Abstract
Background
The real-world safety profile of COVID-19 mRNA vaccines remains incompletely elucidated.
Methods
We performed a nationwide post-market safety surveillance analysis in Singapore, on vacinees aged 5 years and older, through mid-September 2022. Observed-over-expected (O/E) analyses were performed to identify potential safety signals among eight shortlisted adverse events of special interest (AESIs): strokes, cerebral venous thrombosis (CVT), acute myocardial infarction, myocarditis/pericarditis, pulmonary embolism, immune thrombocytopenia, convulsions and appendicitis. Self-controlled case series analyses (SCCS) were performed to validate signals of concern, occurring within 42 days of vaccination.
Findings
Elevated risks were observed on O/E analyses for the following AESIs: myocarditis/pericarditis, [rate ratio (RR): 3.66, 95 % confidence interval (95 % CI): 2.71 to 4.94], appendicitis [RR: 1.14 (1.02 to 1.27)] and CVT [RR: 2.11 (1.18 to 3.77)]. SCCS analyses generated corroborative findings: myocarditis/pericarditis, [relative incidence (RI): 6.96 (3.95 to 12.27) at 1 to 7 days post-dose 2], CVT [RI: 4.30 (1.30 to 14.20) at 22 to 42 days post-dose 1] and appendicitis [RI: 1.31 (1.03 to 1.67) at 1 to 7 days post-dose 1]. Booster dose 1 continued to be associated with higher rates of myocarditis/pericarditis on O/E analysis [RR: 2.30, (1.39 to 3.80) and 1.69, (1.11 to 2.59)] at 21- and 42-days post-booster dose 1, respectively. Males aged 12 to 17 exhibited highest risks of both myocarditis/pericarditis [RI: 6.31 (1.36 to 29.3)] and appendicitis [RI: 2.01 (1.12 to 3.64)] after primary vaccination. Similarly, CVT was also predominantly observed in males aged above 50 (11 out of 16 cases), within 42-days of vaccination.
Interpretation
Our data suggest that myocarditis/pericarditis, appendicitis and CVT are associated with primary vaccination using COVID-19 mRNA vaccines. Males at specific ages exhibit higher risks for all three AEs identified. The risk of myocarditis/pericarditis continues to be elevated after booster dose 1.
Keywords: Pharmacovigilance, Active surveillance, Vaccine safety, Covid-19 mRNA vaccines, Signal detection, Signal validation
Introduction
In Singapore, the BNT162b2 (Pfizer-BioNTech/ Comirnaty) COVID-19 vaccine was first rolled out on 30 December 2020, followed by the mRNA-1273 (Moderna/ Spikevax) COVID-19 vaccine on 12 March 2021.
In late 2020, the Health Sciences Authority (HSA) and the Ministry of Health (MOH) embarked on an initiative to augment the safety surveillance of COVID-19 vaccines by leveraging electronic health records (EHRs) of public health institutions. Collectively, these institutions provide over 80 % of tertiary care in Singapore.[1] Here we report safety signals detected from nationwide surveillance activities of selected adverse events of special interest (AESIs) following mRNA COVID-19 vaccination through 31 July 2022 in the population aged 5 and above, for both the primary series and first booster doses.
Given the low levels of COVID-19 transmission in 2020 and the majority of 2021, Singapore serves as a relatively unique setting to study the real-world safety profile of mRNA vaccines without interference of intercurrent infection on the development of AESIs.[2].
Methods
In part 1, the general approach towards post-market safety signal detection adopted by HSA is described including additional safeguards created for COVID-19 vaccines. Part 2 describes specific analyses performed and presented for all eight AESIs.
Part 1: Data sources, surveillance methods and adverse events of special interest (AESIs)
AE reporting has been the cornerstone pharmacovigilance tool for signal detection in Singapore. Singapore has consistently ranked amongst the top 10 countries with the highest individual case safety reports per million inhabitants (unpublished, World Health Organization-Uppsala Monitoring Centre). All COVID-19 vaccine related reports assessed as serious by reporters were actively followed up on for additional information for assessment. Quantitative data mining methods (e.g. Reporting Odds Ratio, Sequential Probability Ratio Test and Gamma Poisson Shrinker) were applied on the spontaneous AE database to detect disproportionately reported vaccine-AE pairs.[3] Additionally, literature searches, case series reviews, international workgroup discussions and alerts by other regulators were performed to identify all possible AEs related to COVID-19 vaccines. Periodic consultations with clinical expert panels appointed by HSA were also performed.
Special attention was paid to AEs prioritized by the Brighton Collaboration and the US Food & Drug Administration (US FDA) for COVID-19 vaccines.[4], [5] Eight AESIs [strokes, cerebral venous thrombosis (CVT), acute myocardial infarction, myocarditis/pericarditis, pulmonary embolism, immune thrombocytopenia, convulsions and appendicitis] were eventually shortlisted based on reporting volume, public concern and feasibility of investigation with the data available. The AESIs were subjected to active surveillance using deidentified EHRs, covering more than 80 % of the population in terms of tertiary care provision. These include inpatient admission and discharge details, diagnosis codes, laboratory test records, unstructured clinical notes, medication and vaccination records.[6].
These data allowed for observed-over-expected (O/E) analyses to be performed on various AESIs. The decision to embark on an O/E analysis for a given safety concern was typically multifactorial, considering the prevailing evidence and the clinical importance of the concern. When the lower bound 95 % CI of the O/E rate ratio (RR) exceeds 1, or if an AE was of public concern, robust pharmacoepidemiology studies were performed [namely, self-controlled case series (SCCS) analyses for COVID-19 vaccines].
SCCS analyses make key assumptions on the absence of event dependence of exposure probability and observation time.[7] As such, the analyses are evaluated for violation of these assumptions and if present, modified SCCS analyses are conducted instead. Chart reviews are performed to ensure only true cases are included. If infeasible due to volume, chart reviews are performed on a sample of cases to estimate the degree of misclassification from false positive cases, for which adjustments may be performed to yield minimally biased relative incidence (RI) estimates.[8].
Part 2: Observed-over-expected and self-controlled case series analyses
Expected (E) event rates were estimated for all 8 AESIs, using annual population data [9] and diagnosis records [Systematized Nomenclature of Medicine Clinical Terms (SNOMED-CT) codes] of incident events from 2018, 2019 and 2020, averaged over three years. Data from 2020 were included given the limited community spread of COVID-19 in Singapore in 2020 (approximately 1 % of population infected in 2020).[10] We additionally excluded patients if they were diagnosed with a SARS-CoV-2 infection within 42 days before the AESI diagnosis.
Observed (O) event rates were similarly estimated using the same databases. Each vaccinee contributed 21 or 42 days of post-vaccination observation time and incident events occurring within these time periods were included. Poisson regression was applied to estimate incidence rate ratios. Interim O/E analyses were performed on a subset of AESIs during the early phases of primary series vaccination[11], based on AE reports or potential signals identified overseas. Subsequently O/E analyses were conducted for all eight AESIs for primary series vaccinations up till November 30, 2021 to confirm our interim O/E findings and to detect any new safety signals. Finally, O/E analyses of selected AEs were performed in children aged 5 to 11 years, in whom vaccination commenced on December 27, 2021, as well as O/E analyses for first booster doses, which included vaccinations received up till July 31, 2022 for individuals 12 years and above. The study end date for O/E analyses was 42 days from July 31, i.e. mid September 2022.
Signals exceeding the lower-bound O/E threshold of 1.00 or of public concern were subjected to SCCS analyses. SCCS analyses were conducted for strokes, CVT, myocarditis/pericarditis and appendicitis. Supplementary Fig. 1 provides an overview of the standard and modified SCCS methods that were used to investigate these four AESIs. Standard SCCS analyses can generate upwardly biased estimates when AESIs delay subsequent vaccination. If delays are short-lived (as is assumed for appendicitis), a pre-exposure period can be excluded from baseline event rate estimation. However, when events lead to permanent cancelation or contraindicate post-event vaccination, a modified SCCS for event-dependent exposure, that considers only post-exposure events is necessary (applied for strokes, CVT and myocarditis/pericarditis).[12] Only events occurring after the pre-defined risk periods are used for baseline event rate estimation.
For all SCCS analyses, observation start date was December 30, 2020 when vaccinations were launched; study end dates for myocarditis/pericarditis (September 30, 2021), stroke and CVT (October 31, 2021), and appendicitis (March 31, 2021) varied because these signals emerged at different time-points (Supplementary Fig. 2 and Supplementary Table 1). The day of admission was used as the event onset date. The study end dates for myocarditis and appendicitis were later than that of the counterpart interim O/E analyses (conducted earlier in response to an emerging signal). This was to allow for a sufficient proportions of the population to be vaccinated and observed for 42 days, before conducting the SCCS analyses. For all AESIs, consistent post-vaccination risk periods were adopted after each dose: Day 0, 1–7, 8–14, 15–21, and 22–42-days. In event of overlapping risk periods arising from a subsequent dose administered during risk periods of a preceding dose, precedence is given to the most recent dose and risk periods are accordingly reassigned.[13] Chart reviews were conducted in all potential cases (identified through diagnosis codes) for CVT and myocarditis, and a random sample of 5 % were chart-reviewed for appendicitis and strokes. As AESI recurrences can be influenced by earlier events thus violating the requirement of event independence, the first incident AESI occurrence was considered in all analyses under the assumption that the AESIs are adequately rare, even for the most frequent AESIs (acute myocardial infarction and strokes).[14], [15], [16] In all SCCS analyses, unvaccinated cases were included. While this is non-essential for the standard SCCS analysis applied for appendicitis, excluding unvaccinated cases can result in biased estimates when applying the modified SCCS for event-dependent exposures because vaccinations (and their timing) that occur after events are potentially influenced by the timing of the event. By extension, the absence of vaccination may be informative about the timing of the event. [12], [13] Therefore all unvaccinated cases were included in the analysis of myocarditis/pericarditis, CVT and stroke.
Given the relatively short observation periods, age effects were not considered but seasonality was controlled for in 30-day intervals. Likewise, associations between a positive SARS-CoV-2 test and each AE were assessed similarly to compare effect estimates of vaccination against infection.
All analyses were retrospective and were conducted as part of public health surveillance, in accordance with the Declaration of Helsinki.
Results
By November 30, 2021, 85 % of the 5.5 million population had received at least one dose and 82 % had completed the primary series vaccination. By July 31, 2022, 77 % had received their first booster dose and 75 % of children aged five to 11 had completed primary series vaccination (Supplementary Table 2). These two dates served as milestones at which O/E analyses were conducted for primary series and booster doses, respectively, in addition to the interim O/E analyses perfomed for each signal at distinct timepoints prior. BNT162b2 comprised over 75 % of all doses administered and was the only vaccine administered to those aged five to 17 (Supplementary Table 1). The signals identified through nationwide safety surveillance presented here represent the evidence on each signal at the time of analysis.
Elevated risks were identified for three AESIs during their respective interim O/E analyses; myocarditis/pericarditis [rate ratio (RR): 2.33, 95 % confidence interval: 1.54 to 3.52] and CVT [RR: 2.19, (1.22 to 3.91)] and appendicitis [RR: 1.14 (1.02 to 1.27)] 42 days post-dose 1 (Supplementary Fig. 3). An elevated risk was observed only for CVT [RR: 2.27, (1.04 to 4.96)] at 21 days post-dose 2. SCCS analyses that these interim O/E findings triggered had generated corroborating relative incidence (RI) estimates of increased risks of all three AESIs; myocarditis/pericarditis [RI: 6.96, (3.95 to 12.27) at 1 to 7 days after dose 2], CVT [RI: 4.30 (1.30 to 14.20) at 22 to 42 days after dose 1] and appendicitis [RI: 1.31 (1.03 to 1.67) at 1 to 7 days and RI: 1.39 (1.10 to 1.77) at 15 to 21 days post-dose 1] (Table 1).
Table 1.
Self-controlled case series analysis for COVID-19 mRNA vaccination and positive SARS-Cov-2 on risk of myocarditis/pericarditis, cerebral venous thrombosis, strokes and appendicitis.
| AESI and Time Period |
D1 Vaccination |
D2 Vaccination |
B1 Vaccination* |
COVID-19 Infection |
||||
|---|---|---|---|---|---|---|---|---|
| D1 Cases | D1 Relative Incidence (95 % CI) | D2 Cases | D2 Relative Incidence | B1 Cases | B1 Relative incidence (95 % CI) | Infection Cases | Infection Relative Incidence (95 %CI) | |
| Myocarditis/Pericarditis, 12 years & above(through September 30, 2021, modified SCCS analysis) | ||||||||
| Baseline | 101 | 1.00 | 101 | 1.00 | NA^ | 2 | Not estimated | |
| 0 day | 1 | 1.37 [0.17 to 10.92] | 1 | 1.46 [0.20 to 10.68] | 0 | NA^ | 0 | Not estimated |
| 1–7 days | 10 | 1.90 [0.84 to 4.27] | 32 | 6.96 [3.95 to 12.27] | 0 | NA^ | 1 | Not estimated |
| 8–14 days | 10 | 1.83 [0.90 to 3.72] | 5 | 1.16 [0.45 to 2.99] | 0 | NA^ | 0 | Not estimated |
| 15–21 days | 4 | 0.72 [0.25 to 2.11] | 4 | 0.95 [0.33 to 2.75] | 0 | NA^ | 0 | Not estimated |
| 22–42 days | 2 | 0.17 [0.04 to 0.74] | 6 | 0.55 [0.22 to 1.33] | 0 | NA^ | 0 | Not estimated |
| Cerebral Venous Thrombosis, 18 years & above(through October 31, 2021, modified SCCS analysis) | ||||||||
| Baseline | 51 | 1.00 | NA^ | |||||
| 0 day | 0 | NA | 0 | NA | 0 | NA^ | 0 | Not estimated |
| 1–7 days | 0 | NA | 2 | 2.11 [0.48 to 9.33] | 0 | NA^ | 0 | Not estimated |
| 8–14 days | 2 | 1.57 [0.40 to 6.37] | 2 | 2.19 [0.48 to 10.01] | 1 | NA^ | 1 | Not estimated |
| 15–21 days | 1 | 0.91 [0.12 to 6.84] | 0 | NA | 0 | NA^ | 0 | Not estimated |
| 22–42 days | 5 | 4.30 [1.30 to 14.20] | 3 | 1.14 [0.31 to 4.27] | 0 | NA^ | 0 | Not estimated |
| Strokes, 18 years & above(through October 31, 2021, modified SCCS analysis) | ||||||||
| Baseline | 5931 | 1.00 | 7361 | 1.00 | ||||
| 0 day | 7 | 0.29 [0.14 to 0.61] | 0 | NA | 0 | NA^ | 16 | 28.0 [16.7 to 47.0] |
| 1–7 days | 122 | 0.73 [0.60 to 0.87] | 124 | 0.78 [0.65 to 0.95] | 50 | NA^ | 11 | 3.06 [1.65 to 5.66] |
| 8–14 days | 153 | 0.91 [0.77 to 1.07] | 135 | 0.86 [0.72 to 1.02] | 44 | NA^ | 9 | 3.04 [1.54 to 5.99] |
| 15–21 days | 134 | 0.94 [0.78 to 1.12] | 116 | 0.75 [0.62 to 0.91] | 44 | NA^ | 8 | 3.51 [1.71 to 7.22] |
| 22–42 days | 97 | 0.70 [0.56 to 0.86] | 416 | 0.93 [0.84 to 1.04] | 57 | NA^ | 8 | 2.31 [1.11 to 4.82] |
| Appendicitis, 12 years & above(through March 31, 2022, standard SCCS analysis) | ||||||||
| Baseline | 2339 | 1 | NA | |||||
| −28 days to −1 days | 116 | 0.56 [0.47 to 0.68] | NA | NA | 98 | 0.58 [0.47 to 0.71] | NA | NA |
| 0 day | 1 | 0.13 [0.02 to 0.96] | 0 | NA | 0 | NA | 15 | 10.15 [6.07 to 16.97] |
| 1–7 days | 68 | 1.31 [1.03 to 1.67] | 55 | 1.09 [0.84 to 1.43] | 35 | 0.83 [0.59 to 1.15] | 9 | 0.88 [0.46 to 1.71] |
| 8–14 days | 48 | 0.93 [0.70 to 1.23] | 48 | 0.96 [0.72 to 1.27] | 40 | 0.95 [0.70 to 1.31] | 12 | 1.21 [0.68 to 2.15] |
| 15–21 days | 69 | 1.39 [1.10 to 1.77] | 55 | 1.10 [0.84 to 1.44] | 42 | 1.01 [0.74 to 1.37] | 9 | 0.99 [0.51 to 1.93] |
| 22–42 days | 55 | 1.03 [0.79 to 1.36] | 146 | 0.97 [0.82 to 1.15] | 99 | 0.82 [0.67 to 1.00] | 20 | 0.98 [0.63 to 1.55] |
Booster doses rolled out on September 14, 2021.
Analysis not conducted as too few events as at observation end dates indicated in parentheses next to adverse event of special interest (AESI) within table.
For myocarditis/pericarditis, the highest risks were observed after dose 2, in males aged 12 to 17 [RI: 6.31 (1.36 to 29.3), Supplementary Table 3]. For CVT, the majority of cases were observed in males aged 50 and above (10 out of the 16 cases in total, five cases each after dose 1 and dose 2, Supplementary Table 3).
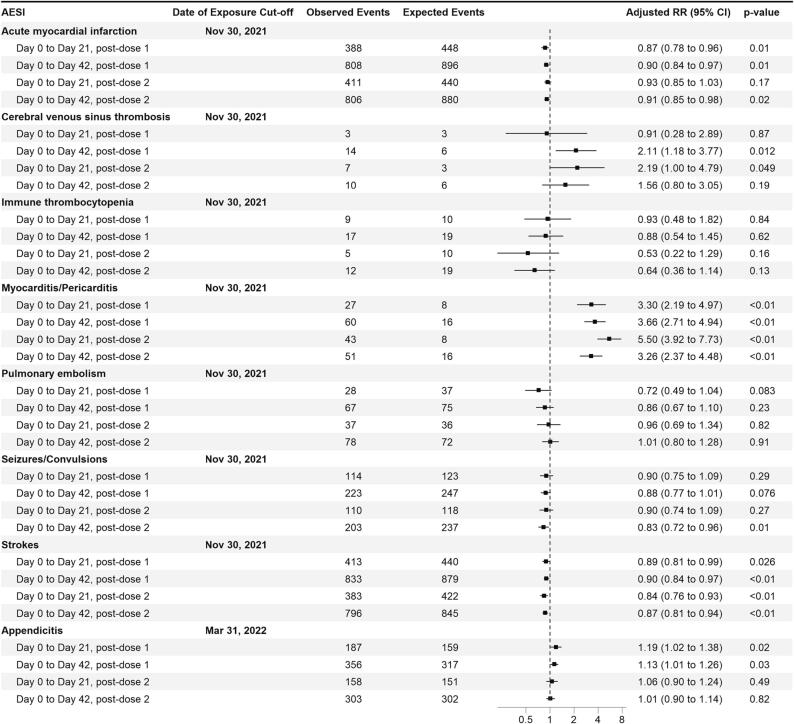
For the signals of myocarditis and appendicitis, the interim O/E analyses were conducted three and four months earlier than the corresponding SCCS analysis, respectively. With receipt of more local AE reports, subsequent O/E analyses were conducted through November 2021 (for all AESIs besides appendicitis) and March 2022 (for appendicitis) which continued to show elevated risks for myocarditis/pericarditis, CVT and appendicitis (Fig. 1).
Fig. 1.
Observed/Expected analyses for selected AESIs with mRNA vaccination Primary series for vaccines aged 12 years and above with two distinct risk period definitions. All cases in the ‘Day 0 to Day 21′ analyses are also included in the ‘Day 0 to Day 42′ analyses.
With regard to SCCS analyses, for myocarditis/pericarditis and CVT associated with SARS-CoV-2 infection, only 1 case each was observed, precluding meaningful comparisons as that infection rates were very low during the observation periods; an increased risk of appendicitis was not observed up to 42-days post-infection (Table 1, end observation date for appendicitis: March 31, 2022). Conversely for strokes, an increased risk was observed up to 42-days post-infection, but not observed post-vaccination (Table 1). This negative finding for strokes corroborated with the O/E analyses which revealed no increased risk up to 42 days post vaccination (Fig. 1, Fig. 2).
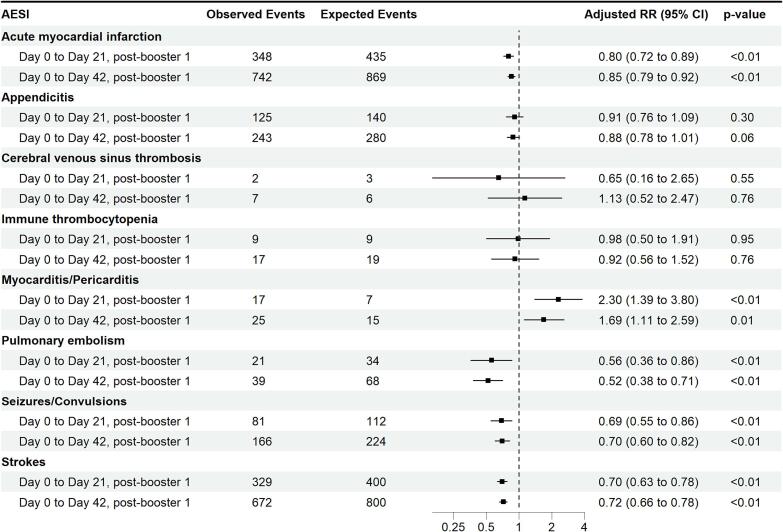
Fig. 2.
Observed/Expected analyses for selected AESIs with mRNA vaccination Booster Dose 1 through 31 July 2022 for vaccinees aged 12 years and above with two distinct risk period definitions. All cases in the ‘Day 0 to Day 21′ analyses are also included in the ‘Day 0 to Day 42′ analyses.
For booster dose 1 evaluated through July 2022 via O/E analysis, an elevated risk was found for myocarditis/pericarditis, with an RR of 2.30, (1.39 to 3.80)] at the 21-day risk window and RR: 1.69, 1.11 to 2.59 at 42-day risk window (Fig. 2). For booster doses, no AESI met the requirement for a potential signal warranting further validation.
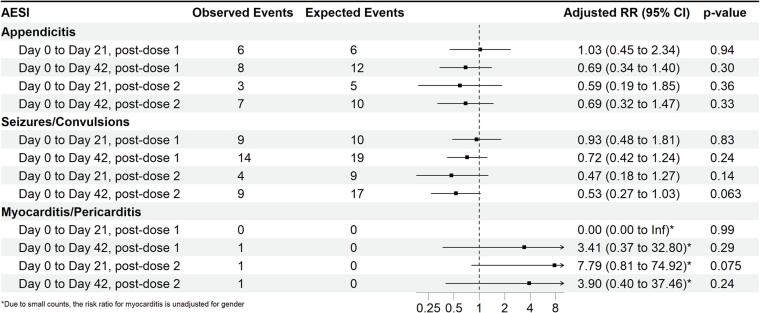
Among children aged five to 11 years, only three AESIs (myocarditis/pericarditis, appendicitis and seizures/convulsions) had been identified as potential safety concerns based on AE reports received and active surveillance at the largest paediatric hospital in Singapore.[17] None of the observed events rates exceeded the expected (Fig. 3).
Fig. 3.
Observed/Expected analyses for selected AESIs with mRNA vaccination through July 31, 2022, in children aged five to 11 years of age with two distinct risk period definitions. All cases in the ‘Day 0 to Day 21′ analyses are also included in the ‘Day 0 to Day 42′ analyses.
Discussion
Our nationwide post-market safety surveillance of mRNA COVID-19 vaccines in over 5 million vaccinated persons aged 5 and above has identified three safety concerns that were not detected prior to market authorization.
Myocarditis/pericarditis with mRNA COVID-19 vaccines following primary series vaccination was first identified in Israel and the US in mid-2021.[18], [19], [20] We had similarly observed a higher than expected reporting rate in young males at the time.[21] Our findings suggest an elevated risk particularly after dose 2 in the overall population [RI: 6.96 (3.95 to 12.27), at 1 to 7 days post-dose 2] with males aged 12 to 17 having the highest risk (Supplementary Table 3). This approximately translates to 1 additional case per 80,000 and 20,000 persons following primary series mRNA vaccination, in the overall population and in males aged 12 to 17 years, respectively.
Based on O/E analysis, an elevated risk of myocarditis/pericarditis remains apparent with booster dose 1 at both 21-day and 42-day risk windows [RR: 2.30 (1.39 to 3.80) and 1.69 (1.11 to 2.59)], respectively. Notably, the magnitude of the rate ratios was lower than that observed for Dose 2 of the primary series. Post-marketing studies on boosters from Canada and England suggest that this risk remains elevated.[22], [23] Notably, vaccine-associated myocarditis/pericarditis has been predominantly mild. Data on the short-term clinical trajectories of patients appear reassuring although long-term outcomes remain unclear, warranting continued surveillance.[24] COVID-19 infections carry substantial risks of myocarditis/pericarditis as well, even with Omicron sub-variants.[25], [26], [27] Given the low disease prevalence in Singapore over the course of the national primary series vaccination campaign, a calendar time-aligned comparison of the risks between primary series vaccination and infection could not be conducted; internationally however, the published literature suggest a higher risk of myocarditis following COVID-19 infection as compared to vaccination, when assessed at the overall population.[26], [28], [29] The benefit-risk balance of added doses should be reassessed periodically, given the emergence of novel viral (sub)variants, and are likely to hinge on factors such as age, gender, co-morbidities and prior vaccination and infection.
CVT, with thrombocytopenia, first surfaced with the ChAdOx1 vaccine in March 2021.[30], [31] A possible risk with mRNA vaccines was first detected after dose 1, via an SCCS analysis performed on a UK population.[32] Notably, this study applied the standard SCCS method, not accounting for long-term event dependent exposure and thus includes baseline periods before vaccination (Supplementary Fig. 1). On sensitivity analysis however using just the post-vaccination period – approximating the modified SCCS for event-dependent exposures – no significant increase was observed. In our data, in addition to being flagged via O/E analysis, the CVT signal persisted despite applying the modified SCCS for event-dependent exposures.[12].
Separately, Tu et al had estimated the crude incidence rate of CVT after mRNA vaccination in Singapore to be 2.59 (1.19 to 4.92), twice as high as the estimated historical incidence of 1.30 per 100,000 person-years, translating to approximately one additional CVT case per 500,000 persons vaccinated.[33] Out of the 16 CVT cases we detected within 42-days of vaccination, (10 of whom were males aged 50 years and above, Supplementary Table 3), the majority had occurred between one and two weeks post-dose 2 or four to five weeks post-dose 1, suggesting a longer onset time or a possible post-dose 2 risk exacerbation effect with CVT. However, we had used the date of admission as the date of CVT onset in our analysis because it was not possible to clearly ascertain the actual date of symptom onset in all cases despite chart review. As CVT typically presents with non-specific symptoms such as headache which may delay patients’ presentation and clinical work up, it’s possible that the actual event date precedes the admission date which could have led to an apparent extended latency.
Several other studies had either found inconclusive or no association for CVT with mRNA vaccination, possibly due to methodological differences (in population size, case definitions, control group selection and risk interval length applied) and possible inherent differences in CVT predisposition.[34], [35], [36], [37], [38] These studies were conducted in settings where adenovirus vector COVID-19 vaccines were also used alongside mRNA vaccines. Arguably, some degree of outcome displacement through competing exposures may be a remote explanation although methodological differences probably play a larger factor. In Singapore, adenovirus vector COVID-19 vaccines were not available. Likewise, communal spread of COVID-19 was also very low during the months of mass vaccination in Singapore[39]; As with adenovirus vector COVID-19 vaccines, COVID-19 infection is associated with increased risks of CVT.[31], [32], [40], [41] The difference in methodology (application of two varied study designs) could explain the detection of an association in our study, but not with previous reports. Nonetheless, further studies with standardized methodologies applied on similar data settings as ours are necessary to further elucidate the risk of CVT following mRNA vaccination.
Studies on ischemic and hemorrhagic strokes have mostly showed no increased risk[19], [42], [43], [44], [45] except for one SCCS analysis performed in the UK[33] which found a small increased risk of ischemic strokes after dose 1 BNT162b (which similarly disappeared on sensitivity analysis using only the post-vaccination period, as described with CVT above). Another SCCS study, similarly applying the standard SCCS, involving 3 Nordic countries[34] found an increased risk of cerebrovascular disease and intracranial hemorrhage with BNT162b2 and mRNA-1273 vaccines in the 28-day period following vaccination. Two other SCCS studies from the UK [46] and Hong Kong[47] have also detected an increased risk of hemorrhagic strokes following BNT162b2 vaccination. Notably, the Hong Kong study applied the modified SCCS method for event dependent exposures, whereas the UK study had applied the standard SCCS method with a pre-specified pre-exposure period removed from the baseline. [12].
As with CVT, methodological factors and population-specific differences may explain the varied findings. The difference in COVID-19 epidemiology in different countries and their testing frequency may have contributed to varying degrees of misattribution to vaccines instead of undiagnosed infections. Acute ischemic stroke risks can be elevated during the convalescent period of a COVID-19 infection without respiratory symptoms.[48] Another US study demonstrated that COVID-19 infection was associated with an increased risk of cerebrovascular disorders beyond the acute phase of the infection.[32].
Overall, a causal association between mRNA vaccines and strokes remains undetermined. Our data did not suggest an elevated risk. However, stratification by hemorrhagic and ischemic strokes would help to confirm our findings. Importantly, the incidence of stroke post-infection appears substantially greater than that post-vaccination in many studies[32], [44], [46] as with ours presented here.
A numerically higher number of appendicitis cases were first observed in the vaccine arm of BNT162b2′s phase III trial (12 versus 8).[49] An Israeli post-marketing safety identified elevated risks of appendicitis with BNT162b2.[19] The Upsala Monitoring Centre had also identified appendicitis as a possible safety signal with mRNA vaccines, following disproportionality analysis of the global pharmacovigilance database.[50] However, a subsequent Danish cohort study[51] did not detect any increased risk relative to unvaccinated controls. Notably, the background incidence of appendicitis was much higher in the Danish study (approximately 150 per 100,000 person-years) compared to the Israeli study (64 per 100,000 person-years). Our background incidence of appendicitis was similar to Israel’s.
Lymphadenopathy and lymphoid hyperplasia are plausible mechanisms leading to appendicitis.[50] Lymphadenopathy is an adverse effect of mRNA vaccines, particularly in younger populations.[52] Our SCCS analysis suggest a possible bi-modal risk distribution (Day 1 to 7 and Day 15 to Day 21, Table 1) although there may have been a lack of statistical power to detect an increase between Day 8 and 14. We observed modest but statistically significant risk elevations in 12 to 17 year-old males post-dose 1 and in 12 to 17 year old females, post-dose 2, translating to 1 additional case per 50,000 adolescents aged 12 to 17 years while that for the overall population is 1 additional case per 350,000 persons vaccinated. No increased risk was observed with boosters. For 18 to 29 year-old males, the increased risk was less certain as the SCCS results were not consistent with age-stratified O/E analyses (data not shown). Further studies are needed to confirm this finding.
Based on O/E, CVT and appendicitis observed with primary series were not observed with booster dose 1. This may be partly attributed to the very low risk of these events occurring in susceptible individuals. A lower AE incidence with booster dose 1 is possibly attributable to the “healthy vaccinee effect” – individuals who had already developed the AE after primary series may avoid mRNA boosters.
This analysis was conducted in a population with limited COVID-19 infections, with less than 2 % infected through end September 2021. By end August 2021, 80 % of the population had already completed primary series vaccinations. This, coupled with the high testing frequency, arguably minimized misclassification bias arising through undetected or asymptomatic SARS-CoV-2 infections, which have known associations with the AESIs studied. Secondly, the SCCS analyses conducted for myocarditis/pericarditis, CVT and strokes account for possible event dependent exposures. The three safety signals identified had persisted despite two distinct analytical methods [O/E (using external, historical controls) and SCCS (self-controlled)] applied. Corroborative findings were also observed in the negative direction for strokes by both methods.
Nonetheless, the following limitations are noteworthy. Firstly, we rely on accuracy of coded diagnoses to identify AESIs, which may vary by setting. This is partially addressed through chart reviews. Secondly, the use of hospital admissions to identify events possibly underestimates AE incidence if patients are managed at outpatient or emergency settings (e.g. for myocarditis/pericarditis and immune thrombocytopenia). Thirdly, the association of thromboses and thrombocytopenia with the ChAdOx1 vaccine made known in April 2021 could have led to spill-over diagnostic biases for other COVID-19 vaccines. Clinicians may be more inclined to perform diagnostic tests for thrombovascular disorders such as CVT, leading to differential detection rates after April 2021. We however did not observe any suggestive evidence of such an effect on trending CVT diagnoses by calendar time in 2021. Lastly, COVID-19 had led to changes in healthcare utilization, including postponed elective surgeries which could affect the incidence of pulmonary embolism. Future studies that take healthcare utilisation changes into consideration would be useful.
Conclusion
Our nationwide safety surveillance of COVID-19 mRNA vaccines has identified three salient safety signals. At a population-wide level, these risks are very rare; following primary series vaccination, we observe one additional case of myocarditis/pericarditis, CVT and appendicitis in 80,000, 350,000 and 500,000 vaccinated persons, respectively. Myocarditis/pericarditis occurs most frequently in males aged 12 to 17, in whom one additional case is observed per 20,000 vaccinated while one additional case of appendicitis is observed in every 50,000 vaccinated adolescents (male and female) aged 12 to 17 years. Notably, the risk of myocarditis/pericarditis appears to persist with booster doses on O/E analyses. No increase in risk of acute myocardial infarction and stokes have been observed. Contextualizing the risks and against the benefits of vaccination in appropriately stratified populations serve to inform vaccination strategies and preserve public trust in national vaccination programmes.
Additional contributions
We thank our expert panel members: Dr Lim Toon Wei, A/Prof Lim Yean Teng, A/Prof Ding Zee Pin and Dr Jonathan Choo, Prof Lo Yew Long, A/Prof Kevin Tan, A/Prof Umapathi N Thirugnanam, A/Prof Derrick Chan, A/Prof Ong Hian Tat, Dr Phuah Huan Kee, Dr Narayanaswamy Venketasubramanian Ramani and Dr Tu Tian Ming, Dr Thong Jiun Fong, Dr Yeo Seng Beng, A/Prof Yuen Heng Wai, A/Prof Lee Lai Heng, Dr Tan Chuen Wen, Dr Yap Eng Soo, A/Prof Bernard Yu-Hor Thong, Dr Amelia Santosa, Prof Edmund Lee Jon Deoon, Prof Hugo Van Bever, A/Prof Lee Haur Yueh, A/Prof Sum Chee Fang, Dr Tan Teck Choon and A/Prof Thaschawee (Tash) Arkachaisri, A/Prof Jason Choo Chon Jun, Dr Chua Horng Ruey, A/Prof Yung Chee Fu and Prof Cheung Yin Bun. These individuals were not paid for their contributions. We thank A/Prof Thoon Kok Cheng and Ms Diana Oh Bee Khim from the KK Woman’s and Children’s Hospital Sentinel Site. We also thank Ms Patricia Ng, Ms Amelia Suen, Ms Celine Loke and Ms Tan Siew Har from the Vigilance and Compliance Branch, Health Sciences Authority for lending support to the safety surveillance programme.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jvacx.2023.100419.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Data availability
The data for this paper arise from public health surveillance activities and not research. we are thus unable to provide access to the raw data on request unfortunately.
References
- 1.Tan C.C., Lam C.S.P., Matchar D.B., Zee Y.K., Wong J.E.L. Singapore's health-care system: key features, challenges, and shifts. Lancet. 2021;398(10305):1091–1104. doi: 10.1016/S0140-6736(21)00252-X. [DOI] [PubMed] [Google Scholar]
- 2.Fisher D., Mak K. Exiting the pandemic: Singapore style. BMC Med. 2021;19(1):238. doi: 10.1186/s12916-021-02117-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan C.L., Soh S., Tan S.H., et al. Quantitative data mining in signal detection: the Singapore experience. Expert Opin Drug Saf. 2020;19(5):633–639. doi: 10.1080/14740338.2020.1734559. [DOI] [PubMed] [Google Scholar]
- 4.Brighton collaboration. Priority List of Adverse Events of Special Interest. 2020. https://brightoncollaboration.us/priority-list-aesi-COVID/ (accessed 12.8.2022.
- 5.US Food & Drug Administration: Center for Biologics Evaluation and Research. CBER Surveillance Program: COVID-19 Vaccine Safety Surveillance: Active Monitoring Master Protocol. 2021.
- 6.Health Promotion Board (Singapore). National Immunisation Registry. https://www.nir.hpb.gov.sg/nirp/eservices/login (accessed 12.09.2022.
- 7.Whitaker H.J., Ghebremichael-Weldeselassie Y., Douglas I.J., Smeeth L., Farrington C.P. Investigating the assumptions of the self-controlled case series method. Stat Med. 2018;37(4):643–658. doi: 10.1002/sim.7536. [DOI] [PubMed] [Google Scholar]
- 8.Xu S., Clarke C.L., Newcomer S.R., Daley M.F., Glanz J.M. Analyzing self-controlled case series data when case confirmation rates are estimated from an internal validation sample. Biom J. 2018;60(4):748–760. doi: 10.1002/bimj.201700088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Department of Statistics (Singapore). Population Trends 2022.
- 10.Ministry of Health (Singapore). COVID-19 Situation Report (31st Dec 2020). 2020. https://www.moh.gov.sg/docs/librariesprovider5/2019-ncov/ceg_20201231_daily_report_on_covid-19_cabinet.pdf (accessed 11.11.22.
- 11.Ministry of Health (Singapore). Vaccination Statistics https://www.moh.gov.sg/covid-19/vaccination/statistics (accessed 15.11.2022.
- 12.Ghebremichael-Weldeselassie Y., Jabagi M.J., Botton J., et al. A modified self-controlled case series method for event-dependent exposures and high event-related mortality, with application to COVID-19 vaccine safety. Stat Med. 2022;41(10):1735–1750. doi: 10.1002/sim.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farrington CP, WHItaker HJ, Ghebremichael-Weldeselassie Y. Self-Controlled Case Series Studies: A Modelling Guide with R; 2018.
- 14.Whitaker H.J., Steer C.D., Farrington C.P. Self-controlled case series studies: Just how rare does a rare non-recurrent outcome need to be? Biom J. 2018;60(6):1110–1120. doi: 10.1002/bimj.201800019. [DOI] [PubMed] [Google Scholar]
- 15.National Registry of Diseases Office. Singapore Stroke Registry Annual Report 2020. 2020. https://nrdo.gov.sg/publications/stroke (accessed 11.1.2023.
- 16.National Registry of Diseases Office. Singapore Myocardial Infarction Registry Annual Report 2020. 2020. https://nrdo.gov.sg/publications/ami (accessed 11.1.2023.
- 17.Thoon K.C., Soh S.B., Liew W.K., et al. Active surveillance of adverse events following childhood immunization in Singapore. Vaccine. 2014;32(39):5000–5005. doi: 10.1016/j.vaccine.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Gargano J.W.W.M., Hadler S.C., Langley G., et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: Update from the advisory committee on immunization practices — United States. Morb Mortal Wkly Rep. June 2021;70(27):977–982. doi: 10.15585/mmwr.mm7027e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barda N., Dagan N., Ben-Shlomo Y., et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2021;385(12):1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ministry of Health (Israel). Surveillance of Myocarditis (Inflammation of the Heart Muscle) Cases Between December 2020 and May 2021 (Including). 2021. https://www.gov.il/en/departments/news/01062021-03 (accessed 15.09.2022.
- 21.Health Sciences Authority (Singapore). HSA Safety Update No. 3 on COVID-19 Vaccines, July 5, 2020 https://www.hsa.gov.sg/COVID19-vaccines-safety-updates (accessed 15.09.2022.
- 22.Naveed Z., Li J., Spencer M., et al. Observed versus expected rates of myocarditis after SARS-CoV-2 vaccination: a population-based cohort study. CMAJ. 2022;194(45):E1529–E1536. doi: 10.1503/cmaj.220676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stowe J., Miller E., Andrews N., Whitaker H.J. Risk of myocarditis and pericarditis after a COVID-19 mRNA vaccine booster and after COVID-19 in those with and without prior SARS-CoV-2 infection: A self-controlled case series analysis in England. PLoS Med. 2023;20(6):e1004245. doi: 10.1371/journal.pmed.1004245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oster M.E., Shay D.K., Su J.R., et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331–340. doi: 10.1001/jama.2021.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menni C., Valdes A.M., Polidori L., et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID study. Lancet. 2022;399(10335):1618–1624. doi: 10.1016/S0140-6736(22)00327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patone M., Mei X.W., Handunnetthi L., et al. Risk of myocarditis after sequential doses of COVID-19 vaccine and SARS-CoV-2 infection by age and sex. Circulation. 2022;146(10):743–754. doi: 10.1161/CIRCULATIONAHA.122.059970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fishman B., Goitein O., Berkovitch A., Rahav G., Matetzky S. First report of myocarditis in two patients with COVID-19 Omicron variant: case report. Eur Heart J Case Rep. 2022;6(10):ytac407. doi: 10.1093/ehjcr/ytac407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Block J.P., Boehmer T.K., Forrest C.B., et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination - PCORnet, United States, January 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(14):517–523. doi: 10.15585/mmwr.mm7114e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patone M., Mei X.W., Handunnetthi L., et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28(2):410–422. doi: 10.1038/s41591-021-01630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pottegard A., Lund L.C., Karlstad O., et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 2021;373 doi: 10.1136/bmj.n1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.European Medicines Agency., Pharmacovigilance Risk Assessment Committee. Signal assessment report on embolic and thrombotic events (SMQ) with COVID-19 Vaccine (ChAdOx1-S [recombinant]) – COVID-19 Vaccine AstraZeneca (Other viral vaccines). 2021 (accessed 13/09/2023.
- 32.Hippisley-Cox J., Patone M., Mei X.W., et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ. 2021;374 doi: 10.1136/bmj.n1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tu T.M., Yi S.J., Koh J.S., et al. Incidence of cerebral venous thrombosis following SARS-CoV-2 infection vs mRNA SARS-CoV-2 vaccination in Singapore. JAMA Netw Open. 2022;5(3):e222940. doi: 10.1001/jamanetworkopen.2022.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dag Berild J., Bergstad Larsen V., Myrup Thiesson E., et al. Analysis of thromboembolic and thrombocytopenic events after the AZD1222, BNT162b2, and MRNA-1273 COVID-19 vaccines in 3 Nordic Countries. JAMA Netw Open. 2022;5(6):e2217375. doi: 10.1001/jamanetworkopen.2022.17375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simpson C.R., Shi T., Vasileiou E., et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med. 2021;27(7):1290–1297. doi: 10.1038/s41591-021-01408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andrews N.J., Stowe J., Ramsay M.E., Miller E. Risk of venous thrombotic events and thrombocytopenia in sequential time periods after ChAdOx1 and BNT162b2 COVID-19 vaccines: A national cohort study in England. Lancet Reg Health Eur. 2022;13 doi: 10.1016/j.lanepe.2021.100260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kerr S., Joy M., Torabi F., et al. First dose ChAdOx1 and BNT162b2 COVID-19 vaccinations and cerebral venous sinus thrombosis: A pooled self-controlled case series study of 11.6 million individuals in England, Scotland, and Wales. PLoS Med. 2022;19(2):e1003927. doi: 10.1371/journal.pmed.1003927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.See I., Lale A., Marquez P., et al. Case series of thrombosis with thrombocytopenia syndrome after COVID-19 vaccination-United States, December 2020 to August 2021. Ann Intern Med. 2022;175(4):513–522. doi: 10.7326/M21-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization. COVID-19 in Singapore Situation Report 35 (10 September 2021). 2021. https://www.who.int/singapore/internal-publications-detail/covid-19-in-singapore-situation-report-35.
- 40.Dakay K., Cooper J., Bloomfield J., et al. Cerebral venous sinus thrombosis in COVID-19 infection: A case series and review of the literature. J Stroke Cerebrovasc Dis. 2021;30(1) doi: 10.1016/j.jstrokecerebrovasdis.2020.105434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCullough-Hicks M.E., Halterman D.J., Anderson D., Cohen K., Lakshminarayan K. High incidence and unique features of cerebral venous sinus thrombosis in hospitalized patients with COVID-19 infection. Stroke. 2022;53(9):e407–e410. doi: 10.1161/STROKEAHA.122.038955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simpson C.R., Kerr S., Katikireddi S.V., et al. Second-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Commun. 2022;13(1):4800. doi: 10.1038/s41467-022-32264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein N.P., Lewis N., Goddard K., et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021;326(14):1390–1399. doi: 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stefanou M.I., Palaiodimou L., Aguiar de Sousa D., et al. Acute arterial ischemic stroke following COVID-19 vaccination: A systematic review and meta-analysis. Neurology. 2022 doi: 10.1212/WNL.0000000000200996. [DOI] [PubMed] [Google Scholar]
- 45.Jabagi M.J., Botton J., Bertrand M., et al. Myocardial infarction, stroke, and pulmonary embolism after BNT162b2 mRNA COVID-19 vaccine in people aged 75 years or older. JAMA. 2022;327(1):80–82. doi: 10.1001/jama.2021.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patone M., Handunnetthi L., Saatci D., et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat Med. 2021;27(12):2144–2153. doi: 10.1038/s41591-021-01556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chui C.S.L., Fan M., Wan E.Y.F., et al. Thromboembolic events and hemorrhagic stroke after mRNA (BNT162b2) and inactivated (CoronaVac) covid-19 vaccination: A self-controlled case series study. EClinicalMedicine. 2022;50 doi: 10.1016/j.eclinm.2022.101504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tu T.M., Seet C.Y.H., Koh J.S., et al. Acute ischemic stroke during the convalescent phase of asymptomatic COVID-2019 infection in men. JAMA Netw Open. 2021;4(4):e217498. doi: 10.1001/jamanetworkopen.2021.7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.US Food & Drug Administration: Center for Biologics Evaluation and Research. FDA Briefing Document: Vaccines and Related Biological Products Advisory Committee Meeting, Pfizer-BioNTech COVID-19 Vaccine. 2020. https://www.fda.gov/media/144245/download.
- 50.Mitchell J., Yue Q.Y. Appendicitis as a possible safety signal for the COVID-19 vaccines. Vaccine X. 2021;9 doi: 10.1016/j.jvacx.2021.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kildegaard H., Ladebo L., Andersen J.H., et al. Risk of appendicitis after mRNA COVID-19 vaccination in a Danish population. JAMA Intern Med. 2022;182(6):684–686. doi: 10.1001/jamainternmed.2022.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park J.Y., Lee J.Y., Yi S.Y. Axillary lymphadenopathy on ultrasound after COVID-19 vaccination and its influencing factors: A single-center study. J Clin Med. 2022;11(1) doi: 10.3390/jcm11010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data for this paper arise from public health surveillance activities and not research. we are thus unable to provide access to the raw data on request unfortunately.