Abstract
An extracellular electron carrier excreted into the growth medium by cells of Geobacter sulfurreducens was identified as a c-type cytochrome. The cytochrome was found to be distributed in about equal amounts in the membrane fraction, the periplasmic space, and the surrounding medium during all phases of growth with acetate plus fumarate. It was isolated from periplasmic preparations and purified to homogeneity by cation-exchange chromatography, gel filtration, and hydrophobic interaction chromatography. The electrophoretically homogeneous cytochrome had a molecular mass of 9.57 ± 0.02 kDa and exhibited in its reduced state absorption maxima at wavelengths of 552, 522, and 419 nm. The midpoint redox potential determined by redox titration was −0.167 V. With respect to molecular mass, redox properties, and molecular features, this cytochrome exhibited its highest similarity to the cytochromes c of Desulfovibrio salexigens and Desulfuromonas acetoxidans. The G. sulfurreducens cytochrome c reduced ferrihydrite (Fe(OH)3), Fe(III) nitrilotriacetic acid, Fe(III) citrate, and manganese dioxide at high rates. Elemental sulfur, anthraquinone disulfonate, and humic acids were reduced more slowly. G. sulfurreducens reduced the cytochrome with acetate as an electron donor and oxidized it with fumarate. Wolinella succinogenes was able to reduce externally provided cytochrome c of G. sulfurreducens with molecular hydrogen or formate as an electron donor and oxidized it with fumarate or nitrate as an electron acceptor. A coculture could be established in which G. sulfurreducens reduced the cytochrome with acetate, and the reduced cytochrome was reoxidized by W. succinogenes in the presence of nitrate. We conclude that this cytochrome can act as iron(III) reductase for electron transfer to insoluble iron hydroxides or to sulfur, manganese dioxide, or other oxidized compounds, and it can transfer electrons to partner bacteria.
The reduction of ferric iron to ferrous iron is an important process in sediments and hydromorphic soils (38). The process and the involvement of bacteria in it have been studied with defined bacterial cultures for more than 4 decades (4, 7, 12, 22). Iron reduction has gained interest again recently with the discovery of specifically iron-reducing bacteria which can couple this process to the oxidation of a broad range of substrates (27–30). Several species of specifically iron-reducing bacteria are known today, including Shewanella (Alteromonas) putrefaciens (32), Geobacter metallireducens (30, 33), Geobacter sulfurreducens (11), and several strains of unknown taxonomic affiliation. Several nitrate-reducing or -fermenting bacteria can reduce iron(III) facultatively as an additional method of electron release.
Iron is a transition element that easily changes between the redox states Fe(II) and Fe(III). The standard redox potential of the Fe3+-Fe2+ couple (−770 mV) is applicable only in strongly acidic solution (pH <2.5), in which both ions are well soluble. At neutral pH, the redox transition occurs mainly between, e.g., Fe(OH)3 (ferrihydrite) and the Fe2+ ion at a redox potential around +150 mV (52). Thus, the redox potential at which neutrophilic iron reducers release their electrons is in the same range as that of the fumarate-succinate couple (+30 mV). The main problem with iron reduction under these conditions is the low solubility of iron(III) hydroxides. The free Fe3+ ion concentration in a Fe(OH)3-saturated neutral solution is around 10−19 M; with other iron oxohydroxides (goethite, hematite, and lepidocrocite), the free Fe3+ concentration is even lower (47). Thus, iron-reducing bacteria have to deliver their electrons to an essentially insoluble acceptor system, and the question of how cells can accomplish this at sufficient rates has not yet been answered convincingly. It was suggested recently (35) that humic compounds could act as redox mediators between iron-reducing bacterial cells and iron hydroxides; however, the efficiency of this coupling with natural humic compounds still needs to be evaluated. In the present communication, we report on the purification and characterization of a periplasmically localized c-type cytochrome of G. sulfurreducens that can act as an extracellular iron reductase and that can transfer electrons as well to other acceptor systems.
MATERIALS AND METHODS
Sources of strains.
G. sulfurreducens PCA (ATCC 51573) was obtained from D. Lovley, Amherst, Mass. Wolinella succinogenes DSM 1740 was obtained from A. Kröger, Frankfurt, Germany. Pelobacter acetylenicus WoAcy1 (DSM 3246), Pelobacter propionicus DSM 2379, Clostridium homopropionicum DSM 5847, and Methanospirillum hungatei SK (DSM 3595), were from our own culture collection. All strains were checked for purity at regular intervals by phase-contrast microscopy after growth in mineral medium or in complex medium (AC-medium; Difco Laboratories, Detroit, Mich.) (diluted 1:10).
Media and growth conditions.
G. sulfurreducens and W. succinogenes were grown in carbonate-buffered, cysteine-reduced mineral medium (50) containing a seven-vitamin solution (50), selenite-tungstate solution (49), and the trace element solution SL10 (51). The final pH of the medium was adjusted to 7.2 to 7.4. The growth temperature was 28°C. Substrates were added from sterile, neutralized stock solutions. Mass cultures of G. sulfurreducens cells were grown in 5-liter carboys with 10 mM acetate plus 40 mM fumarate as substrates. Cells were harvested by centrifugation at 10,000 × g for 25 min.
Localization experiments.
Cells were harvested at the end of the logarithmic-growth phase by 25 min of centrifugation at 10,000 × g, washed once, and resuspended in 50 mM Tris-HCl (pH 7.0). Sucrose (20% [wt/vol]), 2 mM EDTA, and 1 mg of lysozyme per ml (21,500 U · mg of protein−1) were added, and the suspension was incubated for at least 1 h at 28°C. Spheroplast formation was monitored microscopically. Spheroplasts were removed by 30 min of centrifugation at 5,000 × g, and the supernatant was cleared of cell debris afterwards by 30 min of centrifugation at 45,000 × g to give the periplasmic fraction. Malate dehydrogenase activity was monitored in the periplasmic fraction and culture supernatant and served as a tracer of the cytoplasmic fraction by measuring NADH oxidation with oxaloacetate (45).
Purification of cytochrome c.
Purification started with the periplasmic fraction, which was applied to a cation-exchange column (0.6 by 5 cm) (Mono S, prepacked; Pharmacia, Uppsala, Sweden) preequilibrated with 25 mM sodium phosphate buffer (pH 6.3) as the eluent. In a linear gradient up to 1 M NaCl, the cytochrome eluted at a 150 mM (±10 mM) concentration of NaCl. The fraction containing cytochrome c (detected by its reduced absorption spectrum) was loaded onto a gel filtration column (1.25 by 30 cm; Superose 12 prepacked; Pharmacia) run with 0.15 M ammonium acetate buffer (pH 6.3). If necessary, the fraction containing the cytochrome was lyophilized before further ammonium acetate was added, to give a 1.7 M ammonium acetate concentration, and loaded onto a hydrophobic interaction column (1.25 by 10.5 cm; phenyl Superose prepacked; Pharmacia). The cytochrome eluted with 1.7 M ammonium acetate, whereas the remaining contaminating proteins were retained and eluted at lower ionic strength. The cytochrome fraction was lyophilized, diluted in two steps with 10 volumes of distilled water each, and lyophilized to give a concentrated solution.
Characterization of cytochrome c.
For sodium dodecyl sulfate (SDS) gel electrophoresis, the method of Laemmli (24) was applied with 12 or 14% polyacrylamide resolving gels and 4% stacking gels. Samples were diluted in sample buffer containing 60 mM Tris-HCl, 2% (wt/vol) SDS, 10% (wt/vol) glycerol, 0.025% (wt/vol) bromophenol blue, and no mercaptoethanol. Electrophoresis was carried out in a dual slab cell (Mini-Protean II; Bio-Rad, Richmond, Calif.) with Tris-glycine-SDS buffer (25 mM, 250 mM, 0.1% [wt/vol], respectively), starting at 30 mA until samples entered the resolving gel and separating at 40 mA.
Heme staining in SDS gels was performed as described earlier (48) with modifications by Goodhew et al. (20). Silver staining of proteins was performed at room temperature according to the following procedure: gels were incubated in an aqueous solution of 50% (vol/vol) methanol, 12% (vol/vol) acetic acid, and 0.5 ml of 37% formaldehyde liter−1 for 1 h and washed three times in 50% (vol/vol) ethanol–water for 20 min. The next incubation was in an aqueous solution of 0.2 g of Na2S2O3 · 5H2O for exactly 1 min, followed by washing with distilled water three times for 20 s each. Afterwards, gels were incubated in an aqueous solution of 2 g of AgNO3 liter−1 plus 0.75 ml of 37% formaldehyde liter−1 for exactly 20 min and washed with distilled water twice for exactly 20 each. Color development in an aqueous solution of 20 g of sodium carbonate liter−1 0.5 ml of 37% formaldehyde liter−1, and 4 mg of Na2S2O3 · 5H2O liter−1 was stopped immediately when the first bands appeared. Gels were washed twice with distilled water for 2 min and incubated in an aqueous solution of 50% (vol/vol) methanol plus 12% (vol/vol) acetic acid for 10 min. The last washing step was in 50% methanol–water for 20 min, and gels were stored afterwards in the same solution at −4°C.
The molecular mass of the purified cytochrome c was estimated by SDS-polyacrylamide gel electrophoresis (PAGE) (24) and by matrix-assisted laser ionization desorption mass spectroscopy (MALDI) in a biflex linear time of flight setup (Bruker, Billerica, Mass.). The midpoint redox potential (E0′) was determined by titration with 100 μM flavin mononucleotide (FMN) (E0′ = −190 mV) and 100 μM indigodisulfonate (E0′ = −125 mV) as redox indicators, dithionite as reductant, and air as oxidant. Absorptions of dyes and cytochrome were recorded with a double-beam UV-visible light (UV/VIS) spectrophotometer (Uvikon 860; Kontron, Zurich, Switzerland).
Analytical methods.
Cytochrome c was quantified by determining the redox difference absorption spectra of dithionite-reduced minus air- or hydrogen peroxide-oxidized preparations. Spectra were recorded with double-beam UV/VIS spetrophotometers (Uvikon 860 or 930; Kontron) at room temperature. The glass and quartz cuvettes used had a 1-ml total volume and a 1-cm light path and were sealed with rubber stoppers and gassed with nitrogen for anoxic measurements. Peak heights were measured relative to a baseline drawn between the troughs at 530 to 535 and 565 to 570 nm. Protein was quantified according to the method of Bradford (6).
Chemicals.
All chemicals were analytical or reagent grade and were obtained from Biomol (Ilvesheim, Germany), Boehringer (Mannheim, Germany), Eastman Kodak (Rochester, N.Y.), Fluka (Neu-Ulm, Germany), Merck (Darmstadt, Germany), Pharmacia (Freiburg, Germany), Serva (Heidelberg, Germany), and Sigma (Deisenhofen, Germany). Gases were purchased from Messer-Griesheim (Darmstadt, Germany) and Sauerstoffwerke Friedrichshafen (Friedrichshafen, Germany).
RESULTS
Cytochrome contents of cells and cell subfractions.
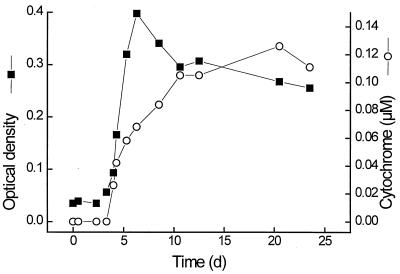
Cells of G. sulfurreducens contained cytochromes which stained colonies in deep-agar cultures pinkish-red, as observed earlier (11). Redox difference spectra of cell extracts exhibited absorption bands at wavelengths of 552, 522, and 419 nm typical of those of a c-type cytochrome. During growth with acetate plus fumarate in liquid medium, 29% of this cytochrome was found in the culture supernatant, 28% was found in the periplasmic space, and 21% was associated with the membrane fraction (Table 1). No cytochrome was detected in the cytoplasm. The membrane-associated cytochrome c could not be solubilized with 0.2 M KCl, but was resolved from the membranes nearly quantitatively with 1% Triton X-100. The comparably high proportion of cytochrome found in the extracellular space was not caused by excessive aging of the culture in the stationary phase: the proportion of cytochrome distribution in the various compartments was stable throughout the entire period of growth with acetate plus fumarate (Fig. 1). Malate dehydrogenase as a tracer enzyme of cytoplasmic contaminations was distributed at a ratio of 91%/4%/5% between the cytoplasmic, membrane, and periplasmic fractions. No malate dehydrogenase activity was found in the culture supernatant, indicating that there was no major spill of cytoplasmic proteins into the growth medium due to cell lysis. Similar amounts of cytochrome were also excreted in cultures grown with acetate plus ferrihydrite.
TABLE 1.
Distribution of cytochromes in G. sulfurreducens cultures
| Cell fraction | Amt of:
|
Cytochrome content (μg · mg−1) | ||||
|---|---|---|---|---|---|---|
| Protein
|
Cytochrome
|
|||||
| μg · ml−1 | % | nmol · ml−1 | μg · ml−1 | %a | ||
| Total | 260 | 100 | 0.30 | 3.37 | 100 | 13 |
| Supernatant | 15 | 6 | 0.09 | 0.98 | 29 | 66 |
| Cytoplasm | 81 | 31 | 0.00 | 0.00 | 0 | 0 |
| Periplasm | 110 | 42 | 0.08 | 0.93 | 28 | 17 |
| Membranes | 54 | 21 | 0.06 | 0.70 | 21 | 13 |
Note that membrane-associated cytochrome c could not be solubilized with 0.2 M KCl, but was resolved from the membranes nearly quantitatively (32 to 80%) with 1% Triton X-100.
FIG. 1.
Growth and simultaneous release of cytochrome c into the growth medium by cells of G. sulfurreducens. ▪, optical density at 570 nm; ○, extracellular cytochrome c.
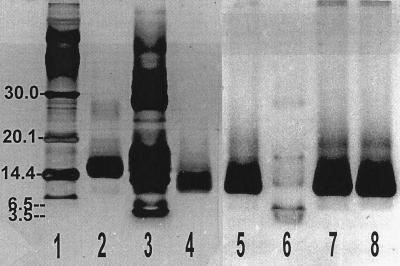
The cytochrome was most easily accessible at a high concentration in the periplasmic fraction, and therefore enrichment and isolation started from this fraction. Part of the contaminating proteins was bound to an anion exchanger, DE 52, in 50 mM Tris-HCl buffer (pH 7.0), which did not bind the cytochrome. A Mono S cation exchanger equilibrated with 25 mM sodium phosphate (pH 7.0) bound 83% of the cytochrome, which was eluted subsequently with a linear NaCl gradient at a 0.17 M concentration. Gel filtration on Sepharose 12 and hydrophobic interaction chromatography yielded a homogeneous cytochrome preparation which exhibited only one protein band after SDS-PAGE and silver staining (Table 2 and Fig. 2). This band was also stained with high sensitivity by specific heme staining. If the cytochrome of the crude cell extract was subjected to the same enrichment procedure, about 40% of the total cytochrome present bound to the anion exchanger. Obviously, there were other cytochromes present in the cells that exhibited binding properties different from those of the periplasmic cytochrome c, and the appearance of more than one heme-staining band in gel electrophoresis (Table 2) indicated the presence of more than one cytochrome in this bacterium. However, in the extracellular and the periplasmic fractions, only one type of cytochrome was found which exhibited identical properties with respect to binding, spectral absorption, and electrophoretic mobility.
TABLE 2.
Purification protocol for periplasmic cytochrome c of G. sulfurreducens
| Purification step | Total amt of cytochrome (μg) | Yield (%) | Total amt of protein (mg) | Specific cytochrome content (μg · mg−1) | Enrich- ment factor |
|---|---|---|---|---|---|
| Periplasm | 6,285 | 100.0 | 23.05 | 272.6 | 1.00 |
| Cation exchange | 1,472 | 23.4 | 1.02 | 1,441.0 | 5.29 |
| Gel filtration | 1,256 | 20.0 | 1.46 | 858.8 | 3.15 |
| Hydrophobic interaction | 1,061 | 16.9 | 1.25 | 848.4 | 3.11 |
FIG. 2.
SDS-PAGE of purified cytochrome c. Lanes 1 to 4 were silver stained; lanes 5 to 8 were identical runs stained for hemes. Lanes 1, 3, and 6, commercial protein standards (lane 1, lactalbumin, 14.4 kDa, trypsin inhibitor, 20.1 kDa, carbonic anhydrase, 30.0 kDa [higher masses not distinguishable]; lane 3, insulin β chain, 3.5 kDa; lane 6, aprotinin, 6.5 kDa [higher masses not distinguishable]), lane 2; commercial horse heart cytochrome c; lanes 4, 5, 7, and 8, periplasmic cytochrome c of G. sulfurreducens. Lanes 1, 2, 4, and 5 contained 1.2 to 1.5 μg of protein per band; lanes 3, 6, 7, and 8 contained 2 to 3 μg of protein per band. Bands with higher molecular masses appearing in heme-stained gels loaded with large amounts of protein (lanes 7 and 8) were not detected in protein sequencing or MALDI measurements, which are very sensitive to impurities.
Since the periplasmic cytochrome tended to bind to ultrafiltration membranes, we avoided such steps in the purification procedure. If necessary, cytochrome solutions were concentrated by lyophilization in ammonium acetate buffer (pH 6.2) to avoid excessive buffer salt concentrations.
Characterization of the periplasmic cytochrome c.
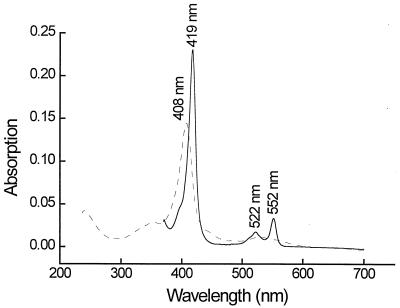
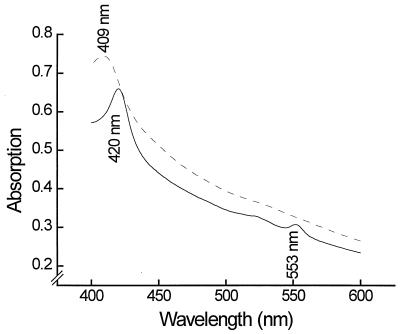
A first estimation of the molecular mass of the electrophoretically homogeneous cytochrome c of G. sulfurreducens by SDS-PAGE in Tris-glycine or Tris-tricine buffer indicated a molecular mass of 11.2 ± 0.3 kDa in comparison with standard proteins with molecular masses of 1.4 to 26.6 kDa. A more exact determination by MALDI revealed a molecular mass of 9.57 ± 0.02 kDa. Absorption spectra of the cytochrome c in its oxidized or reduced form are shown in Fig. 3. The reduced cytochrome had absorption maxima at 552, 522, and 418 nm, and the oxidized form had a maxim um at 407 nm. The specific absorption of the α band of the reduced form of the cytochrome at a wavelength of 552 nm was 32.5 mM−1 · cm−1, calculated on the basis of the MALDI-determined molecular mass.
FIG. 3.
Absorption spectra of purified cytochrome c. Dashed line, air oxidized; solid line, dithionite reduced.
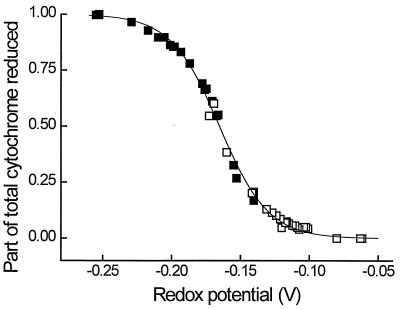
The midpoint redox potential of the isolated cytochrome c was determined by redox titration with FMN (E0′ = −0.190 V) and indigodisulfonate (E0′ = −0.125 V) as redox indicators. The titration was performed in both directions, either with dithionite as reductant or with oxygen as oxidant. The titration curve (Fig. 4) exhibited only one inflection point, and a midpoint redox potential of −0.167 V ± 1 mV was calculated.
FIG. 4.
Redox titration of purified cytochrome c with dithionite and air. Indicator dyes were indigodisulfonate (□) (E0′ = −0.125 V) and FMN (▪) (E0′ = −0.190 V). The line was fit to the curve according to the Nernst equation. The midpoint potential of the cytochrome for assumed independent electron transfers was calculated to be −0.167 V.
Electron donors and acceptors.
In the absence of additional mediators, the periplasmic cytochrome c was reduced with dithionite, H2S, or FeSO4 at high rates. It was oxidized quickly (>12 μmol · min−1 · mg of protein−1) by O2, Fe(OH)3, or MnO2, and more slowly by colloidal sulfur (S0), anthraquinone disulfonate, and humic acids (Sigma). In the presence of phenazine methosulfate, the cytochrome could also be reduced by NADH or be oxidized by FMN. Flavin adenine dinucleotide did not oxidize the cytochrome, even in the presence of phenazine methosulfate.
The cytochrome could also be reduced with hydrogen in the presence of a palladium charcoal catalyst or with hydrogen or formate in the presence of crude cell extracts of W. succinogenes (0.1 nmol · min−1 · mg of protein−1). W. succinogenes cell extracts also oxidized the reduced cytochrome in the presence of fumarate (0.5 nmol · min−1 · mg of protein−1).
Coupling of electron transfer from G. sulfurreducens to external electron acceptors.
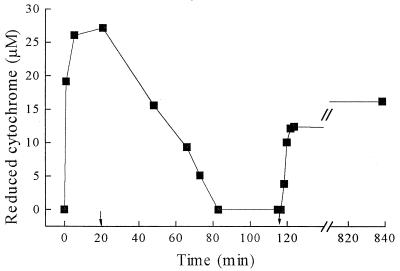
G. sulfurreducens cells grown with acetate plus fumarate reduced or oxidized the extracellular cytochrome, depending on the relative electron availability. With excess acetate over fumarate, the cytochrome was quantitatively reduced; with excess fumarate, it was quantitatively oxidized (Fig. 5). The reduced or oxidized state of the culture could even be recognized by the naked eye, changing between pink-orange (reduced) and yellow (oxidized). The reduction and oxidation of G. sulfurreducens cytochrome c could also be catalyzed by cell suspensions of other bacteria, such as W. succinogenes, in the presence of excess hydrogen or formate or excess fumarate, as documented in Fig. 6.
FIG. 5.
Absorption spectra of G. sulfurreducens cultures grown under different substrate supply situations. Dashed line, 10 mM acetate and 60 mM fumarate; solid line, 10 mM acetate and 30 mM fumarate.
FIG. 6.
Reduction and oxidation of G. sulfurreducens cytochrome c by washed intact cells of G. sulfurreducens and W. succinogenes (each at about 0.02 mg of protein per ml) in the presence of excess nitrate (2 mM [added after 20 min]) or excess acetate (3 mM [added after 117 min]).
G. sulfurreducens and W. succinogenes could be coupled in an interspecies electron transfer in which acetate was oxidized by Geobacter and the electrons were transferred to cytochrome c; the latter was reoxidized by W. succinogenes with nitrate as the terminal electron acceptor. Such a syntrophic coculture has been described in a separate paper (13). We checked various bacteria described previously to be active in interspecies electron transfer for possible interaction with the cytochrome preparation. P. acetylenicus, P. propionicus, Clostridium homopropionicum, and M. hungatei neither reduced nor oxidized the G. sulfurreducens periplasmic cytochrome.
DISCUSSION
Comparison of the G. sulfurreducens periplasmic cytochrome with other known cytochromes.
Compared to known tri- and tetraheme class III cytochromes (39) from mostly sulfate- and sulfur-reducing bacteria, the cytochrome c described here has a rather low molecular mass and a comparably high midpoint redox potential. Cytochromes of Desulfovibrio vulgaris Miyazaki (43, 54), D. vulgaris Hildenborough (1, 10, 14–16), Desulfovibrio salexigens Benghazi (17), D. gigas (3, 25, 53), D. desulfuricans Norway (8, 9), D. desulfuricans Essex 6 (15), and S. putrefaciens (36) have molecular masses of 13 kDa or higher and standard redox potentials below −0.2 V. Desulfobulbus elongatus has a cytochrome with a standard redox potential (−0.165 V) similar to that of G. sulfurreducens, but which also is of high molecular mass (13.7 kDa) (42). All cytochromes mentioned have nearly identical absorption maxima (±2-nm wavelength) in both their reduced and oxidized forms. The cytochrome most similar to the periplasmic cytochrome c of G. sulfurreducens is the one isolated from Desulfuromonas acetoxidans, with standard redox potentials of the three hemes reported to be −0.102, −0.177, and −0.177 V (18) or −0.140, −0.210, and −0.240 V (10) and with a molecular mass of 9.8 kDa (40). This cytochrome c has been characterized as a triheme cytochrome (2). The specific absorption coefficient of the α band of the reduced G. sulfurreducens cytochrome was 32.5 mM−1 · cm−1, similar to that of the three-heme cytochrome c of D. acetoxidans (30.8 mM−1 · cm−1) (40), and 2.5 times as high as that of the monoheme horse heart cytochrome c. These findings, together with the low molecular mass, suggest that the G. sulfurreducens cytochrome contains three hemes, and further biochemical and sequence data obtained recently confirm this assumption (18a). The structural similarities between these two c-type cytochromes are in accordance with the apparent 16S rRNA sequence similarities between D. acetoxidans and G. sulfurreducens (11) and with the observation that D. acetoxidans can reduce ferric iron as well (41).
Physiological function and ecological significance of the periplasmic cytochrome c.
The cytochrome described here can be oxidized by an unusually broad variety of electron acceptors, such as oxygen, Fe(OH)3, Fe(III) citrate, Fe(III) nitrilotriacetic acid, MnO2, and sulfur, as well as by humic acids and anthraquinone. The midpoint redox potential (−0.167 V) is in the same range as that of the sulfur-sulfide couple (−0.24 V) and is slightly lower than those of the various iron(III) hydroxide reduction reactions (0 to +0.2 V) (47). It can accept electrons, e.g., from an nNADH-oxidizing redox system described recently (19), and transfer them to the various acceptor systems mentioned. With its rather unspecific reactivity, this cytochrome lends itself as a carrier for electron transfer to extracellular electron acceptors, especially since it appears to be excreted at a significant amount into the surrounding medium. Its comparably small size may be a prerequisite for this excretion across the outer cell membrane. In none of the above-mentioned publications was a significant release of cytochrome into the growth medium mentioned. This phenomenon had been reported so far only for the fermenting bacterium Malonomonas rubra (23). The cytochrome c3 of D. vulgaris was reported to also reduce chromate or uranium(VI) compounds (31, 34), but these acceptors are highly soluble, and their reduction does not depend on an extracellular electron carrier.
The possibility of electron transfer to insoluble acceptors such as iron(III) oxohydroxides was discussed for S. putrefaciens MR-1 (37). According to this hypothesis, electrons are transferred by a cytochrome which is present in large amounts and which is tightly bound to the outer membrane. Accordingly, iron-reducing bacteria would have to operate in immediate contact with the iron(III) mineral in order to secure an effective electron transfer. In the present study, we found a cytochrome c in the periplasmic space as well as in the membrane fraction, which after the preparation procedure was applied, includes the cytoplasmic and the outer membrane (44), as well as the surrounding medium. Based on our findings concerning the distribution and reactivity of the periplasmic cytochrome c of G. sulfurreducens, we propose that this cytochrome may act as an iron(III) reductase and may also be involved in electron transfer to the other acceptor systems mentioned. At first sight, excretion of a cytochrome into the medium appears to be an excessively expensive mechanism of electron transfer, taking into account that approximately 750 to 800 mol of ATP is needed for the synthesis of 1 mol of protein with a molecular mass of 9.5 kDa (46). Nonetheless, an up to 200 nM concentration of this cytochrome, as observed in our culture medium in the stationary phase, is equivalent to about 5% of the total protein content of the culture, and therefore is in an affordable range of investment for the cells. This situation is to some extent comparable to that of, e.g., cellulose degradation by extracellular cellulases. These proteins are also excreted, and this energy investment has to be covered by a sufficient return of oligosaccharides.
Obviously, the excretion of 200 nM cytochrome as we observed it in our cultures could be afforded by the cells concomitant with good growth. With the following assumptions—a cell size of 0.5 by 2.5 μm, a cell surface area of 4.3 μm2, a concentration of 108 cells per ml, a diffusion constant (D) for the cytochrome of 1.2 × 10−6 cm2 · s−1 (26), and a cytochrome concentration of 200 nM—the electron transport rate via diffusion of reduced cytochrome (transporting three electrons with three hemes) would be in the range of 18 nmol · min−1 · mg of protein−1 along a diffusion distance of 10 μm to the acceptor mineral, would be 10 times as high along a distance of 1 μm, and thus would be well within the range of the metabolic activity of actively growing cells. The efficiency of electron transport through extracellular cytochromes could even be enhanced by the known ability of certain c-type cytochromes to transport electrons by intermolecular electron transfer (5, 21), and the near-neutral isoelectric point of our cytochrome would favor such a transfer in neutral solution. If we realize further that iron-reducing bacteria in nature grow mainly in colonies of several hundred or thousand cells in close association with iron mineral and that the excreted cytochrome molecules are used and returned by such a community much more efficiently than in our suspended culture, the assumption appears realistic that an extracellular cytochrome can act as a dissolved electron carrier and iron reductase in bacterial iron reduction.
In a separate paper, we reported that G. sulfurreducens can grow and oxidize acetate in syntrophic association with W. succinogenes when nitrate is used as a terminal electron acceptor (13). Our original assumption that this syntrophic cooperation was based on interspecies hydrogen transfer turned out to be unlikely because of the extremely small hydrogen concentrations measured in these cultures (0.02 to 0.04 nM), which could not account for the observed electron flux. Also, these cocultures contained free cytochromes at the same concentration as that in pure cultures of G. sulfurreducens. Based on the calculations described above, and taking into account that the average distance between single cells in a suspension of 108 cells per ml is around 20 μm, the cytochrome at the observed concentration could contribute significantly to this interspecies electron transfer, especially if convective transport in the free liquid and active swimming of both partners enhance the exchange of reduced and oxidized cytochrome.
We conclude that the periplasmic cytochrome of G. sulfurreducens can act as an iron(III) reductase and also as a redox carrier between the cell and various extracellular electron acceptor systems, including humic acids or partner bacteria. Whether this concept of electron transfer can be generalized as well for other iron-reducing bacteria still needs to be examined. Further experiments on the impact of free cytochrome c on electron transfer and interaction between bacterial cells and with insoluble electron acceptors are in progress in our laboratory.
ACKNOWLEDGMENTS
We are indebted to Claudio Luchinat, Florence, Italy, for helpful comments on the biochemistry of cytochromes and to Michael Przybylski and his coworkers, Konstanz, Germany, for determination of the molecular mass of our cytochrome by MALDI.
Support through a grant of the Deutsche Forschungsgemeinschaft, Bonn-Bad Godesberg, Germany, on “Energetics of syntrophic associations” is gratefully acknowledged.
REFERENCES
- 1.Ambler R P. The amino acid sequence of cytochrome c3 from Desulfovibrio vulgaris. Biochem J. 1968;109:47–48. doi: 10.1042/bj1090047pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambler R P. The amino acid sequence of cytochrome c-551.5 (cytochrome c7) from the green photosynthetic bacterium Chloropseudomonas ethylica. FEBS Lett. 1971;18:351–353. doi: 10.1016/0014-5793(71)80484-2. [DOI] [PubMed] [Google Scholar]
- 3.Ambler R P, Bruschi M, Le Gall J. The structure of cytochrome c′3 from Desulfovibrio gigas (NCIB 9332) FEBS Lett. 1969;5:115–117. doi: 10.1016/0014-5793(69)80308-x. [DOI] [PubMed] [Google Scholar]
- 4.Balashova V V, Zavarzin G A. Anaerobic reduction of ferric iron by hydrogen bacteria. Microbiology. 1980;48:635–639. [PubMed] [Google Scholar]
- 5.Bertini I, Gaudemer A, Luchinat C, Piccioli M. Electron self-exchange in high-potential iron-sulfur proteins. Characterization of protein I from Ectothiorhodospira vacuolata. Biochemistry. 1993;32:12887–12893. doi: 10.1021/bi00210a042. [DOI] [PubMed] [Google Scholar]
- 6.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Bromfield S M. Reduction of ferric compounds by soil bacteria. J Gen Microbiol. 1954;11:1–6. doi: 10.1099/00221287-11-1-1. [DOI] [PubMed] [Google Scholar]
- 8.Bruschi M. The primary structure of the tetraheme cytochrome c3 from Desulfovibrio desulfuricans (strain Norway 4). Description of a new class of low-potential cytochromes c. Biochim Biophys Acta. 1981;671:219–224. [Google Scholar]
- 9.Bruschi M, Hatchikian C E, Golovleva L A, Le Gall J. Purification and characterization of cytochrome c3, ferredoxin, and rubredoxin isolated from Desulfovibrio desulfuricans Norway. J Bacteriol. 1977;129:30–38. doi: 10.1128/jb.129.1.30-38.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruschi M, Loutfi M, Bianco P, Haladjian J. Correlation studies between structural and redox properties of cytochromes c3. Biochem Biophys Res Commun. 1984;120:384–389. doi: 10.1016/0006-291x(84)91265-8. [DOI] [PubMed] [Google Scholar]
- 11.Caccavo F, Jr, Lonergan D J, Lovley D R, Davis M, Stolz J F, McInerney M J. Geobacter sulfurreducens sp. nov., a hydrogen- and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl Environ Microbiol. 1994;60:3752–3759. doi: 10.1128/aem.60.10.3752-3759.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coleman M L, Hedrick D B, Lovley D R, White D C, Pye K. Reduction of Fe(III) in sediments by sulphate-reducing bacteria. Nature. 1993;361:436–438. [Google Scholar]
- 13.Cord-Ruwisch R, Lovley D R, Schink B. Growth of Geobacter sulfurreducens with acetate in syntrophic cooperation with hydrogen-oxidizing anaerobic partner bacteria. Appl Environ Microbiol. 1998;64:2232–2236. doi: 10.1128/aem.64.6.2232-2236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dervartanian D V, Xavier A V, Le Gall J. EPR determination of the oxidation-reduction potentials of the hemes in cytochrome c3 from Desulfovibrio vulgaris. Biochimie. 1978;60:321–325. doi: 10.1016/s0300-9084(78)80829-3. [DOI] [PubMed] [Google Scholar]
- 15.Drucker H, Campbell L L. Electrophoretic and immunological differences between the cytochrome c3 of Desulfovibrio desulfuricans and that of Desulfovibrio vulgaris. J Bacteriol. 1969;100:358–364. doi: 10.1128/jb.100.1.358-364.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drucker H, Trousil E B, Campbell L L, Barlow G H, Margoliash E. Amino acid composition, heme content, and molecular weight of cytochrome c3 of Desulfovibrio desulfuricans and Desulfovibrio vulgaris. Biochemistry. 1970;9:1515–1518. doi: 10.1021/bi00809a006. [DOI] [PubMed] [Google Scholar]
- 17.Drucker H, Trousil E B, Campbell L L. Purification and properties of cytochrome c3 of Desulfovibrio salexigens. Biochemistry. 1970;9:3395–3400. doi: 10.1021/bi00819a016. [DOI] [PubMed] [Google Scholar]
- 18.Fiechtner M D, Kassner J R. The redox properties and heme environment of cytochrome c-551.5 from Desulfuromonas acetoxidans. Biochim Biophys Acta. 1979;579:269–278. doi: 10.1016/0005-2795(79)90054-0. [DOI] [PubMed] [Google Scholar]
- 18a.Foerster, S., and P. Kroneck. Personal communication.
- 19.Gaspard, S., F. Vazquez, and C. Holliger. Localization and solubilization of the iron(III)-reductase of Geobacter sulfurreducens. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 20.Goodhew C F, Brown K R, Pettigrew G W. Haem staining in gels, a useful tool in the study of bacterial c-type cytochromes. Biochim Biophys Acta. 1986;852:288–294. [Google Scholar]
- 21.Gupta R K. Electron transfer in cytochrome c. Role of the polypeptide chain. Biochim Biophys Acta. 1973;292:291–295. doi: 10.1016/0005-2728(73)90274-0. [DOI] [PubMed] [Google Scholar]
- 22.Kamura T, Takai Y, Ishikawa K. Microbial reduction mechanism of ferric iron in paddy soils. Part 1. Soil Sci Plant Nutr. 1963;9:171–175. [Google Scholar]
- 23.Kolb S. Physiologie und Biochemie des anaeroben Abbaus von Malonsäure und Phthalsäure. Ph.D. thesis. Tübingen, Germany: University of Tübingen; 1995. [Google Scholar]
- 24.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.LeGall J, Mazza G, Dragoni N. Le cytochrome c3 de Desulfovibrio gigas. Biochim Biophys Acta. 1965;99:385–387. [PubMed] [Google Scholar]
- 26.Lehninger A L. Biochemistry. New York, N.Y: Worth Publishers; 1970. [Google Scholar]
- 27.Lovley D R. Organic matter mineralization with the reduction of ferric iron: a review. Geomicrobiol J. 1987;5:375–399. [Google Scholar]
- 28.Lovley D R. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev. 1991;55:259–287. doi: 10.1128/mr.55.2.259-287.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lovley D R. Dissimilatory metal reduction. Annu Rev Microbiol. 1993;47:263–290. doi: 10.1146/annurev.mi.47.100193.001403. [DOI] [PubMed] [Google Scholar]
- 30.Lovley D R, Phillips E J P. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol. 1988;54:1472–1480. doi: 10.1128/aem.54.6.1472-1480.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lovley D R, Phillips E J P. Reduction of chromate by Desulfovibrio vulgaris and its c3 cytochrome. Appl Environ Microbiol. 1994;60:726–728. doi: 10.1128/aem.60.2.726-728.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lovley D R, Phillips E J P, Lonergan D J. Hydrogen and formate oxidation coupled to dissimilatory reduction of iron or manganese by Alteromonas putrefaciens. Appl Environ Microbiol. 1989;55:700–706. doi: 10.1128/aem.55.3.700-706.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lovley D R, Giovannoni S J, White D C, Champine J E, Phillips E J P, Gorby Y A, Goodwin S. Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals. Arch Microbiol. 1993;159:336–344. doi: 10.1007/BF00290916. [DOI] [PubMed] [Google Scholar]
- 34.Lovley D R, Widman P K, Woodward J C, Phillips E J P. Reduction of uranium by cytochrome c3 of Desulfovibrio vulgaris. Appl Environ Microbiol. 1993;59:3572–3576. doi: 10.1128/aem.59.11.3572-3576.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lovley D R, Coates J D, Blunt-Harris E L, Phillips E J P, Woodward J C. Humic substances as electron acceptors for microbial respiration. Nature. 1996;382:445–448. [Google Scholar]
- 36.Morris C J, Black A C, Pealing S L, Manson F D C, Chapman S K, Reid G A, Gibson D M, Ward F B. Purification and properties of a novel cytochrome: flavocytochrome c from Shewanella putrefaciens. Biochem J. 1994;302:587–593. doi: 10.1042/bj3020587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myers C R, Myers J M. Localization of cytochromes to the outer membrane of anaerobically grown Shewanella putrefaciens MR-1. J Bacteriol. 1992;174:3429–3438. doi: 10.1128/jb.174.11.3429-3438.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neilands J B. Iron and its role in microbial physiology. In: Neilands J B, editor. Microbial iron metabolism. New York, N.Y: Academic Press; 1974. pp. 3–34. [Google Scholar]
- 39.Pettigrew G W, Moore G R. Cytochromes c—biological aspects. New York, N.Y: Springer; 1987. [Google Scholar]
- 40.Probst I, Bruschi M, Pfennig N, LeGall J. Cytochrome c-551.5 from Desulfuromonas acetoxidans. Biochim Biophys Acta. 1977;460:58–64. doi: 10.1016/0005-2728(77)90151-7. [DOI] [PubMed] [Google Scholar]
- 41.Roden E E, Lovley D R. Dissimilatory Fe(III) reduction by the marine microorganism Desulfuromonas acetoxidans. Appl Environ Microbiol. 1993;59:734–742. doi: 10.1128/aem.59.3.734-742.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samain E, Albagnac G, Le Gall J. Redox studies of the tetraheme cytochrome c3 isolated from the propionate-oxidizing, sulfate-reducing bacterium Desulfobulbus elongatus. FEBS Lett. 1986;204:247–250. [Google Scholar]
- 43.Sokol W F, Evans D H, Niki K, Yagi T. Reversible voltammetric response for a molecule containing four non-equivalent redox sites with application to cytochrome c3 of Desulfovibrio vulgaris, strain Miyazaki. J Electroanal Chem. 1980;108:107–115. [Google Scholar]
- 44.Sprott G D, Koval S F, Schnaitman C A. Cell fractionation. In: Gerhardt P, Murray R G E, Wood W A, Krieg N R, editors. Methods for general and molecular bacteriology. Washington, D.C: American Society for Microbiology; 1994. pp. 72–103. [Google Scholar]
- 45.Stams A J M, Kremer D R, Nicolay K, Weenk G H, Hansen T A. Pathway of propionate formation in Desulfobulbus propionicus. Arch Microbiol. 1984;137:329–337. [Google Scholar]
- 46.Stouthamer A H. The search for correlation between theoretical and experimental growth yields. Int Rev Biochem Microbiol Biochem. 1979;21:1–47. [Google Scholar]
- 47.Stumm W, Morgan J J. Aquatic chemistry. New York, N.Y: John Wiley & Sons; 1981. [Google Scholar]
- 48.Thomas P E, Ryan D, Levin W. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal Biochem. 1976;75:168–176. doi: 10.1016/0003-2697(76)90067-1. [DOI] [PubMed] [Google Scholar]
- 49.Tschech A, Pfennig N. Growth yield increase linked to caffeate reduction in Acetobacterium woodii. Arch Microbiol. 1984;137:163–167. [Google Scholar]
- 50.Widdel F, Pfennig N. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. I. Isolation of a new sulfate-reducer enriched with acetate from saline environments. Description of Desulfobacter postgatei gen. nov. sp. nov. Arch Microbiol. 1981;129:395–400. doi: 10.1007/BF00406470. [DOI] [PubMed] [Google Scholar]
- 51.Widdel F, Kohring G W, Mayer F. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. III. Characterization of the filamentous gliding Desulfonema limicola gen. nov. sp. nov., and Desulfonema magnum sp. nov. Arch Microbiol. 1983;134:286–294. [Google Scholar]
- 52.Widdel F, Schnell S, Heising S, Ehrenreich A, Aßmus B, Schink B. Anaerobic ferrous iron oxidation by anoxygenic phototrophs. Nature (London) 1993;362:834–836. [Google Scholar]
- 53.Xavier A V, Moura J J G, Le Gall J, Dervartanian D V. Oxidation-reduction potentials of the hemes in cytochrome c3 from Desulfovibrio gigas in the presence and absence of ferredoxin by EPR spectroscopy. Biochimie. 1979;61:689–695. doi: 10.1016/s0300-9084(79)80167-4. [DOI] [PubMed] [Google Scholar]
- 54.Yagi T, Maruyama K. Purification and properties of cytochrome c3 of Desulfovibrio vulgaris, Miyazaki. Biochim Biophys Acta. 1971;243:214–224. doi: 10.1016/0005-2795(71)90078-x. [DOI] [PubMed] [Google Scholar]