Abstract
OBJECTIVE
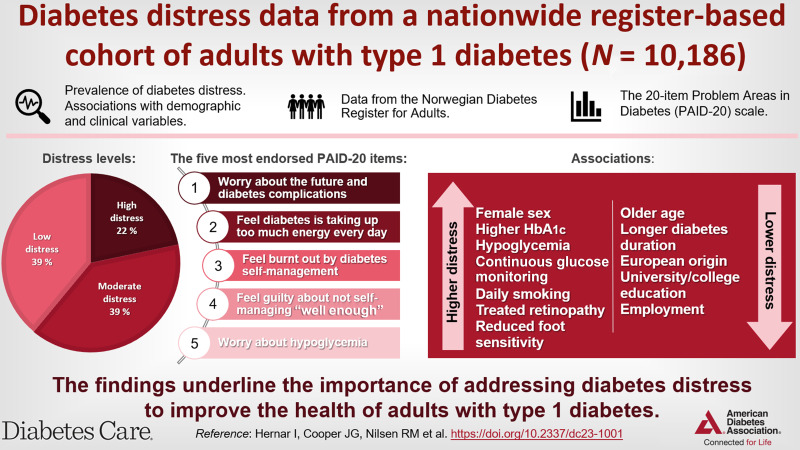
To estimate diabetes distress prevalence and associations with demographic and clinical variables among adults with type 1 diabetes in Norway.
RESEARCH DESIGN AND METHODS
In this nationwide population-based registry study, the 20-item Problem Areas in Diabetes (PAID-20) questionnaire was sent to 16,255 adults with type 1 diabetes. Linear regression models examined associations of demographic and clinical variables with distress.
RESULTS
In total, 10,186 individuals (62.7%) completed the PAID-20, with a mean score of 25.4 (SD 18.4) and 21.7% reporting high distress. Respondents endorsed worrying about the future and complications as the most problematic item (23.0%). Female sex, younger age, non-European origin, primary education only, unemployment, smoking, continuous glucose monitoring use, more symptomatic hypoglycemia, reduced foot sensitivity, treated retinopathy, and higher HbA1c were associated with higher distress.
CONCLUSIONS
Diabetes distress is common among adults with type 1 diabetes and associated with clinically relevant factors, underlining that regular care should include efforts to identify and address distress.
Graphical Abstract
Introduction
Diabetes distress reflects the emotional responses to the challenges of living with diabetes (1,2) and impacts on self-management (3). Consequently, routine diabetes distress monitoring is recommended in clinical guidelines (e.g., using the 20-item Problem Areas in Diabetes [PAID-20]) (4–6). However, we currently lack data from population-based studies regarding the prevalence of clinically significant diabetes distress that may require additional support, because existing estimates are derived from samples obtained in clinical studies (1,7). Therefore, in this nationwide population-based registry study, we aimed to 1) calculate PAID-20 scores and the proportion of respondents reporting high diabetes distress, 2) describe the distribution of PAID-20 item scores to identify the most reported problem areas, and 3) examine associations of demographic and clinical variables with PAID-20 scores.
Research Design and Methods
We conducted a nationwide population-based study using data from the Norwegian Diabetes Register for Adults (NDR-A), which consists of data about adults (age ≥18 years) with diabetes who attend outpatient clinics. The Norwegian Regional Committee for Medical and Health Research Ethics (REK Vest 171685) and data protection officials approved the study.
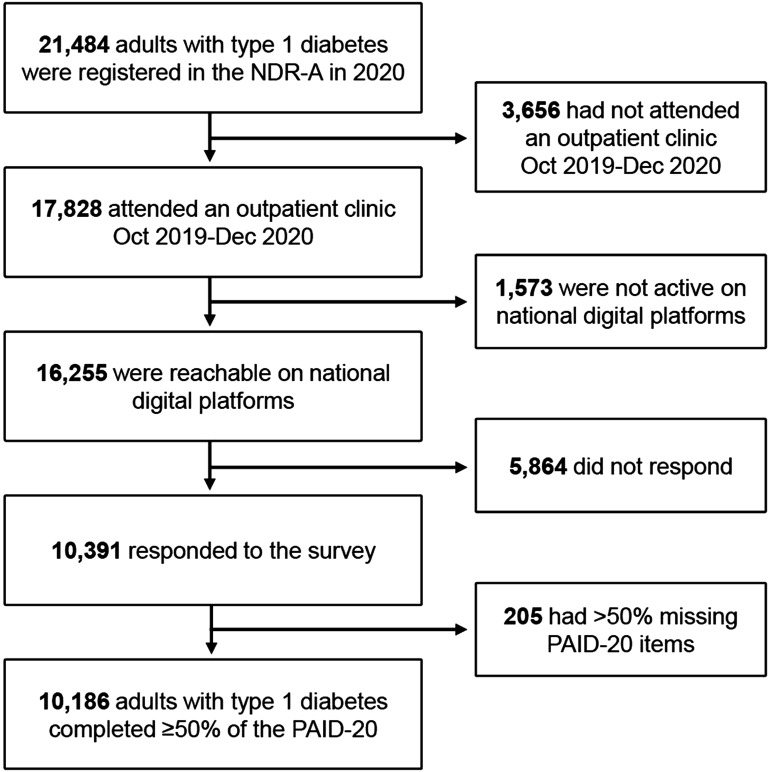
In 2020, 21,484 adults with type 1 diabetes (from 51 of 52 diabetes clinics) were registered in the NDR-A. We included the 17,828 (83.0%) who attended consultations between October 2019 and December 2020 (Fig. 1). In May 2021, NDR-A administrators sent a digital questionnaire to 16,255 individuals reachable on national digital platforms, and 10,391 (63.9%) responded. Diabetes distress was assessed with the PAID-20, measuring common diabetes-related problems scored from 0 (not a problem) to 4 (serious problem) (8). Scores are transformed to a 0–100 scale, where ≥40 is the established cut point for clinically significant high distress (1,7). Recently, moderate distress (score 17–39) was suggested (9). Furthermore, we extracted demographic variables (sex, age, ethnic origin, educational level, work, and cohabitation status), clinical process variables (diabetes duration, insulin regimen, continuous glucose monitoring [CGM], BMI, and smoking habits), and clinical outcome variables (hemoglobin A1c [HbA1c] and acute and long-term complications) from the NDR-A database.
Figure 1.
Flowchart for the cohort of study participants with type 1 diabetes from the NDR-A.
Analyses
We conducted all statistical analyses using Stata SE (version 17.0). We calculated the PAID-20 total score, the proportion reporting high distress, and the distribution of the PAID-20 response options for all items. We identified the five items with the highest endorsement of “somewhat serious problem” or “serious problem” (score 3 or 4). Among PAID-20 completers (<50% missing items), we used person-mean substitution for missing item scores in 240 individuals (2.4%) (Supplementary Table 1).
We used linear regression models to examine associations of demographic and clinical variables with distress scores. We imputed missing data using a chained equations algorithm and applied Rubin combinations rules in pooling β and B coefficients with 95% CIs across 100 imputed data sets. We report regression coefficients (B) with 95% CIs, signifying the change in PAID-20 score associated with a one-unit increase in the exposure variables, and standardized coefficients (β), indicating the strength and relative importance of each coefficient. We performed regression analyses, crude and with adjustment in a hierarchical manner, mutually adjusting the demographic variables for one another (model 1), the clinical process variables for one another and the demographics (model 2), and clinical outcome variables for one another, the demographic and clinical process variables (model 3). We interpreted the coefficients in models 1 to 3 block diagonally. Using Spearman, tetrachoric, and polychoric correlations and variance inflation factors, we found no collinearity.
Data and Resource Availability
Norwegian legislation prevents patient-level data sharing in public repositories. Data requestors must submit a proposal to the NDR-A (noklus@noklus.no), sign a data access agreement, and obtain necessary approvals to gain access.
Results
We analyzed PAID-20 data from 10,186 participants (Fig. 1), of whom 54.0% were male (Table 1); mean age was 46.7 years (SD 15.3), mean diabetes duration was 22.9 years (SD 14.4), 35.6% received pump treatment, 69.7% used CGM, and mean was HbA1c 59 mmol/mol (SD 12.7). Furthermore, 14.7% had treated retinopathy, 12.9% had an elevated urine albumin-to-creatinine ratio, and 10.9% had reduced foot sensitivity.
Table 1.
Diabetes distress scores and characteristics of adults (age ≥18 years) with type 1 diabetes completing the PAID-20 scale (N = 10,186)
| Variable | n * | Results† |
|---|---|---|
| PAID-20 | ||
| Score (0–100) | 10,186 | |
| Mean | 25.4 ± 18.4 | |
| Median | 22.5 (0–100) | |
| Score level | 10,186 | |
| 0–16 (low) | 3,970 (39.0) | |
| 17–39 (moderate) | 4,002 (39.3) | |
| ≥40 (high) | 2,214 (21.7) | |
| Demographics | ||
| Sex | 10,186 | |
| Male | 5,502 (54.0) | |
| Female | 4,684 (46.0) | |
| Age, years | 10,186 | 46.7 ± 15.3 |
| European origin | 9,491 | 9,258 (97.5) |
| Educational level | 10,023 | |
| Primary school | 732 (7.3) | |
| Secondary school | 4,637 (46.3) | |
| University/college | 4,654 (46.4) | |
| Working/studying | 9,950 | 7,286 (73.2) |
| Living alone | 10,025 | 1,949 (19.4) |
| Clinical processes | ||
| Diabetes duration, years | 10,175 | 22.9 ± 14.4 |
| CSII | 10,005 | 3,558 (35.6) |
| CGM use | 9,950 | 6,938 (69.7) |
| BMI, kg/m2 | 7,847 | 27.1 ± 5.0 |
| Daily smoking | 9,644 | 1,030 (10.7) |
| Clinical outcomes | ||
| HbA1c, % | 10,055 | 7.6 ± 1.2 |
| HbA1c, mmol/mol | 10,055 | 59 ± 12.7 |
| Acute complications | ||
| Symptomatic hypoglycemic events in previous month | 8,609 | 7.9 ± 9.6 |
| History of severe hypoglycemia | 8,749 | 4,041 (46.2) |
| History of DKA | 8,454 | 1,857 (22.0) |
| Long-term complications | ||
| Treated retinopathy | 8,839 | 1,299 (14.7) |
| Reduced foot sensitivity | 8,116 | 885 (10.9) |
| eGFR <60 mL/min/1.73 m2 | 9,382 | 419 (4.5) |
| Urine ACR ≥3 mg/mmol | 8,043 | 1,034 (12.9) |
| Stroke | 8,747 | 187 (2.1) |
| Coronary heart disease | 8,726 | 588 (6.7) |
| Two or more complications | 9,220 | 822 (8.9) |
ACR, albumin-to-creatinine ratio; CSII, continuous subcutaneous insulin infusion; DKA, diabetic ketoacidosis; eGFR, estimated glomerular filtration rate.
n of participants with available data for the variables.
Data are mean ± SD, median (min–max), or n (%).
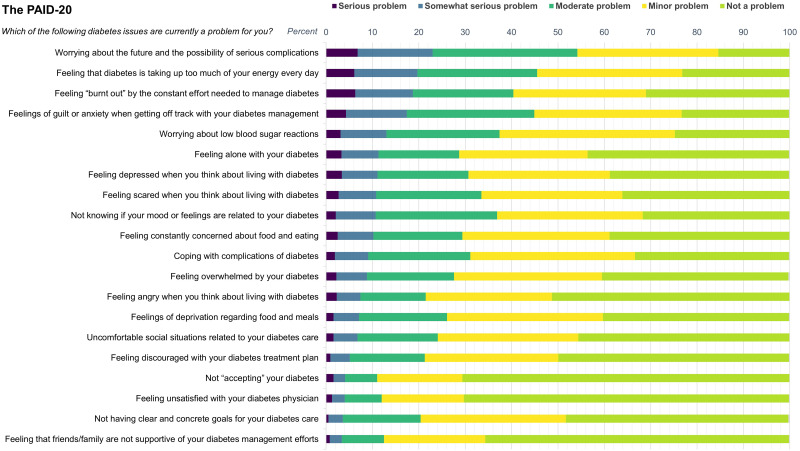
The mean PAID-20 score was 25.4 (SD 18.4), and 21.7% reported high distress (Table 1). The 20-item score distribution is shown in Fig. 2 and Supplementary Table 2. The five items most endorsed as problematic were “worrying about the future and the possibility of serious complications” (23.0%), “feeling that diabetes is taking up too much of your mental and physical energy every day” (19.7%), “feeling burnt out by the constant effort needed to manage diabetes” (18.7%), “feelings of guilt or anxiety when you get off track with your diabetes management” (17.4%), and “worrying about low blood sugar reactions” (13.0%). In total, 42.2% reported that at least one item was a somewhat serious or serious problem (Supplementary Table 3). Among participants with a PAID-20 score <40, this proportion was 20.9%.
Figure 2.
Score distribution of the 20 items in the PAID-20 scale among 10,186 adults with type 1 diabetes by problem areas endorsed as most problematic (i.e., the response options “somewhat serious problem” and “serious problem”).
In the fully adjusted regression (model 3), female sex, CGM use, daily smoking, symptomatic hypoglycemia, HbA1c, treated retinopathy, and reduced foot sensitivity were all associated with higher PAID-20 scores (Table 2), whereas older age, longer diabetes duration, European origin, university/college education, and working/studying were associated with lower PAID-20 scores. In examining these associations, sex displayed the largest effect (β) on PAID-20 scores, followed by age and HbA1c. The associations observed for the demographic and clinical process variables remained consistent across the models, except for university/college education, which was attenuated in model 3.
Table 2.
Associations of demographic, clinical process, and clinical outcome variables with PAID-20 scores in adults (age ≥18 years) with type 1 diabetes (N = 10,186)
| Variable | Crude | Model 1 | Model 2 | Model 3 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | 95% CI for B |
P | B | 95% CI for B |
β | P | B | 95% CI for B |
β | P | B | 95% CI for B |
β | P | |
| Demographics* | |||||||||||||||
| Female sex | 7.6 | 6.9, 8.3 | <0.001 | 7.3 | 6.6, 8.0 | 0.20 | <0.001 | 7.0 | 6.3, 7.7 | 0.19 | <0.001 | 7.0 | 6.3, 7.7 | 0.19 | <0.001 |
| Age, years | −0.2 | −0.21, −0.16 | <0.001 | −0.2 | −0.25, −0.20 | −0.19 | <0.001 | −0.2 | −0.21, −0.15 | −0.15 | <0.001 | −0.2 | −0.19, −0.13 | −0.14 | <0.001 |
| European origin | −8.6 | −11.0, 6.3 | <0.001 | −6.7 | −9.0, −4.4 | −0.06 | <0.001 | −6.8 | −9.1, −4.5 | −0.06 | <0.001 | −6.5 | −8.7, −4.2 | −0.05 | <0.001 |
| Living alone | 1.7 | 0.8, 2.6 | <0.001 | 1.4 | 0.5, 2.3 | 0.03 | 0.002 | 1.4 | 0.5, 2.3 | 0.03 | 0.002 | 1.1 | 0.2, 1.9 | 0.02 | 0.015 |
| Educational level | |||||||||||||||
| Secondary school | −1.9 | −3.3, −0.5 | 0.01 | −1.2 | −2.5, 0.2 | −0.03 | 0.10 | −0.9 | −2.3, 0.5 | −0.02 | 0.21 | −0.4 | −1.7, 1.0 | −0.10 | 0.59 |
| University/college | −4.2 | −5.7, −2.8 | <0.001 | −3.5 | −4.9, −2.1 | −0.10 | <0.001 | −3.0 | −4.5, −1.6 | −0.08 | <0.001 | −1.8 | −3.2, −0.4 | −0.05 | 0.013 |
| Working or studying | −1.8 | −2.6, −1.0 | <0.001 | −4.1 | −5.0, −3.3 | −0.10 | <0.001 | −4.1 | −5.0, −3.2 | −0.10 | <0.001 | −3.3 | −4.2, −2.4 | −0.08 | <0.001 |
| Clinical processes† | |||||||||||||||
| Diabetes duration, years | −0.1 | −0.16, −0.11 | <0.001 | −0.1 | −0.10, −0.05 | −0.06 | <0.001 | −0.1 | −0.15, −0.08 | −0.09 | <0.001 | ||||
| CGM use | 3.8 | 3.0, 4.5 | <0.001 | 3.1 | 2.3, 3.9 | 0.08 | <0.001 | 2.9 | 2.1, 3.7 | 0.07 | <0.001 | ||||
| CSII | 3.0 | 2.2, 3.7 | <0.001 | 1.1 | 0.3, 1.9 | 0.03 | 0.005 | 0.8 | 0.1, 1.6 | 0.02 | 0.04 | ||||
| Daily smoking | 3.4 | 2.2, 4.6 | <0.001 | 2.7 | 1.5, 3.8 | 0.05 | <0.001 | 1.6 | 0.5, 2.8 | 0.03 | 0.005 | ||||
| Clinical outcomes‡ | |||||||||||||||
| Symptomatic hypoglycemic events, n | 0.1 | 0.08, 0.17 | <0.001 | 0.1 | 0.06, 0.14 | 0.05 | <0.001 | ||||||||
| HbA1c, mmol/mol | 0.3 | 0.23, 0.28 | <0.001 | 0.2 | 0.18, 0.24 | 0.14 | <0.001 | ||||||||
| History of DKA | 4.0 | 3.1, 5.0 | <0.001 | 0.5 | −0.4, 1.5 | 0.01 | 0.27 | ||||||||
| History of severe hypoglycemia | −0.6 | −1.3, 0.2 | 0.15 | −0.1 | −0.9, 0.7 | −0.003 | 0.75 | ||||||||
| Treated retinopathy | 0.8 | −0.3, 1.8 | 0.17 | 2.6 | 1.4, 3.7 | 0.05 | <0.001 | ||||||||
| Reduced foot sensitivity | −0.4 | −1.6, 0.9 | 0.59 | 1.9 | 0.6, 3.2 | 0.03 | 0.005 | ||||||||
| Reduced kidney function§ | 0.5 | −0.6, 1.6 | 0.33 | 0.7 | −0.4, 1.8 | 0.01 | 0.21 | ||||||||
| Coronary heart disease | −1.7 | −3.2, −0.1 | 0.033 | 0.5 | −1.1, 2.0 | 0.006 | 0.55 | ||||||||
CSII, continuous subcutaneous insulin infusion; DKA, diabetic ketoacidosis; eGFR, estimated glomerular filtration rate.
Male sex, non-European origin, not living alone, primary education, and not working/studying.
Self-monitoring of blood glucose, insulin pen use, and nonsmoker.
No history of DKA, no retinopathy or nontreated retinopathy, normal foot sensitivity, normal kidney function (eGFR >60 mL/min/1.73 m2 and urine albumin-to-creatinine ratio <3 mg/mmol), and no coronary heart disease.
Reduced kidney function defined as eGFR <60 mL/min/1.73 m2 and urine albumin-to-creatinine ratio ≥3 mg/mmol.
Conclusions
In this large population-based cohort of adults with type 1 diabetes and PAID-20 data, we found that 21.7% of respondents reported high distress. The most common distress sources were concerns about complications, self-management burdens, and feelings of guilt or anxiety when self-management efforts fell short. Additionally, we identified associations between higher diabetes distress and sex, age, non-European origin, primary education only, not working/studying, shorter diabetes duration, CGM use, more symptomatic hypoglycemia, higher HbA1c, retinopathy, reduced foot sensitivity, and smoking.
We found that one in five participants reported high distress, confirming results from smaller studies reporting 17% to 24% (7,9,10). The most endorsed problem areas related to worries about complications and diabetes self-management are consistent with previous research in health care settings (9,10). The associations of higher distress with female sex and younger age also confirm previous studies (7,9,11). Surprisingly, CGM use was associated with a higher PAID-20 score. One could assume that some choose CGM because of distress and that using CGM would positively affect and lower distress. However, in a recent review, only two of nine randomized controlled trials reported distress reduction after initiation of CGM (12), possibly explained by devices serving as constant reminders of disease or contributing to information overload. Prospective studies are needed to examine the CGM–distress mechanisms.
Our results support the relationship between poorer HbA1c and higher distress (9,11,13). The association could be bidirectional, since previous studies have found that high distress is associated with higher HbA1c (14,15). Not reaching the recommended glycemic target can be a potential source of diabetes distress, and distress can contribute to less effective self-management, typically increasing HbA1c (3,16). However, individuals reaching target HbA1c might also experience high distress, and distress sources may vary by HbA1c level. Consequently, the link between HbA1c and distress requires further investigation.
Diabetes distress is an expected part of living with diabetes that can arise from many sources (1,2,10). Elevated diabetes distress should be recognized as clinically important, since research indicates associations with psychological and somatic variables (1,2,9,12,14). If unaddressed, diabetes distress tends to increase or persist (17,18). Therefore, clinicians should be able to address the multifaceted nature of living with diabetes in routine care (1,4,5). Individuals with diabetes typically want to talk to clinicians about their challenges (10,19). Discussing distress can reduce its impacts on living with diabetes, thereby improving self-management and glycemic control (2,3,7,10,18). Clinicians should pay particular attention to those who report feeling guilty or anxious about not succeeding with self-management, worrying about complications, and consequently feeling exhausted from everyday demands. Addressing specific problem areas with elevated scores is probably more relevant than examining total scores. However, many clinicians report lack of training, confidence, and resources to address distress (20). Health care services clearly need to allocate resources for this purpose. Diabetes teams should have access to assistance from behavioral health specialists and specialist follow-up of patients with very high distress.
Our population-based study contributes to an increased understanding of diabetes distress among adults with type 1 diabetes. Major strengths are the real-world setting, sample size (the largest reported to date), and high response rate. The national population-based data add weight to smaller studies in health care settings that may have been biased by participant selection. Furthermore, we used the PAID-20, recommended for research and clinical settings (1,6). However, timing and technology literacy may have affected the response rate. Therefore, diabetes distress rates might have been underestimated. Also, the study design prohibits causal inference.
To conclude, diabetes distress is common among adults with type 1 diabetes in Norway and associated with clinically relevant factors, warranting greater attention in regular diabetes care, which should include efforts to identify and address diabetes distress. By doing so, we may help improve health outcomes for individuals with type 1 diabetes.
This article contains supplementary material online at https://doi.org/10.2337/figshare.24397879.
Article Information
Funding. This study was funded by the Western Norway University of Applied Sciences, the Norwegian Nursing Association, and grant F-12610 from the Western Norway Regional Health Authority (Helse Vest).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. I.H. wrote the initial draft of the manuscript, with assistance from J.G.C., R.M.N., T.C.S., R.B.S., M.M.I., M.G., K.F.L., T.V.M., S.S.L., D.A.R., G.Å.U., and A.H. I.H. and A.H. designed the study, with input from R.M.N., T.C.S., R.B.S., M.M.I., and M.G. I.H. and T.E. conducted the statistical analyses, under the supervision of R.M.N. All authors contributed revisions to the paper and approved the final version. I.H. and A.H. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This study was presented in part in poster form at the International Diabetes Federation World Diabetes Congress, Lisbon, Portugal, 5–8 December 2022.
Funding Statement
This study was funded by the Western Norway University of Applied Sciences, the Norwegian Nursing Association, and grant F-12610 from the Western Norway Regional Health Authority (Helse Vest).
References
- 1. Skinner TC, Joensen L, Parkin T.. Twenty-five years of diabetes distress research. Diabet Med 2020;37:393–400 [DOI] [PubMed] [Google Scholar]
- 2. Fisher L, Polonsky WH, Hessler D.. Addressing diabetes distress in clinical care: a practical guide. Diabet Med 2019;36:803–812 [DOI] [PubMed] [Google Scholar]
- 3. Hessler D, Strycker L, Fisher L.. Reductions in management distress following a randomized distress intervention are associated with improved diabetes behavioral and glycemic outcomes over time. Diabetes Care 2021;44:1472–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Holt RIG, DeVries JH, Hess-Fischl A, et al. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2021;44:2589–2625 [DOI] [PubMed] [Google Scholar]
- 5. ElSayed NA, Aleppo G, Aroda VR, et al. ; American Diabetes Association . 5. Facilitating positive health behaviors and well-being to improve health outcomes: Standards of Care in Diabetes—2023. Diabetes Care 2023;46(Suppl. 1):S68–S96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nano J, Carinci F, Okunade O, et al. ; Diabetes Working Group of the International Consortium for Health Outcomes Measurement (ICHOM) . A standard set of person-centred outcomes for diabetes mellitus: results of an international and unified approach. Diabet Med 2020;37:2009–2018 [DOI] [PubMed] [Google Scholar]
- 7. Dennick K, Sturt J, Hessler D, et al. High rates of elevated diabetes distress in research populations: a systematic review and meta-analysis. Int Diabetes Nurs 2016;12:93–107 [Google Scholar]
- 8. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care 1995;18:754–760 [DOI] [PubMed] [Google Scholar]
- 9. de Wit M, Pouwer F, Snoek FJ.. How to identify clinically significant diabetes distress using the Problem Areas in Diabetes (PAID) scale in adults with diabetes treated in primary or secondary care? Evidence for new cut points based on latent class analyses. BMJ Open 2022;12:e056304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hendrieckx C, Halliday JA, Russell-Green S, et al. Adults with diabetes distress often want to talk with their health professionals about it: findings from an audit of 4 Australian specialist diabetes clinics. Can J Diabetes 2020;44:473–480 [DOI] [PubMed] [Google Scholar]
- 11. Fisher L, Hessler D, Polonsky W, Strycker L, Masharani U, Peters A.. Diabetes distress in adults with type 1 diabetes: prevalence, incidence and change over time. J Diabetes Complications 2016;30:1123–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Speight J, Choudhary P, Wilmot EG, et al. Impact of glycaemic technologies on quality of life and related outcomes in adults with type 1 diabetes: a narrative review. Diabet Med 2023;40:e14944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Joensen LE, Almdal TP, Willaing I.. Associations between patient characteristics, social relations, diabetes management, quality of life, glycaemic control and emotional burden in type 1 diabetes. Prim Care Diabetes 2016;10:41–50 [DOI] [PubMed] [Google Scholar]
- 14. Nagel KE, Dearth-Wesley T, Herman AN, Smith HG, Whitaker RC.. Diabetes distress and glycaemic control in young adults with type 1 diabetes: associations by use of insulin pumps and continuous glucose monitors. Diabet Med 2021;38:e14660. [DOI] [PubMed] [Google Scholar]
- 15. Strandberg RB, Graue M, Wentzel-Larsen T, Peyrot M, Rokne B.. Relationships of diabetes-specific emotional distress, depression, anxiety, and overall well-being with HbA1c in adult persons with type 1 diabetes. J Psychosom Res 2014;77:174–179 [DOI] [PubMed] [Google Scholar]
- 16. Hessler DM, Fisher L, Polonsky WH, et al. Diabetes distress is linked with worsening diabetes management over time in adults with type 1 diabetes. Diabet Med 2017;34:1228–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ehrmann D, Kulzer B, Haak T, Hermanns N.. Longitudinal relationship of diabetes-related distress and depressive symptoms: analysing incidence and persistence. Diabet Med 2015;32:1264–1271 [DOI] [PubMed] [Google Scholar]
- 18. Hessler D, Fisher L, Polonsky W, et al. There is value in treating elevated levels of diabetes distress: the clinical impact of targeted interventions in adults with type 1 diabetes. Diabet Med 2020;37:71–74 [DOI] [PubMed] [Google Scholar]
- 19. Hernar I, Graue M, Strandberg RB, et al. Young adults with type 1 diabetes and their experiences with diabetes follow-up and participation in the DiaPROM pilot trial: a qualitative study. Diabet Med 2021;38:e14535. [DOI] [PubMed] [Google Scholar]
- 20. Byrne JL, Davies MJ, Willaing I, et al. Deficiencies in postgraduate training for healthcare professionals who provide diabetes education and support: results from the Diabetes Attitudes, Wishes and Needs (DAWN2) study. Diabet Med 2017;34:1074–1083 [DOI] [PubMed] [Google Scholar]