Abstract
Objective
To assess the feasibility of a 24-week, center-based, aerobic exercise program plus duloxetine to treat symptomatic knee osteoarthritis (OA) and major depression.
Design
Patients with symptomatic knee OA and major depression were recruited between August 2021 and November 2022 from the University of Maryland and VA Maryland Health Care Systems and Baltimore metropolitan area using medical records and advertisements. The intervention included 1) supervised treadmill walking 3 times weekly and 2) duloxetine starting at 30 mg each day and titrating up to the optimal dosage of 60 mg daily. Data collection occurred at baseline and 12- and 24-weeks follow-up. Feasibility was evaluated from recruitment rates, reasons for drop out, and treatment adherence. Clinical measures included the Knee Injury and Osteoarthritis Outcome Score (KOOS) and the Hamilton Depression Rating Scale (HAM-D).
Results
Among 377 interested participants, 9 patients were enrolled, and 1 completed treatment. The most common reason reported for not prescreening was time commitment (n = 39), many patients did not satisfy depression screening criteria (n = 45), and most enrolled participants were not experiencing a major depressive episode (n = 6). The single treated participant was 100 % adherent to duloxetine and depression severity decreased (HAM-D = 25 to 1), but compliance to supervised exercise was only 26 %, and knee pain severity changed little (KOOS = 41.7 to 44.4).
Conclusions
This intervention had low feasibility. Time commitment to supervised exercise sessions reduced accessibility, and depression defined by diagnostic criteria precluded knee OA patients with depressive symptoms not a meeting case-level diagnosis from receiving treatment.
Clinical trial registration number
Keywords: Knee osteoarthritis, Depression, Duloxetine, Exercise, Feasibility
1. Introduction
Knee osteoarthritis (OA) is the most common joint disorder that impacts over 30 % of older American adults [1]. Depressive symptoms are prevalent among 20 % of knee OA patients and are a significant barrier to clinical management [2]. However, medical care guidelines do not advise how to treat both conditions contemporaneously [3]. Prior clinical research has evaluated depression interventions in knee OA patients, but these studies focused on treating depressive symptoms, rather than managing their co-occurrence [4]. Recent research also recommends combining treatments that affect symptoms of both conditions to maximize intervention efficacy [4]. Current evidence-based treatment guidelines advise exercise training for knee OA patients, but engagement in physical activity in this population is low and exacerbated by comorbid depression [5]. Duloxetine is an FDA-approved antidepressant medication indicated to treat both major depression and musculoskeletal pain and is a viable complement to exercise training [6]. Thus, the objective was to assess the feasibility of a 24-week, center-based, aerobic exercise program plus duloxetine to treat symptomatic knee OA and major depression. It was hypothesized that duloxetine plus a supervised aerobic exercise program would be feasible regarding participant recruitment, enrollment, and screening, as well as acceptable to patients experiencing the treatment protocol.
2. Method
2.1. Sample
Knee OA patients were recruited between August 2021 and November 2022 from the University of Maryland and VA Maryland Health Care Systems and Baltimore metropolitan area using medical records and study advertisements. Inclusion criteria were: English speaking; age 40 years or older; symptomatic knee OA satisfying ACR criteria [7]; major depression according to the SCID-5 (Structured Clinical Interview for DSM-V, ruling out history of bipolar disorder or psychotic symptoms; substance abuse disorder or suicidal ideation in the last year) [8]; no plans for knee surgery; and ability to walk on a treadmill. Exclusions included: exercising at least twice per week; taking duloxetine, antipsychotics, benzodiazepines, or opioid analgesics; cognitive impairment; comorbidity precluding exercise; and pregnant or lactating women. The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000, and detailed protocol information is provided in Appendix A. This study was reviewed and approved by the University of Maryland Baltimore Institutional Review Board (HP-00089160).
2.2. Design & intervention
This single-site and -group, open label feasibility study, evaluated a combined treatment protocol that included six months of aerobic exercise plus duloxetine. As it not possible to accurately estimate effect sizes from feasibility research, the sampling strategy was driven by grounded theory framework [9,10]. Grounded theory involves an iterative process of identifying thematic patterns surrounding a phenomenon of interest, which is not focused on obtaining a generalizable (i.e., sample to population) group of participants, but rather information richness (i.e., depth and breadth) to understand protocol strengths and weakness [9]. Given the homogeneity of the patient subgroup and research aim specificity, it was anticipated that 10 participants would be sufficient to evaluate protocol feasibility. Exercise training consisted of a treadmill walking program, and sessions took place three times per week under the supervision of a trained exercise physiologist within the VA Maryland Health Care System at the Geriatric Research Education and Clinical Center Senior Exercise and Rehabilitation Center. Exercise training plans were individualized based upon participants’ peak heart rate achieved during a graded exercise test. Duloxetine is a potent and selective inhibitor of 5H-T and NE reuptake in vitro and in vivo in the central nervous system and was prescribed and monitored by a clinical psychiatrist [6]. Starting duloxetine dosage was 30 milligrams per day (mg/day), and patients were titrated up to an optimal dose of 60 mg/day, as tolerated [6]. There was no cost to participants for any portion of the multimodal intervention.
2.3. Screening & data collection
Interested participants were initially assessed via telephone pre-screening, and eligible patients were invited to complete two in-person screening visits. These visits involved obtaining written informed consent, self- and interviewer-administered surveys that evaluated mental and physical health, history and physical evaluation with a clinical geriatrician, and fasting blood draw to assess metabolic parameters. After screening, data collection occurred over three separate visits at baseline and 12- and 24-weeks follow-up. During these visits, participants completed survey questionnaires, physical performance tests, qualitative interviews, and graded exercise tests. Furthermore, patients met with the clinical psychiatrist to evaluate mental health status and obtain medication prescriptions. Biweekly medication telephone assessments were performed by study staff to evaluate adherence and unexpected adverse effects. Physical health and training program progression were evaluated on a weekly basis by exercise physiologists.
2.4. Measures & analyses
Sociodemographic factors were assessed at study enrollment. Primary feasibility outcomes were assessed using recruitment and screening rates, reasons for non-participation and drop out, as well as treatment adherence to aerobic exercise and duloxetine [10]. Secondary clinical measures for knee OA and major depression were pain severity and depressive symptoms evaluated at baseline and follow-up visits, respectively. Depressive symptoms were measured using the Hamilton Depression Rating Scale (HAM-D) [11]. Pain severity was evaluated with the Knee Injury and Osteoarthritis Outcome Score (KOOS) [12]. The KOOS pain subscale was rescaled to range from 0 to 100. Higher scores on the HAM-D and KOOS indicate more depressive symptoms and greater pain severity, respectively [11,12]. Sociodemographic information, feasibility outcomes, and clinical measures were summarized using frequencies and percentages and means and standard deviations for categorical and continuous data, respectively.
3. Results
3.1. Recruitment
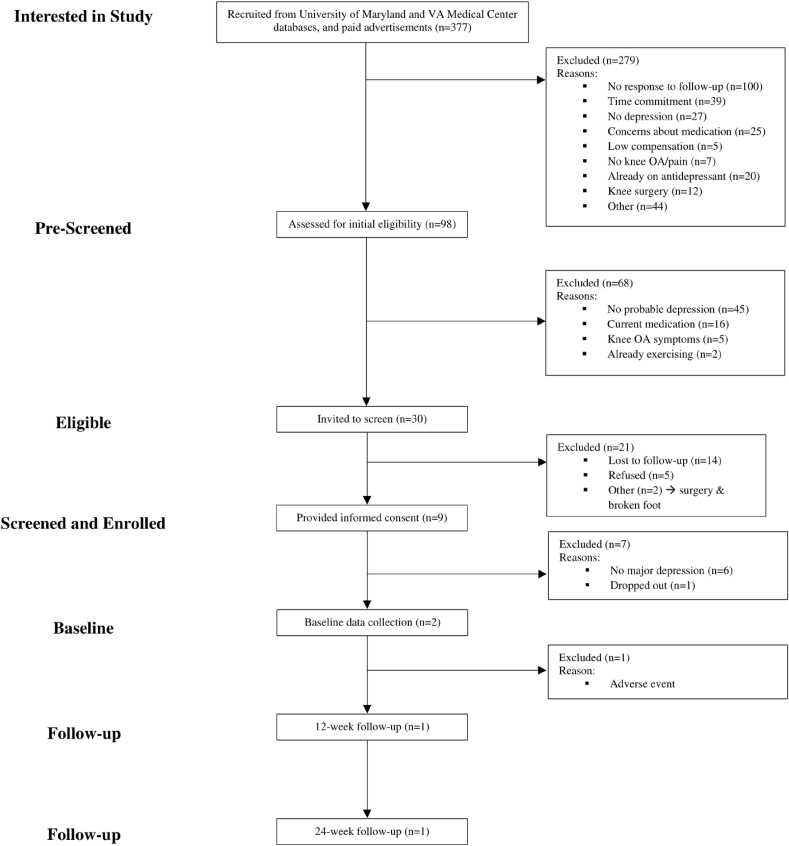
Among 377 interested participants, 98 of them completed telephone pre-screening (Fig. 1). Common reasons for not pre-screening included non-response to follow-up calls (n = 100), time commitment (n = 39), no depression (n = 27), concerns about the medication (n = 25), and already taking antidepressants (n = 20). Of the 98 patients who pre-screened, there were 68 ineligible participants who did not satisfy the screening criteria for probable depression (n = 45), concomitant medications (n = 16), knee OA symptoms (n = 5), and exercise (n = 2). There were 30 eligible patients invited to in-person screening visits, of which 21 participants were not enrolled because of loss to follow-up (n = 14), refusal to participate (n = 5), and other medical events (n = 2).
Fig. 1.
Study sample flow diagram.
3.2. Sample characteristics
There were 9 older patients (mean age = 64.7) enrolled who provided written informed consent (Table 1). The sample was predominantly female (n = 7) and patients who identified as Black/African American (n = 8). Most participants (n = 7) also attended and/or graduated from college, but less than half were married (n = 3) or retired (n = 4). During in-person screening, 7 participants were excluded. The majority of patients (n = 6) were restricted because they did not satisfy DSM-V criteria for major depression. Among 3 individuals with a current major depressive episode, one voluntarily withdrew during screening. Two eligible patients with major depression completed screening, but another participant subsequently withdrew during baseline data collection due to a non-study related adverse event.
Table 1.
Study sample sociodemographic characteristics.
| Variables | Mean or N | SD or % |
|---|---|---|
| Age (years) | 64.7 | 7.4 |
| Sex | ||
| Females | 7 | 77.70 % |
| Ethnicity | ||
| Not Hispanic or Latino | 9 | 100.00 % |
| Race | ||
| White | 1 | 11.00 % |
| Black or African American | 8 | 88.90 % |
| Education | ||
| Some High School | 1 | 11.00 % |
| High School Graduate | 1 | 11.00 % |
| Some College or Degree | 7 | 77.70 % |
| Marital Status | ||
| Never Married | 2 | 22.00 % |
| Currently Married | 3 | 33.00 % |
| Marriage-like Relationship | 1 | 11.00 % |
| Divorced/Separated | 2 | 22.00 % |
| Widowed | 1 | 11.00 % |
| Work Status | ||
| Full-time | 2 | 22.00 % |
| Part-time | 1 | 11.00 % |
| Retired | 4 | 44.00 % |
| Disability | 2 | 22.00 % |
3.3. Treatment adherence & outcomes
A single patient experienced the 24-week intervention, completed the treatment protocol, and had two non-study related adverse events. This participant was almost 100 % adherent to duloxetine and had a reduction in depressive symptoms (HAM-D = 25 to 1) from baseline to 24 weeks follow-up. By contrast, exercise training compliance was low and only 26 % of supervised sessions were attended. There was also little change in knee pain severity (KOOS = 41.7 to 44.4) from baseline to 24-weeks follow-up.
4. Discussion
Feasibility results demonstrated low recruitment rates and poor retention after enrollment, and throughout each stage of the study, there were major design challenges and significant attrition among potential participants. During the initial stage of the recruitment process, time commitment to the intervention was a frequent barrier (n = 39) to pursuing treatment. In addition, many prescreened participants (n = 45) and enrolled patients (n = 6) did have not have case-level mood disorder. Despite inquiries from 377 interested individuals, only 2 participants satisfied study eligibility criteria and finished screening procedures, and 1 patient completed the treatment protocol and data collection. Collectively, findings highlight the difficulties of developing a combined intervention to address the co-occurrence of physical and mental morbidity associated with knee OA and depression.
The largest barrier to feasibility was the many interested patients that chose not to participate after initially speaking to research staff to learn more about the study. Among those reasons reported by participants for not prescreening, the most common was concerns about time commitment to a center-based exercise program. This finding is consistent with previous research showing that adherence to exercise training among older adults is approximately two times higher (68 % versus 36 %) for home-than center-based programs [13]. During the 24-week study period, patients were required to travel to downtown Baltimore approximately 83 times: 2 times for in-person screening visits; 3 times per week for supervised aerobic exercise training sessions; and 9 times for data collection visits at baseline and 12- and 24-weeks follow-up. In addition, adherence to the medication component was approximately four times greater compared to the exercise training portion (100 % versus 26 %, respectively) in the 1 participant who completed the protocol. Thus, the time commitment required for the treatment protocol and other study procedures may have been too burdensome, especially during the COVID-19 pandemic, and alternative tele-health intervention designs that can be done remotely utilizing at-home exercise sessions may be more optimal.
Another pervasive issue impeding access to treatment among interested patients were depressive symptoms not meeting screening scores for self-reported symptomology (9-Item Patient Health Questionnaire score ≥10) and/or depression not satisfying interviewer-assessed diagnostic criteria for case-level mood disorder [8]. “Melancholic” depressive symptoms are consistent with the DSM-V definition for major depressive disorder and the most severe among the subgroups of the “depression” knee OA clinical phenotype [8,14]. Symptomology that comports with diagnostic criteria for case-level mood disorder is the least common among knee OA patients, and there are other clinically relevant depression subgroups that have milder and/or different constellations of depressive symptoms [14]. There is a paucity of clinical research that has developed and tested prevention strategies for affective disorders in this population, and knee OA patients with depressive symptoms who are at-risk for major depression may benefit from preventive intervention. A more feasible version of the combined intervention assessed in the current study could be used for both depression prevention and treatment, particularly as research indicates that adding duloxetine to usual care is cost effective among knee OA patients with moderate pain, regardless of depressive symptoms [15].
Findings must be interpreted in relation to study limitations. Foremost, generalizability may be limited by geographic location of the Baltimore metropolitan area and time period regarding the COVID-19 pandemic. This issue is further exacerbated because the study focused on a subset of patients within a specific clinical phenotype of the general knee OA population using an extensive screening and data collection process that made it difficult to recruit and enroll eligible patients. Lastly, this small study enrolled 9 patients, and due to the described challenges, only 1 participant experienced the treatment protocol, which further limits the potential implications of the results. Nonetheless, this research represents the first empirical evaluation of an intervention combining a core non-pharmacological treatment with secondary adjunctive pharmacotherapy to treat the co-occurrence of symptomatic knee OA and major depression. Additional research is required to determine whether it is possible to develop a feasible implementation of an intervention that combines treatments affecting symptoms related to both physical and mental health in a way that optimizes effectiveness by simultaneously intervening on knee OA pain and depressive symptoms.
In conclusion, a center-based aerobic exercise program plus duloxetine to treat symptomatic knee OA and major depression may have low feasibility. Future research must address the noted intervention challenges to develop a more feasible treatment protocol that is tailored to the “depression” knee OA phenotype [14].
Author contributions
Alan M. Rathbun obtained funding, participated in study conception and design and data acquisition and assembly, conducted statistical analyses, interpreted results, and wrote the manuscript. Rhea Mehta participated in study conception and design and data acquisition and assembly and provided administrative, technical, and logistic support. Alice S. Ryan, Yu Dong, and Brock Beamer participated in study conception and design and data acquisition, interpreted results, and critically revised the manuscript for important intellectual content. Justine Golden participated in study conception and design and data acquisition and assembly and conducted statistical analyses. Joseph J. Gallo, Mark Luborsky, Michelle D. Shardell, Jason E. Peer, and Marc C. Hochberg participated in study conception and design, interpreted results, and critically revised the manuscript for important intellectual content. Alan M. Rathbun (arathbun@som.umaryland.edu) takes responsibility for the integrity of the work as a whole from inception to finished article. All authors read and approved the final version of the manuscript for publication.
Role of the funding source
This study was supported by grants from the National Institute on Aging (K01 AG064041, P30 AG028747, T32 AG000262). The sponsor had no role in the study design, collection, analysis, and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of competing interest
Marc C. Hochberg is the president of Rheumcon and receives consulting fees from Bone Therapeutics, Bristol-Myers Squibb, Eli Lilly, Galapagos, IBSA Insititut Biotechniq SA, Novartis Pharma AG, Pfizer, Samumed LLC, Theralogix LLC, and Kolon TissueGene. Alan M. Rathbun, Rhea Mehta, Alice S. Ryan, Yu Dong, Brock Beamer, Justine Golden, Joseph J. Gallo, Mark Luborsky, Michelle D. Shardell, and Jason E. Peer have no conflicts of interest to declare.
Acknowledgements
Research reported in this publication was supported by National Institute on Aging of the National Institutes of Health under award numbers: K01 AG064041, P30 AG028747, and T32 AG000262. This material is also based on upon work supported (or supported in part) by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, VA Maryland Health Care System, and Baltimore VA Medical Center. The authors would like to thank the VA Maryland Health Care System Geriatric Research Education and Clinical Center staff who made this work possible.
Handling Editor: Professor H Madry
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ocarto.2023.100426.
Contributor Information
Alan M. Rathbun, Email: arathbun@som.umaryland.edu.
Rhea Mehta, Email: rhea.mehta@som.umaryland.edu.
Alice S. Ryan, Email: aryan@som.umaryland.edu.
Yu Dong, Email: yu.dong@som.umaryland.edu.
Brock Beamer, Email: brock.beamer@va.gov.
Justine Golden, Email: jgolden@som.umaryland.edu.
Joseph J. Gallo, Email: jgallo2@jhu.edu.
Mark Luborsky, Email: mluborsky@wayne.edu.
Michelle D. Shardell, Email: mshardell@som.umaryland.edu.
Jason E. Peer, Email: jason.peer@va.gov.
Marc C. Hochberg, Email: marc.hochberg@va.gov.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 2013;21(9):1145–1153. doi: 10.1016/j.joca.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stubbs B., Aluko Y., Myint P.K., Smith T.O. Prevalence of depressive symptoms and anxiety in osteoarthritis: a systematic review and meta-analysis. Age Ageing. 2016;45(2):228–235. doi: 10.1093/ageing/afw001. 1. [DOI] [PubMed] [Google Scholar]
- 3.Cg N. National Institute for Health and Care Excellence; London: 2014. Osteoarthritis Care and Management in Adults. [PubMed] [Google Scholar]
- 4.Rathbun A.M., Shardell M.D., Stuart E.A., Yau M.S., Gallo J.J., Schuler M.S., et al. Pain severity as a mediator of the association between depressive symptoms and physical performance in knee osteoarthritis. Osteoarthritis Cartilage. 2018;26(11):1453–1460. doi: 10.1016/j.joca.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gay C., Eschalier B., Levyckyj C., Bonnin A., Coudeyre E. Motivators for and barriers to physical activity in people with knee osteoarthritis: a qualitative study. Joint Bone Spine. 2018;85(4):481–486. doi: 10.1016/j.jbspin.2017.07.007. 1. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues-Amorim D., Olivares J.M., Spuch C., Rivera-Baltanás T. A systematic review of efficacy, safety, and tolerability of duloxetine. Front. Psychiatr. 2020;11 doi: 10.3389/fpsyt.2020.554899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altman R., Asch E., Bloch D., Bole G., Borenstein D., Brandt K., et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 8.Shankman S.A., Funkhouser C.J., Klein D.N., Davila J., Lerner D., Hee D. Reliability and validity of severity dimensions of psychopathology assessed using the Structured Clinical Interview for DSM-5 (SCID) Int. J. Methods Psychiatr. Res. 2018;27(1) doi: 10.1002/mpr.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crabtree B.F., Miller W.L., editors. Annual North American Primary Care Research Group Meeting, 19th, May, 1989. Sage Publications, Inc; Quebec, PQ, Canada: 1992. Doing qualitative research. [Google Scholar]
- 10.Arain M., Campbell M.J., Cooper C.L., Lancaster G.A. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med. Res. Methodol. 2010;10(1):1–7. doi: 10.1186/1471-2288-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faries D., Herrera J., Rayamajhi J., DeBrota D., Demitrack M., Potter W.Z. The responsiveness of the Hamilton depression rating Scale. J. Psychiatr. Res. 2000;34(1):3–10. doi: 10.1016/s0022-3956(99)00037-0. [DOI] [PubMed] [Google Scholar]
- 12.Roos E.M., Lohmander L.S. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual. Life Outcome. 2003;1(1):1–8. doi: 10.1186/1477-7525-1-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashworth N.L., Chad K.E., Harrison E.L., Reeder B.A., Marshall S.C. Home versus center based physical activity programs in older adults. Cochrane Database Syst. Rev. 2005;(1) doi: 10.1002/14651858.CD004017.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rathbun A.M., Schuler M.S., Stuart E.A., Shardell M.D., Yau M.S., Gallo J.J., et al. Depression subtypes in individuals with or at risk for symptomatic knee osteoarthritis. Arthritis Care Res. 2020;72(5):669–678. doi: 10.1002/acr.23898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lenhard N.K., Sullivan J.K., Ross E.L., Song S., Edwards R.R., Hunter D.J., et al. Does screening for depressive symptoms help optimize duloxetine use in knee OA patients with moderate pain? A cost-effectiveness analysis. Arthritis Care Res. 2022;74(5):776–789. doi: 10.1002/acr.24519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.