Graphical Abstract
Graphical Abstract.
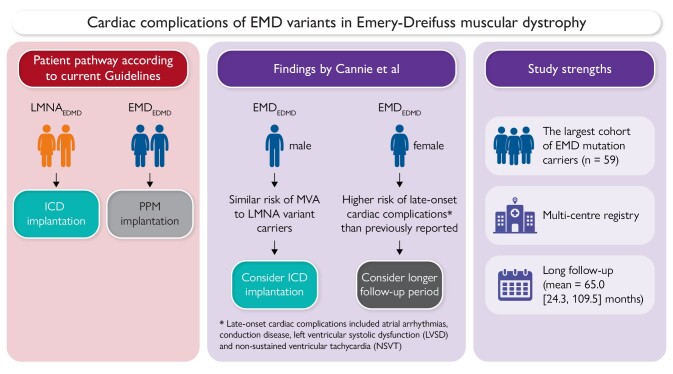
A comparative overview of current guideline-recommended patient pathways vs. findings by Cannie et al.5
This editorial refers to ‘Emery–Dreifuss muscular dystrophy Type 1 is associated with a high risk of malignant ventricular arrhythmias and end-stage heart failure’, by D.E. Cannie et al., https://doi.org/10.1093/eurheartj/ehad561.
Emery–Dreifuss muscular dystrophy (EDMD) first gained distinct recognition in the 1960s through studies that differentiated it from other forms of muscular dystrophy such as Duchenne and Becker.1 Most documented cases of EDMD fall into one of two categories: emerinopathy (EDMD1), associated with variants in the EMD gene which is inherited in a recessive X-linked manner, or laminopathy (EDMD2) caused by pathogenic variants in the LMNA gene which generally follows an autosomal dominant inheritance pattern. EDMD affects both genders, although males are obviously predominantly affected in X-linked forms. The disease presents a complex ‘triad within a triad’ of symptoms: early contractures appearing in the first decade, progressive muscle weakness becoming evident by the second or third decade, and significant cardiac complications.
These cardiac complications often first manifest as atrial tachyarrhythmias and atrial standstill, but often progress to ventricular tachyarrhythmias and cardiomyopathy. Symptoms such as palpitations, pre-syncope, syncope, and exercise intolerance usually start as early as the second decade of life, often preceding the onset of significant skeletal muscle weakness. While skeletal muscle symptoms in EDMD1 predominantly affect men due to its X-linked inheritance, both genders are impacted in EDMD2.2 Cardiac complications, including atrioventricular (AV) conduction defects and atrial arrhythmias, are common to both types. However, dilated cardiomyopathy (DCM) and fatal ventricular arrhythmias have typically been reported more frequently in patients with LMNA variants.3 Current guidelines reflect these differences, recommending implantable cardioverter defibrillators (ICDs) for LMNA variant carriers, while patients with EMD-related bradyarrhythmia are often considered for permanent pacemakers (PPMs) alone. Despite these guidelines, there is a notable lack of evidence supporting these practices, particularly concerning the potential use of ICDs in EMD variant carriers.4
In this issue of the European Heart Journal, Cannie and colleagues perform a multi-centre study that aims to fill this gap by comparing the incidence of malignant ventricular arrhythmia (MVA) and end-stage heart failure (ESHF) in both male and female carriers of both EMD and LMNA variants.5 The study demonstrates that, quite contrary to current thinking, males with pathogenic EMD variants face a risk of MVA comparable with those with pathogenic LMNA variants, with incidence rates of 4.8 and 6.6 per 100 person-years for EMD and LMNA variant carriers, respectively (P = .49). This finding challenges the existing guidelines, which have primarily advocated for pacemaker implantation as a preventive measure for EMD variant carriers. Given the similar MVA risk profiles between EMD and LMNA variants, the study strongly recommends considering early ICD implantation in male EMD patients.
However, the risk profiles for MVA between EMD and LMNA variants, while similar, are not identical. Male EMD variant carriers tend to have a higher incidence of neuromuscular involvement than LMNA variant carriers (70% vs. 12%). For the remaining 30% of EMD variant carriers who do not show neuromuscular symptoms, the term ‘cardiac emerinopathy’ has been discussed to characterize their distinct cardiac phenotype. These patients also tend to present earlier with cardiac symptoms and maintain a higher left ventricular ejection fraction (LVEF) over time, resulting in fewer cases with left ventricular systolic dysfunction compared with LMNA carriers. Interestingly, current guidelines for ICD implantation in LMNA variant carriers often hinge on the presence of reduced left ventricular systolic function as one of the primary prognostic stratifiers for ICD implantation in patients with non-ischaemic DCM. However, recent studies challenge the adequacy of considering LVEF for this purpose. For instance, the DANISH trial did not highlight any survival benefit among non-ischaemic DCM patients who underwent prophylactic ICD implantation based solely on reduced LVEF.6 Moreover, research by Gatzoulis et al.7 and Escobar-Lopez et al.8 suggests that even patients with LVEF >35% may benefit from ICD intervention. Incorporating genetic variants and other clinical markers could offer a more nuanced understanding of arrhythmic risks, thereby informing more effective ICD implantation strategies.
Interestingly, even though ICD implantation is not part of current guideline recommendations for EMD variant carriers, no significant differences were observed in this study regarding the rates of ICD implantation between EMD and LMNA carriers, both at baseline and during follow-up. This finding suggests that decisions regarding ICD implantation may be influenced by factors other than established guidelines, particularly when made in centres with specialized expertise. This deviation from standard protocols underscores the need for a more nuanced approach to risk stratification. It raises the question of whether current protocols are sufficiently comprehensive and adaptable to account for the complexities of individual cases. The observation also highlights the importance of revisiting and refining these protocols, incorporating insights from specialized centres and recent research to ensure that they are as effective and tailored as possible.
Two other findings from this study are particularly notable. The secondary ESHF endpoint was more often reached in LMNA variant carriers, though this was not statistically significant given the modest sample sizes of the study (P = .09). Nevertheless, the substantial proportion of EMD variant carriers developing ESHF (15.2%) suggests that the left ventricular function of EDMD patients should be regularly monitored regardless of the underlying genotype. Secondly, cardiac disease in female EMD variant carriers was much less severe than in males, as expected for an X-linked disorder. However, 42.9% of female patients did develop late-onset (median 58.6 years) cardiac complications which included atrial arrhythmias, conduction disease, left ventricular systolic dysfunction, and non-sustained ventricular tachycardia. The proportion of affected females was higher than in previous reports,9 probably reflecting the longer follow-up time and older age of the current cohort, and highlights the need for continuing clinical monitoring of female EMD variant carriers in middle age and beyond.
The study by Cannie et al. highlights the increasing importance of developing evidence-based clinical guidelines that are tailored to key characteristics of individual patients, such as genetic status and sex. For example, increasing cohort sizes for DCM are enabling the specific characteristics of the underlying causative genotypes to be elucidated in more detail.8 For less prevalent diseases, however, and even rarer genetic subtypes of these diseases, it can be extremely challenging to design studies that are powered to derive these insights. Indeed, one of the inherent challenges in advancing our understanding of cardiac complications in EMD variant carriers with EDMD lies in its rarity. The low prevalence (0.13:100 000) of this condition significantly hampers the ability to conduct large-scale, statistically robust studies.10 This limitation leads to an underevaluation of patient prognosis and cardiac outcomes. Consequently, the evidence regarding cardiac complications is based on case reports and low-numbered longitudinal case studies.3,11,12
While multi-centre studies and reviews present a viable solution to these challenges, they often suffer from a shortfall in ongoing quality control and sustainability. One notable issue is the inconsistency in coding variables across different centres, which can lead to data heterogeneity and compromise the integrity of the study. Additionally, varying interpretations and understandings of the disease among different healthcare institutions can further skew results and conclusions. Multi-centre registries, on the other hand, can collect standardized data from various healthcare institutions, offering a broader view of the clinical landscape and enabling researchers to draw more generalizable conclusions.13
Currently, Europe is home to >700 rare disease registries, a significant achievement made possible through the collaborative efforts of Orphanet and European Reference Networks (ERNs). Orphanet serves as a comprehensive database for rare diseases and orphan drugs, while ERNs focus on specialized care for complex or rare conditions. ERN GUARD-Heart is a specialized department within ERNs that concentrates on heart-related diseases. These registries are invaluable for collecting patient data, understanding disease patterns, and facilitating research which would be difficult or impossible to achieve with single-centre approaches.14,15 Beyond their academic and medical utility, these registries have also proven to be instrumental in the legal arena. For instance, new evidence from the CALM registry was crucial in re-evaluating a significant legal case, leading to a re-examination of a long-standing conviction.16
In conclusion, the study by Cannie et al. offers crucial insights into the management of EDMD, particularly in relation to EMD variant carriers. It challenges existing guidelines by revealing that males with EMD variants face a similar risk of MVA to those with LMNA variants. The study also sheds light on the cardiac complications in female EMD variant carriers, emphasizing the need for ongoing clinical monitoring in this demographic as well. Furthermore, the study highlights the indispensable role of multi-centre studies and registries in enhancing our understanding and treatment of rare diseases.
Contributor Information
Daria Kramarenko, Department of Experimental Cardiology, Heart Centre, Amsterdam UMC location, University of Amsterdam, Meibergdreef 9, Amsterdam, The Netherlands.
Roddy Walsh, Department of Experimental Cardiology, Heart Centre, Amsterdam UMC location, University of Amsterdam, Meibergdreef 9, Amsterdam, The Netherlands.
Declarations
Disclosure of Interest
D.K. is a member of the European Reference Network for Rare, Low Prevalence and Complex Diseases of the Heart (ERNGUARD-Heart), Amsterdam, The Netherlands. The other author declare no conflict of interest for this contribution.
References
- 1. Emery AE, Dreifuss FE. Unusual type of benign X-linked muscular dystrophy. J Neurol Neurosurg Psychiatry 1966;29:338–342. 10.1136/jnnp.29.4.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heller SA, Shih R, Kalra R, Kang PB. Emery–Dreifuss muscular dystrophy. Muscle Nerve 2020;61:436–448. 10.1002/mus.26782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang S, Peng D. Cardiac involvement in Emery–Dreifuss muscular dystrophy and related management strategies. Int Heart J 2019;60:12–18. 10.1536/ihj.17-604 [DOI] [PubMed] [Google Scholar]
- 4. Wilde AAM, Semsarian C, Márquez MF, Sepehri Shamloo A, Ackerman MJ, Ashley EA, et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) expert consensus statement on the state of genetic testing for cardiac diseases. Heart Rhythm 2022;19:e1–e60. 10.1016/j.hrthm.2022.03.1225 [DOI] [PubMed] [Google Scholar]
- 5. Cannie DE, Syrris P, Protonotarios A, Bakalakos A, Pruny J-F, Ditaranto R, et al. Emery–Dreifuss muscular dystrophy Type 1 is associated with a high risk of malignant ventricular arrhythmias and end-stage heart failure. Eur Heart J 2023;44:5064–5073. 10.1093/eurheartj/ehad561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Køber L, Thune JJ, Nielsen JC, Haarbo J, Videbæk L, Korup E, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 2016;375:1221–1230. 10.1056/NEJMoa1608029 [DOI] [PubMed] [Google Scholar]
- 7. Gatzoulis KA, Vouliotis A-I, Tsiachris D, Salourou M, Archontakis S, Dilaveris P, et al. Primary prevention of sudden cardiac death in a nonischemic dilated cardiomyopathy population: reappraisal of the role of programmed ventricular stimulation. Circ Arrhythm Electrophysiol 2013;6:504–512. 10.1161/CIRCEP.113.000216 [DOI] [PubMed] [Google Scholar]
- 8. Escobar-Lopez L, Ochoa JP, Mirelis JG, Espinosa MÁ, Navarro M, Gallego-Delgado M, et al. Association of genetic variants with outcomes in patients with nonischemic dilated cardiomyopathy. J Am Coll Cardiol 2021;78:1682–1699. 10.1016/j.jacc.2021.08.039 [DOI] [PubMed] [Google Scholar]
- 9. Viggiano E, Madej-Pilarczyk A, Carboni N, Picillo E, Ergoli M, Gaudio SD, et al. X-linked Emery–Dreifuss muscular dystrophy: study of X-chromosome inactivation and its relation with clinical phenotypes in female carriers. Genes (Basel) 2019;10:919. 10.3390/genes10110919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Norwood FLM, Harling C, Chinnery PF, Eagle M, Bushby K, Straub V. Prevalence of genetic muscle disease in Northern England: in-depth analysis of a muscle clinic population. Brain 2009;132:3175–3186. 10.1093/brain/awp236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buckley AE, Dean J, Mahy IR. Cardiac involvement in Emery Dreifuss muscular dystrophy: a case series. Heart 1999;82:105–108. 10.1136/hrt.82.1.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boriani G, Gallina M, Merlini L, Bonne G, Toniolo D, Amati S, et al. Clinical relevance of atrial fibrillation/flutter, stroke, pacemaker implant, and heart failure in Emery–Dreifuss muscular dystrophy: a long-term longitudinal study. Stroke 2003;34:901–908. 10.1161/01.STR.0000064322.47667.49 [DOI] [PubMed] [Google Scholar]
- 13. Hageman IC, Van Rooij IALM, De Blaauw I, Trajanovska M, King SK. A systematic overview of rare disease patient registries: challenges in design, quality management, and maintenance. Orphanet J Rare Dis 2023;18:106. 10.1186/s13023-023-02719-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Orphanet report series—rare disease registries in Europe—May 2018. Available online: http://www.orpha.net/orphacom/cahiers/docs/GB/Registries.pdf. (15 September 2023).
- 15. Condition-specific registries ERN GUARD. Available online: https://guardheart.ern-net.eu/condition-specific-registries/. (15 September 2023).
- 16. Brohus M, Arsov T, Wallace DA, Jensen HH, Nyegaard M, Crotti L, et al. Infanticide vs. inherited cardiac arrhythmias. Europace 2021;23:441–450. 10.1093/europace/euaa272 [DOI] [PMC free article] [PubMed] [Google Scholar]