Abstract
Background and Aims
Emery–Dreifuss muscular dystrophy (EDMD) is caused by variants in EMD (EDMD1) and LMNA (EDMD2). Cardiac conduction defects and atrial arrhythmia are common to both, but LMNA variants also cause end-stage heart failure (ESHF) and malignant ventricular arrhythmia (MVA). This study aimed to better characterize the cardiac complications of EMD variants.
Methods
Consecutively referred EMD variant-carriers were retrospectively recruited from 12 international cardiomyopathy units. MVA and ESHF incidences in male and female variant-carriers were determined. Male EMD variant-carriers with a cardiac phenotype at baseline (EMDCARDIAC) were compared with consecutively recruited male LMNA variant-carriers with a cardiac phenotype at baseline (LMNACARDIAC).
Results
Longitudinal follow-up data were available for 38 male and 21 female EMD variant-carriers [mean (SD) ages 33.4 (13.3) and 43.3 (16.8) years, respectively]. Nine (23.7%) males developed MVA and five (13.2%) developed ESHF during a median (inter-quartile range) follow-up of 65.0 (24.3–109.5) months. No female EMD variant-carrier had MVA or ESHF, but nine (42.8%) developed a cardiac phenotype at a median (inter-quartile range) age of 58.6 (53.2–60.4) years. Incidence rates for MVA were similar for EMDCARDIAC and LMNACARDIAC (4.8 and 6.6 per 100 person-years, respectively; log-rank P = .49). Incidence rates for ESHF were 2.4 and 5.9 per 100 person-years for EMDCARDIAC and LMNACARDIAC, respectively (log-rank P = .09).
Conclusions
Male EMD variant-carriers have a risk of progressive heart failure and ventricular arrhythmias similar to that of male LMNA variant-carriers. Early implantable cardioverter defibrillator implantation and heart failure drug therapy should be considered in male EMD variant-carriers with cardiac disease.
Keywords: Emery–Dreifuss muscular dystrophy, Emerin, Cardiomyopathy, Sudden death, Ventricular arrhythmia, Heart failure
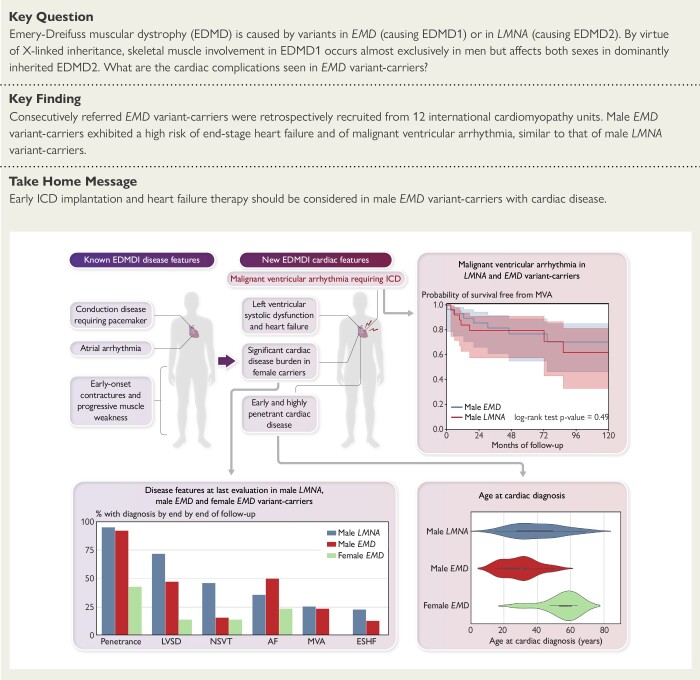
Structured Graphical Abstract
Structured Graphical Abstract.
Graphic depiction of new cardiac disease features demonstrated by this study (top left) with arrows to relevant analyses. Kaplan Meier survival analysis for the primary malignant ventricular arrhythmia endpoint during follow-up for male EMD and male LMNA variant-carriers with a baseline cardiac phenotype (top right). Cardiac disease features at last evaluation for male LMNA, male EMD and female EMD variant-carriers (bottom left). Violin plots comparing age at cardiac diagnosis for male EMD, female EMD and male LMNA variant-carriers (bottom right). Abbreviations: AF, atrial fibrillation; EDMD1, Emery–Dreifuss muscular dystrophy Type 1; ESHF, end-stage heart failure; LVSD, left ventricular systolic dysfunction; MVA, malignant ventricular arrhythmia; NSVT, non-sustained ventricular tachycardia.
See the editorial comment for this article ‘Emery–Dreifuss muscular dystrophy: a closer look at cardiac complications’, by D. Kramarenko and R. Walsh, https://doi.org/10.1093/eurheartj/ehad735.
Introduction
Emery–Dreifuss muscular dystrophy (EDMD) is a rare, progressive genetic disorder characterized by a triad of early joint contractures, progressive muscle atrophy, and cardiac abnormalities.1,2 In most cases, EDMD is inherited as an X-linked or autosomal dominant disease caused by variants in genes encoding one or more of the proteins comprising the nuclear envelope. The two most commonly implicated genes are EMD (EDMD1) and LMNA (EDMD2).3,4
By virtue of X-linked inheritance, skeletal muscle involvement in EDMD1 occurs almost exclusively in men but affects both sexes in dominantly inherited EDMD2. With respect to cardiac manifestations, atrioventricular (AV) conduction defects and atrial arrhythmia are common to both EDMD1 and 2, but dilated cardiomyopathy and fatal ventricular arrhythmia are more frequently reported in LMNA variant-carriers.5,6 In recognition of these differences, current guidelines for patients with LMNA variants and cardiac disease recommend placement of an implantable cardioverter defibrillator (ICD) irrespective of the need for cardiac pacing or the presence of skeletal muscle disease, whereas patients with EMD-related bradyarrhythmia are considered for permanent pacemakers (PPMs) alone in the absence of documented or suspected ventricular arrhythmia.7 However, given the rarity of EMD variants, there is a lack of evidence to support this practice, with no systematic analysis of life-threatening ventricular arrhythmia in patients carrying EMD variants. In this multi-centre study, we sought to compare and contrast the incidence of malignant ventricular arrhythmia (MVA) and end-stage heart failure (ESHF) in male and female carriers of EMD and LMNA variants.
Methods
Study design
This was a multi-centre, retrospective, longitudinal cohort study. The study conforms to the principles outlined in the Declaration of Helsinki,8 and participants provided written informed consent for genetic testing. The authors from each centre guaranteed the integrity of data from their institution and received approval for anonymized patient data collation and analysis from local ethics committees and institutional review boards.
Cohort composition and genetic testing
Patients and relatives with EMD variants were recruited from 12 cardiomyopathy centres in Europe and the USA. A proband was defined as an index patient presenting with features consistent with either EDMD or cardiac disease associated with a likely pathogenic or pathogenic (LP/P) variant in EMD. Relatives carrying EMD variants were recruited irrespective of disease expression.
The different modes of EMD and LMNA inheritance precluded a simple comparison between all EMD and all LMNA variant-carriers. Male EMD variant-carriers were therefore compared with a cohort of consecutive male LP/P LMNA variant-carriers, recruited from the Barts Heart Centre/University College London cardiomyopathy database. Female LMNA variant-carriers were not included in the main analysis as comparison between a cohort of males and females and one of males alone would introduce significant bias given recognized sex differences in the risk of adverse events in LMNA variant-carriers.6,9
Genetic testing in probands was undertaken using Sanger sequencing or next-generation targeted panels at participating centres or accredited diagnostic laboratories. Variants were classified using the American College of Medical Genetics and Genomics criteria.10 Where additional evidence of pathogenicity (e.g. segregation data) was available from the contributing centre, variants were reclassified accordingly. Only LP/P variants were included. Patients with additional LP/P variants in other genes associated with cardiomyopathy were excluded. Sanger sequencing was used for cascade screening of relatives.
Baseline clinical assessment
Baseline demographics, comorbidities, symptoms, 12-lead electrocardiogram (ECG), transthoracic echocardiogram, and ambulatory ECG data were collected from clinical records. Data were recorded using RedCap, a secure web application, ensuring uniformity and validity of data collection from each centre.11 A cardiac phenotype in males was defined as early-onset atrial arrhythmia (<40 years of age), high-degree AV block or sinoatrial (SA) node disease requiring a PPM, non-sustained ventricular tachycardia (NSVT, defined as three consecutive ventricular ectopic beats at a rate of >120 b.p.m.), premature ventricular complex (PVC) count >500/24 h, or left ventricular systolic dysfunction [LVSD; left ventricular ejection fraction (LVEF) <50%]. The same definitions applied to females with the exception of the age criterion for atrial arrhythmia, which was removed to ensure accurate reporting of later-onset phenotypes.
Study endpoints
Follow-up time for each patient was calculated from date of first evaluation to date of most recent evaluation, heart transplantation, or death from any cause. The primary endpoint was MVA defined as sudden cardiac death (SCD), aborted SCD, appropriate ICD shock or anti-tachycardia pacing, or sustained ventricular tachycardia (VT). A secondary endpoint of ESHF consisted of left ventricular assist device implantation, heart transplantation, or heart failure-related death. The term conduction disease was defined as high-degree AV block (Mobitz Type II fixed AV block or complete heart block) or SA node disease (including atrial standstill) that required PPM implantation. Patients were censored at the time of their first endpoint event during follow-up or at their last evaluation.
Statistical analysis
All data were anonymized, and statistical analyses were performed using the Python programming language (Version 3.8, Python Software Foundation).12 Continuous variables were tested for normality of distribution by visual inspection of histograms and statistical normality tests (Shapiro–Wilk). Normally distributed variables are expressed as mean ± SD and non-normally distributed variables as median (25th–75th percentiles). Categorical variables are reported as counts and percentages, as appropriate. The TableOne library was used for the construction of summary statistics tables and for all statistical comparisons.13 The Seaborn and Matplotlib libraries were utilized for data visualization.14
The Lifelines library was used for all time-to-event analyses.15 Kaplan–Meier plots were used to display the cumulative probability of the occurrence of endpoints and to compare male EMD and LMNA cohorts. The log-rank test was used to compare survival. The impact of competing risk from ESHF outcomes on the incidence of MVA was assessed using Aalen–Johansen and Fine–Gray estimates.16 The zEpid library was used to calculate incidence rates.17
Cox regression was used to assess the association of baseline variables with the primary endpoint after confirmation that the proportional hazards assumption was supported by the data. The robust sandwich estimator was used to obtain standard errors to adjust for correlations within families. P-values <.05 were considered significant.
Results
Sixty-four LP/P EMD variant-carriers were identified (41 males and 23 females) (Figure 1 and Supplementary data online, Table S1). Five variant-carriers (three males and two females) were excluded due to insufficient baseline and follow-up data; two of the excluded males had undergone heart transplantation. The cohort for analysis therefore comprised 38 males and 21 females from 29 families. Twenty six of 38 males (68.4%) were probands with no female probands. Year of baseline assessment ranged between 1995 and 2022.
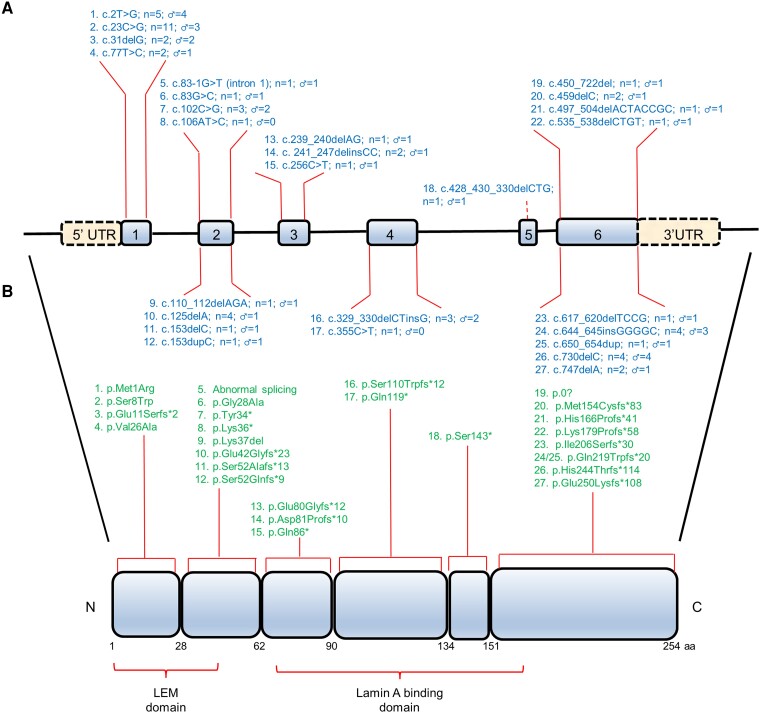
Figure 1.
Schematic representation of the EMD gene (A) and emerin protein (B) structure. Numbered boxes represent EMD exons. Functional domains containing variants in dilated cardiomyopathy patients in this study are shown under the EMD protein. The position of variants identified in our study, the total number of individuals, and male patients carrying each variant are depicted along length of the gene and protein. aa; amino acid residues; C, C-terminus; LEM domain, LAP2, emerin, MAN1 domain; N, N-terminus; UTR, untranslated region.
Comparator cohort
Thirty-nine consecutive male LP/P LMNA variant-carriers [15/39 (38.5%) probands] were recruited for comparison with male EMD variant-carriers (see Supplementary data online, Table S2). For comparison of the primary and secondary endpoints, only those with a baseline cardiac phenotype (LMNACARDIAC) were compared with male EMD variant-carriers with a baseline cardiac phenotype (EMDCARDIAC). Comparison of EMDCARDIAC males with a cohort of all consecutive LMNACARDIAC variant-carriers (males and females) can be found in the Supplementary data online (Supplementary data online, Tables S3–S5 and Figure S1).
Baseline characteristics
Baseline characteristics of male and female EMD variant-carriers are shown in Table 1. Mean (SD) age for males at first cardiac evaluation was 33.4 (13.3). Thirty-three of 38 (86.8%) had a cardiac phenotype at baseline evaluation. Two males (5.3%) had MVA prior to baseline, and 28/38 (73.7%) had neuromuscular involvement.
Table 1.
Baseline characteristics of male and female EMD variant-carriers
| Missing | Female | Male | |
|---|---|---|---|
| n | 21 | 38 | |
| Proband, n (%) | 0 | 0 | 26 (68.4) |
| Presence of cardiac phenotype, n (%) | 0 | 3 (14.3) | 33 (86.8) |
| Age at first evaluation, mean (SD) | 0 | 43.3 (16.8) | 33.4 (13.3) |
| White ethnicity, n (%) | 1 | 21 (100.0) | 35 (94.6) |
| MVA prior to baseline, n (%) | 0 | 0 | 2 (5.3) |
| Prior history of heart failure, n (%) | 0 | 0 | 4 (10.5) |
| Hypertension, n (%) | 0 | 5 (23.8) | 2 (5.3) |
| Diabetes mellitus, n (%) | 0 | 0 | 2 (5.3) |
| Prior conduction disease, n (%) | 0 | 1 (4.8) | 16 (42.1) |
| Device implanted by baseline, n (%) | 0 | 1 (4.8) | 17 (44.7) |
| ICD implanted by baseline, n (%) | 0 | 0 | 8 (21.1) |
| Prior atrial arrhythmia, n (%) | 0 | 0 | 16 (42.1) |
| Previous CVA, n (%) | 0 | 0 | 1 (2.6) |
| Neuromuscular involvement, n (%) | 0 | 0 | 28 (73.7) |
| Cardiac symptoms at baseline assessment, n (%) | 0 | 7 (33.3) | 16 (42.1) |
| Shortness of breath, n (%) | 0 | 2 (9.5) | 9 (23.7) |
| NYHA Class III or IV, n (%) | 0 | 0 | 2 (5.3) |
| Syncope, n (%) | 0 | 0 | 2 (5.3) |
| Palpitations, n (%) | 0 | 6 (28.6) | 5 (13.2) |
| On cardiac medications at baseline, n (%) | 1 | 6 (28.6) | 20 (54.1) |
| Sinus rhythm, n (%) | 1 | 19 (95.0) | 17 (44.7) |
| Baseline ECG paced, n (%) | 0 | 1 (4.8) | 11 (28.9) |
| PR interval (ms), median (Q1–Q3) | 30 | 162.5 (136.5–178.8) | 192.0 (154.0–224.5) |
| QRS duration (ms), median (Q1–Q3) | 17 | 85.0 (84.5–102.5) | 106.0 (96.0–115.0) |
| LBBB, n (%) | 19 | 0 | 2 (7.4) |
| LV internal diameter (diastole) (mm), median (Q1–Q3) | 7 | 46.0 (43.5–49.0) | 53.0 (48.0–58.0) |
| LV ejection fraction (%), median (Q1–Q3) | 1 | 62.0 (57.5–65.0) | 57.5 (45.0–64.0) |
| LA diameter (mm), median (Q1–Q3) | 23 | 32.0 (30.0–35.0) | 40.0 (35.0–48.8) |
| RV dilatation, n (%) | 0 | 0 | 3 (8.8) |
| PVCs/24 h on first Holter, median (Q1–Q3) | 30 | 40.0 (6.8–185.2) | 80.0 (1.5–1083.0) |
CVA, cerebrovascular accident; ECG, electrocardiogram; ICD, implantable cardioverter defibrillator; LA, left atrium; LBBB, left bundle branch block; LV, left ventricular; MVA, malignant ventricular arrhythmia; NYHA, New York Heart Association; PVC, premature ventricular complex; RV, right ventricular.
No females were probands, and none had features of neuromuscular disease. Three females had cardiac disease consistent with an EMD phenotype at baseline evaluation (one with AV block requiring a PPM in her seventh decade, one with a history of frequent PVCs and LVSD at baseline, and one with atrial tachycardia on baseline Holter monitor).
Clinical follow-up and first features of cardiac disease
Median [inter-quartile range (IQR)] follow-up time for EMD variant-carriers was 65.0 (24.3–109.5) months. Follow-up data are shown in Table 2. Median (IQR) age at diagnosis of cardiac disease for males was 30.5 (19.5–34.4) years.
Table 2.
Outcomes for male and female EMD variant-carriers
| Female | Male | |
|---|---|---|
| n | 21 | 38 |
| Presence of LVSD by end follow-up, n (%) | 3 (14.3) | 18 (47.4) |
| Cardiac phenotype by end follow-up, n (%) | 9 (42.9) | 35 (92.1) |
| Age at cardiac phenotype, median (Q1–Q3) | 58.6 (53.2–60.4) | 30.5 (19.5–34.4) |
| Atrial fibrillation or flutter, n (%) | 5 (23.8) | 19 (50.0) |
| Non-sustained VT, n (%) | 3 (14.3) | 6 (15.8) |
| Cerebrovascular accident, n (%) | 2 (9.5) | 3 (7.9) |
| Pacemaker only in situ, n (%) | 2 (9.5) | 9 (23.7) |
| ICD in situ, n (%) | 2 (9.5) | 21 (55.3) |
| Inappropriate shock, n (%) | 0 | 3 (14.3) |
| Appropriate shock, n (%) | 0 | 4 (19.0) |
| Anti-tachycardia pacing only, n (%) | 0 | 3 (14.3) |
| Sustained VT or VF, n (%) | 0 | 8 (21.1) |
| Presence conduction disease by end follow-up, n (%) | 2 (9.5) | 20 (52.6) |
| New-onset conduction disease, n (%) | 1 (4.8) | 4 (10.5) |
| Cardiac arrest, n (%) | 0 | 1 (2.6) |
| Heart failure admission, n (%) | 0 | 6 (15.8) |
| LV assist device implantation, n (%) | 0 | 1 (2.6) |
| Heart transplantation, n (%) | 0 | 3 (7.9) |
| Heart failure death, n (%) | 0 | 1 (2.6) |
| Sudden cardiac death, n (%) | 0 | 1 (2.6) |
| Death, n (%) | 0 | 3 (7.9) |
LV, left ventricular; LVSD, left ventricular systolic dysfunction; ICD, implantable cardioverter defibrillator; VT, ventricular tachycardia; VF, ventricular fibrillation.
The most common initial features of cardiac disease in male carriers were atrial arrhythmia (17/38, 45%) and conduction disease (14/38, 37%) (5 had both atrial arrhythmia and conduction disease). Six of 14 patients with conduction disease had isolated SA node disease, 4 had isolated high-degree AV block, and 6 had both SA node disease and high-degree AV block.
Non-sustained ventricular tachycardia, PVC count >500 over 24 h, and heart failure were the first features of cardiac disease in 5/38 (13%), 2/38 (5.3%), and 6/38 (16%) individuals, respectively. Two male carriers were found to have LVSD on screening in the absence of symptoms. One male in his fourth decade had sustained ventricular arrhythmia and cardiac arrest at baseline having had no prior cardiac phenotype; his contemporary LVEF was 35%. By the end of follow-up, 35/38 (92%) male carriers had features of cardiac disease consistent with an EMD phenotype and 18/38 (47.4%) had LVSD. Thirty individuals (78.9%) had an implanted cardiac device (9 PPMs and 21 ICDs).
Nine of 38 (23.7%) males had MVA during follow-up at a median (IQR) age of 41.1 (37.4–62.1) years. The male carrier with cardiac arrest at presentation had further sustained ventricular arrhythmia with multiple appropriate ICD shocks. A male in his fifth decade with a PPM in situ died suddenly. A post-mortem pacemaker download showed rapid, sustained VT concurrent with collapse, and an echocardiogram 2 weeks prior had shown an LVEF of 43%. Neither of the two males with MVA events prior to baseline evaluation suffered further MVA during follow-up.
Univariable Cox regression identified multiple variables associated with the primary MVA endpoint including LVEF {HR 1.08 per 1% decrement [95% confidence interval (CI) 1.06–1.11], P < .001} and left ventricular internal diameter at end diastole [hazard ratio (HR) 1.22 per 1 mm increase (95% CI 1.11–1.33), P < .001] (see Supplementary data online, Table S6). Age at first evaluation, proband status, and NSVT were not associated with the primary MVA endpoint.
Female EMD variant-carriers
No females reached the primary MVA outcome or secondary ESHF outcome. Nine of 21 (42.9%) female variant-carriers developed cardiac disease consistent with an EMD phenotype. The median (IQR) age at first detection of a cardiac abnormality was 58.6 (53.2–60.4) years.
Three females had LVSD by the end of follow-up. Female 1, previously noted to have frequent PVCs, developed LVSD with regional wall motion abnormalities (RWMAs) in her seventh decade. She also required a PPM for SA disease and had atrial fibrillation (AF) in the same year. Female 2 was found to have mild LVSD and RWMAs in her fourth decade during pregnancy. This persisted post-partum with the development of left bundle branch block (LBBB) on electrocardiogram (ECG). Female 3 was found to have mild LVSD with RWMAs in her sixth decade while attending for screening, and NSVT was identified on Holter monitor. An ICD was implanted, and device interrogation demonstrated atrial tachycardia and AF.
Female 4, from the same family as female 3, had normal left ventricular systolic function but was found to have symptomatic and prolonged episodes of NSVT and had an ICD implanted in her fifth decade.
In total, five female variant-carriers developed AF during follow-up, all over the age of 55 years. An additional female developed atrial tachycardia in her sixth decade. Two females had ischaemic strokes at the ages of 59 and 63 years (only one with documented AF). Seven of nine females with a cardiac phenotype were carriers of EMD variants resulting in a truncated protein. The remaining two females carried missense variants.
EMD males compared with LMNA males
The 38 male EMD variant-carriers were compared with 39 male LMNA variant-carriers with respect to age at cardiac diagnosis and age at onset of conduction disease.
Median (IQR) age at cardiac diagnosis was 30.5 (19.5–34.4) years for male EMD variant-carriers and 36.9 (28.4–49.0) years for male LMNA variant-carriers (P = .003) (Figure 2). At last evaluation, 35/38 male EMD variant-carriers and 38/39 male LMNA variant-carriers had evidence of a cardiac phenotype. Of the four males without a cardiac phenotype by last evaluation, only one was older than 21 years of age, a 70-year-old EMD variant-carrier with a neuromuscular phenotype who did not meet criteria for a cardiac phenotype with AF onset at 65 years of age.
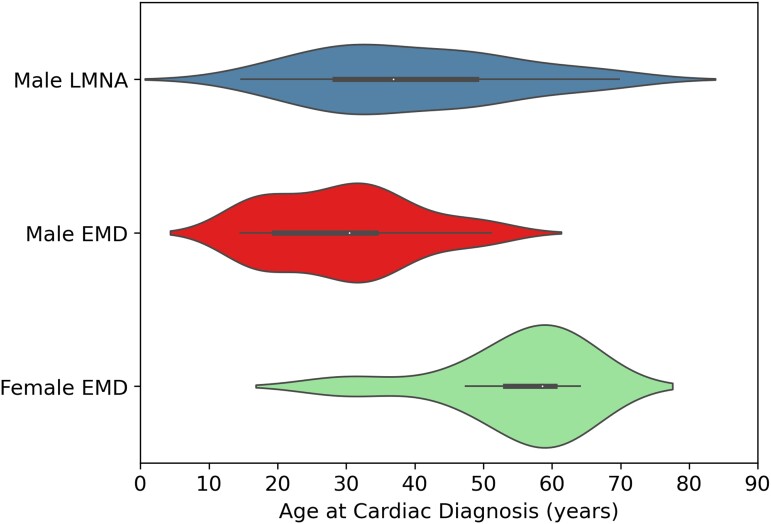
Figure 2.
Violin plots comparing age at cardiac diagnosis for male EMD variant-carriers, female EMD variant-carriers, and male LMNA variant-carriers. Male EMD variant-carriers had cardiac disease features at an earlier age than male LMNA variant-carriers. Female EMD variant-carriers demonstrate late-onset cardiac disease.
Median (IQR) age at onset of conduction disease was 32.4 (23.8–40.8) for male EMD variant-carriers and 44.4 (34.8–50.0) years for male LMNA variant-carriers (P = .001) (see Supplementary data online, Figure S2). At last evaluation, 20/38 (52.6%) male EMD variant-carriers and 16/39 (41.0%) male LMNA variant-carriers had developed conduction disease (P = .43).
Outcomes for the 33 male EMD variant-carriers with a baseline cardiac phenotype (EMDCARDIAC) were compared with those of 26 male LMNA variant-carriers with a baseline cardiac phenotype (LMNACARDIAC). Baseline characteristics and outcomes are shown in Supplementary data online, Tables S7 and S8. Median (IQR) follow-up time for LMNACARDIAC was 48.5 (30–117) months, and for EMDCARDIAC, it was 71 (29–110) months. Male EMD variant-carriers with a baseline cardiac phenotype were younger at presentation, and more had neuromuscular disease. Male LMNA variant-carriers with a baseline cardiac phenotype had significantly lower LVEFs at baseline evaluation.
More LMNACARDIAC had an LVEF < 50% by the end of follow-up; however, this did not translate into more heart failure admissions. NSVT was more frequent in the LMNACARDIAC cohort. The EMDCARDIAC cohort had more SA node disease requiring pacing. Three EMDCARDIAC males and one LMNACARDIAC male had MVA at or prior to baseline and were therefore excluded from survival analysis of the primary MVA endpoint.
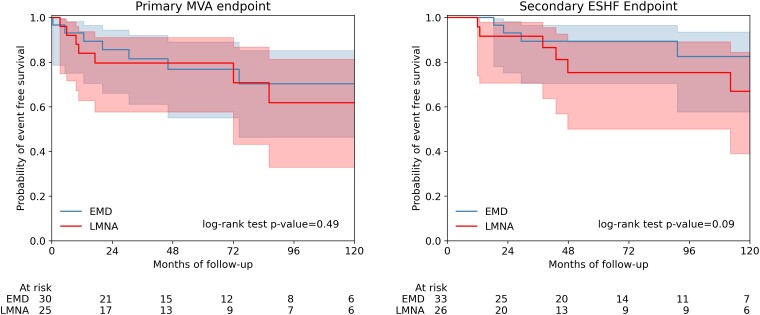
The primary MVA endpoint was reached by 9/25 (36%) of LMNACARDIAC vs. 8/30 (26.7%) of EMDCARDIAC; log-rank test P = .49 (Figure 3). The incidence of the primary MVA endpoint in the EMDCARDIAC cohort was 4.8 per 100 person-years vs. 6.6 per 100 person-years for the LMNACARDIAC cohort [incidence rate ratio of 0.72 (0.28–1.86)].
Figure 3.
Kaplan–Meier survival analysis for the primary malignant ventricular arrhythmia (left) and secondary end-stage heart failure (right) endpoints during follow-up for male EMD and LMNA variant-carriers with a baseline cardiac phenotype. Males with prior or baseline malignant ventricular arrhythmia were excluded from survival analysis of the primary malignant ventricular arrhythmia endpoint but included for the secondary end-stage heart failure endpoint.
Univariable Cox regression showed that having a LMNA variant conferred no significant additional hazard to having an EMD variant for the primary MVA endpoint [HR 1.4 (95% CI 0.55–3.5), P = .48]. LMNA variants showed no association with the primary MVA endpoint in a multivariable model including age at first evaluation [HR 1.39 (95% CI 0.53–3.6], P = .50]. There was no significant impact on the primary outcome from the competing risk of ESHF events (Fine–Gray P = .55; Supplementary data online, Figure S3).
Of the eight EMDCARDIAC cohort that reached the primary MVA endpoint, only one had previously documented NSVT, and this was recorded 2 weeks prior to SCD. Data on LVEF contemporary to MVA (within 1 year) were limited to four patients, all of whom had an LVEF ≥ 35%.
The ESHF endpoint was reached by 9/26 (34.6%) LMNACARDIAC vs. 5/33 (15.2%) of EMDCARDIAC; log rank test P = .09 (Figure 3). Incidence rates for ESHF endpoint were 2.4 per 100 person-years for EMDCARDIAC and 5.9 per 100 person-years for LMNACARDIAC [incidence rate ratio 0.42 (0.14–1.23)].
Discussion
We present the first longitudinal cohort study of EMD variant-carriers derived from tertiary cardiac centres and show that males with EMD variants are prone to life-threatening ventricular arrhythmia and progressive heart failure (Structured Graphical Abstract). These findings have important implications for the management of this patient group and provide further evidence of the utility of genetic testing in risk stratification.18
Prevention of sudden cardiac death in EMD variant-carriers
The cardinal features of EMD-related heart disease are reported to be early-onset conduction disease and atrial arrhythmia. Sudden cardiac death is described, but as the predominant mechanism is thought to be bradyarrhythmia,19 practice guidelines continue to emphasize pacemaker implantation rather than early ICD implantation for primary prevention.7 This is in marked contrast to recommendations for other nuclear envelopathies, in particular those caused by LMNA or TMEM43 variants, where early ICD implantation is advised based upon data showing a high risk of fatal ventricular arrhythmia.20
A previous single-centre study comparing EMD and LMNA variant-carriers showed an association between LMNA variants and all-cause mortality but did not compare MVA or ESHF outcomes or stratify the cohorts by sex.21 A single male EMD variant-carrier was reported as having MVA. A second, single-centre and cross-sectional study of male EMD variant-carriers did not report any MVA.22
In this study, we show that the risk of MVA is similar in patients with cardiac disease caused by variants in EMD and LMNA, despite the fact that more LMNA variant-carriers had LVSD. The discrepancy between the incidence of MVA in EMD variant-carriers in this cohort compared with those previously reported could be related to multiple factors. A difference in the age of participants is likely important. Previous cohorts were, on average, ∼10 or more years younger, and therefore, only a small proportion of patients might be expected to reach the median (IQR) age of MVA we report of 41.1 (37.4–62.1) years by last evaluation. Previous cohorts were also only representative of a small number of families from single centres in comparison with the 29 families from 12 international centres reported here.
EMD variant-carriers developed conduction disease earlier than LMNA variant-carriers, and 42% of all male EMD variant-carriers had a device implanted prior to first evaluation at a specialist cardiac unit. By last evaluation, and in contrast to current guidelines, just over two-thirds of male EMD variant-carriers with a device had an ICD, likely reflecting the expertise of the centres participating in this study. On balance, the high rate of MVA observed in EMD variant-carriers suggests that, as in patients with cardiac laminopathy, early ICD implantation should be strongly considered in individuals with cardiac expression and should be preferred over pacemakers alone in patients with conventional pacing indications.
Progressive heart failure in EMD variant-carriers
Dilated cardiomyopathy and heart failure in EMD variant-carriers are described in case reports.19,23 In our study, LVSD was observed in just under half of all male EMD variant-carriers, and 13% developed ESHF suggesting that regular monitoring of left ventricular function should be routine. It was also notable that over a quarter of male EMD variant-carriers with cardiac involvement had no reported neuromuscular disease, supporting recent work by Ishikawa et al.24 who suggested the term ‘cardiac emerinopathy’ to describe those patients with an isolated cardiac phenotype.
Cardiac disease in female carriers
Viggiano et al.25 reported a cross-sectional cohort of 30 female EMD variant-carriers with one-third over 50 years of age. Seventeen per cent had cardiac involvement (first- or second-degree AV block not requiring a pacemaker), all of whom were over 50 years of age. The proportion of cardiac disease in females was considerably higher in our study (43%), likely reflecting the older age of the cohort with nearly two-thirds of females over 50 years old by last evaluation. Atrial arrhythmia was the most frequent abnormality, but conduction disease, NSVT, and LVSD were also observed.
The mechanisms underlying disease expression in female carriers are not well understood. Skewed X-inactivation is common in the general population,26 increases with age,27 and has been shown to correlate with development of symptoms in female carriers in Duchenne’s and Becker’s muscular dystrophies.28,29 Marked reduction in the emerin content of lymphocytes and lymphoblastoid cell lines have been described in a female carrier,30 but in a study of 28 symptomatic and asymptomatic female carriers, skewed X-inactivation in EMD did not correlate with cardiac symptoms.25 The authors acknowledged that the X-inactivation pattern in lymphocytes may not reflect that in cardiac tissues. More recent work in a symptomatic female carrier of a truncating EMD variant demonstrated a mixed population of emerin-positive and emerin-negative myoblasts, ruling out skewed X-inactivation as a mechanism. Preferential proliferation of emerin-negative myoblasts and a high rate of spontaneous differentiation in both cell populations were reported and with time, this would lead to a depletion of emerin-positive satellite cells and might explain a later onset EMD phenotype.31
Irrespective of the mechanism, the presence of cardiac disease in female EMD variant-carriers, at a higher prevalence than in the general population, highlights the need for regular clinical review.32,33
Limitations
As EDMD is a rare disease, this study is limited by the small cohort size. The use of RedCap11 aimed to mitigate against the unstandardized data collection that can be a flaw in retrospective, multi-centre studies. As data were collected from cardiomyopathy units only, there is limited information on neuromuscular phenotypes. Many patients were paced by a cardiac device at first evaluation leading to a significant proportion of missingness of ECG parameters. Future studies combining adult and paediatric cardiology and neurology services are required.
Conclusions
In addition to the well-established features of atrial arrhythmia and conduction disease, the cardiac phenotype of male EMD variant-carriers includes progressive heart failure and ventricular arrhythmias. The risk of MVA in males is similar to that of male LMNA variant-carriers. Female EMD variant-carriers have a high frequency of cardiac disease after the fifth decade of life.
Supplementary Material
Acknowledgements
The authors are grateful to the patients and their families whose contributions have made this work possible. This study was supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
Contributor Information
Douglas E Cannie, Institute of Cardiovascular Science, University College London, London, UK; Department of Inherited Cardiovascular Diseases, Barts Heart Centre, St Bartholomew’s Hospital, London, UK.
Petros Syrris, Institute of Cardiovascular Science, University College London, London, UK; Department of Inherited Cardiovascular Diseases, Barts Heart Centre, St Bartholomew’s Hospital, London, UK.
Alexandros Protonotarios, Institute of Cardiovascular Science, University College London, London, UK; Department of Inherited Cardiovascular Diseases, Barts Heart Centre, St Bartholomew’s Hospital, London, UK.
Athanasios Bakalakos, Institute of Cardiovascular Science, University College London, London, UK; Department of Inherited Cardiovascular Diseases, Barts Heart Centre, St Bartholomew’s Hospital, London, UK.
Jean-François Pruny, APHP, Sorbonne Université, Centre de Référence pour les Maladies Cardiaques Héréditaires ou rares, ICAN Institute, Hôpital Pitié-Salpêtrière, Paris, France.
Raffaello Ditaranto, Cardiology Unit, Cardiac Thoracic and Vascular Department, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy; European Reference Network for Rare and Low Prevalence Complex Diseases of the Heart (ERN-GUARDHEART).
Cristina Martinez-Veira, Unidad de Cardiopatías Familiares, Complexo Hospitalario Universitario de A Coruña, Instituto de Investigación Biomédica de A Coruña (INIBIC/CIBERCV), Servizo Galego de Saúde (SERGAS), Universidade da Coruña, A Coruña, Spain.
Jose M Larrañaga-Moreira, Unidad de Cardiopatías Familiares, Complexo Hospitalario Universitario de A Coruña, Instituto de Investigación Biomédica de A Coruña (INIBIC/CIBERCV), Servizo Galego de Saúde (SERGAS), Universidade da Coruña, A Coruña, Spain.
Kristen Medo, Cardiovascular Institute and Adult Medical Genetics Program, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
Francisco José Bermúdez-Jiménez, Cardiology Department, Hospital Universitario Virgen de las Nieves, Instituto de Investigación Biosanitaria IBS Granada, Granada, Spain.
Rabah Ben Yaou, APHP-Sorbonne Universite, Centre de Référence des Maladies Neuromusculaires, Inserm, Centre de Recherche en Myologie, Institut de Myologie, Hopital Pitie-Salpetriere, Paris, France.
France Leturcq, APHP, Cochin Hospital, Department of Genomic Medicine and Systemic Diseases, University of Paris, Paris, France.
Ainhoa Robles Mezcua, Heart Failure and Familial Cardiomyopathies Unit, Department of Cardiology, IBIMA, Málaga. Spain; Ciber-Cardiovascular, Instituto de Salud Carlos III, Madrid, Spain.
Chiara Marini-Bettolo, Department of Cardiology, Newcastle upon Tyne Hospitals NHS Trust, Newcastle upon Tyne, UK; The John Walton Muscular Dystrophy Research Centre, Translational and Clinical Research Institute, Newcastle University and Newcastle Hospitals NHS Foundation Trust, Newcastle upon Tyne, United Kingdom.
Eva Cabrera, European Reference Network for Rare and Low Prevalence Complex Diseases of the Heart (ERN-GUARDHEART); Heart Failure and Inherited Cardiac Diseases Unit, Department of Cardiology, Hospital Universitario Puerta de Hierro, IDIPHISA, CIBERCV, Madrid, Spain.
Chloe Reuter, Stanford Center for Inherited Cardiovascular Disease, Division of Cardiovascular Medicine, Department of Medicine, Stanford University School of Medicine, 291 Campus Drive, Stanford, CA 94305, USA.
Javier Limeres Freire, European Reference Network for Rare and Low Prevalence Complex Diseases of the Heart (ERN-GUARDHEART); Ciber-Cardiovascular, Instituto de Salud Carlos III, Madrid, Spain; Inherited Cardiovascular Diseases Unit, Department of Cardiology, Hospital Universitari Vall d´Hebron, Vall d’Hebron Institut de Recerca (VHIR), Universitat Autònoma de Barcelona, Barcelona, Spain.
José F Rodríguez-Palomares, European Reference Network for Rare and Low Prevalence Complex Diseases of the Heart (ERN-GUARDHEART); Ciber-Cardiovascular, Instituto de Salud Carlos III, Madrid, Spain; Inherited Cardiovascular Diseases Unit, Department of Cardiology, Hospital Universitari Vall d´Hebron, Vall d’Hebron Institut de Recerca (VHIR), Universitat Autònoma de Barcelona, Barcelona, Spain.
Luisa Mestroni, Cardiovascular Institute and Adult Medical Genetics Program, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
Matthew R G Taylor, Cardiovascular Institute and Adult Medical Genetics Program, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
Victoria N Parikh, Stanford Center for Inherited Cardiovascular Disease, Division of Cardiovascular Medicine, Department of Medicine, Stanford University School of Medicine, 291 Campus Drive, Stanford, CA 94305, USA.
Euan A Ashley, Stanford Center for Inherited Cardiovascular Disease, Division of Cardiovascular Medicine, Department of Medicine, Stanford University School of Medicine, 291 Campus Drive, Stanford, CA 94305, USA.
Roberto Barriales-Villa, Unidad de Cardiopatías Familiares, Complexo Hospitalario Universitario de A Coruña, Instituto de Investigación Biomédica de A Coruña (INIBIC/CIBERCV), Servizo Galego de Saúde (SERGAS), Universidade da Coruña, A Coruña, Spain.
Juan Jiménez-Jáimez, Cardiology Department, Hospital Universitario Virgen de las Nieves, Instituto de Investigación Biosanitaria IBS Granada, Granada, Spain.
Pablo Garcia-Pavia, European Reference Network for Rare and Low Prevalence Complex Diseases of the Heart (ERN-GUARDHEART); Heart Failure and Inherited Cardiac Diseases Unit, Department of Cardiology, Hospital Universitario Puerta de Hierro, IDIPHISA, CIBERCV, Madrid, Spain; Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid, Spain.
Philippe Charron, APHP, Sorbonne Université, Centre de Référence pour les Maladies Cardiaques Héréditaires ou rares, ICAN Institute, Hôpital Pitié-Salpêtrière, Paris, France; European Reference Network for Rare and Low Prevalence Complex Diseases of the Heart (ERN-GUARDHEART).
Elena Biagini, Cardiology Unit, Cardiac Thoracic and Vascular Department, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy; European Reference Network for Rare and Low Prevalence Complex Diseases of the Heart (ERN-GUARDHEART).
José M García Pinilla, Heart Failure and Familial Cardiomyopathies Unit, Department of Cardiology, IBIMA, Málaga. Spain; Ciber-Cardiovascular, Instituto de Salud Carlos III, Madrid, Spain; Departamento de Medicina y Dermatología, Universidad de Malaga, Malaga, Spain.
John Bourke, Department of Cardiology, Newcastle upon Tyne Hospitals NHS Trust, Newcastle upon Tyne, UK; The John Walton Muscular Dystrophy Research Centre, Translational and Clinical Research Institute, Newcastle University and Newcastle Hospitals NHS Foundation Trust, Newcastle upon Tyne, United Kingdom.
Konstantinos Savvatis, Institute of Cardiovascular Science, University College London, London, UK; Department of Inherited Cardiovascular Diseases, Barts Heart Centre, St Bartholomew’s Hospital, London, UK; William Harvey Institute, Queen Mary University London, London, United Kingdom; National Institute for Health Research, University College London Hospitals Biomedical Research Centre, London, United Kingdom.
Karim Wahbi, AP-HP, Pitié-Salpêtrière Hospital, Reference Center for Muscle Diseases Paris-Est, Myology Institute, Paris, France; AP-HP, Cochin Hospital, Cardiology Department, Paris Cedex, France; Université de Paris, Paris, France; Paris Cardiovascular Research Center (PARCC), INSERM Unit 970, Paris, France.
Perry M Elliott, Institute of Cardiovascular Science, University College London, London, UK; Department of Inherited Cardiovascular Diseases, Barts Heart Centre, St Bartholomew’s Hospital, London, UK.
Supplementary data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
E.A.A.: Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: Personalis, Novartis, Deepcell, Foresite Capital, Bristol Myers Squibb, Sequence Bio, Svexa, Takeda, AstraZeneca, Medical Excellence Capital, Apple, NuevoCor, RCD CO, Sequence Bio, and Parameter Health. C.M.-B.: Funding from Biogen and Roche towards a grant for Adult Spinal muscular atrophy network and database. Consultancy honoraria from Biogen, Roche, and Novartis to present at meetings. Travel to London supported by Novartis to present at meeting. J.B.: Consulting fees from Sarepta Advisory Board Member for gene therapy trials in DMD and EspeRare Foundation, Switzerland, Advisory Board Member. Invited speaker 23rd National Congress of Italian Muscle Society, June 2023 and Parent Project Muscular Dystrophy, New Orleans, USA—Duchenne Cardiac Care Meeting, March 2023, with expenses paid. Participation on a Data Safety Monitoring Board or Advisory Board: Sarepta gene therapy studies DMC member. D.E.C.: British Heart Foundation Clinical Research Training Fellowship (FS/CRTF/20/24022). P.C.: Consulting fees from Pfizer, Amicus, Bristol-Myers Squibb, and Sanofi and support to attend meetings from Pfizer and Sanofi. P.M.E.: Research grant from Sarepta. Consulting fees from Pfizer, BMS, Biomarin, Novonordisk, and Cytokinetics. Payment or Honoria for lectures from Pfizer and Sanofi. Patents planned for novel high-throughput, multiplex, targeted proteomic plasma assay (UK Patent: GB1815111) and gene therapy compositions and treatment of right ventricular arrhythmogenic cardiomyopathy (US Pat No 62/903,103). Trial steering committee member for Cytokinetics. Board member of European Society Cardiology, president of Cardiomyopathy UK, Chairman of International Cardiomyopathy Network. L.M.: Support for present manuscript: NIH/NCATS Colorado CTSA Grant Numbers UL1 TR002535, NIH/NHLBI R01HL164634, and NIH/NHLBI R01HL153325 (payments to institution). Additional grants or contracts from Greenstone bioscience and SHaRe DCM (payments to institution). V.N.P.: NHLBI K08HL143185. Consulting fees from Nuevocor, Viz.ai, Biomarin, and Lexeo therapeutics. Stocks or stock options with Lexeo Therapeutics and Constantiam. A.P.: British Heart Foundation Clinical Research Training Fellowship (FS/18/82/34024).
Data Availability
Patient-level data will not be made available as consent was not sought for public dissemination, and due to concerns, that information could be used to identify individuals.
Funding
The work reported in this publication was funded by: a British Heart Foundation Clinical Research Training Fellowship to D.E.C. (FS/CRTF/20/24022); a British Heart Foundation Clinical Research Training fellowship to A.P. (FS/18/82/34024); The Ministry of Health, Italy, project RC-2022-2773270 to E.B.; the National Institutes of Health (NIH) (R01HL69071, R01HL116906, R01HL147064, NIH/NCATS UL1 TR002535, and UL1 TR001082) to L.M.; and support from the Rose Foundation for K.M.
Ethical Approval
The study conforms to the principles outlined in the Declaration of Helsinki, and participants provided written informed consent for genetic testing. The authors from each centre guaranteed the integrity of data from their institution and received approval for anonymized patient data collation and analysis from local ethics committees and institutional review boards.
Pre-registered Clinical Trial Number
None supplied.
References
- 1. Emery AE. Emery-Dreifuss syndrome. J Med Genet 1989;26:637–41. 10.1136/JMG.26.10.637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mah JK, Korngut L, Fiest KM, Dykeman J, Day LJ, Pringsheim T, et al. A systematic review and meta-analysis on the epidemiology of the muscular dystrophies. Can J Neurol Sci 2016;43:163–77. 10.1017/CJN.2015.311 [DOI] [PubMed] [Google Scholar]
- 3. Bione S, Maestrini E, Rivella S, Mancini M, Regis S, Romeo G, et al. Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat Genet 1994;8:323–7. 10.1038/NG1294-323 [DOI] [PubMed] [Google Scholar]
- 4. Bonne G, Di Barletta MR, Varnous S, Bécane HM, Hammouda EH, Merlini L, et al. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat Genet 1999;21:285–8. 10.1038/6799 [DOI] [PubMed] [Google Scholar]
- 5. Boriani G, Gallina M, Merlini L, Bonne G, Toniolo D, Amati S, et al. Clinical relevance of atrial fibrillation/flutter, stroke, pacemaker implant, and heart failure in Emery-Dreifuss muscular dystrophy: a long-term longitudinal study. Stroke 2003;34:901–8. 10.1161/01.STR.0000064322.47667.49 [DOI] [PubMed] [Google Scholar]
- 6. Wahbi K, Ben Yaou R, Gandjbakhch E, Anselme F, Gossios T, Lakdawala NK, et al. Development and validation of a new risk prediction score for life-threatening ventricular tachyarrhythmias in laminopathies. Circulation 2019;140:293–302. 10.1161/circulationaha.118.039410 [DOI] [PubMed] [Google Scholar]
- 7. Wilde AAM, Semsarian C, Márquez MF, Sepehri Shamloo A, Ackerman MJ, Ashley EA, et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) expert consensus statement on the state of genetic testing for cardiac diseases. Heart Rhythm 2022;19:e1–e60. 10.1016/J.HRTHM.2022.03.1225 [DOI] [PubMed] [Google Scholar]
- 8. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–4. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 9. van Rijsingen IAW, Nannenberg EA, Arbustini E, Elliott PM, Mogensen J, Hermans-van Ast JF, et al. Gender-specific differences in major cardiac events and mortality in lamin A/C mutation carriers. Eur J Heart Fail 2013;15:376–84. 10.1093/EURJHF/HFS191 [DOI] [PubMed] [Google Scholar]
- 10. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–24. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/J.JBI.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Rossum G. Python 3 Reference Manual. Scotts Valley: CA CreateSpace; 2009. [Google Scholar]
- 13. Pollard TJ, Johnson AEW, Raffa JD, Mark RG. Tableone: an open source Python package for producing summary statistics for research papers. JAMIA Open 2018;1:26–31. 10.1093/JAMIAOPEN/OOY012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Waskom ML. Seaborn: statistical data visualization. J Open Source Softw 2021;6:3021. 10.21105/JOSS.03021 [DOI] [Google Scholar]
- 15. Davidson-Pilon C. Lifelines: survival analysis in Python. J Open Source Softw 2019;4:1317. 10.21105/JOSS.01317 [DOI] [Google Scholar]
- 16. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 17. Zivich P. zEpid. https://github.com/pzivich/zEpid (24 January 2023, date last accessed).
- 18. Tayal U, Ware JS, Lakdawala NK, Heymans S, Prasad SK. Understanding the genetics of adult-onset dilated cardiomyopathy: what a clinician needs to know. Eur Heart J 2021;42:2384–96. 10.1093/EURHEARTJ/EHAB286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sakata K, Shimizu M, Ino H, Yamaguchi M, Terai H, Fujino N, et al. High incidence of sudden cardiac death with conduction disturbances and atrial cardiomyopathy caused by a nonsense mutation in the STA gene. Circulation 2005;111:3352–8. 10.1161/CIRCULATIONAHA.104.527184 [DOI] [PubMed] [Google Scholar]
- 20. Zeppenfeld K, Tfelt-Hansen J, De Riva M, Winkel BG, Behr ER, Blom NA, et al. 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2022;43:3997–4126. 10.1093/EURHEARTJ/EHAC262 [DOI] [PubMed] [Google Scholar]
- 21. Marchel M, Madej-Pilarczyk A, Steckiewicz R, Stolarz P, Peller M, Tymińska A, et al. Predictors of mortality and cardiovascular outcomes in Emery-Dreifuss muscular dystrophy in a long-term follow-up. Kardiol Pol 2021;79:1335–42. 10.33963/KP.A2021.0159 [DOI] [PubMed] [Google Scholar]
- 22. Yunisova G, Ceylaner S, Oflazer P, Deymeer F, Parman YG, Durmus H. Clinical and genetic characteristics of Emery-Dreifuss muscular dystrophy patients from Turkey: 30 years longitudinal follow-up study. Neuromuscul Disord 2022;32:718–27. 10.1016/J.NMD.2022.07.397 [DOI] [PubMed] [Google Scholar]
- 23. Finsterer J, Stöllberger C, Sehnal E, Rehder H, Laccone F. Dilated, arrhythmogenic cardiomyopathy in Emery-Dreifuss muscular dystrophy due to the emerin splice-site mutation c.449 + 1G > A. Cardiology 2015;130:48–51. 10.1159/000368222 [DOI] [PubMed] [Google Scholar]
- 24. Ishikawa T, Mishima H, Barc J, Takahashi MP, Hirono K, Terada S, et al. Cardiac emerinopathy: a nonsyndromic nuclear envelopathy with increased risk of thromboembolic stroke due to progressive atrial standstill and left ventricular noncompaction. Circ Arrhythm Electrophysiol 2020;13:1165–74. 10.1161/CIRCEP.120.008712 [DOI] [PubMed] [Google Scholar]
- 25. Viggiano E, Madej-Pilarczyk A, Carboni N, Picillo E, Ergoli M, Del Gaudio S, et al. X-Linked Emery–Dreifuss muscular dystrophy: study of X-chromosome inactivation and its relation with clinical phenotypes in female carriers. Genes (Basel) 2019;10:919. 10.3390/GENES10110919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shvetsova E, Sofronova A, Monajemi R, Gagalova K, Draisma HHM, White SJ, et al. Skewed X-inactivation is common in the general female population. Eur J Hum Genet 2018;27:455–65. 10.1038/s41431-018-0291-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Busque L, Mio R, Mattioli J, Brais E, Biais N, Lalonde Y, et al. Nonrandom X-inactivation patterns in normal females: lyonization ratios vary with age. Blood 1996;88:59–65. 10.1182/blood.v88.1.59.bloodjournal88159 [DOI] [PubMed] [Google Scholar]
- 28. Viggiano E, Picillo E, Ergoli M, Cirillo A, Del Gaudio S, Politano L. Skewed X-chromosome inactivation plays a crucial role in the onset of symptoms in carriers of Becker muscular dystrophy. J Gene Med 2017;19:e2952. 10.1002/jgm.2952 [DOI] [PubMed] [Google Scholar]
- 29. Viggiano E, Ergoli M, Picillo E, Politano L. Determining the role of skewed X-chromosome inactivation in developing muscle symptoms in carriers of Duchenne muscular dystrophy. Hum Genet 2016;135:685–98. 10.1007/S00439-016-1666-6 [DOI] [PubMed] [Google Scholar]
- 30. Manilal S, Recan D, Sewry CA, Hoeltzenbein M, Llense S, Leturcq F, et al. Mutations in Emery-Dreifuss muscular dystrophy and their effects on emerin protein expression. Hum Mol Genet 1998;7:855–64. 10.1093/hmg/7.5.855 [DOI] [PubMed] [Google Scholar]
- 31. Meinke P, Schneiderat P, Srsen V, Korfali N, Lê Thành P, Cowan GJM, et al. Abnormal proliferation and spontaneous differentiation of myoblasts from a symptomatic female carrier of X-linked Emery-Dreifuss muscular dystrophy. Neuromuscul Disord 2015;25:127–36. 10.1016/J.NMD.2014.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alonso A, Alam AB, Kamel H, Subbian V, Qian J, Boerwinkle E, et al. Epidemiology of atrial fibrillation in the all of us research program. PLoS One 2022;17:e0265498. 10.1371/JOURNAL.PONE.0265498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics—2021 update: a report from the American Heart Association. Circulation 2021;143:E254–743. 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Patient-level data will not be made available as consent was not sought for public dissemination, and due to concerns, that information could be used to identify individuals.