Key Points
Question
What constituted meaningful within-patient score improvement on the Psoriasis Symptoms and Signs Diary (PSSD) in a phase 3 trial among patients with psoriasis?
Findings
In this predefined analysis of 666 patients using data from the phase 3 POETYK PSO-1 clinical trial, the patient-perceived meaningfulness of a minimum 15-point improvement in the PSSD score was supported by the Patient Global Impression of Change (PGI-C), and the meaningfulness of a 25-point change was supported by both the PGI-C and the Patient Global Impression of Severity. A 30-point threshold identified patients who experienced greater improvements in their psoriasis symptoms and signs.
Meaning
These findings suggest that patient-reported, anchor-based methods established meaningful change thresholds for PSSD score changes from baseline, enabling better interpretation of results with this instrument.
Abstract
Importance
Change from baseline score on the validated Psoriasis Symptoms and Signs Diary (PSSD) is a widely used, patient-reported end point in clinical trials for psoriasis. Meaningful score change thresholds anchored to patient-reported assessments have not been established in a clinical trial setting.
Objective
To evaluate meaningful within-patient score change thresholds for the PSSD using data from the phase 3 Program to Evaluate the Efficacy and Safety of Deucravacitinib, a Selective TYK2 Inhibitor (POETYK), PSO-1 clinical trial, which compared the efficacy and safety of deucravacitinib vs placebo and apremilast among adults with moderate to severe plaque psoriasis.
Design, Setting, and Participants
In this predefined analysis using data from the POETYK PSO-1 multicenter, randomized, double-blind, placebo-controlled phase 3 clinical trial, conducted from August 7, 2018, to September 2, 2020, 666 adults with moderate to severe plaque psoriasis completed the PSSD daily throughout the trial. Meaningful change thresholds were derived by anchoring mean PSSD score change from baseline to week 16 to category improvements on the Patient Global Impression of Change (PGI-C) and the Patient Global Impression of Severity (PGI-S).
Interventions
Deucravacitinib, 6 mg, once daily; placebo; or apremilast, 30 mg, twice daily.
Main Outcome and Measures
The main outcome was score change from baseline to week 16 on the PSSD, anchored to the PGI-C and PGI-S.
Results
The trial included 666 patients (mean [SD] age, 46.1 [13.4] years; 453 men [68.0%]). Three thresholds were identified using an analysis set of 609 patients. Score improvement of at least 15 points from baseline reflected meaningful within-patient change anchored to the PGI-C. Score improvements of 25 points were supported by both the PGI-C and the PGI-S, while a 30-point score change identified patients with greater improvements in their psoriasis symptoms and signs.
Conclusions and Relevance
This analysis suggests that PSSD score improvements of 15, 25, or 30 points represent increasing improvements in disease burden that are meaningful to patients with psoriasis.
This predefined analysis uses data from the phase 3 POETYK PSO-1 clinical trial to evaluate meaningful within-patient score change thresholds for the Psoriasis Symptoms and Signs Diary.
Introduction
The Psoriasis Symptoms and Signs Diary (PSSD) is a validated patient-reported outcome instrument that assesses patient-perceived psoriasis sign and symptom severity.1,2,3 A PSSD score change of −40 points from baseline, derived with reference to clinician-assessed anchors, has been proposed as a threshold reflecting clinically meaningful improvement.2 However, regulatory guidelines, including recent draft guidance issued by the US Food and Drug Administration, recommend that thresholds defining responders on patient-reported outcome instruments be derived using patient-reported, rather than clinician-rated, anchor-based methods4,5 and suggest the Patient Global Impression of Change (PGI-C) and the Patient Global Impression of Severity (PGI-S) as suitable anchors.5 A meaningful within-patient change threshold (MWPCT)6 for the PSSD using patient-reported anchors has not been identified in clinical trials, to our knowledge. We describe the determination of MWPCTs for the PSSD incorporating patient perspectives using pooled, treatment-blinded PSSD, PGI-S, and PGI-C data from baseline to week 16 in the Program to Evaluate the Efficacy and Safety of Deucravacitinib, a Selective TYK2 Inhibitor (POETYK), PSO-1,7 a recent phase 3 clinical trial in plaque psoriasis.
Methods
POETYK PSO-1 was conducted from August 7, 2018, to September 2, 2020, in accordance with the Declaration of Helsinki.8 Independent institutional review board approval was obtained from all 154 sites, and all participants provided written informed consent (NCT03624127) (trial protocols and statistical analysis plans in Supplement 1), which applies to this analysis. The clinical trial collected patients’ baseline demographics, including race and ethnicity (patients were categorized as Asian, Black, White, or other). The secondary analysis presented here did not stratify patients by race or ethnicity. Patients (N = 666; eFigure 1 in Supplement 2) completed the PSSD daily throughout the trial, rating 5 skin symptoms (itch, pain, stinging, burning, and tightness) and 6 skin signs (dryness, cracking, scaling, shedding or flaking, redness, and bleeding) associated with psoriasis on an 11-point scale from 0 (absent) to 10 (worst imaginable). If more than 3 items were missing, the domain score was considered missing. To obtain symptom and sign scores, responses within each domain were averaged and multiplied by 10. Domain summary scores were averaged to derive total scores ranging from 0 to 100; higher scores indicated greater disease burden.1,9 Weekly scores were calculated by averaging daily scores for each 7-day period; baseline PSSD scores were calculated from daily diary data collected during screening. If more than 3 days of the weekly period were missing, that week’s score was set to missing.
Any patient who completed at least 1 PSSD item at baseline and a postbaseline visit was included in the derivation analysis (n = 609; deucravacitinib, n = 303; placebo, n = 150; apremilast, n = 156). At week 16, patients responded to the PGI-C question, “Since you started taking the study medication, how would you rate the overall impact of psoriasis on your life currently?” on a 7-point scale ranging from very much better to very much worse. At baseline and week 16, patients responded to the PGI-S question, “How severe are your psoriasis symptoms currently?” using a scale from 0 (absent) to 3 (severe).
With all treatment groups combined, patients were grouped according to their responses on the anchors. Polyserial correlations were calculated between category changes on the anchors and change from baseline in the PSSD domain and total scores at week 16; a coefficient of at least 0.4 was considered suitable for use.10 Within each anchor group, the mean (SD) PSSD score change from baseline, the 95% CI, and the standardized effect size (SES) were calculated. The smallest improvement category on each anchor with an SES of at least 0.5 and a significant P value was identified (P ≤ .05 [2-sided] from a paired [within-samples] t test). The lowest point outside the overlap in the 95% CI between the anchor group with no change and the improved group constituted the preliminary estimate. All analyses were performed using SAS, version 9.4 or higher (SAS Institute Inc).
Anchor-based and distribution-based estimates (0.5 SDs and standard error of measurement [SEM]) were triangulated. Distribution-based approaches yield the lowest score change exceeding that expected to occur by chance; they cannot determine patient-perceived meaningfulness. Thus, anchor-based analysis formed the primary approach, while distribution-based methods were supportive. This process was repeated for the change from baseline in each individual PSSD item.
Cumulative distribution functions were generated for each anchor category. Any given point on the continuous line indicated the cumulative proportion of patients who reported that degree of score change from baseline or lower. Derived MWPCTs were applied to unblinded data from POETYK PSO-1 after database lock to compare the cumulative distribution of patients achieving meaningful PSSD responses at week 16 by treatment group. These cumulative distribution functions permit visualization of between-group differences in within-patient PSSD score changes from baseline.
Results
The trial included 666 patients (mean [SD] age, 46.1 [13.4] years; 453 men [68.0%]). Of the 601 patients who completed the PGI-S and PSSD at baseline, 481 patients completed the PGI-S and PSSD at week 16, and 486 patients completed the PGI-C and PSSD at week 16. Of the 666 included patients, 121 (18.2%) were Asian, 6 (0.9%) were Black, 534 (80.2%) were White, and 5 (0.8%) identified their race as “other.” The secondary analysis did not collect data on race or ethnicity. Correlation coefficients between week 16 change from baseline in each PSSD domain, and each anchor (0.541-0.606) indicated suitability for MWPCT derivation (eTable 1 in Supplement 2). The “a little better” PGI-C category (mean [SD] change from baseline: PSSD symptom score, –18.7 [19.9] [95% CI, –32.5 to 23.7]; P < .001; PSSD sign score, –19.1 [18.2] [95% CI, –22.8 to –15.5]; P < .001; PSSD total score, –18.9 [18.6] [95% CI, –22.7 to –15.2]; P < .001) and the 1-point improvement PGI-S category (mean [SD] change from baseline: PSSD symptom score, –26.5 [19.6] [95% CI, –29.3 to –23.6]; P < .001; PSSD sign score, –27.7 [18.5] [95% CI, –30.4 to –25.0]; P < .001; PSSD total score, –27.1 [18.6] [95% CI, –29.8 to –24.4]; P < .001) each showed significant within-group improvements in week 16 PSSD score, with medium to large SES (Table). Changes from baseline from −14.7 to −15.5 points were the lowest levels of change falling outside the 95% CI for the no-change group and within that of the improved group on the PGI-C; changes from baseline from −23.6 to −25.0 points were the lowest levels of change falling outside the 95% CI for the no-change group and within that of the improved group on the PGI-S (Table). Cumulative distribution function curves for change from baseline in PSSD total score, using each anchor, showed clear separation between the no-change groups and each improvement category, indicating that the response categories on the anchor scale adequately discerned the difference in scores (Figure 1). eFigures 2 and 3 in Supplement 2 show similar cumulative distribution function curves for PSSD domain scores.
Table. Anchored Mean PSSD Domain and Total Score Change From Baseline at Week 16 in the POETYK PSO-1 Trial.
| Change category | PSSD symptom score | PSSD sign score | PSSD total sore | |||
|---|---|---|---|---|---|---|
| Mean (SD) CFB [95% CI] | SES | Mean (SD) CFB [95% CI] | SES | Mean (SD) CFB [95% CI] | SES | |
| Patient Global Impression of Change | ||||||
| Very much better (n = 187)a | −36.3 (24.4) [−39.9 to −32.8] | −1.42 | −39.9 (23.2) [−43.3 to −36.6] | −1.80 | −38.1 (23.2) [−41.5 to −34.8] | −1.64 |
| Moderately better (n = 87)a | −27.9 (21.4) [−32.5 to −23.7] | −1.09 | −29.7 (19.4) [−33.8 to −25.6] | −1.34 | −28.8 (19.6) [−33.0 to −24.6] | −1.24 |
| A little better (n = 96)a | −18.7 (19.9) [−22.8 to −14.7] | −0.73 | −19.1 (18.2) [−22.8 to −15.5] | −0.86 | −18.9 (18.6) [−22.7 to −15.2] | −0.81 |
| No change (n = 69) | −5.7 (21.5) [−10.9 to −0.6] | −0.22 | −6.1 (22.2) [−11.5 to −0.8] | −0.28 | −5.9 (21.6) [−11.1 to −0.8] | −0.25 |
| A little worse (n = 15) | −8.5 (21.0) [−20.1 to 3.1] | −0.33 | −8.3 (18.9) [−18.7 to 2.2] | −0.37 | −8.4 (19.7) [−19.3 to 2.5] | −0.36 |
| Moderately worse (n = 13) | 1.8 (23.6) [−12.5 to 16.0] | 0.07 | −0.1 (19.4) [−11.8 to 11.6] | −0.01 | 0.8 (20.6) [−11.6 to 13.3] | 0.03 |
| Very much worse (n = 19) | 2.1 (14.3) [−4.8 to 9.0] | 0.08 | 2.9 (14.4) [−4.0 to 9.9] | 0.13 | 2.5 (13.0) [−3.8 to 8.8] | 0.11 |
| Patient Global Impression of Severity | ||||||
| 3-Point improvement (n = 21)a | −48.2 (26.9) [−60.4 to −35.9] | −1.88 | −51.1 (25.3) [−62.6 to −39.6] | −2.00 | −49.6 (25.6) [−61.3 to −38.0] | −1.94 |
| 2-Point improvement (n = 124)a | −38.2 (26.3) [−42.8 to −33.5] | −1.49 | −41.6 (24.4) [−45.9 to −37.2] | −1.62 | −39.9 (24.6) [−44.2 to −35.5] | −1.56 |
| 1-Point improvement (n = 186)a | −26.5 (19.6) [−29.3 to −23.6] | −1.03 | −27.7 (18.5) [−30.4 to −25.0] | −1.08 | −27.1 (18.6) [−29.8 to −24.4] | −1.06 |
| No change (n = 132) | −5.2 (17.7) [−8.2 to −2.2] | −0.20 | −6.3 (17.7) [−9.3 to −3.2] | −0.24 | −5.7 (17.1) [−8.7 to −2.8] | −0.22 |
| 1-Point worsening (n = 17) | −1.1 (21.4) [−12.1 to 9.9] | −0.04 | −1.5 (21.7) [−12.6 to 9.7] | −0.06 | −1.3 (21.5) [−12.3 to 9.8] | −0.05 |
| 2-Point worsening (n = 0) | NA | NA | NA | NA | NA | NA |
| 3-Point worsening (n = 1) | NA | NA | NA | NA | NA | NA |
Abbreviations: CFB, change from baseline; NA, not applicable; PSSD, Psoriasis Symptoms and Signs Diary; SES, standardized effect size.
P < .001 from a paired (within-samples) t test.
Figure 1. Cumulative Distribution Function for Psoriasis Symptoms and Signs Diary (PSSD) Total Score Change From Baseline to Week 16 With All Treatment Groups Combined.
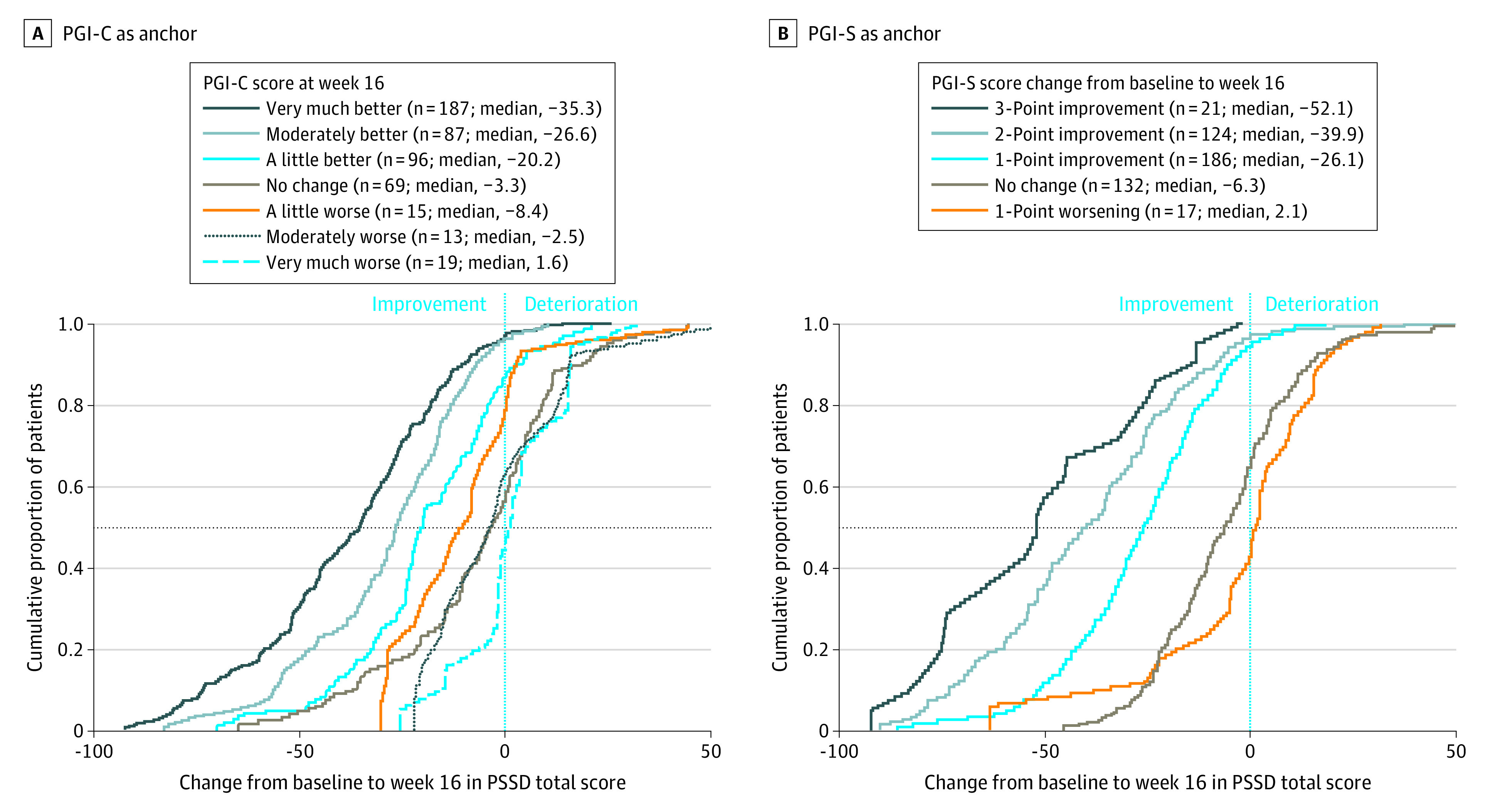
A, Anchored to the Patient Global Impression of Change (PGI-C). B, Anchored to the Patient Global Impression of Severity (PGI-S). The horizontal dashed line indicates the median. The vertical line at zero indicates no change.
Patient Global Impression of Change anchor-based analysis supports an MWPCT for improvement of 15 points for domain and total scores. A higher MWPCT of 25 points represents improvements on both the PGI-C and PGI-S anchors, including SES for within-group changes that exceeded 1.0. An MWPCT of 30 points identified patients who improved higher than “a little” on the PGI-C. These anchor-based estimates were all higher than the distribution-based estimates represented by 0.5 SDs, which ranged from 11.1 to 12.8, and the SEM, which ranged from 4.1 to 5.8 (eTable 2 in Supplement 2). Owing to small sample sizes and nonsignificant effect sizes within worsened disease anchor groups, no MWPCTs for deterioration were identified.
eTables 3 and 4 in Supplement 2 report the anchored change for individual PSSD items. The meaningfulness of a score improvement of 2 points on individual PSSD items is supported by the PGI-C and 0.5 SDs (eTable 5 in Supplement 2).
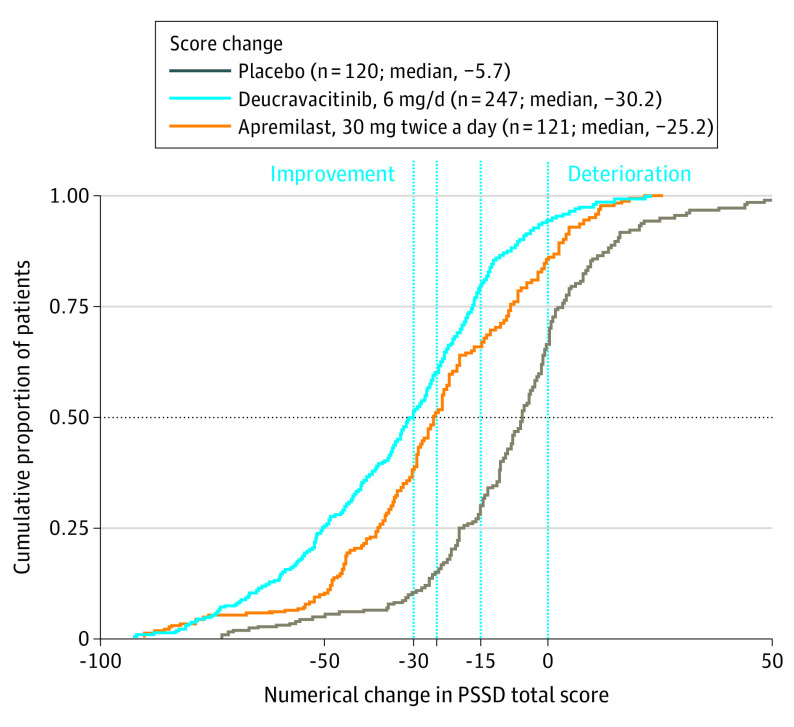
Figure 2 depicts cumulative distribution function curves for the PSSD total score change from baseline at week 16 by treatment group, with the deucravacitinib group consistently above both comparators at each MWPCT. eFigures 4 and 5 in Supplement 2 show similar cumulative distribution functions for week 16 change from baseline in symptom and sign scores. These curves indicate that, at any of the 3 thresholds, greater proportions of patients receiving deucravacitinib experienced meaningful improvement in their psoriasis burden at week 16 than those receiving apremilast or placebo.
Figure 2. Cumulative Proportion of Patients Reporting Any Change From Baseline in Psoriasis Symptoms and Signs Diary (PSSD) Total Score at Week 16 of POETYK PSO-1, by Treatment Group.
The horizontal dashed line indicates the median. The vertical line at zero indicates no change. The vertical lines at –15, –25, and –30 indicate meaningful change.
Discussion
The interpretability of patient-reported outcome end points is best established with reference to MWPCTs derived from patient-reported anchors rather than clinician assessments.10 Armstrong et al2 recommended a −40-point change from baseline threshold on the PSSD to indicate a minimum clinically meaningful improvement, derived from clinician-rated anchors; however, smaller changes in score may be meaningful to patients themselves. The use of the PGI-C and PGI-S as anchors for determining patient-relevant change measured by the PSSD therefore constitutes an important advance in interpreting changes in PSSD scores. Moreover, this anchored analysis conforms to published credibility criteria11 and regulatory guidelines for determining MWPCTs.
Limitations
This study has some limitations. The lowest MWPCT identified, −15 points, corresponds to a rating of change rather than the rating of concept recommended by some researchers to overcome present-state bias.12,13,14,15 In addition, the applicability of MWPCTs for improvement to worsening psoriasis symptoms or signs has not been examined.16
Conclusions
In this analysis, data from the POETYK PSO-1 trial anchored to responses on the PGI-C established PSSD domain and total score decreases of 15 points as meaningful to patients with psoriasis. The patient-perceived meaningfulness of a −25-point change from baseline is supported by both the PGI-C and the PGI-S, while a −30-point MWPCT identified patients who reported higher improvement on the PGI-C. The determination of MWPCTs allows for responder analyses of the PSSD score change from baseline, enhancing the patient-relevant interpretation of this widely used instrument in clinical trials of psoriasis.
Trial Protocols and Statistical Analysis Plans
eFigure 1. Patient Disposition Throughout the POETYK PSO-1 Trial
eFigure 2. Cumulative Distribution Function for PSSD Symptom Score Change From Baseline to Week 16 With All Treatment Arms Combined, Anchored to (A) the Patient Global Impression of Change and (B) the Patient Global Impression of Severity
eFigure 3. Cumulative Distribution Function for PSSD Sign Score Change From Baseline to Week 16 With All Treatment Arms Combined, Anchored to (A) the Patient Global Impression of Change and (B) the Patient Global Impression of Severity
eFigure 4. Cumulative Proportion of Patients Reporting Any Change From Baseline in PSSD Symptom Score at Week 16 of POETYK PSO-1, by Treatment Arm
eFigure 5. Cumulative Proportion of Patients Reporting Any Change From Baseline in PSSD Sign Score at Week 16 of POETYK PSO-1, by Treatment Arm
eTable 1. Polyserial Correlations Between Anchor Variables and Change From Baseline at Week 16 in PSSD Domain and Total Scores
eTable 2. Distribution-Based Meaningful Within-Patient Change Threshold Estimates at Baseline for PSSD Domain and Total Scores
eTable 3. PGI-C–Anchored Mean Individual PSSD Item Score Change From Baseline at Week 16 in POETYK PSO-1
eTable 4. PGI-S–Anchored Mean Individual PSSD Item Score Change From Baseline at Week 16 in POETYK PSO-1
eTable 5. Distribution-Based Meaningful Within-Patient Change Threshold Estimates at Baseline for Individual PSSD Items
Data Sharing Statement
References
- 1.Mathias SD, Feldman SR, Crosby RD, Colwell HH, McQuarrie K, Han C. Measurement properties of a patient-reported outcome measure assessing psoriasis severity: the Psoriasis Symptoms and Signs Diary. J Dermatolog Treat. 2016;27(4):322-327. doi: 10.3109/09546634.2015.1114567 [DOI] [PubMed] [Google Scholar]
- 2.Armstrong A, Puig L, Langley R, et al. Validation of psychometric properties and development of response criteria for the Psoriasis Symptoms and Signs Diary (PSSD): results from a phase 3 clinical trial. J Dermatolog Treat. 2019;30(1):27-34. doi: 10.1080/09546634.2017.1364694 [DOI] [PubMed] [Google Scholar]
- 3.Feldman SR, Mathias SD, Schenkel B, et al. Development of a patient-reported outcome questionnaire for use in adults with moderate-to-severe plaque psoriasis: the Psoriasis Symptoms and Signs Diary. J Dermatol Dermatol Surg. 2016;20(1):19-26. doi: 10.1016/j.jdds.2015.07.004 [DOI] [Google Scholar]
- 4.U.S. Food and Drug Administration. Patient-reported outcome measures: use in medical product development to support labeling claims: guidance for industry. December 2009. Accessed June 14, 2022. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims
- 5.U.S. Food and Drug Administration. Patient-focused drug development: incorporating clinical outcome assessments into endpoints for regulatory decision-making. April 2023. Accessed September 7, 2023. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-focused-drug-development-incorporating-clinical-outcome-assessments-endpoints-regulatory
- 6.Hudgens S, Floden L, Blackowicz M, et al. Meaningful change in depression symptoms assessed with the Patient Health Questionnaire (PHQ-9) and Montgomery-Åsberg Depression Rating Scale (MADRS) among patients with treatment resistant depression in two, randomized, double-blind, active-controlled trials of esketamine nasal spray combined with a new oral antidepressant. J Affect Disord. 2021;281:767-775. doi: 10.1016/j.jad.2020.11.066 [DOI] [PubMed] [Google Scholar]
- 7.Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88(1):29-39. doi: 10.1016/j.jaad.2022.07.002 [DOI] [PubMed] [Google Scholar]
- 8.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 9.Papp KA, Blauvelt A, Kimball AB, et al. Patient-reported symptoms and signs of moderate-to-severe psoriasis treated with guselkumab or adalimumab: results from the randomized VOYAGE 1 trial. J Eur Acad Dermatol Venereol. 2018;32(9):1515-1522. doi: 10.1111/jdv.14910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coon CD, Cook KF. Moving from significance to real-world meaning: methods for interpreting change in clinical outcome assessment scores. Qual Life Res. 2018;27(1):33-40. doi: 10.1007/s11136-017-1616-3 [DOI] [PubMed] [Google Scholar]
- 11.Devji T, Carrasco-Labra A, Qasim A, et al. Evaluating the credibility of anchor based estimates of minimal important differences for patient reported outcomes: instrument development and reliability study. BMJ. 2020;369:m1714. doi: 10.1136/bmj.m1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crosby RD, Kolotkin RL, Williams GR. Defining clinically meaningful change in health-related quality of life. J Clin Epidemiol. 2003;56(5):395-407. doi: 10.1016/S0895-4356(03)00044-1 [DOI] [PubMed] [Google Scholar]
- 13.Nixon A, Doll H, Kerr C, Burge R, Naegeli AN. Interpreting change from patient reported outcome (PRO) endpoints: patient global ratings of concept versus patient global ratings of change, a case study among osteoporosis patients. Health Qual Life Outcomes. 2016;14:25. doi: 10.1186/s12955-016-0427-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmitt JS, Abbott JH. Patient global ratings of change did not adequately reflect change over time: a clinical cohort study. Phys Ther. 2014;94(4):534-542. doi: 10.2522/ptj.20130162 [DOI] [PubMed] [Google Scholar]
- 15.Terluin B, Griffiths P, Trigg A, Terwee CB, Bjorner JB. Present state bias in transition ratings was accurately estimated in simulated and real data. J Clin Epidemiol. 2022;143:128-136. doi: 10.1016/j.jclinepi.2021.12.024 [DOI] [PubMed] [Google Scholar]
- 16.Coon CD, Cappelleri JC. Interpreting change in scores on patient-reported outcome instruments. Ther Innov Regul Sci. 2016;50(1):22-29. doi: 10.1177/2168479015622667 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocols and Statistical Analysis Plans
eFigure 1. Patient Disposition Throughout the POETYK PSO-1 Trial
eFigure 2. Cumulative Distribution Function for PSSD Symptom Score Change From Baseline to Week 16 With All Treatment Arms Combined, Anchored to (A) the Patient Global Impression of Change and (B) the Patient Global Impression of Severity
eFigure 3. Cumulative Distribution Function for PSSD Sign Score Change From Baseline to Week 16 With All Treatment Arms Combined, Anchored to (A) the Patient Global Impression of Change and (B) the Patient Global Impression of Severity
eFigure 4. Cumulative Proportion of Patients Reporting Any Change From Baseline in PSSD Symptom Score at Week 16 of POETYK PSO-1, by Treatment Arm
eFigure 5. Cumulative Proportion of Patients Reporting Any Change From Baseline in PSSD Sign Score at Week 16 of POETYK PSO-1, by Treatment Arm
eTable 1. Polyserial Correlations Between Anchor Variables and Change From Baseline at Week 16 in PSSD Domain and Total Scores
eTable 2. Distribution-Based Meaningful Within-Patient Change Threshold Estimates at Baseline for PSSD Domain and Total Scores
eTable 3. PGI-C–Anchored Mean Individual PSSD Item Score Change From Baseline at Week 16 in POETYK PSO-1
eTable 4. PGI-S–Anchored Mean Individual PSSD Item Score Change From Baseline at Week 16 in POETYK PSO-1
eTable 5. Distribution-Based Meaningful Within-Patient Change Threshold Estimates at Baseline for Individual PSSD Items
Data Sharing Statement