Abstract
Humans are exposed to complex mixtures of phthalates. Gestational exposure to phthalates has been linked to preeclampsia and preterm birth through potential pathways such as endocrine disruption, oxidative stress, and inflammation. Eicosanoids are bioactive signaling lipids that are related to a variety of homeostatic and inflammatory processes. We investigated associations between urinary phthalates and their mixtures with plasma eicosanoid levels during pregnancy using the PROTECT cohort in Puerto Rico (N=655). After adjusting for covariates, we estimated pair-wise associations between the geometric mean of individual phthalate metabolite concentrations across pregnancy and eicosanoid biomarkers using multivariable linear regression. We used bootstrapping of adaptive elastic net regression (adENET) to evaluate phthalate mixtures associated with eicosanoids and subsequently create environmental risk scores (ERS) to represent weighted sums of phthalate exposure for each individual. After adjusting for false-discovery, in single-pollutant analysis, 14 of 20 phthalate metabolites or parent compound indices showed significant and primarily negative associations with multiple eicosanoids. In our mixture analysis, associations with several metabolites of low molecular weight phthalates – DEP, DBP, and DIBP – became prominent. Additionally, MEHHTP and MECPTP, metabolites of a new phthalate replacement, DEHTP, were selected as important predictors for determining the concentrations of multiple eicosanoids from different pathway groups. A unit increase in phthalate ERS derived from bootstrapping of adENET was positively associated with several eicosanoids mainly from Cytochrome P450 pathway. For example, an increase in ERS was associated with 11(S)-HETE (β=1.6, 95% CI: 0.020, 3.180), (±)11,12-DHET (β = 2.045, 95% CI: 0.250, 3.840), 20(S)-HETE (β=0.813, 95% CI: 0.147, 1.479), and 9s-HODE (β=2.381, 95% CI: 0.657, 4.104). Gestational exposure to phthalates and phthalate mixtures were associated with eicosanoid levels during pregnancy. Results from the mixture analyses underscore the complexity of physiological impacts of phthalate exposure and call for further in-depth studies to examine these relationships.
Keywords: phthalates, phthalate replacements, mixture analysis, eicosanoids
1. Introduction
Phthalates are a class of chemicals widely used in plastics, consumer products and various personal care products such as lotions, perfumes, and deodorants, making exposure to phthalates in the general population ubiquitous. Since phthalates can work as endocrine and metabolic disruptors, phthalate exposure has been linked to several diseases including reduced fertility, endometriosis, metabolic syndrome, diabetes, and more [Benjamin et al., 2017, Eales et al., 2022]. Gestational exposure to phthalates is associated with adverse birth outcomes, fetal developmental, and child neurodevelopment disorders [Santos et al., 2021, Zhong et al., 2021, Cathey et al., 2022, Pollard et al., 2017, Nidens et al., 2021]. Studies have shown endocrine disruption, oxidative stress or inflammation during pregnancy may mediate phthalate exposure and pregnancy- or development-related endpoints. Numerous reports have highlighted these concerns, prompting global regulatory measures and controls on commonly used phthalate plasticizers. Consequently, new chemicals such as di-(2-ethylhexyl) terephthalate (DEHTP), a structural isomer of di-(2-ethylhexyl) phthalate (DEHP), have been adopted as replacements for various industrial applications [Schütze et al., 2014, Lessmann et al., 2019, Schmidtkunz et al., 2019]. Indeed, exposure to phthalate replacements is also increasing in the US population and Puerto Rico [Silva et al., 2017,2019, Rodríguez-Carmona et al., 2020]. Although limited information is available, a few studies have suggested that phthalate replacement chemicals also have reproductive toxicity, developmental toxicity, and endocrine disrupting activity [Kambia et al., 2019, Lee et al., 2020, Yun et al., 2023].
Recent advances in targeted lipidomics have allowed comprehensive analysis of eicosanoids, a class of bioactive lipids, and related polyunsaturated fatty acids (PUFAs). Increasing attention is being drawn to eicosanoids as they are involved in the pathophysiology of both inflammation and oxidative stress [Dennis et al., 2015]. Eicosanoids are derived from polyunsaturated fatty acids (PUFAs) that are related to a variety of homeostatic and inflammatory processes in the human body [Khanapure 2007]. Specifically, eicosanoids are metabolites of parent fatty acid compounds – arachidonic acid (AA), docosahexaenoic acid (DHA), and eicosapentaenoic acid (EPA) – via three enzymatic pathways – cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450. Eicosanoids regulate multiple important physiological responses, including immune response to infection, homeostasis, and inflammation [Dennis et al., 2015]. Importantly, eicosanoid production is known to be increased with the activation of inflammation, and COX and LOX pathways are operative in such activation [Dennis et al., 2015]. Although most studies have linked eicosanoids to pro-inflammatory responses, as eicosanoid signaling is similar to pro-inflammatory cytokine signaling and inflammasome formation [Lawrence et al., 2002, Shinomiya et al., 2001], recent studies also suggest some anti-inflammatory activities of eicosanoids [Chiang et al., 2005, Dennis et al., 2015].
While still not well understood, eicosanoids may play crucial roles during pregnancy and parturition [Peiris et al., 2017]. The parturition process is a complex physiological phenomenon that is mediated by two independent signaling pathways – fetal endocrine signals and fetal growth-related signals [Challis et al., 2005, Mesiano, 2019]. Both pathways may promote the production of multiple eicosanoids, which suggest the potential role of abnormal eicosanoid levels during pregnancy on adverse birth outcomes [Keirse., 2020, Mosaad et al., 2020]. For example, prostaglandin E2 (PGE2) and prostaglandin F2a (PGF2a) are known to mediate myometrial contractibility at term [Egarter et al., 1992, Li et al., 2021]. Other studies of the amniotic fluid showed the onset of labor was accompanied by increased levels of thromboxanes [Ölund et al., 1980, Romero et al., 1996] and prostaglandins (PGE2, PGD2, PGF2a, and others) [Berryman et al., 1987, Romero et al., 1994]. In addition, growing evidence suggests eicosanoid levels during pregnancy are associated with a variety of adverse pregnancy outcomes such as preeclampsia [Jiang et al., 2013, Dalle et al., 2016], preterm birth [Hong et al., 2016, Aung et al., 2019], and possibly fetal growth disorders [Pearson et al., 2010, Welch et al., 2020].
Given the potential role of eicosanoids in the etiology of adverse birth outcomes in combination with results from our previous preliminary study in the Boston LIFECODES cohort showing strong relationships between urinary phthalate concentrations and plasma eicosanoid levels (Aung et al., 2021), in this study we aimed to examine the impact of phthalate exposure on eicosanoid levels during gestation in a larger number of pregnant women from the Puerto Rico PROTECT cohort using both single pollutant and multi-pollutant approaches.
2. Methods
2.1. Study participants
Participants were women enrolled in the ongoing PROTECT prospective birth cohort in Puerto Rico. The PROTECT study was initiated in 2010 and recruits women at approximately 14 ± 2 weeks of gestation with the following inclusion criteria: 1) between 18 to 40 years old, 2) lived in the Northern karst region, 3) did not use oral contraceptives three months before pregnancy, 4) did not report major obstetrical or medical complications, and 5) did not use in vitro fertilization to achieve the pregnancy [Cantonwine et al., 2014]. Prenatal urine samples were collected during study visits conducted up to three times at approximately 18 (visit 1), 22 (visit 2), and 26 (visit 3) weeks of gestation. Demographic/socioeconomic information of study participants was collected through a series of detailed questionnaires [Meeker et al., 2013]. At the time of this analysis, a total of 1,576 PROTECT women had at least one prenatal phthalate metabolite measurement from at least one of three study visits, and 655 of those had plasma eicosanoids measured at visit 3 and were included in the present analysis. Among 655 participants, 540 (82.4%) have three urinary phthalate metabolites measures, while 98 participants (15.0%) have two measures and 17 (2.6%) have only one measure across gestation. This study was approved by the research and ethics committees of the University of Michigan School of Public Health, Northeastern University, the University of Puerto Rico, and participating hospitals and clinics. Study participants received a full description of the study and provided full informed consent prior to participation.
2.2. Phthalate measurements
Phthalate metabolites were measured in maternal urine samples collected during each prenatal study visit up to three times during gestation. All samples were frozen at −80°C and shipped on dry ice to the Centers for Disease Control and Prevention for analysis. Urinary concentrations of 15 phthalates and 2 metabolites of a recent phthalate replacement, di-2-ethylhexyl terephthalate (DEHTP), were measured using solid-phase extraction HPLC-isotope dilution tandem mass spectrometry as previously described [Kato et al., 2005, Silva et al., 2007, Silva et al., 2019]. Measured phthalate metabolites were mono (3-carboxypropyl) phthalate (MCPP), monocarboxyoctyl phthalate (MCOP), mono-oxoisononyl phthalate (MONP), mono- isobutyl phthalate (MiBP) and mono-2-hydroxy-iso-butyl phthalate (MHiBP), mono-ethyl phthalate (MEP), mono(2-ethyl-5-oxohexyl) phthalate(MEOHP), mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-carboxypentyl) phthalate (MECPP), monocarboxynonyl phthalate (MCNP), mono-3-hydroxybutyl phthalate (MHBP), mono-n-butyl phthalate(MBP), mono-benzyl phthalate(MBzP),mono-isononyl phthalate (MNP), and two DEHTP metabolites, mono-2-ethyl-5-carboxypentyl terephthalate (MECPTP) and mono-2-ethyl-5-hydroxyhexyl terephthalate (MEHHTP). Most phthalates were detected in more than 80% of samples, with the exceptions of MECPTP (72.50%), MEHHTP (72.83%), and MONP (72.72%). The number of participants with measurements for these three metabolites was lower since those metabolites were added to the analytical panel in more recent years. Pearson correlation coefficients were calculated on natural log-transformed phthalate metabolite concentrations to create a heat map of correlation coefficients between metabolites across the study duration. We calculated the summary measures of phthalate parent compounds by adding the molar fractions of their metabolites. Specifically, the metabolites used for DEHP included MEHP, MEHHP, MEOHP, and MECPP; for DBP, MBP and MHBP were used; for DIBP, MIBP and MHIBP were used; and for DEHTP, MECPTP and MEHHTP were used. We then multiplied the summary measures by the molecular weight of the primary metabolite for unit comparability (ng/ml); MEHP (278.348 g/mol) for DEHP; MBP (222.24 g/mol) for DBP; MIBP (222.24 g/mol) for DIBP; MEHHTP (294.34 g/mol) for DEHTP, as previously described [Rodríguez-Carmona et al., 2020].
Values below the limit of detection (LOD) were substituted by LOD divided by the square root of 2. This imputation method has been shown to produce nonbiased means and standard deviations when the percent of samples below the LOD is low, and is justified since all phthalate metabolites in our analysis were detected in more than 70% of urine samples collected across the study visits [Hornung & Reed 1990]. To account for urinary dilution, urinary phthalate concentrations were corrected for specific gravity using the following formula Pc = P * [(SGm - 1) / (SGi - 1)], where Pc is the specific gravity corrected concentration (ng/ml), P is the measured urinary concentration, SGm is the median specific gravity across study population (1.019), and SGi is the specific gravity for each participant [Meeker et al., 2009]. Geometric means of specific gravity-corrected phthalate metabolite levels across pregnancy were used in both single-pollutant and multi-pollutant analyses.
2.3. Eicosanoids measurements
2.3.1. Extraction of Eicosanoids from Plasma
Aliquots of 100 μL human plasma were diluted to 1 mL with 50mM phosphate salt buffer (PH7.4) spiked with 200 picomol/L of deuterated internal standards as described previously (Afshinnia et al., 2020). The eicosanoids were extracted using Strata-X Polymeric SPE columns (Phenomenex, Inc, Torrance, CA). The columns were preconditioned with 3 mL of 100% methanol, then equilibrated with 3 mL of water, and the plasma containing internal standards were loaded into columns, followed by 3 ml of 10% methanol washing, then eluted with 1 mL of 100% methanol and stored at −80 °C. All authentic eicosanoids and their deuterated forms were purchased from Cayman Chemical (Ann Arbor, MI).
2.3.2. HPLC/MS/MS analysis.
Prior to analysis, the eluants were dried under vacuum, redissolved in 100 μL of solvent A (water/acetonitrile/acetic acid, 60:40:0.02; v/v/v) for HPLC/MS/MS analysis. Samples were subjected to reverse phase chromatography utilizing Luna C18 LC column (100 Å, 3 μm, 150 x 2 mm; Phenomenex, Inc, Torrance, CA) with solvent A (water/acetonitrile (60:40) with 0.02% acetic acid) and solvent B (acetonitrile/isopropyl alcohol (50:50)) utilizing an Agilent 1200 LC system (Santa Clara, CA). Mass spectrometric experiments were performed using an Agilent 6490 Triple Quadrupole system equipped with iFunnel technology. Eicosanoid identification was based on unique fragmentation and corresponding compound-specific retention time with no isobaric overlap in MS1. Quantification of eicosanoids and their precursors were performed by comparing peak areas of the analyte of interest and their corresponding isotopically labeled internal standards. Units are μmol/L for parent compound – Arachidonic Acid, Docosahexaenoic Acid, Eicosapentaenoic Acid, Linoleic Acid, and α-Linolenic Acid – and nmol/L, otherwise.
2.3.3. Quality control
Sequential dilution of each internal standard was performed in duplicate to establish linearity, identify the lower limit of detection (LOD), and to estimate the coefficient of variation (CV) of the measurements at various concentrations. CVs and LODs of eicosanoids are summarized in Supplementary Table 6. In addition, pooled samples were analyzed at the beginning of each batch and then after each 10 unknown samples during mass spectrometry to assess drift in measurements over time as well as the batch-to-batch variability.
Nearly all eicosanoids were detected above the LOD in more than 75% of samples. We utilized machine-read values for samples below the LOD, except when the eicosanoid concentrations were negative. In cases where the eicosanoid concentration was negative, it was excluded from the analysis. However, negative values occurred in very few instances. This approach was implemented to prevent an excessive number of data points with identical values, which can adversely affect distribution, standard errors, and other statistical measures. A similar methodology has been employed in previous studies [Bloom et al., 2014, Eskenazi et al., 2017, Zhang et al., 2022; Taibl et al., 2022].
2.4. Statistical analysis
2.4.1. Single pollutant analysis
Distributions of urinary exposure biomarkers and plasma eicosanoids were assessed prior to analysis, and both were natural log-transformed and were included as continuous variables in regression models. The associations between urinary concentrations of phthalate and replacement metabolites and plasma eicosanoid levels were examined using multivariable regression models, adjusting for potential confounders. The confounders were primarily selected based on a priori knowledge and included in the final model after examining their impacts on the main effect estimate (>10%). Potential confounders considered were maternal age, maternal education, pre-pregnancy BMI, marital status, environmental tobacco smoke, alcohol consumption, and employment status (Directed acyclic graph in Supplementary Figure 3). All final models were adjusted for maternal age (continuous), maternal education (categorical; GED or less, some college, Bachelors or higher), and maternal pre-pregnancy BMI (continuous). Separate models were fitted for each phthalate metabolite and each eicosanoid. Regression results are presented as the difference in eicosanoid level from the plasma sample (95% confidence interval) per natural log-unit (ng/ml) increase in urinary phthalate metabolite level. The Benjamini and Hochberg method was used to control for the false discovery rate (FDR q-value < 0.05) [Benjamini et al., 1995]. To conduct a sensitivity analysis, we performed single pollutant analysis using a subset of the population (N=540) with complete phthalate measurements across three visits throughout gestation.
2.4.2. Multipollutant analysis
The subset of 530 women who had all 16 urinary phthalate and 54 plasma eicosanoids measures was included in the mixture analysis. The adaptive elastic net (adENET) was utilized to calculate environmental risk scores (ERS), a weighted sum of an individual’s exposure to a mixture of phthalates (Park et al., 2014, 2017). Elastic net (ENET) is a combination of two variable selection approaches, LASSO and Ridge regression, to overcome the limitations of LASSO with highly correlated data [Zou and Hastie 2005, Tibshirani 1996]. While both LASSO and Ridge regression are regularized regression that include penalty terms to shrink unimportant predictors, ridge regression does not shrink unimportant predictors to zero while LASSO randomly selects one predictor from highly correlated predictors and shrinks the others to zero [Tibshirani 2011]. Thus, ridge regression and ENET, a hybrid of Ridge and LASSO, is better equipped to handle data with highly correlated predictors. Additionally, the adaptive elastic net (adENET), proposed by Zou and Zhang provides additional shrinkage of unimportant predictors and allows for hypothesis testing of selected predictors and provides standard error (SEs) and p-values. Two optimal tuning parameters, λ1 and λ2, were decided by 5-fold cross-validations to minimize prediction errors. We did not penalize the coefficients of covariates. To avoid potential overfitting issues in constructing ERS, we ran adENET with a 70% randomly selected subset. Additionally, we utilized 100 bootstrap replications (B=100) of resampling and adENET to account for variability due to random sampling. The phthalate metabolites that were selected for greater than or equal to 50% of the replications were used to construct ERS by averaging their coefficients over the replications and using them as the weights to reflect the relative importance in the prediction of the outcome. Entire dataset was used to calculate ERS, subsequently, ERS was calculated as a weighted sum of each individual in the sample. The calculated ERS were then used as predictor variables in multivariable linear regressions to test for associations between exposure to phthalate metabolite mixtures and each outcome. The R package gcdnet (R version 4.0.4) was used.
3. Results
3.1. Demographics and biomarker distributions
The demographic characteristics of study participants (N=655) are summarized in Table 1. The majority of demographic information had missing values of less than 1%, with the exception of pre-pregnancy BMI (4.58%), and annual household income (12.98%). In general, most women in the analysis were under the age 30 (72.5%), had received a college degree (71.6%), were not single (79.5%), were currently employed (55.3%), had an annual household income under 30k (61.2%), had a pre-pregnancy BMI under 30 kg/m2 (73.6%), did not ever consume alcohol (58.8%), and had < 2 total pregnancies (78.6%). The distributions of maternal urinary phthalate metabolite concentrations averaged across gestation, as well as plasma eicosanoid concentrations measured at visit 3, are presented in Table 2. Additionally, the distributions of maternal urinary phthalate metabolite by each visit are presented in Supplementary Table 1. The figure displayed in Supplementary Figure 1 illustrates a heat map depicting the Pearson correlation coefficients between phthalate metabolite concentrations, which were adjusted for specific gravity and averaged over gestation. As anticipated, metabolites originating from the same parent compound exhibited moderate to strong correlations (R = 0.67 - 0.97), while metabolites originating from different parent compounds demonstrated low to moderate correlations (R = 0.01 - 0.57).
Table 1.
Demographic characteristics for 655 women in PROTECT.
| N | % | ||
|---|---|---|---|
| Maternal Age (years) | 18-24 | 274 | 41.83 |
| 25-29 | 201 | 30.69 | |
| 30-34 | 108 | 16.49 | |
| 35-41 | 72 | 10.99 | |
| Maternal Education | GED or less | 182 | 27.79 |
| Some college | 226 | 34.5 | |
| Bachelors or higher | 243 | 37.1 | |
| Missing | 4 | 0.61 | |
| Marital Status | Single | 127 | 19.39 |
| Married | 287 | 43.82 | |
| Cohabitating | 234 | 35.73 | |
| Missing | 7 | 1.07 | |
| Currently Employed | No | 288 | 43.97 |
| Yes | 362 | 55.27 | |
| Missing | 5 | 0.76 | |
| Annual Household Income | <10k | 256 | 39.08 |
| 10k - <30k | 145 | 22.14 | |
| 30k - <50k | 113 | 17.25 | |
| ≥50k | 56 | 8.55 | |
| Missing | 85 | 12.98 | |
| Pre-pregnancy BMI | (0, 25] | 304 | 46.41 |
| (25, 29.9] | 178 | 27.18 | |
| (29.9, 51] | 143 | 21.83 | |
| Missing | 30 | 4.58 | |
| Alcohol Use | Never | 385 | 58.78 |
| Yes, before pregnancy | 235 | 35.88 | |
| Yes, currently | 29 | 4.43 | |
| Missing | 6 | 0.92 | |
| Number of Children | 0 | 300 | 45.8 |
| 1 | 215 | 32.82 | |
| 2-5 | 137 | 20.92 | |
| Missing | 3 | 0.46 |
Table 2.
Distribution of specific gravity-adjusted phthalate metabolite and eicosanoid biomarker concentrations (N=655). Unit is ng/mL for phthalate. For eicosanoid, units are μmol/L for Docosahexaenoic Acid, Eicosapentaenoic Acid, Linoleic Acid, and α-Linolenic Acid, and nmol/L, otherwise.
| Phthalate / Replacement* | |||||
|---|---|---|---|---|---|
| Parent Compound | Metabolite | GM (25th, 75th) | Parent Compound | Metabolite | GM (25th, 75th) |
|
| |||||
| Multiple HW | MCPP | 1.13 (0.68, 1.77) | DEP | MEP | 36.6 (12.9, 88.12) |
| DNP/DINP | MCOP | 7.24 (4.19, 11.24) | DEHP | MEHP | 2.12 (1.32, 3.24) |
| DINP | MONP | 2.2 (1.4, 3.27) | MEHHP | 6.23 (4.04, 9.33) | |
| DIBP | MIBP | 9.8 (5.77, 16.58) | MEOHP | 5.7 (3.7, 8.36) | |
| MHIBP | 3.93 (2.38, 6.34) | MECPP | 11.3 (7.78, 16.17) | ||
| DBP | MBP | 16.7 (9.38, 28.35) | BzBP | MBZP | 2.32 (1.16, 4.26) |
| MHBP | 1.68 (0.93, 2.88) | DEHTP * | MECPTP | 23.74 (13.19, 36.98) | |
| DDP | MCNP | 1.38 (0.92, 1.97) | MEHHTP | 3.85 (2.18, 6.33) | |
|
| |||||
| Eicosanoid | |||||
| Enzymatic Pathway | eicosanoid | GM (25th, 75th) | Enzymatic Pathway | eicosanoid | GM (25th, 75th) |
|
| |||||
| Cyclooxygenase | 13,14-dihydro-15-keto Prostaglandin D2 | 4.38 (2.06, 11.25) | Lipoxygenase | Leukotriene D4 | 6.23 (2.41, 16.09) |
| 13,14-dihydro-15-keto Prostaglandin E2 | 3.75 (2.02, 7.22) | Leukotriene E4 | 0.18 (0.05, 0.6) | ||
| 13,14-dihydro-15-keto Prostaglandin F2α | 12.26 (7.84, 18.04) | Resolvin D1 | 13.45 (10.59, 23.3) | ||
| 13,14-dihydro-15-keto Prostaglandin J2 | 2.13 (0.71, 8.18) | Resolvin D2 | 7.78 (4.51, 12.71) | ||
| 15-deoxy-Δ12,14-Prostaglandin J2 | 2.18 (0.8, 5.42) | Cytochrome P450 | (±)11,12-DHET | 1.08 (0.74, 1.35) | |
| 9-OxoODE | 121.08 (111.6, 186.76) | (±)12,13-DiHOME | 17.64 (10.5, 28.5) | ||
| Bicyclo Prostaglandin E1 | 1.51 (0.78, 2.86) | (±)18-HETE | 2.57 (1.4, 5.78) | ||
| Bicyclo Prostaglandin E2 | 0.72 (0.24, 1.38) | (±)5,6-DHET | 1.15 (0.51, 3.09) | ||
| Prostaglandin A2 | 15.82 (15.39, 20.84) | (±)8,9-DHET | 1.69 (0.67, 3.68) | ||
| Prostaglandin B2 | 7.71 (2.96, 19.46) | (±)9,10-DiHOME | 12.95 (7.61, 21.08) | ||
| Prostaglandin D2 | 6.82 (2.55, 15.84) | 11(12)-EET | 2.42 (1.12, 5.36) | ||
| Prostaglandin D3 | 3.34 (1.3, 7.66) | 11(S)-HETE | 1.45 (0.57, 3.84) | ||
| Prostaglandin E1 (power) | 5.28 (2.08, 12.11) | 12(13)-EpOME | 21.35 (13.11, 37.52) | ||
| Prostaglandin E2 | 1.87 (1.08, 2.84) | 14(15)-EET | 3.24 (1.62, 6.51) | ||
| Prostaglandin E3 | 1.06 (0.48, 2.28) | 16(S)-HETE | 3.79 (1.1, 11.89) | ||
| Prostaglandin J2 | 12.32 (8.82, 18.98) | 17(S)-HETE | 3.25 (1.46, 7.97) | ||
| Thromboxane B2 | 2.73 (1.14, 5.94) | 20-carboxy Arachidonic Acid | 17.1 (13.29, 77.34) | ||
| Lipoxygenase | 12-OxoETE | 6.69 (2.42, 17.23) | 20(S)-HETE | 189.89 (150, 298.34) | |
| 12(S)-HETE | 2.93 (1.36, 5) | 5(6)-EET | 16.2 (5.71, 33.62) | ||
| 13-OxoODE | 26.57 (17.89, 36.81) | 8(9)-EET | 7.87 (3.5, 19.25) | ||
| 13S-HODE | 24.21 (15.64, 36.39) | 9(10)-EpOME | 10.9 (7.72, 16.34) | ||
| 15-OxoETE | 12.43 (4.03, 33.74) | 9s-HODE | 14.84 (9.41, 23.98) | ||
| 15(S)-HETE | 1.79 (0.83, 3.41) | Parent Compound | Arachidonic Acid | 10.11 (6.54, 16.02) | |
| 5-OxoETE | 16.11 (6.48, 39.86) | Docosahexaenoic Acid | 4.9 (3.84, 9.95) | ||
| 5(S)-HETE | 5.38 (1.98, 12.1) | Eicosapentaenoic Acid | 1.58 (0.81, 2.55) | ||
| 8(S)-HETE | 1.16 (0.53, 2.33) | Linoleic Acid | 74.11 (48.79, 161.3) | ||
| Leukotriene B4 | 0.71 (0.3, 1.58) | α-Linolenic Acid | 396.89 (300.12, 1160.82) | ||
indicates the recent phthalate replacement, DEHTP.
3.2. Single pollutant analyses with eicosanoids
After adjusting for multiple comparisons, we found that 14 of the 20 phthalate metabolites or parent compound indices showed significant associations with at least one eicosanoid except for 13,14-dihydro-15-keto Prostaglandin F2α (Cyclooxygenase), 5(6)-EET (Cytochrome P450), (±)11,12-DHET (Cytochrome P450), and Leukotriene C4 methyl ester (Lypoxygenase), and 75% of which were negative (Figure 1, Supplementary Table 2). For example, parent compounds – arachidonic acid, docosahexaenoic acid, eicosapentaenoic acid, linoleic acid, and α-linolenic acid – predominantly showed negative associations with phthalate metabolites except for one positive association between MBZP and arachidonic acid. We observed some trends in associations related to the molecular weight of phthalates; metabolites of high molecular weight phthalates more frequently showed associations with multiple eicosanoids than low molecular weight phthalates. Among others, three phthalate metabolites (MCPP, MCOP, and MCNP) were associated with the majority of the measured eicosanoids across cyclooxygenase, cytochrome P450, and lipoxygenase pathway groups, and the parent lipid compound group. DEHP metabolites had negative associations with 13 eicosanoids, while positive associations were observed with 6 eicosanoids including 13,14-dihydro-15-keto Prostaglandin D2 (cyclooxygenase), Prostaglandin A2 (cyclooxygenase), 12(13)-EpOME (cytochrome P450), 8(9)-EET (cytochrome P450), 12(S)-HETE (lipoxygenase), and Leukotriene B4 (lipoxygenase). While negative trends of associations were dominant, a few eicosanoids consistently showed positive associations with multiple phthalate metabolites and parent compounds. Specifically, 13,14-dihydro-15-keto Prostaglandin D2 (cyclooxygenase), 12(13)-EpOME (cytochrome P450), and Leukotriene B4 (lipoxygenase) were positively associated with MCPP, MCOP, MCNP, and DEHP metabolites. Additionally, we observed that 12(13)-EpOME (cytochrome P450) was also positively associated with DIBP metabolites and MEP. Additional information of the effect estimates is summarized in Supplementary Table 2. The results of the sensitivity analysis, conducted with a subset of participants (N=540) who had complete phthalate measurements across three visits, showed that the trends of associations remained consistent. The findings from a subset of participants with complete phthalate measurements across three study visits (N=540) are presented in Supplementary Figure 4.
Figure 1.
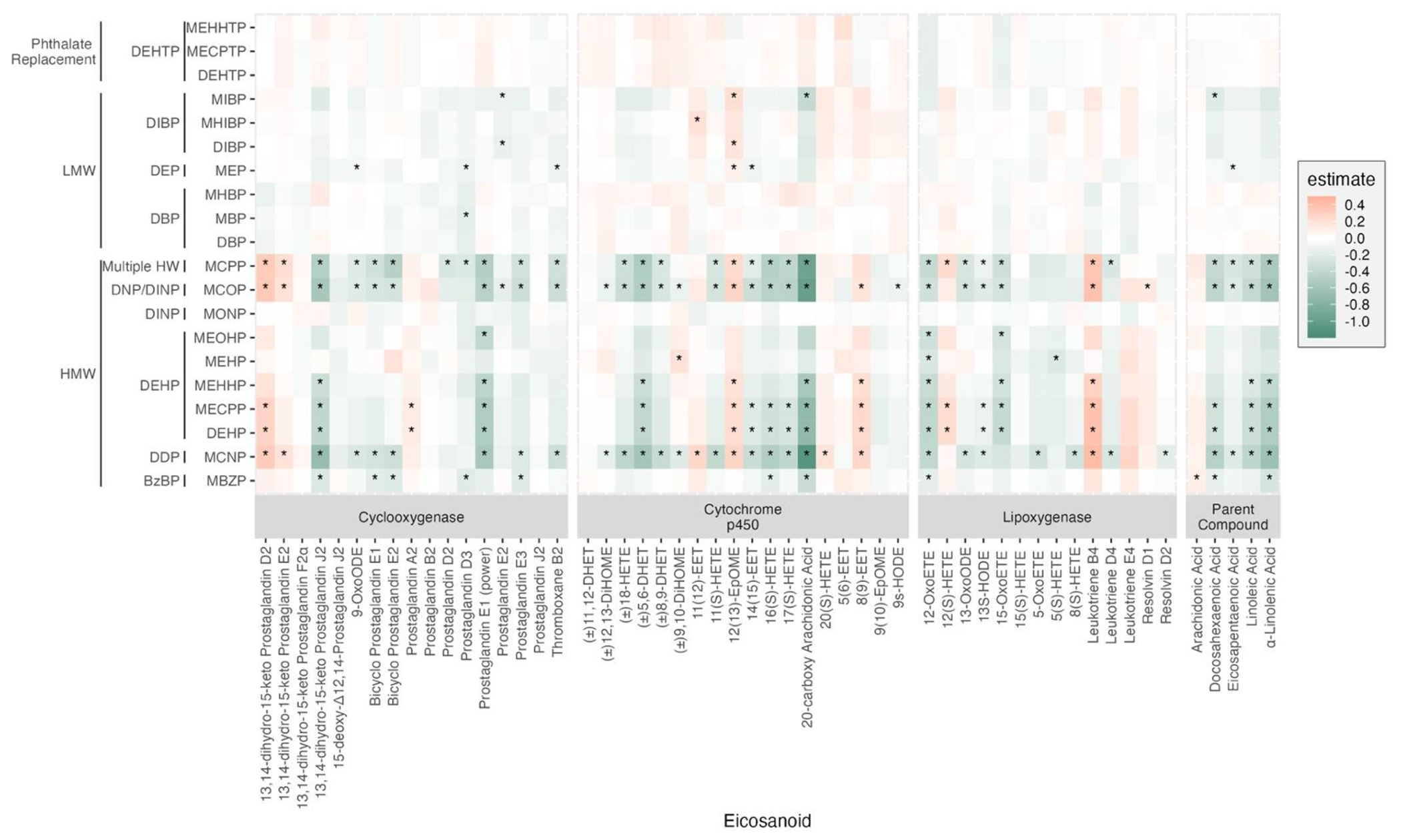
Heat map of pair-wise associations between phthalate exposure analytes and eicosanoids biomarkers estimated using multiple linear regression (N=655). Both phthalate and eicosanoids were natural log-transformed. Models adjusted for maternal age, education, pre-pregnancy BMI, and specific gravity. P-values were adjusted for multiple comparisons (FDR q < 0.05).
3.3. Multipollutant analyses with eicosanoids
In order to provide a comprehensive interpretation of our mixtures analyses results, we begin by offering contextual information. We present the weights derived from the adENET analyses, which represent the β estimate assigned by adENET. These weights indicate the relative importance of each predictor in determining the outcome. A large positive weight indicates a strong positive association between the predictor and the outcome, while a weight of zero signifies that the predictor was not selected.
The weights derived from adENET are then utilized to construct an Environmental Risk Score (ERS). The ERS represents a weighted sum of an individual’s overall phthalate exposure profile, taking into account the individual phthalate associations with the outcome of interest. In the subsequent multivariable linear regression, where ERS is employed as a predictor, the β estimate for ERS serves as an indicator of the collective impact of the predictor mixture on the outcome.
3.3.1. Bootstrapping of adaptive elastic net (adENET)
Bootstrapping of adENET selected more than one phthalate metabolite (≥50% from B=100) for 43 of 54 eicosanoids (cyclooxygenase n=13; cytochrome P450 n=16; lipoxygenase n=10; parent compound n=4) and the selected signals for each phthalate are shown in Figure 2 and Supplementary Table 3. We observed several differences in comparison to the single pollutant results. Firstly, metabolites of low molecular weight phthalates – DEP, DBP, and DIBP metabolites – became prominent in the mixture analysis result. For example, MHIBP, MEP, MBP, and MHBP were selected for multiple eicosanoids, unlike the results of the single pollutant analysis. Similarly, some phthalates that were null in single pollutant analysis became prominent in multipollutant analysis, mainly showing stronger positive weights and receding negative signals. For example, MEHP was selected for ten eicosanoids, mostly showing positive signals. In addition, we observed a new positive trend in the signals for Prostaglandin E1 with MHIBP, MEP, and MHBP, while the negative associations observed with MCPP, and MCOP in the single pollutant analysis faded in the mixture analysis. Most phthalate metabolites that were positively associated with Leukotriene B4 in the single pollutant analysis were not selected from the adENET bootstrapping, instead, negative signals were detected from MEP and two DBP metabolites, MHBP and MBP. Interestingly, MEHHTP and MECPTP, the metabolites of a new phthalate replacement, DEHTP, were selected for multiple eicosanoids from different pathway groups. Specifically, both MEHHTP and MECPTP showed negative weights for prostaglandin A2, while positive weights were observed for prostaglandin E1. Moreover, MEHHTP was selected for four cytochrome P450 eicosanoids – (±)8,9-DHET, 9(10)-EpOME, 12(13)-EpOME, and 5(6)-EET – but the directions of the weights were not consistent. In contrast, MECPTP showed consistent directions of weights by the enzymatic pathways of eicosanoids. For example, MECPTP was selected for three cytochrome P450 eicosanoids - (±)11,12-DHET, (±)9,10-DiHOME, and (±)12,13-DiHOME – and showed positive weights, but then showed negative weights for two lipoxygenase eicosanoids, 12-OxoETE and15-OxoETE. However, there were some similarities with the single pollutant analysis. For instance, similar to the single pollutant analysis, MCNP was most frequently selected (n=17) for eicosanoids from four pathway groups, mainly showing negative weights except for two eicosanoids from cytochrome P450 group – 11(12)-EET and 20(S)-HETE. MBZP also displayed a similar trend of associations as in the single pollutant analysis with multiple eicosanoids except for a few additional positive signals with (±)8,9-DHET (cytochrome P450), (±)11,12-DHET (cytochrome P450), and Leukotriene E4 (lipoxygenase). Additional information of the effect estimates is summarized in Supplementary Table 3.
Figure 2.
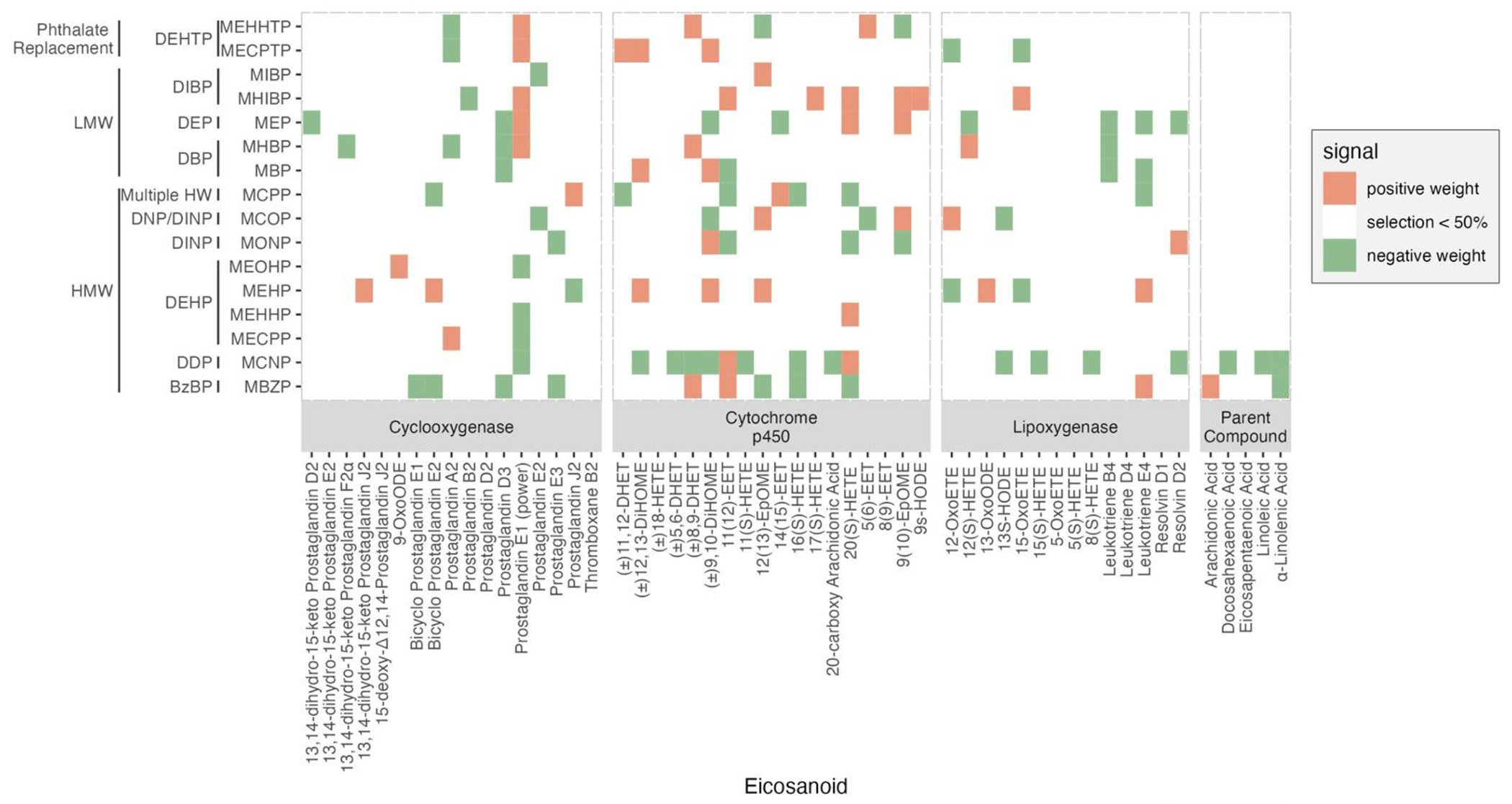
Heat map of multipollutant associations between phthalate exposure analytes and eicosanoids biomarkers selected by bootstrapping (B=100) of adaptive elastic net (≥ 50% selection) (N=530). Both phthalate and eicosanoids were natural log-transformed. Models adjusted for maternal age, education, pre-pregnancy BMI, and specific gravity.
3.3.2. Associations of environmental risk score (ERS) with eicosanoid
We observed that environmental risk score (ERS) was associated with ten eicosanoids from three different pathways – Cyclooxygenase, Lipoxygenase, and Cytochrome P450 – but not with eicosanoid parent compound (Figure 3 and Supplementary Table 4). In Figure 3, the β estimate for ERS serves as an indicator of the collective impact of the phthalate mixture on the individual eicosanoid levels. Interestingly, most observed associations were with eicosanoids from Cytochrome P450 pathway. For example, an increase in ERS was associated with a unit change in natural-log-transformed 11(S)-HETE (β=1.6, 95% CI: 0.020, 3.180), (±)11,12-DHET (β = 2.045, 95% CI: 0.250, 3.840), 17(S)-HETE (β= 2.136, 95% CI: 0.233, 4.039), 20(S)-HETE (β=0.813, 95% CI: 0.147, 1.479), 9(10)-EpOME (β=0.806, 95% CI: 0.248, 1.363), 9s-HODE (β=2.381, 95% CI: 0.657, 4.104), and (±)9,10-DiHOME (β=0.55, 95% CI: 0.155, 0.944). In addition, an increase in ERS was associated with two eicosanoids from the Cyclooxygenase pathway – Bicyclo Prostaglandin E2 (β=1.237, 95% CI: 0.516, 1.958) and Prostaglandin D3 (β=1.21, 95% CI: 0.404, 2.015). Moreover, we observed that one eicosanoid from the lipoxygenase pathway group were also positively associated with ERS. Specifically, an increase in ERS was associated with 15-OxoETE by 1.425 (95% CI: 0.186, 2.663). No significant negative associations were observed. Additional information of the effect estimates is summarized in Supplementary Table 4.
Figure 3.
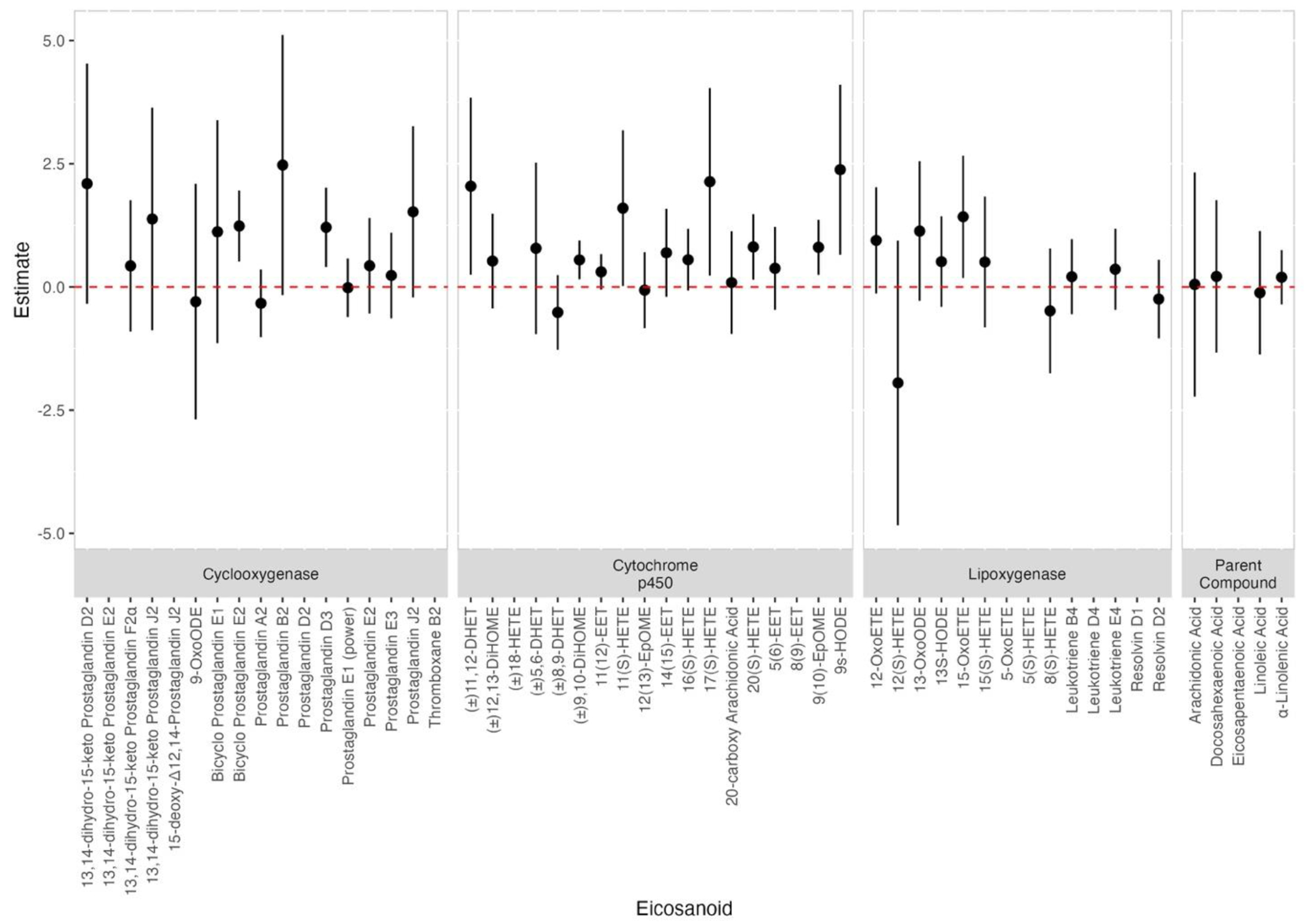
Associations and 95% confidence intervals between eicosanoid and ERS derived from 100 iterations of adaptive elastic net (N=530). Both phthalate and eicosanoids were natural log-transformed. Models adjusted for maternal age, education, pre-pregnancy BMI, and specific gravity.
Discussion
This study provides two primary contributions to the existing literature by (1) assessing the associations between phthalate metabolites and eicosanoid biomarkers of pregnant women in a multipollutant framework as well as single pollutant analysis, and (2) reducing selection variability by random chance by utilizing the bootstrapping methods in the adaptive elastic net framework. In single-pollutant analysis, we observed numerous significant associations between eicosanoid and phthalate metabolites and distinct patterns by molecular weight. Of note, in the single pollutant analysis, high molecular weight phthalate metabolites –MCNP, MCPP, MCOP, and two DEHP metabolites, MEHHP and MECPP – consistently showed negative associations with most eicosanoids. In the multipollutant analysis, however, metabolites of low molecular weight phthalates – MEP, DBP metabolites, and DIBP metabolites – also became prominent. We also observed that the direction of associations for high molecular weight varied, showing both positive and/or negative directions of association with eicosanoids. Additionally, we observed several associations of DEHTP metabolites – MEHHTP and MECPTP – with multiple eicosanoids from different enzymatic pathways in multipollutant analysis unlike no association observed in single pollutant analysis. The dissimilar trends observed in the multipollutant analysis, as opposed to the single pollutant analysis, can potentially be attributed to the substantial correlations among multiple phthalate metabolites, as demonstrated in Supplementary Figure 2. These correlations indicate the presence of multicollinearity concerns. Single pollutant models only account for marginal effects, whereas conditional models account for complex dependence structures among pollutants and potential confounding by co-pollutant. Considering this, it is reasonable to expect changes in effect size or direction when analyzing the individual pollutant one at a time compared to analyzing mixtures of pollutants. The differences in the results of single pollutant analysis and multipollutant analysis highlight the importance of examining the effect of phthalate exposure in mixture framework.
Our results provide important clues to understanding the health implications of phthalate exposure concerning altered eicosanoid levels during gestation. Eicosanoids are involved in multiple crucial physiological pathways such as inflammation, immune response, and oxidative stress [Basu 2010, Esser-von Bieren 2017], and thus may play important roles to mediate parturition [Mitchell 1987, Mosaad 2020, Keirse 2020]. For example, prostaglandins (PGs), leukotrienes (LTs), and hydroxy eicosatetraenoic acids (HETEs) are known to be pro-inflammatory eicosanoids, whereas some eicosapentaenoic acid (EPA) driven eicosanoids – lipoxins (LXs), epoxyeicosatrienoic acids (EETs), and resolvins – have anti-inflammatory activities. In addition, studies showed eicosanoids control key immune responses such as chemotaxis, antigen presentation, and phagocytes [Esser-von Bieren 2017]. Gestational phthalate exposure has been linked to adverse birth outcomes and developmental problems in the fetus [Qian et al., 2020, Zhong et al., 2020, Santos et al., 2021, Radke et al., 2019, Park et al., 2023]. Specifically, a recent study with 16 US cohorts has found that MBP, MIBP, MECPP, and MCPP are linked to higher odds of preterm birth [Welch et al., 2022]. Similarly, Wang et al. (2022) conducted a multinational cohorts study and observed a positive association between MBP, DEHP, and MCPP with preterm birth, and higher odds of spontaneous preterm birth related to MBP, MEP, MIBP, and MCPP. In addition, phthalate mixture was associated with ultrasound-derived fetal growth, reduced birthweight, and increased odds of preterm birth [Stevens et al., 2022, Cathey at al., 2021]. Previous research has implicated inflammation and oxidative stress as potential mechanisms underlying these associations [Basu 2010; Melville et al., 2013; Christian et al., 2012; Cappelletti et al., 2016, Ferguson et al., 2017]. Furthermore, our recent study with the LIFECODES birth cohort in Boston identified multiple eicosanoids, particularly from the lipoxygenase and cytochrome P450 pathways, were associated with preterm birth [Aung et al., 2019]. Eicosanoids not only have close relationships with intermediate pathways such as inflammation and oxidative stress but may also represent important biological pathways linking phthalate exposure to adverse birth outcomes. Understanding the impact of gestational phthalate exposure on eicosanoid concentrations during pregnancy is crucial for unraveling the etiology of adverse birth outcomes and fetal developmental problems associated with phthalate exposure. Our findings could have significant implications for informing interventions and diagnostics with the potential to improve public health outcomes.
While limited information on eicosanoids in relation to phthalate exposure has been reported, a small number of studies are relevant to our analysis. Zhou et al., (2018) observed that arachidonate, the free fatty acid (FFA) precursor for eicosanoid biosynthesis, measured in pregnant women was positively associated with multiple metabolites of both low molecular weight phthalates – MEP, MBP, MIBP – and high molecular weight phthalates – DEHP metabolites and the high molecular weight sum of MBZP, MCPP, MCOP, and MCNP. In the LIFECODES study of pregnant women in Boston (N = 173), unlike results from the present study, Aung et al., (2021) reported primarily positive associations between eicosanoids and the measured phthalate metabolites. In a multipollutant analysis, they reported several positive signals with eicosanoids from the cyclooxygenase, cytochrome P450, and lipoxygenase pathways. Specifically, in their single pollutant analysis, Aung et al., (2021) reported 13S-HODE (Lipoxygenase) was positively associated with some high molecular weight phthalates – DEHP metabolites and MCPP – while we observed negative associations in the present study. Similarly, in the LIFECODES study, Prostaglandin E1 (Cyclooxygenase) was positively associated with DEHP metabolites, while our analysis showed negative associations. Still, there are some consistencies with our study. First, 8(9)-EET (Cytochrome P450) was positively associated with the DEHP metabolite MEHHP, and 14(15)EET (Cytochrome P450) was negatively associated with the DEHP metabolite MECPP in both studies. In addition, from the multipollutant analysis, MBZP was selected for 20(S)-HETE (Cytochrome P450) with a negative direction in both studies. Although these similarities, there were more discrepancies between the present study and the LIFECODES study in our multipollutant analysis results. The differences might be attributed to 1) some phthalate metabolites in this study, specifically, MCOP, MONP, MCNP and DEHTP metabolites, were not included in the LIFECODES study which could be influencing residual confounding in the exposure matrix, 2) subsequently different combinations of predictors possibly leading to different selection patterns. There were also differences in cohort demographics, study design, exposure levels, and other factors which may contribute to different results between studies. As an illustration, the LIFECODES study predominantly comprised participants who were over 30 years old and of White ethnicity, whereas the current PROTECT study primarily included Latina participants who were under 30 years old. Furthermore, it is worth noting that the Puerto Rican population, in general, exhibits a higher poverty rate (40.6%) compared to the population of Massachusetts (17.6%) [U.S. Census Bureau, 2022]. The composition of the study population, in terms of demographics, socioeconomic status, and various lifestyle factors, may have influenced the observed associations. Additionally, the present study and the LIFECODES study differ in their study designs. Specifically, in the Aung et al. study (2021), a subset of LIFECODES participants was selected, prioritizing the proportion of preterm birth cases (<37 weeks gestation) and controls at a ratio of 1:2, whereas our study population was not based on a case-control design. Moreover, the present study benefits from a larger sample size (N=655) compared to the preliminary study (N=173), thereby increasing the statistical power and reinforcing the robustness of the current findings. Lastly, it is important to consider that variations in exposure levels may have contributed to the divergent results observed between the two studies. The LIFECODES analysis involved participants who were recruited between 2006 and 2008, while the PROTECT study included participants from 2010 to the present. During this time period, exposure levels to many phthalates have been shown to decline. Specifically, when comparing the participants in the LIFECODES study to those in the PROTECT study, it was found that the exposure levels of all phthalate metabolites were higher in the LIFECODES study participants, ranging from at least 1.3 times to as much as 5.7 times higher. A summary of the comparison between exposure and outcome profiles in the two studies can be found in Supplementary Table 5.
Previous research may aid in our understanding of the potential biological mechanisms underlying the results of our study. Phthalates can affect eicosanoid levels by disrupting the metabolic pathways that act upon the fatty acid parent compounds. A previous in vitro study reported inhibitory effects of DBP and DEP on cytochrome P450 activities [Ozaki., 2016], and an animal study demonstrated that DBP exposure to pregnant rats was associated with a significant reduction in cytochrome P450 family gene expressions [Struve et al., 2009]. Several in vitro studies have also shown that DEHP and its metabolite MEHP significantly increased cytosolic cyclooxygenase-2 expression [Wong et al., 2019, Onorato et al., 2008] and can subsequently increase prostaglandin E2 (PGE2) levels [Tetz et al., 2015]. Furthermore, Rakkestad et al. (2010) showed that MEHP exposure impacted the lipoxygenase pathway by decreasing lipoxygenase inhibitor NDGA and observed a subsequent increase in leukotriene LTB4 levels. Interestingly, MEHP was not associated with PEG2 or LTB4 both from single pollutant and multipollutant analysis. In addition, inhibition of 15-Lipoxygenase (15-LOX) by DEHP has been reported [Malterud et al., 1999], and 15-LOX enzyme plays a role in producing 15-HETE eicosanoid [Kuhn & Borngraber 1999], which can be converted to 15-oxo-ETE by oxidation [Wei et al., 2009]. While limited information exists, a recent animal study has shown the association of DEHTP with altered cytochrome P450 enzyme transcription [Yun & Ji., 2023]. Align with this, in our mixture analysis we observed a metabolite of DEHP, MEHP, was selected for 15-oxo-ETE, showing negative weight. Although limited information is available regarding the impact of phthalate exposure on altered eicosanoid levels and potential pathways, both commonalities and discrepancies with our study exist. Future translational studies on this association may help bridge these uncertainties.
Furthermore, altered levels of eicosanoids can affect each other, as shown by the example where 11,12-EET (Cytochrome P450 pathway) can be converted by an epoxide hydrolase to 11,12-DHET [Fang et al., 1996]. Our analysis found a positive association between both 11,12-EET and 11,12-DHET and ERS. Although the association between ERS and 11,12-EET did not reach statistical significance, the instability of 11,12-EET may explain this result, as it rapidly converts to 11,12-DHET [Imig, 2012]. Similarly,15-oxo-ETE (Lipoxygenase pathway) can be produced by the oxidation of 15(S)-HETE [Wei et al., 2009]. Our study found a positive association between ERS and 15-oxo-ETE but not with 15(S)-HETE, which could be due to the consumption of 15(S)-HETE for 15-oxo-ETE production. In addition, other eicosanoids that were positively associated with ERS in our study, such as 9S-HODE and 20(S)-HETE, are known to have pro-inflammatory effects [Shinto et al., 2022; Medhora et al., 2008]. Inflammation and eicosanoids are involved in a complex feedback loop, where inflammation can increase the production of eicosanoids such as prostaglandins and leukotrienes, while elevated levels of eicosanoids further increase the expression of proinflammatory cytokines and other genes involved in inflammation [Hellewell & Williams, 1988; Calder, 2006; Theken et al., 2011]. Thus, further studies compressively examining the interaction between inflammation and altered eicosanoid levels concerning phthalate exposure are needed for mechanistically understanding these relationships.
This study has several strengths. Firstly, we utilized the PROTECT birth cohort in Puerto Rico, which provided valuable data on a population facing a high burden of environmental contamination, poverty, and adverse health outcomes. Our research aimed to inform the development of actionable preventive measures for this vulnerable population and support efforts to improve their health. In terms of methodology, our analysis included a large sample size (N= 655) and extensive panels of both phthalate metabolites and eicosanoid biomarkers. Furthermore, our statistical approach was innovative, as we utilized multivariable regression analysis adjusted for the false-discovery rate to examine the independent impact of single pollutants. We also utilized adaptive elastic net to examine individual phthalate effects in the context of concurrent exposure to other phthalates in a mixture. To reduce selection variability in the mixture analysis, we bootstrapped the adaptive elastic net. Finally, we investigated phthalate mixture effects on eicosanoids through multivariable regression analysis of environmental risk score (ERS) and eicosanoid levels. By employing this multipollutant approach, our study revealed notable differences when compared to single pollutant analyses. This is particularly relevant as individuals are not exposed to a single phthalate in isolation, but rather to complex mixtures of phthalates from multiple exposure routes. As a result, our findings hold significant implications for public health considerations. Understanding the collective effects of these mixtures is crucial for accurately assessing the potential risks associated with phthalate exposure and implementing appropriate interventions. By considering the complexity of phthalate mixtures, our study contributes valuable insights that can inform strategies to safeguard public health.
However, this study also had several limitations that need to be addressed in future research. For instance, we used phthalate metabolite concentrations averaged over gestation, which meant that we were unable to examine potential windows of susceptibility for these associations at specific time points in pregnancy. Similarly, we were limited to a single time point measurement of eicosanoid biomarkers during pregnancy, which prevented us from evaluating the time-course changes in eicosanoid levels during gestation. Additionally, possible interactions with other co-pollutants in addition to phthalates were not examined, as it was beyond the scope of this study, but remains a goal in our future analyses. Lastly, the potential impact of underlying maternal medical conditions, such as polycystic ovary syndrome or endometriosis, which were not considered in our exclusion criteria, might exist. Due to limited access to this information, we were unable to fully account for their effects. Future studies that incorporate comprehensive data on these conditions would contribute to a deeper understanding of their associations with phthalate exposure and eicosanoid levels.
In conclusion, this study leveraged multiple state-of-the-art biomarker measurements and statistical methods to examine eicosanoids associated with individual phthalate metabolites and with phthalate mixtures. We found that linear associations between eicosanoid levels and exposure to individual phthalates during gestation do not necessarily align with the signal observed for the overall phthalate mixture. This underscores the complexity of phthalate mixtures on their physiological impact and calls for further in-depth studies to examine these relationships.
Supplementary Material
Highlights.
Largest study to examine phthalate and eicosanoid associations in single and multipollutant frameworks.
First study to investigate the impact of a recent phthalate replacement on altered levels of eicosanoids during pregnancy.
Metabolites of low molecular phthalates and a recent replacement became prominent in multipollutant analysis.
Weighted sum of phthalate exposure positively associated with multiple eicosanoids, particularly those from the Cytochrome P450 pathway.
Acknowledgements
This study was supported by the Superfund Research Program of the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH; grant number P42ES017198). Additional support was provided from NIEHS grant numbers R01ES032203, P30ES017885, R01ES031591 and the Environmental influences on Child Health Outcomes (ECHO) program grant number UH3OD023251. Dr. Aung was supported in part by NIEHS core grant P30ES007048, U2COD023375 OIF subaward, and the JPB Environmental Health Fellowship. ECHO is a nationwide research program supported by the NIH, Office of the Director to enhance child health. We would like to extend our gratitude to all PROTECT study participants and their families. We also thank the nurses and research staff who participated in cohort recruitment and follow up, as well as the Federally Qualified Health Centers (FQHC) and clinics in Puerto Rico who facilitated participant recruitment, including Morovis Community Health Center (FQHC), Prymed: Ciales Community Health Center (FQHC), Camuy Health Services, Inc. (FQHC), and the Delta OBGyn (Prenatal Clinic). We thank Prof. Sung Kyun Park for helpful discussions on mixture modeling.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Afshinnia F, Zeng L, Byun J, Wernisch S, Deo R, Chen J, Hamm L, Miller ER, Rhee EP, Fischer MJ, Sharma K, Feldman HI, Michailidis G, & Pennathur S (2020). Elevated lipoxygenase and cytochrome P450 products predict progression of chronic kidney disease. Nephrol Dial Transplant, 35(2), 303–312. 10.1093/ndt/gfy232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aung MT, Yu Y, Ferguson KK, Cantonwine DE, Zeng L, McElrath TF, Pennathur S, Mukherjee B, & Meeker JD (2019). Prediction and associations of preterm birth and its subtypes with eicosanoid enzymatic pathways and inflammatory markers. Scientific reports, 9(1), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aung MT, Yu Y, Ferguson KK, Cantonwine DE, Zeng L, McElrath TF, Pennathur S, Mukherjee B, & Meeker JD (2021). Cross-sectional estimation of endogenous biomarker associations with prenatal phenols, phthalates, metals, and polycyclic aromatic hydrocarbons in single-pollutant and mixtures analysis approaches. Environmental health perspectives, 129(3), 037007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basu S (2010). Bioactive eicosanoids: Role of prostaglandin F and F-isoprostanes in inflammation and oxidative stress related pathology. Molecules & Cells (Springer Science & Business Media BV; ), 30(5). [DOI] [PubMed] [Google Scholar]
- 5.Benjamin S, Masai E, Kamimura N, Takahashi K, Anderson RC, & Faisal PA (2017). Phthalates impact human health: Epidemiological evidences and plausible mechanism of action. Journal of hazardous materials, 340, 360–383. [DOI] [PubMed] [Google Scholar]
- 6.Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological), 57(1), 289–300. [Google Scholar]
- 7.Berryman GK, Strickland DM, Hankins GD, & Mitchell MD (1987). Amniotic fluid prostaglandin D2 in spontaneous and augmented labor. Life sciences, 41(13), 1611–1614. [DOI] [PubMed] [Google Scholar]
- 8.Bloom MS, Jansing RL, Kannan K, Rej R, & Fitzgerald EF (2014). Thyroid hormones are associated with exposure to persistent organic pollutants in aging residents of upper Hudson River communities. International journal of hygiene and environmental health, 217(4-5), 473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong Q, Liu H.-l., Fu H, Niu Q.-s., Wu H.-b., Huang F, 2021. Prenatal exposure to phthalates with preterm birth and gestational age: A systematic review and meta-analysis. Chemosphere 282, 130991. [DOI] [PubMed] [Google Scholar]
- 10.Bureau, U. S. C. (2022). Population Estimates Program (PEP).
- 11.Calder PC (2006). Polyunsaturated fatty acids and inflammation. Prostaglandins, leukotrienes and essential fatty acids, 75(3), 197–202. [DOI] [PubMed] [Google Scholar]
- 12.Cantonwine DE, Cordero JF, Rivera-González LO, Del Toro LVA, Ferguson KK, Mukherjee B, Calafat AM, Crespo N, Jiménez-Vélez B, & Padilla IY (2014). Urinary phthalate metabolite concentrations among pregnant women in Northern Puerto Rico: distribution, temporal variability, and predictors. Environment international, 62, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cappelletti M, Della Bella S, Ferrazzi E, Mavilio D, & Divanovic S (2016). Inflammation and preterm birth. Journal of leukocyte biology, 99(1), 67–78. [DOI] [PubMed] [Google Scholar]
- 14.Cathey AL, Eaton JL, Ashrap P, Watkins DJ, Rosario ZY, Vega CV, Alshawabkeh AN, Cordero JF, Mukherjee B, & Meeker JD (2021). Individual and joint effects of phthalate metabolites on biomarkers of oxidative stress among pregnant women in Puerto Rico. Environment international, 154, 106565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cathey AL, Watkins DJ, Rosario ZY, Vélez C, Mukherjee B, Alshawabkeh AN, Cordero JF, & Meeker JD (2022). Biomarkers of exposure to phthalate mixtures and adverse birth outcomes in a Puerto Rico birth cohort. Environmental health perspectives, 130(3), 037009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Challis JR, Bloomfield FH, Bocking AD, Casciani V, Chisaka H, Connor K, Dong X, Gluckman P, Harding JE, & Johnstone J (2005). Fetal signals and parturition. The journal of obstetrics and gynaecology research, 31(6), 492–499. [DOI] [PubMed] [Google Scholar]
- 17.Chiang N, Arita M, & Serhan CN (2005). Anti-inflammatory circuitry: lipoxin, aspirin-triggered lipoxins and their receptor ALX. Prostaglandins, leukotrienes and essential fatty acids, 73(3-4), 163–177. [DOI] [PubMed] [Google Scholar]
- 18.Christian LM (2012). Psychoneuroimmunology in pregnancy: Immune pathways linking stress with maternal health, adverse birth outcomes, and fetal development. Neuroscience & Biobehavioral Reviews, 36(1), 350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalle Vedove F, Fava C, Jiang H, Zanconato G, Quilley J, Brunelli M, Guglielmi V, Vattemi G, & Minuz P (2016). Increased epoxyeicosatrienoic acids and reduced soluble epoxide hydrolase expression in the preeclamptic placenta. Journal of hypertension, 34(7), 1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dennis EA, & Norris PC (2015). Eicosanoid storm in infection and inflammation. Nature Reviews Immunology, 15(8), 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eales J, Bethel A, Galloway T, Hopkinson P, Morrissey K, Short RE, & Garside R (2022). Human health impacts of exposure to phthalate plasticizers: An overview of reviews. Environment international, 158, 106903. [DOI] [PubMed] [Google Scholar]
- 22.Egarter C, & Husslein P (1992). 4 Biochemistry of myometrial contractility. Baillière’s clinical obstetrics and gynaecology, 6(4), 755–769. [DOI] [PubMed] [Google Scholar]
- 23.Eskenazi B, Rauch SA, Tenerelli R, Huen K, Holland NT, Lustig RH, Kogut K, Bradman A, Sjödin A, Harley KG, 2017. In utero and childhood DDT, DDE, PBDE and PCBs exposure and sex hormones in adolescent boys: The CHAMACOS study. International journal of hygiene and environmental health 220 (2), 364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esser-von Bieren J (2017). Immune-regulation and-functions of eicosanoid lipid mediators. Biological chemistry, 398(11), 1177–1191. [DOI] [PubMed] [Google Scholar]
- 25.Fang X, Kaduce TL, Weintraub NL, VanRollins M, & Spector AA (1996). Functional implications of a newly characterized pathway of 11, 12-epoxyeicosatrienoic acid metabolism in arterial smooth muscle. Circulation research, 79(4), 784–793. [DOI] [PubMed] [Google Scholar]
- 26.Ferguson KK, Chen Y-H, VanderWeele TJ, McElrath TF, Meeker JD, & Mukherjee B (2017). Mediation of the relationship between maternal phthalate exposure and preterm birth by oxidative stress with repeated measurements across pregnancy. Environmental health perspectives, 125(3), 488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hellewell P, & Williams T (1988). Interactions between eicosanoids and other mediators of inflammation. Eicosanoids in Inflammatory Conditions of the Lung, Skin and Joints, 43–66. [Google Scholar]
- 28.Hong J-S, Romero R, Lee D-C, Than NG, Yeo L, Chaemsaithong P, Ahn S, Kim J-S, Kim CJ, & Kim YM (2016). Umbilical cord prostaglandins in term and preterm parturition. The Journal of Maternal-Fetal & Neonatal Medicine, 29(4), 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hornung RW, & Reed LD (1990). Estimation of average concentration in the presence of nondetectable values. Applied occupational and environmental hygiene, 5(1), 46–51. [Google Scholar]
- 30.Imig JD (2012). Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiological reviews, 92(1), 101–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang H, McGiff JC, Fava C, Amen G, Nesta E, Zanconato G, Quilley J, & Minuz P (2013). Maternal and fetal epoxyeicosatrienoic acids in normotensive and preeclamptic pregnancies. American journal of hypertension, 26(2), 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kambia NK, Séverin I, Farce A, Moreau E, Dahbi L, Duval C, Dine T, Sautou V, & Chagnon MC (2019). In vitro and in silico hormonal activity studies of di-(2-ethylhexyl) terephthalate, a di-(2-ethylhexyl) phthalate substitute used in medical devices, and its metabolites. Journal of Applied Toxicology, 39(7), 1043–1056. [DOI] [PubMed] [Google Scholar]
- 33.Kato K, Silva MJ, Needham LL, & Calafat AM (2005). Determination of 16 phthalate metabolites in urine using automated sample preparation and on-line preconcentration/high-performance liquid chromatography/tandem mass spectrometry. Analytical chemistry, 77(9), 2985–2991. [DOI] [PubMed] [Google Scholar]
- 34.Keirse MJ (2020). Eicosanoids in human pregnancy and parturition. Eicosanoids in reproduction, 199–222. [Google Scholar]
- 35.Khanapure SP, Garvey DS, Janero DR, & Gordon Letts L (2007). Eicosanoids in inflammation: biosynthesis, pharmacology, and therapeutic frontiers. Current topics in medicinal chemistry, 7(3), 311–340. [DOI] [PubMed] [Google Scholar]
- 36.Kuhn H, & Borngraber S (1999). Part I: Enzymology, molecular biology, and biological functions of mammalian lipoxygenases-mammalian 15-lipoxygenases: Enzymatic properties and biological implications. Advances in Experimental Medicine and Biology, 447, 5–28. [PubMed] [Google Scholar]
- 37.Lawrence T, Willoughby DA, & Gilroy DW (2002). Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nature Reviews Immunology, 2(10), 787–795. [DOI] [PubMed] [Google Scholar]
- 38.Lee G, Kim S, Bastiaensen M, Malarvannan G, Poma G, Casero NC, Gys C, Covaci A, Lee S, & Lim J-E (2020). Exposure to organophosphate esters, phthalates, and alternative plasticizers in association with uterine fibroids. Environmental research, 189, 109874. [DOI] [PubMed] [Google Scholar]
- 39.Lessmann F, Kolossa Gehring M, Apel P, Rüther M, Pälmke C, Harth V, Brüning T, Koch HM, 2019. German Environmental Specimen Bank: 24-hour urine samples from 1999 to 2017 reveal rapid increase in exposure to the para-phthalate plasticizer di (2-ethylhexyl) terephthalate (DEHTP). Environment international 132, 105102. [DOI] [PubMed] [Google Scholar]
- 40.Li W.-j., Lu J. w., Zhang C.-y., Wang W.-s., Ying H, Myatt L, Sun K, 2021. PGE2 vs PGF2α in human parturition. Placenta 104, 208–219. [DOI] [PubMed] [Google Scholar]
- 41.Malterud KE, Rydland KM, & Haugli T (1999). Inhibition of 15-Lipoxygenase by Phthalate Plasticizers. Bulletin of Environmental Contamination and Toxicology, 62(3), 352–355. 10.1007/s001289900881 [DOI] [PubMed] [Google Scholar]
- 42.Medhora M, Chen Y, Gruenloh S, Harland D, Bodiga S, Zielonka J, Gebremedhin D, Gao Y, Falck JR, & Anjaiah S (2008). 20-HETE increases superoxide production and activates NAPDH oxidase in pulmonary artery endothelial cells. American Journal of Physiology-Lung Cellular and Molecular Physiology, 294(5), L902–L911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meeker JD, Cantonwine DE, Rivera-González LO, Ferguson KK, Mukherjee B, Calafat AM, Ye X, Anzalota Del Toro LV, Crespo-Hernández N, & Jiménez-Vélez B (2013). Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in Puerto Rico. Environmental science & technology, 47(7), 3439–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meeker JD, Hu H, Cantonwine DE, Lamadrid-Figueroa H, Calafat AM, Ettinger AS, Hernandez-Avila M, Loch-Caruso R, & Téllez-Rojo MM (2009). Urinary phthalate metabolites in relation to preterm birth in Mexico City. Environmental health perspectives, 117(10), 1587–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Melville JM, & Moss TJ (2013). The immune consequences of preterm birth. Frontiers in neuroscience, 7, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mesiano S (2019). Endocrinology of human pregnancy and fetal-placental neuroendocrine development. In Yen and Jaffe’s reproductive endocrinology (pp. 256–284. e259). Elsevier. [Google Scholar]
- 47.Mitchell M (1987). Regulation of eicosanoid biosynthesis during pregnancy and parturition. Eicosanoids and Reproduction, 108–127. [Google Scholar]
- 48.Mosaad E, Peiris HN, Holland O, Morean Garcia I, & Mitchell MD (2020). The role (s) of eicosanoids and exosomes in human parturition. Frontiers in Physiology, 11, 594313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nidens N, Vogel M, Körner A, & Kiess W (2021). Prenatal exposure to phthalate esters and its impact on child development. Best Practice & Research Clinical Endocrinology & Metabolism, 35(5), 101478. [DOI] [PubMed] [Google Scholar]
- 50.Ölund A, Kindahl H, Oliw E, Jan ÅL, & Larsson B (1980). Prostaglandins and thromboxanes in amniotic fluid during rivanol-induced abortion and labour. Prostaglandins, 19(5), 791–803. [DOI] [PubMed] [Google Scholar]
- 51.Onorato TM, Brown PW, & Morris PL (2008). Mono-(2-ethylhexyl) Phthalate Increases Spermatocyte Mitochondrial Peroxiredoxin 3 and Cyclooxygenase 2. Journal of andrology, 29(3), 293–303. [DOI] [PubMed] [Google Scholar]
- 52.Ozaki H, Sugihara K, Watanabe Y, Ohta S, & Kitamura S (2016). Cytochrome P450-inhibitory activity of parabens and phthalates used in consumer products. The Journal of toxicological sciences, 41(4), 551–560. [DOI] [PubMed] [Google Scholar]
- 53.Park SK, Tao Y, Meeker JD, Harlow SD, Mukherjee B, 2014. Environmental risk score as a new tool to examine multi-pollutants in epidemiologic research: an example from the NHANES study using serum lipid levels. PLoS One 9 (6), e98632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park SK, Zhao Z, & Mukherjee B (2017). Construction of environmental risk score beyond standard linear models using machine learning methods: application to metal mixtures, oxidative stress and cardiovascular disease in NHANES. Environ Health, 16(1), 102. 10.1186/s12940-017-0310-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park S, Zimmerman E, Huerta-Montañez G, Rosario-Pabón Z, Vélez-Vega CM, Cordero JF, Alshwabekah A, Meeker JD, & Watkins DJ (2023). Gestational exposure to phthalates and phthalate replacements in relation to neurodevelopmental delays in early childhood. Toxics, 11(1), 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pearson T, Zhang J, Arya P, Warren AY, Ortori C, Fakis A, Khan RN, & Barrett DA (2010). Measurement of vasoactive metabolites (hydroxyeicosatetraenoic and epoxyeicosatrienoic acids) in uterine tissues of normal and compromised human pregnancy. Journal of hypertension, 28(12), 2429–2437. [DOI] [PubMed] [Google Scholar]
- 57.Peiris H, Vaswani K, Almughlliq F, Koh Y, & Mitchell M (2017). Eicosanoids in preterm labor and delivery: potential roles of exosomes in eicosanoid functions. Placenta, 54, 95–103. [DOI] [PubMed] [Google Scholar]
- 58.Pollard SH, & Porucznik CA (2017). Impact of periconceptional exposure to phthalates on pregnancy, birth, and neonatal outcomes. Current Epidemiology Reports, 4, 199–210. [Google Scholar]
- 59.Qian Y, Shao H, Ying X, Huang W, & Hua Y (2020). The endocrine disruption of prenatal phthalate exposure in mother and offspring. Frontiers in Public Health, 8, 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Radke EG, Glenn BS, Braun JM, & Cooper GS (2019). Phthalate exposure and female reproductive and developmental outcomes: a systematic review of the human epidemiological evidence. Environment international, 130, 104580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rakkestad KE, Holme JA, Paulsen RE, Schwarze PE, & Becher R (2010). Mono (2-ethylhexyl) phthalate induces both pro-and anti-inflammatory responses in rat alveolar macrophages through crosstalk between p38, the lipoxygenase pathway and PPARα. Inhalation toxicology, 22(2), 140–150. [DOI] [PubMed] [Google Scholar]
- 62.Rodríguez-Carmona Y, Ashrap P, Calafat AM, Ye X, Rosario Z, Bedrosian LD, Huerta-Montanez G, Vélez-Vega CM, Alshawabkeh A, & Cordero JF (2020). Determinants and characterization of exposure to phthalates, DEHTP and DINCH among pregnant women in the PROTECT birth cohort in Puerto Rico. Journal of exposure science & environmental epidemiology, 30(1), 56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Romero R, Gonzalez R, Baumann P, Behnke E, Rittenhouse L, Barberio D, Cotton D, & Mitchell M (1994). Topographic differences in amniotic fluid concentrations of prostanoids in women in spontaneous labor at term. Prostaglandins, leukotrienes and essential fatty acids, 50(2), 97–104. [DOI] [PubMed] [Google Scholar]
- 64.Romero R, Munoz H, Gomez R, Parra M, Polanco M, Valverde V, Hasbun J, Garrido J, Ghezzi F, & Mazor M (1996). Increase in prostaglandin bioavailability precedes the onset of human parturition. Prostaglandins, leukotrienes and essential fatty acids, 54(3), 187–191. [DOI] [PubMed] [Google Scholar]
- 65.Santos S, Sol CM, van Zwol–Janssens C, Philips EM, Asimakopoulos AG, Martinez-Moral M-P, Kannan K, Jaddoe VW, & Trasande L (2021). Maternal phthalate urine concentrations, fetal growth and adverse birth outcomes. A population-based prospective cohort study. Environment international, 151, 106443. [DOI] [PubMed] [Google Scholar]
- 66.Schmidtkunz C, Gries W, Weber T, Leng G, Kolossa-Gehring M, 2019. Internal exposure of young German adults to di (2-propylheptyl) phthalate (DPHP): Trends in 24-h urine samples from the German Environmental Specimen Bank 1999–2017. International journal of hygiene and environmental health 222 (3), 419–424. [DOI] [PubMed] [Google Scholar]
- 67.Schütze A, Kolossa-Gehring M, Apel P, Brüning T, Koch HM, 2014. Entering markets and bodies: increasing levels of the novel plasticizer Hexamoll® DINCH® in 24 h urine samples from the German Environmental Specimen Bank. International journal of hygiene and environmental health 217 (2–3), 421–426. [DOI] [PubMed] [Google Scholar]
- 68.Shinomiya S, Naraba H, Ueno A, Utsunomiya I, Maruyama T, Ohuchida S, Ushikubi F, Yuki K, Narumiya S, & Sugimoto Y (2001). Regulation of TNFα and interleukin-10 production by prostaglandins I2 and E2: studies with prostaglandin receptor-deficient mice and prostaglandin E-receptor subtype-selective synthetic agonists. Biochemical pharmacology, 61(9), 1153–1160. [DOI] [PubMed] [Google Scholar]
- 69.Shinto LH, Raber J, Mishra A, Roese N, & Silbert LC (2022). A Review of Oxylipins in Alzheimer’s Disease and Related Dementias (ADRD): Potential Therapeutic Targets for the Modulation of Vascular Tone and Inflammation. Metabolites, 12(9), 826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Silva MJ, Samandar E, Preau JL Jr, Reidy JA, Needham LL, & Calafat AM (2007). Quantification of 22 phthalate metabolites in human urine. Journal of Chromatography B, 860(1), 106–112. [DOI] [PubMed] [Google Scholar]
- 71.Silva MJ, Wong L-Y, Samandar E, Preau JL, Calafat AM, & Ye X (2017). Exposure to di-2-ethylhexyl terephthalate in a convenience sample of US adults from 2000 to 2016. Archives of toxicology, 91, 3287–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Silva MJ, Wong L-Y, Samandar E, Preau JL Jr, Jia LT, & Calafat AM (2019). Exposure to di-2-ethylhexyl terephthalate in the US general population from the 2015–2016 National Health and Nutrition Examination Survey. Environment international, 123, 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stevens DR, Bommarito PA, Keil AP, McElrath TF, Trasande L, Barrett ES, Bush NR, Nguyen RH, Sathyanarayana S, & Swan S (2022). Urinary phthalate metabolite mixtures in pregnancy and fetal growth: Findings from the infant development and the environment study. Environment international, 163, 107235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Struve MF, Gaido KW, Hensley JB, Lehmann KP, Ross SM, Sochaski MA, Willson GA, & Dorman DC (2009). Reproductive toxicity and pharmacokinetics of di-n-butyl phthalate (DBP) following dietary exposure of pregnant rats. Birth Defects Research Part B: Developmental and Reproductive Toxicology, 86(4), 345–354. [DOI] [PubMed] [Google Scholar]
- 75.Taibl KR, Schantz S, Aung MT, Padula A, Geiger S, Smith S, Park J-S, Milne GL, Robinson JF, & Woodruff TJ (2022). Associations of per-and polyfluoroalkyl substances (PFAS) and their mixture with oxidative stress biomarkers during pregnancy. Environment international, 169, 107541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tetz LM, Aronoff DM, & Loch-Caruso R (2015). Mono-ethylhexyl phthalate stimulates prostaglandin secretion in human placental macrophages and THP-1 cells. Reproductive Biology and Endocrinology, 13, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Theken KN, Deng Y, Kannon MA, Miller TM, Poloyac SM, & Lee CR (2011). Activation of the acute inflammatory response alters cytochrome P450 expression and eicosanoid metabolism. Drug metabolism and disposition, 39(1), 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tibshirani R (1996). Regression shrinkage and selection via the lasso. Journal of the Royal statistical society: series B (Methodological), 58(1), 267–288. [Google Scholar]
- 79.Tibshirani R (2011). Regression Shrinkage and Selection via The Lasso: A Retrospective. Journal of the Royal Statistical Society Series B: Statistical Methodology, 73(3), 273–282. 10.1111/j.1467-9868.2011.00771.x [DOI] [Google Scholar]
- 80.Wang X, Wang L.-l., Tian Y.-k., Xiong S.-m., Liu Y.-j., Zhang H.-n., Shen X.-b., & Zhou Y.-z. (2022). Association between exposures to phthalate metabolites and preterm birth and spontaneous preterm birth: A systematic review and meta-analysis. Reproductive Toxicology. [DOI] [PubMed] [Google Scholar]
- 81.Wei C, Zhu P, Shah SJ, & Blair IA (2009). 15-oxo-Eicosatetraenoic acid, a metabolite of macrophage 15-hydroxyprostaglandin dehydrogenase that inhibits endothelial cell proliferation. Molecular pharmacology, 76(3), 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Welch BM, Keil AP, Buckley JP, Calafat AM, Christenbury KE, Engel SM, O’Brien KM, Rosen EM, James-Todd T, & Zota AR (2022). Associations between prenatal urinary biomarkers of phthalate exposure and preterm birth: a pooled study of 16 US cohorts. JAMA pediatrics, 176(9), 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Welch BM, Keil AP, van ‘t Erve TJ, Deterding LJ, Williams JG, Lih FB, Cantonwine DE, McElrath TF, & Ferguson KK (2020). Longitudinal profiles of plasma eicosanoids during pregnancy and size for gestational age at delivery: A nested case-control study. PLoS Medicine, 17(8), e1003271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wong JH, Wang YS, Nam S, Ho KH, Chang CM, Chen KC, Chen YF, & Chang WC (2019). Phthalate plasticizer di (2-ethyl-hexyl) phthalate induces cyclooxygenase-2 expression in gastric adenocarcinoma cells. Environmental toxicology, 34(11), 1191–1198. [DOI] [PubMed] [Google Scholar]
- 85.Yun K, & Ji K (2023). Effects of di-(2-ethylhexyl) terephthalate on hypothalamus-pituitary-gonad axis in adult zebrafish. Reproductive Toxicology, 108408. [DOI] [PubMed] [Google Scholar]
- 86.Zhang M, Yu CH, Wang G, Buckley JP, Hong X, Pearson C, Adams WG, Fan ZT, & Wang X (2022). Longitudinal trajectories and determinants of plasma per-and polyfluoroalkyl substance (PFAS) levels from birth to early childhood and metabolomic associations: A pilot study in the Boston Birth Cohort. Precision Nutrition, 1(1), 10.1097. [PMC free article] [PubMed] [Google Scholar]
- 87.Zhong Q, Liu H.-l., Fu H, Niu Q.-s., Wu H.-b., & Huang F (2021). Prenatal exposure to phthalates with preterm birth and gestational age: A systematic review and meta-analysis. Chemosphere, 282, 130991. [DOI] [PubMed] [Google Scholar]
- 88.Zou H, & Hastie T (2005). Regularization and variable selection via the elastic net. Journal of the royal statistical society: series B (statistical methodology), 67(2), 301–320. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


