mainwaring RD, Ibrahimiye AN, Hanley FL. Surgical technique for repair of peripheral pulmonary artery stenosis and other complex peripheral reconstructions. Ann Thorac Surg 2016; 102: e181–e183. doi: 10.1016/j.athoracsur.2016.03.028 [DOI] [PubMed] [Google Scholar]
- 13.Mainwaring RD, Collins RT. 2nd, Patrick WL, et al. . Surgical repair of coronary artery ostial stenosis in patients with Williams and elastin arteriopathy syndromes. J Thorac Cardiovasc Surg 2021; 162: 212–219. doi: 10.1016/j.jtcvs.2020.08.070 [DOI] [PubMed] [Google Scholar]
- 14.Gay BB Jr, French RH, Shuford WH, et al. . The roentgenologic features of single and multiple coarctations of the pulmonary artery and branches. Am J Roentgenol Radium Ther Nucl Med 1963; 90: 599–613. [PubMed] [Google Scholar]
- 15.Hosseini Z, Firouzi A, Mohebbi B, et al. . The treatment dilemma in adult patients with peripheral pulmonary artery stenosis of diverse etiologies. Egypt Heart J 2021; 73: 65. doi: 10.1186/s43044-021-00190-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tonelli AR, Ahmed M, Hamed F, et al. . Peripheral pulmonary artery stenosis as a cause of pulmonary hypertension in adults. Pulm Circ 2015; 5: 204–210. doi: 10.1086/679727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Humbert M, Kovacs G, Hoeper MM, et al. . 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2023; 61: 2200879. doi: 10.1183/13993003.00879-2022 [DOI] [PubMed] [Google Scholar]
- 18.Tahara N, Sugiyama Y, Tahara A, et al. . Reverse remodeling of small pulmonary arteries and right ventricle in pulmonary arterial hypertension. J Nucl Cardiol 2022; 29: 3615–3618. doi: 10.1007/s12350-021-02727-2 [DOI] [PubMed] [Google Scholar]
- 19.Papamatheakis DG, Poch DS, Fernandes TM, et al. . Chronic thromboembolic pulmonary hypertension: JACC Focus Seminar. J Am Coll Cardiol 2020; 76: 2155–2169. doi: 10.1016/j.jacc.2020.08.074 [DOI] [PubMed] [Google Scholar]
- 20.Wang M, Ma R, Wu D, et al. . Value of lung perfusion scintigraphy in patients with idiopathic pulmonary arterial hypertension: a patchy pattern to consider. Pulm Circ 2019; 9: 2045894018816968. doi: 10.1177/2045894018816968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiely DG, Levin D, Hassoun P, et al. . EXPRESS: statement on imaging and pulmonary hypertension from the Pulmonary Vascular Research Institute (PVRI). Pulm Circ 2019; 9: 2045894019841990. doi: 10.1177/2045894019841990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galiè N, McLaughlin VV, Rubin LJ, et al. . An overview of the 6th World Symposium on Pulmonary Hypertension. Eur Respir J 2019; 53: 1802148. doi: 10.1183/13993003.02148-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moradi F, Morris TA, Hoh CK. Perfusion scintigraphy in diagnosis and management of thromboembolic pulmonary hypertension. Radiographics 2019; 39: 169–185. doi: 10.1148/rg.2019180074 [DOI] [PubMed] [Google Scholar]
- 24.Takahashi K, Nakamura J, Sakiyama S, et al. . A histopathological report of a 16-year-old male with peripheral pulmonary artery stenosis and moyamoya disease with a homozygous RNF213 mutation. Respir Med Case Rep 2019; 29: 100977. doi: 10.1016/j.rmcr.2019.100977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamura Y, Kumamaru H, Satoh T, et al. . Effectiveness and outcome of pulmonary arterial hypertension-specific therapy in Japanese patients with pulmonary arterial hypertension. Circ J 2017; 82: 275–282. doi: 10.1253/circj.CJ-17-0139 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary tables ERJ-00763-2023.SUPPLEMENT (151KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-00763-2023.Shareable (1,006.5KB, pdf)