Abstract
Extracellular vesicles (EVs) carry DNA, RNA, protein, and other substances involved in intercellular crosstalk and can be used for the targeted delivery of drugs. Triple-negative breast cancer (TNBC) is rich in recurrent and metastatic disease and lacks therapeutic targets. Studies have proved the role of EVs in the different stages of the genesis and development of TNBC. Cancer cells actively secrete various biomolecules, which play a significant part establishing the tumor microenvironment via EVs. In this article, we describe the roles of EVs in the tumor immune microenvironment, metabolic microenvironment, and vascular remodeling, and summarize the application of EVs for objective delivery of chemotherapeutic drugs, immune antigens, and cancer vaccine adjuvants. EVs-based therapy may represent the next-generation tool for targeted drug delivery for the cure of a variety of diseases lacking effective drug treatment.
Keywords: Triple-negative breast cancer, vesicle, tumor microenvironment/host-tumor interactions, liquid biopsy, drug resistance/biochemistry of membrane metabolism and transportion
Graphical abstract
The mechanism of the occurrence and development of TNBC is quite complicated. Zhao and colleagues summarized the role of EVs in the formation of TNBC and the potential value of EVs in the treatment of TNBC. They discussed the role of EVs in TNBC.
Introduction
Breast cancer (BC) has replaced lung cancer to be the malignant tumor with the highest occurrence and deaths in females across the world.1 Triple-negative breast cancer (TNBC) is identified by hardly expression of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 and the intracellular and extracellular signaling paths involved in the pathogenesis are more complex. Extracellular vesicles (EVs) especially tumor-derived exosomes (TDEs), play a unique role in the occurrence, development, and therapies of TNBC.2 In addition to carrying material, transmembrane proteins on the EV surface are also involved in the development of TNBC, such as metastasis. Understanding the complex role between EVs and TNBC is helpful to further develop a new treatment method for TNBC. So we sum up the role of EVs in TNBC from the perspectives of the tumor microenvironment (TME), distant metastasis, drug resistance, and clinical application in this passage.
Overview of EVs
EVs are a kind of ordered assembly of molecules with a closed bilayer structure, also known as liposomes.3 EVs have a complex structure consisting of transmembrane proteins and lipid bilayers wrapped in soluble hydrophilic components and contain substances that originated from the cytoplasm of the donor cell.4 EVs have also been sorted according to their cell origin. EVs have been sorted into exosomes, microsomes, apoptotic bodies, and oncosomes according to their diameter, mode of abscission, and inclusion.5 Exosomes are EVs within the scope of 40 to 160 nm in diameter and represent a subtype of EVs formed through the plasma membrane by budding or entosis and exocytosis.6 Exosomes can be released by almost all kinds of cells and are widely distributed in body fluids.7 Exosomes contain many cellular components such as DNA, RNA, lipids, metabolites, and cytoplasmic and cell surface proteins.8,9 Microparticles (MPs) are produced by outbudding off the cell membrane with diameters ranging from 100 to 1,000 nm.10 Apoptosis also leads to the production of membrane-bound EVs, which are referred to as apoptotic bodies.11 Studies have indicated that EVs can transport useful materials to healthy cells (e.g., autoantigens).12,13 Cancer bodies are EVs with a diameter of 1 to 10 μm released by tumor cells.14 Tumor-associated exosomes are exosomes released by cancer.15 The bioactive substances in tumor-associated exosomes can influence the recipient cells in two major avenues. The first is the direct contact with the receptor cells. The second is that vesicles enter the receptor cells by plasmalemma fusion and internalization.
EVs in the TME
Exosomes from normal cells play a positive part while exosomes from pathological cells play a negative part.16 Kalluri and LeBleu described the significance of EVs in cell survival, metastasis, metabolic recombination, and angiogenesis.17 EVs play a significant part in intercellular communication (especially in immune cells) through direct or indirect means, such as receptor-ligand interactions, direct fusion with the plasma membrane, and endocytosis.18 Attachment of an EV to the target cell can induce signals through receptor-ligand interaction, or lead to transfer of its contents to the cytoplasm.19 Exosomes secreted by cancer-associated fibroblasts (CAFs) promote proliferation, invasion, migration, and epithelial mesenchymal transformation (EMT) and inhibit apoptosis of BC cells.20 In vivo experiments have indicated that microvesicles released by BC cells can cause transformation and acquisition of malignant features, such as enhanced proliferation and survival induction, by transferring tissue glutamintransferase and fibronectin to surrounding fibroblasts.21
EVs in the immune microenvironment
As a communication medium between cancer and immune cells, EVs have been considered to be an important modulator of immune response during tumor progression.22,23,24 TDEs can influence antitumor activity through delivery of inhibitory cargo.25 EVs can influence the activation, differentiation or polarization, recruitment, cytokine production, and various other effector functions of innate immune cells through delivery of mediators (bioactive lipids, acute phase proteins, and cytokines), enzymes, and RNAs, thus playing a proinflammatory role, including antigen presentation and activation of immune cells.26,27
Macrophage
Necrotic apoptosis EV phagocytosis by macrophages can regulate cytokine and chemokine secretion.28 After EV uptake, interferon (IFN) β induces interferon stimulator genes in the recipient macrophages through IFN-α/β receptors and can induce differentiation of monocytes into macrophages. High expression of this feature is related to T cell infiltration and perpetuates patient existence.29,30 Macrophage growth factor CSF-1 binds to TNBC-derived EV to promote the differentiation of M2 macrophages.31 TDEs can regulate macrophages that express programmed cell death ligand 1 (PD-L1). Therefore, they are supposed as promising predictive landmarks of tumor progression and potential marks for therapeutic immune tolerance.32
Natural killer cells
Studies have shown that exosome pretreatment of mouse cancer cells can reduce the quantity of natural killer (NK) cells.33 Liu et al., after exosome pretreatment with mouse BC cell lines, found that not only the number of NK cells in mice was reduced, but also the cytotoxicity was impaired.34 Experiments with BC cell line MDA231 have shown that exosomes can significantly prevent interleukin (IL)-2-induced NK cell proliferation, leading to immune escape and cancer progression.35
T cells
T cell-derived exosomes show efficient antitumor responses through stimulating CD8+ T cells and strengthening cytotoxicity.36 EVs derived from tumor cells inhibit the antitumor ability of T cells and block the genesis of EVs, thus promoting the activation, proliferation, and antitumor capacity of T cells.37
Reactive oxygen species
Reactive oxygen species (ROS) take part in REDOX response in vivo as modulators of cell function and signal transduction.38 The increase of ROS greatly downregulates the expression of exosomal miR-155-5p secreted by tumor; in other words, ROS can induce the downregulation of EVs that produce a favorable TME for tumor.39
Tumor-associated fibroblasts
Exosomes derived from CAFs cause increased expression of PD-L1 through miR-92 notably promote apoptosis of T cells and block the function of NK cells, thereby inhibiting immune cell function in BC.40 ATP-binding box (ABC) transporters regulate the intracellular levels of assorted cytokines and chemokines by regulating the transmembrane transport of cytokines in the tumor immune microenvironment (TIME) through EVs, and control the distinction, removal, and function of immune cells to affect the TIME.41,42
Besides, EVs carrying tumor cell-derived PD-L1 may interact with T cells producing programmed death 1 (PD-1), thereby reducing the response of TNBC patients to immune checkpoint blocking drugs. Lee et al. demonstrated in cellular animal experiments that macitentan improves the antitumor immune response by inhibiting the secretion of these EVs.43
EVs in metabolic microenvironment
The Warburg effect is a major characteristic of carcinoma metabolism, where tumor cells still resort to lactate glycolysis even under conditions of adequate oxygen.44 Compared with normal tissues, TNBC samples showed significant upregulation of oxidative phosphorylation.45 MicroRNAs (miRNAs) contained in EVs in the microenvironment of BC can alter the metabolic level of cancer cells. For example, BC-derived vesicles miR-122 can be transferred to stromal cells of neutrophils in the brain and lungs to alter glucose metabolism, thereby improving the metastasis efficiency of BC cells.46 Morrissey et al. demonstrated that TDEs augment PD-L1 expression by way of metabolic reprogramming led by NF-κB-dependent glycolysis, and lead to increased glucose uptake through TDE signaling of NF-κB.47
TDEs restrain mitochondrial oxidative phosphorylation and augment the transformation of pyruvate to lactic acid. Lactate acid feeds back NF-κB pathway and arguments PD-L1 ulteriorly, thereby polarizing macrophages into an immunosuppressive phenotype TDEs47 (Figure 1). In BC, CAF-derived exosomes can act as a molecular “sponge” to up-control the expression of the PKM pathway, restrain mitochondrial oxidative phosphorylation, augment glycolysis carboxylation, and enhance multiplication of BC cells.48
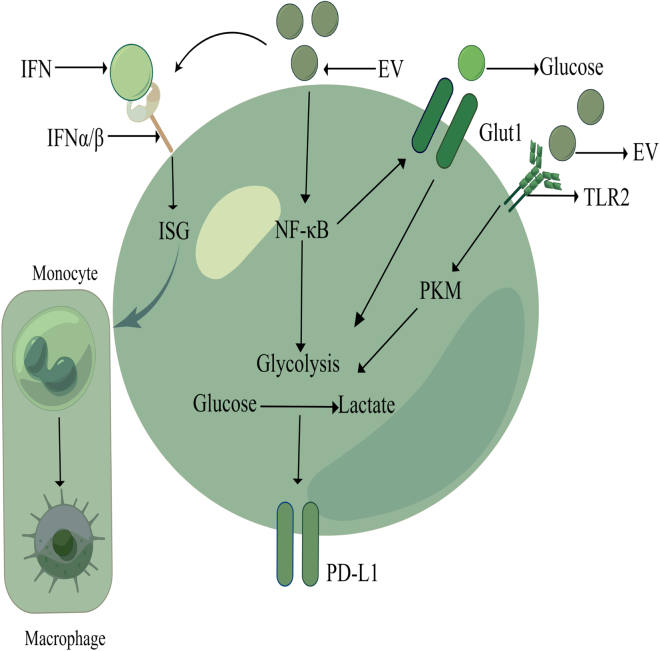
Figure 1.
Role of exosomes in tumor microenvironment
After EV uptake, interferon (IFN) β induces IFN stimulating genes in recipient macrophages via IFN α/β receptors, and it can induce monocytes to differentiate into macrophages, which can extend the life span of cancer patients. TDE increases PD-L1 expression through NF-κB-dependent glycolytic-led metabolic reprogramming, and promotes glucose to lactate conversion through the PKM pathway via TLR2, the lactic acid is increased, lactic acid further feeds back NF-κB, thereby increasing PD-L1, thereby polarizing macrophages to an immunosuppressive phenotype.
In addition, macropinocytosis refers to the formation of an endocytic vesicle driven by actin on the plasma membrane that deforms the plasma membrane.49,50 By stimulating the giant pinocytosis pathway, autophagy-inhibited cancer cells meet their energy requirements through degradation of proteins external to the lysosome, such as albumin or extracellular matrix components.51,52 Tu et al. found that the central Rab GAP cascade of protein PripA-TbcrA complex promotes macropinocytosis.53
EVs in vascular remodeling
Hypoxia has been indicated to further angiogenesis pathways by secretion of cancer-associated EVs.54 Exosome-promoted angiogenesis is mediated by up-control of protease-activated receptor 2 in epithelial cells.55 BC-derived EVs contain nucleoside diphosphokinase B, whose expression and phosphotransferase activity are enriched in EVs, promoting vascular endothelial cell migration and destroying monolayer integrity.56 Delta-like 4 (Dll4), Notch ligand, Dll4-containing exosomes mediate Dll4-Notch signaling and can cross collagen matrix and bind to endothelial cells leading to increased cell motility.57 Exosomes act as “sponges” that absorb miR-106 and control the expression of angiogenic factor that activate signaling in mesenchymal and endothelial cells, thereby promoting angiogenesis.58,59 Exosomes activate AMPK signaling through a typical forward signaling pathway dependent on ligin Ephrin A1.60 VEDG is a main angiogenic factor in BC.61 Overexpression of VEGF often occurs prior to the invasive growth of BC cells.62 Interleukin-3 promotes angiogenesis through EVs. The experimental results showed that targeted blocking of EVs could not only induce tumor vessel regression of TNBC cells, but also increase tumor cell apoptosis and decrease cell activity and migration ability.63
EVs in distant metastatic implantation
Zomer et al. demonstrated that EVs enhanced the migration and metastasis capability of tumor through the Cre-LoxP system.64 It was found that the EVs derived from TNBC modified the mechanical properties of MCF7 cells, a kind of non-metastatic TNBC cells, by reducing cell rigidity and rearranging cytoskeleton, make it metastatic.65
CAF-derived EVs
By regulating CD44 expression, CAF-derived EVs transfer circHIF1A into BC cells during hypoxia and strengthen the plasticity of tumor stem cells by regulating CD44 expression.66 Chen et al. demonstrated that miR-500a-5p in EVs released by CAF promotes the proliferation and metastasis of BC cells through reducing the expression of ubiquitin-specific peptidase 28.67 Exosome-mediated miR-9 is up-control in several BC cell lines and has been recognized as a metastasis-promoting miRNA, affecting the characteristics of breast fibroblasts and increasing the conversion to CAF phenotype to promote tumor growth and metastasis.68,69
Tumor cell-derived EVs
BC cells can enhance the arousal of fibroblasts through secreting EVs containing abundant Survivin protein.70 TDEs were shown to promote TNBC tumorigenesis, metastasis, and migration via the miR-637/Akt1 axis.71 In the BC microenvironment, miR-10b in vesicles can urge the invasion of non-metastatic cancer cells through inhibiting protein levels of target genes, such as KLF4 and HOXD10.72,73 After co-culturing two TNBC cell lines with dissimilar levels of glycolytic activity, Kang found that in the process of transforming the phenotypes of other subtypes into invasive phenotypes induced by invasive cancer cells, EVs carry key protein of phosphorylated PKM2 to activate glucose metabolism.74
Pre-translational niche
As a microenvironment fitting for the existence and growth of tumor in the distal metastatic site, the pre-metastatic niche can also be reshaped through miRNA in EVs. For example, miR-186 and miR-205 in EVs were found to increase the formation of niches in liver, thus providing a beneficial environment for the growth of metastatic cancer cells.75,76,77
Chemotherapy
Although chemotherapy may reduce tumor size, it may increase the danger of metastasis.78 Doxorubicin (DXR) therapy upregulates the secretion of primary breast tumor EVs, and intake of these DXR-EVs in other organs (mainly the lung) to initiate the pre-metastatic niche and create suitable conditions for the colonization of metastatic BC cells.79
Epithelial mesenchymal transformation
FUS/circEZH2/KLF5/feedback loops contribute to metastasis of CXCr4-induced breast cancer to liver by enhancing EMT.80 EVs transfer miR-221 to recipient cells, thereby promoting EMT.81 In BC, the pluripotent factors Lin28A and Lin28B promote lung metastasis by inhibiting the pre-metastatic niche through EVs.82,83
Brain metastasis
Although the BBB (blood-brain barrier) does not allow passive entry of anything sized larger than 400 D into the brain, EVs can mediate BC metastasis to the brain.84,85 EVs induce endothelial cells in the BBB to phagocytose and absorb EVs, a standard biological pathway referred to as subendocytosis.86,87 Studies have shown that EVs migrate to and colonize the cerebral vascular niches in the brain to induce proinflammatory states and promote the colonization of cancer cells in the brain.88
Liver metastasis
The liver is one of the most common organs for TNBC metastasis and is related with prognosis and survival.89,90 Circulating cancer cells and bone marrow resident cancer cells migrate as "metastatic seeds" along the circulation to organs, causing liver metastases.91,92 Aspartate β hydroxylase can initiate the organ metastasis of BC cells by directing the synthesis and secretion of EVs rich in metalloproteinases.93 The EVs isolated from TNBC patients contained higher levels of transforming growth factor β 1 (TGFβ1). TGFβ1 can upregulate fibronectin in sinusoidal endothelial cells. It is an extracellular matrix protein that promotes adhesion between cancer cells and the liver microenvironment.94
EVs released by macrophage
In lung adenocarcinoma, EVs released by M2 macrophage boost the formation of TME and strengthen the invasion and metastasis ability of tumor cells through capacity delivering miR-942.95,96 In colorectal cancer, EVs activate the large tumor suppressor kinase 2-yes associated protein-matrix metalloproteinase 7 axis, exosome miRNA-106b-5p activates EMT cancer cells and interacts with TAM, and exosome miR-934 promotes the occurrence of liver metastasis by inducing macrophage M2 polarization.97,98,99
Surface membrane protein
MPs and EVs are different subtypes of EVs. Three tumor progression-related EV surface membrane proteins (CD9, Slc29a1) are present on both surfaces, which enable the binding of EVs to specific receptor cells. Experiments on mice have indicated that reduced expression of these surface membrane proteins reduces the lung tropism of metastasized TNBC cells.100 CD81, which is a kind of tetraspanin transmembrane protein on the EVs, has been newly identified as a factor of TNBC dryness and metastasis. Their extracellular regions have been found to interact with CD44 in human and mouse models, so as to promote TNBC lung metastasis.101
Besides, it was shown that the EVs derived from circulating cancer endothelial cells promoted the formation of new blood vessels in TNBC lung metastasis and depleted T cells. That is to say, the EVs could not only promote the growth of lung metastases, but also cause extensive immunosuppression.102
EVs in mediating drug action
There are two main effects of EVs in TNBC. First EVs participate in drug resistance; second by virtue of their properties, they can act as carriers of drugs for therapeutic purposes.
EVs in mediated resistance
EVs can assist drug efflux pumps to transport products from drug-resistant cancer cells to drug-sensitive cells, or help maintain tumor cells healthy by effecting inhibitory substances through EVs.103,104 Lehuédé et al. found that tumor-associated adipocytes increased the amount of EVs released by cancer cells and promoted the direct efflux of Adriamycin, rendering the BC cells resistant to Adriamycin.105 EVs directly participate in drug efflux through various drug transporters in a targeted manner.106 P-glycoprotein is a transporter on the ABC on EV membranes, which can use ATP as energy to export various compounds, including various anticancer drugs, thereby enhancing drug resistance.107,108 BC resistance protein can promote the transfer of chemotherapy drugs through the PI3K-Akt signaling pathway, and enhance the resistance of cancer cells to mitoxantrone.109 Xia et al. demonstrated the involvement of TAM in conferring resistance to most TNBCs by secreting EVs containing non-coding RNAs (ncRNAs), including circular RNAs (circRNAs), long non-coding RNAs (lncRNAs), and miRNAs.110 ncRNAs carried by EVs released by CAFs can enhance chemotherapy resistance through controlling signaling pathways (PI3K/AKT and ER) via target organs or targeted ion channels CAFs.111 EVs can restrain the migration and proliferation of T cells and differentiation of macrophages, thus participating in cancer immune escape.112 Mixed lineage kinase 4 has been shown to promote the chemoresistance of TNBC by modulating pro-survival response to DNA damage therapy.113 Histone chaperone proteins regulate cell proliferation and chemical resistance in TNBC by the YAP1/NDRG1 transcriptional axis.114 Lin et al. found that ubiquitin-specific protease 7 induced chemical resistance of TNBC through deubiquitination and stabilization of ABCB1.115 Activation of the mitochondrial apoptosis pathway in tumor cells is an important pathway of cell death after chemotherapy, and changes in this pathway can induce the progression of drug resistance in cancer cells.116 EVs released by tumor can “trap” anti-PD-L2 antibodies, leading to a decrease in drug concentration in the body, and faster clearance of the bound anti-PD-L1 antibodies. This results in insufficient time for drug action, impairing the antitumor ability and subsequent drug resistance.117
Clinical application of EVs
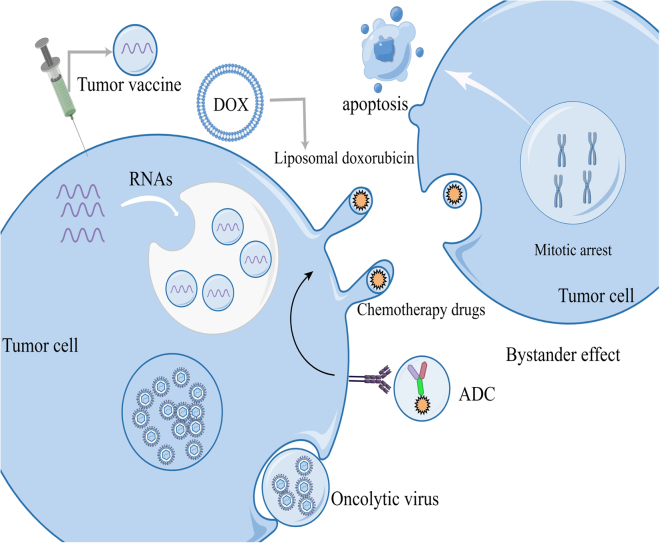
Due to high biocompatibility, low immunogenicity, intrinsic cell targeting, and ease of chemical and genetic manipulation, vesicles are considered effective vectors for biotherapy (Figure 2).
Figure 2.
Vesicles as drug loading route for tumor treatment
At present, the clinical application of vesicles is mainly reflected in tumor vaccine, liposome drug, ADC drug, and oncolytic virus and so on. Tumor vaccine is a kind of biological immunotherapy that contains DNA, RNA polypeptide, and other tumor antigens by vesicles. Liposome drugs including liposome paclitaxel, liposome Adriamycin, and liposome carboplatin have been used in clinical treatment. ADC drugs are loaded with vesicles, which transport therapeutic drugs targeted to tumor cells, and can enhance tumor killing ability through the bystander effect. In oncolytic virus therapy, the vesicles transport the virus into the tumor cells for therapeutic purposes by replicating and spreading the virus from the tumor cells to death.
EVs as transport carriers
Based on EV secretion and gene transfer by macrophages, nanoparticles can be used for delivery of active drugs to cells.118 Nanoparticles of tumor exosomes are effective carriers for chemotherapeutic agents.119 Disease-related miRNA mimics or inhibitors can be loaded into the EVs of patients by using the carrier properties of exosomes and their capability to cross the BBB for therapeutic purposes.120 circRNA-CREIT disrupts the stability of PKR protein and its overexpression significantly increases chemo-induced apoptosis, thus it can be packed into EVs to significantly strengthen the chemosensitivity of TNBC through exosomal delivery of stress-inhibiting particles.121
Antibody-drug conjugates
Antibody-drug conjugates (ADCs) have been approved for targeted tumor therapy.122 These are designed to selectively deliver payloads to target tumor cells and mitigate the adverse effects on non-malignant cells.123 ADC drugs are internalized for intracellular processing and payload release.124 Most ADCs exhibit cytotoxic effects on adjacent cancer cells that lack target antigen expression through their ability to cross cell membranes with a payload.125 ADC target antigens are usually expressed on EVs to some extent.126 EVs can deliver ADC to adjacent tumor cells that lack antibody target proteins, resulting in the bystander effect that can enhance the anticancer effectiveness of ADC.127 TROP2 target ADC drugs (Goxstuzumab) developed for TNBC have been utilized in clinical practice.128 The progress in the research and development of other ADC drugs is summarized in Table 1.
Table 1.
ADC drugs
| Drug | Target spot | Stage | Number | Identifier |
|---|---|---|---|---|
| Sacrituzumab | TROP-2 | II | 51 | NCT04230109 |
| Govitecan | ||||
| Datopotamab | TROP-2 | I | NCT03401385 | |
| Deruxtecan | II | 118 | NCT05460273 | |
| PF-06664178 | TROP-2 | I | 31 | NCT02122146 |
| ZEN003694 | I | 75 | NCT02711956 | |
| Talazoparib | TROP-2 | II | 179 | NCT03901469 |
| SKB264 | TROP-2 | I/II | 430 | NCT04152499 |
| Enfortumab Vedotin | Nectin-4 | II | 288 | NCT04225117 |
| PF-06650808 | Notch3 | I | 40 | NCT02129205 |
| PF-06647263 | I | 60 | NCT02078752 | |
| PF-06647020 | I | 138 | NCT02222922 | |
| PF-06263507 | I | 26 | NCT01891669 | |
| BAY94-9343 | I | 12 | NCT02485119 | |
| Cofetuzumab Pelidotin | PTK7 | I | 18 | NCT03243331 |
| AVID100 | I | 49 | NCT01891669 | |
| CAB-RC48-ADC | EGFR | I/II | 49 | NCT01891669 |
| SAR566658 | Ror2 | I/II | 420 | NCT03504488 |
| RC48-ADC | CA6 | II | 23 | NCT02984683 |
| RC108 | II | 64 | NCT05331326 | |
| SKB264 | I | 32 | NCT05331326 | |
| MRG002 | II | 95 | NCT05445908 | |
| ARX788 | II | 66 | NCT04742153 | |
| DS-8201a | I | 106 | NCT03255070 | |
| ADCT-502 | I | 75 | NCT03366428 | |
| ADCT-301 | HER-2 | I | 21 | NCT03125200 |
| PF-06804103 | I | 78 | NCT03621982 | |
| A166 | HER-2 | 95 | NCT03284723 | |
| XB002 | I/II | 49 | NCT03602079 | |
| BAY94-9343 | I | 524 | NCT04925284 | |
| TORL-2-307-ADC | I | 12 | NCT02485119 | |
| TORL-1-23 | I | 70 | NCT05156866 | |
| CAB-AXL-ADC | I | 70 | NCT05103683 | |
| FDA018-ADC | II | 240 | NCT05103683 | |
| TR1801-ADC | I | 78 | NCT05174637 | |
| F0002-ADC | I | 40 | NCT03859752 | |
| OBT076 | I | 23 | NCT03894150 | |
| HS-20089 | I | 150 | NCT04064359 | |
| STRO-002 | I | 177 | NCT05263479 | |
| FOR46 | I | 160 | NCT03748186 | |
| MYTX-011 | I | 56 | NCT03575819 | |
| SYD1875 | I | 150 | NCT05652868 | |
| REGN5093-M114 | I | 31 | NCT04202705 | |
| SOT102 | I/II | 81 | NCT04982224 | |
| STI-6129 | I/II | 269 | NCT05525286 | |
| FDA022-BB05 | I/II | 64 | NCT05565807 | |
| I | 107 | NCT05564858 |
Liposomal drugs
Liposomal drugs are also an embodiment of the clinical application of vesicles, such as paclitaxel, liposomal Adriamycin, and liposomal carboplatin.129,130,131,132 Chemical or biological modification of EVs through genetic or chemical modification can alter or enhance their therapeutic ability.133,134 Tumor cell-derived MPs carry chemotherapy drugs to reverse M2 macrophages into M1 macrophages by activating lysozyme somatic pigment P2 and nuclear hnRNPA1B450, thereby enhancing antitumor immunity.135
Genetic engineering
Current studies have developed different EV labeling and imaging methods that facilitate the use of EVs in genetic engineering.136 EVs with antibody targeting groups and immunoregulatory proteins displayed on the membrane surface were genetically engineered to not only activate T cells to kill epidermal growth factor receptor (EGFR)-positive TNBC cells, but also induce antitumor immunity.31 Silva et al. suggested that the new EVs sorting protein could enrich the target protein substance into vesicles thus widening the prospects for the usage of EVs as delivery carriers for more complicated therapeutic proteins.137 Cas9 EVs modified by genetic engineering can be used as a novel gene editing tool.138 EVs can also serve as multifunctional ribonucleoprotein delivery vectors for effective and secure gene editing.139
Tumor vaccine
mRNAs coated with lipid nanoparticles can be used as tumor vaccines, protein replacement therapies, and gene editing components for rare genetic diseases.140,141,142 Human neutrophil elastase and Hylton alcohol (TLR3 agonist) were loaded into alpha-whey protein engineered EVs released by TNBC to produce dendritic cell (DC) vaccines that were effective in both mouse experiments and patient trials.143 Preclinical models and mouse experiments demonstrated that combination with anti-PD-L1 ligand-dependent corepressor (LCOR)-mRNA which was delivered to tumor cells via EVs to restore LCOR expression, could overcome TNBC resistance and eliminate cancer metastasis.144
EVs in combination with other treatments
Tian et al. found that the combined use of vesicles loaded with small interfering RNA targeting PD-L1 and radiotherapy notably inhibited tumor growth and improved mouse survival.145 EVs secreted by gamma-δT cells were found to effectively destroy nasopharyngeal carcinoma cells as well as boost T cell activity in the immunosuppressed condition. Moreover, it showed a synergistic effect with radiotherapy.146 Recently, Yu et al. found that exosomes inhibit docetaxel resistance in lung adenocarcinoma by chelating ubiquitin-specific protease 5 to destabilize upstream transcription factor 1 proteins.147
EVs as biomarkers
EVs can be applied as functional biomarkers for diagnosis and prognostic assessment.148 The transmembrane proteins CD147 and A33 contained in fecal EVs were dissimilar between colorectal tumor patients and healthy people. Thus, it is a novel non-invasive marker for colorectal cancer screening and prognosis.149 Liquid biopsy is a minimally invasive and repeatable method for tumor diagnosis and treatment.150 EVs have advantages such as easy access, stable structure, and rich information constracted with circulating tumor cells and circulating tumor DNA.151 Jafari et al. found that EVs can be utilized as a non-invasive tool of diagnosis for glioblastoma.152 The use of EVs can improve the sensitivity of detection of EGFR mutations in plasma from patients with lung cancer.153 Thanks to the characteristics and metabolic analysis of EVs, there are clear differences between patients with early gastric cancer and healthy control group, which provides a new idea for monitoring early gastric cancer.154 Other aspects of EVs as markers of diagnosis and therapy and prognostic screening for tumor are shown in Table 2.
Table 2.
Tumor-derived exosomes as diagnostic biomarkers
| Cancer | Source | Biomarker | Number | Reg. NO |
|---|---|---|---|---|
| Gastric | Plasma | Exosome | 80 | NCT01779583 |
| Cancer | Serum | MiR-15b-3p | Wei et al., 2020155 | |
| Hepatocellular | Serum | miRNA-21 | Lee et al., 2019156 | |
| Carcinoma | Urine | Phospholipid | ChiCTR2200065653 | |
| Colorectal | Serum | Has-circ-0004771 | Pan et al., 2019157 | |
| Cancer | Blood | EXOSCOL01 | 80 | NCT04394572 |
| Non-small cell | Plasma | CD63; EGFR; | Fan et al., 2020158 | |
| Lung Cancer | Blood | CD8+; EGFR | ChiCTR2200061697 | |
| Plasma | 150 | NCT05587114 | ||
| Serum | LncRNA | 1000 | NCT03830619 | |
| Small Cell | Plasma | Non-coding RNA | ChiCTR2200064246 | |
| Lung Cancer | ||||
| Prostatic | Urine | MiR-196a | Rodríguez et al., 2017159 | |
| Cancer | ||||
| Cholangiocarcinoma | Plasma | NcRNA | 80 | NCT03102268 |
| Ovarian Cancer | Plasma | CA125; CD24 | 90 | Im et al., 2014160 |
| Breast Cancer | Blood | CA153 | 90 | Wang et al., 2017161 |
| Plasma | 100 | NCT01344109 | ||
| Osteosarcoma | Blood | RNA | 50 | NCT03108677 |
| Clear cell | Urine | CD9+ | 74 | NCT04053855 |
| Renal Cancer | ||||
| Thyroid | Urine | Thyroglobulin | 30 | NCT05463107 |
| Cancer | Urine | Galectin −3 | NCT03488134 | |
| Saliva | HPV | NCT02147418 | ||
| Oropharyngeal Cancer |
Oncolytic virus therapy
Oncolytic virus treatment for cancer is an active area of research.162 Oncolytic virus therapy entails transporting the virus to tumor cells through vesicles where they replicate and spread resulting in the death of cancer cells.163 In a clinical trial of pediatric high-grade glioma therapy with herpes simplex virus type 1 (HSV-1) G207 was found to significantly improve median survival.164 Oncolytic reovirus selectively induces autophagy in KRAS-mutated colorectal cancer and further promotes apoptosis.165 The successful application of oncolytic viruses in other cancer species also boosted the study of the role of oncolytic viruses in TNBC. At present, the study has entered phase 1–2 clinical trials. The clinical trial conducted by Soliman et al. preliminarily confirmed that oncolytic virus therapy enhanced the efficacy of neoadjuvant chemotherapy in TNBC patients (ClinicalTrials.gov ID: NCT02779855).166 Two mumps virus (MuV) isolates, MUV-UA and MUV-UC, strongly killed a set of established human BC cell lines in vitro, significantly extending the survival of nude mice with MD-MB-231 tumor xenografts, and showed significant killing activity against BC patient-derived xenograft cell lines grown as 3D organoids, including from patients resistant to anthracycline and taxane chemotherapy.167
EVs in combination with other treatments
Cell-derived EVs carry proteins, RNAs, and lipids, which are involved in intercellular communication, regulation of inflammation, and promotion of vascular repair to urge wound healing in diabetic patients.168 In the mouse experimental model, vesicles secreted by cardiosphere-derived cells were found to mediate anti-inflammatory, immunomodulatory and anti-myocardial fibrosis, improve cardiac function, and inhibit arrhythmia.169 Experiments in rats have shown that EVs containing LncRNA TUG1 can be potential therapeutic targets for myocardial infarction.170 In terms of assessing endometrial receptivity, biopsies of EVs originating from the endometrium can avoid endometrial injury associated with traditional biopsy methods.171 Brain-derived EVs released by the central nervous system have been considered as diagnostic and prognostic biomarkers for Alzheimer’s disease.172 In animal studies of acute kidney injury, stem cell-derived EVs were found to improve renal function and inflammatory response status, and reduce apoptosis.173 Mesenchymal stromal cell-derived EVs reduce inflammation in lung diseases such as pulmonary fibrosis, asthma, and chronic obstructive pulmonary disease, regulate immunity, and improve organ function.174 EVs released by mesenchymal stem cells mediate signal transduction through paracrine pathways to regulate systemic inflammation and promote liver repair.175 Implantation of mRNA encoding extracellular matrix α1I collagen from human dermal fibroblasts EVs was found to reduce dermal wrinkles in mice.176 Experiments on mice show that timely treatment of waste in the body can delay aging, which provides new ideas for anti-aging in humans.177 Liu et al. demonstrated the participation of EVs in the humoral coordination of bacterial infection.178 EVs bind to circulating iron-containing proteins, regulate systemic iron metabolism, and prevent bacteria from obtaining iron, leading to bacteriostatic effects.178
Summary and outlook
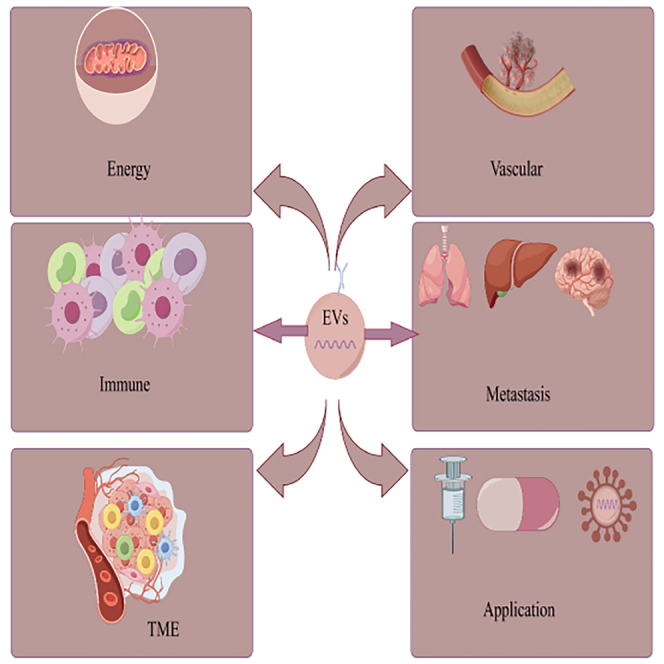
BC, especially TNBC, is a malignant cancer with high incidence and lacking prognosis. Due to the lack of related receptors, chemotherapy is the mainstay of therapy for TNBC. Although PARP inhibitors or immunotherapy can be used in some cases of TNBC, treatment needs are still far from being met. In recent years, preclinical and clinical studies have indicated promising prospects for use of EVs for treatment of various types of cancer and aging-related diseases. EVs were earlier thought to act as “garbage bags,” carrying metabolic waste and apoptotic components. However, there is growing evidence that EVs play a significant part in intercellular crosstalk through transfer of materials. TDEs play a critical part in inducing host immunosuppression. These EVs can transport antitumor drugs from tumor cells via their own membrane proteins, thereby interfering with immunotherapy and inducing chemotherapeutic resistance. EVs of cancer cells after genotoxic stress and epigenetic drug therapy or radiotherapy enhance antitumor effects. The special components of the EVs originating from tumor cells and the specific membrane proteins make them new biomarkers. Unlike conventional cancer testing, liquid biopsies are inexpensive, repeatable, minimally invasive, relatively acceptable to patients, and easier to apply for large-scale cancer screening. EVs (mainly exosomes) are also used for drug and gene delivery. For example, through genetic engineering modification, antibody coupling, and genetic or chemical modification, the characteristics of vesicle lipid or membrane-specific proteins can be used to selectively enter tumor cells, thus inhibiting the development of cancer and achieving anticancer effect. EVs containing immune checkpoint components can be used as new targets for immunotherapy. However, further research is required for in-depth characterization of the molecular mechanisms by which EVs regulate immunity, metabolism, and drug resistance. Moreover there is a need to further refine the methods for the extraction, purification, and storage of EVs; in addition, their potential effects on the host immune system also need to be confirmed. Understanding the pathological effects and mechanisms of EV-mediated TNBC will further enhance the role of immunotherapy and other precision therapies in the treatment of cancer (Figure 3).
Figure 3.
Role of EVs in the genesis and development of TNBC
The EVs from TNBC contained higher levels of transforming growth factor β 1. This factor can upregulate fibronectin on sinusoidal endothelial cells and promote adhesion of cancer cells to the liver microenvironment. EVs carrying bioactive substances participate in the change of TME, and play a bridge role in the process of tumor development. EVs shuttle through the immune system to regulate the proliferation and differentiation of various immune cells, which facilitates the escape of tumor cells from immune surveillance. TDEs inhibit mitochondrial oxidative phosphorylation, increase glycolytic carboxylation, and enhance TNBC cell proliferation. EVs act as "sponges" that absorb and regulate the expression of angiogenic factors, thereby promoting tumor angiogenesis.
Acknowledgments
Throughout the writing of this dissertation I have received a great deal of support and assistance. I would first like to thank my supervisor, Xi-He Zhao, whose expertise was invaluable in formulating the manuscript. Her insightful feedback pushed me to sharpen my thinking and brought my work to a higher level. I would like to acknowledge my teammates, Chun-Yan Yan and Meng-Lu Zhao, for their wonderful collaboration and patient support. Figures were created by Figdraw (www.figdraw.com). This study was funded by the National Natural Science Foundation of China (No.81802760, 81702402), Science and Technology Project of Liaoning (No.20170520027), and 345 talent plan project of Shengjing Hospital (X.-H.Z., Lei Liu).
Author contributions
Conception and design: X.-H.Z.; Administrative support: X.-H.Z.; Collection and assembly of data: All authors; Manuscript writing: All authors; Final approval of manuscript: All authors.
Declaration of interests
The authors declare no competing interests.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA. Cancer J. Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Paskeh M.D.A., Entezari M., Mirzaei S., Zabolian A., Saleki H., Naghdi M.J., Sabet S., Khoshbakht M.A., Hashemi M., Hushmandi K., et al. Emerging role of exosomes in cancer progression and tumor microenvo. J. Hematol. Oncol. 2022;15:83. doi: 10.1186/s13045-022-01305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu Y., Allegri G., Huskens J. Vesicle-based artificial cells: materials, construction methods and applications. Mater. Horiz. 2022;9:892–907. doi: 10.1039/d1mh01431e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu W., Hurley J., Roberts D., Chakrabortty S.K., Enderle D., Noerholm M., Breakefield X.O., Skog J.K. Exosome-based liquid biopsies in cancer: opportunities and challenges. Ann. Oncol. 2021;32:466–477. doi: 10.1016/j.annonc.2021.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Niel G., D’Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 6.Mathieu M., Martin-Jaular L., Lavieu G., Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 7.Yang L., Shi P., Zhao G., Xu J., Peng W., Zhang J., Zhang G., Wang X., Dong Z., Chen F., Cui H. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target. Ther. 2020;5:8. doi: 10.1038/s41392-020-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Biology, Function, and Biomedical Applications of Exosomes | Science. https://www.science.org/doi/10.1126/science.aau6977 [DOI] [PMC free article] [PubMed]
- 9.Bebelman M.P., Smit M.J., Pegtel D.M., Baglio S.R. Biogenesis and function of extracellular vesicles in cancer. Pharmacol. Ther. 2018;188:1–11. doi: 10.1016/j.pharmthera.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Lee T.H., D’Asti E., Magnus N., Al-Nedawi K., Meehan B., Rak J. Microvesicles as mediators of intercellular communication in cancer--the emerging science of cellular “debris.”. Semin. Immunopathol. 2011;33:455–467. doi: 10.1007/s00281-011-0250-3. [DOI] [PubMed] [Google Scholar]
- 11.Nagata S. Apoptosis and Clearance of Apoptotic Cells. Annu. Rev. Immunol. 2018;36:489–517. doi: 10.1146/annurev-immunol-042617-053010. [DOI] [PubMed] [Google Scholar]
- 12.Xu X., Lai Y., Hua Z.-C. Apoptosis and apoptotic body: disease message and therapeutic target potentials. Biosci. Rep. 2019;39 doi: 10.1042/BSR20180992. BSR20180992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Debbi L., Guo S., Safina D., Levenberg S. Boosting extracellular vesicle secretion. Biotechnol. Adv. 2022;59:107983. doi: 10.1016/j.biotechadv.2022.107983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minciacchi V.R., Freeman M.R., Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin. Cell Dev. Biol. 2015;40:41–51. doi: 10.1016/j.semcdb.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Z., Chen Y., Ma L., Chen Y., Liu J., Guo Y., Yu T., Zhang L., Zhu L., Shu Y. Role of exosomal non-coding RNAs from tumor cells and tumor-associated macrophages in the tumor microenvironment. Mol. Ther. 2022;30:3133–3154. doi: 10.1016/j.ymthe.2022.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Record M., Subra C., Silvente-Poirot S., Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem. Pharmacol. 2011;81:1171–1182. doi: 10.1016/j.bcp.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.St-Denis-Bissonnette F., Khoury R., Mediratta K., El-Sahli S., Wang L., Lavoie J.R. Applications of Extracellular Vesicles in Triple-Negative Breast Cancer. Cancers. 2022;14:451. doi: 10.3390/cancers14020451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tkach M., Théry C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell. 2016;164:1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 20.Wang H., Wei H., Wang J., Li L., Chen A., Li Z. MicroRNA-181d-5p-Containing Exosomes Derived from CAFs Promote EMT by Regulating CDX2/HOXA5 in Breast Cancer. Mol. Ther. Nucleic Acids. 2020;19:654–667. doi: 10.1016/j.omtn.2019.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antonyak M.A., Li B., Boroughs L.K., Johnson J.L., Druso J.E., Bryant K.L., Holowka D.A., Cerione R.A. Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc. Natl. Acad. Sci. USA. 2011;108:4852–4857. doi: 10.1073/pnas.1017667108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie Q.-H., Zheng J.-Q., Ding J.-Y., Wu Y.-F., Liu L., Yu Z.-L., Chen G. Exosome-Mediated Immunosuppression in Tumor Microenvironments. Cells. 2022;11:1946. doi: 10.3390/cells11121946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marar C., Starich B., Wirtz D. Extracellular vesicles in immunomodulation and tumor progression. Nat. Immunol. 2021;22:560–570. doi: 10.1038/s41590-021-00899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olejarz W., Dominiak A., Żołnierzak A., Kubiak-Tomaszewska G., Lorenc T. Tumor-Derived Exosomes in Immunosuppression and Immunotherapy. J. Immunol. Res. 2020;2020:6272498. doi: 10.1155/2020/6272498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang Q., Yang S., He G., Zheng H., Zhang S., Liu J., Wei S., Fan Q., Peng X., Li X., et al. Tumor-derived exosomes in the cancer immune microenvironment and cancer immunotherapy. Cancer Lett. 2022;548:215823. doi: 10.1016/j.canlet.2022.215823. [DOI] [PubMed] [Google Scholar]
- 26.Buzas E.I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 2023;23:236–250. doi: 10.1038/s41577-022-00763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Brien K., Breyne K., Ughetto S., Laurent L.C., Breakefield X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020;21:585–606. doi: 10.1038/s41580-020-0251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shlomovitz I., Erlich Z., Arad G., Edry-Botzer L., Zargarian S., Cohen H., Manko T., Ofir-Birin Y., Cooks T., Regev-Rudzki N., Gerlic M. Proteomic analysis of necroptotic extracellular vesicles. Cell Death Dis. 2021;12:1059. doi: 10.1038/s41419-021-04317-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Budden C.F., Gearing L.J., Kaiser R., Standke L., Hertzog P.J., Latz E. Inflammasome-induced extracellular vesicles harbour distinct RNA signatures and alter bystander macrophage responses. J. Extracell. Vesicles. 2021;10:e12127. doi: 10.1002/jev2.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tkach M., Thalmensi J., Timperi E., Gueguen P., Névo N., Grisard E., Sirven P., Cocozza F., Gouronnec A., Martin-Jaular L., et al. Extracellular vesicles from triple negative breast cancer promote pro-inflammatory macrophages associated with better clinical outcome. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2107394119. e2107394119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng Q., Dai Z., Smbatyan G., Epstein A.L., Lenz H.-J., Zhang Y. Eliciting anti-cancer immunity by genetically engineered multifunctional exosomes. Mol. Ther. 2022;30:3066–3077. doi: 10.1016/j.ymthe.2022.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo C., Xin H., Zhou Z., Hu Z., Sun R., Yao N., Sun Q., Borjigin U., Wu X., Fan J., et al. Tumor-derived exosomes induce immunosuppressive macrophages to foster intrahepatic cholangiocarcinoma progression. Hepatol. Baltim. Md. 2022;76:982–999. doi: 10.1002/hep.32387. [DOI] [PubMed] [Google Scholar]
- 33.Liu C., Yu S., Zinn K., Wang J., Zhang L., Jia Y., Kappes J.C., Barnes S., Kimberly R.P., Grizzle W.E., Zhang H.G. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J. Immunol. 2006;176:1375–1385. doi: 10.4049/jimmunol.176.3.1375. [DOI] [PubMed] [Google Scholar]
- 34.Liu C., Yu S., Zinn K., Wang J., Zhang L., Jia Y., Kappes J.C., Barnes S., Kimberly R.P., Grizzle W.E., Zhang H.G. Murine Mammary Carcinoma Exosomes Promote Tumor Growth by Suppression of NK Cell Function1. J. Immunol. 2006;176:1375–1385. doi: 10.4049/jimmunol.176.3.1375. [DOI] [PubMed] [Google Scholar]
- 35.Hosseini R., Sarvnaz H., Arabpour M., Ramshe S.M., Asef-Kabiri L., Yousefi H., Akbari M.E., Eskandari N. Cancer exosomes and natural killer cells dysfunction: biological roles, clinical significance and implications for immunotherapy. Mol. Cancer. 2022;21:15. doi: 10.1186/s12943-021-01492-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin S., Jung I., Jung D., Kim C.S., Kang S.-M., Ryu S., Choi S.-J., Noh S., Jeong J., Lee B.Y., et al. Novel antitumor therapeutic strategy using CD4+ T cell-derived extracellular vesicles. Biomaterials. 2022;289:121765. doi: 10.1016/j.biomaterials.2022.121765. [DOI] [PubMed] [Google Scholar]
- 37.Lim A.R., Rathmell W.K., Rathmell J.C. 2020. The Tumor Microenvironment as a Metabolic Barrier to Effector T Cells and Immunotherapy eLife. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lennicke C., Cochemé H.M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell. 2021;81:3691–3707. doi: 10.1016/j.molcel.2021.08.018. [DOI] [PubMed] [Google Scholar]
- 39.Li X., Wang S., Mu W., Barry J., Han A., Carpenter R.L., Jiang B.-H., Peiper S.C., Mahoney M.G., Aplin A.E., et al. Reactive oxygen species reprogram macrophages to suppress antitumor immune response through the exosomal miR-155-5p/PD-L1 pathway. J. Exp. Clin. Cancer Res. 2022;41:41. doi: 10.1186/s13046-022-02244-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dou D., Ren X., Han M., Xu X., Ge X., Gu Y., Wang X. Cancer-Associated Fibroblasts-Derived Exosomes Suppress Immune Cell Function in Breast Cancer via the miR-92/PD-L1 Pathway. Front. Immunol. 2020;11:2026. doi: 10.3389/fimmu.2020.02026. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Fan J., To K.K.W., Chen Z.-S., Fu L. ABC transporters affects tumor immune microenvironment to regulate cancer immunotherapy and multidrug resistance. Drug Resist. Updat. 2023;66:100905. doi: 10.1016/j.drup.2022.100905. [DOI] [PubMed] [Google Scholar]
- 42.Dong H., Wang M., Li Q. Exosomal miR-4488 and miR-1273g-5p inhibit the epithelial-mesenchymal transition of transforming growth factor β2-mediated retinal pigment epithelial cells by targeting ATP-binding cassette A4. Bioengineered. 2021;12:9693–9706. doi: 10.1080/21655979.2021.1987068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee C.-H., Bae J.-H., Choe E.-J., Park J.-M., Park S.-S., Cho H.J., Song B.-J., Baek M.-C. Macitentan improves antitumor immune responses by inhibiting the secretion of tumor-derived extracellular vesicle PD-L1. Theranostics. 2022;12:1971–1987. doi: 10.7150/thno.68864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warburg O. On the Origin of Cancer Cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 45.Gong J., Luk F., Jaiswal R., George A.M., Grau G.E.R., Bebawy M. Microparticle drug sequestration provides a parallel pathway in the acquisition of cancer drug resistance. Eur. J. Pharmacol. 2013;721:116–125. doi: 10.1016/j.ejphar.2013.09.044. [DOI] [PubMed] [Google Scholar]
- 46.Fong M.Y., Zhou W., Liu L., Alontaga A.Y., Chandra M., Ashby J., Chow A., O’Connor S.T.F., Li S., Chin A.R., et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 2015;17:183–194. doi: 10.1038/ncb3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morrissey S.M., Zhang F., Ding C., Montoya-Durango D.E., Hu X., Yang C., Wang Z., Yuan F., Fox M., Zhang H.g., et al. Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metab. 2021;33:2040–2058.e10. doi: 10.1016/j.cmet.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y., Zhao Z., Liu W., Li X. SNHG3 Functions as miRNA Sponge to Promote Breast Cancer Cells Growth Through the Metabolic Reprogramming. Appl. Biochem. Biotechnol. 2020;191:1084–1099. doi: 10.1007/s12010-020-03244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buckley C.M., Pots H., Gueho A., Vines J.H., Munn C.J., Phillips B.A., Gilsbach B., Traynor D., Nikolaev A., Soldati T., et al. Coordinated Ras and Rac Activity Shapes Macropinocytic Cups and Enables Phagocytosis of Geometrically Diverse Bacteria. Curr. Biol. 2020;30:2912–2926.e5. doi: 10.1016/j.cub.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Junemann A., Filić V., Winterhoff M., Nordholz B., Litschko C., Schwellenbach H., Stephan T., Weber I., Faix J. A Diaphanous-related formin links Ras signaling directly to actin assembly in macropinocytosis and phagocytosis. Proc. Natl. Acad. Sci. USA. 2016;113:E7464–E7473. doi: 10.1073/pnas.1611024113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Su H., Yang F., Fu R., Li X., French R., Mose E., Pu X., Trinh B., Kumar A., Liu J., et al. Cancer cells escape autophagy inhibition via NRF2-induced macropinocytosis. Cancer Cell. 2021;39:678–693.e11. doi: 10.1016/j.ccell.2021.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mondal G., Debnath J. NRF2 activates macropinocytosis upon autophagy inhibition Cancer Cell. Cancer Cell. 2021;39:596–598. doi: 10.1016/j.ccell.2021.03.011. [DOI] [PubMed] [Google Scholar]
- 53.Tu H., Wang Z., Yuan Y., Miao X., Li D., Guo H., Yang Y., Cai H. The PripA-TbcrA complex-centered Rab GAP cascade facilitates macropinosome maturation in Dictyostelium. Nat. Commun. 2022;13:1787. doi: 10.1038/s41467-022-29503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Svensson K.J., Kucharzewska P., Christianson H.C., Sköld S., Löfstedt T., Johansson M.C., Mörgelin M., Bengzon J., Ruf W., Belting M. Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2-mediated heparin-binding EGF signaling in endothelial cells. Proc. Natl. Acad. Sci. USA. 2011;108:13147–13152. doi: 10.1073/pnas.1104261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Azmi A.S., Bao B., Sarkar F.H. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis. Rev. 2013;32:623–642. doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duan S., Nordmeier S., Byrnes A.E., Buxton I.L.O. Extracellular Vesicle-Mediated Purinergic Signaling Contributes to Host Microenvironment Plasticity and Metastasis in Triple Negative Breast Cancer. Int. J. Mol. Sci. 2021;22:E597. doi: 10.3390/ijms22020597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharghi-Namini S., Tan E., Ong L.-L.S., Ge R., Asada H.H. Dll4-containing exosomes induce capillary sprout retraction in a 3D microenvironment. Sci. Rep. 2014;4:4031. doi: 10.1038/srep04031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Behera J., Kumar A., Voor M.J., Tyagi N. Exosomal lncRNA-H19 promotes osteogenesis and angiogenesis through mediating Angpt1/Tie2-NO signaling in CBS-heterozygous mice. Theranostics. 2021;11:7715–7734. doi: 10.7150/thno.58410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Z., Li Y., Li Y., Ren K., Li X., Han X., Wang J. Long non-coding RNA H19 promotes the proliferation and invasion of breast cancer through upregulating DNMT1 expression by sponging miR-152. J. Biochem. Mol. Toxicol. 2017;31:e21933. doi: 10.1002/jbt.21933. [DOI] [PubMed] [Google Scholar]
- 60.Han B., Zhang H., Tian R., Liu H., Wang Z., Wang Z., Tian J., Cui Y., Ren S., Zuo X., et al. Exosomal EPHA2 derived from highly metastatic breast cancer cells promotes angiogenesis by activating the AMPK signaling pathway through Ephrin A1-EPHA2 forward signaling. Theranostics. 2022;12:4127–4146. doi: 10.7150/thno.72404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ribatti D., Nico B., Ruggieri S., Tamma R., Simone G., Mangia A. Angiogenesis and Antiangiogenesis in Triple-Negative Breast cancer. Transl. Oncol. 2016;9:453–457. doi: 10.1016/j.tranon.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ayoub N.M., Jaradat S.K., Al-Shami K.M., Alkhalifa A.E. Targeting Angiogenesis in Breast Cancer: Current Evidence and Future Perspectives of Novel Anti-Angiogenic Approaches. Front. Pharmacol. 2022;13:838133. doi: 10.3389/fphar.2022.838133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lopatina T., Grange C., Cavallari C., Navarro-Tableros V., Lombardo G., Rosso A., Cedrino M., Pomatto M.A.C., Koni M., Veneziano F., et al. Targeting IL-3Rα on tumor-derived endothelial cells blunts metastatic spread of triple-negative breast cancer via extracellular vesicle reprogramming. Oncogenesis. 2020;9:1–14. doi: 10.1038/s41389-020-00274-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zomer A., Maynard C., Verweij F.J., Kamermans A., Schäfer R., Beerling E., Schiffelers R.M., de Wit E., Berenguer J., Ellenbroek S.I.J., et al. In Vivo Imaging Reveals Extracellular Vesicle-Mediated Phenocopying of Metastatic Behavior. Cell. 2015;161:1046–1057. doi: 10.1016/j.cell.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Senigagliesi B., Samperi G., Cefarin N., Gneo L., Petrosino S., Apollonio M., Caponnetto F., Sgarra R., Collavin L., Cesselli D., et al. Triple negative breast cancer-derived small extracellular vesicles as modulator of biomechanics in target cells. Nanomedicine. 2022;44:102582. doi: 10.1016/j.nano.2022.102582. [DOI] [PubMed] [Google Scholar]
- 66.Zhan Y., Du J., Min Z., Ma L., Zhang W., Zhu W., Liu Y. Carcinoma-associated fibroblasts derived exosomes modulate breast cancer cell stemness through exonic circHIF1A by miR-580-5p in hypoxic stress. Cell Death Discov. 2021;7:141. doi: 10.1038/s41420-021-00506-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen B., Sang Y., Song X., Zhang D., Wang L., Zhao W., Liang Y., Zhang N., Yang Q. Exosomal miR-500a-5p derived from cancer-associated fibroblasts promotes breast cancer cell proliferation and metastasis through targeting USP28. Theranostics. 2021;11:3932–3947. doi: 10.7150/thno.53412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baroni S., Romero-Cordoba S., Plantamura I., Dugo M., D’Ippolito E., Cataldo A., Cosentino G., Angeloni V., Rossini A., Daidone M.G., Iorio M.V. Exosome-mediated delivery of miR-9 induces cancer-associated fibroblast-like properties in human breast fibroblasts. Cell Death Dis. 2016;7:e2312. doi: 10.1038/cddis.2016.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma L., Young J., Prabhala H., Pan E., Mestdagh P., Muth D., Teruya-Feldstein J., Reinhardt F., Onder T.T., Valastyan S., et al. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat. Cell Biol. 2010;12:247–256. doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li K., Liu T., Chen J., Ni H., Li W. Survivin in breast cancer-derived exosomes activates fibroblasts by up-regulating SOD1, whose feedback promotes cancer proliferation and metastasis. J. Biol. Chem. 2020;295:13737–13752. doi: 10.1074/jbc.RA120.013805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang S.-J., Wang D.-D., Zhong S.-L., Chen W.-Q., Wang F.-L., Zhang J., Xu W.-X., Xu D., Zhang Q., Li J., et al. Tumor-derived exosomal circPSMA1 facilitates the tumorigenesis, metastasis, and migration in triple-negative breast cancer (TNBC) through miR-637/Akt1/β-catenin (cyclin D1) axis. Cell Death Dis. 2021;12:420. doi: 10.1038/s41419-021-03680-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singh R., Pochampally R., Watabe K., Lu Z., Mo Y.-Y. Exosome-mediated transfer of miR-10b promotes cell invasion in breast cancer. Mol. Cancer. 2014;13:256. doi: 10.1186/1476-4598-13-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tian X.-P., Wang C.-Y., Jin X.-H., Li M., Wang F.-W., Huang W.-J., Yun J.-P., Xu R.-H., Cai Q.-Q., Xie D. Acidic Microenvironment Up-Regulates Exosomal miR-21 and miR-10b in Early-Stage Hepatocellular Carcinoma to Promote Cancer Cell Proliferation and Metastasis. Theranostics. 2019;9:1965–1979. doi: 10.7150/thno.30958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kang S.Y., Lee E.J., Byun J.W., Han D., Choi Y., Hwang D.W., Lee D.S. Extracellular Vesicles Induce an Aggressive Phenotype in Luminal Breast Cancer Cells Via PKM2 Phosphorylation. Front. Oncol. 2021;11:785450. doi: 10.3389/fonc.2021.785450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dioufa N., Clark A.M., Ma B., Beckwitt C.H., Wells A. Bi-directional exosome-driven intercommunication between the hepatic niche and cancer cells. Mol. Cancer. 2017;16:172. doi: 10.1186/s12943-017-0740-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang H., Ou J., Jian Z., Ou Y. miR-186 modulates hepatocellular carcinoma cell proliferation and mobility via targeting MCRS1-mediated Wnt/β-catenin signaling. J. Cell. Physiol. 2019;234:23135–23145. doi: 10.1002/jcp.28878. [DOI] [PubMed] [Google Scholar]
- 77.Plantamura I., Cataldo A., Cosentino G., Iorio M.V. miR-205 in Breast Cancer: State of the Art. Int. J. Mol. Sci. 2020;22:27. doi: 10.3390/ijms22010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Karagiannis G.S., Pastoriza J.M., Wang Y., Harney A.S., Entenberg D., Pignatelli J., Sharma V.P., Xue E.A., Cheng E., D’Alfonso T.M., et al. Neoadjuvant chemotherapy induces breast cancer metastasis through a TMEM-mediated mechanism. Sci. Transl. Med. 2017;9:eaan0026. doi: 10.1126/scitranslmed.aan0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wills C.A., Liu X., Chen L., Zhao Y., Dower C.M., Sundstrom J., Wang H.-G. Chemotherapy-Induced Upregulation of Small Extracellular Vesicle-Associated PTX3 Accelerates Breast Cancer Metastasis. Cancer Res. 2021;81:452–463. doi: 10.1158/0008-5472.CAN-20-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu P., Wang Z., Ou X., Wu P., Zhang Y., Wu S., Xiao X., Li Y., Ye F., Tang H. The FUS/circEZH2/KLF5/feedback loop contributes to CXCR4-induced liver metastasis of breast cancer by enhancing epithelial-mesenchymal transition. Mol. Cancer. 2022;21:198. doi: 10.1186/s12943-022-01653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Das K., Paul S., Singh A., Ghosh A., Roy A., Ansari S.A., Prasad R., Mukherjee A., Sen P. Triple-negative breast cancer-derived microvesicles transfer microRNA221 to the recipient cells and thereby promote epithelial-to-mesenchymal transition. J. Biol. Chem. 2019;294:13681–13696. doi: 10.1074/jbc.RA119.008619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qi M., Xia Y., Wu Y., Zhang Z., Wang X., Lu L., Dai C., Song Y., Xu K., Ji W., Zhan L. Lin28B-high breast cancer cells promote immune suppression in the lung pre-metastatic niche via exosomes and support cancer progression. Nat. Commun. 2022;13:897. doi: 10.1038/s41467-022-28438-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Piskounova E., Polytarchou C., Thornton J.E., LaPierre R.J., Pothoulakis C., Hagan J.P., Iliopoulos D., Gregory R.I. Lin28A and Lin28B inhibit let-7 microRNA biogenesis by distinct mechanisms. Cell. 2011;147:1066–1079. doi: 10.1016/j.cell.2011.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sweeney M.D., Zhao Z., Montagne A., Nelson A.R., Zlokovic B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019;99:21–78. doi: 10.1152/physrev.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brightman M.W., Reese T.S. Junctions between intimately apposed cell membranes in the vertebrate brain. J. Cell Biol. 1969;40:648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morad G., Carman C.V., Hagedorn E.J., Perlin J.R., Zon L.I., Mustafaoglu N., Park T.-E., Ingber D.E., Daisy C.C., Moses M.A. Tumor-Derived Extracellular Vesicles Breach the Intact Blood-Brain Barrier via Transcytosis. ACS Nano. 2019;13:13853–13865. doi: 10.1021/acsnano.9b04397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arnold J., Schattschneider J., Blechner C., Krisp C., Schlüter H., Schweizer M., Nalaskowski M., Oliveira-Ferrer L., Windhorst S. Tubulin Tyrosine Ligase Like 4 (TTLL4) overexpression in breast cancer cells is associated with brain metastasis and alters exosome biogenesis. J. Exp. Clin. Cancer Res. 2020;39:205. doi: 10.1186/s13046-020-01712-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rodrigues G., Hoshino A., Kenific C.M., Matei I.R., Steiner L., Freitas D., Kim H.S., Oxley P.R., Scandariato I., Casanova-Salas I., et al. Tumour exosomal CEMIP protein promotes cancer cell colonization in brain metastasis. Nat. Cell Biol. 2019;21:1403–1412. doi: 10.1038/s41556-019-0404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Agarwal G., Nanda G., Lal P., Mishra A., Agarwal A., Agrawal V., Krishnani N. Outcomes of Triple-Negative Breast Cancers (TNBC) Compared with Non-TNBC: Does the Survival Vary for All Stages? World J. Surg. 2016;40:1362–1372. doi: 10.1007/s00268-016-3422-4. [DOI] [PubMed] [Google Scholar]
- 90.Wyld L., Gutteridge E., Pinder S.E., James J.J., Chan S.Y., Cheung K.L., Robertson J.F.R., Evans A.J. Prognostic factors for patients with hepatic metastases from breast cancer. Br. J. Cancer. 2003;89:284–290. doi: 10.1038/sj.bjc.6601038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alix-Panabières C., Riethdorf S., Pantel K. Circulating tumor cells and bone marrow micrometastasis. Clin. Cancer Res. 2008;14:5013–5021. doi: 10.1158/1078-0432.CCR-07-5125. [DOI] [PubMed] [Google Scholar]
- 92.Joosse S.A., Gorges T.M., Pantel K. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol. Med. 2015;7:1–11. doi: 10.15252/emmm.201303698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lin Q., Chen X., Meng F., Ogawa K., Li M., Song R., Zhang S., Zhang Z., Kong X., Xu Q., et al. ASPH-notch Axis guided Exosomal delivery of Prometastatic Secretome renders breast Cancer multi-organ metastasis. Mol. Cancer. 2019;18:156. doi: 10.1186/s12943-019-1077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim J., Lee C., Kim I., Ro J., Kim J., Min Y., Park J., Sunkara V., Park Y.-S., Michael I., et al. Three-Dimensional Human Liver-Chip Emulating Premetastatic Niche Formation by Breast Cancer-Derived Extracellular Vesicles. ACS Nano. 2020;14:14971–14988. doi: 10.1021/acsnano.0c04778. [DOI] [PubMed] [Google Scholar]
- 95.Wei K., Ma Z., Yang F., Zhao X., Jiang W., Pan C., Li Z., Pan X., He Z., Xu J., et al. M2 macrophage-derived exosomes promote lung adenocarcinoma progression by delivering miR-942. Cancer Lett. 2022;526:205–216. doi: 10.1016/j.canlet.2021.10.045. [DOI] [PubMed] [Google Scholar]
- 96.Jiang C., Zhang N., Hu X., Wang H. Tumor-associated exosomes promote lung cancer metastasis through multiple mechanisms. Mol. Cancer. 2021;20:117. doi: 10.1186/s12943-021-01411-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sun H., Meng Q., Shi C., Yang H., Li X., Wu S., Familiari G., Relucenti M., Aschner M., Wang X., Chen R. Hypoxia-Inducible Exosomes Facilitate Liver-Tropic Premetastatic Niche in Colorectal Cancer. Hepatol. Baltim Md. 2021;74:2633–2651. doi: 10.1002/hep.32009. [DOI] [PubMed] [Google Scholar]
- 98.Zhao S., Mi Y., Guan B., Zheng B., Wei P., Gu Y., Zhang Z., Cai S., Xu Y., Li X., et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J. Hematol. Oncol. 2020;13:156–219. doi: 10.1186/s13045-020-00991-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang C., Dou R., Wei C., Liu K., Shi D., Zhang C., Liu Q., Wang S., Xiong B. Tumor-derived exosomal microRNA-106b-5p activates EMT-cancer cell and M2-subtype TAM interaction to facilitate CRC metastasis. Mol. Ther. 2021;29:2088–2107. doi: 10.1016/j.ymthe.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Magoling BJA, Wu AY-T, Chen Y-J, Wong WW-T, Chuo ST-Y, Huang H-C, Sung Y-C, Hsieh HT, Huang P, Lee K-Z, et al. Membrane protein modification modulates big and small extracellular vesicle biodistribution and tumorigenic potential in breast cancers in vivo Adv. Mater. n/a, 2208966. [DOI] [PubMed]
- 101.Ramos E.K., Tsai C.-F., Jia Y., Cao Y., Manu M., Taftaf R., Hoffmann A.D., El-Shennawy L., Gritsenko M.A., Adorno-Cruz V., et al. Machine learning-assisted elucidation of CD81–CD44 interactions in promoting cancer stemness and extracellular vesicle integrity. eLife. 2022;11:e82669. doi: 10.7554/eLife.82669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Koni M., Lopatina T., Grange C., Sarcinella A., Cedrino M., Bruno S., Buffolo F., Femminò S., Camussi G., Brizzi M.F. Circulating extracellular vesicles derived from tumor endothelial cells hijack the local and systemic anti-tumor immune response: Role of mTOR/G-CSF pathway. Pharmacol. Res. 2023;195:106871. doi: 10.1016/j.phrs.2023.106871. [DOI] [PubMed] [Google Scholar]
- 103.Jaiswal R., Raymond Grau G.E., Bebawy M. Cellular communication via microparticles: role in transfer of multidrug resistance in cancer. Future Oncol. 2014;10:655–669. doi: 10.2217/fon.13.230. [DOI] [PubMed] [Google Scholar]
- 104.Chen C., Yu H., Han F., Lai X., Ye K., Lei S., Mai M., Lai M., Zhang H. Tumor-suppressive circRHOBTB3 is excreted out of cells via exosome to sustain colorectal cancer cell fitness. Mol. Cancer. 2022;21:46. doi: 10.1186/s12943-022-01511-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lehuédé C., Li X., Dauvillier S., Vaysse C., Franchet C., Clement E., Esteve D., Longué M., Chaltiel L., Le Gonidec S., et al. Adipocytes promote breast cancer resistance to chemotherapy, a process amplified by obesity: role of the major vault protein (MVP) Breast Cancer Res. 2019;21:7. doi: 10.1186/s13058-018-1088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fletcher J.I., Williams R.T., Henderson M.J., Norris M.D., Haber M. ABC transporters as mediators of drug resistance and contributors to cancer cell biology. Drug Resist. Updat. 2016;26:1–9. doi: 10.1016/j.drup.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 107.Nedeljković M., Damjanović A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer—How We Can Rise to the Challenge. Cells. 2019;8:957. doi: 10.3390/cells8090957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yalcin-Ozkat G. Molecular Modeling Strategies of Cancer Multidrug Resistance. Drug Resist. Updat. 2021;59:100789. doi: 10.1016/j.drup.2021.100789. [DOI] [PubMed] [Google Scholar]
- 109.Goler-Baron V., Sladkevich I., Assaraf Y.G. Inhibition of the PI3K-Akt signaling pathway disrupts ABCG2-rich extracellular vesicles and overcomes multidrug resistance in breast cancer cells. Biochem. Pharmacol. 2012;83:1340–1348. doi: 10.1016/j.bcp.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 110.Xia M., Zu X., Chen Z., Wen G., Zhong J. Noncoding RNAs in triple negative breast cancer: Mechanisms for chemoresistance. Cancer Lett. 2021;523:100–110. doi: 10.1016/j.canlet.2021.09.038. [DOI] [PubMed] [Google Scholar]
- 111.Li C., Teixeira A.F., Zhu H.-J., Ten Dijke P. Cancer associated-fibroblast-derived exosomes in cancer progression. Mol. Cancer. 2021;20:154. doi: 10.1186/s12943-021-01463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huang M., Peng X., Yang L., Yang S., Li X., Tang S., Li B., Jin H., Wu B., Liu J., Li H. Non-coding RNA derived from extracellular vesicles in cancer immune escape: Biological functions and potential clinical applications. Cancer Lett. 2021;501:234–246. doi: 10.1016/j.canlet.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 113.Mehlich D., Łomiak M., Sobiborowicz A., Mazan A., Dymerska D., Szewczyk Ł.M., Mehlich A., Borowiec A., Prełowska M.K., Gorczyński A., et al. MLK4 regulates DNA damage response and promotes triple-negative breast cancer chemoresistance. Cell Death Dis. 2021;12:1111. doi: 10.1038/s41419-021-04405-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mao M., Jia Y., Chen Y., Yang J., Xu L., Zhang X., Zhou J., Li Z., Chen C., Ju S., Wang L. HJURP regulates cell proliferation and chemo-resistance via YAP1/NDRG1 transcriptional axis in triple-negative breast cancer. Cell Death Dis. 2022;13:396. doi: 10.1038/s41419-022-04833-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lin Y.-T., Lin J., Liu Y.-E., Chen Y.-C., Liu S.-T., Hsu K.-W., Chen D.-R., Wu H.-T. USP7 Induces Chemoresistance in Triple-Negative Breast Cancer via Deubiquitination and Stabilization of ABCB1. Cells. 2022;11:3294. doi: 10.3390/cells11203294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kale J., Kutuk O., Brito G.C., Andrews T.S., Leber B., Letai A., Andrews D.W. Phosphorylation switches Bax from promoting to inhibiting apoptosis thereby increasing drug resistance. EMBO Rep. 2018;19:e45235. doi: 10.15252/embr.201745235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen J., Yang J., Wang W., Guo D., Zhang C., Wang S., Lu X., Huang X., Wang P., Zhang G., et al. Tumor extracellular vesicles mediate anti-PD-L1 therapy resistance by decoying anti-PD-L1. Cell. Mol. Immunol. 2022;19:1290–1301. doi: 10.1038/s41423-022-00926-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Griffiths G., Gruenberg J., Marsh M., Wohlmann J., Jones A.T., Parton R.G. Nanoparticle entry into cells; the cell biology weak link. Adv. Drug Deliv. Rev. 2022;188:114403. doi: 10.1016/j.addr.2022.114403. [DOI] [PubMed] [Google Scholar]
- 119.Fu S., Li G., Zang W., Zhou X., Shi K., Zhai Y. Pure drug nano-assemblies: A facile carrier-free nanoplatform for efficient cancer therapy. Acta Pharm. Sin. B. 2022;12:92–106. doi: 10.1016/j.apsb.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ullah M., Kodam S.P., Mu Q., Akbar A. Microbubbles versus Extracellular Vesicles as Therapeutic Cargo for Targeting Drug Delivery. ACS Nano. 2021;15:3612–3620. doi: 10.1021/acsnano.0c10689. [DOI] [PubMed] [Google Scholar]
- 121.Wang X., Chen T., Li C., Li W., Zhou X., Li Y., Luo D., Zhang N., Chen B., Wang L., et al. CircRNA-CREIT inhibits stress granule assembly and overcomes doxorubicin resistance in TNBC by destabilizing PKR. J. Hematol. Oncol. 2022;15:122. doi: 10.1186/s13045-022-01345-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tarantino P., Carmagnani Pestana R., Corti C., Modi S., Bardia A., Tolaney S.M., Cortes J., Soria J.-C., Curigliano G. Antibody–drug conjugates: Smart chemotherapy delivery across tumor histologies. CA. Cancer J. Clin. 2022;72:165–182. doi: 10.3322/caac.21705. [DOI] [PubMed] [Google Scholar]
- 123.Chau C.H., Steeg P.S., Figg W.D. Antibody-drug conjugates for cancer. Lancet Lond. Engl. 2019;394:793–804. doi: 10.1016/S0140-6736(19)31774-X. [DOI] [PubMed] [Google Scholar]
- 124.Jin Y., Schladetsch M.A., Huang X., Balunas M.J., Wiemer A.J. Stepping forward in antibody-drug conjugate development. Pharmacol. Ther. 2022;229:107917. doi: 10.1016/j.pharmthera.2021.107917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Colombo R., Rich J.R. The therapeutic window of antibody drug conjugates: A dogma in need of revision. Cancer Cell. 2022;40:1255–1263. doi: 10.1016/j.ccell.2022.09.016. [DOI] [PubMed] [Google Scholar]
- 126.Barok M., Puhka M., Yazdi N., Joensuu H. Extracellular vesicles as modifiers of antibody-drug conjugate efficacy. J. Extracell. Vesicles. 2021;10:e12070. doi: 10.1002/jev2.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Duro-Sánchez S., Nadal-Serrano M., Lalinde-Gutiérrez M., Arenas E.J., Bernadó Morales C., Morancho B., Escorihuela M., Pérez-Ramos S., Escrivá-de-Romaní S., Gandullo-Sánchez L., et al. Therapy-Induced Senescence Enhances the Efficacy of HER2-Targeted Antibody-Drug Conjugates in Breast Cancer. Cancer Res. 2022;82:4670–4679. doi: 10.1158/0008-5472.CAN-22-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu X., Deng J., Yuan Y., Chen W., Sun W., Wang Y., Huang H., Liang B., Ming T., Wen J., et al. Advances in Trop2-targeted therapy: Novel agents and opportunities beyond breast cancer. Pharmacol. Ther. 2022;239:108296. doi: 10.1016/j.pharmthera.2022.108296. [DOI] [PubMed] [Google Scholar]
- 129.Large D.E., Abdelmessih R.G., Fink E.A., Auguste D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021;176:113851. doi: 10.1016/j.addr.2021.113851. [DOI] [PubMed] [Google Scholar]
- 130.Keenan T.E., Tolaney S.M. Role of Immunotherapy in Triple-Negative Breast Cancer. J. Natl. Compr. Canc. Netw. 2020;18:479–489. doi: 10.6004/jnccn.2020.7554. [DOI] [PubMed] [Google Scholar]
- 131.Zhang N., Shu G., Qiao E., Xu X., Shen L., Lu C., Chen W., Fang S., Yang Y., Song J., et al. DNA-Functionalized Liposomes In Vivo Fusion for NIR-II/MRI Guided Pretargeted Ferroptosis Therapy of Metastatic Breast Cancer. ACS Appl. Mater. Inter. 2022;14:20603–20615. doi: 10.1021/acsami.2c01105. [DOI] [PubMed] [Google Scholar]
- 132.Schneeweiss A., Michel L.L., Möbus V., Tesch H., Klare P., Hahnen E., Denkert C., Kast K., Pohl-Rescigno E., Hanusch C., et al. Survival analysis of the randomised phase III GeparOcto trial comparing neoadjuvant chemotherapy of intense dose-dense epirubicin, paclitaxel, cyclophosphamide versus weekly paclitaxel, liposomal doxorubicin (plus carboplatin in triple-negative breast cancer) for patients with high-risk early breast cancer. Eur. J. Cancer. 2022;160:100–111. doi: 10.1016/j.ejca.2021.10.011. [DOI] [PubMed] [Google Scholar]
- 133.Zhang C., Shang Y., Chen X., Midgley A.C., Wang Z., Zhu D., Wu J., Chen P., Wu L., Wang X., et al. Supramolecular Nanofibers Containing Arginine-Glycine-Aspartate (RGD) Peptides Boost Therapeutic Efficacy of Extracellular Vesicles in Kidney Repair. ACS Nano. 2020;14:12133–12147. doi: 10.1021/acsnano.0c05681. [DOI] [PubMed] [Google Scholar]
- 134.Richter M., Vader P., Fuhrmann G. Approaches to surface engineering of extracellular vesicles. Adv. Drug Deliv. Rev. 2021;173:416–426. doi: 10.1016/j.addr.2021.03.020. [DOI] [PubMed] [Google Scholar]
- 135.Wei K., Zhang H., Yang S., Cui Y., Zhang B., Liu J., Tang L., Tan Y., Liu S., Chen S., et al. Chemo-drugs in cell microparticles reset antitumor activity of macrophages by activating lysosomal P450 and nuclear hnRNPA2B1. Signal Transduct. Target. Ther. 2023;8:22. doi: 10.1038/s41392-022-01212-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Betzer O., Barnoy E., Sadan T., Elbaz I., Braverman C., Liu Z., Popovtzer R. Advances in imaging strategies for in vivo tracking of exosomes. WIREs Nanomedicine Nanobiotechnology. 2020;12:e1594. doi: 10.1002/wnan.1594. [DOI] [PubMed] [Google Scholar]
- 137.Silva A.M., Lázaro-Ibáñez E., Gunnarsson A., Dhande A., Daaboul G., Peacock B., Osteikoetxea X., Salmond N., Friis K.P., Shatnyeva O., Dekker N. Quantification of protein cargo loading into engineered extracellular vesicles at single-vesicle and single-molecule resolution. J. Extracell. Vesicles. 2021;10:e12130. doi: 10.1002/jev2.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Osteikoetxea X., Silva A., Lázaro-Ibáñez E., Salmond N., Shatnyeva O., Stein J., Schick J., Wren S., Lindgren J., Firth M., et al. Engineered Cas9 extracellular vesicles as a novel gene editing tool. J. Extracell. Vesicles. 2022;11:e12225. doi: 10.1002/jev2.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yao X., Lyu P., Yoo K., Yadav M.K., Singh R., Atala A., Lu B. Engineered extracellular vesicles as versatile ribonucleoprotein delivery vehicles for efficient and safe CRISPR genome editing. J. Extracell. Vesicles. 2021;10:e12076. doi: 10.1002/jev2.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Eygeris Y., Gupta M., Kim J., Sahay G. Chemistry of Lipid Nanoparticles for RNA Delivery. Acc. Chem. Res. 2022;55:2–12. doi: 10.1021/acs.accounts.1c00544. [DOI] [PubMed] [Google Scholar]
- 141.Qiu M., Li Y., Bloomer H., Xu Q. Developing Biodegradable Lipid Nanoparticles for Intracellular mRNA Delivery and Genome Editing. Acc. Chem. Res. 2021;54:4001–4011. doi: 10.1021/acs.accounts.1c00500. [DOI] [PubMed] [Google Scholar]
- 142.Wang C., Zhang Y., Dong Y. Lipid Nanoparticle-mRNA Formulations for Therapeutic Applications. Acc. Chem. Res. 2021;54:4283–4293. doi: 10.1021/acs.accounts.1c00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Huang L., Rong Y., Tang X., Yi K., Qi P., Hou J., Liu W., He Y., Gao X., Yuan C., Wang F. Engineered exosomes as an in situ DC-primed vaccine to boost antitumor immunity in breast cancer. Mol. Cancer. 2022;21:45. doi: 10.1186/s12943-022-01515-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pérez-Núñez I., Rozalén C., Palomeque J.Á., Sangrador I., Dalmau M., Comerma L., Hernández-Prat A., Casadevall D., Menendez S., Liu D.D., et al. LCOR mediates interferon-independent tumor immunogenicity and responsiveness to immune-checkpoint blockade in triple-negative breast cancer. Nat. Cancer. 2022;3:355–370. doi: 10.1038/s43018-022-00339-4. [DOI] [PubMed] [Google Scholar]
- 145.Tian T., Liang R., Erel-Akbaba G., Saad L., Obeid P.J., Gao J., Chiocca E.A., Weissleder R., Tannous B.A. Immune Checkpoint Inhibition in GBM Primed with Radiation by Engineered Extracellular Vesicles. ACS Nano. 2022;16:1940–1953. doi: 10.1021/acsnano.1c05505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang X., Zhang Y., Mu X., Tu C.R., Chung Y., Tsao S.W., Chan G.C.-F., Leung W.-H., Lau Y.L., Liu Y., Tu W. Exosomes derived from γδ-T cells synergize with radiotherapy and preserve antitumor activities against nasopharyngeal carcinoma in immunosuppressive microenvironment. J. Immunother. Cancer. 2022;10:e003832. doi: 10.1136/jitc-2021-003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yu Z., Tang H., Chen S., Xie Y., Shi L., Xia S., Jiang M., Li J., Chen D. Exosomal LOC85009 inhibits docetaxel resistance in lung adenocarcinoma through regulating ATG5-induced autophagy. Drug Resist. Updat. 2023;67:100915. doi: 10.1016/j.drup.2022.100915. [DOI] [PubMed] [Google Scholar]
- 148.KATOH M. Therapeutics targeting angiogenesis: Genetics and epigenetics, extracellular miRNAs and signaling networks (Review) Int. J. Mol. Med. 2013;32:763–767. doi: 10.3892/ijmm.2013.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang Z., Liu X., Yang X., Jiang Y., Li A., Cong J., Li Y., Xie Q., Xu C., Liu D. Identification of faecal extracellular vesicles as novel biomarkers for the non-invasive diagnosis and prognosis of colorectal cancer. J. Extracell. Vesicles. 2023;12:12300. doi: 10.1002/jev2.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cai X., Janku F., Zhan Q., Fan J.-B. Accessing Genetic Information with Liquid Biopsies. Trends Genet. 2015;31:564–575. doi: 10.1016/j.tig.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 151.Ye Q., Ling S., Zheng S., Xu X. Liquid biopsy in hepatocellular carcinoma: circulating tumor cells and circulating tumor DNA. Mol. Cancer. 2019;18:114. doi: 10.1186/s12943-019-1043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jafari D., Tiyuri A., Rezaei E., Moradi Y., Jafari R., Jokar Shoorijeh F., Barati M. Diagnostic accuracy of cerebrospinal fluid and serum-isolated extracellular vesicles for glioblastoma: a systematic review and meta-analysis. Expert Rev. Mol. Diagn. 2020;20:1075–1085. doi: 10.1080/14737159.2020.1844006. [DOI] [PubMed] [Google Scholar]
- 153.Krug A.K., Enderle D., Karlovich C., Priewasser T., Bentink S., Spiel A., Brinkmann K., Emenegger J., Grimm D.G., Castellanos-Rizaldos E., et al. Improved EGFR mutation detection using combined exosomal RNA and circulating tumor DNA in NSCLC patient plasma. Ann. Oncol. 2018;29:700–706. doi: 10.1093/annonc/mdx765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen H., Huang C., Wu Y., Sun N., Deng C. Exosome Metabolic Patterns on Aptamer-Coupled Polymorphic Carbon for Precise Detection of Early Gastric Cancer. ACS Nano. 2022;16:12952–12963. doi: 10.1021/acsnano.2c05355. [DOI] [PubMed] [Google Scholar]
- 155.Wei S., Peng L., Yang J., Sang H., Jin D., Li X., Chen M., Zhang W., Dang Y., Zhang G. Exosomal transfer of miR-15b-3p enhances tumorigenesis and malignant transformation through the DYNLT1/Caspase-3/Caspase-9 signaling pathway in gastric cancer. J. Exp. Clin. Cancer Res. 2020;39:32. doi: 10.1186/s13046-019-1511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lee Y.R., Kim G., Tak W.Y., Jang S.Y., Kweon Y.O., Park J.G., Lee H.W., Han Y.S., Chun J.M., Park S.Y., Hur K. Circulating exosomal noncoding RNAs as prognostic biomarkers in human hepatocellular carcinoma. Int. J. Cancer. 2019;144:1444–1452. doi: 10.1002/ijc.31931. [DOI] [PubMed] [Google Scholar]
- 157.Pan B., Qin J., Liu X., He B., Wang X., Pan Y., Sun H., Xu T., Xu M., Chen X., et al. Identification of Serum Exosomal hsa-circ-0004771 as a Novel Diagnostic Biomarker of Colorectal Cancer. Front. Genet. 2019;10:1096. doi: 10.3389/fgene.2019.01096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fan Y., Duan X., Zhao M., Wei X., Wu J., Chen W., Liu P., Cheng W., Cheng Q., Ding S. High-sensitive and multiplex biosensing assay of NSCLC-derived exosomes via different recognition sites based on SPRi array. Biosens. Bioelectron. 2020;154:112066. doi: 10.1016/j.bios.2020.112066. [DOI] [PubMed] [Google Scholar]
- 159.Rodríguez M., Bajo-Santos C., Hessvik N.P., Lorenz S., Fromm B., Berge V., Sandvig K., Linē A., Llorente A. Identification of non-invasive miRNAs biomarkers for prostate cancer by deep sequencing analysis of urinary exosomes. Mol. Cancer. 2017;16:156. doi: 10.1186/s12943-017-0726-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Im H., Shao H., Park Y.I., Peterson V.M., Castro C.M., Weissleder R., Lee H. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat. Biotechnol. 2014;32:490–495. doi: 10.1038/nbt.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang W., Xu X., Tian B., Wang Y., Du L., Sun T., Shi Y., Zhao X., Jing J. The diagnostic value of serum tumor markers CEA, CA19-9, CA125, CA15-3, and TPS in metastatic breast cancer. Clin. Chim. Acta. 2017;470:51–55. doi: 10.1016/j.cca.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 162.Hemminki O., Dos Santos J.M., Hemminki A. Oncolytic viruses for cancer immunotherapy. J. Hematol. Oncol. 2020;13:84. doi: 10.1186/s13045-020-00922-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ran L., Tan X., Li Y., Zhang H., Ma R., Ji T., Dong W., Tong T., Liu Y., Chen D., et al. Delivery of oncolytic adenovirus into the nucleus of tumorigenic cells by tumor microparticles for virotherapy. Biomaterials. 2016;89:56–66. doi: 10.1016/j.biomaterials.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 164.Friedman G.K., Johnston J.M., Bag A.K., Bernstock J.D., Li R., Aban I., Kachurak K., Nan L., Kang K.-D., Totsch S., et al. Oncolytic HSV-1 G207 Immunovirotherapy for Pediatric High-Grade Gliomas. N. Engl. J. Med. 2021;384:1613–1622. doi: 10.1056/NEJMoa2024947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jiffry J., Thavornwatanayong T., Rao D., Fogel E.J., Saytoo D., Nahata R., Guzik H., Chaudhary I., Augustine T., Goel S., Maitra R. Oncolytic Reovirus (pelareorep) Induces Autophagy in KRAS-mutated Colorectal Cancer. Clin. Cancer Res. 2021;27:865–876. doi: 10.1158/1078-0432.CCR-20-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Soliman H., Hogue D., Han H., Mooney B., Costa R., Lee M.C., Niell B., Williams A., Chau A., Falcon S., et al. Oncolytic T-VEC virotherapy plus neoadjuvant chemotherapy in nonmetastatic triple-negative breast cancer: a phase 2 trial. Nat. Med. 2023;29:450–457. doi: 10.1038/s41591-023-02210-0. [DOI] [PubMed] [Google Scholar]
- 167.Behrens M.D., Stiles R.J., Pike G.M., Sikkink L.A., Zhuang Y., Yu J., Wang L., Boughey J.C., Goetz M.P., Federspiel M.J. Oncolytic Urabe mumps virus: A promising virotherapy for triple-negative breast cancer. Mol. Ther. Oncolytics. 2022;27:239–255. doi: 10.1016/j.omto.2022.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Qiao Z., Wang X., Zhao H., Deng Y., Zeng W., Yang K., Chen H., Yan Q., Li C., Wu J., Chen Y. The effectiveness of cell-derived exosome therapy for diabetic wound: A systematic review and meta-analysis. Ageing Res. Rev. 2023;85:101858. doi: 10.1016/j.arr.2023.101858. [DOI] [PubMed] [Google Scholar]
- 169.Lin Y.-N., Mesquita T., Sanchez L., Chen Y.-H., Liu W., Li C., Rogers R., Wang Y., Li X., Wu D., et al. Extracellular vesicles from immortalized cardiosphere-derived cells attenuate arrhythmogenic cardiomyopathy in desmoglein-2 mutant mice. Eur. Heart J. 2021;42:3558–3571. doi: 10.1093/eurheartj/ehab419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dang Y., Hua W., Zhang X., Sun H., Zhang Y., Yu B., Wang S., Zhang M., Kong Z., Pan D., et al. Anti-angiogenic effect of exo-LncRNA TUG1 in myocardial infarction and modulation by remote ischemic conditioning. Basic Res. Cardiol. 2023;118:1–21. doi: 10.1007/s00395-022-00975-y. [DOI] [PubMed] [Google Scholar]
- 171.Giacomini E., Scotti G.M., Vanni V.S., Lazarevic D., Makieva S., Privitera L., Signorelli S., Cantone L., Bollati V., Murdica V., et al. Global transcriptomic changes occur in uterine fluid-derived extracellular vesicles during the endometrial window for embryo implantation. Hum. Reprod. 2021;36:2249–2274. doi: 10.1093/humrep/deab123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kim K.Y., Shin K.Y., Chang K.-A. Brain-Derived Exosomal Proteins as Effective Biomarkers for Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Biomolecules. 2021;11:980. doi: 10.3390/biom11070980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liu C., Wang J., Hu J., Fu B., Mao Z., Zhang H., Cai G., Chen X., Sun X. Extracellular vesicles for acute kidney injury in preclinical rodent models: a meta-analysis. Stem Cell Res. Ther. 2020;11:11. doi: 10.1186/s13287-019-1530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tieu A., Hu K., Gnyra C., Montroy J., Fergusson D.A., Allan D.S., Stewart D.J., Thébaud B., Lalu M.M. Mesenchymal stromal cell extracellular vesicles as therapy for acute and chronic respiratory diseases: A meta-analysis. J. Extracell. Vesicles. 2021;10:e12141. doi: 10.1002/jev2.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Psaraki A., Ntari L., Karakostas C., Korrou-Karava D., Roubelakis M.G. Extracellular vesicles derived from mesenchymal stem/stromal cells: The regenerative impact in liver diseases. Hepatology. 2022;75:1590–1603. doi: 10.1002/hep.32129. [DOI] [PubMed] [Google Scholar]
- 176.You Y., Tian Y., Yang Z., Shi J., Kwak K.J., Tong Y., Estania A.P., Cao J., Hsu W.-H., Liu Y., et al. Intradermally delivered mRNA-encapsulating extracellular vesicles for collagen-replacement therapy. Nat. Biomed. Eng. 2023;7:887–900. doi: 10.1038/s41551-022-00989-w. [DOI] [PubMed] [Google Scholar]
- 177.Zou W., Lai M., Jiang Y., Mao L., Zhou W., Zhang S., Lai P., Guo B., Wei T., Nie C., et al. Exosome Release Delays Senescence by Disposing of Obsolete Biomolecules. Adv. Sci. 2023;10:2204826. doi: 10.1002/advs.202204826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kuang H., Dou G., Cheng L., Wang X., Xu H., Liu X., Ding F., Yang X., Liu S., Bao L., et al. Humoral regulation of iron metabolism by extracellular vesicles drives antibacterial response. Nat. Metab. 2023;5:111–128. doi: 10.1038/s42255-022-00723-5. [DOI] [PubMed] [Google Scholar]