Abstract
The ftsE(Ts) mutation of Escherichia coli causes defects in cell division and cell growth. We expressed alkaline phosphatase (PhoA) fusion proteins of KdpA, Kup, and TrkH, all of which proved functional in vivo as K+ ion pumps, in the mutant cells. During growth at 41°C, these proteins were progressively lost from the membrane fraction. The reduction in the abundance of these proteins inversely correlated with cell growth, but the preformed proteins in the membrane were stable at 41°C, indicating that the molecules synthesized at the permissive temperature were diluted in a growth-dependent manner at a high temperature. Pulse-chase experiments showed that KdpA-PhoA was synthesized, but the synthesized protein did not translocate into the membrane of the ftsE(Ts) cells at 41°C and degraded very rapidly. The loss of KdpA-PhoA from the membrane fractions of ftsE(Ts) cells was suppressed by a multicopy plasmid carrying the ftsE+ gene. While cell growth stopped when the abundance of these proteins decreased 15-fold, the addition of a high concentration of K+ ions specifically alleviated the growth defect of ftsE(Ts) cells but not cell division, and the cells elongated more than 100-fold. We conclude that one of the causes of growth cessation in the ftsE(Ts) mutants is a defect in the translocation of K+-pump proteins into the cytoplasmic membrane.
Gene disruption studies show that many cell division genes of Escherichia coli, such as ftsZ, zipA, ftsN, and ftsI, are essential for cell growth (4, 6, 13, 14), but the essential functions affected by mutations in these genes have not been identified. In this study, we analyzed the mechanism of cell growth arrest in the ftsE(Ts) mutant and found that the mutation caused a defect in the translocation of K+-pump protein–alkaline phosphatase (PhoA) fusion proteins into the cytoplasmic membrane and that the addition of a high concentration of K+ partially restores cell growth but not cell division in the mutant.
The ftsE(Ts) mutant strain grows normally at 30°C but stops dividing and loses viability immediately upon a temperature shift to 41°C (29), and DNA sequence analysis shows that FtsE(Ts) has a missense substitution of Ser for 135Pro (10). FtsE is localized to the cytoplasmic side of the inner membrane (12). Therefore, it is hypothesized that FtsE may act at the inner membrane in a “septalsome” complex. While ftsE is the second gene in the ftsYEX operon at 76 min on the E. coli chromosomal map, its product belongs to the superfamily of ABC (ATP-binding cassette) transporters (11). HlyB, a member of this family, directs the nonconventional protein export, which does not require an N-terminal signal sequence or the cellular Sec machinery (19). FtsY shows sequence homology with a signal recognition particle (SRP) receptor subunit (SRα) of eukaryotes (30), and it plays a role in the secretion of proteins such as OmpF and β-lactamase (22). More recent work shows that FtsY can promote the cotranslational targeting of a signal sequence bearing nascent chains (22). Therefore, it is speculated that FtsE may also play a role in protein export in conjunction with FtsY. However, the accumulation of precursors of β-lactamase, OmpF, or ribose-binding protein has not been observed in ftsE(Ts) cells, although depletion of FtsY causes an accumulation of these precursor proteins (22).
K+ ions are required for the 50S ribosomal subunit to catalyze the peptidyl transferase reaction (25) and for the 30S ribosomal subunit to bind phenylalanyl-tRNA in vitro (38). Furthermore, K+ forms a membrane potential, which is important for the production of ATP and as a motive force to ingest nutrients (15). Thus, the level of K+ affects cell growth. The K+ concentration is a limiting factor for protein synthesis, since the rate of protein synthesis slows down fourfold when the level of K+ falls below 25 mM (21). The intracellular concentration of K+ is maintained at 200 mM in E. coli cells growing in a medium containing 10 mM K+ (31). The active transport of K+ is mediated by the three K+ ion pumps, Kdp, Kup, and Trk (31). Cells that lose all three K+ ion pumps cannot grow in normal medium containing 10 mM K+, although cells having only one of the pumps can grow (8). However, even cells lacking all the K+ ion pumps can grow in a medium supplemented to more than 115 mM K+ (28). Measurements of K+ uptake show that the triple-mutant cells which lack all three K+ pumps lose almost all of their K+. Transport rates in such strains are linearly dependent on the external K+ concentration up to 105 mM (28). They have suggested that E. coli has a K+ ion channel that allows K+ to flow into the cell by concentration gradient, similar to the process in a eukaryote.
MATERIALS AND METHODS
Bacterial strains and media.
The isogenic pair of strains used in this paper, ftsE(Ts) and ftsE+, were made by the transduction of ftsE(Ts) and ftsE+ into the wild-type strain, MG1655 (32). Transduction with T4GT7 was carried out as described by Wilson et al. (36). ftsE(Ts) and ftsE+ were cotransduced with malT+ from MFT1181 [malT+ ftsE(Ts)] into MG1655 malT::Tn10. The strains used for a complementation test of the plasmids encoding K+ ion pump protein-PhoA fusion proteins, TK2204 (F− thi rha lacZam nagA kdpA4 trkA405 trkD1) and TK2691 [F− thi rha lacZ nadA Δ(kdpFAB)5 trkG92 trkH1 trkD1]), were kindly supplied by W. Epstein (7). Plasmid pJF118HE (9) was kindly supplied by P. W. Postma, and pVP100 (5) was kindly supplied by R. B. Gennis. K10 contains 0.5 g of NaCl/liter, 1 g of NH4Cl/liter, 30.3 g of Na2HPO4 · 12H2O/liter, 2.4 g of NaH2PO4 · 2H2O/liter, 2 mM MgSO4, 0.1 mM CaCl2, 2 g of glucose/liter, and 10 mM KCl. K200 contains 200 mM KCl instead of 10 mM KCl. K10-succinate contains succinate instead of glucose.
Construction of plasmids.
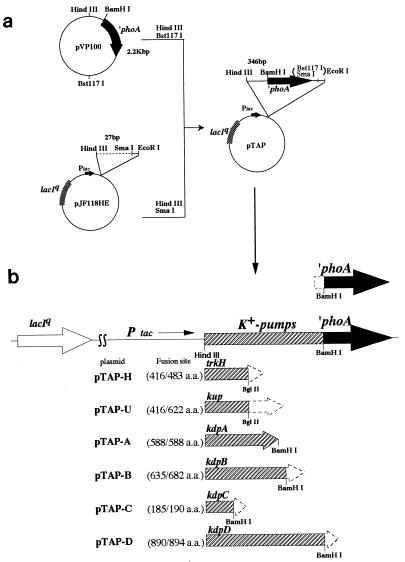
The 2.2-kb HindIII-Bst1107 segment containing phoA without the signal sequence was obtained from pVP100 (5) and ligated with the 5.2-kb HindIII-SmaI segment from pJF118HE (9). The resulting plasmid, pTAP, carried lacIq and phoA, with phoA inserted just downstream of the tac promoter (Fig. 1). The PCR products containing kdpA, kdpC, kdpD, kup, and trkH, respectively, were prepared by the method of Innis et al. (16) with the following primers. The forward primers had the 5′ flanking sequences of the corresponding genes, including the Shine-Dalgarno sequence and the HindIII recognition sequence (kdpA, 5′-TTTAAGCTTATGCGGAGGCGTTCTGATGG-3′; kdpC, 5′-TTTAAGCTTGGTCTGGTGTGAGGTTTACC-3′; kdpD, 5′-GGGAAGCTTGGCGCTGGATAAACTTGATG-3′; trkH, 5′-AAAAAGCTTAAGGAAGCGGCAGAGATGC-3′; and kup, 5′-TTTAAGCTTTTTTGTGGGCCAAGGGAC-3′). The reverse primers had 3′ flanking sequences that were interrupted either by the BamHI recognition sequence (kdpA, 5′-TTAGGATCCGAGAGATATTCCGCCACCGG-3′; kdpC, 5′-AAAGGATCCAGCGCCAGATTGAGTTCAAC-3′; and kdpD, 5′-GGGGGATCCCGTGGGGCGATAAAAAAGAG-3′) or the BglII recognition sequence (trkH, 5′-AAAAGATCTATAATCGCCAGCATACTGAC-3′; and kup, 5′-AAAAGATCTAGGTTAGCGGTGAACAATGG-3′). The PCR products were cut with HindIII and then cut further with BamHI or BglII and cloned into the HindIII-BamHI site of pTAP. As a result, the phoA sequence was fused in frame to one of the K+-pump genes and placed under the control of the tac promoter.
FIG. 1.
(a) Construction of the ′phoA plasmid (pTAP). pTAP is a pJF118HE derivative that contains a ′phoA fragment lacking the sequence for the phoA signal sequence. The construction of this vector is described in Materials and Methods. (b) Structure of the plasmid encoding PhoA fusion protein. Truncated sequences from the C-terminal region of each K+-pump protein were subcloned into the BamHI site of pTAP. This construction creates an in-frame K+-pump protein–PhoA fusion protein under the control of the tac promoter. The fusion site of each K+-pump protein–PhoA fusion protein is shown in parentheses (fusion site/total amino acids [a.a.]).
Western blotting analysis.
The membrane fraction was prepared by the method of Yamada et al. (37). Proteins were then solubilized with sodium dodecyl sulfate and analyzed by Western blotting; PhoA fusion proteins of the K+ pump were detected with anti-PhoA antiserum and the ECL Western blotting detection kit (Amersham). Glucose dehydrogenase (GDH) was detected with an anti-GDH antiserum. Protein concentrations were determined by the method of Lowry et al. (20), and a fixed amount of protein was used for quantitative analysis.
RESULTS
High concentration of KCl in the medium partially restores growth of strain ftsE(Ts) at the nonpermissive temperature.
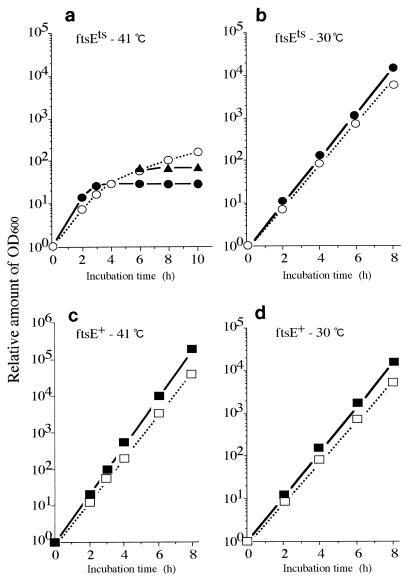
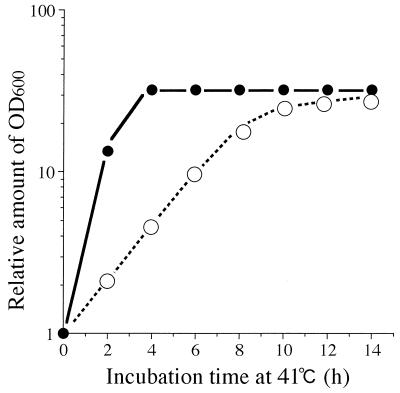
We analyzed the effect of high concentrations of K+ in the medium on the growth (as measured by the increase in optical density at 600 nm [OD600]) and division of a pair of isogenic strains, ftsE(Ts) and ftsE+. Bacteria were grown exponentially in K10 medium containing 10 mM of KCl at 30°C. The cultures were then diluted with K10 or K200 (200 mM KCl) medium to an appropriate dilution to avoid growth saturation and possible depletion of K+ ions and incubated at 30 or 41°C. The OD600 value of each sample was corrected for the dilution factor. In K10, the growth of ftsE(Ts) stopped completely 4 h after the shift to 41°C (Fig. 2a). During this period, the OD600 value increased 32-fold. However, in K200, the growth of ftsE(Ts) continued at least up to 10 h after the shift to 41°C (Fig. 2a), although the growth rate was very low compared to that at 30°C (Fig. 2b). We also found that the amount of protein in each culture was proportional to the OD600 value (data not shown). Therefore, the OD600 value represents the protein mass. The growth of ftsE+ at 41°C (Fig. 2c), or the growth of either strain at 30°C (Fig. 2b and d), was not affected by high concentration of K+. When K200-grown ftsE(Ts) cells were washed and recultured in K10 at 41°C, growth stopped again (Fig. 2a). Therefore, the recovery of cell growth of ftsE(Ts) in K200 is distinct from nonspecific growth enhancement. High concentrations of NaCl (100 to 300 mM) did not restore the growth of ftsE(Ts) at 41°C (data not shown). The KCl effect, therefore, is not a general salt effect. We also found that ftsE(Ts) formed colonies on the K200 agar plate at 41°C but not on the K10 plate. Cell division of ftsE(Ts) stopped completely within 20 min after exposure to a temperature of 41°C, irrespective of the KCl concentration (Fig. 3).
FIG. 2.
Growth of ftsE(Ts) and ftsE+ cells in the presence of 10 or 200 mM KCl. Bacterial cultures growing exponentially in K10 medium at 30°C were diluted 10- to 104-fold with fresh K10 or K200 medium and incubated at 30 or 41°C. The OD600 values (actual readings, 0.013 to 0.042) were corrected for the dilution factors and plotted as values relative to that of the 0-h sample, (a) ftsE(Ts) grown at 41°C in K10 (•), in K200 (○), and with a change from K200 to K10 at 6 h (▴). (b) ftsE(Ts) grown at 30°C in K10 (•) and in K200 (○). (c) ftsE+ grown at 41°C in K10 (▪) and in K200 (□). (d) ftsE+ grown at 30°C in K10 (▪) and in K200 (□).
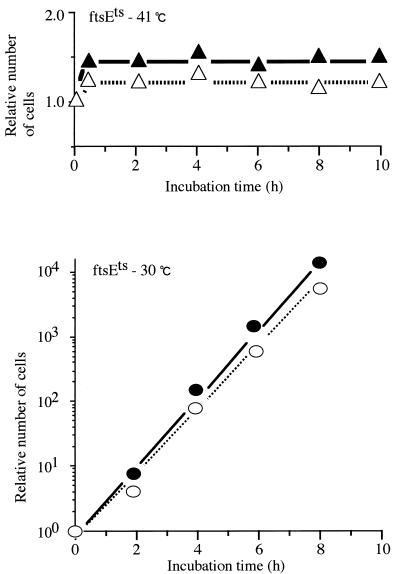
FIG. 3.
Cell division of ftsE(Ts) in K200 and K10 media. Numbers of cells in the ftsE(Ts) cultures shown in Fig. 2 were measured by using a Coulter counter. The numbers were corrected for the dilution factors and plotted relative to the numbers of the 0-h samples. ▴, ftsE(Ts) grown in K10 at 41°C; ▵, ftsE(Ts) grown in K200 at 41°C; •, ftsE(Ts) grown in K10 at 30°C; ○, ftsE(Ts) grown in K200 at 30°C.
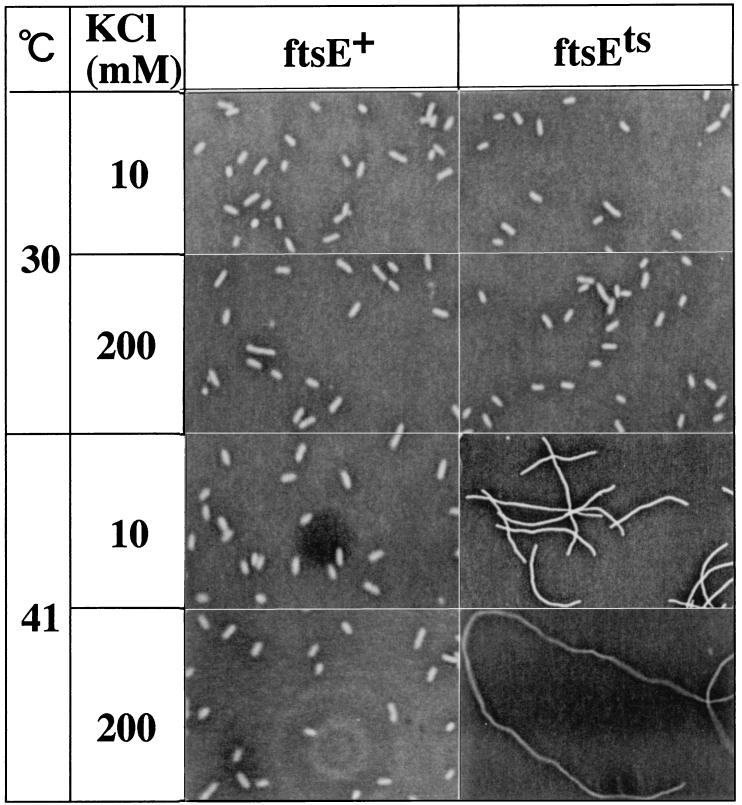
Recovery of cell growth without cell division in response to high concentrations of K+ was also shown by microscopical observation of cells from the same cultures (Fig. 4). The average length of ftsE(Ts) cells cultured in K200 at 41°C increased with time, and after 10 h, the cells elongated to 308 μm, which corresponded to a 97-fold elongation over the original cells. However, in K10, elongation stopped completely at 75 μm after 4 h of incubation at 41°C. This value is not in conflict with that expected based on the ratio of the OD600 value to the number of cells. The addition of high concentrations of K+ affected neither the cell division of ftsE(Ts) at 30°C nor that of ftsE+ at either temperature; cells in these cases were rod shaped (Fig. 4). Microscope observations of DAPI (4′, 6-diamidino-2-phenylindole)-stained cells from the same cultures also showed that the addition of high concentrations of K+ did not affect the DNA synthesis and partition of the nucleoids (data not shown).
FIG. 4.
Phase-contrast micrographs of ftsE(Ts) and ftsE+ (the 8-h cultures shown in Fig. 2) grown in different concentrations of KCl. Magnification, ×400 [ftsE(Ts) at 41°C] and ×800 (others).
These results indicate that the growth defect of strain ftsE(Ts) at 41°C is specifically alleviated by the addition of high concentrations of K+ into the medium. Cell division remains defective under such conditions, however.
The proteins constituting the K+ pumps are missing in the membrane fraction of the ftsE(Ts) mutant at 41°C.
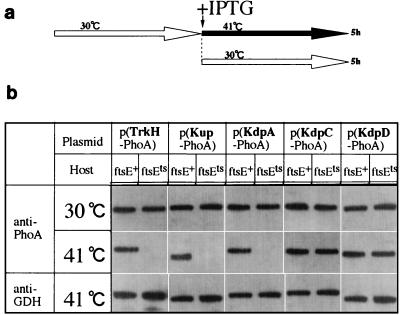
The results described above suggested that the inhibition of cell growth of ftsE(Ts) at 41°C may have been caused by a reduction in the intracellular K+ level. The K+ level of the wild-type cell is controlled by three sets of K+ pumps, Kdp, Kup, and Trk (31). Therefore, we analyzed the effect of the ftsE(Ts) mutation on the amounts of five membrane proteins of the K+ pumps, KdpA, KdpC, KdpD, Kup, and TrkH. We constructed a series of plasmids encoding fusion proteins between each of these proteins and PhoA, with a fusion junction located near the C terminus of the protein. Various transformants of ftsE(Ts) and ftsE+ by these plasmids were grown to an exponential phase at 30°C in K10, diluted to 1/200 with K10, and further incubated in the presence of 50 μM IPTG (isopropyl-β-d-thiogalactopyranoside) at 41 or 30°C for 5 h. Membrane fractions were prepared and subjected to Western blotting analysis with anti-PhoA serum (23). As shown in Fig. 5, in the case of ftsE+, all the fusion proteins tested were detected irrespective of the incubation temperature. However, in the case of ftsE(Ts) cultured at 41°C, three fusion proteins, KdpA-PhoA, TrkH-PhoA, and Kup-PhoA, were not detected in the membranes. At 30°C, these proteins were present in the membrane fractions. In contrast, KdpC-PhoA and KdpD-PhoA were detected in the membrane fractions irrespective of the ftsE genotype of the host strain or the growth temperature. GDH, used as an internal control, was detected in similar amounts in all the samples examined. Therefore, the loss of the three K+-pump proteins from the membrane fractions of ftsE(Ts) cells at 41°C cannot be explained in terms of membrane instability of the sick cells. The three fusion proteins which were lost from the membranes of ftsE(Ts) cells were detected in the membranes of ftsE(Ts) cells carrying a multicopy plasmid bearing ftsE+ (unpublished data) and cultured at 41°C.
FIG. 5.
Western blot analysis of the PhoA fusion proteins of the K+-pump proteins. Cultures of transformants of ftsE(Ts) and ftsE+ carrying one of the plasmids encoding KdpA-PhoA, KdpC-PhoA, KdpD-PhoA, Kup-PhoA, and TrkH-PhoA were grown exponentially at 30°C and diluted 200-fold with K10 medium containing IPTG, followed by incubation for 5 h at 30 or 41°C. Cells were collected from each culture, and the membrane fractions were isolated. Membrane proteins (10 μg each) were electrophoresed in a sodium dodecyl sulfate–12% polyacrylamide gel and analyzed by Western blotting with anti-PhoA antiserum or anti-GDH antiserum.
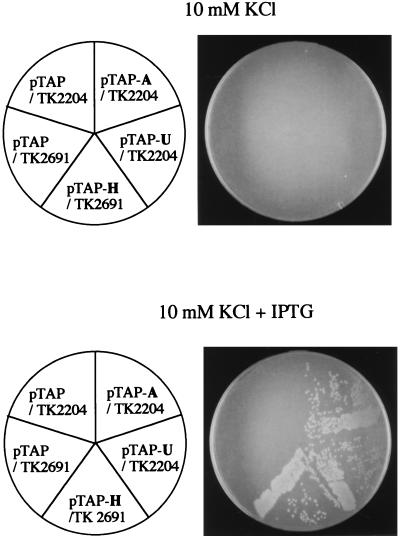
To determine whether the three fusion proteins affected by ftsE(Ts) were functional, the plasmid encoding each of them was introduced into a strain that contained an appropriate mutant allele (kdpA, kup [equivalent to trkD], or trkH) as well as mutations preventing the function of the other two K+ pumps, because one of the three pumps alone can support cell growth under low K+ concentrations (8, 28). As shown in Fig. 6, all three plasmids complemented the corresponding mutants in the presence of 50 μM IPTG. Therefore, our conclusion from the experimental results with PhoA fusion proteins may be extended to the natural phenomena that actually occur in the ftsE(Ts) cells.
FIG. 6.
Complementation tests of the plasmids encoding the PhoA fusion proteins. Transformants of TK2204 and TK2691 by one of the plasmids encoding PhoA fusion proteins were streaked on K10 agar plates with or without IPTG, and the plates were incubated at 37°C for 24 h. pTAP was used as the vector; the plasmid pTAP-A encodes KdpA-PhoA, pTAP-U encodes Kup-PhoA, and pTAP-H encodes TrkH-PhoA. TK2204 has the mutations kdpA4, trkA405, and trkD1, which cause defects in the Kdp, Trk, and Kup pumps, respectively. TK2691 has the mutations Δ(kdpFAB)5, trkG92 and trkH1, and trkD1, which cause defects in the Kdp, Trk, and Kup pumps, respectively. Cells that lose the functions of all three K+ pumps cannot grow in K10, whereas cells having one of the three pumps can grow in K10. Note that the Trk pumps are composed of either TrkA, -E, and -G or TrkA, -E, and -H.
Growth-dependent dilution of the KdpA-PhoA, TrkH-PhoA, and Kup-PhoA proteins in the membranes of ftsE(Ts) cells at 41°C.
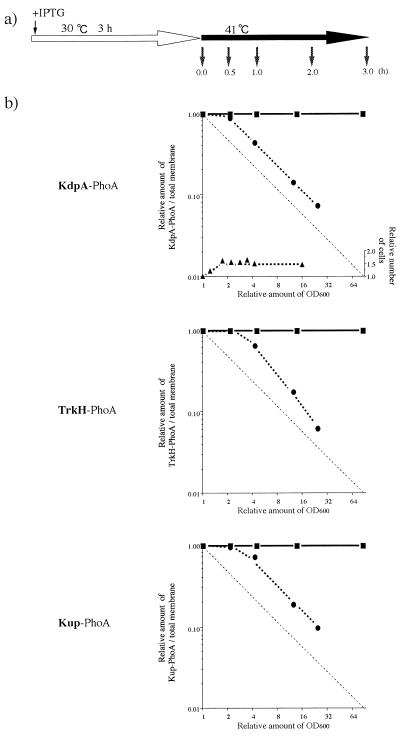
To elucidate the mechanism by which the three K+-pump proteins are specifically lost from the ftsE(Ts) cell membrane, we analyzed the time courses of their disappearances after a temperature shift to 41°C. The ftsE(Ts) and ftsE+ cells, carrying the plasmids encoding the three fusion proteins, were grown at 30°C for 3 h in K10 containing 50 μM IPTG. The cultures were then diluted 1/40 with the same medium and incubated at 41°C for an additional 3 h. At the indicated times (Fig. 7a), samples were withdrawn for quantitative analysis of the fusion proteins by Western blotting. The relative intensities of the proteins in a fixed amount of membrane preparation were plotted against the increase in OD600 of the cultures. As shown in Fig. 7b, the abundance of the three fusion proteins in the membrane did not change in ftsE+. However, the specific content of each of the fusion proteins in the membranes of ftsE(Ts) cells remained unchanged for the first doubling of OD600 and then decreased. The half-life of this disappearance coincided well with the time required for one doubling of OD600. Using parts of the same cultures, we analyzed the levels of mRNA for the three fusion genes by the competitive reverse transcription method (2) and found no significant differences between ftsE(Ts) and ftsE+ (data not shown). Under the same culture conditions, cell division of ftsE(Ts) stopped completely after one doubling of OD600 (Fig. 7b). Thus, the fusion proteins stopped accumulating in the membrane soon after cell division was inhibited by the ftsE(Ts) mutation; thereafter, they were simply diluted as the cell mass increased.
FIG. 7.
Growth-dependent dilution of the KdpA-PhoA, Kup-PhoA, and TrkH-PhoA proteins. (a) Cultures of various transformants of ftsE(Ts) and ftsE+ by the plasmid encoding KdpA-PhoA, Kup-PhoA, or TrkH-PhoA were grown exponentially at 30°C in K10 medium containing 50 μM IPTG (open arrow) and diluted 1- to 200-fold with the same medium, followed by further incubation at 41°C (solid arrow). At each time point (small arrows), OD600, the number of cells, and the amounts of PhoA fusion proteins were measured. (b) The OD600 values of each sample, 0.17 to 0.4, were corrected for the dilution factors and are shown relative to the value of the 0-h sample. Cell numbers (▴) were measured by a Coulter counter. The proteins (10 μg each) of the membrane fractions were analyzed by Western blotting, and the relative amounts of the PhoA fusion proteins were plotted against the relative increase in OD600. •, ftsE(Ts) transformants; ▪, ftsE+ transformants.
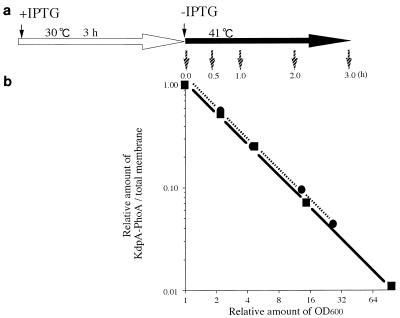
We further examined whether KdpA-PhoA synthesized at 30°C is destabilized at 41°C. The ftsE(Ts) and ftsE+ cells, carrying the plasmid encoding KdpA-PhoA, were grown exponentially in K10 medium containing 50 μM IPTG at 30°C for 3 h. The cells were then collected, washed, resuspended in K10 without IPTG, and further incubated at 41°C. The relative amounts of the KdpA-PhoA protein were measured (Fig. 8a) at intervals. In both the ftsE(Ts) and ftsE+ cells, the abundance of the fusion protein decreased in proportion to the increase in OD600 (Fig. 8b). Moreover, the amount decreased in inverse proportion to the increase in OD600. These results indicate that in both ftsE(Ts) and ftsE+ cells, the fusion protein, once localized in the membrane at 30°C, is stable at 41°C for at least 3 h.
FIG. 8.
Preformed KdpA-PhoA in the membrane fractions of ftsE(Ts) and ftsE+ transformants. (a) Overnight cultures of ftsE(Ts) and ftsE+ transformants by the plasmid encoding KdpA-PhoA were diluted 100-fold with K10 medium containing 50 μM IPTG and incubated at 30°C for 3 h (open arrow). The OD600 values of ftsE(Ts) and ftsE+ transformants were 0.32 and 0.31, respectively, at that point. Cells were washed with K10 without IPTG and diluted 1- to 100-fold with fresh K10 (solid arrow). The cultures were then incubated at 41°C for 0, 0.5, 1, 2, and 3 h (small arrows). After this step, the analysis procedure was the same as that described in the legend to Fig. 7. The relative amounts of KdpA-PhoA were plotted against the relative increase in OD600. •, ftsE(Ts) transformant; ▪, ftsE+ transformant.
The ftsE(Ts) mutation affects the translocation of KdpA-PhoA.
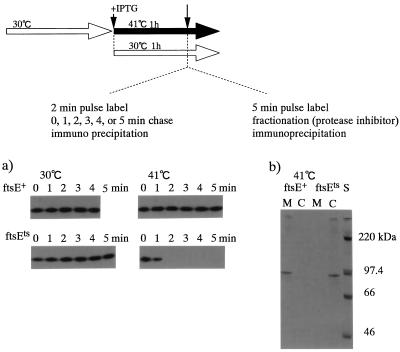
The results described above suggest that ftsE(Ts) affects the translocation of the three fusion proteins into the membrane and that the fusion proteins which have failed to translocate into the membrane might be degraded. We performed a pulse-chase experiment by the method of Ito et al. (18) to test this hypothesis. The ftsE(Ts) and ftsE+ cells carrying the KdpA-PhoA plasmid were grown exponentially in K10 medium at 30°C. The cultures were then diluted 1/40 with K10 containing 50 μM IPTG and incubated at 30 or 41°C for 1 h. The cells were labeled with [3H]leucine for 2 min and chased with unlabeled leucine for 0 to 5 min. As shown in Fig. 9a, KdpA-PhoA was synthesized in similar amounts in all the cultures irrespective of the ftsE genotype or the growth temperature, but the protein synthesized in ftsE(Ts) disappeared during a 2-min chase at 41°C.
FIG. 9.
(a) Pulse-chase of KdpA-PhoA. Overnight cultures of ftsE(Ts) and ftsE+ transformants by the plasmid encoding KdpA-PhoA were grown exponentially at 30°C in K10 medium (short open arrow in top diagram) and diluted 40-fold with K10 containing 50 μM IPTG (+ IPTG), followed by incubation for 1 h at 41 or 30°C (long arrows). The cells were pulse labeled for 2 min with [3H]leucine and chased with unlabeled leucine for 5 min at each temperature. At the indicated time points, trichloroacetic acid (TCA) was added to a final concentration of 5%, and KdpA-PhoA in a TCA-insoluble fraction was analyzed as described by Ito et al. (18), although protein A-Sepharose (Pharmacia) was used for immunoprecipitation instead of heat-treated Staphylococcus aureus cells. (b) Localization of labeled KdpA-PhoA. In another experiment, cells cultured for 1 h at 41°C were labeled for 5 min with [3H]leucine. Protease inhibitor was added as described by Ito (17), the cells were fractionated into cytoplasmic (lanes C) and membrane (lanes M) fractions, and KdpA-PhoA in a TCA-insoluble fraction of each was analyzed as described for panel (a). A 14C-labeled molecular mass marker (CFA626; Amersham) was also loaded in the righthand lane.
To determine whether this instability is due to an insertion problem or occurs because the PhoA fusion protein synthesized at 41°C is incorporated into the membrane but does not assemble into a higher-order structure and consequently is degraded, we examined the localization of the labeled KdpA-PhoA. In another experiment, the 0-min samples, cultured and labeled similarly, were fractionated into membrane and cytoplasmic fractions in the presence of protease inhibitor, and 3H-labeled KdpA-PhoA was immunoprecipitated (Fig. 9b). In the ftsE(Ts) cells, the KdpA-PhoA synthesized at 41°C was detected only in the cytoplasmic fraction, although in the case of the ftsE+ cells, it was detected only in the membrane fraction. These results indicate that FtsE affects the translocation of KdpA-PhoA into the membrane.
The absence of K+-pump biogenesis may be responsible for the growth cessation of ftsE(Ts).
To investigate the relation between the inhibition of cell growth and the loss of K+ pumps in the membranes of ftsE(Ts) cells, we followed cell growth at 41°C in two different media. A culture of ftsE(Ts) growing exponentially in K10-succinate at 30°C was diluted at 0 h with K10 or K10-succinate to an appropriate dilution to avoid growth saturation, and subsequent cell growth at 41°C was followed by measuring the OD600 of the cultures, with appropriate corrections for the dilution (Fig. 10). Doubling times were 24 min with K10 and 110 min with K10-succinate. The growth of both cultures stopped when the OD600 increased 32-fold. Thus, the timing of growth cessation was determined by the extent of cell mass increase but not by the time spent at 41°C. The results presented in Fig. 7 show that KdpA-PhoA, TrkH-PhoA, and Kup-PhoA decreased to 1/15.4, 1/14.9, and 1/13.5, respectively, when the OD600 of ftsE(Ts) increased 32-fold at 41°C. Thus, there seem to be critical concentrations of the K+-pump proteins below which the ftsE(Ts) cells cannot grow at 41°C. In the present plasmid-based expression systems, these values may be about 1/15 of the fully induced levels.
FIG. 10.
Growth of ftsE(Ts) at 41°C in poor or rich medium. A bacterial culture, growing exponentially at 30°C, was diluted with K10 (•) or K10-succinate (○) medium and incubated at 41°C for 14 h. The OD600 measured at each point was 0.012 to 0.09. The values were corrected for the dilution factors, and the corrected values were plotted relative to that of the 0-h sample.
DISCUSSION
The PhoA fusion proteins of the components of three K+ pumps, KdpA, Kup, and TrkH, were missing in the membrane fraction of the ftsE(Ts) mutant at 41°C. The fusion proteins, once localized in the membranes of ftsE(Ts) cells at 30°C, were stable even after a 6-h cultivation at 41°C, but they were diluted in a growth-dependent manner. The loss of the fusion proteins from the membrane fraction was suppressed by a multicopy plasmid carrying ftsE+. A pulse-labeling experiment showed that K+-pump protein–PhoA fusion proteins were synthesized in the ftsE(Ts) mutant cells at 41°C, but the synthesized fusion proteins did not translocate into the membrane at 41°C and degraded rapidly. Thus, the ftsE(Ts) mutation affects the translocation of PhoA-fused K+-pump proteins into the membrane.
The ftsE(Ts) mutant first elongates and then stops growing at the nonpermissive temperature. We showed here that the defect in cell growth is specifically alleviated in the presence of high concentrations of K+. However, since the defect in cell division remained impaired, the cells elongated more than 100-fold. It is known that the triple mutants, which lack all three K+ pumps, lose almost all of their K+, retaining less than 10% after 2 h at 37°C, but the level is recovered by the addition of high concentrations of K+ into the medium (28). K+ is essential for protein synthesis, so that a cell cannot grow when the level of K+ is lowered below a critical level (21). Under different growth rate conditions, the ftsE(Ts) cells always stopped growing when the concentrations of K+-pump protein–PhoA fusion proteins in the membrane decreased 15-fold. It seems that the arrest in cell growth of the ftsE(Ts) cells is due to a defect in the de novo formation of all three K+ pumps in the membrane. Moreover, plasmids encoding the PhoA fusion proteins complemented the mutants having a defect in the corresponding proteins. Therefore, we extended our conclusion from the experimental results with the PhoA fusion proteins to the biogenesis of natural K+ pumps, and we conclude that the ftsE(Ts) mutation affects the translocation of K+-pump proteins into the cytoplasmic membrane. The defect in cell growth of ftsE(Ts) is, at least partially, ascribable to the loss of all three K+ pumps in the membrane.
FtsE is speculated to have a function in protein translocation in conjunction with Ffh-4.5S ribonucleoprotein (SRP) and FtsY (10, 22), the bacterial counterpart of the mammalian SRP system (24). SRP and FtsY indeed promote the cotranslational targeting of signal sequence-bearing nascent chains (26). Ulbrandt et al. (34) have screened genes that cause synthetic lethality upon overexpression (a Slo phenotype) in E. coli cells with limiting SRP activity and have suggested that FtsE requires SRP for its membrane insertion. However, it is equally possible that FtsE participates in the translocation facilitation of some proteins. Thus, its overproduction may cause some unbalance in the SRP-related translocation machinery. We have shown here that the fusion proteins are certainly synthesized, but fail to assemble into the membrane and degrade very rapidly in the ftsE(Ts) mutant cells. Thus, FtsE actually mediates protein translocation. However, the accumulation of precursors of β-lactamase, OmpF, or ribose-binding protein has not been observed in ftsE(Ts), although the depletion of FtsY causes an accumulation of these precursor proteins (22). Moreover, no mutations have been mapped in ftsY, despite localized mutagenesis of the ftsYEX gene cluster (10), although the depletion of Ffh gives rise to an elongated morphological phenotype (22). A homolog of FtsY has been identified in an archaebacterium, but the relevant gene is not flanked by ftsE or ftsX (27) and sequence analysis of Mycobacterium tuberculosis DNA reveals open reading frames homologous with those of ftsX and ftsE but not ftsY (33). These results suggest that the molecular mechanism of FtsE action may be different from that of FtsY, although both may act to facilitate the translocation of proteins in conjunction with SRP.
All of the three proteins, KdpA, TrkH, and Kup, which were affected by the ftsE(Ts) mutation have more than eight membrane-spanning segments. In contrast, KdpC and KdpD, which were not affected by the ftsE(Ts) mutation, have only one and four membrane-spanning segments, respectively. We found that the tetA+ transductant of ftsE(Ts) showed a decreased resistance to tetracycline even at the permissive temperature (unpublished data). However, TetA, having 12 membrane-spanning segments (3), accumulated normally in the membrane fractions of ftsE(Ts) cells carrying the tetA gene (data not shown). Therefore, a specific number of membrane-spanning segments may not be a necessary feature.
The defect in cell division of ftsE(Ts) could be explained if some proteins essential for cell division are translocated into the membrane by FtsE. This speculation is not in conflict with the fact that ftsE(Ts) recovers cell division at 30°C in the presence of chloramphenicol after 60 min of incubation at 41°C (29). The ftsE(Ts) mutation may affect the late stage of the cell division cycle because the FtsZ ring can be detected in ftsE(Ts) cells at 41°C (unpublished observation). FtsQ, which is not required for FtsZ ring formation (1), is known to be cross-linked to the SRP subunit Ffh (35), suggesting that FtsQ might be a candidate substrate for FtsE action. Further studies will elucidate the molecular mechanism of FtsE action in cell division.
ACKNOWLEDGMENTS
We are grateful to Robert K. Fujimura and Shigeki Moriya for a critical reading of the manuscript. We are indebted to Akihito Yamaguchi and Yuichi Someya for providing anti-TetA antiserum and the plasmid pLGT2 encoding TetA.
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science, Sports, and Culture of Japan.
REFERENCES
- 1.Addinall S G, Bi E, Lutkenhaus J. FtsZ ring formation in fts mutants. J Bacteriol. 1996;178:3877–3884. doi: 10.1128/jb.178.13.3877-3884.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiba H, Adhya S, Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981;256:11905–11910. [PubMed] [Google Scholar]
- 3.Allard J D, Bertrand K P. Membrane topology of the pBR322 tetracycline resistance protein. J Biol Chem. 1992;267:17809–17819. [PubMed] [Google Scholar]
- 4.Bi E, Dai K, Subbarao S, Beall B, Lutkenhaus J. FtsZ and cell division. Res Microbiol. 1991;142:249–252. doi: 10.1016/0923-2508(91)90037-b. [DOI] [PubMed] [Google Scholar]
- 5.Chepuri V, Gennis R B. The use of gene fusions to determine the topology of all of the subunits of the cytochrome o terminal oxidase complex of Escherichia coli. J Biol Chem. 1990;265:12978–12986. [PubMed] [Google Scholar]
- 6.Dai K, Xu Y, Lutkenhaus J. Topological characterization of the essential Escherichia coli cell division protein FtsN. J Bacteriol. 1996;178:1328–1334. doi: 10.1128/jb.178.5.1328-1334.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dosch D C, Helmer G L, Sutton S H, Salvacion F F, Epstein W. Genetic analysis of potassium transport loci in Escherichia coli: evidence for three constitutive systems mediating uptake of potassium. J Bacteriol. 1991;173:687–696. doi: 10.1128/jb.173.2.687-696.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epstein W, Kim B S. Potassium transport loci in Escherichia coli K-12. J Bacteriol. 1971;108:639–644. doi: 10.1128/jb.108.2.639-644.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fürste J P, Pansegrau W, Frank R, Blocker H, Scholz P, Bagdasarian M, Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48:119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- 10.Gibbs T W, Gill D R, Salmond G P C. Localized mutagenesis of fts YEX operon: conditionally lethal missense substitutions in the FtsE cell division protein of Escherichia coli are similar to those found in the cystic fibrosis transmembrane conductance regulator protein (CFTR) of human patients. Mol Gen Genet. 1992;234:121–128. doi: 10.1007/BF00272353. [DOI] [PubMed] [Google Scholar]
- 11.Gill D R, Hatfull G F, Salmond G P C. A new cell division operon in Escherichia coli. Mol Gen Genet. 1986;205:134–145. doi: 10.1007/BF02428043. [DOI] [PubMed] [Google Scholar]
- 12.Gill D R, Salmond G P C. The Escherichia coli cell division proteins FtsY, FtsE and FtsX are inner membrane-associated. Mol Gen Genet. 1987;210:504–508. doi: 10.1007/BF00327204. [DOI] [PubMed] [Google Scholar]
- 13.Hale C A, de Boer P A J. Direct binding of FtsZ to ZipA, an essential component of the septal ring structure that mediates cell division in E. coli. Cell. 1997;88:175–185. doi: 10.1016/s0092-8674(00)81838-3. [DOI] [PubMed] [Google Scholar]
- 14.Hara H, Park J T. A far upstream region is required for expression of the ftsI gene coding for penicillin-binding protein 3 of Escherichia coli. In: De Pedro M A, Loffelhardt W, Holtje J V, editors. Bacterial growth and lysis: metabolism and structure of the bacterial sacculus. New York, N.Y: Plenum Press; 1993. pp. 303–308. [Google Scholar]
- 15.Harold F M, Maloney P C. Energy transduction by ion currents. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: American Society for Microbiology; 1996. pp. 283–306. [Google Scholar]
- 16.Innis M A, Myambo K B, Gelfand D H, Brow M A D. DNA sequencing with Thermus aquaticus DNA polymerase and direct sequencing of polymerase chain reaction amplified DNA. Proc Natl Acad Sci USA. 1988;85:9436–9440. doi: 10.1073/pnas.85.24.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito K. Identification of the secA (prlA) gene product involved in protein export in Escherichia coli. Mol Gen Genet. 1984;197:204–208. doi: 10.1007/BF00330964. [DOI] [PubMed] [Google Scholar]
- 18.Ito K, Bassford P J, Beckwith J. Protein localization in E. coli: is there a common step in the secretion of periplasmic and outer-membrane proteins? Cell. 1981;24:707–717. doi: 10.1016/0092-8674(81)90097-0. [DOI] [PubMed] [Google Scholar]
- 19.Koronakis V, Hughes C. Bacterial signal peptide-independent protein export: HlyB directed secretion of haemolysin. Semin Cell Biol. 1993;4:7–15. doi: 10.1006/scel.1993.1002. [DOI] [PubMed] [Google Scholar]
- 20.Lowry O H, Rosebrough N J, Farr A I, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 21.Lubin M, Ennis H L. On the role of intracellular potassium in protein synthesis. Biochim Biophys Acta. 1964;80:614–631. doi: 10.1016/0926-6550(64)90306-8. [DOI] [PubMed] [Google Scholar]
- 22.Luirink J, ten Hagen-Jongman C M, van der Weijden C C, Oudega B, High S, Dobberstein B, Kusters R. An alternative protein targeting pathway in Escherichia coli: studies on the role of FtsY. EMBO J. 1994;13:2289–2296. doi: 10.1002/j.1460-2075.1994.tb06511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 24.Miller J D, Bernstein H D, Walter P. Interaction of E. coli Ffh/4.5S ribonucleoprotein and FtsY mimics that of mammalian signal recognition particle and its receptor. Nature. 1994;367:657–659. doi: 10.1038/367657a0. [DOI] [PubMed] [Google Scholar]
- 25.Miskin R, Zamir A, Elson D. Inactivation and reactivation of ribosomal subunits: the peptidyl transferase activity of the 50S subunit of Escherichia coli. J Mol Biol. 1970;54:355–378. doi: 10.1016/0022-2836(70)90435-3. [DOI] [PubMed] [Google Scholar]
- 26.Powers T, Walter P. Co-translational protein targeting catalyzed by the Escherichia coli signal recognition particle and its receptor. EMBO J. 1997;16:4880–4886. doi: 10.1093/emboj/16.16.4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramirez C, Matheson A T. A gene in the archaebacterium Sulfolobus solfataricus that codes for a protein equivalent of the alpha subunit of the signal recognition particle receptor in eukaryotes. Mol Microbiol. 1991;5:1687–1693. doi: 10.1111/j.1365-2958.1991.tb01916.x. [DOI] [PubMed] [Google Scholar]
- 28.Rhoads D B, Waters F B, Epstein W. Cation transport in Escherichia coli. VIII. Potassium transport mutants. J Gen Physiol. 1976;67:325–341. doi: 10.1085/jgp.67.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ricard M, Hirota Y. Process of cellular division in Escherichia coli: physiological study on thermosensitive mutants defective in cell division. J Bacteriol. 1973;116:314–322. doi: 10.1128/jb.116.1.314-322.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Römisch K, Webb J, Herz J, Prehn S, Frank R, Vingron M, Dobberstein B. Homology of 54K protein of signal-recognition particle, docking protein and two E. coli proteins with putative GTP-binding domains. Nature. 1989;340:478–482. doi: 10.1038/340478a0. [DOI] [PubMed] [Google Scholar]
- 31.Silver S. Transport of inorganic cations. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: American Society for Microbiology; 1996. pp. 1091–1102. [Google Scholar]
- 32.Singer M, Baker T A, Schnizler G, Deischel S M, Goel M, Dove W, Jaacks K J, Grossman A, Erickson J W, Gross C A. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev. 1989;53:1–24. doi: 10.1128/mr.53.1.1-24.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tyagi J S, Das T K, Kinger A K. An M. tuberculosis DNA fragment contains genes encoding cell division proteins ftsX and ftsE, a basic protein and homologues of PemK and Small protein B. Gene. 1996;177:59–67. doi: 10.1016/0378-1119(96)00271-5. [DOI] [PubMed] [Google Scholar]
- 34.Ulbrandt N D, Newitt J A, Bernstein H D. The E. coli signal recognition particle is required for the insertion of a subset of inner membrane proteins. Cell. 1997;88:187–196. doi: 10.1016/s0092-8674(00)81839-5. [DOI] [PubMed] [Google Scholar]
- 35.Valent Q A, Gier J L, Heijne G, Kendall D A, Jongman C M, Oudega B, Luilink J. Nascent membrane and presecretory proteins synthesized in Escherichia coli associate with signal recognition particle and trigger factor. Mol Microbiol. 1997;25:53–64. doi: 10.1046/j.1365-2958.1997.4431808.x. [DOI] [PubMed] [Google Scholar]
- 36.Wilson G G, Young K K Y, Edlin G J. High-frequency generalized transduction by bacteriophage T4. Nature. 1979;280:80–82. doi: 10.1038/280080a0. [DOI] [PubMed] [Google Scholar]
- 37.Yamada M, Sumi K, Matsushita K, Adachi O, Yamada Y. Topological analysis of quinoprotein glucose dehydrogenase in Escherichia coli and its ubiquinone-binding site. J Biol Chem. 1993;268:12812–12817. [PubMed] [Google Scholar]
- 38.Zamir A, Miskin R, Elson D. Inactivation and reactivation of ribosomal subunits: amino acyl-transfer RNA binding activity of the 30S subunit of Escherichia coli. J Mol Biol. 1971;60:347–364. doi: 10.1016/0022-2836(71)90299-3. [DOI] [PubMed] [Google Scholar]