Abstract
The massive scale-up of HIV treatment and prevention over the past two decades has resulted in important reductions in new infections and mortality globally. Reduction in HIV incidence, however, has been unequal, with worsening epidemics in regions where the reach and scale of HIV control programmes have been insufficient, especially in eastern Europe, central Asia, the Middle East, north Africa, and Latin America where HIV epidemics are concentrated among key populations, including people who inject drugs, men who have sex with men, transgender people, and some minority racial and ethnic groups. The global state of the HIV pandemic highlights disparities in HIV control efforts and provides a roadmap for what should be done, including investment to better implement the effective HIV prevention and treatment tools that are available, but whose adoption and scale-up are not yet sufficient to get us close to an AIDS-free generation. To achieve the full potential of global HIV control, we call for urgent, evidence-informed implementation at scale of our existing and novel HIV prevention and treatment strategies in ways that are better, faster, more efficient, and cost-effective, especially in key populations and regions where the HIV pandemic continues to expand.
Introduction
The HIV pandemic and its consequences have caused over 40 million deaths since it was first recognised over 40 years ago. Despite the many successes in HIV prevention and treatment, global efforts to control the pandemic are in jeopardy. In 2021, an estimated 1·5 million adults and 160 000 children became infected with HIV and 552 000 adults and 98 000 children died.1,2 38 million adults and 1·7 million children younger than 15 years have HIV. The negative effects of HIV on individuals, health-care systems, communities, economics, and politics, are felt in nearly every society globally. The early optimism for controlling the HIV pandemic that arose from advances in diagnostics, treatment, and prevention of HIV has been tempered by the continued spread of the virus and the challenge of providing lifelong antiretroviral therapy (ART) and supportive care to a very large number people with HIV. Global ART service scale-up increased from coverage of under 1·0 million people with HIV in 2000 to 7·5 million in 2010; due to concerted effort, commitment, and funding from multilateral, bilateral, and domestic sources, the number of people with HIV on ART increased to 28·7 million in 2021. Consequently, a staggering 16·5 million HIV-related deaths were averted between 2001 and 2021.1,2 In 2022, over US$18 billion was allocated for HIV treatment and prevention, mostly in low-income and middle-income countries (LMICs), from the United States President’s Emergency Plan for AIDS Relief (PEPFAR) and the Global Fund Against AIDS, Tuberculosis and Malaria (GFATM).3,4 To control the HIV pandemic, the 2030 UNAIDS 95-95-95 targets specify that 95% of all people with HIV should know their HIV status, 95% of all people with diagnosed HIV infection should be on ART, and 95% of all people receiving ART should have viral suppression. These 2030 UNAIDS targets note that there is considerable room for improvement to achieve these goals.1
Some countries have responded to these goals more effectively than others. The good news is that massive ART scaleup worldwide has resulted in effective HIV treatment as prevention causing a 45% decline in mortality since 2010 and a large reduction in HIV transmission.1,2 Globally, the estimated number of new HIV infections in 2021 was 54% lower than in 2001. The greatest effect of ART scale-up was observed in sub-Saharan Africa, which has the largest number of people with HIV worldwide (roughly 60% of the world’s total). From 2010 to 2021, the number of new HIV infections in sub-Saharan Africa decreased by about 40%. However, the number of new infections increased alarmingly by 33% in eastern Europe and central Asia, where the epidemic is concentrated in people who inject drugs. During the same period, new HIV infections increased by 27% in the Middle East and north Africa and by 11% in Latin America (figure 1).1,2 In western and central Europe, although there have been declines in new diagnoses over the past decade, incident cases of AIDS more than halved, with an estimated one in eight people with HIV remaining undiagnosed. In the UK, 20% of people with HIV have a transmissible viral load and an estimated 25% of these people are undiagnosed.1 Meanwhile, new HIV infections have decreased by 16% in the USA since 2010 (but only 3% since 2016).
Figure 1:
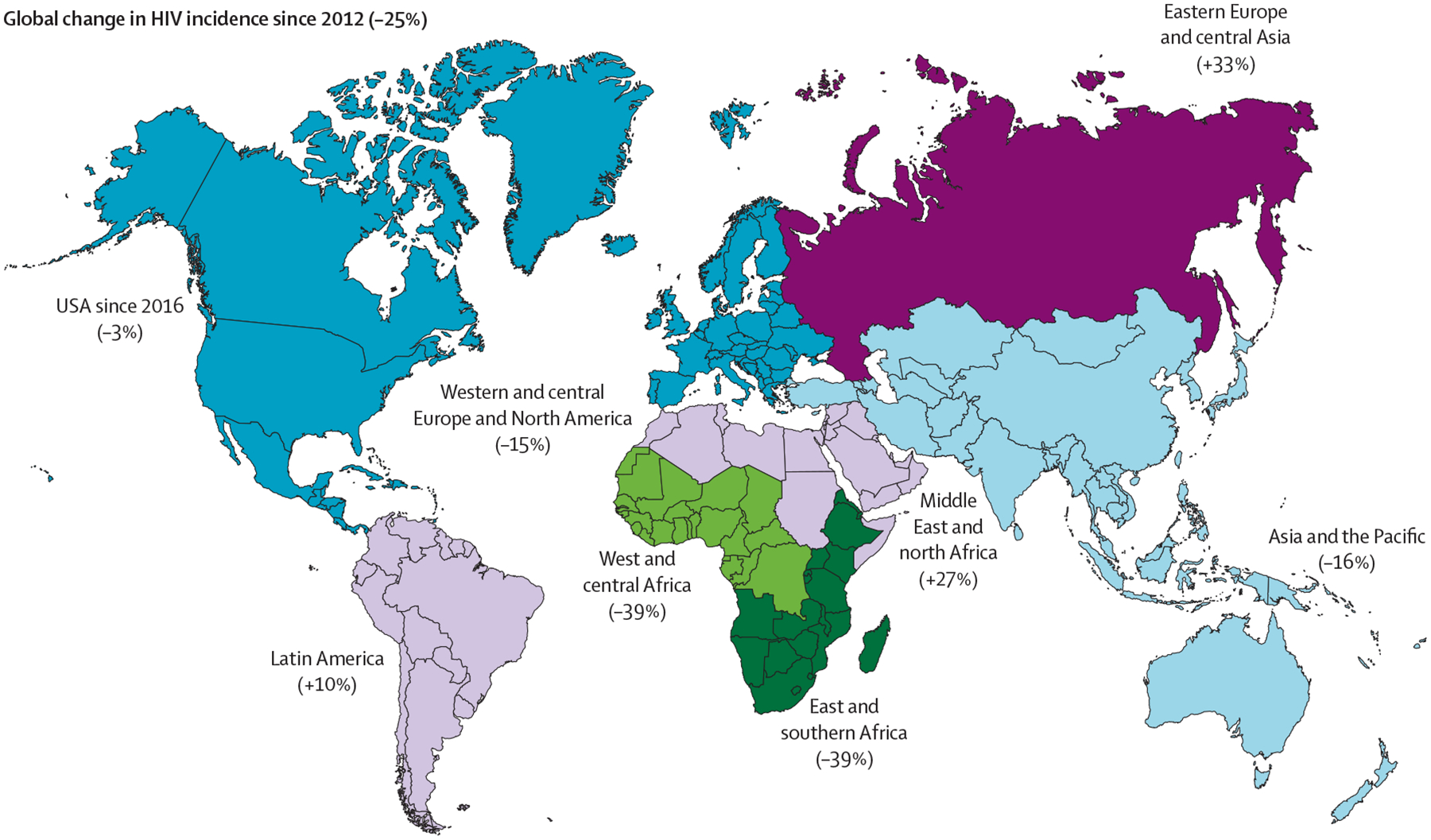
UNAIDS 2022 estimates of percentage change in HIV incidence since 20121
Global disparities in response
Sub-Saharan Africa has been substantially more successful in achieving HIV treatment targets among adults than eastern Europe and central Asia (figure 2A–C),1,2 due to successful HIV prevention and treatment programmes among people with HIV and their sexual partners. As of 2022, several GFATM or PEPFAR-supported countries in sub-Saharan Africa (eg, Burundi, Botswana, Eswatini, Malawi, Namibia, Rwanda, and Zimbabwe) have neared or achieved the UNAIDS 95-95-95 treatment targets. Botswana was the first country to be certified by WHO as reaching the agency’s silver level of achievement on the path to elimination of HIV, with documented ART coverage of more than 95% among pregnant women in 2021 (up from 77% in 2010) and a mother-to-child transmission rate of 2·2% in 2022 (down from 40% in 1999).1,2 Successful ART outcomes among adults in African nations were attributed to public health approaches, such as comprehensive and innovative systems for community outreach, including rapid ART initiation; differentiated service delivery models of HIV care; roll-out of voluntary medical male circumcision that reduces the risk of female-to-male HIV transmission by about 60%; and high levels of commitment from national leaders and international funders.3–6 However, obstacles to optimal HIV control in sub-Saharan Africa persist. These obstacles include demographics (eg, 90% of global paediatric HIV infections still occur in sub-Saharan Africa), and persistent gaps in HIV testing among children compared with adults (59% in children vs 86% in adults), ART coverage (52% in children vs 76% in adults), and HIV viral suppression (41% in children vs 70% in adults).1,2 Furthermore, HIV incidence in sub-Saharan Africa is still three-times higher among adolescent girls and young women (aged 10–24 years) compared with boys and young men (aged 10–24 years). Additional challenges include economic factors, socio-cultural barriers, and political issues, in particular poverty, stigma, and criminalisation of LGBTQ+ people and lifestyles (table).1,2 Data from 65 South African primary care clinics showed that ART provision was generally maintained during the 2020 COVID-19 lockdown. HIV testing and ART initiation decreased by 46·2% during the first week of lockdown; however, as restrictions eased, these services returned to before-lockdown levels.7 The resilience of ART programmes despite the COVID-19 pandemic indicates that the infrastructure and operations are strong and resilient, but they should still be expanded to currently underserved populations.
Figure 2: UNAIDS 2022 estimates of HIV testing and treatment cascade.
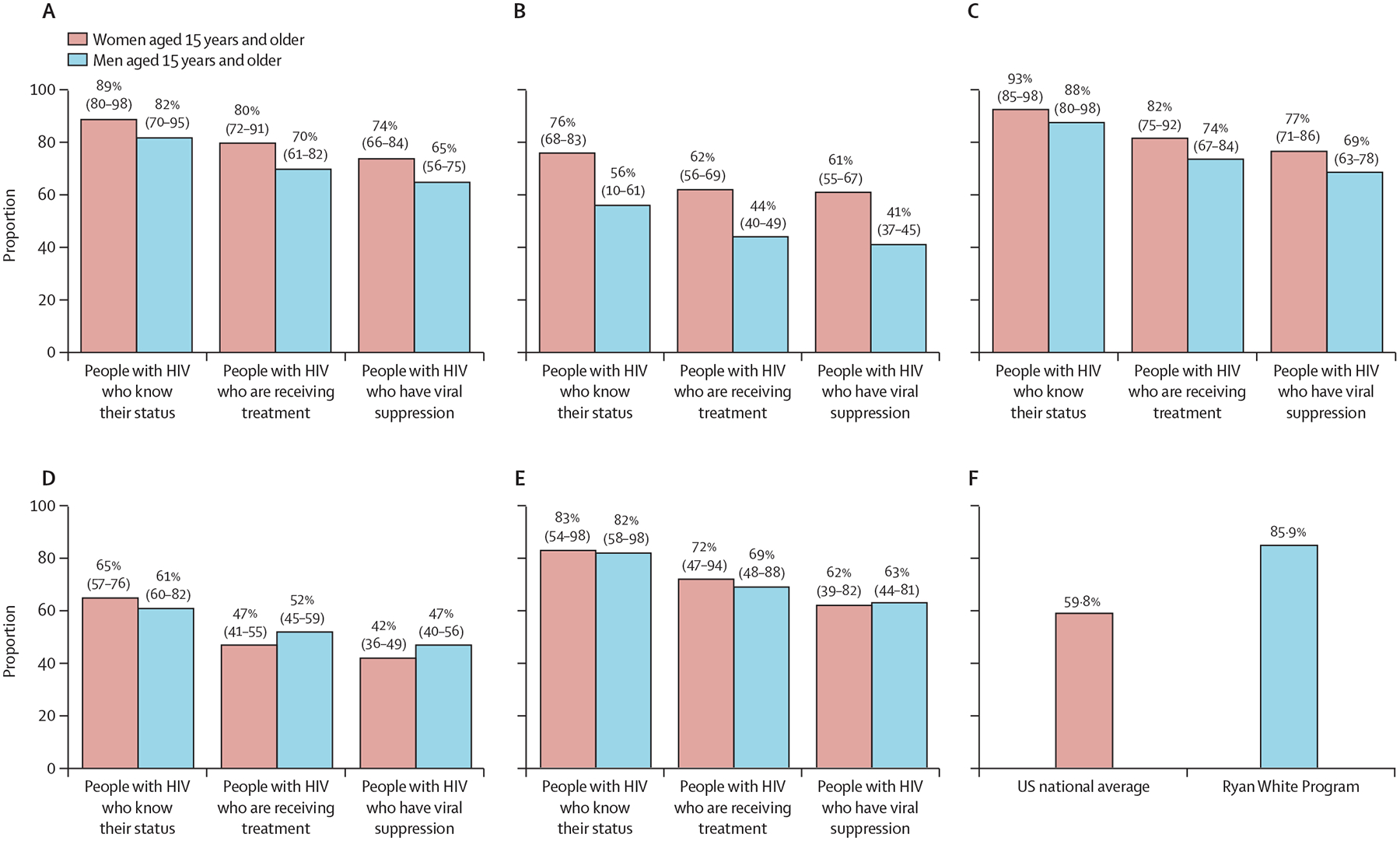
(A) Global. (B) Eastern Europe and central Asia. (C) Eastern and southern Africa. (D) Middle East and north Africa. (E) Latin America. (F) Rate of viral suppression in people with HIV in the USA. Numeric data are point estimate (95% CI). Source: Health Resources and Services Administration.
Table:
Global HIV control—target regions, remaining challenges, and solutions2
| Remaining challenges | Priority solutions | |
|---|---|---|
| Sub-Saharan Africa (25·6 million people with HIV) | Gaps in HIV testing between children and adults, ART coverage, and viral load suppression; HIV incidence is still 3-fold higher among adolescent girls vs boys and young men; missed opportunities for early infant diagnosis; insufficient paediatric antiretroviral drug formulations and paediatric ART coverage; poor antepartum and postpartum adherence and retention; rebound in viraemia, particularly during postpartum period; fear of HIV status disclosure; low social and male-partner support; postpartum depression; criminalisation of LGBT people; and stigma | Implement novel interventions to address social, cultural, and structural barriers to HIV service access, uptake, and continuity; provide point-of-care virological diagnostic testing; improve access to newer antiretroviral drugs in paediatric formulations; prioritise viral load testing in pregnant and breastfeeding women; provide peer-based psychosocial support; assist with disclosure and improving male-partner involvement; screen for depression and substance misuse; provide case management; provide long-acting antiretroviral agents; advocate for human rights; and scale up PrEP and male circumcision |
| Western and central Europe and North America (2·3 million people with HIV) | Reaching men who have sex with men (40%), heterosexual men (19%), and migrants (from sub-Saharan Africa, Latin America and Caribbean); PrEP underused; and stigma. In the USA: racism; fragmented heath care; housing and food insecurity; poverty; mental illness; and substance misuse. | Improve prevention, treatment, and care interventions targeting men who have sex with men at risk of infection need to be scaled up and strengthened together with linkage to immediate care and ART for those found to be positive; improve testing, prevention, and care services for migrants with HIV or who are at risk of HIV; provide long-acting antiretroviral agents; and scale up PrEP. In the USA: advocate for human rights focused on early diagnosis; scale up PrEP; provide long-acting antiretroviral agents; expand the Ryan White Program; scale up opioid agonist therapies; and scale up syringe services programmes |
| Latin America (2’2 million people with HIV) | Reaching men who have sex with men, transgender people, sex workers, and people who inject drugs; stigma; discrimination; and criminalisation of LGBTQ+ people | Advocate for human rights; improve the HIV testing and treatment and prevention programmes including scaling up PrEP; enable safer injection practices; integrate and link HIV and drug misuse treatment; and provide long-acting antiretroviral agents |
| Eastern Europe and central Asia (1·8 million people with HIV) | Insufficient access to sterile injecting equipment and the unavailability of opioid substitution therapy; stigma; and criminalisation of key populations making them targets of violence in some countries; and armed conflicts (eg, Ukraine) | Make safer sex possible; advocate for human rights; produce decentralised, community-based differentiated service delivery models including testing, access to ART, adherence counselling, and viral load monitoring; scale up PrEP and long-acting antiretroviral agents; scale up opioid agonist therapies; and scale up syringe services programmes |
| Middle East and north Africa (180 000 people with HIV) | Reaching key populations; stigma; and criminalisation of key populations in some countries | Advocate for human rights; adapt services including outreach for testing and ART; make adherence support targeted; integrate and link HIV and drug misuse treatment; scale up PrEP; scale up opioid agonist therapies; and provide long-acting antiretroviral agents |
ART=antiretroviral therapy. PrEP=pre-exposure prophylaxis.
In contrast to sub-Saharan Africa, the HIV epidemic in eastern Europe and central Asia has continued to expand, reflecting structural impediments to evidence-based HIV prevention, the substantial stigma and discrimination towards people who inject drugs, suboptimal political leadership, and low domestic investment in national HIV responses across much of the region (figure 1). Regional trends are strongly influenced by Russia, which is home to 70% of the people with HIV in the region.1 Russia has banned the distribution of sterile injecting equipment and opioid agonist therapies such as methadone and buprenorphine, which are essential tools for controlling HIV epidemics concentrated in people who inject drugs.8,9 Furthermore, the ongoing armed conflict has disrupted the provision of testing, prevention, and treatment services in Ukraine.1,9 Also, the growing HIV epidemics among LGBTQ+ individuals are understudied and unrecognised by several national HIV responses in eastern Europe and central Asia. Russia legislatively banned engagement by governmental and non-governmental agencies with the LGBTQ+ community as they viewed this as promoting homosexuality, with other eastern European and central Asian countries now considering similar legislation. Political, legal, and technical barriers in many national HIV programmes are delaying or preventing the use of new, innovative HIV control approaches and tools. The unique potential of community outreach organisations must be enhanced to reach marginalised populations heavily affected by the epidemic (table).
In the Middle East and north Africa, the HIV epidemic is largely hidden, despite a 20% increase in HIV incidence over the past decade (figure 1, figure 2D).1,2 New HIV infections are largely occurring among populations that face high levels of stigma, discrimination, and criminalisation, such as men who have sex with men and people who inject drugs. Almost two-thirds of new HIV infections in this region were in Egypt, Iran, and Sudan. Special efforts are now needed to expand and improve access to HIV testing and to treatment and prevention programmes, including pre-exposure prophylaxis (PrEP) for these populations at high risk.
In Latin America, no countries have reached the 2020 UNAIDS 90-90-90 targets (figure 2E).10,11 In most of this region, the HIV epidemic remains concentrated in large urban centres and in vulnerable populations, with men who have sex with men and transgender people having the highest burden.1,2 Between 2016 and 2020, HIV prevalence in the region was estimated at 13·9% for men who have sex with men and 25·9% for transgender people, with differences among and within countries.12,13 Barriers to HIV control include inadequate access to HIV services that are not decentralised, stigma, discrimination, and human rights violations (table). The HIV continuum of care in Latin American countries has slowly improved, including expanded ART coverage, which has resulted in modest decreases in AIDS-related mortality, but retention in care efforts have been thwarted by highly centralised treatment services. However, over a third of people with HIV are still diagnosed with advanced HIV infection, and AIDS-related conditions remain the leading causes of death among this group. Despite high PrEP awareness and willingness to use it, its adoption and scale-up is suboptimal and access and availability are generally low. This region represents only 10% of PrEP users globally, most of whom are in Brazil, which has the largest PrEP programme.
In the USA, continued challenges for HIV diagnosis, treatment, and prevention include barriers at the societal, health-care system, and individual levels, such as stigma, racism, fragmented care, housing and food insecurity, poverty, mental illness, and substance use (table).14 Major health inequities result in HIV bearing a disproportionate effect on specific US populations and, despite considerable resources, HIV prevention and treatment programmes do not adequately reach the most vulnerable groups, such as men who have sex with men, people who inject drugs, transgender people, and minority racial or ethnic groups. The proportion of people with HIV who are members of minority racial or ethnic groups represents an increasing share of all people with HIV and of people newly diagnosed with HIV. Although African Americans represent only 13·6% of the US population, 45% of all HIV-associated deaths occur in this population, the highest of all sub-groups.1 Further, the intertwined opioid and HIV epidemics have the potential to undermine several years of successful prevention. Mitigating these large health disparities and reducing drug epidemics through scale-up of opioid agonist therapies and syringe services programmes are essential for further control of HIV.15
In 2019, a new US HIV strategy was launched to reduce infections by 75% in 5 years and by 90% in 10 years, termed Ending the HIV epidemic: a plan for America. This plan is based on early diagnosis, rapid and effective treatment, multipronged prevention, and outbreak response.14 The provision of HIV care in the USA, however, is dependent on medical insurance status and health-care access. To address this gap, the Ryan White HIV Program provides a comprehensive system of primary HIV medical care, medication, and essential support services to people with HIV, including a specific focus on health equity, addressing social determinants of health, and supporting community needs. In 2020, 89·4% of those who received continuous HIV care through the Ryan White HIV Program had suppressed HIV viral loads, compared with 65·5% of patients receiving care outside of the programme (figure 2F). Ryan White services, however, are not fully accessible to all who need them, especially in rural settings. Innovative approaches like the Ryan White HIV Program, which provide wrap-around services and whole person care, need expansion to many more people with HIV if we are to succeed in further reducing HIV deaths and transmission in the USA.
HIV prevention gap
Globally, there is an urgent need to address the HIV prevention gap arising from underuse of PrEP and the challenges associated with long-term adherence to both PrEP and ART. Intramuscular injections of long-acting cabotegravir and long-acting rilpivirine are a novel ART combination approved for use as a fully suppressive regimen for people with HIV (table) by the US Food and Drug Administration and other regulatory agencies in high-income countries and a few LMICs.16–19 Long-acting cabotegravir and rilpivirine ART has substantially reduced the required dosing frequency from once daily to monthly or bimonthly injections, resulting in low (<2%) virological failure rates in clinical trials.20 However, data from real-world clinical experience of long-acting cabotegravir and rilpivirine are needed and demonstration projects that include populations at risk for poor ART adherence are ongoing.21–23 Long-acting cabotegravir alone is approved for PrEP in a variety of populations after confirming its superiority relative to oral PrEP.24,25 The effectiveness of long-acting cabotegravir as PrEP outside of high-income settings will be challenging to evaluate due to its prohibitive annual cost (US$22 200), over 185-times higher than the $60–119 estimated cost-effectiveness threshold for LMICs.26 Following civil society pressure, ViiV Healthcare provided access to generic versions of ART medication in 90 countries with the Medicines Patent Pool, including all African nations. However, a real challenge is expanding access to many other LMICs outside Africa, where the HIV epidemic is continuously expanding. Therefore, for long-acting cabotegravir to have a significant global impact on the HIV pandemic, its affordability needs to be expanded to all in need.27 WHO added long-acting cabotegravir to its HIV prevention guidelines in July, 2022, but combined long-acting cabotegravir and rilpivirine has not been added to its global antiretroviral treatment guidelines, perhaps partly due to cost considerations.28
Conclusion
In summary, the early successes in scale-up of HIV treatment and prevention, which changed the global inflection point on HIV-related mortality and transmission, have slowed in the past 10 years, with unequal progress depending on the geographic setting, at-risk population, or demographic groups. Although current projections and the absence of an effective HIV vaccine and cure do not support the possibility of complete HIV eradication in the near future,29,30 new HIV infections can be reduced in African countries and other regions with an increased focus on sustainable HIV treatment and prevention services. Whereas several tools for HIV prevention and treatment are effective, their adoption and scale-up has been inadequately implemented in some regions and for some populations, especially those who experience social and health inequities that make effective implementation challenging.31 Our future efforts should be reinvigorated through the expansion of targeted strategies that can be effectively implemented in each context, especially for those at increased risk for health inequities. In our vision for the future, we call on public-health and political leaders to urgently intensify global efforts to scale-up safe, convenient, and effective options for ART (including for pregnant women, children, and hard to reach populations); expand access to syringe services programmes to support safer injection practices and opioid agonist therapies that are key to controlling the HIV epidemic among people who inject drugs; implement a range of combination prevention strategies, including PrEP; and continue research towards an effective HIV vaccine and a durable ART-free method for immune control of HIV. Guided implementation of these evidence-based strategies should more quickly reach a larger number of individuals with or at risk for HIV, work better than the current strategies, and remain cost-effective. With evidence-based, general-isable, and cost-effective patient-based and community-based innovative HIV/AIDS programmes, implemented and sustained at scale and for all affected populations, including in sub-Saharan Africa where the largest number of people with HIV reside, through compassionate and effective leadership, the world could finally realise its full potential to reduce HIV incidence and the human burden of HIV. Without unwavering commitment to achieve this goal, the glass will remain half empty for too many people with HIV and for those at risk of HIV long into the future.
Search strategy and selection criteria.
We searched PubMed using MeSH. We focused on HIV-related terms (MeSH terms “HIV infections” and “HIV”). We used a person keywords search to capture specific populations that included “adolescent”, “adult”, “children”, and “pregnant women” AND “global epidemiology”. The term AND was used to combine the HIV and persons search. We sorted results by region. We added terms about key populations, health equity, and health disparities with a focus on HIV prevention, testing, and antiretroviral treatment (MeSH terms “anti-retroviral agents”, “antiretroviral therapy”, “highly active”, “Anti-HIV agents” including sub-headings on “therapeutic use”, “therapy”, and “control”). We reviewed articles that were published in English from database conception until December, 2022. Authors also recommended specific articles to review. The final reference list was generated based on originality of the articles and relevance to the broad scope of this Viewpoint.
Acknowledgments
The authors thank John L Johnson, (Case Western Reserve University, Cleveland, OH, USA), Mark J Siedner (Harvard Medical School, Boston, MA, USA), Philip J Rosenthal (University of California, San Francisco, CA, USA), and Michel P Hermans (Cliniques Universitaires St-Luc, Université Catholique de Louvain, Brussels, Belgium), for critical review and helpful advice. JBN is supported by the US National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID), and Fogarty International Center (FIC; grant numbers NIH/FIC R25TW011217; NIH/FIC D43TW010937; NIH/FIC D43TW011827-01A1; NIH/FIC R21TW011706-0; and NIH/NIAID U01AI096299-13). JWM is supported by US NIH/NIAID grants (numbers UM1 AI069494, UM1 AI106701, UM1 AI126603, UM1 AI164556, and UM1 AI164565), and National Cancer Institute contract (number 75N91019D00024). FLA is supported by NIH/National Institute on Drug Abuse (grant numbers R21DA041953, R01DA045384, R01 DA043125, R01DA033679, R01DA029910, R01DA054703, and R01DA054851), and NIH/FIC (grants R01TW012674, R21 TW011663, D43TW011324, and D43TW012492). TCQ is supported by the Division of Intramural Research, NIH/NIAID, Bethesda, MD, USA. This content is solely the responsibility of the authors and does not necessarily represent the official views or policies of the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, or the Department of Health and Human Services, nor does mention of trade names, commercial products, or organisations imply endorsement by the US Government.
Declaration of interests
AP has research grants from Gilead, ViiV, Merck, and Janssen, paid to European treatment network for HIV, Hepatitis and Global Infectious Diseases Foundation, and has received speaker fees from Gilead, ViiV, Merck, and Janssen. FLA has received speaker fees from Gilead Sciences. JWM is employed by the University of Pittsburgh; is a consultant to AlloVir, Infectious Disease Connect, and Gilead Sciences; has received grant funding from Gilead Sciences to the University of Pittsburgh; receives compensation from Abound Bio that are unrelated to the current work; and holds share options in Galapagos, Infectious Disease Connect, and MingMed Biotechnology that are unrelated to the current work.
References
- 1.UNAIDS. In danger: UNAIDS global AIDS update 2022. 2022. https://www.aidsdatahub.org/sites/default/files/resource/2022-global-aids-update-en.pdf (accessed Jan 16, 2023).
- 2.UNAIDS. Global HIV & AIDS statistics—fact sheet. 2021. https://www.unaids.org/en/resources/fact-sheet (accessed Nov 16, 2022).
- 3.US Department of State. PEPFAR: 2022 annual report to Congress. 2022. https://www.state.gov/wp-content/uploads/2022/05/PEPFAR2022.pdf (accessed June 10, 2023).
- 4.Nachega JB, Sam-Agudu NA, Mofenson LM, Schechter M, Mellors JW. Achieving viral suppression in 90% of people living with human immunodeficiency virus on antiretroviral therapy in low- and middle-income countries: progress, challenges, and opportunities. Clin Infect Dis 2018; 66: 1487–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaolathe T, Wirth KE, Holme MP, et al. Botswana’s progress toward achieving the 2020 UNAIDS 90-90-90 antiretroviral therapy and virological suppression goals: a population-based survey. Lancet HIV 2016; 3: e221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loevinsohn G, Kigozi G, Kagaayi J, et al. Effectiveness of voluntary medical male circumcision for human immunodeficiency virus prevention in Rakai, Uganda. Clin Infect Dis 2021; 73: e1946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorward J, Khubone T, Gate K, et al. The impact of the COVID-19 lockdown on HIV care in 65 South African primary care clinics: an interrupted time series analysis. Lancet HIV 2021; 8: e158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan J, Altice FL, Madden LM, Zelenev A. Effect of expanding opioid agonist therapies on the HIV epidemic and mortality in Ukraine: a modelling study. Lancet HIV 2020; 7: e121–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altice FL, Bromberg DJ, Dvoriak S, et al. Extending a lifeline to people with HIV and opioid use disorder during the war in Ukraine. Lancet Public Health 2022; 7: e482–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.PAHO. HIV Prevention in the Spotlight. An analysis from the perspective of the health sector in Latin America and the Caribbean, 2017. 2017. https://iris.paho.org/handle/10665.2/34381 (accessed July 9, 2023).
- 11.Crabtree-Ramírez B, Belaunzarán-Zamudio PF, Cortes CP, et al. The HIV epidemic in Latin America: a time to reflect on the history of success and the challenges ahead. J Int AIDS Soc 2020; 23: e25468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coelho LE, Torres TS, Veloso VG, et al. The prevalence of HIV among men who have sex with men (MSM) and young MSM in Latin America and the Caribbean: a systematic review. AIDS Behav 2021; 25: 3223–37. [DOI] [PubMed] [Google Scholar]
- 13.Stutterheim SE, van Dijk M, Wang H, Jonas KJ. The worldwide burden of HIV in transgender individuals: an updated systematic review and meta-analysis. PLoS One 2021; 16: e0260063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azar A. Ending the HIV epidemic: a plan for America. 2019. https://www.hiv.gov/blog/ending-hiv-epidemic-plan-america (accessed Oct 1, 2022).
- 15.Hodder SL, Feinberg J, Strathdee SA, et al. The opioid crisis and HIV in the USA: deadly synergies. Lancet 2021; 397: 1139–50. [DOI] [PubMed] [Google Scholar]
- 16.Swindells S, Andrade-Villanueva J-F, Richmond GJ, et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med 2020; 382: 1112–23. [DOI] [PubMed] [Google Scholar]
- 17.Orkin C, Arasteh K, Górgolas Hernández-Mora M, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med 2020; 382: 1124–35. [DOI] [PubMed] [Google Scholar]
- 18.Orkin C, Bernal Morell E, Tan DHS, et al. Initiation of long-acting cabotegravir plus rilpivirine as direct-to-injection or with an oral lead-in in adults with HIV-1 infection: week 124 results of the open-label phase 3 FLAIR study. Lancet HIV 2021; 8: e668–78. [DOI] [PubMed] [Google Scholar]
- 19.Scarsi KK, Swindells S. The promise of improved adherence with long-acting antiretroviral therapy: what are the data? J Int Assoc Provid AIDS Care 2021; 20: 23259582211009011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orkin C, Schapiro JM, Perno CF, et al. Expanded multivariable models to assist patient selection for long-acting cabotegravir and rilpivirine treatment: clinical utility of a combination of patient, drug concentration, and viral factors associated with virologic failure over 152 weeks. HIV Drug Therapy Glasgow; Oct 23–26, 2022. (abstr 044). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.US FDA. Cabenuva (cabotegravir extended-release injectable suspension; rilpivirine extended-release injectable suspension), co-packaged for intramuscular use. 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/212888s005s006lbl.pdf (accessed Oct 18, 2022).
- 22.US FDA. Apretude (cabotegravir extended-release injectable suspension), for intramuscular use. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215499s000lbl.pdf (accessed Oct 18, 2022).
- 23.Nachega JB, Scarsi KK, Gandhi M, et al. Long-acting antiretrovirals and HIV treatment adherence. Lancet HIV 2023; 10: e332–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landovitz RJ, Donnell D, Clement ME, et al. Cabotegravir for HIV prevention in cisgender men and transgender women. N Engl J Med 2021; 385: 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delany-Moretlwe S, Hughes JP, Bock P, et al. Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomised clinical trial. Lancet 2022; 399: 1779–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phillips AN, Bansi-Matharu L, Cambiano V, et al. The potential role of long-acting injectable cabotegravir–rilpivirine in the treatment of HIV in sub-Saharan Africa: a modelling analysis. Lancet Glob Health 2021; 9: e620–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pepperrell T, Cross S, Hill A. Cabotegravir-global access to long-acting pre-exposure prophylaxis for HIV. Open Forum Infect Dis 2022; 10: ofac673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO. New WHO guidelines advise countries to deliver long-acting cabotegravir as part of comprehensive approach to HIV prevention. https://www.who.int/news/item/28-07-2022-who-recommends-long-acting-cabotegravir-for-hiv-prevention (accessed Jan 21, 2023).
- 29.Lee JH, Crotty S. HIV vaccinology: 2021 update. Semin Immunol 2021; 51: 101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chun TW, Moir S, Fauci AS. HIV reservoirs as obstacles and opportunities for an HIV cure. Nat Immunol 2015; 16: 584–89. [DOI] [PubMed] [Google Scholar]
- 31.Bromberg DJ, Mayer KH, Altice FL. Identifying and managing infectious disease syndemics in patients with HIV. Curr Opin HIV AIDS 2020; 15: 232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]


