Abstract
Introduction
N-terminal pro-B-type natriuretic peptide (NT-proBNP) and cardiac troponin T (cTnT) measurements are recommended in patients with acute dyspnea. We aimed to assess the prognostic merit of cTnT compared to NT-proBNP for 30-day readmission or death in patients hospitalized with acute dyspnea.
Methods
We measured cTnT and NT-proBNP within 24 h in 314 patients hospitalized with acute dyspnea and adjudicated the cause of the index admission. Time to first event of readmission or death ≤30 days after hospital discharge was recorded, and cTnT and NT-proBNP measurements were compared head-to-head.
Results
Patients who died (12/314) or were readmitted (71/314) within 30 days had higher cTnT concentrations (median: 32.6, Q1–Q3: 18.4–74.2 ng/L vs. median: 19.4, Q1–Q3: 8.4–36.1 ng/L; p for comparison <0.001) and NT-proBNP concentrations (median: 1,753.6, Q1–Q3: 464.2–6,862.0 ng/L vs. median 984, Q1–Q3 201–3,600 ng/L; for comparison p = 0.027) compared to patients who survived and were not readmitted. cTnT concentrations were associated with readmission or death within 30 days after discharge both in the total cohort (adjusted hazard ratio [aHR]: 1.64, 95% confidence interval [CI]: 1.30–2.05) and in patients with heart failure (HF) (aHR: 1.58, 95% CI: 1.14–2.18). In contrast, NT-proBNP concentrations were not associated with short-term events, neither in the total cohort (aHR: 1.10, 95% CI: 0.94–1.30) nor in patients with adjudicated HF (aHR: 1.06, 95% CI: 0.80–1.40).
Conclusion
cTnT concentrations are associated with 30-day readmission or death in patients hospitalized with acute dyspnea, as well as in patients adjudicated HF.
Keywords: Cardiac troponin T, N-terminal pro-B-type natriuretic peptide, Acute dyspnea, Readmission, Heart Failure
Introduction
Acute dyspnea is a common cause of hospitalization in the emergency department (ED) and refers to a subjective patient experience of breathing discomfort [1]. It is a frequent manifestation of diseases in the cardiovascular and respiratory systems, such as congestive heart failure (HF), acute myocardial infarction, chronic obstructive pulmonary disease (COPD), and pulmonary thromboembolism [2, 3]. Patients with dyspnea have among the highest incidences of 30-day readmission or mortality in the hospital setting [4].
The standard diagnostic assessment of patients with acute dyspnea includes medical history, physical examination, and imaging with chest X-ray, in combination with measurement of biomarker concentrations. Cardiac troponins are the biomarkers of choice for diagnosis, risk stratification, and selection of treatment strategy in patients with suspected acute coronary syndrome [5]; however, cardiac troponins also provide diagnostic and prognostic information in patients with HF [6–8]. Similarly, N-terminal pro-B-type natriuretic peptide (NT-proBNP) is essential for diagnosis, risk stratification, and follow-up of patients with HF [9]. NT-proBNP concentrations are also included as a criterion to enter contemporary clinical trials for HF and serve as a proxy metric to demonstrate the effect of interventions on HF morbidity and mortality [10]. Previous studies have compared cardiac troponins and NT-proBNP “head-to-head” as prognostic markers in patients with coronary heart disease [11], elderly hospitalized patients [12], and patients with cardiogenic stroke [13]. In contrast, less is known about the different properties of cardiac troponin and NT-proBNP in predicting short-term prognosis in unselected patients with acute dyspnea. Determining the superior prognostic biomarker in this regard could potentially improve risk assessment, reduce readmission rates, and ultimately reduce the costs associated with readmissions in this vulnerable patient group. Therefore, we aimed to compare the merit of NT-proBNP to those of cardiac troponin T (cTnT) for predicting short-term events in patients hospitalized with acute dyspnea.
Methods
Akershus Cardiac Examination Study 2
The Akershus Cardiac Examination (ACE) 2 Study was conducted at Akershus University Hospital over a period of approximately 17 months, from June 2009 to November 2010. ACE 2 was a prospective single-center study, and the details of the study have been reported previously [14]. Patients admitted to the ED at Akershus University Hospital with acute dyspnea were assessed for inclusion in the study. Inclusion criteria were acute dyspnea as the main cause of index hospitalization, age ≥18 years, ability to provide informed consent, and the possibility to obtain patient approval and draw blood samples of sufficient quantity and quality within 24 h of admission. Exclusion criteria were dementia or other conditions that made informed consent challenging, myocardial infarction, coronary intervention, or major surgery during the last 14 days, or known disseminated cancer or other somatic diseases associated with short life expectancy.
Data Collection
Patients were included in the study from Monday to Thursday from 08:00 to 14:00. Dedicated study personnel completed a standardized clinical questionnaire where the information was either obtained directly from the patients or from the treating physicians from the ED. All blood samples were obtained within 24 h of admission. The medical records of the patients were used to collect information on clinical variables, which included blood pressure, heart rate, body temperature, electrocardiogram, and previous medical history. Coronary artery disease was defined as history of previous myocardial infarction or any coronary intervention. Paroxysmal, persistent, or chronic atrial fibrillation were classified together as AF as previously reported [8]. Body mass index was calculated as (weight in kilograms) divided by (height in meters)2, the values of height and weight were determined either directly from the patient or collected from the medical records. Left ventricular ejection fraction (LVEF) and additional echocardiographic indices were determined by echocardiography as part of clinical routine. Consequently, not all patients in whom HF was considered improbable had echocardiograms, corresponding with the approach used in previous studies of unselected patients with acute dyspnea [15, 16].
Adjudication of Diagnosis and Follow-Up Data
Two senior physicians independently reviewed the patients’ electronic health records, including results of additional examinations and follow-up data with median follow-up of 464 days (interquartile range: 304–705 days) from index hospitalization to adjudication. The patients were classified as either suffering from dyspnea due to HF or non-HF-related dyspnea. Most common non-HF etiologies of acute dyspnea were COPD exacerbation, asthma, and pneumonia, and less common non-HF causes of acute dyspnea were pulmonary embolism, sepsis, and a few cases of anaphylactic reactions. The diagnosis of HF was established according to the criteria outlined by the European Society of Cardiology [17]. HF was characterized as symptoms and clinical signs of HF and evidence of myocardial structural/functional impairment or injury. LVEF was calculated by echocardiography as part of clinical routine and was available in all patients with acute dyspnea due to HF (n = 143). Patients with HF and reduced LVEF were classified as having heart failure with reduced ejection fraction (HFrEF), and patients considered to have symptoms and signs of HF, but with estimated LVEF of ≥50%, were classified as having heart failure with preserved ejection fraction (HFpEF) if there was evidence of structural (left ventricular hypertrophy, left atrial enlargement) or functional (for example, inverted E/A ratio) myocardial dysfunction on echocardiography.
The index hospitalization diagnoses adjudicated by the two senior physicians were concordant in 95% of the cases. If there was any discrepancy, it was resolved by consensus.
Patients’ electronic health records were used to collect information on all-cause mortality during hospitalization or within 30 days of discharge and readmission within 30 days of discharge after the index hospitalization. If patients were readmitted at a facility other than Akershus University Hospital, the hospital received a copy of the discharge documents, which were then scanned into the electronic patient journal available to us at our center. We had access to patients’ “Summary Care Record,” where we could track if they had been admitted at other facilities during the first 30 days of discharge.
“Summary Care Record” is a digital platform, where patients’ health records with information on admission/discharge, independent of the health care facility, are available for the treating physician. Mortality status is available at all Norwegian hospitals, as these records are synchronized with Statistics Norway on a regular basis.
Biomarker Quantification: cTnT and NT-proBNP
Blood samples were obtained within 24 h of admission, centrifuged, and serum was stored immediately at –80°C. Biochemical analysis was conducted without preceding freeze-thaw cycles, ensuring stability for both analytes, as previously reported [18].
For cTnT concentrations, a high-sensitivity assay (Elecsys TnT hs STAT, Roche Diagnostics, Penzberg, Germany) on a Cobas 8000 Platform (Roche Diagnostics) at Akershus University Hospital was used, as previously reported [19]. The range of detection of this assay is from 3 to 10,000 ng/L. cTnT values lower than the limit of detection were assigned the value of 3 ng/L. NT-proBNP was measured with the proBNP II assay (Roche Diagnostics) on the Cobas 8000 Platform at Akershus University Hospital. The range of detection for this assay is from 5 to 35,000 ng/L. Epidemiology Collaboration (CKD-EPI) equation was used to calculate the estimated glomerular filtration rate [20].
Statistical Analysis
All continuous variables are presented as median (interquartile range) and compared with the Mann-Whitney U test; categorical variables are presented as absolute numbers and percentages (%) and compared with the χ2 test. As a result of the right skewed distributions, we transformed concentrations of cTnT and NT-proBNP by the natural logarithm before using them as continuous variables in prognostic models. We assessed the prognostic accuracy of the cardiac biomarkers in predicting 30-day readmission and mortality using receiver operating characteristic curve (ROC) analysis with the area under the curve (AUC). Kaplan-Meier plots were used to illustrate the associations of quartiles (Q) of cTnT and NT-proBNP with 30-day readmission or death, and the plots were compared using the log-rank test. Cox proportional hazards models were used to assess time to first event of 30-day readmission or death. We compared the Cox proportional hazards models by use of the Akaike information criterion and the relative likelihoods of the two models. The survival models were adjusted for sex, age, body mass index, systolic blood pressure, history of HF, coronary artery disease, atrial fibrillation, diabetes mellitus,COPD, and estimated glomerular filtration rate.
We did additional analysis with patients stratified according to concentrations of cTnT and NT-proBNP below and above the median (median cTnT 23 ng/L and median NT-proBNP 1,094 ng/L) – (1) cTnT below median and NT-proBNP below median, (2) cTnT above median and NT-proBNP below median, (3) cTnT below median and NT-proBNP above median, (4) cTnT above median and NT-proBNP above median. Kaplan-Meier plots were used to demonstrate associations of the different groups with 30-day readmissions and death, and groups were compared using the log-rank test.
We assumed statistical significance at p < 0.05. All analyses were performed using Stata 17 (StataCorp LP, College Station, TX, USA).
Results
Baseline Characteristics
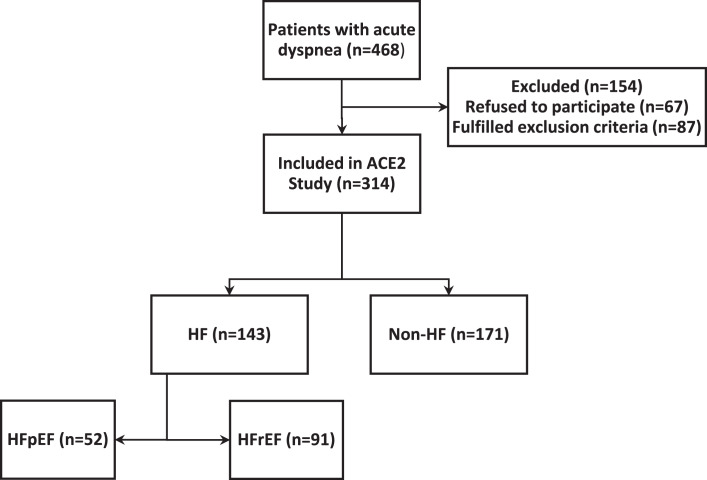
Out of 468 patients hospitalized with acute dyspnea as chief complaint, 67 patients refused to participate in the study and 87 patients fulfilled exclusion criteria, and we ended up with a cohort of total 314 patients (Fig. 1). The median age of the patients was 73 (Q1–Q3: 63–81) years, and 164 (52.2%) of the patients were male. Seventy-one patients (23%) were readmitted within 30 days, and 12 patients (4%) died either during index hospitalization or within 30 days after hospital discharge. Forty-four (53%) patients who were readmitted or died within 30 days of discharge had adjudicated HF as the cause of index hospitalization. The proportion of patients who were readmitted or died within 30 days was not statistically different between HF and non-HF patients (p = 0.11; Table 1).
Fig. 1.
OS Study flowchart.
Table 1.
Baseline characteristics according to 30-day readmission or death after discharge
| Readmission or death within 30 days | p value | |||
|---|---|---|---|---|
| total | no | yes | ||
| N | 314 | 231 | 83 | |
| Male sex, n (%) | 164 (52.2) | 119 (51.5) | 45 (54.2) | 0.67 |
| Age, years | 73.0 (63.0–81.0) | 72.0 (62.0–81.0) | 74 (64.0–80.0) | 0.43 |
| Body mass index, kg/m2 | 25.7 (21.7–30.1) | 26.5 (22.3–30.3) | 23.4 (20.7–28.1) | 0.001 |
| Heart rate, bpm | 90 (78–108) | 90 (76–108) | 90 (80–106) | 0.56 |
| Systolic blood pressure, mm Hg | 142 (127–161) | 143 (130–162) | 137 (119–160) | 0.009 |
| Diastolic blood pressure, mm Hg | 78 (69–90) | 79 (69–91) | 76 (65–89) | 0.14 |
| History of | ||||
| HF, n (%) | 101 (32.2) | 66 (28.6) | 35 (42.2) | 0.023 |
| Coronary artery disease, n (%) | 111 (35.4) | 75 (32.5) | 36 (43.4) | 0.08 |
| Diabetes mellitus, n (%) | 68 (21.7) | 48 (20.8) | 20 (24.1) | 0.53 |
| COPD, n (%) | 155 (49.4) | 113 (48.9) | 42 (50.6) | 0.79 |
| Atrial fibrillation, n (%) | 96 (30.6) | 64 (27.7) | 32 (38.6) | 0.07 |
| eGFR, mL/min/1.73 m2 | 76.5 (60.2–98.9) | 77.4 (60.5–99.1) | 73.5 (57.1–98.7) | 0.32 |
| NT-proBNP, ng/L | 1,093.9 (253.3–3,875.1) | 984.3 (201.4–3,599.8) | 1,753.6 (464.2–6,862.0) | 0.027 |
| cTnT, ng/L | 23.1 (10.4–42.1) | 19.4 (8.4–36.1) | 32.6 (18.4–74.2) | <0.001 |
| HF cause of index admission, n (%) | 143 (45.5) | 99 (42.9) | 44 (53) | 0.11 |
| HFrEF cause of index admission, n (%) | 91 (29.0) | 64 (27.7) | 27 (32.5) | 0.41 |
| HFpEF cause of index admission, n (%) | 52 (16.6) | 35 (15.2) | 17 (20.5) | 0.26 |
Data are presented as median (IQR) for continuous variables, and n (%) for categorical variables.
IQR, interquartile range; eGFR, estimated glomerular filtration rate.
Cardiac Biomarkers and Risk of 30-Day Readmission or Death
Patients with Acute Dyspnea
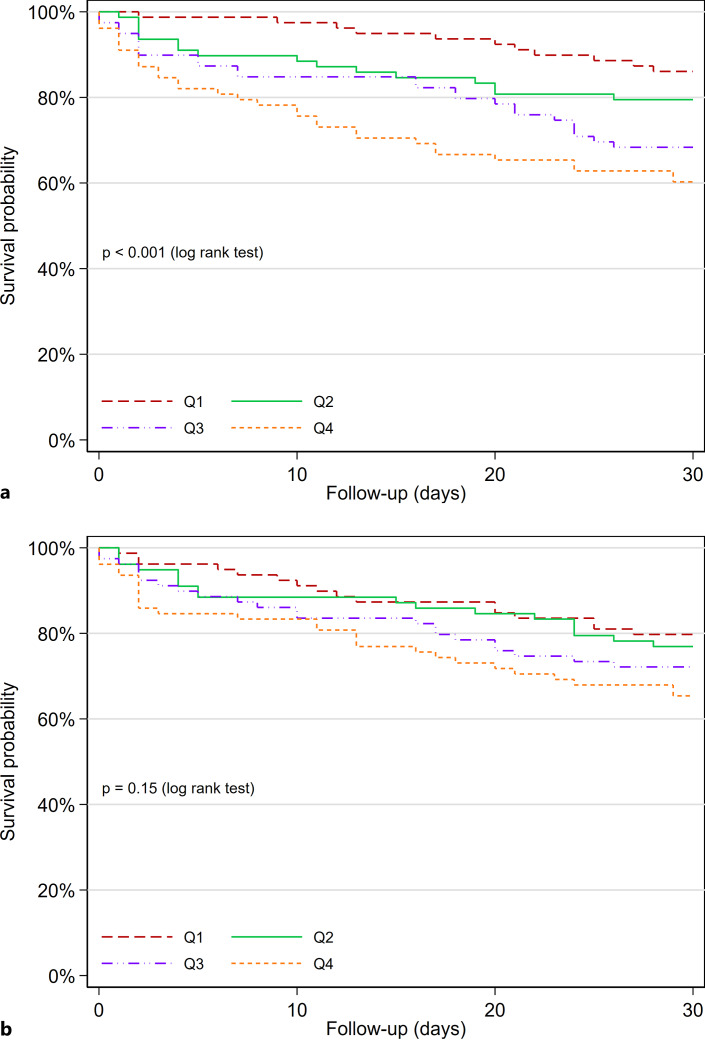
In the unadjusted analysis, patients who died or were readmitted within 30 days had higher cTnT concentrations (median: 32.6, Q1–Q3: 18.4–74.2 ng/L) as compared to patients who survived and were not readmitted (median: 19.4, Q1–Q3: 8.4–36.1 ng/L; p for comparison <0.001). NT-proBNP concentrations were also higher for patients who had an event (median: 1,753.6, Q1–Q3: 464.2–6,862.0 ng/L) compared to patients with no events (median: 984.3, Q1–Q3: 201.4–3,599.8 ng/L). In adjusted analyses, concentrations of cTnT were associated with outcome (adjusted hazard ratio [aHR]: 1.64, 95% confidence interval [CI]: 1.30–2.05), but NT-proBNP concentrations were not (aHR: 1.10, 95% CI: 0.94–1.30; p for comparison of HRs <0.001; Table 2). ROC-AUC in the total cohort for the prediction of 30-day readmission or death was higher for cTnT (0.66, 95% CI: 0.59–0.72) compared to NT-proBNP (0.58, 95% CI: 0.51–0.65; p for comparison = 0.022). Stratifying patients by cTnT quartiles separated patients with a poor and favorable outcome (p for log-rank test <0.001; Fig. 2a), while no such differences were observed for NT-proBNP concentrations (p for log-rank test = 0.15; Fig. 2b).
Table 2.
Associations of cTnT and NT-proBNP with 30-day readmission or death
| cTnT | NT-proBNP | p for comparison | |
|---|---|---|---|
| Acute dyspnea (n = 314) | |||
| Unadjusted HR (95% CI) | 1.51 (1.26–1.81) | 1.17 (1.04–1.32) | 0.001 |
| aHR (95% CI)* | 1.64 (1.30–2.05) | 1.10 (0.94–1.30) | <0.001 |
| AUC (95% CI) | 0.66 (0.59–0.72) | 0.58 (0.51–0.65) | 0.022 |
| Acute HF (n = 143) | |||
| Unadjusted HR (95% CI) | 1.56 (1.18–2.04) | 1.19 (0.94–1.51) | 0.023 |
| aHR (95% CI)* | 1.58 (1.14–2.18) | 1.06 (0.80–1.40) | 0.025 |
| AUC (95% CI) | 0.66 (0.56–0.75) | 0.57 (0.43–0.66) | 0.10 |
| HFrEF (n = 91) | |||
| Unadjusted HR (95% CI) | 1.53 (1.07–2.19) | 1.29 (0.94–1.77) | 0.28 |
| aHR (95% CI)* | 1.52 (0.97–2.38) | 1.07 (0.76–1.51) | 0.19 |
| AUC (95% CI) | 0.66 (0.54–0.77) | 0.63 (0.49–0.77) | 0.74 |
| HFpEF (n = 52) | |||
| Unadjusted HR (95% CI) | 1.60 (1.05–2.44) | 1.15 (0.78–1.68) | 0.13 |
| aHR (95% CI)* | 2.04 (1.08–3.87) | 1.16 (0.67–2.01) | 0.08 |
| AUC (95% CI) | 0.67 (0.51–0.83) | 0.52 (0.35–0.69) | 0.08 |
| Non-HF (n = 171) | |||
| Unadjusted HR (95% CI) | 1.60 (1.16–2.20) | 1.14 (0.94–1.38) | 0.038 |
| aHR (95% CI)* | 1.74 (1.09–2.79) | 1.02 (0.80–1.32) | 0.07 |
| AUC (95% CI) | 0.64 (0.54–0.73) | 0.58 (0.48–0.68) | 0.23 |
AUC, area under the curve; CI, confidence interval; cTnT, cardiac troponin T; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
*Adjusted for sex, age, BMI, systolic blood pressure, HF, CAD, AF, eGFR, COPD, and diabetes mellitus.
Fig. 2.
Kaplan-Meier plot for the endpoint of 30-day readmission or death in patients with acute dyspnea. Participants stratified according to quartiles of cTnT (a) and NT-proBNP (b).
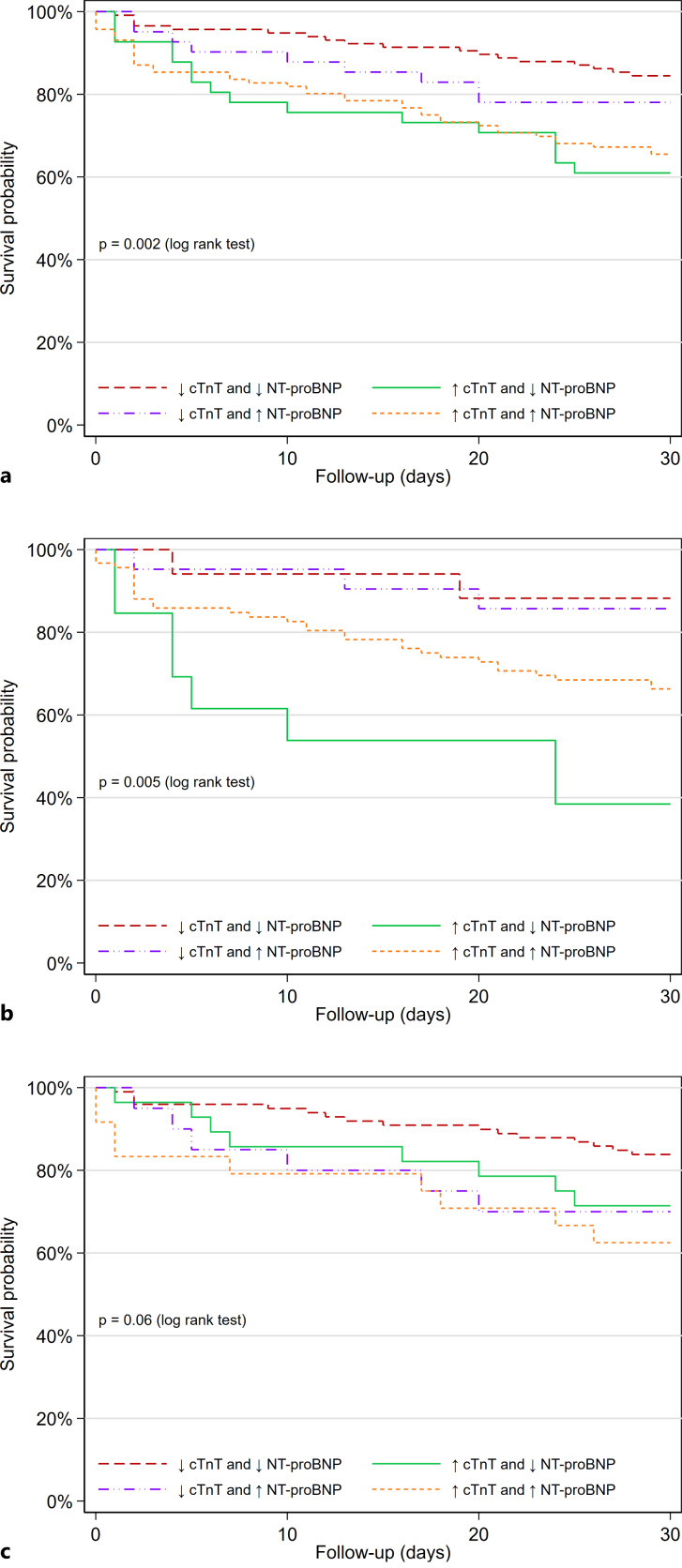
We further compared the predictive ability of different subgroups according to concentrations of cTnT and NT-proBNP below and above the median values (median cTnT 23 ng/L and median NT-proBNP 1,094 ng/L), and patients with concentrations of both cTnT and NT-proBNP below the median had the most favorable prognosis (Fig. 3a; p for log-rank test = 0.002). In adjusted analysis, cTnT concentrations above the median value were associated with outcome in the group with NT-proBNP below the median value (aHR: 3.21, 95% CI: 1.50–6.88) and in the group with NT-proBNP concentrations above the median value (aHR: 2.32, 95% CI: 1.11–4.82) (online suppl. Table 1; for all online suppl. material, see https://doi.org/10.1159/000533266).
Fig. 3.
Kaplan-Meier plot for the endpoint of 30-day readmission or death in patients with acute dyspnea. Patients are stratified according to concentrations of cTnT and NT-proBNP above and below the median values. a Total cohort. b Patients with HF as the cause of acute dyspnea. c Patients with non-HF cause of acute dyspnea.
Patients with HF
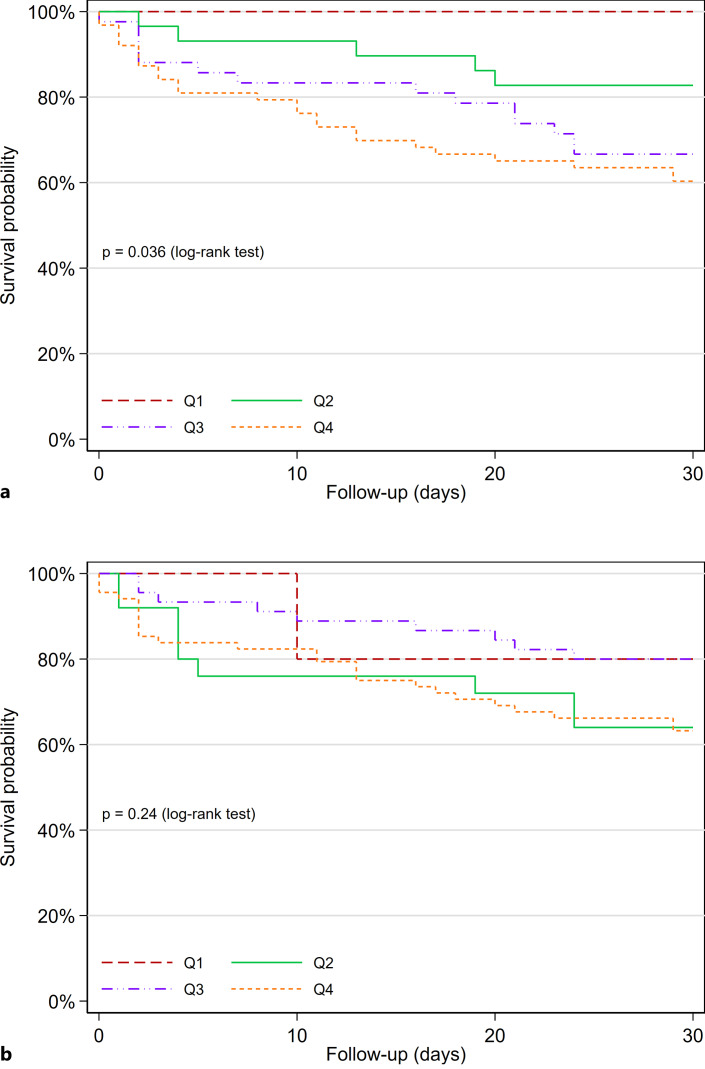
As previously reported [8], the subgroup of patients with adjudicated HF had higher concentrations of cTnT (median 38 ng/L vs. median 13 ng/L) and NT-proBNP (median 3,600 ng/L vs. median 348 ng/L) compared to the non-HF group. cTnT concentrations (aHR: 1.58, 95% CI: 1.14–2.18) but not NT-proBNP concentrations (aHR: 1.06, 95% CI: 0.80–1.40) were associated with short-term outcomes (p for comparison of HRs = 0.025). The ROC-AUC of cTnT was 0.66 (95% CI: 0.56–0.75) and the ROC-AUC of NT-proBNP was 0.57 (95% CI: 0.43–0.66; p for comparison = 0.10; Table 2). In patients with HF, quartiles of cTnT separated between patients with and without short-term outcomes (p for log-rank test = 0.036; Fig. 4a), while quartiles of NT-proBNP did not separate patients with a poor and favorable outcome (p for log-rank test = 0.24; Fig. 4b).
Fig. 4.
Kaplan-Meier plot for the endpoint of 30-day readmission or death in patients with acute HF. Participants stratified according to quartiles of cTnT (a) and NT-proBNP (b).
In adjusted analyses, concentrations of cTnT and NT-proBNP did not associate with short-term outcomes in patients with HFrEF (Table 2). For HFpEF, concentrations of cTnT (aHR: 2.04, 95% CI: 1.08–3.87) associated with short-term outcomes but concentrations of NT-proBNP did not (aHR: 1.16, 95% CI: 0.67–2.01; p for comparison of HRs = 0.08). In patients with HFrEF, the ROC-AUC of cTnT was 0.66 (95% CI: 0.54–0.77) and the ROC-AUC of NT-proBNP was 0.63 (95% CI: 0.49–0.77; p for comparison = 0.74). In patients with HFpEF, the ROC-AUC of cTnT was 0.67 (95% CI: 0.51–0.83) and the ROC-AUC of NT-proBNP was 0.52 (95% CI: 0.35–0.69; p for comparison = 0.08). Quartiles of cTnT and NT-proBNP did not separate patients according to short-term outcomes when we investigated HFrEF (online suppl. Fig. 1a, b) and HFpEF (online suppl. Fig. 2a, b) separately.
As for the total cohort, we compared the predictive ability of different subgroups according to concentrations of cTnT and NT-proBNP below and above the median values for patients with HF. Patients with concentrations of both cTnT and NT-proBNP below the median had the most favorable prognosis (Fig. 3b; p for log-rank test = 0.005). In adjusted analysis, cTnT concentrations above the median value were associated with outcome (aHR: 6.90, 95% CI: 1.37–34.81) in the group with NT-proBNP below the median value; however, there was no significant association (aHR: 2.20, 95% CI: 0.48–10.02) in the group with NT-proBNP concentrations above the median value (online suppl. Table 1).
Patients with Non-HF Cause of Dyspnea
cTnT concentrations (aHR: 1.74, 95% CI: 1.09–2.79) but not NT-proBNP concentrations (aHR: 1.02, 95% CI: 0.80–1.32) were associated with short-term outcomes (p for comparison of HRs = 0.07) in patients with non-HF cause of dyspnea. The ROC-AUC of cTnT was 0.64 (95% CI 0.54–0.73) and the ROC-AUC of NT-proBNP was 0.58 (95% CI 0.48–0.68; p for comparison = 0.23) (Table 2). In patients with non-HF causes of dyspnea, quartiles of cTnT and NT-proBNP did not separate patients according to short-term outcomes (online suppl. Fig. 3a, b). Neither did we find statistically significant association with outcomes according to concentrations of cTnT and NT-proBNP below and above the median values (online suppl. Table 1; Fig. 3c; p for log-rank test = 0.06).
Discussion
The main result of this study is that early cTnT measurements predict short-term outcomes in patients with acute dyspnea presenting to the ED, irrespective of the cause of dyspnea. Patients who met the clinical endpoint in the study (readmission or mortality within 30 days after discharge) had significantly higher concentrations of cTnT and NT-proBNP, and concentrations of cTnT were independently associated with prognosis. Measurement of cTnT concentrations during the early phases of hospitalization could potentially identify patients who are at higher risk of readmission and death after discharge. Our results reinforce cTnT as a strong prognostic marker in patients with acute dyspnea, which is in line with earlier studies [21].
Elevated cTnT concentrations have been associated with increased mortality in patients with acute and chronic HF [7, 22], and also in patients with noncardiac conditions [23]. Elevated cardiac troponin I concentrations have also been shown to have a prognostic value comparable to that of cTnT in the general population [24], in patients with noncardiac cause of cardiac troponin elevation [25], and in patients with acute HF [7].
In patients with cTnT elevation and no apparent acute cardiac disease, cTnT concentrations should be considered as a marker of comorbidities with low organ reserve [8, 23]. Our results demonstrating superiority of cTnT over NT-proBNP for the prediction of short-term outcomes are however limited to the setting of ED, as we have used biomarker concentrations obtained in the acute phase (≤24 h of admission) and have not analyzed changes of biomarker concentrations under hospitalization. The latter could have provided further insight to the current investigation, as earlier studies have demonstrated that changes in NT-proBNP concentrations during hospitalization for HF correlate with short-term events [26]. It should be noted that the prognostic value of change in biomarker concentrations under hospitalization also applies to cTnT [27]. In the guidelines from the American Heart Association, it is recommended to take a pre-discharge measurement of NT-proBNP to establish post-discharge prognosis in patients with HF [9]. NT-proBNP in the ED more closely reflects the volume status in the acute phase and not the severity of the underlying disease, possibly explaining the weaker association with prognosis compared to cTnT in our study.
In patients with adjudicated HF, we observed a trend comparable to the total population, where cTnT concentrations had a stronger association with short-term outcomes as compared to NT-proBNP concentrations. In the subgroup analysis of HF patients according to LVEF (HFpEF and HFrEF), cTnT and NT-proBNP did not predict short-term outcomes for HFrEF, but cTnT predicted short-term outcomes in HFpEF patients. As cTnT predicted short-term outcomes in both the total study population and in the subgroup of patients with HF, the association of cTnT with outcomes in HF patients appears to be driven by strong associations in patients with HFpEF. However, due to the moderate size of the cohort and only minor differences in hazard ratios for HFrEF and HFpEF, our results could represent a sample size problem. cTnT concentrations are primarily associated with structural changes in the left ventricle and not systolic function [28, 29], and subsequently with the degree of increased filling pressures and LV hypertrophy. A possible explanation for the stronger prognostic impact of cTnT in HFpEF as compared to HFrEF, can indeed be structural changes such as left ventricular hypertrophy, and co-morbidity-driven pathomechanisms such as endothelial dysfunction and chronic inflammation closely associated with disease severity and cTnT release [30–33]. The origin of cTnT in HFrEF is possibly associated with subclinical ischemic heart disease [34], which probably does not have a major impact on the prognosis.
Strengths of the current investigation are that all relevant data were collected by dedicated personnel, and cTnT and NT-proBNP were measured at a core laboratory as a batch, largely avoiding variation in laboratory calibration over time [8]. There was excellent agreement between the adjudicators in diagnosing the cause of acute dyspnea, increasing the external validity of our results. A limitation to our study is the single-center design, with a moderate cohort of 314 patients. Due to the moderate sample size, particularly for the subgroup with acute HF, the study could have been underpowered to examine the prognostic value of cTnT and NT-proBNP, especially in patients with acute HF, HFrEF, and HFpEF, and data should be interpreted in this context. Another limitation to our study is the lack of data on exact timings for measurement of cTnT and NT-proBNP concentrations. There is a possibility that certain patients could have received therapy prior to measurement of biomarkers, and especially those with successful compensation of HF could have experienced changes in cTnT and NT-proBNP concentrations. There is lack of generalizability in our results, and our results may not be applicable beyond the setting of an ED of a hospital in Northern Europe. We did not have designated personnel to perform echocardiography; therefore, echocardiography was only performed if the treating physician found it necessary, with no standardized protocol for echocardiography. There is a possibility that some patients could have wrongly been adjudicated as non-HF cause of dyspnea, and the diagnosis of HF and especially HFpEF could have been missed [35].
Conclusion
cTnT is superior to NT-proBNP in predicting short-term events in unselected patients with acute dyspnea, as well as patients with acute HF. The association of cTnT with outcomes in HF may have been driven by strong associations of cTnT in patients with HFpEF.
Statement of Ethics
This study involves human participants and was approved by the Regional Committee for Medical Research Ethics in Southeast Norway. Reference number for ethics approval: S-08824d 2008/21288 09/418. Participants gave written informed consent to participate in the study before taking part.
Conflict of Interest Statement
The authors declare no conflict of interest.
Funding Sources
This work was supported by a research grant from the Norwegian Research Council (197992/B-07029) to T.O. and H.R. and by internal grants from Akershus University Hospital (41810/340104). Roche Diagnostics supported the study by providing reagents at a reduced price to Akershus University Hospital.
Author Contributions
R.B., K.B., and M.N.L. had access to all of the data in the study and take full responsibility for the overall content as guarantors. Study conception and design, analysis and interpretation of data, and drafting of the manuscript: R.B., K.B., H.R., and M.N.L. Acquisition of data: K.B., J.B., R.R., G.C., A.D.H., T.O., and H.R. Critical revision of the manuscript for important intellectual content: K.B., J.B., R.R., G.C., A.D.H., H.R., T.O., and M.N.L. Final approval of manuscript: R.B., K.B., J.B., R.R., G.C., A.D.H., T.O., H.R., and M.N.L.
Funding Statement
This work was supported by a research grant from the Norwegian Research Council (197992/B-07029) to T.O. and H.R. and by internal grants from Akershus University Hospital (41810/340104). Roche Diagnostics supported the study by providing reagents at a reduced price to Akershus University Hospital.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material files. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
References
- 1. Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435–52. 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frostad A, Soyseth V, Haldorsen T, Andersen A, Gulsvik A. Respiratory symptoms and long-term cardiovascular mortality. Respir Med. 2007;101(11):2289–96. 10.1016/j.rmed.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 3. Ho SF, O’Mahony MS, Steward JA, Breay P, Buchalter M, Burr ML. Dyspnoea and quality of life in older people at home. Age Ageing. 2001;30(2):155–9. 10.1093/ageing/30.2.155. [DOI] [PubMed] [Google Scholar]
- 4. Sorensen SF, Ovesen SH, Lisby M, Mandau MH, Thomsen IK, Kirkegaard H. Predicting mortality and readmission based on chief complaint in emergency department patients: a cohort study. Trauma Surg Acute Care Open. 2021;6(1):e000604. 10.1136/tsaco-2020-000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Erhardt L, Herlitz J, Bossaert L, Halinen M, Keltai M, Koster R, et al. Task force on the management of chest pain. Eur Heart J. 2002;23(15):1153–76. 10.1053/euhj.2002.3194. [DOI] [PubMed] [Google Scholar]
- 6. Januzzi JL Jr, Filippatos G, Nieminen M, Gheorghiade M. Troponin elevation in patients with heart failure: on behalf of the third universal definition of myocardial infarction global task force: heart failure section. Eur Heart J. 2012;33(18):2265–71. 10.1093/eurheartj/ehs191. [DOI] [PubMed] [Google Scholar]
- 7. Peacock WF, De Marco T, Fonarow GC, Diercks D, Wynne J, Apple FS, et al. Cardiac troponin and outcome in acute heart failure. N Engl J Med. 2008;358(20):2117–26. 10.1056/nejmoa0706824. [DOI] [PubMed] [Google Scholar]
- 8. Berge K, Lyngbakken MN, Myhre PL, Brynildsen J, Røysland R, Strand H, et al. High-sensitivity cardiac troponin T and N-terminal pro-B-type natriuretic peptide in acute heart failure: data from the ACE 2 study. Clin Biochem. 2021;88:30–6. 10.1016/j.clinbiochem.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 9. Yancy, CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American heart association task force on clinical practice guidelines and the heart failure society of America. Circulation. 2017;136(6):e137–61. 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 10. Vaduganathan M, Claggett B, Packer M, McMurray JJV, Rouleau JL, Zile MR, et al. Natriuretic peptides as biomarkers of treatment response in clinical trials of heart failure. JACC Heart Fail. 2018;6(7):564–9. 10.1016/j.jchf.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 11. Nikorowitsch J, Ojeda F, Lackner KJ, Schnabel RB, Blankenberg S, Zeller T, et al. Head-to-Head comparison of the incremental predictive value of the three established risk markers, Hs-troponin I, C-reactive protein, and NT-proBNP, in coronary artery disease. Biomolecules. 2020;10(3):394. 10.3390/biom10030394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen JR, Wang Q, Wu W, Zhang SJ. Comparison of prognostic values of high-sensitivity cardiac troponin T and N-terminal prohormone brain natriuretic peptide to assess mortality in elderly inpatients. Clin Interv Aging. 2019;14:81–90. 10.2147/CIA.S187757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Otaki Y, Watanabe T, Sato N, Shirata T, Tsuchiya H, Wanezaki M, et al. Direct comparison of prognostic ability of cardiac biomarkers for cardiogenic stroke and clinical outcome in patients with stroke. Heart Vessels. 2019;34(7):1178–86. 10.1007/s00380-019-01345-w. [DOI] [PubMed] [Google Scholar]
- 14. Rosjo H, Dahl MB, Jørgensen M, Røysland R, Brynildsen J, Cataliotti A, et al. Influence of glycosylation on diagnostic and prognostic accuracy of N-terminal pro-B-type natriuretic peptide in acute dyspnea: data from the Akershus Cardiac Examination 2 Study. Clin Chem. 2015;61(8):1087–97. 10.1373/clinchem.2015.239673. [DOI] [PubMed] [Google Scholar]
- 15. Maisel AS, McCord J, Nowak RM, Hollander JE, Wu AHB, Duc P, et al. Bedside B-Type natriuretic peptide in the emergency diagnosis of heart failure with reduced or preserved ejection fraction. Results from the Breathing Not Properly Multinational Study. J Am Coll Cardiol. 2003;41(11):2010–7. 10.1016/s0735-1097(03)00405-4. [DOI] [PubMed] [Google Scholar]
- 16. O’Donoghue M, Chen A, Baggish AL, Anwaruddin S, Krauser DG, Tung R, et al. The effects of ejection fraction on N-terminal ProBNP and BNP levels in patients with acute CHF: analysis from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) study. J Card Fail. 2005;11(5 Suppl):S9–14. 10.1016/j.cardfail.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 17. McMurray JJV, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787–1847. 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 18. Mansour M, Clark L, Kavsak PA. Effect of freeze-thaw and refrigeration conditions on high-sensitivity troponin T concentrations. Ann Clin Biochem. 2012;49(Pt 1):101–2. 10.1258/acb.2011.011204. [DOI] [PubMed] [Google Scholar]
- 19. Hoiseth AD, Brynildsen J, Hagve TA, Christensen G, Søyseth V, Omland T, et al. The influence of heart failure co-morbidity on high-sensitivity troponin T levels in COPD exacerbation in a prospective cohort study: data from the Akershus cardiac examination (ACE) 2 study. Biomarkers. 2016;21(2):173–9. 10.3109/1354750X.2015.1126645. [DOI] [PubMed] [Google Scholar]
- 20. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–70. 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 21. van Wijk S, Jacobs L, Eurlings LW, van Kimmenade R, Lemmers R, Broos P, et al. Troponin T measurements by high-sensitivity vs conventional assays for risk stratification in acute dyspnea. Clin Chem. 2012;58(1):284–92. 10.1373/clinchem.2011.175976. [DOI] [PubMed] [Google Scholar]
- 22. Latini R, Masson S, Anand IS, Missov E, Carlson M, Vago T, et al. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation. 2007;116(11):1242–9. 10.1161/CIRCULATIONAHA.106.655076. [DOI] [PubMed] [Google Scholar]
- 23. Kaura A, Panoulas V, Glampson B, Davies J, Mulla A, Woods K, et al. Association of troponin level and age with mortality in 250 000 patients: cohort study across five UK acute care centres. BMJ. 2019;367:l6055. 10.1136/bmj.l6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van der Linden N, Klinkenberg LJJ, Bekers O, Loon LJC, Dieijen-Visser MP, Zeegers MP, et al. Prognostic value of basal high-sensitive cardiac troponin levels on mortality in the general population: a meta-analysis. Medicine. 2016;95(52):e5703. 10.1097/MD.0000000000005703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arnadottir A, Falk Klein C, Iversen K. Head-to-head comparison of cardiac troponin T and troponin I in patients without acute coronary syndrome: a systematic review. Biomarkers. 2017;22(8):701–8. 10.1080/1354750X.2017.1335779. [DOI] [PubMed] [Google Scholar]
- 26. Bettencourt P, Azevedo A, Pimenta J, Friões F, Ferreira S, Ferreira A. N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation. 2004;110(15):2168–74. 10.1161/01.cir.0000144310.04433.be. [DOI] [PubMed] [Google Scholar]
- 27. Felker GM, Mentz RJ, Teerlink JR, Voors AA, Pang PS, Ponikowski P, et al. Serial high sensitivity cardiac troponin T measurement in acute heart failure: insights from the RELAX-AHF study. Eur J Heart Fail. 2015;17(12):1262–70. 10.1002/ejhf.341. [DOI] [PubMed] [Google Scholar]
- 28. de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304(22):2503–12. 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosjo H, Andreassen J, Edvardsen T, Omland T. Prognostic usefulness of circulating high-sensitivity troponin T in aortic stenosis and relation to echocardiographic indexes of cardiac function and anatomy. Am J Cardiol. 2011;108(1):88–91. 10.1016/j.amjcard.2011.02.346. [DOI] [PubMed] [Google Scholar]
- 30. Konerman MC, Greenberg JC, Kolias TJ, Corbett JR, Shah RV, Murthy VL, et al. Reduced myocardial flow reserve is associated with diastolic dysfunction and decreased left atrial strain in patients with normal ejection fraction and epicardial perfusion. J Card Fail. 2018;24(2):90–100. 10.1016/j.cardfail.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, et al. Phenotype-specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation. 2016;134(1):73–90. 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fudim M, Ambrosy AP, Sun JL, Anstrom KJ, Bart BA, Butler J, et al. High-sensitivity troponin I in hospitalized and ambulatory patients with heart failure with preserved ejection fraction: insights from the heart failure clinical research network. J Am Heart Assoc. 2018;7(24):e010364. 10.1161/JAHA.118.010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Srivaratharajah K, Coutinho T, deKemp R, Liu P, Haddad H, Stadnick E, et al. Reduced myocardial flow in heart failure patients with preserved ejection fraction. Circ Heart Fail. 2016;9(7):e002562. 10.1161/CIRCHEARTFAILURE.115.002562. [DOI] [PubMed] [Google Scholar]
- 34. Vedin O, Lam CSP, Koh AS, Benson L, Teng THK, Tay WT, et al. Significance of ischemic heart disease in patients with heart failure and preserved, midrange, and reduced ejection fraction: a nationwide cohort study. Circ Heart Fail. 2017;10(6):e003875. 10.1161/CIRCHEARTFAILURE.117.003875. [DOI] [PubMed] [Google Scholar]
- 35. Wong CW, Tafuro J, Azam Z, Satchithananda D, Duckett S, Barker D, et al. Misdiagnosis of heart failure: a systematic review of the literature. J Card Fail. 2021;27(9):925–33. 10.1016/j.cardfail.2021.05.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material files. Further inquiries can be directed to the corresponding author.