Abstract
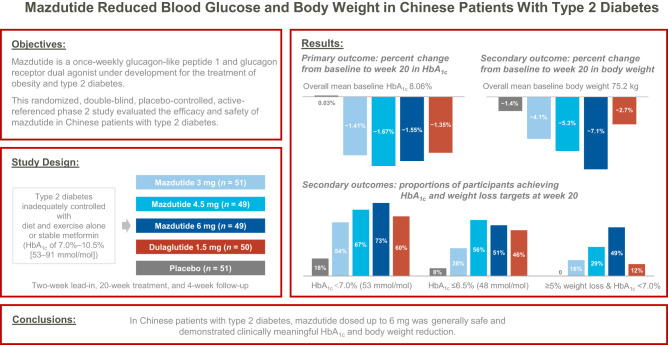
OBJECTIVE
We conducted a randomized, double-blind, placebo-controlled phase 2 trial to evaluate the efficacy and safety of mazdutide, a once-weekly glucagon-like peptide 1 and glucagon receptor dual agonist, in Chinese patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Adults with type 2 diabetes inadequately controlled with diet and exercise alone or with stable metformin (glycated hemoglobin A1c [HbA1c] 7.0–10.5% [53–91 mmol/mol]) were randomly assigned to receive 3 mg mazdutide (n = 51), 4.5 mg mazdutide (n = 49), 6 mg mazdutide (n = 49), 1.5 mg open-label dulaglutide (n = 50), or placebo (n = 51) subcutaneously for 20 weeks. The primary outcome was change in HbA1c from baseline to week 20.
RESULTS
Mean changes in HbA1c from baseline to week 20 ranged from −1.41% to −1.67% with mazdutide (−1.35% with dulaglutide and 0.03% with placebo; all P < 0.0001 vs. placebo). Mean percent changes in body weight from baseline to week 20 were dose dependent and up to −7.1% with mazdutide (−2.7% with dulaglutide and −1.4% with placebo). At week 20, participants receiving mazdutide were more likely to achieve HbA1c targets of <7.0% (53 mmol/mol) and ≤6.5% (48 mmol/mol) and body weight loss from baseline of ≥5% and ≥10% compared with placebo-treated participants. The most common adverse events with mazdutide included diarrhea (36%), decreased appetite (29%), nausea (23%), vomiting (14%), and hypoglycemia (10% [8% with placebo]).
CONCLUSIONS
In Chinese patients with type 2 diabetes, mazdutide dosed up to 6 mg was generally safe and demonstrated clinically meaningful HbA1c and body weight reductions.
Graphical Abstract
Introduction
Diabetes has become a serious global public health problem, with increasing prevalence. According to the International Diabetes Federation Atlas, China accounts for one in four of 537 million adults living with diabetes worldwide, most of whom have type 2 diabetes (1). Type 2 diabetes is often accompanied by obesity and other cardiometabolic risk factors, such as hypertension and dyslipidemia, underlining the importance of disease management beyond glycemic control (2,3).
Glucagon-like peptide 1 (GLP-1) receptor agonists, used in combination with oral antidiabetic drugs, achieved desirable glycated hemoglobin A1c (HbA1c) targets, improved multiple metabolic parameters, and conferred cardiovascular protections in patients with type 2 diabetes (4,5). Tirzepatide, a GLP-1 and glucose-dependent insulinotropic polypeptide receptor dual agonist, demonstrated superior efficacy over semaglutide in both HbA1c and body weight reductions (6). Other endeavors in the modulation of proglucagon-derived peptides involve targeting the glucagon receptor. However, because of the potential counteractive effect of glucagon and GLP-1 on glucose reduction, the strengths of GLP-1 and glucagon receptor dual agonists are believed to be largely limited to body weight reduction and resolution of nonalcoholic steatohepatitis (3). Several attempts to take advantage of this coagonism have yielded modest or no improvements in glycemic control in patients with overweight or obesity and/or type 2 diabetes (7–10).
Mazdutide (also known as IBI362 or LY3305677) is a synthetic peptide analog of mammalian oxyntomodulin and a once-weekly GLP-1 and glucagon receptor dual agonist. In a 12-week multiple-ascending-dose phase 1b study in Chinese patients with type 2 diabetes (clinical trial reg. no. NCT04466904, clinicaltrials.gov), mean changes in HbA1c ranged from −1.46% to −2.23% with mazdutide (−1.98% with 1.5 mg dulaglutide and −0.87% with placebo). Mean changes in body weight were up to −5.4% with mazdutide, −0.9% with dulaglutide, and −1.1% with placebo (11). A 12- to 16-week phase 1b study in Western patients with type 2 diabetes (clinical trial reg. no. NCT03928379, clinicaltrials.gov) reported mean changes in HbA1c up to −2.16% with mazdutide (−0.70% with placebo) and mean changes in body weight up to −11.2 kg with mazdutide (−2.0 kg with placebo) (12). In both studies, mazdutide was well tolerated, with gastrointestinal adverse events most frequently reported (11,12).
In this study, we further assessed the efficacy and safety of mazdutide in Chinese patients with type 2 diabetes.
Research Design and Methods
Study Design and Procedures
This phase 2 study adopted a randomized, double-blind, placebo-controlled, active-referenced (1.5 mg dulaglutide) design and included a 1-week screening period, a 2-week lead-in period, a 20-week treatment period, and a 4-week safety follow-up period (Supplementary Fig. 1).
Participants were enrolled from 32 hospitals in China. The study was conducted in accordance with local laws, the International Conference on Harmonisation Good Clinical Practice guidelines, and the ethical principles outlined in the Declaration of Helsinki. The clinical study protocol, the protocol amendment, and the informed consent forms were approved by the ethics committee of each participating hospital. All participants provided written informed consent before study entry.
Eligible participants were adults aged 18–75 years (both inclusive) who had been diagnosed at least 6 months earlier with type 2 diabetes that was inadequately controlled with diet and exercise alone or with stable metformin therapy 3 months before screening, with HbA1c of 7.0% to 10.5% (53–91 mmol/mol; both inclusive) and BMI of ≥20 and <40 kg/m2 at screening and with stable body weight (change no more than 5%) during the previous 12 weeks before screening, and who agreed to maintain stable diet and exercise during the study.
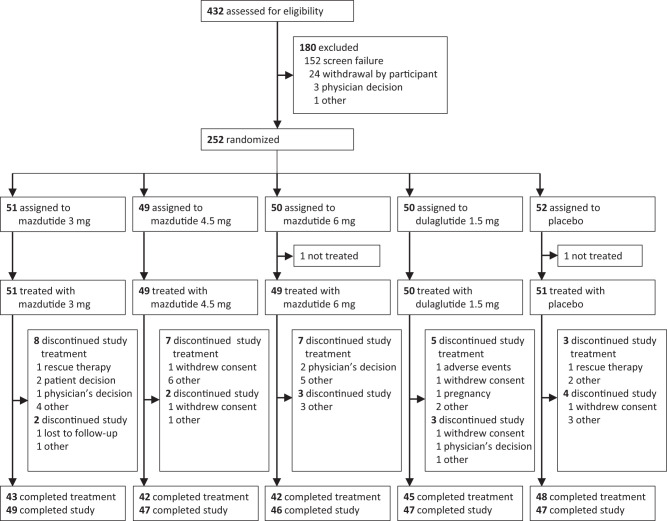
We randomly assigned eligible participants 1:1:1:1:1 to receive 3 mg mazdutide, 4.5 mg mazdutide, 6 mg mazdutide, 1.5 mg open-label dulaglutide, or placebo using an interactive web response system, stratified by previous use of metformin (yes or no) (participant flow is shown in Fig. 1). The randomization list was generated by an independent statistician who was not involved in the clinical operations of the study. Mazdutide and placebo were identically labeled and indistinguishable in appearance. As such, the participants, investigators, study site personnel involved in treating and assessing participants, and sponsor personnel were masked to mazdutide and placebo allocation. Participants who were on metformin before enrollment in this trial were required to continue their previous dose of metformin throughout the trial.
Figure 1.
Participant flow.
All treatments were administered subcutaneously once a week. Mazdutide was administered at one of three doses: 3 mg (1.5 mg weeks 1–4 and 3 mg weeks 5–20), 4.5 mg (1.5 mg weeks 1–4, 3 mg weeks 5–8, and 4.5 mg weeks 9–20), or 6 mg (2 mg weeks 1–4, 4 mg weeks 5–8, and 6 mg weeks 9–20). Dulaglutide was administered at a fixed dose of 1.5 mg. The treatment groups and dose-escalation schedules of mazdutide are shown in Supplementary Fig. 1.
Study visits occurred at screening; start of lead-in; days −1 (baseline) and 1 (randomization); and weeks 1, 2, 4, 5, 8, 9, and 12 and every 4 weeks thereafter through week 24.
Body weight, vital signs, 12-lead electrocardiogram, and adverse events were monitored at every visit. Waist circumference was measured at screening, start of lead-in, baseline, and day 1 and every 4 weeks thereafter through week 24. Local laboratory parameters were monitored at screening, baseline, day 1, week 12, and week 20. Seven-point self-measured blood glucose was monitored at day 1 and every 4 weeks thereafter through week 20. HbA1c was tested at screening and day 1 and every 4 weeks thereafter through week 20 in the central laboratory (except for screening). Chemistry panel, lipid panel, serum calcitonin, lipase, and amylase were tested at baseline and week 20 in the central laboratory. A mixed-meal tolerance test (MMTT; instant noodles) was performed at baseline and week 20, with fasting plasma glucose and 2-h postprandial plasma glucose tested in the central laboratory. Pharmacodynamic parameters (fasting plasma glucose, insulin, and C-peptide) were monitored at day 1 and every 4 weeks thereafter through week 20 in the central laboratory. Immunogenicity was monitored at day 1, week 2, and week 4 and every 4 weeks thereafter through week 24 in the central laboratory.
Participants who discontinued the study treatment before week 20 were requested to attend the remaining visits. Participants who discontinued the study before week 20 were requested to undergo the same end-of-study procedures as those who completed the study.
Outcomes
The primary outcome was change from baseline to week 20 in HbA1c, assessed in the modified intent-to-treat (mITT) population, defined as all participants who received at least one dose of the study drug and had both baseline and at least one postbaseline HbA1c measurement.
The secondary efficacy outcomes, assessed in the mITT population, included proportion of participants achieving HbA1c targets (<7.0% [53 mmol/mol], ≤6.5% [48 mmol/mol], and <5.7% [39 mmol/mol]) at week 20; change from baseline to week 20 in fasting plasma glucose, 2-h post-MMTT plasma glucose, and 7-point self-measured blood glucose; change from baseline to week 20 in body weight, waist circumference, and BMI; proportion of participants achieving weight loss of ≥5% at week 20; proportion of participants achieving weight loss of ≥5% and HbA1c of <7.0% (53 mmol/mol) at week 20; and change from baseline to week 20 in systolic and diastolic blood pressure, lipids, serum uric acid, ALT, and AST levels. Post hoc analysis of proportion of participants achieving weight loss of ≥10% was performed. Pharmacodynamic outcomes included change in fasting plasma glucose, insulin, and C-peptide, assessed in all participants who received at least one dose of the study drug and had both baseline and at least one postbaseline measurement.
Safety outcomes, assessed in all participants receiving at least one dose of the study drug (safety population), included adverse events and abnormalities in physical examinations, laboratory tests, vital signs, and 12-lead electrocardiogram. Treatment-emergent adverse events were monitored from day 1 to the safety follow-up period. The severity of treatment-emergent adverse events (mild, moderate, or severe) and the association between an event and the study drug were assessed by investigators based on prespecified criteria. Hypoglycemia was classified according to the American Diabetes Association 2013 classification of hypoglycemia as severe, documented symptomatic, asymptomatic, probable symptomatic, or pseudohypoglycemic (13). Immunogenicity was monitored by titers of antidrug antibodies and neutralizing antibodies.
Exploratory outcomes included change from baseline to week 20 in HOMAs of β-cell function and insulin resistance.
Statistical Analysis
The sample size was determined based on the results of a phase 1b study of mazdutide in Chinese patients with type 2 diabetes. Assuming mean changes from baseline to week 20 in HbA1c of −1.7% with 3 mg mazdutide, −1.9% with 4.5 mg mazdutide, −2.0% with 6 mg mazdutide, −1.28% with 1.5 mg dulaglutide, and −0.8% with placebo and a common SD of 1.2%, the sample size of 240 participants (48 in each treatment group) would provide 95% power to show the superiority of at least one of the mazdutide doses over placebo in terms of HbA1c change from baseline to week 20 and 80% power to show the superiority of the optimal mazdutide dose over 1.5 mg dulaglutide in terms of HbA1c change from baseline to week 20, at a two-sided significance level of 0.05. No adjustment for multiplicity was performed.
The primary analysis for the primary outcome was conducted using a mixed-effect model for repeated measures, with treatment, visit, treatment-by-visit interaction, and stratification factor as fixed effects and baseline HbA1c as a covariate. An unstructured covariance matrix was used to model the relationship of within-participant errors. In case of nonconvergence, matrix-like variance components and compound symmetry were tested in subsequent order until model convergence was achieved. A sensitivity analysis of the primary outcome was performed using an ANCOVA model, with treatment and stratification factor as fixed effects and baseline HbA1c value as a covariate. Missing data at week 20 were imputed using the last observation carried forward method. Other continuous efficacy outcomes were analyzed using the same method as the primary analysis for the primary outcome, with corresponding baseline values as covariates. For continuous efficacy outcomes, treatment difference versus placebo and dulaglutide are provided, with corresponding 95% CIs and P values.
For categorical outcomes of body weight and HbA1c, the Clopper-Pearson method was used for within-group CI calculation, and the Mantel-Haenszel method was used for between-group CI calculation and statistical testing; missing data at week 20 were imputed using the multiple imputation method. Odds ratios versus placebo and dulaglutide are provided, with corresponding 95% CIs and P values.
Safety data are summarized descriptively. All statistical analyses were performed using SAS software (version 9.4).
Data and Resource Availability
Individual deidentified participant data underlying the results reported in this article will be made available to investigators whose proposed use of the data is approved by the corresponding author (W.Y.).
Results
Between 17 August 2021 and 12 November 2021, 432 participants were screened for eligibility, of whom 252 were enrolled. A total of 250 participants (mean age 53.5 years, 148 men [59.2%], mean baseline BMI 27.4 kg/m2, mean HbA1c 8.06% [65 mmol/mol], mean diabetes duration 4.9 years, and 66.4% on metformin) received at least one dose of 3 mg mazdutide (n = 51), 4.5 mg mazdutide (n = 49), 6 mg mazdutide (n = 49), 1.5 mg dulaglutide (n = 50), or placebo (n = 51) and were included in the safety population (n = 250). Thirty participants (12.0%) discontinued the study treatment and remained in the study. Fourteen participants (5.6%) discontinued the study prematurely (participant flow is shown in Fig. 1). Two participants without any postbaseline HbA1c or pharmacodynamic parameter measurements because of premature discontinuation were excluded from the mITT and pharmacodynamic population (n = 248). Demographic and baseline characteristics were balanced between treatment groups (Table 1).
Table 1.
Demographics and baseline characteristics
| Mazdutide | Dulaglutide | Placebo (n = 51) | |||
|---|---|---|---|---|---|
| 3 mg (n = 51) | 4.5 mg (n = 49) | 6 mg (n = 49) | 1.5 mg (n = 50) | ||
| Age, years | 52.5 (13.4) | 55.1 (10.7) | 54.4 (9.8) | 52.8 (12.7) | 52.6 (11.1) |
| Sex, n (%) | |||||
| Male | 26 (51.0) | 32 (65.3) | 36 (73.5) | 26 (52.0) | 28 (54.9) |
| Female | 25 (49.0) | 17 (34.7) | 13 (26.5) | 24 (48.0) | 23 (45.1) |
| Asian race, n (%) | 51 (100) | 49 (100) | 49 (100) | 50 (100) | 51 (100) |
| HbA1c, % | 8.11 (0.84) | 8.11 (0.91) | 7.94 (0.93) | 7.94 (0.92) | 8.16 (0.91) |
| HbA1c, mmol/mol | 65.18 (9.19) | 65.09 (10.00) | 63.31 (10.20) | 63.32 (10.06) | 65.69 (9.91) |
| Fasting plasma glucose, mmol/L | 9.3 (2.5) | 9.3 (1.9) | 9.6 (2.2) | 8.7 (2.0) | 9.2 (2.2) |
| Fasting insulin, mU/L | 8.8 (5.8–14.7) | 9.0 (6.0–12.8) | 11.0 (7.6–15.7) | 8.7 (6.8–11.0) | 8.6 (5.4–13.1) |
| Body weight, kg | 78.1 (18.7) | 75.3 (13.8) | 76.3 (13.2) | 72.3 (12.8) | 73.9 (14.4) |
| BMI, kg/m2 | 28.0 (4.8) | 27.3 (3.8) | 27.2 (3.4) | 26.7 (3.2) | 27.5 (3.5) |
| Waist circumference, cm | 96.8 (11.0) | 95.5 (9.3) | 94.7 (9.2) | 93.5 (8.8) | 95.0 (9.0) |
| Systolic blood pressure, mmHg | 128.5 (13.1) | 130.5 (11.2) | 128.9 (11.7) | 125.6 (13.3) | 128.7 (12.8) |
| Diastolic blood pressure, mmHg | 82.3 (8.6) | 82.6 (7.9) | 82.6 (8.1) | 80.9 (8.6) | 82.0 (6.8) |
| Diabetes duration, years | 3.6 (1.9–5.9) | 4.4 (2.0–7.3) | 3.3 (1.4–5.4) | 3.4 (2.4–6.4) | 3.8 (1.4–8.0) |
| Metformin use, n (%) | 35 (68.6) | 33 (67.3) | 32 (65.3) | 33 (66.0) | 33 (64.7) |
| Heart rate, bpm | 70.8 (12.0) | 71.2 (10.8) | 72.5 (9.5) | 73.6 (10.7) | 73.9 (9.0) |
Data are mean (SD) or median (interquartile range), unless otherwise indicated.
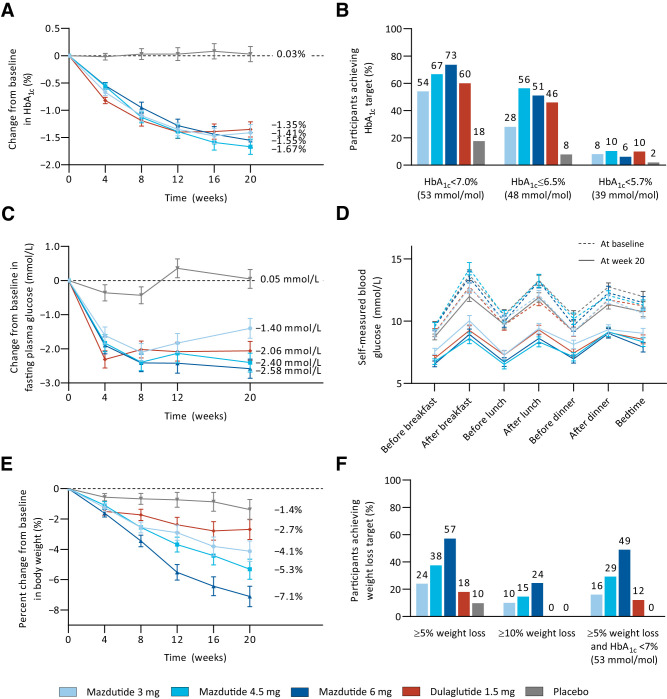
In the primary analysis using the mixed-effect model for repeated measures, the reductions in HbA1c from baseline to week 20 were greater with mazdutide (mean changes of −1.41% to −1.67% across different doses) and dulaglutide (−1.35%) compared with placebo (0.03%) (Fig. 2A and Supplementary Table 1). The estimated treatment differences versus placebo were −1.44% (95% CI −1.84, −1.04) with 3 mg mazdutide, −1.70% (−2.09, −1.30) with 4.5 mg mazdutide, −1.58% (−1.98, −1.18) with 6 mg mazdutide, and −1.38% (−1.77, −0.98) with 1.5 mg dulaglutide (all P < 0.0001). Treatment differences versus dulaglutide ranged from −0.06% to −0.32% across mazdutide doses. The results of a sensitivity analysis using the ANCOVA model were essentially consistent with those of the primary analysis. In both models, 4.5 mg mazdutide had the greatest HbA1c reduction (Supplementary Table 1).
Figure 2.
Changes in HbA1c, fasting plasma glucose (FPG), and body weight over time; proportion of participants achieving HbA1c and weight loss targets; and self-measured blood glucose (SMBG) profiles. A: Change from baseline in HbA1c over time. B: Proportion of participants reaching HbA1c targets. C: Change from baseline in FPG over time. D: SMBG profiles at baseline and week 20. E: Percent change from baseline in body weight over time. F: Proportion of participants reaching weight loss targets. Data in A, C, D, and E are plotted as least squares mean ± SE from an MMRM analysis of the mITT population. Proportion of participants reaching HbA1c (B) or weight loss (F) targets was obtained by dividing the number of participants reaching the respective target at week 20 by the number of participants in the mITT population. Participants numbers were as follows: 3 mg mazdutide n = 50, 4.5 mg mazdutide n = 48, 6 mg mazdutide n = 49, placebo n = 51, and dulaglutide n = 50.
At week 20, 54.0–73.5% of participants treated with mazdutide achieved the HbA1c target of <7.0% (53 mmol/mol; 60.0% with dulaglutide and 17.6% with placebo), and 28.0–56.3% achieved the HbA1c target of ≤6.5% (48 mmol/mol; 46.0% with dulaglutide and 7.8% with placebo). Normoglycemia (HbA1c level of <5.7% [39 mmol/mol]) was achieved in 6.1–10.4% of participants with mazdutide across different doses, compared with 10.0% with dulaglutide and 2.0% with placebo (Fig. 2B and Supplementary Table 1).
Mean changes in fasting plasma glucose from baseline to week 20 were greater with mazdutide (−1.40 to −2.58 mmol/L) and dulaglutide (−2.06 mmol/L) compared with placebo (0.05 mmol/L) (Fig. 2C and Supplementary Table 1). At week 20, greater reductions in 2-h postprandial plasma glucose after MMTT were achieved with all mazdutide doses and dulaglutide versus placebo (Supplementary Table 1). Reductions in fasting and postprandial glucose were also evidenced from overall improvements in 7-point self-measured blood glucose profiles at week 20 with all mazdutide doses and dulaglutide (Fig. 2D). HOMA of β-cell function increased from baseline to week 20 with all mazdutide doses and dulaglutide versus placebo. Reductions in fasting insulin and decrease in HOMA of insulin resistance were more prominent with 4.5 and 6 mg mazdutide (Supplementary Table 2).
Mean percent changes in body weight from baseline to week 20 ranged from −4.12% to −7.11% with mazdutide in a dose-dependent manner, compared with −2.69% with dulaglutide and −1.38% with placebo (Fig. 2E and Supplementary Table 1). The estimated treatment differences versus placebo were −2.74% (95% CI −4.62, −0.86) with 3 mg mazdutide, −3.92% (−5.78, −2.06) with 4.5 mg mazdutide, −5.73% (−7.61, −3.85) with 6 mg mazdutide, and −1.31% (−3.18, 0.55) with 1.5 mg dulaglutide. The reductions in body weight were greater with 4.5 and 6 mg mazdutide than with dulaglutide (Supplementary Table 1). Body weight loss was generally associated with HbA1c reduction at all mazdutide doses. HbA1c reduction was numerically greater in participants with weight loss of ≥5% compared with those with weight loss of <5% (Supplementary Fig. 2).
At week 20, 24.0–57.1% of participants treated with mazdutide achieved body weight loss of ≥5% (18.0% with dulaglutide and 9.8% with placebo), and 16.0–49.0% achieved body weight loss of ≥10% (none with dulaglutide or placebo) (Fig. 2F). Moreover, 16.0–49.0% of participants treated with mazdutide achieved body weight loss of ≥5% and HbA1c of <7.0% (53 mmol/mol; 12.0% with dulaglutide and none with placebo) (Fig. 2F and Supplementary Table 1). Greater reductions in waist circumference were achieved with mazdutide and dulaglutide compared with placebo (Supplementary Table 3 and Supplementary Fig. 3). Systolic and diastolic blood pressure were reduced with all mazdutide doses and dulaglutide, with the greatest reduction achieved with 4.5 mg mazdutide (Supplementary Table 3 and Supplementary Fig. 4).
Reductions in total cholesterol, LDL cholesterol, and triglycerides were dose dependent with mazdutide and greater with all mazdutide doses versus placebo (Supplementary Table 4). Reductions in ALT, AST, and serum uric acid were observed with all mazdutide doses (Supplementary Table 4).
Treatment-emergent adverse events of any grade were reported in 79.9% of participants receiving mazdutide, 76.0% receiving dulaglutide, and 64.7% receiving placebo. Severe events considered to be related to the study treatment by the investigators included decreased appetite, asthenia, and nausea reported in one participant receiving 4.5 mg mazdutide and diarrhea reported in one participant receiving 6 mg mazdutide. Serious adverse events considered to be related to the study treatment by the investigators included chronic gastritis and enteritis reported in one participant receiving dulaglutide and nausea and gastrooesophageal reflux disease reported in another participant receiving dulaglutide. Events leading to treatment discontinuation were reported in one participant treated with dulaglutide, which were judged unrelated to the study treatment by the investigator (Table 2).
Table 2.
Treatment-emergent adverse events
| Mazdutide | Dulaglutide | Placebo (n = 51) |
|||
|---|---|---|---|---|---|
| 3 mg (n = 51) | 4.5 mg (n = 49) | 6 mg (n = 49) | 1.5 mg (n = 50) | ||
| Any | 43 (84.3) | 37 (75.5) | 39 (79.6) | 38 (76.0) | 33 (64.7) |
| Mild | 32 (62.7) | 25 (51.0) | 20 (40.8) | 22 (44.0) | 26 (51.0) |
| Moderate | 11 (21.6) | 11 (22.4) | 18 (36.7) | 15 (30.0) | 6 (11.8) |
| Severe | 0 | 1 (2.0) | 1 (2.0) | 1 (2.0) | 1 (2.0) |
| Serious | 0 | 0 | 3 (6.1) | 4 (8.0) | 4 (7.8) |
| Leading to treatment discontinuation | 0 | 0 | 0 | 1 (2.0) | 0 |
| Occurring in at least 10% of participants in one or more treatment groups* | |||||
| Diarrhea | 19 (37.3) | 20 (40.8) | 14 (28.6) | 8 (16.0) | 4 (7.8) |
| Decreased appetite | 15 (29.4) | 13 (26.5) | 15 (30.6) | 9 (18.0) | 4 (7.8) |
| Nausea | 11 (21.6) | 12 (24.5) | 12 (24.5) | 15 (30.0) | 3 (5.9) |
| Vomiting | 6 (11.8) | 7 (14.3) | 8 (16.3) | 7 (14.0) | 1 (2.0) |
| Hypoglycemia | 3 (5.9) | 4 (8.2) | 8 (16.3) | 1 (2.0) | 4 (7.8) |
| Upper respiratory tract infection | 3 (5.9) | 4 (8.2) | 6 (12.2) | 8 (16.0) | 2 (3.9) |
| Abdominal distension | 5 (9.8) | 2 (4.1) | 6 (12.2) | 5 (10.0) | 1 (2.0) |
| Asthenia | 6 (11.8) | 3 (6.1) | 2 (4.1) | 1 (2.0) | 1 (2.0) |
| Urinary tract infection | 3 (5.9) | 3 (6.1) | 1 (2.0) | 5 (10.0) | 1 (2.0) |
| Others of clinical interest | |||||
| Severe hypoglycemia† | 0 | 0 | 0 | 0 | 0 |
| Hypoglycemia (blood glucose <54 mg/dL [3.0 mmol/L]) | 0 | 0 | 1 (2.0) | 0 | 1 (2.0) |
| Injection site reaction‡ | 0 | 3 (6.1) | 2 (4.1) | 1 (2.0) | 2 (3.9) |
| Hypersensitivity§ | 0 | 0 | 0 | 1 (2.0) | 1 (2.0) |
| Cardiac disorders* | |||||
| Supraventricular extrasystoles | 2 (3.9) | 0 | 3 (6.1) | 1 (2.0) | 2 (3.9) |
| Sinus tachycardia | 2 (3.9) | 0 | 0 | 2 (4.0) | 0 |
| Sinus bradycardia | 0 | 1 (2.0) | 1 (2.0) | 0 | 0 |
| Atrioventricular block, first degree | 0 | 1 (2.0) | 0 | 1 (2.0) | 0 |
| Sinoatrial block | 0 | 0 | 1 (2.0) | 0 | 1 (2.0) |
| Sinus arrhythmia | 0 | 0 | 1 (2.0) | 0 | 0 |
Data are n (%) and reflect safety population.
By the Medical Dictionary for Regulatory Activities (MedDRA; version 24.0) preferred terms.
Hypoglycemia episodes were classified according to American Diabetes Association 2013 classification of hypoglycemia.
Includes the MedDRA preferred terms of injection site pain, injection site hemorrhage, and injection site hypersensitivity.
Includes the MedDRA preferred terms of hypersensitivity.
Gastrointestinal symptoms (diarrhea, nausea, and vomiting) and decreased appetite were the most common adverse events, with no clear association with mazdutide dose (Table 2). Diarrhea, nausea, and vomiting were mostly mild or moderate in severity and more frequent during dose escalation, with incidence declining gradually over time (Supplementary Fig. 5).
Hypoglycemic episodes, mostly asymptomatic, increased with mazdutide dose. Hypoglycemic episodes with glucose level <54 mg/dL (3.0 mmol/L) occurred in one participant receiving 6 mg mazdutide and one receiving placebo. There were no reports of severe hypoglycemia (Table 2 and Supplementary Table 5).
Cardiac disorders of clinical interest included supraventricular arrhythmias and cardiac conduction disorders, all revealed by electrocardiogram, which were mild in severity, asymptomatic, and transient. Heart rate increase, accompanied by reduction in systolic and diastolic blood pressure, was noted with mazdutide and dulaglutide during the treatment period, with end-of-treatment mean change from baseline ranging from 6.4 to 6.6 bpm with mazdutide (7.5 bpm with dulaglutide and 1.7 bpm with placebo) (Supplementary Fig. 4).
Transaminase, lipase, and amylase remained mostly within three times the upper limit of normal during the study. No one had calcitonin exceeding 20 ng/L during the study (Supplementary Table 6). No investigator-suspected pancreatitis, thyroid tumors, neoplasms, or C-cell hyperplasia events were reported.
Treatment-induced anti-mazdutide antibodies were detected in 9.8–20.4% of participants across different mazdutide doses (Supplementary Table 6). No significant differences in HbA1c or body weight reduction or incidence of adverse events were observed between participants with treatment-induced antimazdutide antibodies and those without (data not shown).
Conclusions
In this phase 2 trial in Chinese patients with type 2 diabetes, 20-week treatment with mazdutide demonstrated clinically meaningful glycemic control, dose-dependent body weight reduction, and improvements in multiple cardiometabolic risk factors, together with overall favorable tolerability and safety profile. These results provide important evidence supporting the strength of GLP-1 and glucagon receptor dual agonists in the treatment of type 2 diabetes and related metabolic disorders.
Despite the well-established potency of GLP-1 receptor agonism in glycemic control, coagonism at the glucagon receptor has widely been believed to counterbalance this effect, thus posing a great challenge to the development of GLP-1 and glucagon receptor dual agonists. Cotadutide and SAR425899, two pioneering once-daily GLP-1 and glucagon receptor dual agonists, demonstrated glycemic effects comparable to those of 1.8 mg liraglutide, with modest body weight loss effect (10,14). Several once-weekly GLP-1 and glucagon receptor dual agonists showed no benefits in glycemic parameters or even impaired glucose tolerance, probably because of excessive stimulation of the glucagon receptors (7,8). Nevertheless, body weight loss induced by these long-acting dual agonists was comparable with GLP-1 receptor agonists (7,8). Of note, in a phase 2 study in type 2 diabetes, BI 456906, a GLP-1 and glucagon receptor dual agonist, achieved a 0.93–1.88% HbA1c reduction and a 1.9–6.7% body weight reduction at week 16 with weekly dosing, compared with a 1.47% HbA1c reduction and a 5.4% body weight reduction with 1.0 mg semaglutide (15,16). Furthermore, retatrutide, a glucose-dependent insulinotropic polypeptide, GLP-1, and glucagon receptor agonist, achieved HbA1c reduction up to 2.02% and body weight reduction up to 14.2% at week 24 in a phase 2 study in U.S. patients with type 2 diabetes (17). These results, together with similar findings of a 1.41–1.67% HbA1c reduction and a 4.1–7.1% body weight reduction at week 20 with mazdutide in our study, exemplify optimal tuning of GLP-1 and glucagon receptor agonism for simultaneous and effective glycemic and weight control.
We observed a numerically greater HbA1c reduction with 4.5 and 6 mg mazdutide versus dulaglutide, although superiority was not established. In the primary analysis, the treatment difference versus dulaglutide was within the 0.4% upper limit of the 95% interval. Nevertheless, body weight loss with 4.5 and 6 mg mazdutide was significantly greater than that with dulaglutide. The noninferiority of mazdutide versus dulaglutide for HbA1c and superiority for body weight and potentially for HbA1c are currently being evaluated in a phase 3 study of mazdutide versus dulaglutide as add-ons to one to two oral antidiabetic drugs.
In this study, the greatest mean HbA1c reduction of 1.67% was achieved with 4.5 mg mazdutide, compared with a slightly smaller reduction of 1.55% with 6 mg mazdutide. These results were consistent with those observed in the phase 1b study in Chinese patients with type 2 diabetes, in which the greatest 12-week placebo-adjusted HbA1c reduction of 1.35% was achieved with 4.5 mg mazdutide (11). Interestingly, in the phase 2 study of BI 456906, HbA1c reductions at week 16 were 1.88% with 1.8 mg BI 456906 and 1.66% with the highest weekly dose of 2.7 mg (15). Although a clear dose dependency in HbA1c reduction may not be evident, mazdutide titrated to 10 mg achieved HbA1c reduction of 1.9% and weight loss of 12.7% in a phase 1b study in Western patients with type 2 diabetes (12). These results imply that high-dose mazdutide may confer additional weight loss and potentially cumulative benefits in cardiometabolic risk factors, without significantly compromising glycemic effect. This is clinically important in light of the increasing prevalence of obesity and related metabolic disorders comorbid with type 2 diabetes in the Chinese population (18). The potency of high-dose mazdutide in adults with obesity, with or without type 2 diabetes, will be evaluated in future studies.
The association between body weight loss and HbA1c reduction at all mazdutide doses was consistent with the results of a post hoc analysis of the SURPASS clinical trial program (19). Although participants with greater weight loss seemed to have greater HbA1c reduction, a consistent HbA1c reduction of ∼1.0% was observed with all mazdutide doses, supporting the existence of a weight-independent mechanism for mazdutide-induced improvement in glycemic control. The relationship of body weight loss and HbA1c reduction with mazdutide warrants further investigation.
Mazdutide showed favorable tolerability in this study, with no gastrointestinal adverse events leading to treatment discontinuation, which was in sharp contrast to the high adverse event–related treatment discontinuation rates of 10–30% observed with BI 456906 and 10.4–24.5% with JNJ-64565111, another GLP-1 and glucagon receptor dual agonist (7,15). The overall adverse event profile and incidence of gastrointestinal adverse events were similar to those of GLP-1 receptor agonists and coagonists (7,15,20–22). We did not observe a dose-dependent increase in the incidence of diarrhea, nausea, or vomiting in mazdutide-treated participants. Compared with dulaglutide, all three mazdutide dose groups had a lower incidence of nausea and comparable incidence of vomiting. Diarrhea was the most commonly reported gastrointestinal symptom in our study, whereas nausea was the most frequent gastrointestinal symptom reported with GLP-1 receptor agonists and coagonists in the Western population (20,22). Of note, the incidence of diarrhea was higher than that of nausea in patients receiving semaglutide in the SUSTAIN China trial (23). This discrepancy in the upper and lower gastrointestinal adverse events possibly reflects ethnic differences in the response to GLP-1 receptor agonists and may be related to the dietary habits of the different populations.
This study has several limitations. First, the open-label design of the dulaglutide group may introduce biases in the interpretation of the findings. Second, the lack of noninferior hypothesis testing and margin precluded statistically rigorous analysis of the relative glycemic effect of mazdutide versus dulaglutide. Third, the baseline body weight and BMI of enrolled participants were relatively low, which limits the generalizability of the results to patients with obesity and type 2 diabetes. Last, all participants were Chinese, limiting the generalizability of the results to patients of other races or ethnicities.
In summary, in this phase 2 trial in Chinese patients with diabetes, 20-week treatment with mazdutide showed placebo-adjusted HbA1c reduction up to 1.70% and body weight reduction up to 5.7%. The safety profile of mazdutide was generally similar to those of GLP-1 receptor agonists and coagonists. These data support the future development of mazdutide as a promising GLP-1 and glucagon receptor dual agonist for the treatment of type 2 diabetes.
This article contains supplementary material online at https://doi.org/10.2337/figshare.24438229.
Article Information
Acknowledgments. The authors thank all the participants, investigators, and study site staff who were involved in the conduct of this trial.
Funding. This study was sponsored by Innovent Biologics, Inc. B.Z., Z.C., X.Z., D.L., H.J., G.M., X.W., S.G., J.S., P.J., J.Y., B.S., J.M., S.Y., G.W., L.J., X.G., and W.Y. received research funding from Innovent Biologics, Inc., during the conduct of the study. The sponsor of the study was involved in the study design, data collection, data review, data analysis, data interpretation, and writing of the report.
Duality of Interest. J.C., T.Y., P.A., H.D., H.L., L.L., Q.M., and L.Q. were employees of Innovent Biologics, Inc. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. B.Z., Z.C., X.Z., D.L., H.J., G.M., X.W., S.G., J.S., P.J., J.Y., B.S., J.M., S.Y., G.W., L.J., and X.G. conducted the trial and collected the data. J.C., T.Y., P.A., H.D., H.L., L.L., Q.M., L.Q., and W.Y. interpreted the data. J.C., T.Y., P.A., H.L., L.L., and Q.M. analyzed the data. H.D., L.Q., and W.Y. designed the study. Q.M. wrote the manuscript. All authors critically reviewed the manuscript and approved the final manuscript. W.Y. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Statement
This study was sponsored by Innovent Biologics, Inc. B.Z., Z.C., X.Z., D.L., H.J., G.M., X.W., S.G., J.S., P.J., J.Y., B.S., J.M., S.Y., G.W., L.J., X.G., and W.Y. received research funding from Innovent Biologics, Inc., during the conduct of the study. The sponsor of the study was involved in the study design, data collection, data review, data analysis, data interpretation, and writing of the report.
Footnotes
Clinical trial reg. no. NCT04965506, clinicaltrials.gov
B.Z., Z.C., and J.C. contributed equally to this work.
References
- 1. Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045 [published correction appears in Diabetes Res Clin Pract 2023;204:110945]. Diabetes Res Clin Pract 2022;183:109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmad E, Lim S, Lamptey R, Webb DR, Davies MJ.. Type 2 diabetes. Lancet 2022;400:1803–1820 [DOI] [PubMed] [Google Scholar]
- 3. Nauck MA, Wefers J, Meier JJ.. Treatment of type 2 diabetes: challenges, hopes, and anticipated successes. Lancet Diabetes Endocrinol 2021;9:525–544 [DOI] [PubMed] [Google Scholar]
- 4. Nauck MA, Quast DR, Wefers J, Meier JJ.. GLP-1 receptor agonists in the treatment of type 2 diabetes—state-of-the-art. Mol Metab 2021;46:101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dalsgaard NB, Vilsbøll T, Knop FK.. Effects of glucagon-like peptide-1 receptor agonists on cardiovascular risk factors: a narrative review of head-to-head comparisons. Diabetes Obes Metab 2018;20:508–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frías JP, Davies MJ, Rosenstock J, et al. ; SURPASS-2 investigators . Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med 2021;385:503–515 [DOI] [PubMed] [Google Scholar]
- 7. Di Prospero NA, Yee J, Frustaci ME, Samtani MN, Alba M, Fleck P.. Efficacy and safety of glucagon-like peptide-1/glucagon receptor co-agonist JNJ-64565111 in individuals with type 2 diabetes mellitus and obesity: a randomized dose-ranging study. Clin Obes 2021;11:e12433. [DOI] [PubMed] [Google Scholar]
- 8. Friedrichsen MH, Endahl L, Kreiner FF, et al. Results from three phase 1 trials of NNC9204-1177, a glucagon/GLP-1 receptor co-agonist: effects on weight loss and safety in adults with overweight or obesity. Mol Metab 2023;78:101801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Asano M, Sekikawa A, Kim H, et al. Pharmacokinetics, safety, tolerability and efficacy of cotadutide, a glucagon-like peptide-1 and glucagon receptor dual agonist, in phase 1 and 2 trials in overweight or obese participants of Asian descent with or without type 2 diabetes. Diabetes Obes Metab 2021;23:1859–1867 [DOI] [PubMed] [Google Scholar]
- 10. Schiavon M, Visentin R, Göbel B, et al. Improved postprandial glucose metabolism in type 2 diabetes by the dual glucagon-like peptide-1/glucagon receptor agonist SAR425899 in comparison with liraglutide. Diabetes Obes Metab 2021;23:1795–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jiang H, Pang S, Zhang Y, et al. A phase 1b randomised controlled trial of a glucagon-like peptide-1 and glucagon receptor dual agonist IBI362 (LY3305677) in Chinese patients with type 2 diabetes. Nat Commun 2022;13:3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Benson C, Tham LS, Du Y, et al. Oxyntomodulin analog LY3305677 (LY) improves glycemic control and weight loss in healthy volunteers and subjects with type 2 diabetes (T2D). Diabetes 2022;71(Suppl. 1):333-OR [Google Scholar]
- 13. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013;36:1384–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nahra R, Wang T, Gadde KM, et al. Effects of cotadutide on metabolic and hepatic parameters in adults with overweight or obesity and type 2 diabetes: a 54-week randomized phase 2b study. Diabetes Care 2021;44:1433–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rosenstock J, Blueher M, Schmid B, Hoefler J, Hennige A.. Multiple dose-ranging study of the novel glucagon/GLP-1 receptor dual agonist BI 456906 vs placebo and open-label weekly semaglutide reference control in type 2 diabetes. Diabetologia 2022;65: S314–S315 [Google Scholar]
- 16. Rosenstock J, Bluher M, Schoelch C, Hoefler J, Hennige A.. Glucagon/GLP-1 receptor dual agonist BI 456906 reduces body weight in patients with type 2 diabetes. Obesity (Silver Spring) 2022;30(Suppl. 1):30 [Google Scholar]
- 17. Rosenstock J, Frias J, Jastreboff AM, et al. Retatrutide, a GIP, GLP-1 and glucagon receptor agonist, for people with type 2 diabetes: a randomised, double-blind, placebo and active-controlled, parallel-group, phase 2 trial conducted in the USA. Lancet 2023;402:529–544 [DOI] [PubMed] [Google Scholar]
- 18. Pan XF, Wang L, Pan A.. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol 2021;9:373–392 [DOI] [PubMed] [Google Scholar]
- 19. Pedersen SD, Giorgino F, Umpierrez G, et al. Relationship between body weight change and glycaemic control with tirzepatide treatment in people with type 2 diabetes: a post hoc assessment of the SURPASS clinical trial programme. Diabetes Obes Metab 2023;25:2553–2560 [DOI] [PubMed] [Google Scholar]
- 20. Rosenstock J, Wysham C, Frías JP, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet 2021;398:143–155 [DOI] [PubMed] [Google Scholar]
- 21. Sorli C, Harashima S-I, Tsoukas GM, et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol 2017;5:251–260 [DOI] [PubMed] [Google Scholar]
- 22. Aroda VR, Ahmann A, Cariou B, et al. Comparative efficacy, safety, and cardiovascular outcomes with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: insights from the SUSTAIN 1-7 trials. Diabetes Metab 2019;45:409–418 [DOI] [PubMed] [Google Scholar]
- 23. Ji L, Dong X, Li Y, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as add-on to metformin in patients with type 2 diabetes in SUSTAIN China: a 30-week, double-blind, phase 3a, randomized trial. Diabetes Obes Metab 2021;23:404–414 [DOI] [PMC free article] [PubMed] [Google Scholar]