Abstract
Background and Aims
The epidemiology of peripartum cardiomyopathy (PPCM) in Europe is poorly understood and data on long-term outcomes are lacking. A retrospective, observational, population-level study of validated cases of PPCM in Scotland from 1998 to 2017 was conducted.
Methods
Women hospitalized with presumed de novo left ventricular systolic dysfunction around the time of pregnancy and no clear alternative cause were included. Each case was matched to 10 controls. Incidence and risk factors were identified. Morbidity and mortality were examined in mothers and children.
Results
The incidence of PPCM was 1 in 4950 deliveries. Among 225 women with PPCM, obesity, gestational hypertensive disorders, and multi-gestation were found to be associated with having the condition. Over a median of 8.3 years (9.7 years for echocardiographic outcomes), 8% of women with PPCM died and 75% were rehospitalized for any cause at least once. Mortality and rehospitalization rates in women with PPCM were ∼12- and ∼3-times that of controls, respectively. The composite of all-cause death, mechanical circulatory support, or cardiac transplantation occurred in 14%. LV recovery occurred in 76% and, of those who recovered, 13% went on to have a decline in LV systolic function despite initial recovery. The mortality rate for children born to women with PPCM was ∼5-times that of children born to controls and they had an ∼3-times greater incidence of cardiovascular disease over a median of 8.8 years.
Conclusions
PPCM affected 1 in 4950 women around the time of pregnancy. The condition is associated with considerable morbidity and mortality for the mother and child. There should be a low threshold for investigating at-risk women. Long term follow-up, despite apparent recovery, should be considered.
Keywords: Peripartum cardiomyopathy, Incidence, Outcomes, Recovery
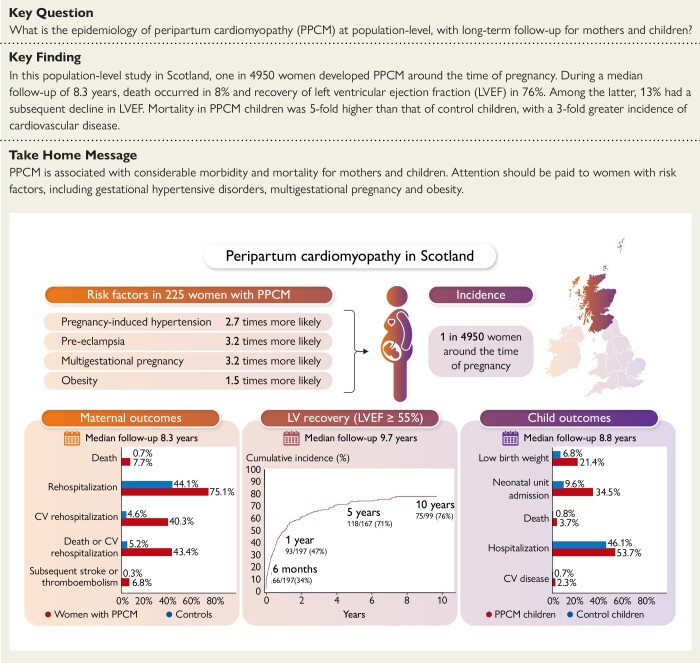
Structured Graphical Abstract
Graphical abstract.
Graphical summary of the main study findings. CV, cardiovascular; LV, left ventricular; LVEF, left ventricular ejection fraction; PPCM, peripartum cardiomyopathy.
See the editorial comment for this article ‘Peripartum cardiomyopathy in Europe: new insights from the UK’, by C. Viljoen et al., https://doi.org/10.1093/eurheartj/ehad724.
Introduction
Peripartum cardiomyopathy (PPCM) is a type of heart failure, secondary to left ventricular (LV) systolic dysfunction (LVSD), identified around the time of pregnancy.1 The incidence of PPCM varies between countries; in White populations in the United States it is reported to be 1 in 1000–4000 and in Black populations, it is reported to be higher at 1 in 1000–2000.2 There are few estimates of how common PPCM is in Europe.3,4 Several factors are associated with the development of PPCM, including hypertensive disorders of pregnancy, multi-parity, and genetics, emphasizing its pathophysiological heterogeneity.5,6 Outcomes for both the mother and child vary according to region and race.7 While true regional variation probably does exist, it is likely that some of the differences in incidence and outcomes are explained by inconsistencies in data sources, definitions of PPCM, inclusion criteria, and study methodologies. There are also gaps in our understanding of long-term outcomes (beyond 5 years). In Western Europe, there are two population studies (Denmark and Sweden) and a multicentre registry (Germany) which include women with PPCM,3,4,8,9 but there are no published studies examining a whole population, with long-term follow-up for both the mother and child. In this retrospective, observational, population-level study, we describe the incidence of and characteristics associated with the development of PPCM in Scotland. In addition, we report outcomes in affected women and their children over 20 years.
Methods
Data sources
In Scotland, everyone has free access to healthcare, both in general practice and hospital, through the National Health Service (NHS). Nearly all healthcare is delivered through the NHS. At birth, every resident receives a unique identifying number and all healthcare contacts thereafter are linked through this, as are death records. The equivalent data for children can be linked to their mother’s record through this identifier. These population-level administrative data were used in the present study, supplemented by data collected from case records across Scotland. The Scottish Morbidity Record (SMR) captures data on hospitalizations and day case attendances in NHS hospitals.10 Demographics, medical conditions, and surgical procedures are included. Each record includes up to six International Classification of Diseases (ICD) diagnostic codes and procedures are recorded using the OPCS Classification of Interventions and Procedures. SMR01 relates to general or acute inpatient and day cases and SMR02 to maternity and deliveries, including obstetric and neonatal data. The accuracy of coding of cardiovascular (CV) conditions in SMR01 is high: e.g. heart failure 91%, myocardial infarction 99%.11 In this study, case records were reviewed to ensure high accuracy. Data sources are listed in full in the Supplementary data online, Appendix.
Study population
Cases
Consecutive cases of PPCM were selected by (i) identifying possible cases through specific discharge codes, and (ii) validating the presumptive diagnosis through review of case records. Women with a discharge diagnosis of PPCM, heart failure, or cardiomyopathy up to 6 months before or 2 years after a pregnancy outcome (delivery or termination) were identified. ICD codes used are listed in the Supplementary data online, Appendix. Inclusion criteria in the validation stage were: impaired LV systolic function [LV ejection fraction (LVEF) on transthoracic echocardiography ≤50% or qualitative assessment reporting LV systolic dysfunction if LVEF unavailable], no clear alternative cause, no pre-existing diagnosis of LVSD or cardiomyopathy, and presentation during pregnancy (excluding the first trimester) to 2 years postpartum. In particular, LVSD associated with specific aetiological processes was excluded (e.g. myocarditis, primary valvular heart disease, severe sepsis). Full inclusion and exclusion criteria are in the Supplementary data online, Appendix. The final study cohort included consecutive women with PPCM from 1998 to 2017. ICD codes in validated cases and non-cases were used to identify the combination of ICD codes (present/absent) resulting in the optimal sensitivity for PPCM (92%). This method was used to identify cases of PPCM in women without records available for validation and is detailed in the Supplementary data online, Appendix. The study consort diagram is in the Supplementary data online, Appendix.
Controls
Each case was matched to 10 controls, except for two cases for whom five controls were available. Controls were women without PPCM/heart failure/cardiomyopathy matched for (i) age at delivery, (ii) year of delivery ±1 year, and (iii) health board of delivery. If matching was limited by smaller health boards or extremes of age, the health board criterion was dropped.
Children
Children of cases and controls who could be linked to their mother were included. In some cases, children were included at the neonatal stage, but not beyond this (i.e. if linkage with their mother beyond delivery was not possible).
Sensitivity analysis
Incidence was calculated in England from 2003 to 2017 using Hospital Episode Statistics, a database of NHS hospital attendance in England.12
Characteristics and outcomes
Demographics, comorbidities, clinical (including laboratory tests, electrocardiographic and echocardiographic data), obstetric, and neonatal data were examined by combining administrative data and data collected from case records. LVEF was obtained from transthoracic echocardiogram reports and, where only qualitative assessment of LV function was provided, LVEF was assigned as the category midpoint (mild, 50%; moderate, 40%; severe, 25%). The following outcomes were analysed: all-cause death, all-cause rehospitalization, CV rehospitalization, a composite of all-cause death, or CV rehospitalization, a composite of subsequent stroke or thromboembolism (not including events that were identified at the index presentation), a composite of all-cause death, intra-aortic balloon pump (IABP), ventricular assist device (VAD) or extracorporeal membrane oxygenation (ECMO), or cardiac transplantation, device therapy [implantable cardioverter defibrillator (ICD) or cardiac resynchronization therapy (CRT)], LV recovery (defined as LVEF ≥55% or normal qualitative assessment) and LV decline after recovery [defined as the first reduction in LVEF of >10% (percentage points) from the LVEF at recovery and to <50% if also present on the subsequent echocardiogram, unless the final echocardiogram]. Neonatal and child outcomes, including death, hospitalization, and disease incidence were analysed. A full list of definitions and sources is in the Supplementary data online, Appendix.
Statistical analysis
Incidence rates were derived using population estimates from the National Records of Scotland Births Time Series Data and Office for National Statistics.13,14 Comparisons were performed using t-tests, Wilcoxon rank-sum test, and chi-squared tests where appropriate. Associations between patient characteristics and the development of PPCM were examined using logistic regression, adjusted for age at delivery, year of delivery (1998–2007 vs. 2008–17), and health board of delivery. The discriminatory ability of each model was assessed using the C-statistic. Event rates were calculated using the start date of the episode of care during which the diagnosis was made (or date of baseline echocardiogram for LV recovery) for women with PPCM, delivery date for controls, and birth date for children, to the start date of the episode of care during which the event occurred, or procedure date, or death or censor date (31 December 2017 for non-echocardiographic outcomes and 31 December 2019 for echocardiographic outcomes) if no event occurred. Cumulative incidences were displayed using Kaplan–Meier curves. Numbers were not displayed if they violated privacy requirements (generally n < 5). Rate ratios were derived using the method by Rothman et al.15 Multivariable Cox and logistic regression using backward selection and P-value <.1 to retain the variable in the model were used to examine variables associated with two outcomes: (i) all-cause death or CV rehospitalization and (ii) LV recovery within 1 year. Women who died before recovery were categorized as unrecovered and as recovered if death occurred after recovery. Missing data were excluded. A two-sided P-value <.05 was considered statistically significant. Analyses were performed using Stata v16 (StataCorp LLC, College Station, TX, USA). Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement guidelines were followed.
Results
Incidence
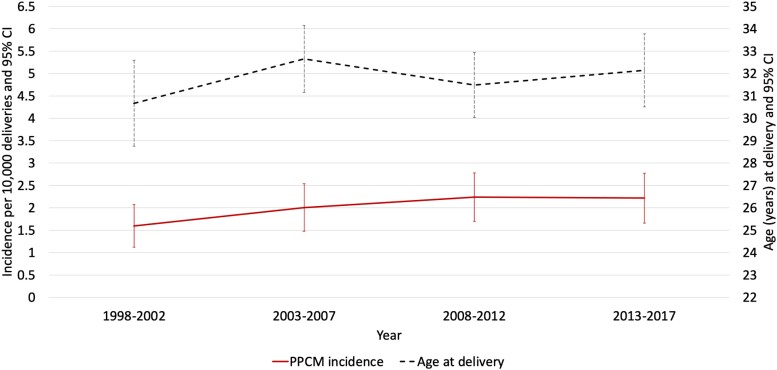
From 1998 to 2017, 225 women in Scotland fulfilled the definition of incident PPCM, with 90% of these validated through individual case record review. The incidence of PPCM was 2.02 (95% CI 1.76–2.29) per 10 000 deliveries (or 1 in 4950) and was similar irrespective of whether an inclusion threshold of 6 months (n = 197, 88% of cases), 1 year (n = 209, 93% of cases), or 2 years (n = 225, all cases) post-delivery was applied (Figure 1, Supplementary data online, Table S1). The incidence was similar in England (2.12 per 10 000 deliveries, 95% CI 2.03–2.21) (see Supplementary data online, Figure S1 and Table S2). The mean age of women with PPCM in Scotland at delivery was 31.9 years (95% CI 31.6–32.1). The incidence of PPCM per 10 000 deliveries was higher in women >32 years than in those aged ≤32 years (3.34, 95% CI 2.90–3.78 vs. 1.45, 95% CI 1.26–1.64) (see Supplementary data online, Figure S2, Supplementary data online, Table S3).
Figure 1.
Incidence of PPCM and age at delivery in Scotland from 1998 to 2017
Patient characteristics
Compared to controls, women with PPCM had worse socioeconomic deprivation, were more often obese (35% vs. 21%), and more frequently had a history of smoking (41% vs. 33%), hypertension (17% vs. 8%) and diabetes (9% vs. 5%) (Table 1). They more often had a pregnancy complicated by pregnancy-induced hypertension (34% vs. 8%), pre-eclampsia (20% vs. 2%), and postpartum haemorrhage (36% vs. 27%), more frequently were multi-parious, had a multi-gestational pregnancy (8% vs. 2%), a shorter estimated gestation (38 weeks vs. 40 weeks) and emergency caesarean section (36% vs. 17%).
Table 1.
Patient characteristics
| Total | PPCM | Controls | P value | Missing | |
|---|---|---|---|---|---|
| n = 2465 | n = 225 | n = 2240 | |||
| Age (years) | 31.9 ± 6.0 | 31.8 ± 6.0 | 31.9 ± 6.0 | .87 | 0 |
| Deprivation quintile | .012 | 5 | |||
| 1 (most deprived) | 596 (24.2) | 76 (33.9) | 520 (23.3) | ||
| 2 | 449 (18.3) | 37 (16.5) | 412 (18.4) | ||
| 3 | 457 (18.6) | 38 (17.0) | 419 (18.7) | ||
| 4 | 470 (19.1) | 35 (15.6) | 435 (19.5) | ||
| 5 | 488 (19.8) | 38 (17.0) | 450 (20.1) | ||
| Body mass index (kg/m2) | 26.5 ± 6.0 | 28.2 ± 7.0 | 26.3 ± 5.8 | <.001 | 852 |
| Body mass index >30 kg/m2 | 369 (22.9) | 68 (34.7) | 301 (21.2) | <.001 | 852 |
| Smoking (current/former) | 796 (33.8) | 91 (41.2) | 705 (33.1) | .016 | 113 |
| Prior hypertension | 217 (8.8) | 38 (16.9) | 179 (8.0) | <.001 | 0 |
| Prior diabetes | 126 (5.1) | 20 (8.9) | 106 (4.7) | .007 | 0 |
| Obstetric factors | |||||
| Gestational diabetes | 51 (2.1) | 7 (3.1) | 44 (2.0) | .25 | 0 |
| Pregnancy-induced hypertension | 259 (10.5) | 77 (34.2) | 182 (8.1) | <.001 | 0 |
| Pre-eclampsia | 94 (3.8) | 44 (19.6) | 50 (2.2) | <.001 | 0 |
| Antepartum haemorrhage | 169 (6.9) | 13 (5.8) | 156 (7.0) | .50 | 0 |
| Postpartum haemorrhage | 696 (28.2) | 81 (36.0) | 615 (27.5) | .007 | 0 |
| Parity >1 | 618 (25.2) | 68 (30.2) | 550 (24.6) | .066 | 8 |
| Parity >2 | 230 (9.4) | 38 (16.9) | 192 (8.6) | <.001 | 8 |
| Parity >3 | 103 (4.2) | 22 (9.8) | 81 (3.6) | <.001 | 8 |
| Multi-gestationa | 59 (2.4) | 18 (8.2) | 41 (1.8) | <.001 | 5 |
| Estimated gestation (weeks)a | 39 (38–40) | 38 (36–40) | 40 (38–40) | <.001 | 9 |
| Estimated gestation <37weeksa | 205 (8.3) | 56 (25.5) | 149 (6.7) | <.001 | 9 |
| Pregnancy outcome | <.001 | 0 | |||
| Live birth | 2448 (99.3) | 216 (96.0) | 2232 (99.6) | ||
| Stillbirth | 12 (0.5) | 4 (1.8) | 8 (0.4) | ||
| Termination | 5 (0.2) | 5 (2.2) | 0 (0.0) | ||
| Induction of laboura | 634 (26.1) | 62 (30.5) | 572 (25.7) | .13 | 38 |
| Mode of delivery, baby 1a | <.001 | 5 | |||
| Vaginal | 1654 (67.2) | 99 (45.0) | 1555 (69.4) | ||
| Elective caesarean | 348 (14.1) | 41 (18.6) | 307 (13.7) | ||
| Emergency caesarean | 458 (18.6) | 80 (36.4) | 378 (16.9) | ||
| Clinical features at index presentation | n = 202 | ||||
| Timing of diagnosis | 0 | ||||
| Postpartum | 165 (81.7) | ||||
| Prepartum | 37 (18.3) | ||||
| Heart rate (bpm) | 108 (90–125) | 16 | |||
| Heart rate >90 bpm | 136 (73.1) | 16 | |||
| Systolic blood pressure (mmHg) | 134 (120–154) | 23 | |||
| Diastolic blood pressure (mmHg) | 85 (73–100) | 25 | |||
| Intravenous diuretic therapy | 132 (70.6) | 15 | |||
| Intensive care | 60 (29.7) | 0 | |||
| Intubation and ventilation | 48 (23.9) | 1 | |||
| Renal replacement therapy | 7 (3.6) | 5 | |||
| Pharmacological haemodynamic support | 29 (14.9) | 7 | |||
| Stroke or thromboembolism | 26 (12.9) | 0 | |||
| LV thrombus | 8 (4.0) | 0 | |||
| Laboratory tests | n = 202 | ||||
| Haemoglobin (g/L) | 114 (101–126) | 11 | |||
| White blood cells (×109/L) | 11.6 (9.1–14.6) | 11 | |||
| Platelets (×109/L) | 283 (205–378) | 12 | |||
| Serum creatinine (μmol/L) | 68 (57–83) | 9 | |||
| Serum albumin (g/L) | 31 (26–35) | 17 | |||
| Electrocardiogram | n = 181 | ||||
| Sinus rhythm | 172 (95.0) | 0 | |||
| QRS duration (ms) | 83 (76–94) | 1 | |||
| Bundle branch block | 26 (14.4) | 1 | |||
| QTc interval (ms) | 451 (425–483) | 4 | |||
| Any electrocardiogram abnormalityb | 138 (77.5) | 3 | |||
| Echocardiogram | n = 202 | ||||
| LV ejection fraction (%), mean | 35 ± 11 | 5 | |||
| LV ejection fraction (%), median | 36 (25–43) | 5 | |||
| LV ejection fraction ≤45% | 155 (78.7) | 5 | |||
| LV end-diastolic diameter (mm) | 57 ± 8 | 28 |
a n = 5 terminations in PPCM group excluded.
bDefined as bundle branch block, QTc interval >470 ms, ventricular ectopy, abnormal R wave progression, or ST-T segment changes.
PPCM was diagnosed after delivery in 82%. Overall, 30% of women with PPCM required admission to an intensive care unit, 13% had a thromboembolic event identified at the index presentation and 4% had a LV thrombus. The majority were in sinus rhythm (95%) and 14% had bundle branch block on electrocardiogram. Baseline LVEF was 35% (±11) and LV end-diastolic diameter (LVEDD) was 57 mm (±8).
Factors associated with the development of PPCM
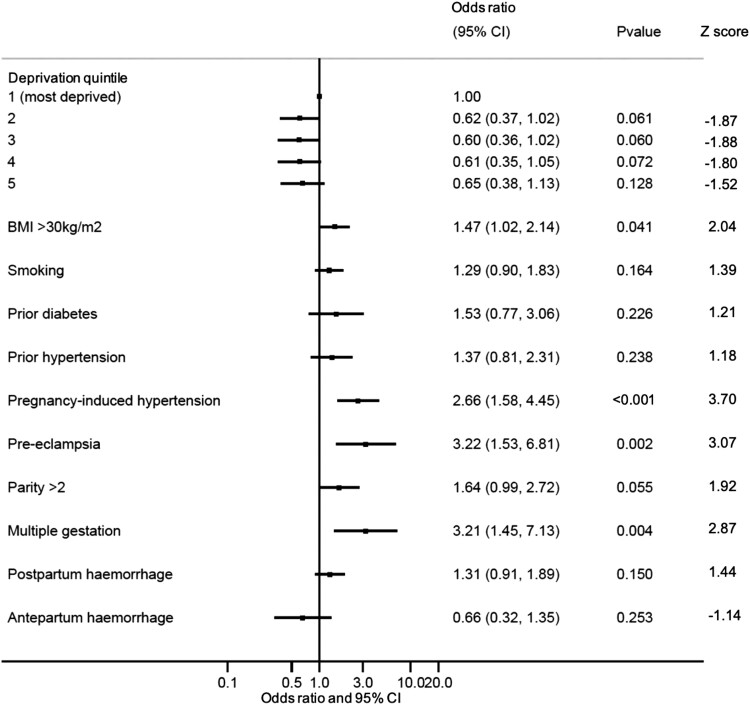
In a multivariable model adjusted for age, year of delivery, and health board, obesity (OR 1.47, 95% CI 1.02–2.14), pregnancy-induced hypertension (OR 2.66, 95% CI 1.58–4.45), pre-eclampsia (OR 3.22, 95% CI 1.53–6.81), and multi-gestation (OR 3.21, 95% CI 1.45–7.13) were independently associated with the development of PPCM (Figure 2). The C-statistic for the model was 0.72 (95% CI 0.68–0.77).
Figure 2.
Factors associated with the development of PPCM (multi-variable). Forest plot displaying maternal characteristics associated with the development of PPCM in a multivariable logistic regression model (also adjusted for maternal age, year of delivery, and health board). Odds ratio and 95% confidence intervals are shown. n = 1582 with complete data in final model
Since certain obstetric/delivery factors may be directly related to the presence of established cardiac disease, an exploration of obstetric associations was conducted separately in 182 women diagnosed postpartum. Independent associations were similar, except for obesity, and with the addition of multi-parity (see Supplementary data online, Figure S3). None of the direct obstetric factors were significant. The C-statistic for the model was 0.74 (95% CI 0.70–0.79).
Outcomes
Maternal outcomes
Linked outcome data were available in 221 women with PPCM with a median follow-up of 8.3 years [interquartile range (IQR) 4.0–12.9], totaling 1911 person-years. Minimum 5- and 10-year follow-up was available in 157 (71%) and 95 (43%), respectively.
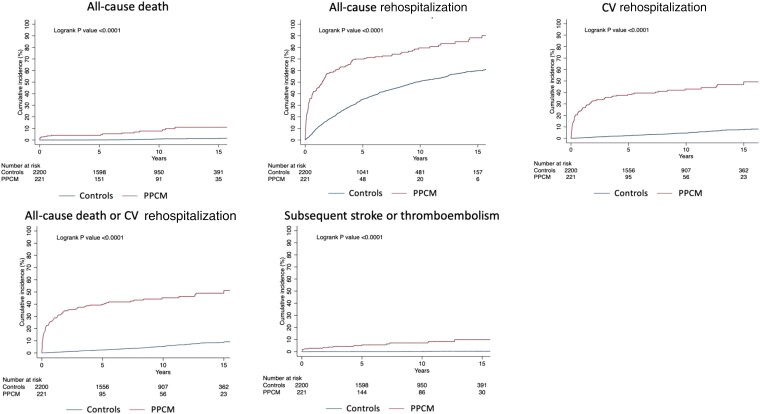
A total of 17 (7.7%, 95% CI 4.8–12.1) women with PPCM and 15 controls (0.7%, 95% CI 0.4–1.1) died from any cause (Table 2). A total of 166 (75.1%, 95% CI 69.0–80.4) women with PPCM and 971 controls (44.1%, 95% CI 42.1–46.2) were rehospitalized for any cause. The causes of the first subsequent non-maternity rehospitalization are shown in Supplementary data online, Figure S4. In women with PPCM, death and rehospitalization occurred at a rate of 12.0- and 3.4-times that of controls, respectively (Table 2, Figure 3).
Table 2.
Maternal outcomes
| PPCM | Controls | |
|---|---|---|
| n = 221 | n = 2210 | |
| All-cause death | ||
| Number | 17 | 15 |
| % (95% CI) | 7.7 (4.8–12.1) | 0.7 (0.4–1.1) |
| Rate per 1000 person-years (95% CI) | 8.9 (5.5–14.3) | 0.7 (0.4–1.2) |
| Rate ratio (95% CI) | 11.98 (5.98–23.99) | |
| All-cause rehospitalization | ||
| Number | 166 | 971 |
| % (95% CI) | 75.1 (69.0–80.4) | 44.1 (42.1–46.2) |
| Rate per 1000 person-years (95% CI) | 243.6 (206.2–283.6) | 72.6 (68.1–77.3) |
| Rate ratio (95% CI) | 3.36 (2.58–4.36) | |
| CV rehospitalization | ||
| Number | 89 | 101 |
| % (95% CI) | 40.3 (34.0–46.9) | 4.6 (3.8–5.5) |
| Rate per 1000 person-years (95% CI) | 70.1 (57.0–86.3) | 5.2 (4.2–6.3) |
| Rate ratio (95% CI) | 13.58 (10.22–18.06) | |
| All-cause death or CV rehospitalization | ||
| Number | 96 | 115 |
| % (95% CI) | 43.4 (37.0–50.1) | 5.2 (4.4–6.2) |
| Rate per 1000 person-years (95% CI) | 75.6 (61.9–92.4) | 5.9 (4.9–7.1) |
| Rate ratio (95% CI) | 12.87 (9.81–16.87) | |
| Subsequent stroke or thromboembolism | ||
| Number | 15 | 7 |
| % (95% CI) | 6.8 (4.1–11.0) | 0.3 (0.2–0.7) |
| Rate per 1000 person-years (95% CI) | 8.2 (5.0–13.7) | 0.3 (0.2–0.7) |
| Rate ratio (95% CI) | 23.79 (9.70–58.34) | |
| All-cause death, IABP, VAD, ECMO, or cardiac transplantation | ||
| Number | 30 | - |
| % (95% CI) | 13.6 (9.6–18.8) | - |
| Rate per 1000 person-years (95% CI) | 16.4 (11.5–23.5) | - |
| Intra-aortic balloon pump | ||
| Number | 16 | - |
| % (95% CI) | 7.2 (4.5–11.5) | - |
| VAD, ECMO or cardiac transplantation | ||
| Number | 8 | - |
| % (95% CI) | 3.6 (1.8–7.1) | - |
| Device therapya (n = 218) | ||
| Number | 20 | - |
| % (95% CI) | 9.2 (6.0–13.8) | - |
| CRT | ||
| Number | 7 | - |
| % (95% CI) | 3.2 (1.5–6.6) | - |
| ICD | ||
| Number | 18 | - |
| % (95% CI) | 8.3 (5.3–12.8) | - |
| ICD type | ||
| Primary prevention—number (%) | 11 (61.1) | - |
| Secondary prevention—number (%) | 7 (38.9) | - |
| Appropriate shock therapy (n = 17 with data) | 5 (29.4) | - |
Outcomes are after diagnosis in women with PPCM and after delivery in controls.
aImplantable cardioverter defibrillator or cardiac resynchronization therapy.
CV, cardiovascular; IABP, intra-aortic balloon pump; VAD, ventricular assist device; ECMO, extracorporeal membrane oxygenation; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator.
Figure 3.
Cumulative incidences of death, rehospitalization, CV rehospitalization, death or CV rehospitalization, and subsequent stroke or thromboembolism in women with PPCM and controls. Kaplan–Meier graphs illustrate the cumulative incidence of outcomes in women with PPCM and controls. Subsequent stroke or thromboembolism excludes events during the index hospital admission
The proportions of women with PPCM who had died at 30 days, 6 months, 1 year, and 5 years were: 2.3% (95% CI 0.9–5.3), 2.8% (95% CI 1.2–6.0), 3.8% (95% CI 1.9–7.4), and 3.8% (95% CI 1.7–8.3), respectively (see Supplementary data online, Table S4).
After the index presentation, there were 894 further hospitalizations among women with PPCM, resulting in a total of 2621 days in hospital (see Supplementary data online, Table S5). Overall, 54% of women had recurrent rehospitalizations (i.e. two or more further hospitalizations).
Not including events during the index hospital admission, subsequent stroke or thromboembolism occurred in 15 (7%, 95% CI 4–11) women with PPCM and in 7 (0.3%, 95% CI 0.2–0.7) controls, with a rate ratio of 23.79 (95% CI 9.70–58.34) (Table 2 and Figure 3).
The composite of death, IABP, VAD, or cardiac transplantation occurred in 30 (14%, 95% CI 10–19) women with PPCM (Table 2). Of 218 women with PPCM with data on device therapy (from years 2000 for ICD and 2006 for CRT), 20 (9%, 95% CI 6–14) received either an ICD or CRT. Of those with an ICD, 17 had device follow-up data and 5 (29%) received appropriate shock therapy at a median of 19 months (IQR 11–63) after device implantation.
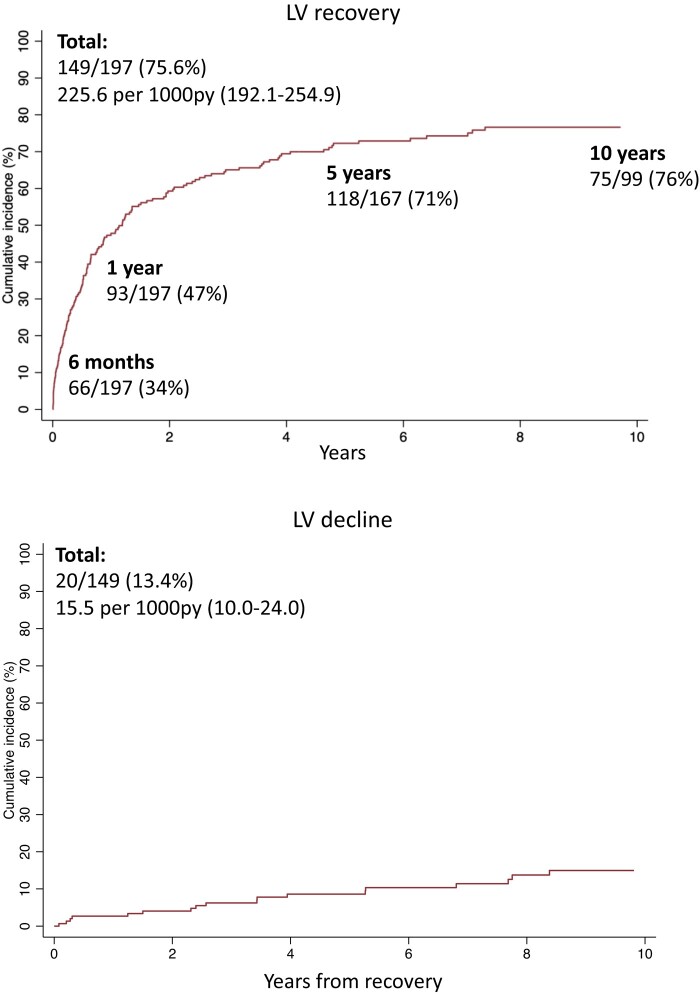
LV recovery
LV recovery was analysed in 197 women with an echocardiogram at baseline and at least one additional echocardiogram (88% of the total cohort/98% of those with a baseline echocardiogram). In these women, data from 1408 echocardiograms were collected over a median echocardiographic follow-up of 9.7 years (IQR 5.8–14.2), totaling 2024 person-years. Quantitative assessment of LVEF was available in 1062 (75%) echocardiograms.
Overall, 149 (76%, 95% CI 67–79) women had LV recovery (LVEF ≥55% or qualitative assessment of preserved/normal) at a median of 214 days (IQR 66–694) (Figure 4, Supplementary data online, Table S6). A total of 34% (95% CI 27–40) women had LV recovery within 6 months, 47% (95% CI 40–54) within 1 year, 71% (95% CI 64–77) within 5 years and 76% (95% CI 67–83) within 10 years (see Supplementary data online, Table S6). LV decline [the first reduction in LVEF of >10% (percentage points) from the LVEF at recovery and to <50% is also present on the subsequent echocardiogram, unless the final echocardiogram] occurred in 20/149 women who had recovered (13%, 95% CI 9–20) at a median of 2.9 years (IQR 1.0–6.0) after recovery. In those women with LV decline, median decline in LVEF was 18.5% (IQR 14–23). As a sensitivity analysis, the definition of LV decline was expanded to capture women with two consecutive echocardiograms meeting these thresholds at any time; this occurred in 22/149 women (15%, 95% CI 10%–21%) at a median of 3.3 years (IQR 1.2–6.1) after recovery.
Figure 4.
Cumulative incidences of LV recovery and LV decline. Kaplan–Meier graphs illustrate the cumulative incidence of LV recovery (from the date of baseline echocardiogram) and of subsequent LV decline (from the date of recovery) in women with PPCM. Proportions shown are of women with complete follow-up at each time point. LV recovery defined as LVEF ≥55% or qualitative assessment of preserved/normal LV function. LV decline defined as the first reduction in LVEF of >10% (percentage points) from the LVEF at recovery and to <50% if also present on the subsequent echocardiogram, unless the final echocardiogram
Predictors of maternal outcomes
The following candidate variables were included: age, smoking, worst deprivation quintile, BMI >30 kg/m2, pre-eclampsia, diabetes (any kind, including gestational in the index or a prior pregnancy) parity >2, heart rate, systolic blood pressure, bundle branch block, QTc interval, LVEF, and LVEDD. In total, 140 women with complete data were in the final models. QTc interval and QRS duration were not strongly correlated (R = .395). QTc interval (per 10 ms increase), smoking, most deprived quintile and bundle branch block were associated with all-cause death or CV rehospitalization [QTc hazard ratio (HR) 1.09, 95% CI 1.02–1.17, smoking HR 1.89, 95% CI, 1.09–3.27, worst deprivation quintile HR 1.77, 95% CI 1.02–3.07, bundle branch block HR 1.93, 95% CI 1.01–3.71]. Only LVEDD (per mm increase) was associated with LV recovery at 1 year with an inverse relationship (OR 0.94, 95% CI 0.89–0.98).
Children
Median follow-up of PPCM children was 8.8 years (IQR 4.2–13.2), totaling 1946 person-years. Neonatal outcomes were available in 239 PPCM children and 2231 control children (pregnancies ending in termination were excluded). Compared to control children, PPCM children were more frequently premature and delivered by caesarean section, had lower birth weights and APGAR scores, and were more often admitted to a neonatal unit (Table 3). An adverse neonatal outcome, defined as a composite of prematurity (estimated gestation <37 weeks), low birth weight (<2500 g), stillbirth, or early neonatal death (death within 7 days of delivery), occurred in 32% of PPCM children and 10% of control children (P < .001).
Table 3.
Neonatal outcomes
| Total | PPCM children | Control children | P value | Missing | |
|---|---|---|---|---|---|
| n = 2470 | n = 239 | n = 2231 | |||
| Boy | 1249 (50.6) | 111 (46.4) | 1138 (51.0) | .18 | 0 |
| Estimated gestation (weeks) | 39 (38–40) | 38 (36–40) | 40 (38–40) | <.001 | 0 |
| Estimated gestation <37weeks | 234 (9.5) | 68 (28.5) | 166 (7.5) | <.001 | 0 |
| Mode of delivery | <.001 | 0 | |||
| Vaginal | 1627 (65.9) | 101 (42.3) | 1526 (68.4) | ||
| Elective caesarean | 371 (15.0) | 49 (20.5) | 322 (14.4) | ||
| Emergency caesarean | 472 (19.1) | 89 (37.2) | 383 (17.2) | ||
| Birthweight (g) | 3410 (3036–3780) | 3145 (2620–3680) | 3430 (3080–3780) | <.001 | 7 |
| Low birth weight (<2500 g) | 202 (8.2) | 50 (21.4) | 152 (6.8) | <.001 | 7 |
| APGAR score 5 min | 9 (9–9) | 9 (9–9) | 9 (9–9) | <.001 | 50 |
| APGAR score 5 min | <.001 | 50 | |||
| 8–10 | 2351 (97.1) | 205 (90.7) | 2146 (97.8) | ||
| 6–7 | 44 (1.8) | 13 (5.8) | 31 (1.4) | ||
| ≤ 5 | 25 (1.0) | 8 (3.5) | 17 (0.8) | ||
| Invasive ventilation | 34 (1.6) | 15 (7.1) | 19 (1.0) | <.001 | 282 |
| Neonatal unit admission | 289 (12.0) | 80 (34.5) | 209 (9.6) | <.001 | 58 |
| Status at maternity discharge | <.001 | 16 | |||
| Discharged | 2268 (92.4) | 173 (77.6) | 2095 (93.9) | ||
| Inpatient | 166 (6.8) | 44 (19.7) | 122 (5.5) | ||
| Stillbirth/early neonatal death | 20 (0.8) | 6 (2.7) | 14 (0.6) | ||
| Adverse neonatal outcomea | 289 (11.8) | 75 (32.2) | 214 (9.6) | <.001 | 6 |
aComposite of prematurity (estimated gestation <37 weeks), low birth weight (<2500 g), stillbirth, or early neonatal death (death within 7 days of delivery).
Long-term data were available for 216 PPCM children and 1985 control children. Including stillbirths and early neonatal deaths (within 7 days), death from any cause occurred in 8 (3.7%, 95% CI 1.9–7.3) PPCM children and 16 (0.8%, 95% CI 0.5–1.3) control children, with a mortality rate ratio of 4.72 (95% CI 2.02–11.02) (Table 4). The incidence of CV disease in PPCM children was 3.40-times (95% CI 1.23–9.44) that of control children.
Table 4.
Child mortality and morbidity
| PPCM children n = 216 | Control children n = 1985 | ||||||
|---|---|---|---|---|---|---|---|
| Number | % (95% CI) | Rate per 1000py (95% CI) | Number | % (95% CI) | Rate per 1000py (95% CI) | Rate ratio (95% CI) | |
| All-cause death | 8 | 3.7 (1.9–7.3) | 4.1 (2.1–8.2) | 16 | 0.8 (0.5–1.3) | 0.9 (0.5–1.4) | 4.72 (2.02–11.02) |
| All-cause hospitalization | 116 | 53.7 (47.0–60.3) | 111.7 (93.1–133.9) | 915 | 46.1 (43.9–48.3) | 78.9 (74.0–84.2) | 1.41 (1.17–1.72) |
| Cardiovascular disease | 5 | 2.3 (1.0–5.5) | 2.6 (1.1–6.3) | 14 | 0.7 (0.4–1.2) | 0.8 (1.3–14.0) | 3.40 (1.23–9.44) |
| Non-cardiovascular congenital anomaly | 15 | 6.9 (4.2–11.2) | 8.2 (5.0–13.7) | 75 | 3.8 (3.0–4.7) | 4.2 (3.4–5.3) | 1.96 (1.13–3.42) |
| Respiratory disease | 48 | 22.2 (17.1–28.3) | 29.8 (22.5–39.6) | 352 | 17.7 (16.1–19.5) | 22.3 (20.1–24.8) | 1.34 (0.99–1.81) |
| Gastrointestinal disease | 37 | 17.1 (12.6–22.8) | 22.4 (16.2–30.9) | 265 | 13.4 (11.9–14.9) | 16.1 (14.3–18.1) | 1.39 (0.99–1.96) |
| Infection | 8 | 3.7 (1.9–7.3) | 4.2 (2.1–8.4) | 72 | 3.6 (2.9–4.5) | 4.0 (3.2–5.1) | 1.04 (0.50–2.17) |
Discussion
In this 20-year population study, PPCM affected 1 in 4950 women around the time of pregnancy. Although around three-quarters of women had LV recovery, adverse outcomes occurred more often in women with PPCM than in healthy controls and around one in eight women had LV decline after apparent recovery. It was notable that cumulative incidences of death and rehospitalization for women with PPCM and controls appeared to stop diverging at around 5 years. Smoking, socioeconomic deprivation, QTc interval, and bundle branch block were most strongly associated with death or CV rehospitalization in women with PPCM, and LVEDD was associated with LV recovery at 1 year. The current study also provides data on long-term outcomes for children, which have never before been reported; mortality was ∼5-times greater in children born to women with PPCM than in those born to controls and incident CV disease was more common (Structured Graphical Abstract).
The incidence of PPCM varies from 1 in 100 to 1 in 20 000 deliveries worldwide.16,17 In Europe, one other small study has reported the incidence of validated PPCM at population-level with an estimate of 1 in 10 149 deliveries.3 Whilst regional differences are likely to exist, discordance may be driven by differences in methods and inclusion criteria (e.g. retrospective vs. prospective; population vs. single/multicentre; consecutive vs. non-consecutive; administrative vs. clinical data; ICD coding vs. validated cases; exclusion criteria vs. none; early vs. late postpartum case identification; LVEF inclusion thresholds). The current study captures consecutive, expertly validated, hospitalized cases in a whole population with rigorous exclusion criteria. PPCM is likely under-reported globally due to asymptomatic LVSD remaining undiagnosed and a failure to recognize symptoms as pathological. More comprehensive diagnostic criteria, with explicit exclusion criteria, are required to define the condition more clearly. Current criteria mandate an arbitrary LVEF of <45%.1 It has been shown that a ‘normal’ LVEF for women is higher than that for men,18 and all women with a reduction in LV systolic function were included in this study. In the future, more comprehensive phenotyping will help advance our understanding of the complex spectrum of LVSD around the time of pregnancy, which includes women with placental and vascular insults, a genetic predisposition to dilated cardiomyopathy (DCM), and other precursors such as hypertension, diabetes, and obesity. Indeed, PPCM with a heart failure and preserved ejection fraction phenotype has also been proposed.19
Obesity, pregnancy-induced hypertension, pre-eclampsia, and multi-gestation were associated with the development of PPCM. Greater maternal age also appeared to be relevant. Specifically, we observed that 20% had a history of pre-eclampsia. There is debate as to whether pre-eclampsia represents a separate process or is an aetiology of PPCM, but previous registries have included women with pre-eclampsia. The prevalence of gestational hypertensive disorders in our study was similar to others.7,20 This may support screening certain at-risk groups of women for LVSD, such as older women with a pregnancy complicated by gestational hypertensive disorders. While LV volumes may increase during a healthy pregnancy, LVEF is not expected to decline.21 In general populations, electrocardiogram (ECG) and natriuretic peptides act as gatekeepers for diagnostic cardiac imaging, and thresholds during pregnancy have been proposed.22 In this cohort, more than three-quarters of women had an abnormal ECG. Screening programmes are underpinned by the notion that pre-clinical identification of a disease can lead to better outcomes through interventions that attenuate risk.23 Earlier identification of cardiac dysfunction may allow modifications to the obstetric plan, such as more judicious intravenous fluid administration and earlier delivery. Treatment of asymptomatic LVSD with disease-modifying therapies reduces the incidence of heart failure and ameliorates LV remodeling.24–26 Furthermore, delayed diagnosis and later presentation are associated with worse outcomes in women with PPCM.27
Around three-quarters of women had ‘LV recovery’ over a median of 9.7 years and recovery of cardiac function, or at least normalization of ejection fraction, continued to occur beyond 1 year. Notably, in 13% of women who had previously recovered, LVEF subsequently declined [by >10% (percentage points) and to below 50%]. It was not possible to understand whether this was explained by withdrawal of treatments, and we now know that there is a risk of deterioration if heart failure therapies are stopped after recovery.28 The current findings suggest that, for some women, recovered PPCM is not ‘cured’ and long-term follow-up may be important. A further consideration relating to LV recovery is its definition. In PPCM, recovery has been based solely on an LVEF threshold. LV remodeling is, of course, more complex than just a decrease in LVEF, with alteration in chamber shape, myocyte hypertrophy and fibrosis, and associated systemic processes such as neurohumoral activation.29 Consequently, recovery of cardiac function cannot be assessed only by an increase in LVEF. In the TRED-heart failure (HF) study, recovery encompassed LVEF, LV volumes, and biomarkers.28 In addition, abnormal longitudinal strain has been shown to persist despite improvements in LVEF in women with PPCM and may be more reliable than LVEF alone.30,31
Finally, we also found that children born to women with PPCM had worse clinical outcomes than those born to controls. In addition to more frequent neonatal complications, death from any cause occurred more often in PPCM children than in control children, with a mortality rate almost five-fold higher, mostly explained by a greater occurrence of stillbirths and early neonatal deaths (within first 7 days). Larger studies are required to explore factors that may be associated with mortality beyond the neonatal period. A further novel finding was the ∼3-times greater incidence of CV disease in PPCM children relative to control children. In the last decade, two studies have shown that ∼15% of women harbour a truncating gene variant associated with DCM, predominantly titin.32,33 It is possible that genetics (including polygenics) and resultant familial cardiomyopathy could explain some of the excess risk seen in children. Genetic testing in women with PPCM is not routinely performed worldwide. Identifying children at greater risk of inheriting a CV phenotype may provide an opportunity for earlier initiation of clinical screening, closer surveillance, and more timely treatment. In this study, privacy constraints limited our ability to describe the disease spectrum in children in detail and larger studies are needed to address this specific question.
There are some important limitations inherent to the retrospective, observational nature of this study. The true incidence of LVSD may be underestimated because it only includes hospitalized patients and, even among those admitted to hospital, heart failure may be unrecognized. Not all women had quantitative assessment of LV function and more sophisticated investigations were not routinely performed, such as cardiac magnetic resonance imaging or biomarker measurement (particularly with respect to defining LV recovery). Other unstudied factors may be relevant to the development of the condition or to outcomes, such as race or genetics. We did not have longitudinal data on medical therapy for the whole cohort and it was not possible to link changes in medical therapy to outcomes.
In summary, the incidence of PPCM in Scotland was 1 in 4950 deliveries. Disease-associations included gestational hypertensive disorders, multi-gestation and obesity. Overall, 8% of women died and 76% had recovery of cardiac function, but 13% of those who recovered had LV decline after apparent recovery. Around a third of children born to women with PPCM had an adverse neonatal outcome and their mortality rate was ∼5-times that of children born to controls, with a ∼3-times greater incidence of CV disease. PPCM is associated with considerable morbidity and mortality in both the mother and child.
Supplementary Material
Contributor Information
Alice M Jackson, BHF Glasgow Cardiovascular Research Centre, Institute of Cardiovascular and Medical Sciences, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK.
Mark Macartney, NHS National Services Scotland, Edinburgh, UK.
Katriona Brooksbank, BHF Glasgow Cardiovascular Research Centre, Institute of Cardiovascular and Medical Sciences, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK.
Carolyn Brown, Dumfries and Galloway Royal Infirmary, Dumfries, UK.
Dana Dawson, Aberdeen Cardiovascular and Diabetes Centre, University of Aberdeen, UK.
Mark Francis, Victoria Hospital, Kirkcaldy, UK.
Alan Japp, BHF Centre for Cardiovascular Science, University of Edinburgh, UK.
Vera Lennie, Aberdeen Royal Infirmary, Aberdeen, UK.
Stephen J Leslie, Raigmore Hospital, Inverness, UK.
Thomas Martin, Ninewells Hospital, Dundee, UK.
Paul Neary, Borders General Hospital, Melrose, UK.
Sowmya Venkatasubramanian, Forth Valley Royal Hospital, Larbert, UK.
Debra Vickers, Western Isles Hospital, Stornoway, UK.
Robin A Weir, Hairmyres Hospital, East Kilbride, UK.
John J V McMurray, BHF Glasgow Cardiovascular Research Centre, Institute of Cardiovascular and Medical Sciences, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK.
Pardeep S Jhund, BHF Glasgow Cardiovascular Research Centre, Institute of Cardiovascular and Medical Sciences, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK.
Mark C Petrie, BHF Glasgow Cardiovascular Research Centre, Institute of Cardiovascular and Medical Sciences, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK.
Supplementary data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
A.M.J. was awarded a BHF Clinical Research Training Fellowship (FS/18/14/33330) to fund this project. A.G.J., C.B., D.D., D.V., M.F., M.M., P.N., R.A.W., S.J.L., S.V., T.M., and V.L. report no disclosures. K.B. reports funding for an open-label, single dose study to assess the safety and efficacy of a novel patch infusor device and novel SUBCUTaneous furosemide formulation combination in patients with Heart Failure: a phase I clinical trial. SUBCUT-HF 1 (SQ Innovation AG), Use of a novel SUBCUTaneous preparation of furosemide to facilitate early supported discharge of patients with Heart Failure: a multi-centre, phase II, randomized, parallel group, active-comparator controlled trial. SUBCUT-HF II (SQ Innovation AG), DAPAgliflozin Versus Thiazide Diuretic in Patients With Heart Failure and Diuretic RESISTance. DAPA-RESIST (AstraZeneca), EMpagliflozin to PRevent worsening of LV volumes and Systolic function after Myocardial Infarction. EMPRESS-MI (Boehringer Ingelheim), Targeted Assessment in High-Risk paTients With dIAbetes to ideNtify Undiagnosed Heart Failure. TARTAN-HF (AstraZeneca), Glasgow Discovery centre for Clinical Excellence in Heart Failure, 2022–27 (ROCHE Framework), Correlation of novel BM of congestion in patients with heart failure with established clinical, invasive and imaging based BM of congestion (CONGESTION-BM), BM in patients with suspected HFpEF (BiopEF). J.J.V. reports that his employer, Glasgow University, has been paid by AstraZeneca (who market dapagliflozin) for time spent as principal/co-principal investigator of the DAPA-HF, DELIVER, DETERMINE trials and DAPA-Resist trial with dapagliflozin in heart failure and Steering Committee member for the DAPA-CKD trial with dapagliflozin in chronic kidney disease; Glasgow University has been paid by Amgen for time spent as Steering Committee member for the ATOMIC-HF, COSMIC-HF, and GALACTIC-HF trials and meetings and other activities related to these trials. Amgen has paid travel and accommodation for some of these meetings/activities; Glasgow University has been paid by Bayer for time spent as co-principal investigator of the FINEARTS trial with finerenone; Glasgow University has been paid by Cardurion for participation in a company advisory board about development in connection with drug development and design of clinical trials; Glasgow University has been paid by Cytokinetics for time spent as Steering Committee member for the GALACTIC-HF trial and meetings and other activities related to this trial. Cytokinetics has paid travel and accommodation for some of these meetings/activities; Glasgow University has been paid by GSK for time spent as Steering Committee member for two trials, ASCEND-D and ASCEND-ND, using daprodustat, and meetings related to these trials. GSK has paid travel and accommodation for some of these meetings; Glasgow University has been paid by KBP Biosciences for time spent as scientific advisor to the company to help guide clinical development in cardio-renal disease, inflammation & infection; Glasgow University has been paid by Novartis for time spent as co-principal investigator for the PARAGON-HF trial and Steering Committee member for PARADISE-MI, PERSPECTIVE and PARACHUTE-HF trials (all with sacubitril/valsartan) and meetings related to these trials. Novartis has paid travel and accommodation for some of these meetings; All these payments were made through a Consultancy with Glasgow University and personal payments were not received in relation to these trials/drugs; payment for participation on George Clinical PTY Ltd; Lecture fees from Abbott, Alkem Metabolics, Astra Zeneca, Blue Ocean Scientific Solutions Ltd., Boehringer Ingelheim, Canadian Medical and Surgical Knowledge, Emcure Pharmaceuticals Ltd., Eris Lifesciences, European Academy of CME, Hikma Pharmaceuticals, Imagica Health, Intas Pharmaceuticals, J.B. Chemicals & Pharmaceuticals Ltd., Lupin Pharmaceuticals, Medscape/Heart.Org., ProAdWise Communications, Radcliffe Cardiology, Sun Pharmaceuticals, The Corpus, Translation Research Group, Translational Medicine Academy; Consultancy fees from Alynylam Pharmaceuticals, Bayer, BMS, Ionis Pharmaceuticals, Novartis, Regeneron Pharmaceuticals, River 2 Renal Corp; Director of Global Clinical Trial Partners Ltd. M.C.P. reports grants from Boehringer Ingelheim, Roche, SQ Innovations, Astra Zeneca, Novartis, Novo Nordisk, Medtronic, Boston Scientific, Horizon, Pharmacosmos; Consulting fees from Boehringer Ingelheim, Novartis, Astra Zeneca, Novo Nordisk, Abbvie, Bayer, Takeda, Corvia, Cardiorentis, Pharmacosmos, Siemens, Vifor; Lecture fees from Boehringer Ingelheim, Novartis, Astra Zeneca, Novo Nordisk, Abbvie, Bayer, Takeda, Corvia, Cardiorentis, Pharmacosmos, Siemens, Vifor; Board participation for Teikoku, Astra Zeneca; Director of Global Clinical Trial Partners Ltd. P.S.J. reports grants from Boehringer Ingelheim, Analog Devices Inc., AstraZeneca, Roche Diagnostics; his employer, Glasgow University, has been remunerated for work on clinical trials with AstraZeneca, Novartis, Novo Nordisk, Bayer; Speaker fees and/or advisory board fees with AztraZeneca, Novartis, Boehringer Ingelheim; Lecture fees from AstraZeneca, Novartis, Inta Pharma, Sun Pharmaceuticals, ProAdwise; Director of Global Clinical Trial Partners Ltd.
Data Availability
Data are not routinely available in line with the NHS National Services Scotland confidentiality policy.
Funding
The study was funded by the British Heart Foundation (BHF) through a BHF Clinical Research Training Fellowship (FS/18/14/33330) awarded to A.M.J. J.J.V., P.S.J., and M.C.P. are funded by the BHF Centre of Research Excellence Grant RE/18/6/34217 and the Vera Melrose Heart Failure Research Fund.
Ethical Approval
Approval was given by the Public Benefit and Privacy Panel of NHS National Services Scotland (Ref:1617-0359), West of Scotland Regional Ethics Committee (Ref:GN18CA603), and Independent Group Advising on the Release of Data (Ref:DARS-NIC-262206-F1P5Z).
Pre-registered Clinical Trial Number
None—not a clinical trial.
References
- 1. Sliwa K, Hilfiker-Kleiner D, Petrie MC, Mebazaa A, Pieske B, Buchmann E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on Peripartum Cardiomyopathy. Eur J Heart Fail 2010;12:767–78. 10.1093/eurjhf/hfq120 [DOI] [PubMed] [Google Scholar]
- 2. Isogai T, Kamiya CA. Worldwide incidence of peripartum cardiomyopathy and overall maternal mortality. Int Heart J 2019;60:503–11. 10.1536/ihj.18-729 [DOI] [PubMed] [Google Scholar]
- 3. Ersbøll AS, Johansen M, Damm P, Rasmussen S, Vejlstrup NG, Gustafsson F. Peripartum cardiomyopathy in Denmark: a retrospective, population-based study of incidence, management and outcome. Eur J Heart Fail 2017;19:1712–20. 10.1002/ejhf.882 [DOI] [PubMed] [Google Scholar]
- 4. Barasa A, Rosengren A, Sandström TZ, Ladfors L, Schaufelberger M. Heart failure in late pregnancy and postpartum: incidence and long-term mortality in Sweden from 1997 to 2010. J Card Fail 2017;23:370–8. 10.1016/j.cardfail.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 5. Davis MB, Arany Z, McNamara DM, Goland S, Elkayam U. Peripartum cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol 2020;75:207–21. 10.1016/j.jacc.2019.11.014 [DOI] [PubMed] [Google Scholar]
- 6. Sliwa K, Bauersachs J, Arany Z, Spracklen TF, Hilfiker-Kleiner D. Peripartum cardiomyopathy: from genetics to management. Eur Heart J 2021;42:3094–102. 10.1093/eurheartj/ehab458 [DOI] [PubMed] [Google Scholar]
- 7. Sliwa K, Petrie MC, van der Meer P, Mebazza A, Hilfiker-Kleiner D, Jackson AM, et al. Clinical presentation, management and 6-month outcomes in women with peripartum cardiomyopathy, an ESC EORP registry. Eur Heart J 2020;41:3787–97. 10.1093/eurheartj/ehaa455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haghikia A, Podewski E, Libhaber E, Labidi S, Fischer D, Roentgen P, et al. Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol 2013;108:366. 10.1007/s00395-013-0366-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moulig V, Pfeffer TJ, Ricke-Hoch M, Schlothauer S, Koenig T, Schwab J, et al. Long-term follow-up in peripartum cardiomyopathy patients with contemporary treatment: low mortality, high cardiac recovery, but significant cardiovascular co-morbidities. Eur J Heart Fail 2019;21:1534–42. 10.1002/ejhf.1624 [DOI] [PubMed] [Google Scholar]
- 10. SMR Datasets | Episode Management | SMR Record Type | ISD Scotland | Data Dictionary [Internet https://www.ndc.scot.nhs.uk/Data-Dictionary/SMR-Datasets/Episode-Management/SMR-Record-Type/ (16 April 2020, date last accessed).
- 11. NHS National Services Scotland . Assessment of SMR01 Data Scotland 2014-2015 [Internet]. https://www.isdscotland.org/Products-and-Services/Data-Quality/docs/Assessment-of-SMR01-Data-2014-15-report-181019.pdf (16 April 2020, date last accessed).
- 12. NHS Digital . Hospital Episode Statistics (HES)—NHS Digital [Internet]. 2019. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics (15 September 2019, date last accessed).
- 13. National Records of Scotland . Birth Time Series Data. [Internet]. National Records of Scotland. https://www.nrscotland.gov.uk/statistics-and-data/statistics/statistics-by-theme/vital-events/births/births-time-series-data (26 April 2021, date last accessed).
- 14. Office for National Statistics . Vital Statistics in the UK: Births, Deaths and Marriages. [Internet]. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/vitalstatisticspopulationandhealthreferencetables (26 April 2021, date last accessed).
- 15. Rothman K, Greenland S, Lash T. Modern Epidemiology [Internet]. 2008. https://www.annemergmed.com/article/S0196-0644(08)01394-2/abstract (3 January 2022, date last accessed).
- 16. Karaye KM, Ishaq NA, Sa’idu H, Balarabe SA, Talle MA, Adamu UG, et al. Incidence, clinical characteristics, and risk factors of peripartum cardiomyopathy in Nigeria: results from the PEACE Registry. ESC Heart Fail 2020;7:236–44. 10.1002/ehf2.12562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kamiya CA, Kitakaze M, Ishibashi-Ueda H, Nakatani S, Murohara T, Tomoike H, et al. Different characteristics of peripartum cardiomyopathy between patients complicated with and without hypertensive disorders. Results from the Japanese Nationwide survey of peripartum cardiomyopathy. Circ J 2011;75:1975–81. 10.1253/circj.CJ-10-1214 [DOI] [PubMed] [Google Scholar]
- 18. Chung AK, Das SR, Leonard D, Peshock RM, Kazi F, Abdullah SM, et al. Women have higher left ventricular ejection fractions than men independent of differences in left ventricular volume: the Dallas heart study. Circulation 2006;113:1597–604. 10.1161/CIRCULATIONAHA.105.574400 [DOI] [PubMed] [Google Scholar]
- 19. Rich MW. Peripartum cardiomyopathy and pregnancy-associated heart failure with preserved ejection fraction: more similar than different. J Card Fail 2021;27:157–8. 10.1016/j.cardfail.2021.01.015 [DOI] [PubMed] [Google Scholar]
- 20. Bello N, Rendon ISH, Arany Z. The relationship between pre-eclampsia and peripartum cardiomyopathy: a systematic review and meta-analysis. J Am Coll Cardiol 2013;62:1715–23. 10.1016/j.jacc.2013.08.717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Savu O, Jurcuţ R, Giuşcǎ S, Van Mieghem T, Gussi I, Popescu BA, et al. Morphological and functional adaptation of the maternal heart during pregnancy. Circ Cardiovasc Imaging 2012;5:289–97. 10.1161/CIRCIMAGING.111.970012 [DOI] [PubMed] [Google Scholar]
- 22. Dockree S, Brook J, Shine B, James T, Vatish M. Pregnancy-specific reference intervals for BNP and NT-pro BNP—changes in natriuretic peptides related to pregnancy. J Endocr Soc 2021;5:1–9. 10.1210/jendso/bvab091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organisation . Screening Programmes: A Short Guide. Increase Effectiveness, Maximize Benefits and Minimize Harm [Internet]. https://apps.who.int/iris/bitstream/handle/10665/330829/9789289054782-eng.pdf? sequence=1&isAllowed=y (14 January 2022, date last accessed).
- 24. SOLVD Investigators; Yusuf S, Pitt B, Davis CE, Hood WB Jr, Cohn JN. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med 1992;327:685–91. 10.1056/NEJM199209033271003 [DOI] [PubMed] [Google Scholar]
- 25. Colucci WS, Kolias TJ, Adams KF, Armstrong WF, Ghali JK, Gottlieb SS, et al. Metoprolol reverses left ventricular remodeling in patients with asymptomatic systolic dysfunction: the Reversal of Ventricular Remodeling with Toprol-XL (REVERT) trial. Circulation 2007;116:49–56. 10.1161/CIRCULATIONAHA.106.666016 [DOI] [PubMed] [Google Scholar]
- 26. Ceconi C, Freedman SB, Tardif JC, Hildebrandt P, McDonagh T, Gueret P, et al. Effect of heart rate reduction by ivabradine on left ventricular remodeling in the echocardiographic substudy of beautiful. Int J Cardiol 2011;146:408–14. 10.1016/j.ijcard.2010.10.125 [DOI] [PubMed] [Google Scholar]
- 27. Lewey J, Levine LD, Elovitz MA, Irizarry OC, Arany Z. Importance of early diagnosis in peripartum cardiomyopathy. Hypertension 2020;75:91–7. 10.1161/HYPERTENSIONAHA.119.13291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Halliday BP, Wassall R, Lota AS, Khalique Z, Jackson R, Rahneva T, et al. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED-HF): an open-label, pilot, randomised trial. Lancet 2019;393:61–73. 10.1016/S0140-6736(18)32484-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol 2000;35:569–82. 10.1016/S0735-1097(99)00630-0 [DOI] [PubMed] [Google Scholar]
- 30. Bortnick AE, von Buchwald C L, Hasani A, Liu C, Berkowitz JL, Vega S, et al. Persistence of abnormal global longitudinal strain in women with peripartum cardiomyopathy. Echocardiography 2021;38:885–91. 10.1111/echo.15071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Briasoulis A, Mocanu M, Marinescu K, Qaqi O, Palla M, Telila T, et al. Longitudinal systolic strain profiles and outcomes in peripartum cardiomyopathy. Echocardiography 2016;33:1354–60. 10.1111/echo.13277 [DOI] [PubMed] [Google Scholar]
- 32. Ware JS, Arany Z, Kealey A, Liu P, Cook SA, Safirstein J, et al. Shared genetic predisposition in peripartum and dilated cardiomyopathies. N Engl J Med 2016;374:233–41. 10.1056/NEJMoa1505517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goli R, Li J, Brandimarto J, Levine LD, Riis V, McAfee Q, et al. Genetic and phenotypic landscape of peripartum cardiomyopathy. Circulation 2021;143:1852–62. 10.1161/CIRCULATIONAHA.120.052395 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are not routinely available in line with the NHS National Services Scotland confidentiality policy.