Abstract
An easily adaptable protocol for the preparation of 5-hydroxy-1H-pyrrol-2(5H)-ones from readily available starting materials has been reported. The reaction of sulfur ylides with carbonyl compounds is a common approach to synthesizing epoxides. Alternatively, we have developed a method with mild reaction conditions wherein sulfur ylide underwent an intramolecular cyclization with a ketonic carbonyl group in a highly efficient way and was followed by 1,3-hydroxy rearrangement to produce 5-hydroxy-1H-pyrrol-2(5H)-ones in excellent yields. The present method offers a straightforward approach to synthesize 5-hydroxy-1H-pyrrol-2(5H)-ones from sulfur ylides without the aid of transition metal in one-pot operation, which involves sequential cyclization and rearrangement reaction. The formation of 5-hydroxy-1H-pyrrol-2(5H)-ones is supported by different spectroscopic techniques, including X-ray crystallographic data and 2D NMR studies (COSY, HSQC, HMBC, and DEPT).
Introduction
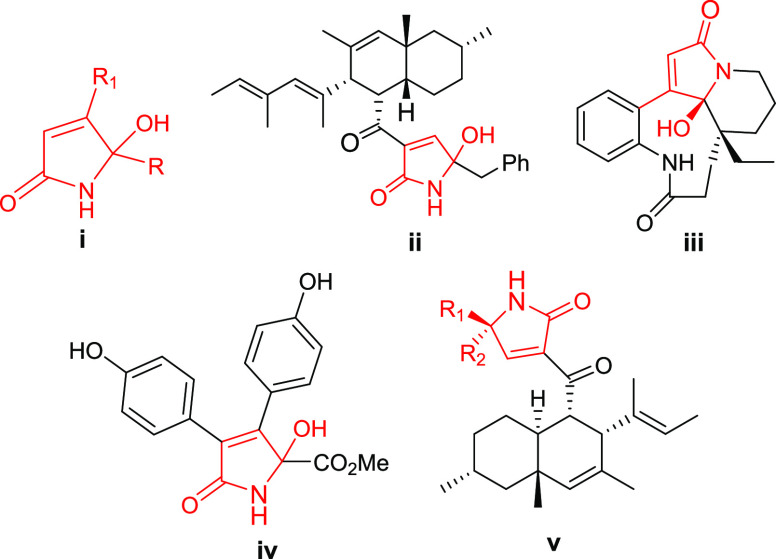
5-Hydroxy-1,5-dihydro-1H-pyrrol-2-ones (i), also referred to as γ-hydroxy-γ-lactams, are highly attractive N-heterocyclic scaffolds found in many natural products1 (Figure 1) and synthetic pharmaceutical molecules.2 In addition, some 5-hydroxy-1H-pyrrol-2-one derivatives are found to exhibit biological activities, namely, oteromycin (ii), a fungi metabolite3 found to be an effective antagonist of the endothelin receptor and can inhibit the integrase activity of the human immunodeficiency virus. (−)-Leuconolam (iii) is a microbial metabolite isolated by Goh from Leuconotis plant Leuconotis griffithii,4 which possesses incredible structural complexity and exhibits excellent anti-inflammatory and antitumor properties. Natural products that have 5-hydroxy-1,5-dihydro-1H-pyrrol-2-ones in their core structures include ianthellidones (iv), isolated from the Ianthella genus, which showed promising anticancer activity.5,6 Myceliothermophins (v), isolated from Myceliophthora thermophila,7 are found to be very good antimicrobial agents.8 In addition, this structural unit is used as a building block in synthetic organic chemistry.9−11 Namely, 5-allyl-5-hydroxy-3-iodo-1H-pyrrol-2(5H)-one is employed as a precursor in the total synthesis of natural product lucilactaene.9
Figure 1.
Substituted 5-hydroxy-1,5-dihydro-1H-pyrrol-2-one motif in bioactive natural products.
Due to widespread appearance of 5-hydroxy-1,5-dihydro-1H-pyrrol-2-ones in natural products and in pharmaceuticals, many synthetic strategies have been documented in the literature for the construction of 5-hydroxy-1H-pyrrol-2(5H)-ones. The primary approaches include (a) the condensation of α,β-diketones and acetamides,12,13 (b) selective reduction followed by addition of an organometallic reagent to maleimides,14−16 (c) oxidation of pyrrolinones,10,17 (d) reaction of chalcones with isonitriles,18 (e) coupling reaction between 2,3-allenamides and aryl iodide catalyzed by Pd(0),19 and (f) ceric ammonium nitrate mediated oxidative 5-endocyclization of enamides.20 Most of the literature reported methods for the preparation of 5-hydroxy-1H-pyrrol-2(5H)-ones go via the enamide intermediate (Figure 2). Moreover, these methods suffer from one or more disadvantages, such as poor chemical yield, low regioselectivity, and limited substrate scope. The development of new methods to synthesize this class of compounds by addressing all the shortcomings of the literature reported methods is the need of the hour.
Figure 2.
Reported synthetic approaches to 5-hydroxy-1H-pyrrol-2(5H)-one derivatives.
Our research group has been involved in developing different synthetic strategies by exploring various reagents and intermediates to access structurally diversified heterocyclic compounds including pyrrolidine derivatives.21 Many other research groups have shown a lot of interest in synthesizing stable sulfonium salts due to their attractive synthetic applications. Sulfonium salt has been proven to be an excellent annulation reagent that is employed in the synthesis of pharmacologically important compounds such as heteroamino compounds, three- to seven-membered heterocycles, and bicyclic and fused compounds. In particular, sulfonium salt is a very good precursor for the diastereoselective synthesis of epoxide-fused heterocycles. In view of the synthetic potential of sulfonium salt, herein we demonstrate a new synthetic route to access functionally diverse novel (2-(benzyl(2-oxo-2-phenylethyl)amino)-2-oxoethyl) dimethylsulfonium salt from commercially available simple starting materials such as phenacyl bromide, benzylamine, and bromoacetyl chloride. In light of the successful synthesis of stable sulfonium salts, we next achieved an efficient base-induced intramolecular cyclization of sulfur ylide, followed by a 1,3-hydroxy shift reaction to produce 5-hydroxy-1H-pyrrol-2-ones. Our investigations in this direction allowed us to access 5-hydroxy-1H-pyrrol-2-one derivatives in good yields under a one-pot operation. Otherwise, the literature reported methods go through two steps with limited substitution patterns on the pyrrole ring.
Materials and Methods
General Information
Reactions requiring anhydrous conditions (refer to the section General Procedure for Synthesis of 5-Hydroxy-1H-pyrrol-2-(5H)-ones) were executed under dry nitrogen or argon atmospheres in glassware that was dried using either a combination of vacuum and heat-gun, oven, or flame drying. Reaction mixtures were stirred magnetically. Air- and moisture-sensitive liquids and solutions were transferred via syringe or cannula into the reaction vessels through rubber septa. All reagents were purchased at the highest commercial quality and used as received. All solvents were either purchased at the highest commercial quality (anhydrous) and used as received or obtained from a purification column composed of activated alumina. Melting points were determined using a melting point apparatus and are uncorrected. Chromatography was performed on aluminum oxide 90 active neutral (70–230 mesh ASTM). TLC was performed on aluminum-backed silica plates (0.2 mm, 60 F254). 1H and 13C NMR spectra were recorded on 400 and 00 MHz instruments. Chemical shifts (δH, δC) are quoted in parts per million (ppm) and referenced to the appropriate NMR solvent peak(s) and are assigned ArH, C, CH, CHH or CHH (diastereotopic protons), and CH2, CH3. COSY, HSQC, and HMBC experiments were used in assigning NMR spectra and are included in the Supporting Information. Spin–spin coupling constants (J) are reported in Hz. Low and high resolution mass spectra were recorded in CI or ESI modes respectively on a FT-MS instrument.
General Procedure for the Synthesis of (2-(Benzyl(2-oxo-2-phenylethyl)amino)-2-oxoethyl)dimethylsulfonium Tetraphenylborates
Benzyl amine (0.2 mmol) in DCM (3 mmol) and base Et3N (0.2 mmol) were taken in a RB flask. Phenacyl bromide (0.2 mmol) dissolved in DCM (2 mmol) was added dropwise into the flask using a dropping funnel for over 5 min. The mixture was stirred for 10 min and an unstable intermediate 2-benzylamino-1-phenylethanone was formed.22 After that, bromoacetyl chloride (1 equiv) dissolved in DCM (2 mmol) was added dropwise over 5 min at 0 °C and yielded a tertiary amine (N-benzyl-2-bromo-N-(2-oxo-2-phenylethyl)acetamide). The solvent was removed under reduced pressure, and the residue was dissolved in dimethyl sulfide (DMS) and stirred for about 10 min at room temperature, affording the corresponding bromide salt as a yellow/white precipitate. The bromide salt formed was not bench-stable, so it was made stable by changing the counterion according to the literature reported method.23 The obtained salt was dissolved in 3.5:1.5 ratio of acetone/water system and stirred for 20 min at room temperature in the presence of sodium tetraphenylborate (1 equiv). Then the reaction mixture was poured into water, and sulfonium tetrapheylborate settled down as a gel, which was dried in an oven overnight to get the corresponding sulfonium salts.
General Procedure for Synthesis of 5-Hydroxy-1H-pyrrol-2-(5H)-ones
Distilled DBU (0.20 mmol) was added dropwise to vinyl sulfonyl salt (0.20 mmol) in anhydrous MeCN (5.0 mL) at 0 °C under nitrogen atmosphere. The reaction mixture was stirred for 10–15 min. After completion of the reaction which is monitored by TLC, the reaction was quenched with a saturated ammonium chloride solution and extracted with DCM (3 × 15 mL). The organic layer was dried over MgSO4 and concentrated under a vacuum. The compound was purified by column chromatography on neutral alumina. Non-polar impurities were eluted with 50% of EtOAc/hexane. Whereas the desired compounds were eluted with DCM and methanol in a 99:1 ratio to yield the 5-hydroxy-1H-pyrrol-2-(5H)-ones.
Results and Discussion
Our initial strategy was to prepare a series of stable sulfonium salts and further utilize them to generate 5-hydroxy-1H-pyrrol-2-one derivatives. Benzyl amine (2) was treated with phenacyl bromide (1) in the presence of mild base and stirred for 20 min at room temperature, which leads to the formation of α-amino ketone monomer 3a as a major product. Whereas the formation of dimer 3b is favored with the longer reaction time. Since the dimer 3b is unwanted for further reactions, we have optimized the reaction condition to get monomer 3a as a predominant product. The required monomer 3a has unstable structural features; immediately, it was acylated with bromoacetyl chloride at 0 °C to convert into stable tertiary amide 4. The sequential addition of DMS to compound 4 results in precipitation after 10 min due to the formation of sulfonium bromide salt 5 (Scheme 1).
Scheme 1. Preparation of Sulfonium Bromide Salt 5a.
Sulfur-based ylides24 are unique reagents which have been used for the construction of epoxy ring systems and for various other rearrangement reactions.25−30 We initiated the investigation of intramolecular cyclization of sulfonium bromide salt with the aid of different organic and inorganic bases in THF solvent at room temperature (Scheme 2, Table 1, Entry 1–7). Interestingly, we have observed the formation of β-hydroxy-γ-lactam 6a with an isolated yield of up to 18% rather than the expected epoxide ring formation. Based on this observation, various polar solvents were subsequently tested (Table 1, Entry 8–11) to improve the product yield. But none of the tested solvents either increased the yield of 6a or produced the epoxide ring. This is probably due to the nucleophilic character of the counterion i.e., the bromide ion. Later, we decided to screen the reaction in the presence of non-nucleophilic counterions to enhance the product yield. The use of other non-nucleophilic counterions like BPh4–, BF4–, and Otf– (Table 1, Entry 12–17) instead of bromide ion under identical conditions afforded β-hydroxy-γ-lactam 6a in appreciable yield rather than the expected epoxide product. The use of BPh4– counterion (Table 1, Entry 14) showed a notable increase in the yield of β-hydroxy-γ-lactam 6a up to 93% in the presence of DBU base. Whereas the use of other bases like DABCO, Et3N, and piperidine decreases the product yield (Table 1, Entry 15–17). In the optimized conditions presented here, the synthesis of β-hydroxy-γ-lactam could be easily scaled up to gram quantities.
Scheme 2. Synthesis of β-Hydroxy-γ-Lactam Derivative 6a.
Table 1. Optimization Condition for the Generation of β-Hydroxy-γ-Lactam 6a.
| entrya | base | solvent | X– | yield of 6a (%) |
|---|---|---|---|---|
| 1 | NaH | THF | Br | 10 |
| 2 | t-BuOK | THF | Br | 5 |
| 3 | NaNH2 | THF | Br | 11 |
| 4 | LDA | THF | Br | 12 |
| 5 | 2,6-lutidine | THF | Br | 5 |
| 6 | KHMDS | THF | Br | 10 |
| 7 | DBU | THF | Br | 18 |
| 8 | DBU | DMF | Br | 15 |
| 9 | DBU | DMSO | Br | 20 |
| 10 | DBU | CH3CN | Br | 25 |
| 11 | DBU | dioxane | Br | 17 |
| 12 | DBU | CH3CN | OTf | 20 |
| 13 | DBU | CH3CN | BF4 | 45 |
| 14 | DBU | CH3CN | BPh4 | 93b |
| 15 | DABCO | CH3CN | BPh4 | 87b |
| 16 | Et3N | CH3CN | BPh4 | 85b |
| 17 | piperidine | CH3CN | BPh4 | 90b |
Reaction conditions: 5 (0.20 mmol), base (0.20 mmol), and solvent (5.0 mL).
Isolated yield after silica gel column chromatography.
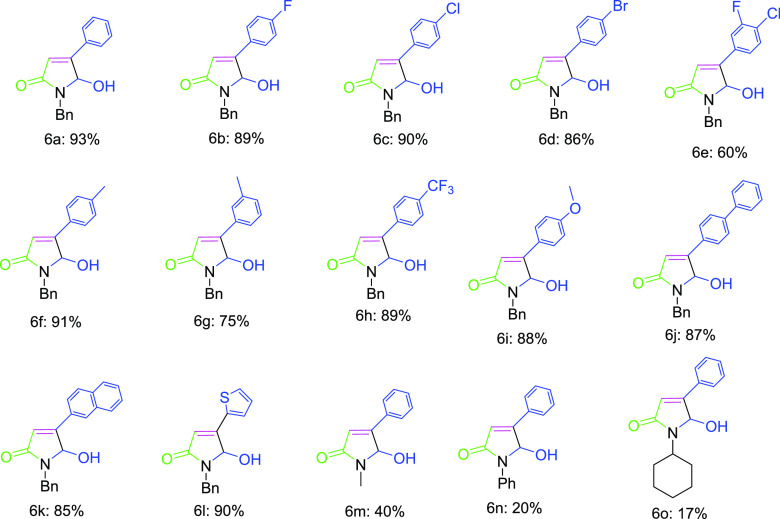
With the optimized reaction conditions in hand, we then explored the generality of the protocol for the substrate scope potential. Bromo acetophenones bearing electron-donating as well as electron-withdrawing groups at para-, meta-, and ortho-positions proved to be productive substrates to generate β-hydroxy-γ-lactam products (characterized by X-ray analysis, Figure 3) in appreciable to high isolated yields (Scheme 3, Figure 4). The presence of electron-withdrawing groups such as fluoro (6b), chloro (6c), bromo (6d), and trifluoromethyl (6h) groups, and electron-donating groups such as methyl (6f) and methoxy (6i) at para position accelerates the formation of 5- hydroxyl lactam product with excellent yields. Any substituent at meta- or ortho-positions slows down the reaction and subsequently decreases the product yield; this may be due to the steric hindrance. Interestingly, the developed protocol also tolerated the thiophene substitution, as illustrated in the synthesis of derivatives 6l. The bulky aromatic moieties, such as naphthyl and biphenyl groups, were also successfully installed into γ-lactam structures 6j and 6k with good yields. Otherwise, the literature reported methods require later-stage modification for their insertion into the γ-lactam moiety. Furthermore, we have attempted the reaction with differently substituted sulfonium salts synthesized by using alkyl, aryl, and cycloalkyl amines instead of using benzyl amines. Unfortunately, none of these attempts gave satisfactory yields of the expected products (6m–o) due to steric hindrance.
Figure 3.
ORTEP diagram for compounds 6a and 6d.
Scheme 3. Scope of Substituted Acetophenones to Synthesize β-Hydroxy γ-Lactams.
Figure 4.
Structurally diversified β-hydroxy-γ-lactams 6.
The plausible mechanism for the construction of β-hydroxy-γ-lactams 6 appears to be simple as depicted in Scheme 4. Sulfonium salt 5, upon treatment with the base, generates sulfur yields A, which in turn undergoes cyclocondensation reactions with the carbonyl group to produce epoxy lactam intermediate B. Furthermore, the base-induced epoxy ring-opening reaction of compound B leads to the formation of enamide intermediate C, which subsequently undergoes the E1cB elimination reaction to generate cyclic iminium intermediate D. Finally, the iminium intermediate, upon reacting with a water molecule, furnishes the final β-hydroxy-γ-lactam product 6. It is noteworthy to mention that the present protocol does not demand any transition metal catalyst to achieve tandem intramolecular cyclization and 1,3-hydroxy rearrangement. The product formation is further supported by different spectroscopic techniques, including X-ray crystallographic data and 2D NMR studies (COSY, HSQC, HMBC, and DEPT).
Scheme 4. Plausible Mechanism for the Construction of β-Hydroxy γ-Lactams.
Conclusions
In summary, we herein disclose a user-friendly, mild, and attractive protocol for a direct and regioselective route for synthetically useful 5-hydroxy, α,β-unsaturated γ-lactams using easily accessible substrates by sequential intramolecular cyclization of sulfur ylide and 1,3-hydroxy rearrangement. This new activation mode therefore holds great promise for a wider range of regioselective hydroxylation reactions under sustainable reaction conditions. The present method allows for the quick construction of different 5-hydroxy, α,β-unsaturated γ-lactams in good yields and is demanded as a first choice for library synthesis and drug discovery research.
Acknowledgments
HDP thanks IOE and the University of Mysore for the research fellowship, and K.S.R. acknowledges CSIR for providing an emeritus scientist fellowship. The authors extend their appreciation to the Researchers Supporting Project number RSP2023R393, King Saud University, Riyadh, Saudi Arabia.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c06866.
Crystal data for C H Br N O (CIF)
Crystal data for C17 H14 N O2 (CIF)
Experimental procedure and 1H COSY spectra of compound 6a and 6d; 13C–1H-COSY spectra of compound 6a; 13C-DEPT spectra of compound 6a and 6d; HMBC spectra of 6a; HSQC spectra of 6a and 6d; and 1H and 13C NMR spectra of all the products (PDF)
Author Contributions
All authors have given approval to the final version of the manuscript
The authors declare no competing financial interest.
Supplementary Material
References
- a Singh S. B.; A M.; Goetz E. T.; Jones G. F.; Bills R. A.; Giacobbe L.; Herranz S.; Stevens-Miles D. L.; Williams D. L. Jr. Oteromycin: A Novel Antagonist of Endothelin Receptor. J. Org. Chem. 1995, 60, 7040–7042. 10.1021/jo00126a071. [DOI] [Google Scholar]; b Kakeya H.; Takahashi I.; Okada G.; Isono K.; Osada H. Epolactaene, a novel neuritogenic compound in human neuroblastoma cells, produced by a marine fungus. J. Antibiot. 1995, 48, 733–735. 10.7164/antibiotics.48.733. [DOI] [PubMed] [Google Scholar]; c Goh S. H.; Wei C.; Ali A. R. M. Strychnos-aspidosperma alkaloids and a novel hydroxydilactam artefact from Leuconotisgriffithii (Apocynaceae). Tetrahedron Lett. 1984, 25, 3483–3484. 10.1016/S0040-4039(01)91054-8. [DOI] [Google Scholar]; d Nay B.; Riache N.; Evanno L. Chemistry and biology of non-tetramic γ-hydroxy-γ-lactams and γ-alkylidene-γ-lactams from natural sources. Nat. Prod. Rep. 2009, 26, 1044–1062. 10.1039/B903905H. [DOI] [PubMed] [Google Scholar]
- a Pereira U. A.; Barbosa L. C. A.; Maltha C. R. A; Demuner A. J.; Masood M. A.; Pimenta A. L. Inhibition of Enterococcus faecalis biofilm formation by highly active lactones and lactams analogues of rubrolides. Eur. J. Med. Chem. 2014, 82, 127–138. 10.1016/j.ejmech.2014.05.035. [DOI] [PubMed] [Google Scholar]; b Kanishchev O. S.; Lavoignat A.; Picot S.; Mdebielle M.; Bouillon J.-P New route to the 5-((arylthio- and heteroarylthio)methylene)-3-(2,2,2-trifluoroethyl)-furan-2(5H)-ones—Key intermediates in the synthesis of 4-aminoquinoline γ-lactams as potent antimalarial compounds. Bioorg. Med. Chem. Lett. 2013, 23, 6167–6171. 10.1016/j.bmcl.2013.08.108. [DOI] [PubMed] [Google Scholar]; c Cornut D.; Lemoine H.; Kanishchev O.; Okada E.; Albrieux F.; Beavogui A. H.; Bienvenu A.-L.; Picot S.; Bouillon J.-P.; Medebielle M. Incorporation of a 3-(2, 2, 2-trifluoroethyl)-γ-hydroxy-γ-lactam motif in the side chain of 4-aminoquinolines. Syntheses and antimalarial activities. J. Med. Chem. 2013, 56, 73–83. 10.1021/jm301076q. [DOI] [PubMed] [Google Scholar]
- Douglas S. A.; Gellai M.; Ezekiel M.; Feuerstein G. Z.; Elliott J. D.; Ohlstein E. H.; Antihypertensive Actions of the Novel NonpeptideEndothelin Receptor Antagonist SB 209670 Hypertension 199525818–822. 10.1161/01.hyp.25.4.818 [DOI] [PubMed] [Google Scholar]
- Izgu E. C.; Hoye T. R. Total synthesis of (±)-leuconolam: intramolecular allylicsilane addition to a maleimidecarbonyl group. Chem. Sci. 2013, 4, 2262. 10.1039/c3sc00056g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X. C.; Xiao X.; Zhang Y. K.; Talele T. T.; Salim A. A.; Chen Z. S.; Capon R. J.; Lamellarin O. A. Pyrrole Alkaloid from an Australian Marine Sponge, Ianthella sp. Reverses BCRP Mediated Drug Resistance in Cancer Cells. Mar. Drugs 2014, 12, 3818–3837. 10.3390/md12073818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Conte M. M.; Huang X. C.; Khalil Z.; Capon R. J. A search for BACE inhibitors reveals new biosynthetically related pyrrolidones, furanones and pyrroles from a southern Australian marine sponge Ianthella sp. Org. Biomol. Chem. 2012, 10, 2656–2663. 10.1039/c2ob06747a. [DOI] [PubMed] [Google Scholar]
- Yang Y. L.; Lu C. P.; Chen M. Y.; Chen K. Y.; Wu Y. C.; Wu S. H. Cytotoxic Polyketides Containing Tetramic Acid Moieties Isolated from the Fungus MyceliophthoraThermophila: Elucidation of the Relationship between Cytotoxicity and Stereoconfiguration. Chem.—Eur. J. 2007, 13, 6985–6991. 10.1002/chem.200700038. [DOI] [PubMed] [Google Scholar]
- Shionozaki N.; Yamaguchi T.; Kitano H.; Tomizawa K. M.; Makino K.; Uchiro H. Total synthesis of myceliothermophins A–E. Tetrahedron Lett. 2012, 53, 5167–5170. 10.1016/j.tetlet.2012.07.058. [DOI] [Google Scholar]
- a Coleman R. S.; Walczak M. C.; Campbell E. L. Total synthesis of lucilactaene, a cell cycle inhibitor active in p53-inactive cells. J. Am. Chem. Soc. 2005, 127, 16038. 10.1021/ja056217g. [DOI] [PubMed] [Google Scholar]; b Uno H.; Yayamn A.; Suzuki H. Perfluoroalkyl migration in the rearrangement of 4-perfluoroalkyl-4-quinols. Tetrahedron 1992, 48, 8353. 10.1016/S0040-4020(01)86584-4. [DOI] [Google Scholar]
- Snider B. B.; Neubert B. J. A Novel Biomimetic Route to the 3-Acyl-5-hydroxy-3-pyrrolin-2-one and 3-Acyl-3,4-epoxy-5-hydroxypyrrolidin-2-one Ring Systems. J. Org. Chem. 2004, 69, 8952. 10.1021/jo048605r. [DOI] [PubMed] [Google Scholar]
- Pin F.; Comesse S.; Garrigues B.; Marchalin S. A.; Daïch A. Intermolecular and intramolecular α-amidoalkylation reactions using bismuth triflate as the catalyst. J. Org. Chem. 2007, 72, 1181–1191. 10.1021/jo062077x. [DOI] [PubMed] [Google Scholar]
- Kakeya H.; Onozuka C.; Sato M.; Arai K.; Osada H. Neuritogenic Effect of Epolactaene Derivatives on Human Neuroblastoma Cells Which Lack High-Affinity Nerve Growth Factor Receptors. J. Med. Chem. 1997, 40, 391. 10.1021/jm960719a. [DOI] [PubMed] [Google Scholar]
- Howard E. G.; Lindsey R. V.; Theobald C. W. Jr Synthesis of 3-substituted 5-hydroxy-3-pyrrolin-2-ones. J. Am. Chem. Soc. 1959, 81, 4355. 10.1021/ja01525a063. [DOI] [Google Scholar]
- a Coleman R. S.; Walczak M. C.; Campbell E. L. Total Synthesis of Lucilactaene, A Cell Cycle Inhibitor Active in p53-Inactive Cells. J. Am. Chem. Soc. 2005, 127, 16038. 10.1021/ja056217g. [DOI] [PubMed] [Google Scholar]; b Uno H.; Yayamn A.; Suzuki H. Perfluoroalkyl migration in the rearrangement of 4-perfluoroalkyl-4-quinols. Tetrahedron 1992, 48, 8353–8368. 10.1016/S0040-4020(01)86584-4. [DOI] [Google Scholar]; c Ananda H.; Sharath Kumar K. S.; Nishana M.; Hegde M.; Srivastava M.; Byregowda R.; Choudhary B.; Raghavan S. C.; Rangappa K. S. Regioselective synthesis and biological studies of novel 1-aryl-3, 5-bis (het) aryl pyrazole derivatives as potential antiproliferative agents. Mol. Cell. Biochem. 2017, 426, 149–160. 10.1007/s11010-016-2887-7. [DOI] [PubMed] [Google Scholar]
- Pin F.; Comesse S.; Garrigues B.; Marchalin S.; Daïch A. Intermolecular and Intramolecular α-Amidoalkylation Reactions Using Bismuth Triflate as the Catalyst. J. Org. Chem. 2007, 72, 1181–1191. 10.1021/jo062077x. [DOI] [PubMed] [Google Scholar]
- a Gavina F.; Costero A. M.; Andreu M. R.; Carda M.; Luis S. V. 2-Aza-2, 4-cyclopentadienone. Existence and reactivity. J. Am. Chem. Soc. 1988, 110, 4017. 10.1021/ja00220a047. [DOI] [Google Scholar]; b Mase N.; Nishi T.; Hiyoshi M.; Ichihara K.; Bessho J.; Yoda H.; Takabe K. Regioselective reduction of maleimide and citraconimide derivatives: general preparation of 5-hydroxy-1,5-dihydropyrrol-2-one. J. Chem. Soc., Perkin Trans. 2002, 1, 707. 10.1039/b200729k. [DOI] [Google Scholar]; c Jagadish S.; Rajeev N.; NaveenKumar S. K.; Sharath Kumar K. S.; Paul M.; Hegde M.; Basappa M. P.; Sadashiva K. S.; Girish K.S.; Rangappa K. S. Platelet protective efficacy of 3,4,5 trisubstitutedisoxazole analogue by inhibiting ROS-mediated apoptosis and platelet aggregation. Mol. Cell. Biochem. 2016, 414, 137–151. 10.1007/s11010-016-2667-4. [DOI] [PubMed] [Google Scholar]
- a Rudler H.; Parlier A.; Ousmer M.; Vaissermann J.. One-Pot Formation of Functionalized Pyrrolinones and 2-Oxohexahydroindoles from Aminocarbene Complexes of Chromium Eur. J. Org. Chem. 199919993315–3321.. [DOI] [Google Scholar]; b Clayden J.; Turnbull R.; Pinto I. Diastereoselective protonation of extended pyrrol-3-en-2-one enolates: an attempted ‘de-epimerisation’. Tetrahedron: Asymmetry 2005, 16, 2235. 10.1016/j.tetasy.2005.05.042. [DOI] [Google Scholar]; c Nirgude S.; Mahadeva R.; Koroth J.; Kumar K. S. S.; Kumar J.; Gopalakrishnan V.; Karki S. S.; Choudhary B. ST09. A Novel Curcumin Derivative, Blocks Cell Migration by Inhibiting Matrix Metalloproteases in Breast Cancer Cells and Inhibits Tumor Progression in EAC Mouse Tumor Models. Molecules 2020, 25, 4499. 10.3390/molecules25194499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adib M.; Mahdavi M.; Noghani M. A.; Bijanzadeh H. R. Reaction between isocyanides and chalcones: an efficient solvent-free synthesis of 5-hydroxy-3,5-diaryl-1,5-dihydro-2H-pyrrol-2-ones. Tetrahedron Lett. 2007, 48, 8056. 10.1016/j.tetlet.2007.09.030. [DOI] [Google Scholar]
- Ma S.; Xie H. Steric Hindrance-Controlled Pd(0)-Catalyzed Coupling–Cyclization of 2,3-Allenamides and Organic Iodides. An Efficient Synthesis of Iminolactones and γ-Hydroxy-γ-lactams. J. Org. Chem. 2002, 67, 6575. 10.1021/jo025967v. [DOI] [PubMed] [Google Scholar]
- Clark A. J.; Dell C. P.; McDonagh J. M.; Geden J.; Mawdsley P. Oxidative 5-Endo Cyclization of Enamides Mediated by Ceric Ammonium Nitrate. Org. Lett. 2003, 5, 2063. 10.1021/ol030045f. [DOI] [PubMed] [Google Scholar]
- a Swaroop T. R.; Sharath Kumar K. S.; Palanivelu M.; Chaitanya S.; Rangappa K. S. A Catalyst free Green Protocol for the Synthesis of Pyranopyrazoles Using Room Temperature Ionic Liquid Choline Chloride urea. J. Heterocycl. Chem. 2014, 51, 1866–1870. 10.1002/jhet.1864. [DOI] [Google Scholar]; b Sharath Kumar K. S.; Ananda H.; Shobith R.; Raghavan S. C.; Rangappa K. S. Regioselective competitive synthesis of 3,5-bis(het) aryl pyrrole-2-carboxylates/carbonitriles vs. β-enaminones from β-thioxoketones. Tetrahedron Lett. 2021, 82, 153373 10.1016/j.tetlet.2021.153373. [DOI] [Google Scholar]; c Ananda H.; Sharath Kumar K. S.; Sudhanva M. S.; Rangappa K. S.; Rangappa S. A trisubstitutedpyrazole derivative reduces DMBA-induced mammary tumor growth in rats by inhibiting estrogen receptor-α expression. Mol. Cell. Biochem. 2018, 449, 137–144. 10.1007/s11010-018-3350-8. [DOI] [PubMed] [Google Scholar]
- a Zhang X.; He Y.; Li J.; Wang R.; Gu L.; Li G. CO2/Photoredox-Cocatalyzed Tandem Oxidative Cyclization of α-Bromo Ketones and Amines To Construct Substituted Oxazoles. J. Org. Chem. 2019, 84 (12), 8225–8231. 10.1021/acs.joc.9b00283. [DOI] [PubMed] [Google Scholar]; b Pradeep N.; Singh D.; Sarah M.; Mandel M.; Robinson Z.; Zhu R.; Franz S.; Bruce Ault D.; Gudmundsdottir A. D. Photolysis of α-Azidoacetophenones: Direct Detection of Triplet Alkyl Nitrenes in Solution. J. Org. Chem. 2003, 68 (21), 7951–7960. 10.1021/jo034674e. [DOI] [PubMed] [Google Scholar]
- Matlock J. V.; Fritz S. P.; Harrison S. A.; Coe D. M.; McGarrigle E. M.; Aggarwal V. K. Synthesis of α-Substituted Vinylsulfonium Salts and Their Application as Annulation Reagents in the Formation of Epoxide- and Cyclopropane-Fused Heterocycles. J. Org. Chem. 2014, 79 (21), 10226–10239. 10.1021/jo501885z. [DOI] [PubMed] [Google Scholar]
- Johnson A. W.; LaCount R. B. Chem. Ind. (Lond.) 1958, 1440–1441. [Google Scholar]
- Corey E. J.; Chaykovsky M. J. DimethyloxosulfoniumMethylide ((CH3)2SOCH2) and DimethylsulfoniumMethylide ((CH3)2SCH2). Formation and Application to Organic Synthesis. J. Am. Chem. Soc. 1965, 87, 1353–1364. 10.1021/ja01084a034. [DOI] [Google Scholar]
- Gololobov Y. G.; Nesmeyanov A. N.; Lysenko V. P.; Boldeskul I. E. Twenty-five years of dimethylsulfoxoniumethylide (corey’s reagent). Tetrahedron 1987, 43, 2609–2651. 10.1016/S0040-4020(01)86869-1. [DOI] [Google Scholar]
- Furukawa N.; Sugihara F.; Fujihara H. Camphoryl sulfide as a chiral auxiliary and a mediator for one-step synthesis of optically active 1,2-diaryloxiranes. J. Org. Chem. 1989, 54, 4222–4224. 10.1021/jo00278a045. [DOI] [Google Scholar]
- Julienne K.; Metzner P.; Henyron V. Asymmetric synthesis of epoxides from aldehydes mediated by (+)-(2 R, 5 R)-2, 5-dimethylthiolane.. J. Chem. Soc., Perkin Trans. 1 1999, 1, 731–735. 10.1039/A807623E. [DOI] [Google Scholar]
- Zanardi J.; Leriverend C.; Aubert D.; Julienne K.; Metzner P. A Catalytic Cycle for the Asymmetric Synthesis of Epoxides Using Sulfur Ylides. J. Org. Chem. 2001, 66, 5620–5623. 10.1021/jo015588m. [DOI] [PubMed] [Google Scholar]
- Winn C. L.; Bellanie B.; Goodman J. M. A highly enantioselective one-pot sulfur ylide epoxidation reaction. Tetrahedron Lett. 2002, 43, 5427–5430. 10.1016/S0040-4039(02)01072-9. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information