Abstract
Posttranscriptional modifications of RNA constitute an emerging regulatory layer of gene expression. The demethylase FTO, an eraser of N6-methyladenosine (m6A), has been shown to play a role in cancer, but its contribution to tumor progression and the underlying mechanisms remain unclear. Here we report widespread FTO downregulation in epithelial cancers, associated with increased invasion, metastasis, and worse clinical outcome. Both in vitro and in vivo, FTO silencing promotes cancer growth, cell motility, and invasion. In patient-derived tumor xenografts (PDXs), its pharmacological inhibition favors tumorigenesis. Mechanistically, we demonstrate that FTO depletion elicits an epithelial-to-mesenchymal transition (EMT) program through increased m6A and altered 3’-end processing of key mRNAs along the Wnt signaling cascade. Accordingly, FTO knockdown acts via EMT to sensitize mouse xenografts to Wnt inhibition. We thus identify FTO as a key regulator, across epithelial cancers, of Wnt-triggered EMT and tumor progression, while revealing a therapeutically exploitable vulnerability of FTO-low tumors.
Growing evidence suggests that chemical modifications on RNA, similar to those on DNA or histones, control gene expression1. More than 100 types of RNA modifications have been reported2, among which N6-methyladenosine (m6A) is the most abundant internal modification3. Transcriptome-wide mappings of m6A identified methylated sites in more than 7,000 mRNA transcripts, many conserved between humans and mice3,4. Marking with m6A occurs on adenosines embedded in the consensus sequence G[G > A] m6ACU, especially in 3’ untranslated regions (UTRs) near stop codons of transcripts5. Installed by the METTL3/METTL14/WTAP methyltransferase complex and erased by the demethylases fat mass- and obesity-associated protein (FTO) and ALKBH56,7, m6A influences fundamental aspects of mRNA metabolism, such as mRNA stability, splicing, transport, and translation, thereby impacting gene expression3–5,8–12.
FTO was found to catalyze m6A demethylation in an Fe(II)- and α-ketoglutarate-dependent enzymatic reaction6,13,14. In addition to an involvement in essential biological processes5,8, FTO has been linked to cancer, being highlighted as an oncoprotein in leukemia15,16. Despite conflicting results by Vu et al., who observed no substantial effect of FTO depletion on AML cell viability17, the development of FTO inhibitors has since shown encouraging results in preclinical settings16,18,19. Similar oncogene functions were assigned to FTO in other cancers, such as glioblastoma and melanoma, highlighting the potential of targeting FTO as a therapeutic strategy against cancer.20,21 However, seemingly conflicting reports, in which a tumor suppressor role was attributed to FTO in other cancers,22–24 called for caution, and the tissue of origin and cancer subtype should be carefully considered when evaluating clinical implications. Strikingly, both gain and loss of expression have been reported in breast cancer, with various impacts on survival 25–27. The situation is thus far from clear, and in-depth studies are needed to disentangle the apparent emerging conundrum about the role of FTO in cancer biology, especially in light of ongoing efforts to develop FTO inhibitors as anticancer drugs18,19. In the present study, we have sought to provide a better understanding of how FTO affects cancer progression, by what mechanisms, and how the knowledge gained might be exploited clinically.
Results
FTO downregulation is widespread in cancers and enhances breast tumorigenesis
To get a broad view of FTO dysregulation in cancers, we first investigated FTO expression in tumor samples from The Cancer Genome Atlas (TCGA) database versus their normal counterparts. We frequently found significant downregulation of FTO in epithelial cancers (bladder, breast, cervix, kidney, lung, prostate, thyroid, and uterus) and a non-epithelial cancer type (glioblastoma) (Fig. 1a). This finding prompted us to explore the consequences of FTO downregulation in breast cancer, one of the most prevalent and clinically challenging cancer types. We first confirmed downregulation of FTO (Extended Data Fig. 1a) and revealed, using mass spectrometry, both reduced m6A methylation in FTO-high versus FTO-low tumor samples (Fig. 1b) and a higher global level of m6A marking in human breast cancers than in normal tissues (Extended Data Fig. 1b). Notably, FTO downregulation occurred in all major molecular subtypes of breast cancer (Fig. 1c).
Figure 1. FTO loss enhances breast tumorigenesis.
a, Downregulation of FTO expression in several cancers (red) versus normal tissues (green) in TCGA. The number of samples (n) is indicated below each tissue type. P-values calculated by two-tailed t-test. Effect size estimated by Cohen’s distance: d(bladder)=0.61, d(breast)=1.39, d(cervix)=1.49, d(glioblastoma)=1.57, d(kidney)=2.22, d(lung)=0.98, d(prostate)=0.90, d(thyroid)=1.06, d(uterine)=1.36. b, Global m6A levels by mass spectrometry in human breast cancer biopsies with low (n=25) or high (n=15) FTO expression. P-value calculated by two-tailed t-test (p=0.0029). c, Downregulation of FTO expression in breast cancer subtypes (red: basal-like; pink: HER2-like; dark blue: luminal A; light blue: luminal B) compared to normal breast (grey) in TCGA. The number of samples (n) is indicated for each group. P-values calculated by two-tailed t-test (versus normal breast), as indicated. d, Effects of FTO knockdown by shRNA on colony-forming capacity (CFU: colony-forming units, left, p=0.0003) and mammosphere-forming capacity (right, p=0.03) in SKBR3 cells. Data from n=3 technical replicates (mean + SD) within a single experiment, representative of 3 biologically independent experiments provided as Source Data. P-values calculated by two-tailed t-test. e, FTO depletion enhances tumor engraftment in nude mice subcutaneously injected with SKBR3 breast cells (n=6 tumors per group; mean ± SEM, p=0.0052, two-way ANOVA). f, Immunochemistry analysis of tumors from SKBR3 xenograft from (e) at endpoint. Representative images of tissue sections are shown, scale bar =250μm. Percent Ki67, CASP3 and CD31 positive cells (n=3 fields × 3 tumors per group; mean + SEM). P-values calculated by two-tailed t-test (pKi67<0.0001; pCASP3=0.5; pCD31=0.0006). g, Effects of FTO knockdown on colony-forming capacity (upper panel, p=0.0004) and mammosphere-forming capacity (lower panel, p=0.006) in MCF7 cells. Data from n=3 technical replicates (mean + SD) within a single experiment, representative of 2 biologically independent experiments provided as Source Data. P-values calculated by two-tailed t-test. h, Nude mice were injected as indicated in panel (e) with MCF7 breast cells (n=6 tumors per group; mean ± SEM, p<0.0001, two-way ANOVA).
Boxes in the box plots of (a), (b), (c) define the interquartile range (IQR) split by the median, with whiskers extending to the most extreme values within 1.5×IQR beyond the box.
We next silenced FTO in the SKBR3 breast cancer cells, a widely-used cellular model of HER2-positive breast cancer. In clonogenic assays, FTO-depleted (RNAi) cells (Extended Data Fig. 1c) showed more pronounced colony and mammosphere formation than control cells (Fig. 1d and Extended Data Fig. 1d, e), and this difference depended on FTO catalytic activity (Extended Data Fig. 1f). In immunocompromised mice, FTO knockdown markedly enhanced engraftment of SKBR3 cells (Fig. 1e) by affecting proliferation (Ki67) and angiogenesis (CD31) but not apoptosis (cleaved caspase 3) (Fig. 1f). To extend our study of the consequences of FTO downregulation to another breast cancer subtype, we conducted functional studies on MCF7, a model of luminal breast cancer. As observed with SKBR3 cells, FTO depletion in MCF7 cells resulted in increased clonogenicity and enhanced engraftment in immunocompromised mice (Fig. 1g, h and Extended Data Fig. 1g). FTO can thus play a tumor-suppressive role in breast cancer cells, and this depends on its m6A demethylation activity.
FTO depletion promotes tumor progression and metastasis in breast cancer
Cancer stem cells can disseminate and have been linked to both tumor relapse and metastatic dissemination28. This, and the increased ability of FTO-knockdown cancer cells to form mammospheres (Fig. 1d, g), led us to speculate that FTO loss might favor breast tumor progression. In real-time chemotaxis assays, we found FTO-depleted SKBR3 cells to display a greater ability to migrate and invade than control cells (Fig. 2a and Extended Data Fig. 2a–c) and this difference was not confounded by differences in proliferation or viability (Extended Data Fig. 2d) but depended on FTO catalytic activity (Extended Data Fig. 2e). We further performed chemotaxis assays with MCF7 breast cancer cells and, as in the case of SKBR3 cells, FTO depletion accelerated migraton of these cells (Fig. 2b).
Figure 2. FTO loss promotes breast cancer cell motility and metastases.
a-b, Real-time chemotactic migration of FTO-depleted (red) and control (green) SKBR3 (a) or MCF7 (b) cells. Data from n=3 technical replicates (mean + SD) within a single experiment, representative of 3 biologically independent experiments. Independent repeats have been deposited on the Figshare repository (DOI: 10.6084/m9.figshare.14602932) c, Breast tumor PDXs in nude mice (pre)-treated with either control vehicle (black) or the FTO inhibitor MA (red, n=3 tumors per group, mean ± SEM, p=0.01, tested by mixed effects model via Restricted Maximum Likelihood). d, Tumor metabolism imaging with 18F-FDG positron emission tomography (PET) of xenograft mouse models bearing RNAi Ctrl (black, n=4) or RNAi FTO (red, n=5) SKBR3 tumors. Representative 18F-FDG PET/CT images (9 weeks after inoculation) are shown (pathological uptake in tumors encircled; physiological uptake in brain (B), kidneys (K), bladder (Bl), brown fat and muscular activity). Quantification of tumor metabolism presented as the 18F-FDG uptake in SUVmax. P-value calculated by two-tailed t-test (p=0.03). e, FTO depletion increases metastatic burden in tail vein injection of SKBR3 cells. Metabolic activity in lung and liver of mice bearing either an RNAi Ctrl (n(lung)=13, n(liver)=14) or a RNAi FTO tumor (n(lung)=14, n(liver)=14) is quantified on the basis of 18F-FDG uptake into SUVmax (uptake in liver (Li), lung (Lu), physiological uptake in heart (H), gallbladder (G)). P-values calculated by two-tailed t-test (plung=0.043; pliver=0.009). f, Box plot displaying median FTO expression according to the tumor grade (left, Kruskal-Wallis test, p=1.33e-8) or to lymph node (LN) invasion (middle, Wilcoxon test, p=6.82e-6). Kaplan-Meier analysis based on FTO expression and distant-metastasis-free survival in breast cancer patients (right; n(high FTO)=594, n(low FTO)=577, logrank test, p=0.0009).
Boxes in the box plots of (d), (e), (f) define the interquartile range (IQR) split by the median, with whiskers extending to the most extreme values within 1.5×IQR beyond the box.
To substantiate these findings in vivo, we assessed tumor engraftment in a patient-derived xenograft (PDX) model of luminal breast cancer, a process linked to clinical parameters of cancer progression29. Strikingly, treatment with the FTO inhibitor meclofenamic acid (MA)30 significantly enhanced primary breast tumor engraftment in this highly relevant preclinical model expressing the FTO protein (Fig. 2c and Extended Data Fig. 2f). We then used positron emission tomography (PET) with fluorine-18 fluorodeoxyglucose (18F-FDG) to measure metabolic activity in mice xenograft tumors, as increased 18 F-FDG uptake has been found to correlate with markers of tumor progression in various cancer types, including breast cancer31,32. We observed higher metabolic activity in FTO-depleted than in control tumors, indicative of a more progressive phenotype (Fig. 2d). We additionally measured increased 18 F-FDG uptake by locoregional subiliac lymph nodes in mice bearing FTO-depleted tumors, as compared to mice with control xenografts (Extended Data Fig. 2g). Consistent with our findings from SKBR3 xenografts, 18F-FDG uptake was increased in the lungs and liver of mice injected into tail vein with FTO-depleted as compared to control SKBR3 cells indicative of the formation of micrometastases (Fig. 2e). Together, our data indicate that downregulation of FTO promotes cancer cell aggressiveness, invasion, and metastasis in vivo.
In keeping with the above results, human breast cancer patients showed an inverse correlation between FTO expression and tumor grade, lymph node (LN) invasion, and distant-metastasis-free survival in meta-analysis of public data (“KM cohort”, see methods section and Fig. 2f). We validated the inverse correlation between FTO expression and survival in the METABRIC breast cancer cohort (Extended Data Fig. 2h) and in several individual datasets of the aggregate KM cohort (Extended Data Fig. 2i).
The role of FTO as tumor suppressor extends to other epithelial cancers
To test whether our findings in breast cancer could be extended to other tumors, we focused on another prevalent cancer type, prostate cancer, in which we found FTO expression to be downregulated (Fig. 1a). In human prostate cancer biopsies, we observed an inverse correlation between FTO loss and high global m6A (Fig. 3a). Silencing FTO in the PC3 prostate cancer cell line (Extended Data Fig. 3a) enhanced tumorigenicity in vitro (Fig. 3b) and accelerated xenograft tumor growth in immunocompromised mice (Extended Data Fig. 3b). As expected, PC3 cell motility was also increased upon FTO knockdown (Fig. 3c and Extended Data Fig. 3c). These observations were further extended to cervical and lung cancer, as FTO-depleted Ca Ski and H1650 cells displayed similar phenotypes (Fig. 3d, e and Extended Data Fig. 3d–f). Consistent with these phenotypes, we observed an inverse correlation between FTO expression and disease progression or clinical outcome in public datasets of prostate cancer (Fig. 3f) or lung and uterus cancers (Extended Data Fig. 3g, h). To further substantiate these data, we performed tail vein injections with PC3 cells in immunocompromised mice to generate a metastatic prostate cancer model. 18F-FDG uptake was increased in the lungs and liver of mice injected with FTO-depleted as compared to control PC3 cells (Fig. 3g). Together, these results echo those obtained in breast cancer. We therefore conclude that low FTO expression correlates with an invasive and metastatic phenotype in various epithelial cancers.
Figure 3. FTO loss promotes tumor progression in several cancers.
a, Global m6A levels by mass spectrometry in human prostate cancer biopsies with low (n=13) or high (n=6) FTO expression. P-value calculated by two-tailed t-test (p=0.017). b, Effects of FTO knockdown on colony-forming capacity (left, p=0.001) and tumorsphere-forming capacity (right, p=0.006) in PC3 prostate cancer cells. P-value calculated by two-tailed t-test. c, Real-time chemotactic migration of FTO-depleted (red) and control (green) PC3 cells. d, Effects of FTO knockdown on colony-forming capacity in Ca Ski cervical cancer cells (left, p=0.022) and H1650 lung cancer cells (right, p=0.016). P-values calculated by two-tailed t-test. e, Real-time chemotactic migration of FTO-depleted (red) and control (green) Ca Ski cells. f, Box plot displaying FTO expression in human biopsies of benign (n=6), localized (n=7), and metastatic primary (n=6) prostate tumors (left, one-way ANOVA, p=0.005). Kaplan-Meier analysis showing relapse-free survival for prostate cancer patients from TCGA, according to the level of tumoral FTO expression (right; n(high FTO)=174, n(low FTO)=174, logrank test, p=0.04). g, FTO depletion increases metastatic burden in tail vein injection of PC3 cells. Metabolic activity in lung and liver of mice bearing either an RNAi Ctrl (n(lung)=14, n(liver)=10) or a RNAi FTO tumor (n(lung)=18, n(liver)=15) quantified on the basis of 18F-FDG uptake into SUVmax (uptake in liver (Li), lung (Lu), physiological uptake in heart (H), gallbladder (G)). P-values calculated by two-tailed t-test (plung=0.002; pliver=0.04).
Boxes in the box plots of (a), (f), (g) define the interquartile range (IQR) split by the median, with whiskers extending to the most extreme values within 1.5×IQR beyond the box. Clonogenicity and motility data in (b-e) are from n=3 technical replicates (mean + SD) within a single experiment, representative of 3 biologically independent experiments, provided as Source Data for clonogenicity experiments. For motility assays in (c), (e), independent repeats have been deposited on the Figshare repository (DOI: 10.6084/m9.figshare.14602944).
FTO loss elicits an EMT program
To expose the mechanism(s) through which FTO loss favors an invasive and metastatic phenotype, we applied Gene Set Enrichment Analysis (GSEA) to data obtained by RNA-seq and identified ‘Epithelial Mesenchymal Transition’ as one of the top overrepresented gene sets in FTO-depleted cells (Fig. 4a and Supplementary Table 1). Accordingly, we found increased expression of mesenchymal genes and decreased expression of epithelial genes in RNAi FTO as compared to RNAi Ctrl SKBR3 cells (Fig. 4b). These results strongly suggest that FTO acts as a modulator of EMT. Following FTO depletion we observed morphological changes associated with EMT (Extended Data Fig. 4a) as well as increased levels of transcripts encoding the mesenchymal markers SNAI2 (SLUG), VIM, FN1, NT5E, SNAI1 (SNAIL), MMP2, and ZEB1 and decreased levels of transcripts encoding the epithelial markers FSTL3, KRT18 and TJP1 (ZO-1) by RT-qPCR (Fig. 4c). Consistently we found, by western blotting, increased expression of FN1, VIM and MMP2 in FTO-depleted SKBR3 cells (Extended Data Fig. 4b). In FTO-depleted tumors from xenografts, western blotting likewise revealed upregulated levels of FN1, VIM and SNAIL proteins (Fig. 4d). We confirmed this finding for FN1 and VIM by IHC (Extended Data Fig. 4c). FTO downregulation thus promotes EMT in both cultured cancer cells and in vivo xenograft tumors. Strikingly, FTO depletion resulted in an EMT phenotype comparable to that induced by TGFβ (Extended Data Fig. 4d, e). We further observed EMT activation upon FTO depletion in MCF7 breast cancer cells and also in prostate and lung cancer cell lines (PC3 and H1650, respectively) (Fig. 4e and Extended Data Fig. 4f, g). Likewise, by IHC we detected increased expression of VIM in FTO-low PC3 xenografts (Extended Data Fig. 4h). In line with the above results, in human tumor biopsies from TCGA, FTO was found to be significantly downregulated in EMT-high breast, prostate and other epithelial cancers (Fig. 4f–h). All these data point to FTO as a previously unrecognized negative regulator of EMT, and this may explain the unfavorable consequences of FTO downregulation in many cancers.
Figure 4. FTO loss elicits an EMT program.
a, GSEA from RNA-seq data (n= 5 per group) in FTO-depleted versus control SKBR3 cells. b, Heatmap showing expression changes (Δ z-score (RNAi FTO – RNAi Ctrl)) for genes of the EMT hallmark signature from panel (a) (with p<0.05 by two-tailed paired t-test) in n=5 paired replicates of SKBR3 cells. c, Relative gene expression of epithelial (upper panel) and mesenchymal (lower panel) EMT marker genes, measured by RT-qPCR in SKBR3 cells following FTO RNAi. Mean + SD, normalized to ACTB and SDHA, n=7 independent replicates (except for n(SLUG)=6; n(ZEB1)=5). P-values calculated by two-tailed t-test, as indicated. d, Western blot of EMT markers in RNAi FTO vs. RNAi Ctrl tumors issued from SKBR3 xenografts in mice (n=4 per group), with β-actin as loading control. e, Relative gene expression of epithelial (left) and mesenchymal (right) EMT marker genes, measured by RT-qPCR in PC3 cells following FTO RNAi. Mean + SD, normalized to ACTB and SDHA, n=5 independent replicates (except for n(TWIST1)=4). P-values calculated by two-tailed t-test. f-h, Box plots displaying FTO expression in human tumors (TCGA) with low or high EMT signature scores in (f) breast cancer (p<0.0001), (g) prostate cancer (p<0.0001) and (h) from left to right: cervical squamous cell carcinoma (n=61 per group); glioblastoma (n=31 per group); lung adenocarcinoma and squamous cell carcinoma (n=205 per group); thyroid carcinoma (n=101 per group); uterine corpus endometrial carcinoma (n=110 per group). The box defines the IQR split by the median, with whiskers extending to the most extreme values within 1.5×IQR beyond the box. P-values calculated by two-tailed t-test, as indicated.
FTO controls Wnt signaling in cancer
To identify critical downstream signaling pathways through which FTO controls EMT, we generated transcriptome-wide m6A profiles of FTO-knockdown and control SKBR3 cells. We identified 1,757 peaks, distributed among 1,338 transcripts, with changed m6A levels in FTO-depleted versus control cells (Extended Data Fig. 5a and Supplementary Table 2). Gene ontology (GO) analysis with identified FTO targets, i.e. transcripts having gained m6A upon FTO depletion, revealed among the top-10 pathways several having a well-established role in breast cancer: Wnt/β-catenin, p53, and TGF-β33,34 (Fig. 5a and Supplementary Table 2). These data suggest that FTO controls critical breast cancer pathways through m6A demethylation of RNA.
Figure 5. FTO controls Wnt signaling in cancer.
a, IPA-performed GO analysis of transcripts that gained m6A upon FTO depletion in SKBR3 cells. Top 10 pathways (by p-value) are shown. b, Schematic representation of m6A/FTO mRNA targets (red asterisks) identified within the Wnt/β-catenin signaling pathway. c, Visualization of the IP m6A signal in RNAi FTO and RNAi Ctrl SKBR3 cells for APC2 and AXIN1 transcripts. d, Representative western blot of total β-catenin in FTO-depleted and control SKBR3 cells (n=3). e, TOPFlash β-catenin/TCF-LEF reporter assay performed on FTO RNAi and control SKBR3 cells (mean + SD, n=6 per group, p-values calculated by two-tailed t-test, as indicated). Wnt3a treatment as positive control for β-catenin-dependent transcriptional activity. f, Heatmap showing the correlation between the expression of Wnt-related genes identified as FTO targets and representative mesenchymal markers of the EMT hallmark signature (shown in Fig. 4b) measured by RNA-seq in 5 pairs of replicates of SKBR3 cells, following FTO RNAi. g, Immunochemistry staining of β-catenin in SKBR3 xenografted tumors (from Fig. 1e at endpoint). Percent β-catenin positive cells (right, n =3 fields × 3 tumors per group; mean + SEM). P-value calculated by two-tailed t-test (p=0.002). h, GO analysis of m6A-enriched transcripts in human breast cancer biopsies. i, Inverse correlation between FTO expression and β-catenin signal intensity in human breast cancer biopsies (n is the number of tumors, two-tailed Mann-Whitney U test, p=0.045). The box defines the IQR split by the median, with whiskers extending to the most extreme values within 1.5×IQR beyond the box. j-k, Relative gene expression of Wnt/β-catenin target genes measure by RT-qPCR, in PC3 (j) or MCF7 (k) cells following RNAi of FTO. Mean + SD from 3 independent experiments, normalized to ACTB and SDHA. P-values calculated by two-tailed t-test, as indicated.
To investigate the molecular mechanisms underlying the observed effects of FTO downregulation (cf. Fig. 1–4), we focused on the Wnt/β-catenin pathway, the most overrepresented among FTO targets, for in-depth analysis. At every level of the canonical β-catenin-dependent Wnt signaling cascade, we detected key transcripts targeted by FTO (Fig. 5b, c and Extended Data Fig. 5b). In contrast to a previous report, CTNNB1 (β-catenin) mRNA was not detected as a direct FTO target35 (Extended Data Fig. 5c and Supplementary Table 2). An FTO target we also identified was WNT5A, a transcript commonly associated with non-canonical Wnt signaling36 but also with β-catenin-dependent signaling activity in various contexts37,38 (Extended Data Fig. 5d). To see if FTO modulates canonical Wnt signaling, we performed western blot analyses and observed, in FTO-knockdown SKBR3 cells, increased stabilization of β-catenin (Fig. 5d and Extended Data Fig. 5e). Western blotting with total β-catenin antibody applied to nuclear and cytoplasmic fractions of SKBR3 cells revealed an increase of the “free” signaling pool of β-catenin as stabilization of β-catenin was observed in nuclear extracts of FTO-depleted versus control cells (Extended Data Fig. 5f). Consistently, we observed by immunofluorescence enhanced levels of nuclear β-catenin in FTO-depleted SKBR3 cells (Extended Data Fig. 5g). In line with these results, FTO depletion caused increased canonical Wnt signaling-dependent transcriptional activity, as evidenced by RT-qPCR quantitation of transcripts of Wnt/β-catenin target genes (Extended Data Fig. 5h) and by increased TOPFlash β-catenin/TCF-LEF reporter activity39 (Fig. 5e). Among our Wnt-related FTO targets, Wnt stimulators (e.g. FZD1, WNT5A, MARK2) correlated positively and Wnt inhibitors (e.g. AXIN1, CSNK1D) inversely with mesenchymal EMT markers (Fig. 5f). This indicates that EMT may be triggered through FTO-mediated upregulation of canonical Wnt signaling. In support of our results on SKBR3 cells, we observed, by western blotting and IHC, stabilization of β-catenin in mouse xenografts grown from SKBR3 FTO-knockdown cells (Fig. 5g and Extended Data Fig. 5i).
We next profiled the m6A mRNA landscape in three human breast tumor biopsies. GO analysis applied to the corresponding m6A peaks (Extended Data Fig. 5j and Supplementary Table 3) revealed Wnt/β-catenin signaling as the top overrepresented pathway (Fig. 5h and Extended Data Fig. 5k). In publicly available data on breast cancer biopsies, FTO expression was found to correlate inversely with the level of β-catenin assessed by immunohistochemistry (IHC)40 (Fig. 5i). The FTO level and canonical Wnt signaling are thus anti-correlated in human breast cancer. When m6A-seq profiling was applied to FTO-knockdown PC3 prostate cancer cells, we again found mRNAs encoding key players of Wnt/β-catenin signaling among the FTO targets (Extended Data Fig. 6a, b and Supplementary Table 4). In line with these data, we observed increased canonical Wnt signaling in FTO-depleted PC3 and MCF7 cells by RT-qPCR and FTO-low PC3 xenografts by IHC (Fig. 5j, k and Extended Data Fig. 6c).
Wnt signaling is regulated by FTO-dependent m6A demethylation
To understand how FTO-dependent m6A demethylation affects mRNA function to control Wnt signaling, we focused particularly on CSNK1D and WNT5A. In contrast to CSNK1D, whose levels decreased upon FTO depletion (Fig. 6a and Extended Data Fig. 6d, e), WNT5A appeared upregulated in FTO RNAi cells (Fig. 6a). Given the role of FTO in 3’ end processing41, we investigated alternative polyadenylation by systematically comparing 3’ UTR lengths in FTO-depleted and control SKBR3 cells. Strikingly, several Wnt-pathway-related FTO targets appeared in the list of transcripts showing a change in 3’ UTR length upon FTO depletion, including SOX11, CSNK1D, and WNT5A mRNAs (Fig. 6b, c and Supplementary Table 1). Transcript stability can be regulated through binding of microRNAs and RNA-binding proteins to the 3’ UTR42. We accordingly observed changes in gene expression for 9 out of 21 Wnt-related FTO targets, partly explainable by changes in mRNA stability (Fig. 6d, 6e). Thus, changes in 3’ UTR length affecting CSNK1D and WNT5A transcripts in FTO-knockdown cells may explain the altered levels of the corresponding transcripts and proteins in these cells. FTO may thus control Wnt signaling through 3’ end processing of several of its key components.
Figure 6. Wnt signaling is regulated by FTO-dependent m6A demethylation.
a, Representative western blot (n=3) showing levels of the proteins encoded by two FTO mRNA targets identified within the Wnt/β-catenin signaling pathway (CSNK1D and WNT5A), in RNAi FTO and RNAi Ctrl SKBR3 cells. HDAC1 and β-actin as loading controls. b, Volcano plot showing the ROAR score versus the p value upon depletion of FTO in SKBR3 cells. Transcripts with differences in 3’ UTR length (p < 0.05; paired Fisher test) upon knockdown of FTO are shown in red (shorter 3’UTR) and green (longer 3’UTR). c, Gene track view of RNA-seq at SOX11, CSNK1D and WNT5A transcripts displaying significant differences in 3’ UTR length upon knockdown of FTO. Alternative polyadenylation sites (Poly A) are indicated by vertical yellow lines. Green horizontal lines represent median expression before and after Poly A site. d, Gene expression regulation (log2 fold-change RNAi FTO/RNAi Ctrl) in SKBR3 cells for FTO targets related to Wnt/β-catenin, p53 and TGFβ (identified in Fig. 4a) measured by RNA-seq in five independent replicates. Transcripts with significant differences in expression (p < 0.05 by two-tailed t-test) are shown in black. Data as log2 fold-change ± lfcSE (standard error of the log2 fold-change). e, Changes in mRNA stability of the FTO targets WNT5A, CSNK1G2 and CSNK1D measured by RT-qPCR following transcription inhibition with actinomycin D in SKBR3 cells upon FTO knockdown (n = 3, data relative to t = 0h, mean ± SEM).
FTO depletion confers sensitivity to pharmacological WNT inhibiton
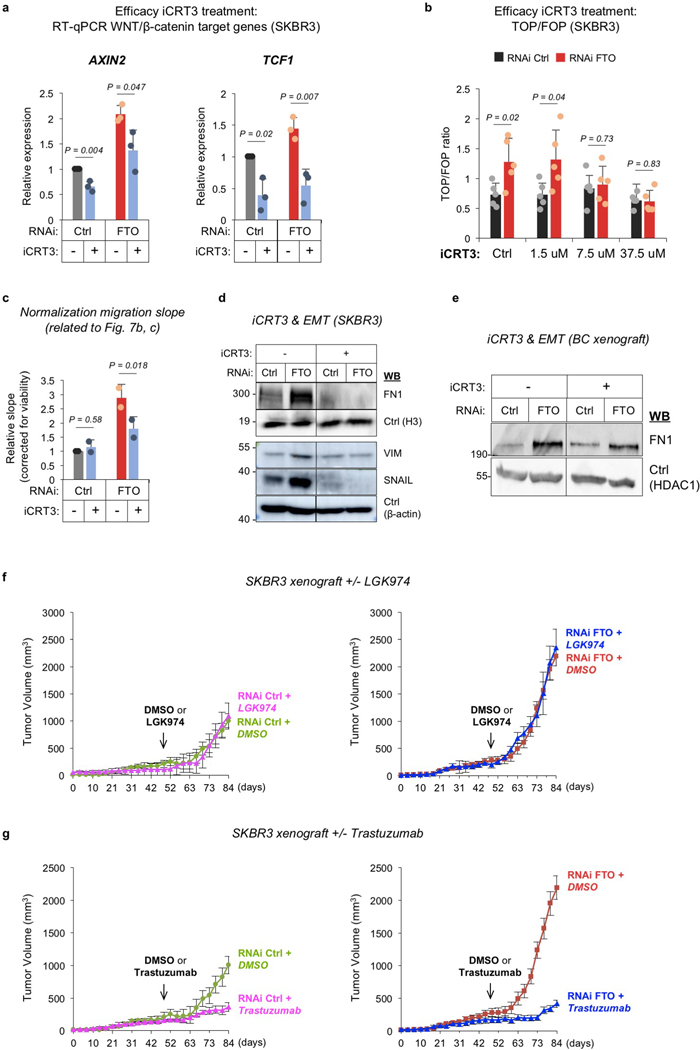
The above data led us to hypothesize that FTO-low tumors might be particularly sensitive to Wnt-pathway antagonists. To test this, we first compared the responses of cultured RNAi FTO and control SKBR3 cells to treatment with a small-molecule inhibitor of nuclear β-catenin called ‘inhibitor of β-catenin responsive transcription-3’ (iCRT3)43. Because iCRT3 inhibits canonical Wnt signaling at the most downstream level by interfering with the T-cell factor (TCF)/β-catenin complex, it enabled us to evaluate the overall effect of FTO on Wnt signaling, resulting from its combined effects on all targets along the canonical Wnt cascade (cf. Fig. 5b). Upon iCRT3 treatment, we observed reduced expression of the Wnt/β-catenin target genes AXIN2 and TCF1 and reduced Wnt signaling activity in both RNAi Ctrl and RNAi FTO cells, confirming the efficacy of Wnt inhibition (Extended Data Fig. 7a, b).
To test whether Wnt inhibition could reverse the phenotype of FTO depletion, we measured mammosphere formation and chemotactic invasion in iCRT3-treated SKBR3 cells. In FTO-depleted but not control cells, iCRT3 did significant reduce mammospheres and invasion (Fig. 7a, b). We further show in MTT assays that cell proliferation and/or viability were affected by Wnt inhibition (Fig. 7c) but importantly, even after correction for observed differences in proliferation/cell viability, RNAi FTO cells treated with iCRT3 exhibited reduced migration (Extended Data Fig. 7c). Together, these results suggest that Wnt activation plays an important role in the phenotype of FTO depletion and that FTO depletion sensitizes SKBR3 cells to Wnt inhibition. Moreover, Wnt inhibition drastically reduced the levels of the mesenchymal marker proteins FN1, VIM, and SNAIL in FTO-depleted cells specifically, suggesting that EMT is triggered through FTO-mediated upregulation of canonical Wnt signaling (Extended Data Fig. 7d). Similar results were obtained upon iCRT3 treatment of FTO-depleted PC3 prostate cancer cells (Fig. 7d). FTO depletion thus enhances the sensitivity of cancer cells to the Wnt inhibitor iCRT3.
Figure 7. In vitro WNT inhibition restores EMT regulation in FTO-depleted cells.
a, Effect of Wnt/β-catenin inhibition by iCRT3 pre-treatment on mammosphere-forming capacity of RNAi Ctrl and RNAi FTO SKBR3 cells. Data from n=3 technical replicates (mean + SD) within a single experiment, representative of 3 biologically independent experiments provided as Source Data. P-values calculated by two-tailed paired t-test (pRNAi Ctrl=0.14; pRNAi FTO=0.0043). b, Effect of iCRT3 vs. vehicle (DMSO) on the invasion capacity of RNAi Ctrl and RNAi FTO PC3 cells. Data from n=3 technical replicates (mean + SD) within a single experiment, representative of 3 biologically independent experiments. Independent repeats have been deposited on the Figshare repository (DOI: 10.6084/m9.figshare.14602953). c, Cell viability following iCRT3 treatment in RNAi Ctrl and RNAi FTO SKBR3 cells, measured by MTT assay. Data as mean + SD, n=5. P-values calculated by two-tailed paired t-test (pRNAi Ctrl=0.01; pRNAi FTO=0.0007). d, Levels of the mesenchymal EMT marker proteins FN1 and SLUG in RNAi Ctrl and RNAi FTO PC3 cells in the absence and presence of iCRT3 (representative of 3 independent replicates) with β-actin as loading control.
FTO-low tumors are sensitive to WNT inhibitor therapy
To confirm the above data in vivo, we injected athymic mice subcutaneously with either FTO-depleted or control SKBR3 cells and, when the tumors reached a measurable size, the mice received intraperitoneal (i.p.) injections of either iCRT3 or DMSO (Fig. 8a). During follow-up, iCRT3-treated control tumors showed only slightly reduced growth as compared to their mock-treated counterparts (Fig. 8b, left panel). FTO-depleted tumors, in remarkable contrast, showed drastically stalled growth in response to treatment (Fig. 8b, right panel). FTO depletion thus strongly potentiated the growth-inhibiting effect of iCRT3 on xenograft tumors. When 18F-FDG PET was used to assess metabolic activity in these tumors, we observed no significant difference between iCRT3-treated and mock-treated control tumors (Fig. 8c, upper left panel), but a significant difference between treated and mock-treated FTO-depleted tumors (lower left panel). Most strikingly, when we measured 18F-FDG uptake by locoregional subiliac lymph nodes (Fig. 8c, right panel) in the different groups of mice, we observed in the mice bearing FTO-depleted tumors a significant decrease (to below the detection limit) in response to iCRT3 treatment (lower right panel), as opposed to no effect in control-tumor-bearing mice (upper right panel). In keeping with the above-established link between FTO and EMT, iCRT3 treatment reduced the level of EMT marker FN1 in FTO-depleted but not control tumors (Extended Data Fig. 7e). To generalize these findings, we treated mice bearing tumors grown from RNAi Ctrl or RNAi FTO PC3 cells with iCRT3. We again observed enhanced sensitivity of FTO-depleted tumors, but not control tumors, to Wnt inhibition (Fig. 8d). Together, these data demonstrate that FTO downregulation makes epithelial cancer cells more vulnerable to Wnt inhibition. This vulnerability is lost with Wnt antagonists targeting the Wnt pathway above the level of FTO, as evidenced by the treatment of SKBR3 xenografts with LGK974 (Extended Data Fig. 7f). Thus, FTO downregulation sensitizes specifically to the iCRT3 mode of action. This is further supported by our observation that treatment of SKBR3 xenografts with the HER2 inhibitor Trastuzumab is equally effective in RNAi Ctrl and RNAi FTO tumors (red line at same height in left and right plot, Extended Data Fig. 7g).
Figure 8. FTO-low tumors are sensitive to WNT inhibitor therapy.
a, Schematic representation of breast cancer xenograft mouse model treated with iCRT3 or DMSO. b, Knockdown of FTO sensitizes breast tumors to Wnt inhibition. Nude mice growing tumors were treated as indicated in panel (a) (n=5 tumors per group, mean ± SEM, pictures of representative tumors are shown for each experimental group, two-way ANOVA, p(RNAi Ctrl) = 0.0116, p(RNAi FTO) < 0.0001). c, Tumor metabolism imaging with 18F-FDG PET/CT, applied to mice bearing RNAi Ctrl or RNAi FTO and treated with or without iCRT3. Representative images are shown for each group (pathological uptake by tumors encircled; physiological uptake in brain (B), kidneys (K), brown fat (F), and muscle (M)). Dotted line represents lower point of imaging scale (detection limit). No tumor is encircled in mouse with iCRT3-treated RNAi FTO tumor, as metabolic activity was below detection limit. Metabolic activity in tumors (middle) and lymph nodes (right) is quantified on the basis of 18F-FDG uptake into SUVmax. The box defines the IQR split by the median, with whiskers extending to the most extreme values within 1.5×IQR beyond the box. The number of samples (n) is indicated for each group. P-values calculated by two-tailed t-test, as indicated. d, Nude mice growing tumors from RNAi Ctrl (left) or RNAi FTO (right) PC3 cells were treated with or without iCRT3 (treatment as indicated in panel (a) for SKBR3 tumors, n=5 tumors per group, mean ± SEM, pictures of representative tumors are shown for each experimental group, two-way ANOVA, p(RNAi Ctrl) > 0.05, p(RNAi FTO) < 0.0001).
Discussion
The present study identifies the m6A demethylase FTO as a previously unrecognized regulator of EMT and cancer progression. Furthermore, we provide proof-of-concept evidence that FTO-low tumors are likely to benefit from Wnt inhibitor treatment. Our focus here has been mainly on breast and prostate cancer, but our conclusions extend to other epithelial cancers as well.
Both promoter and suppressor roles have been attributed to FTO in breast cancer. In contrast to studies proposing an oncogenic role for FTO12,25,27,44, but in line with the observation of Wu et al., we report FTO downregulation in breast cancer. Discrepancy between studies may be due in part to limited cohort sizes, particularly in regards to normal breast controls, as cohorts of large sizes (i.e. TCGA, Oncomine, METABRIC) consistently display FTO downregulation, and its association with poor prognosis, in breast cancer. We further report that loss of FTO occurs in all main breast cancer subtypes, is widespread among epithelial cancers, and promotes Wnt-mediated EMT and tumor progression. Our work not only provides overwhelming evidence for the tumor suppressor function of FTO in epithelial cancers, it also highlights the need to better dissect the dual role of FTO in various cancers.
The control of EMT by FTO evidenced here could provide an explanation for the apparent complex roles observed for FTO in tumors. Rather than being a binary process, emerging evidence indicate that EMT occurs through a spectrum of intermediate states and is often only partially reactivated in cancers45,46. We propose that dynamic fluctuations of FTO levels, according to different tumor types and different stages of progression, may contribute to the context-dependent plastic nature and pleiotropic functions of EMT from cancer initiation to metastasis. Differences in m6A mRNA methylation between tissue types and the versatility of m6A functions further contribute to this complexity, as m6A seems to control EMT in a tissue- and function-specific manner. In cervical cancer, METTL3-mediated m6A methylation at the CDS of Snail transcripts has been shown to trigger protein translation47. In breast cancer, we observed increased m6A methylation of the Wnt pathway, a consequence of FTO downregulation, as an EMT trigger. This appears to occur through 3’-end processing, a mechanism previously reported for FTO41. Changes in 3’UTR length due to alternative poly(A) site usage affect the binding of microRNAs and RNA-binding proteins, thus affecting transcript stability. This mechanism applies to some Wnt transcripts, and it is likely that the other Wnt-related FTO targets are regulated by a different m6A/FTO-mediated mechanism. Furthermore, we have identified additional signaling pathways as FTO targets in breast cancer (e.g. p53, TGF-β), and future studies could provide insights into their regulation by m6A and their potential cross-talk with Wnt signaling in the context of FTO depletion. Collectively, our present work thus reveals that while control of the EMT may be a conserved function of m6A in epithelial tumors, it can occur via different mechanisms in different tissues and cancers.
An emerging and intriguing feature of FTO is its association with a variety of distinct signaling pathways. While we have found FTO to negatively control EMT and tumor progression through regulation of transcripts related to Wnt signalling in breast and prostate cancer, in AML, FTO is thought to regulate stem cell differentiation via the ASB2/RARA axis.15 In melanoma, FTO influences the response to immunotherapy through regulation of cell-intrinsic factors, including PD-1, CXCR4, and SOX10.21 Together this shows that m6A and FTO control a wide variety of cellular processes and this may further explain the intricate dual nature of FTO in cancers, as some pathways may be more dominant in certain tissues, and thus dysregulation of FTO may lead to different phenotypes. This may be further complicated by the dynamics of the various m6A enzymes and readers and their influence on each other as well as the tissue-specific regulation of FTO activity by post-translational modifications, interaction with other proteins, or the presence/absence of cofactors, as shown for epigenetic enzymes with similar functions48–51. Lastly, the balance between the tumor-promoting and tumor-suppressing functions of FTO might be influenced by other levels of regulation, such as mutations and changes in key transcription factors, which are highly dependent on the tissue type and even on the subtype of cancer. In breast and prostate cancers, we have found loss of FTO to support an EMT program, but in tumors wherein the EMT is activated through other means (e.g. mutation in the EMT regulators), loss of FTO might not favor tumor progression in this way52. In conclusion, several molecular mechanisms could shape the activity and distribution of the FTO demethylase and explain discrepancies regarding its functions observed in various cancers.
As previously mentioned, the distribution of m6A is known to be highly tissue-specific.4,53 Accordingly, one would expect FTO also to target different transcripts in different cancer types and subtypes. Indeed, upon mapping m6A in SKBR3 breast cells and PC3 prostate cells, we found only a partial overlap at transcript level (see Supplementary Tables 2 and 4). But despite this target specificity, FTO appears able to maintain certain functions across tissues. In our study, m6A-seq revealed Wnt signaling as a top dysregulated pathway in both breast and prostate cancers, although the targeted transcripts were partly different. In both models, multiple levels of the Wnt signaling cascade were found to be affected by FTO depletion, and increased levels of β-catenin and EMT markers were observed, a finding that was further validated in additional cell lines. Thus, what appears to be conserved is not the identity of affected Wnt-related transcripts but the end point of FTO depletion: EMT activation through β-catenin stabilization. This is not surprising, as it is well documented that the stability of β-catenin is regulated at multiple levels through both positive and negative feedback regulators.54 Hence, what determines β-catenin stabilization is a combination of effects on FTO targets along this cascade. Importantly, since FTO depletion can either increase or decrease transcript stability41, the functional impact of FTO on a given transcript cannot be inferred on the sole basis of the presence of an m6A modification. Thus, FTO depletion can be expected to cause Wnt activation by targeting transcripts of both positive and negative feedback regulators along the Wnt pathway. It can promote EMT in different cancer cells by stabilizing β-catenin via different functional impacts on different Wnt-related transcripts. This concept is further supported by our observations with Wnt inhibition: sensitivity to iCRT3, which targets the Wnt signaling cascade below the level of FTO (i.e. the TCF/β-catenin complex), is maintained in both breast and prostate cancer cells. This suggets that the effect of the inhibitor depends on the overall effect of FTO depletion and not on a specific set of Wnt transcripts targeted by FTO. In keeping with this reasoning, LGK974, which targets the Wnt pathway above the level of FTO, had no effect in xenografts. Thus, the control of Wnt signaling and EMT by FTO are conserved features in epithelial cancers.
Our study extends far beyond the description of FTO as a tumor suppressor, as the present findings have important clinical implications. On the one hand, the above considerations regarding the dual role of FTO in cancer should be reckoned with when attempting to develop therapeutic strategies directly targeting the deposition/erasure of the m6A mark. The oncogenic properties of FTO in various cancers have prompted the development of different, more potent inhibitors for the pharmacological targeting of FTO in clinical settings. The results of two recent preclinical studies in AML provide proof-of-concept that targeting FTO may be a valuable anticancer strategy18,19. Yet our results warrant caution in this regard, as we have found pharmacological inhibition of FTO activity in human PDXs to increase the growth of breast tumors. Therefore, this potential therapeutic approach deserves careful consideration, as distinct cancer types and even subtypes are likely to respond differently.
On the other hand, our findings may raise hopes for additional therapeutic strategies. Firstly, despite encouraging evidence from preclinical studies, Wnt signaling inhibitors have not yet progressed beyond phase II due to limited success.55 However, we demonstrate in our study clear, strong tumor-suppressive effects of Wnt inhibition in an FTO-knockdown xenograft model. This key result suggests that the patients most likely to benefit from Wnt inhibitor treatment, and hence those on whom future trials should focus, are those with FTO-low tumors. A better stratification of patients, combined with the ongoing development of improved inhibitors56, may reveal greater sensitivity to Wnt inhibition for patients. Secondly, from our findings an alternative clinical implication emerges for the recently developed FTO inhibitors18,19. Rather than aiming for single-agent therapy, one might consider combining FTO inhibition with Wnt blockade as a potentially synergistic treatment, regardless of the initial FTO status. Opening this avenue could significantly broaden the reach of this application and this exciting possibility warrants further study.
In conclusion, our work reveals an unexpected mechanistic link between an epigenetic mark on mRNA and key cancer processes. It places the m6A demethylase FTO in a different and surprising light, as a multi-level regulator of Wnt signaling and EMT. The present study tallies with a recent report showing a role for METTL3 in the control of EMT47. Our work further indicates that FTO-low tumors, although they tend to be more aggressive in many cancer contexts, may carry their own nemesis: sensitivity to Wnt inhibitors. The FTO level thus emerges as a potential stratifier for Wnt inhibitor treatment and FTO inhibition in combination with Wnt blockade as a potential synergistic, anticancer treatment. Hence, our discovery of a previously unrecognized regulatory mechanism linking FTO to Wnt signaling and EMT offers encouraging prospects for cancer research and therapy.
Methods
Human biopsies.
Frozen diagnostic samples of breast cancer patients admitted to Saint-Louis Hospital (Paris, France) between 2009 and 2013 were obtained from the biological resource center at Saint-Louis Hospital (agreement n° DC 2009–929), following the Ethics and Legal National French rules for patient information and written consent (ANAES, HAS and INCa).
Human prostate cancer samples were obtained from residual material of a cohort established for a previous study57. Samples came from the local tumor tissue bank, C2RC (Lille, France), after approval by the internal review board (CSTMT-042, 27/07/2009). All patients were informed and written consent was obtained by the referring physician.
Global m6A quantification.
For quantification of m6A in breast and prostate cancers, sample preparation and mass spectrometry analysis was performed as previously described.58 Nucleosides were analyzed by LC-ESI-MS/MS using a Shimadzu LC-20AD HPLC system and an AB 3200 QTRAP mass spectrometer (Applied Biosystems) with an electrospray ionization source (Turbo Ionspray). Target nucleosides were monitored in multiple reaction monitoring (MRM) mode, with the following precursor ion → product ion mass transitions: rA (268.1 → 136.1) and m6A (282.1 → 150.1). Within each cohort, FTO levels were measured by RT-qPCR and samples were divided into two groups (“high” and “low” expression). The cutoff was determined by the best-performing threshold in terms of the p-value between both groups (regardless of the direction of the change in m6A), as determined by t-test.
For quantification of m6A in matched breast biopsies, nucleoside digestion was performed as previously described59. Separation was accomplished by reversed phase chromatography with an Acquity UPLC HSS T3 (Waters) on a VanquishTM Flex Quaternary UHPLC system (Thermo Fisher Scientific). Mass spectrometry was performed on a QuantivaTM triple quadrupole mass spectrometer interfaced with an H-ESI electrospray source (Thermo Fisher Scientific). Data were analyzed with Tracefinder 4.1 (Thermo Fisher Scientific) and Qual browser of Xcalibur 3.0. The mass transitions (precursor → product) for m6A were 282 → 94, 282 → 123, and 282 → 150.
Cell culture and treatments.
All cells were cultured at 37°C under 5% CO2. SK-BR-3 (abbreviated as SKBR3), and MCF7 cells were cultured in DMEM (Gibco), PC-3c (abbreviated as PC3) cells in MEM (Gibco), Ca Ski and H1650 cells were cultured in RPMI (Gibco). All culture media were supplemented with 10% FBS (fetal bovine serum, Gibco) and 1% penicillin and streptomycin (Gibco).
Stable knockdown was done with the pRetroSuper retroviral system (OligoEngine) for FTO (#1: GTGAAAGGGTCTAATATAA, #2: GACAAAGCCTAACCTACTT) or the scramble control (#1: GATCATGTAGATACGCTCA, #2: GAGACCATCACAACCTATT). Replicates of all phenotypic assays were performed with cells generated by two or more independent rounds of infection. All experiments were performed with RNAi FTO and Ctrl #1, except where RNAi FTO and Ctrl #2 are indicated.
Overexpression of FTO in SKBR3 cells was achieved with the T-Rex™ systems (Invitrogen). The coding sequence of wild-type FTO or of its catalytically inactive mutant (H231A/D233A)6 was cloned in the pcDNA™4/TO vector. Then cells were co-transfected with plasmids pcDNA™6/TR and pcDNA™4/TO. Dual selection was performed with 5μg/ml blasticidin (Thermo Fisher Scientific) and 250μg/ml zeocin (Invivogen). To induce FTO expression, cells were incubated with 2μg/ml tetracyclin (Life Technologies) for 24hrs.
In rescue experiments, RNAi Ctrl and RNAi FTO cells were transiently transfected with empty vector (pcDNA3.1+/N-HA) or vector carrying the sequence coding for wildtype or mutant (H231A/D233A) FTO 24hrs before the migration assay.
Clonogenicity assays.
2×104 (SKBR3, MCF7, Ca Ski, H1650) or 1×104 (PC3) cells were seeded in triplicate on pHEMA-coated (Gibco) 6-well plates with 2ml tumorsphere medium [serum-free DMEM/F12 (Gibco) supplemented with B27 (Gibco) and 20ng/ml EGF (Life Technologies)] and grown for 5–7 days. Spheres were then counted in four fields per well and the results averaged. To test for Wnt inhibition, SKBR3 cells were pre-treated with either 37.5μM iCRT3 or vehicle (DMSO) for 24hrs prior to tumorsphere formation.
For colony-formation assays, 5×103 cells were seeded in triplicate in a 6-well plate and grown for 5–10 days in complete medium. Fixation was performed with 10% formaldehyde for 10min. Cells were then washed twice with water and stained with 0.01% (w/v) crystal violet (Sigma-Aldrich) in water for 10min. After 4 water washes, plates were dried upside down. Colonies corresponding to at least 50 cells were counted in each well.
Representative examples are shown for tumorsphere and colony-formation assays. Data are presented as mean ± SD of technical replicates.
Migration and invasion assays.
Migration and invasion assays were performed with the Real-Time Cell Analyzer (RTCA) Dual Plate (DP) system (xCELLigence, ACEA Biosciences) and measured by the cell index. For migration assays, 4×104 cells were seeded into serum-free medium in the upper chamber of the CIM plates. Quantification was performed by measuring the slope of the curve in the linear phase of the migration. For invasion assays, the upper chambers were coated first with 20 μl Matrigel (BD Biosciences) diluted 1:40 in serum-free medium for 4hrs at 37°C. Then 6×104 (SKBR3) or (PC3) cells were seeded. The upper chambers were then assembled with the lower chambers containing medium supplemented with 20% FBS as an attractant, and the system was monitored for 1–2 days. For Wnt inhibition, serum-free medium and Matrigel were supplemented with 37.5 μM iCRT3 (Sigma-Aldrich) or an equal volume of DMSO. For TGFβ treatments, cells were serum-deprived (0.1% FBS) for 24hrs prior to the experiment and the medium of the lower chamber was either supplemented with 10ng/ml TGFβ (R&D Systems) or mock-treated.
For scratch assays, cells grown into monolayers were incubated with 1μg/ml mitomycin C (Sigma-Aldrich) for 2hrs. Then a wound was generated by scratching the monolayer with a 200μ-pipet tip. Cells were washed once with PBS and fresh culture medium was added.
Representative examples of at least three independent replicates of migration and invasion assays are shown.
Proliferation and viability assays.
Proliferation was measured with the Real-Time Cell Analyzer (RTCA) Dual Plate (DP) system (xCELLigence, ACEA Biosciences) and measured by means of the cell index. 104 SKBR3 cells were seeded into RTCA E-Plates and grown for two days in complete medium.
The MTT Assay Kit (Cell Proliferation) (Abcam) was used according to the manufacturer’s instructions to measure SKBR3 cell viability following iCRT3 treatment (24hrs at 37.5μM).
Mouse xenografts.
Female nude (nu/nu) mice of 5–6 weeks of age were inoculated subcutaneously with 10×106 cells per flank in 200μl of 50% Matrigel (Trevigen). For Wnt inhibition, mice were randomized when the tumors reached 200mm3 and injected intraperitoneally daily with vehicle or 50 mg/kg iCRT3 for 5 consecutive days, or given LGK974 (3 mg/kg) by oral gavage for 14 days. Trastuzumab was injected intraperitoneally (4 mg/kg, once weekly) for 6 weeks. For metastasis assays, cells were resuspended in PBS at 10×106 cells/ml and 0.1 ml was injected into tail veins of nude mice. The housing temperature for mice in was between 20–26°C, with 40–60% humidity and 14h-10h light-dark cycles. The experiments were performed in accordance with the European Union Guidelines and validated by the local Animal Ethics Evaluation Committee (CEBEA protocol No: 699N). Values are presented as mean ± SEM.
Small-animal PET-CT imaging and analysis.
Studies were conducted with a μPET-CT scanner (nanoScan®PET/CT, Mediso) on mice injected either subcutaneously or into a tail vein. In subcutaneous xenograft experiments, mice of the control group were scanned twice, once at baseline (4 weeks after inoculation) and once at 9 weeks. Mice of the iCRT3 treatment group were scanned only at the latter time point. In tail vein experiments, mice were scanned 8 or 4 weeks after inoculation. 18F-FDG (4.40 ± 0.32 MBq) was injected into a lateral tail vein and images were acquired 1hr after injection.
PET/CT image analysis and quantification were performed with PMOD software (PMOD Technologies). Three-dimensional spherical volumes of interest (VOI) were drawn on the PET images of tumor sites or of reference organs selected as probable metastatic sites (i.e. lung and liver). A threshold of 40% of the VOI maximum uptake value was applied to assess tracer uptake. Uptake values were normalized to both injected radioactivity and body weight (SUV). The SUV used for the results was the maximum value of the VOI (SUVmax). All acquisitions, measurements, and analyses were conducted under blinding.
Patient-derived xenografts (PDXs).
Experiments on patient-derived xenograft models (PDXs) were performed by Trace, the KU Leuven PDX Platform (Belgium). The PDX model was obtained with informed written patient consent and approval of the UZ Leuven Medical Ethical Committee (S54185). The model was derived from a breast tumor that was positive for the estrogen receptor, negative for the progesterone receptor, and low for the HER2/neu receptor. Experiments were carried out in accordance with the principles of the Declaration of Helsinki. PDX experiments had prior approval from the Animal Care and Ethics Committee (project application P038/2015).
Each fresh tumor was rinsed in PBS (Life Technologies) supplemented with penicillin/streptomycin (10000 IU/ml; 1000μg/ml, Life Technologies) and amphotericin B (Life Technologies, final conc. 1μg/ml). Visible blood clots were removed. The tumor was then cut into small pieces of approximately 3×3×3mm3, and two groups of three pieces each were transferred to two sterile cryotubes containing 500μl Matrigel (BD Biosciences). FTO inhibitor meclofenamic acid (MA, Sigma-Aldrich; 0.25 mg/kg)30 or H2O was then added to the Matrigel in the cryovials. The cryotubes were kept on ice for 3hrs prior to subcutaneous implantation into the interscapular fat pad of anesthetized female NMRI nude mice of minimum 6 weeks of age (Taconic) through a small incision in the skin of the back. Tumors were treated both before and after implantation, because post-implantation MA treatment alone did not yield any difference in tumor size (data not shown). The incision was closed with a sterile suture clip and analgesia (buprenorphine) was delivered post-operatively. Mice xenografted with MA-pretreated tumors also received MA throughout engraftment (0.25 mg/kg in water, intratumoral injection, weekly for 4 weeks); the others were treated with water. Body weight and tumor volume were measured every other day. The mice were sacrificed when the tumor reached 2000mm3 or if showing clear signs of distress. Xenografts were harvested, measured, and processed for further analyses (fresh-frozen and formalin-fixed). Values are presented as means ± SEM.
Association between FTO expression and phenotypic/clinical features in cancer.
Associations between the FTO mRNA level and either distant-metastasis-free survival (DMFS), relapse-free survival (RFS) or overall survival (OS) were evaluated with the Cox proportional hazards regression model, using the Kaplan Meier plotter website (http://kmplot.com/analysis)60 for breast (cohort referred to as the “KM cohort”), lung, and uterine cancers. METABRIC data and TCGA data were also used for breast and prostate cancer, respectively. Statistical significance was assessed by logrank test. In the cohorts from the Kaplan Meier website and METABRIC, “FTO-high” and “FTO-low” groups were generated on the basis of a trichotomized cutoff. For TCGA prostate cancers, samples with the 40% highest and 40% lowest FTO expression were classified as “FTO-high” and “FTO-low” groups, respectively. For breast cancer, cohorts analyzed as an aggregate through the Kaplan Meier plotter website were also tested individually to evaluate the variation between cohorts. Given the smaller size of the individual cohorts, the expression cutoff between “FTO-high” and “FTO-low” groups was determined by the best-performing threshold in terms of the p-value (determined by logrank test) between the two groups in each cohort, regardless of the direction of the hazard ratio.
Associations in breast cancer between the FTO mRNA level and clinical features (tumor grade or LN invasion) were evaluated in the METABRIC cohort61. Node invasion information was binarized into two categories: “no LN invasion”(neg) and “at least one node invaded”(pos). As the Shapiro-Wilk test indicated that the values were not normally distributed, non-parametric tests were used, i.e. the Kruskal-Wallis test for tumor grade and the Wilcoxon test for node positivity.
The association between the FTO transcript level and tumor progression in prostate cancer was evaluated in the dataset GSE332562. One-way ANOVA without equality of variance and the Kruskal-Wallis test were used to measure statistical significance.
For the association between the FTO transcript level and the EMT signature, “TCGA-BRCA” and “TCGA-PRAD” RNAseq data (as FPKM values) were used. A metagene for EMT was generated on the basis of the signature from Rokavec et al.63. For each gene of the EMT signature, we centered the mean at 0 and scaled individual values by the gene standard deviation in order to normalize the variance of all genes. The expression value of each gene for the “Epithelial state” was multiplied by −1, with “Mesenchymal state” genes unchanged. For each sample, the metagene value of EMT was computed as the mean of all scaled gene expression values in Rokavec’s signature. The samples were then stratified between “EMT-low” (samples showing the 20% lowest values for the EMT metagene) and “EMT-high” (20% highest) and t-test was used to evaluate the significance of changes in FTO expression.
RNA extraction and RT-qPCR.
Total RNA was extracted with the RNeasy Kit (Qiagen). For grafted tissue, frozen samples were first homogenized using the FastPrep-24 homogenizer system, with lysing matrix D (MP Biomedicals) in RLT buffer supplemented with β-mercaptoethanol. Removal of residual DNA was performed with the RNase-Free DNase Set (Qiagen). One microgram of total RNA was reverse-transcribed with the First Strand cDNA Synthesis Kit (Roche). Real-time PCR was performed with the LightCycler 480 Probes Master Kit (Roche).
Primer sequences are listed in Supplementary Table 5. Gene expression was normalized by the 2-ΔΔCt method, with Actin and SDHA as reference genes. Values are presented as mean ± SD of n≥3 independent replicates as indicated.
For Wnt inhibition, cells were treated with 37.5 μM iCRT3 for 12hrs before cell collection. For EMT induction, cells were treated with 10ng/ml TGFβ for 6hrs before cell collection. In mRNA stability assays, cells were treated with 5μg/ml actinomycin D (Sigma-Aldrich) for 0, 2, 4 or 6hrs before cell collection. GAPDH, a highly stable transcript, was chosen as reference gene for all stability assays. After RT-qPCR, expression values (relative to t=0) were fitted to an exponential decay model, using R.
Paired-end RNA sequencing.
Library preparation was performed from total RNA with the TruSeq Stranded Total RNA Library Prep Gold (Illumina) and TruSeq RNA Single Indexes (Illumina) according to the manufacturer’s instructions. Reads were filtered against low complexity and adapter content using AfterQC (default parameters)64 and Trimmomatic (ILLUMINACLIP:2:30:10, LEADING:3, TRAILING:3)65 and mapped using STAR (hg19 genome, ensembl transcriptome version 85)66. Differentially expressed genes were identified using a DeSeq2 paired analysis after read counting with HTSeq count tool.67,68 For Gene Set Enrichment Analysis (GSEA)69, the “signed” significance (i.e. -log10 of the differential p-value, multiplied by −1 for genes downregulated in RNAi FTO) was used to rank each genes. This ranked list was submitted to GSEA to identify enriched HALLMARK gene sets from MSigDB (http://software.broadinstitute.org/gsea/msigdb/). For alternative polyA site usage, mapped reads were submitted to the ROAR algorithm, via the ‘roarWrapper_chrBychr.R’ script (https://github.com/vodkatad/roar/blob/master/inst/examples/) using the human polyA sites from polyADB_v270.
Western blotting.
For cultured cells, 50 μg of whole cell extracts were loaded and electrophoresed on 8% SDS–polyacrylamide gels, transferred onto a PVDF membrane (PerkinElmer), and subjected to western blot analysis. Antibodies were diluted in 5% (w/v) nonfat dry milk in PBS containing 0.1% Tween 20 (Supplementary Table 5). Western blots were visualized with the ECL Plus system (Amersham Biosciences).
For nuclear and cytoplasmic extraction, cells were lysed in Cytoplasm Lysis buffer (CLB) (50mM Tris-HCl pH 7.4., 10mM NaCl, 0.5% NP-40, 0.25% Triton X-100, supplemented with cOmplete™ Mini Protease Inhibitor Cocktail) for 15min on ice. After centrifuging for 10min at 3500g, the supernatant was kept as cytoplasmic extract. Pellets were washed 5 times in CLB without NP-40 (centrifuging for 5min at 3500g in-between), then diluted in 2X Laemmli buffer and used as nuclear extracts.
For grafted tissues, frozen samples were first homogenized with the FastPrep-24 homogenizer system with M-PER (Thermo Fischer Scientific) lysis buffer (0.1g/ml) supplemented with Protease Inhibitor Cocktail EDTA-free (Thermo Fischer Scientific) and Phosphatase Inhibitor Cocktail (Thermo Fischer Scientific). Western blots were visualized with the SuperSignal® West Pico Chemiluminescent Substrate (Pierce).
Full scans of all the blots are available as Source Data.
m6A sequencing.
The method for m6A sequencing (m6A-seq) was adapted from a protocol described previously.3 Total RNA was fragmented using a fragmentation buffer (final concentration: 10mM Tris-HCl and 10mM ZnCl2) and incubating at 94°C for 35s. Then, 1/10 volume of 0.5M EDTA was added and the tubes were placed on ice. RNA was then subjected to sodium acetate precipitation and resuspended in RNase-free water. Fragmentation size was assessed by 2100 Bioanalyzer (Agilent). Then, 2–5μg fragmented RNA was kept at −80°C to serve as input. The RNA was denatured by heating (70°C for 5min, then on ice) and the immunoprecipitation mix was prepared as follows: 250μg fragmented RNA from cultured cells or 100μg fragmented RNA from human BC biopsies in 755μl, 10μl (400 units) RNasin® Ribonuclease Inhibitors (Promega), 10μl of 200mM Ribonucleoside Vanadyl Complex (RVC) (NEB), 200μl IP buffer 5x (50mM Tris-HCl, 750mM NaCl, 0.5% (vol/vol) Igepal), 7.8μl anti-m6A antibody (Synaptic System 202003), and 17.2μl RNase-free water. The mix was incubated overnight at 4°C on a rotation wheel. The next day, 60μl G-protein dynabeads (Invitrogen 10004D) were washed twice with IP buffer 1x supplemented with antiproteases (Roche) and blocked by incubating the beads in wash buffer supplemented with 0.5mg/ml BSA for 1hr on a rotation wheel. The beads were washed again twice, then added to the immunoprecipitation mix and incubated for 2hrs at 4°C on a rotation wheel. The beads were washed three times with 1ml washing buffer supplemented with 10μl RNasin® and 10μl RVC, then elution was performed by TriPure (Roche) extraction and RNA was resuspended in 13μl RNase-free water. Reverse transcription of 11μl immunoprecipitated RNA was done with the SuperScript II Reverse Transcriptase Kit (Invitrogen) according to the manufacturer’s instructions. Then, second-strand synthesis was performed from cDNA with NEBNext mRNA Second Strand Synthesis Module (NEB), according to the manufacturer’s instructions. Finally, double-stranded DNA was purified by MinElute PCR purification (QIAGEN) and quantified by Qubit (Thermo Fisher Scientific). A sequence library was prepared with MicroPlex Library Preparation Kit v2 ×12 (Diagenode) for both input and IP samples, which were then sequenced with Illumina NextSeq500. For SKBR3 RNAi FTO and control cells, two replicates (from independent infections) were sequenced.
Bioinformatic analysis of m6A-seq data.
Reads were mapped using STAR66 (hg19 genome, RefSeq transcriptome downloaded in 2012. Peaks (m6A-enriched regions) and differential peaks were identified using the script available on github (https://github.com/martinBizet/m6A_FTO_cancer). Briefly, we used MACS2 to define peaks (using immunoprecipitated (IP) samples and their input counterpart as control) and “expected peaks” (using input as an IP and no control) (q-value < 0.05)71. The “expected genome size” MACS2 parameter was set as the sum of exons and introns lengths (counting once regions shared by several transcripts). To avoid extremely large m6A-enriched regions, the peaks were then resized to 100 bp on both sides of the summit. Then, both peaks and expected peaks were annotated (5’UTR, CDS, 3’UTR or intronic) by intersecting peak center positions with RefSeq annotations. Peaks remaining unannotated were defined as “intergenic”. Peaks associated with more than one region were categorized as “multiple”. m6A enrichment of each annotation region, was evaluated as the ratio between the percentages of m6A peaks and expected peaks in this region. For differential analyses between two conditions, each replicate experiment was analyzed separately by comparing enrichment ratios computed using the following procedure. Positions of the peaks from the two conditions were first merged (using bedtools merge)72 to obtain peaks identified in at least one condition. Bedgraph files, generated from the mapped reads, were used to measure the coverage at each merged peak position. To correct for mapping and transcription biases, the enrichment ratio was defined for each peak for each condition as the ratio of the peak TPM for IP over the maximum of the peak or gene TPM of input. Gene ontology was performed with Ingenuity Pathway Analysis (IPA®, Qiagen).
Motif analysis.
The strand of each m6A and expected peak was attributed by associating it to its RefSeq transcript using “bedtools intersect”72 (Intergenic peaks were ignored and peaks associated to both strands duplicated). Then sequences surrounding by 250 bp, on both sides of the center, the 2500 peaks with the highest MACS2 fold enrichment, were extracted, strandedly, using “bedtools getfasta”.72 The Meme-suite tools (http://meme-suite.org)73 were then used: “Ame” was used to evaluate the enrichment significance of the “DRACH” motif, using a first-order Markov model, generated using the “fasta-get-markov” on input sample sequences, as background model and expected peaks as negative control peaks. The scoring method was “avg” and the test method “ranksum”. “Meme-chip” (DREME and MEME) was used to confirm the presence of “DRACH”-like sequences among the top overrepresented motifs, using same background model and negative peaks as “Ame”. The number of DREME and MEME motifs were restricted to 10, and the MEME searching windows were set between 5 and 12.
TOP/FOP Flash reporter assay.
The vectors Super 8x TOP Flash (Plasmid #12456; a firefly luciferase reporter of β-catenin-mediated transcriptional activation) and Super 8x FOP Flash (Plasmid #12457; a negative mutant control) were purchased from Addgene74. SKBR3 cells were grown to 80% confluency in a 6-well plate, and then TransIT®-LT1 Transfection Reagent (Mirus Bio) was used to co-transfect them with 1.5μg TOP or FOP plasmid and 300ng of a vector coding for the Renilla luciferase (used as an internal control for transfection efficiency and other potential confounding sources of variation). After 2 days, the cells were subjected to mock treatment or 100ng/ml Wnt3a (Abcam) or iCRT3 (Sigma-Aldrich) at doses of 1.5, 7.5 or 37.5μM, for 24hrs. Then luciferase activity was measured with the Dual-Glo® Luciferase Assay System (Promega). Firefly luciferase was first normalized to the Renilla control signal, then the TOP/FOP ratio was calculated. Values are presented as mean ± SD of n≥3 independent replicates.
Immunochemistry.
IHC data on β-catenin levels in human biopsies were obtained previously as part of a series of centrally-reviewed breast cancer cases40, with matching microarray data deposited as GSE88770 on GEO for FTO gene expression75. The probe level intensities were background-adjusted by the Robust multi-array analysis (RMA) method followed by quantile normalization, so that the 2.5% and 97.5% quantiles equaled −1 and +1, respectively. The β-catenin IHC data are provided in Supplementary Table 6. The Mann-Whitney U test was used to evaluate the significance of changes in FTO expression between groups showing low and high β-catenin levels.
In xenograft and PDX experiments, formalin-fixed, paraffin-embedded tissue sections (4 μm) were immunohistochemically stained, with antibodies used are listed in Supplementary Table 5. IHC staining was performed with the Mouse on Mouse Polymer IHC Kit (Abcam) on a Ventana Benchmark XT automated staining instrument (Ventana Medical Systems) for mouse antibodies, and manually with the SignalStain® Boost IHC Detection Reagent (Cell Signaling Technology) for rabbit antibodies. Three regions were selected at random on different parts of the section (3 xenografts per condition) and analyzed with the QuPath program.
For immunofluorescence staining of β-catenin, cells were grown on coverslips were washed 3 times, covered with ice-cold acetone, and incubated for 10min at −20°C. After 3 washes with PBS, slides were incubated with blocking buffer (1% BSA in PBS) for 1hr at room temperature. After removal of PBS, the primary antibody was added to each slide and incubated overnight at 4°C. Slides were washed three times with PBS and incubated with the secondary antibody for 2hrs at room temperature, protected from light. After three additional washes, excess PBS was removed and coverslips were mounted on the slides with ProLong with DAPI (Life Technologies).
Statistics and reproducibility.
Data are presented as means ± SEM or means ± SD as indicated. When box plots are used to visualise data distributions centre line shows median, box limits are upper and lower quartiles, and whiskers represent 1.5x interquartile range. Two-tailed Student’s t-test or one-way ANOVA were used when normal distribution (evaluated through Shapiro-Wilk test) could be assumed; and Wilcoxon or Kruskal-Wallis were used otherwise. Effect size was estimated using Cohen’s d distance. For PDX, differences in tumor volume among groups of mice were tested using a mixed effects model, as implemented in GraphPad Prism, which uses a compound symmetry covariance matrix, and is fit using Restricted Maximum Likelihood (REML). All replicates for in vitro data are derived from independent experiments (with multiple RNAi infections), except for clonogenicity assays for which a representative result (with technical replicates) is shown (with independent experiments presented in the Source Data). Graphs for Figs 2a–b, 3c, 3e, 7b and Extended Data Figs 2a–b, 2d–e, 3c, 3f, 4d were generated by xCELLigence and downloaded directly from the machine, so numerical source data are not available. All independent repeats that support the findings of these experiments are available on the Figshare repository (DOI: 10.6084/m9.figshare.14602932, 10.6084/m9.figshare.14602944, 10.6084/m9.figshare.14602953, 10.6084/m9.figshare.14602965, 10.6084/m9.figshare.14602971 and 10.6084/m9.figshare.14602986). All replicates for in vivo mouse data and human biopsies are from different tumors. No statistical method was used to predetermine sample size. No data were excluded from the analyses. Experiments using cultured cells and mice were randomized. Blinding was exclusively used for PET imaging analyses.
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability.
Sequencing data that support the findings of this study have been deposited in the Gene Expression Omnibus (GEO) repository under accession number GSE128582 (http://ncbi.nlm.nih.gov/geo). Human cancer data (bladder urothelial carcinoma, breast invasive carcinoma, cervical squamous cell carcinoma and endocervical adenocarcinoma, glioblastoma multiforme, kidney chromophobe, lung adenocarcinoma and lung squamous cell carcinoma, prostate adenocarcinoma, thyroid carcinoma, and uterine corpus endometrial carcinoma) were derived from the TCGA Research Network: http://cancergenome.nih.gov/. RNA-seq data from TCGA were obtained as normalised FPKM counts from GDC (https://portal.gdc.cancer.gov/repository) and PAM50 subtype information was extracted from UCSC Xena (https://xenabrowser.net). METABRIC expression and clinical data61 were downloaded through the European Genome-Phenome Archive (EGA) (http://www.ebi.ac.uk/ega/,accessionnumberEGAS00000000083 ). Previously published microarray data that were re-analysed here are available under accession code GSE9195, GSE10780, GSE10810, GSE12276, GSE19615, GSE20711, and GSE21653. Reannotation and processing of Affymetrix Human Genome U133 Plus 2.0 array data was performed as previously detailed76.
The hg19 reference transcriptome (version 85) was obtained from the ensembl portal (ensembl.org). Hallmark gene sets were obtained from MsigDB database (https://www.gsea-msigdb.org/gsea/msigdb). Human polyadenylation sites were obtained from PolyA_DB2 (http://polya.umdnj.edu/PolyA_DB2).
The data underlying Figs 1b, 1d–h, 2c–e, 3a–b, 3d, 3g, 4c, 4e, 5e, 5g, 5j–k, 6e, 7a, 7c, 8b–d and Extended Data Figs 1a–c, 1e–g, 2c–d, 2g, 3a–b, 3d–e, 4c, 4e, 4g–h, 5h, 5n, 6a–b, 7a–c, 7e–f are provided as Source Data files. For motility assays related to Figs 2a–b, 3c, 3e, 7b and Extended Data Figs 2a–b, 2d–e, 3c, 3f, 4d, independent repeats have been deposited on the Figshare repository, under the following DOI: 10.6084/m9.figshare.14602932, 10.6084/m9.figshare.14602944, 10.6084/m9.figshare.14602953, 10.6084/m9.figshare.14602965, 10.6084/m9.figshare.14602971 and 10.6084/m9.figshare.14602986). All other data supporting the findings of this study are available from the corresponding author on reasonable request.
Code availability.
Code supporting this study is available at a dedicated Github repository [https://github.com/martinBizet/m6A_FTO_cancer].
Extended Data
Extended Data Fig. 1. FTO downregulation promotes tumorigenesis in breast cancer.
a, FTO expression in the Affymetrix data meta-analysis cohort (left, 171 normal vs. 812 tumor breast tissues, p=5e-50). RT-qPCR quantification of FTO in an in-house cohort (right, 9 normal vs. 47 tumor breast tissues, p=0.005), normalized to ACTB and SDHA. The box defines the IQR split by the median, with whiskers extending to the most extreme values within 1.5×IQR beyond the box. P values calculated by two-tailed t-test. b, Quantification of m6A levels by mass spectrometry in paired human normal and tumor breast samples (n=6 per group, pairs connected with line, red dots represent the group medians, two-tailed paired t-test, p=0.046). c, Validation of FTO depletion by two shRNAs in SKBR3 cells by RT-qPCR (upper panel, mean + SD, pRNAi#1=6e-10; pRNAi#2=0.0045, nRNAi#1=7; nRNAi#2=3), normalized to ACTB and SDHA) and western blotting (lower panel, representative of n=3 independent replicates with histone H3 as loading control). P values calculated by two-tailed paired t-test. d, Light microscopy imaging of mammospheres formed by RNAi Ctrl and RNAi FTO SKBR3 cells with scale bar of 100μm (representative of n=3 independent experiments). e, Effects of FTO knockdown on colony-forming capacity (left, p=0.001) and mammosphere-forming capacity (right, p=0.02) in SKBR3 cells with a second shRNA (RNAi FTO #2). Data from n=3 technical replicates (mean + SD) within a single experiment, representative of 3 biologically independent experiments provided as Source Data. P values calculated by two-tailed t-test. f, Representative western blot of doxycycline-induced overexpression of wild-type FTO (FTO-WT) and of a catalytically inactive FTO mutant (FTO-MUT) in SKBR3 cells (upper panel) with β-actin as loading control (n=3). Effect of FTO-WT and FTO-MUT overexpression on colony formation and mammosphere formation in SKBR3 cells (bottom). Data from n=3 technical replicates (mean + SD) within a single experiment, representative of 3 and 2 biologically independent experiments, respectively, provided as Source Data. P values calculated by two-tailed t-test, as indicated. g, FTO depletion by shRNA in breast cancer MCF7 cells (n=6) by RT-qPCR. Data as mean + SD, normalized to ACTB and SDHA. P values calculated by two-tailed paired t-test (p=4e-6).
Extended Data Fig. 2. Depletion of FTO promotes breast cancer cell motility and metastases.
a-b, Real-time chemotactic invasion (a) and migration (b) of RNAi FTO (red, shRNA #1 and #2, respectively) and RNAi control SKBR3 cells (green). c, Wound scratch assay performed with RNAi Ctrl and RNAi FTO SKBR3 cells. Representative pictures (left) with quantification of wound closure (as percentage of the width at t=0, right). Data from a single experiment, representative of n=2 biologically independent experiments provided as Source Data. d, Cell viability by MTT assay (left, mean + SD, n=5, two-tailed paired t-test, p=0.37) and real-time proliferation (right) of FTO-depleted and control SKBR3 cells. e, Western blotting of FTO depletion and rescue by overexpression of wild-type FTO (FTO-WT) or a catalytically inactive FTO mutant (FTO-MUT) in SKBR3 cells (upper panel, n=3 with β-actin as loading control) and real-time chemotactic migration (lower panel). f, Expression of FTO in PDX shown by immunochemistry. Representative image of tissue sections with scale bar of 250μm (n=3). g, Metabolism imaging with 18F-FDG PET of subiliac lymph nodes xenograft mice bearing RNAi Ctrl (black, n=4) or RNAi FTO (red, n=5) SKBR3 tumors. Representative images (9 weeks after inoculation) are shown (pathological uptake in LN encircled; physiological uptake in bladder (Bl)). The box defines the IQR split by the median, with whiskers extending to the most extreme values within 1.5×IQR beyond the box. P-value calculated by two-tailed t-test (p=0.0061). h, Kaplan-Meier based on FTO expression and disease-specific survival in the breast cancer METABRIC cohort (n(high FTO)=660, n(low FTO)=661, logrank test, p=7e-4). i, Forest plot showing association between FTO expression and distant-metastasis-free survival in breast cancer. Data shown as hazard ratio (log2), with 95% confidence interval, for both the aggregate KM Plot data set (“overall”, presented in Fig. 2f) and individual cohorts. The number of samples (n) and p values (calculated by two-sided logrank tests, in bold if p < 0.05) are indicated for each cohort.
Data for (a), (b), (d), (e) are from n=3 technical replicates (mean + SD) within a single experiment, representative of 3 biologically independent experiments (except for (e): 2 replicates). Independent repeats have been deposited on the Figshare repository (DOI: 10.6084/m9.figshare.14602965).
Extended Data Fig. 3. Extended Data Fig. 3. FTO depletion in prostate, cervical and lung cancers.
a, FTO depletion by shRNA in PC3 prostate cancer cells by RT-qPCR (left). Mean + SD from 3 independent experiments, normalized to ACTB and SDHA. P value calculated by two-tailed paired t-test (p=0.004). Representative western blot (right) of FTO depletion in PC3 cells (n=3) with β-actin as loading control. b, Nude mice were injected subcutaneously with PC3 prostate cells and tumors was monitored every two days (n=6 tumors per group; mean ± SEM, p=0.0002, two-way ANOVA). c, Real-time chemotactic invasion of RNAi FTO (red) and RNAi control PC3 cells (green). d, FTO depletion by shRNA in Ca Ski cervical cancer cells (left, n=3, p=0.009) and H1650 lung cancer cells (right, n=3, p=0.0004) by RT-qPCR. Data as mean + SD, normalized to ACTB and SDHA. P values calculated by two-tailed paired t-test. e, Effects of FTO knockdown on tumorsphere-forming capacity in Ca Ski (left, p=0.02) and H1650 cells (right, p=0.008). Data from n=3 technical replicates (mean + SD) within a single experiment, representative of 2 biologically independent experiments provided as Source Data. P values calculated by two-tailed t-test. f, Real-time chemotactic migration of FTO-depleted (red) and control (green) H1650 cells. g-h, Kaplan-Meier curves showing the overall survival of patients with either high-FTO or low-FTO lung tumors (n(high FTO)=333, n(low FTO)=326, logrank test, p=0.0073) (g) or uterine tumors (n(high FTO)=310, n(low FTO)=232, logrank test, p=0.048) (h).
Motility data in (c), (f) from n=3 technical replicates (mean + SD) within a single experiment, representative of 2 and 3 biologically independent experiments, respectively. Independent repeats have been deposited on the Figshare repository (DOI: 10.6084/m9.figshare.14602971).
Extended Data Fig. 4. FTO downregulation promotes EMT in several cancers.
a, Representative light microscopy imaging of RNAi Ctrl or RNAi FTO SKBR3 cells (n=3 independent experiments). Cells displaying elongated morphology and extended pseudopodia are indicated with arrows. Scale bars represent 50μm. b, Representative western blot of mesenchymal EMT markers in FTO-depleted (RNAi FTO) and control (RNAi Ctrl) SKBR3 cells (n=3) with HDAC1 as loading control. c, Immunochemistry staining of EMT markers in SKBR3 xenografted tumors (from Fig. 1e at endpoint). Percent VIM and FN1 positive cells (n = 3 fields × 3 tumors per group; mean + SEM). P-values calculated by two-tailed t-test (pVIM=0.0002; pFN1<0.0001). d, Real-time chemotactic migration of FTO-depleted and control SKBR3 cells in the absence and presence of TGFβ. Data from n=3 technical replicates (mean + SD) within a single experiment, representative of 3 biologically independent experiments. Independent repeats have been deposited on the Figshare repository (DOI: 10.6084/m9.figshare.14602986). e, Relative gene expression of mesenchymal (VIM, MMP2) and epithelial (FSTL3) EMT marker genes, as estimated by RT-qPCR in SKBR3 cells following RNAi of FTO, treated or not with TGFβ. Mean + SD from n=3 independent experiments, normalized to ACTB and SDHA. P values calculated by two-tailed paired t-test as indicated. f, Representative western blot of mesenchymal EMT markers FN1 and SLUG in FTO-depleted and control PC3 cells (n=3) with β-actin and histone H3 as loading controls. g, Relative gene expression of mesenchymal EMT marker genes, as estimated by RT-qPCR in MCF7 (left, n=4 except for nFN1=3) and H1650 (right, n=3 except for nSLUG=4) cells following RNAi of FTO. Mean + SD, normalized to ACTB and SDHA. P values calculated by two-tailed t-test. Representative western blot of mesenchymal EMT markers FN1, CDH1 and SLUG in FTO-depleted and control MCF7 (left) and H1650 (right) cells (n=3) with HSP90 and HDAC1 as loading controls. h, Immunochemistry staining of VIM in PC3 xenografted tumors (from Fig. 3c at endpoint). Percent VIM positive cells (n =3 fields × 3 tumors per group; mean + SEM). P-value calculated by two-tailed t-test (p=0.0008). Scale bars represent 250μm.
Extended Data Fig. 5.
FTO loss upregulates W_n_t_/_β-catenin signaling in breast and prostate cancers a, Numbers of m6A peaks and related transcripts identified by m6A-seq in RNAi Ctrl and RNAi FTO SKBR3 cells (upper panel, two independent biological replicates). Scatterplot displaying differentially m6A-methylated peaks (lower panel, hyper and hypo = peaks with increased and decreased m6A in FTO-depleted cells, respectively). b-d, Visualization of the IP m6A signal in RNAi FTO and RNAi Ctrl SKBR3 cells at the DVL3, FZD1, SOX11, MARK2, CSNK1G2 (b), CTNNB1 (c), and WNT5A (d) transcripts. e, Representative western blot of β-catenin in RNAi FTO #2 and RNAi Ctrl #2 SKBR3 cells with β-actin as loading control (n=3). f, Representative western blot of cytoplasmic and nuclear β-catenin in RNAi FTO and RNAi Ctrl SKBR3 cells (n=3). Loading controls: β-Tubulin (cytoplasmic) and histone H3 (nuclear). Wnt3a treatment as positive control for nuclear translocation of β-catenin. g, Representative immunofluorescence staining of β-catenin (red) and DAPI (blue) in RNAi Ctrl and RNAi FTO SKBR3 (n=3). Scale bars represent 20μm. h, Relative gene expression of Wnt/β-catenin targets measured by RT-qPCR in SKBR3 cells. Mean + SD from n=6 independent replicates (except for n(TCF1)=5), normalized to ACTB and SDHA. Two-tailed paired t-tests: pTCF1<0.0001; pAXIN2=0.0001; pNMYC=0.001. i, Western blot of total β-catenin in RNAi FTO vs. RNAi Ctrl from SKBR3 xenografted tumors in mice (n=4 per group) with β-actin as loading control. j, Numbers of m6A peaks and related transcripts identified in three human breast cancer biopsies by m6A-seq (upper left panel). The m6A motif (upper right) retrieved from sample BC1 includes the consensus motif DRACH. Bar graph displaying the distribution of m6A peaks relatively to transcriptomic regions (lower panel, representative of sample BC1). k, m6A IP signal at transcripts related to the Wnt/β-catenin signaling (CSNK1G2, MARK2, AXIN1 and FZD1), in three human breast cancer biopsies.
Extended Data Fig. 6. Decreased expression of Wnt-related FTO target gene CSNK1D.
a, Numbers of m6A peaks and related transcripts identified by m6A-seq in RNAi Ctrl and RNAi FTO PC3 cells (upper panel, two independent biological replicates). Schematic representation of m6A/FTO mRNA targets (red asterisks) identified within the Wnt/β-catenin signaling pathway (lower panel). b, Visualization of the IP m6A signal in RNAi FTO and RNAi Ctrl PC3 cells at the WNT3A and GSK3B transcripts. c, Representative immunochemistry staining of β-catenin in PC3 xenografted tumors with scale bar at 250μm. Percent β-catenin positive cells (right, n =3 fields × 3 tumors per group; mean + SD). Two-tailed t-test, p=0.005. d, Relative quantification of the CSNK1D levels in RNAi FTO and RNAi Ctrl SKBR3 cells displayed in the western blot shown in Fig. 6a. e, Western blot showing the level of CSNK1D in an independent biological replicate of RNAi FTO and RNAi Ctrl SKBR3 cells (left, with HDAC1 as loading control, related to Fig. 6a). Relative quantification of CSNK1D levels (right). Western blot data in (d-e) are representative of n=3 biologically independent experiments and normalized to HDAC1.
Extended Data Fig. 7. FTO-low tumors are sensitive to WNT inhibitor therapy.
a, Relative gene expression of Wnt/β-catenin target genes AXIN2 and TCF1, as estimated by RT-qPCR, in SKBR3 cells following RNAi of FTO, treated or not with iCRT3. Mean + SD from 3 independent experiments, normalized to ACTB and SDHA. P values calculated by two-tailed t-test, as indicated. b, Wnt/β-catenin transcriptional activity, as measured by TOPFlash β-catenin/TCF-LEF reporter assay performed on SKBR3 cells following RNAi of FTO, in the absence and presence of iCRT3 (mean + SD, n=5, iCRT3 doses as indicated). P values calculated by two-tailed t-test, as indicated. c, The invasion capacity, as shown in Fig. 7b, was quantified by the slope of the invasion curves and corrected for cell viability (as measured by MTT assay in Fig. 7c, from 2 biologically independent experiments). P-values calculated by two-tailed t-test (p(RNAi Ctrl)=0.58, p(RNAi FTO)=0.018). d, Levels of the mesenchymal EMT marker proteins FN1, VIM and SNAIL in RNAi Ctrl and RNAi FTO SKBR3 cells in the absence and presence of iCRT3 (n=3) with histone H3 and β-actin as loading controls. e, Levels of FN1 protein in FTO-depleted and control tumors derived from SKBR3 xenografts treated with iCRT3 or vehicle (DMSO), with β-actin as loading control (n=3 independent experiments). f, Nude mice growing tumors from RNAi Ctrl (left) or RNAi FTO (right) SKBR3 cells were treated with LGK974 (n=5 tumors per group, mean ± SEM). P values calculated by two-way ANOVA (p(RNAi Ctrl)=0,69; p(RNAi FTO)=0.48). g, Nude mice growing tumors from RNAi Ctrl (left) or RNAi FTO (right) PC3 cells were treated with or without Trastuzumab (n=5 tumors per group, mean ± SEM). P values calculated by two-way ANOVA, (p(RNAi Ctrl)<0.0001; p(RNAi FTO)<0.0001).
Supplementary Material
Supplementary Table 1. Paired-end RNA sequencing analyses. For DeSeq2-derived data, two-sided paired Wald test and Benjamini-Hochberg adjustment were used to provide the “pvalue” and “padj”, respectively. For GSEA-derived data, two-sided false discovery rate (FDR) was used to provide the adjusted “FDR q val”. For ROAR-derived data, two-sided paired Fisher test was used to provide p values.
Supplementary Table 2. Differential m6A enrichment analysis in SKBR3 breast cancer cells. For Ingenuity-derived data, right-tailed Fisher’s exact test was used.
Supplementary Table 3. m6A enrichment analysis in human breast cancer biopsies. For MACS2-derived peaks, Poisson test with Benjamini-Hochberg adjustment was used to provide q values.
Supplementary Table 4. Differential m6A enrichment analysis in PC3 prostate cancer cells. For Ingenuity-derived data, right-tailed Fisher’s exact test was used.
Supplementary Table 5. List of primers and antibodies.
Supplementary Table 6. Beta-catenin IHC staining in human breast cancer biopsies.
Acknowledgments
J.J. was supported by the Belgian “Fonds de la Recherche Scientifique” (FNRS) postdoctoral fellowship, E.Co. by the L’Oréal “For Women In Science” fellowship and by the Belgian FNRS, and J.Y. by the China Scholarship Council (CSC) postdoctoral fellowship. C.A.W., M.B., B.H., G.D. were supported by the Belgian FNRS. N.S. was supported by the ULB Foundation. F.F. is a ULB Professor. F.F.’s lab was funded by grants from the FNRS and Télévie, the “Action de Recherche Concertée” (ARC)(AUWB-2018–2023 ULB-No 7), Wallon Region grants (U-CAN-REST, INTREPID), a FNRS Welbio grant, the ULB Foundation and the Belgian Foundation Against Cancer (FCC 2016–086 FAF-F/2016/872). P.C. and A.C. are Senior Research Associate and Research Director at the FNRS, respectively. P.A.L. was supported by the National Institutes of Health (GM 058843). Trace is supported by the “Belgian Foundation Against Cancer” and as part of the EurOPDX infrastructure, by EDIReX a Horizon 2020 grant agreement #731105. PET/CT acquisition were performed at the nuMix-CMMI, which is supported by the European Regional Development Fund (ERDF) and the Walloon Region.
Footnotes
Competing Interests Statement
F.F. is a co-founder of Epics Therapeutics, Belgium. The other authors declare no competing interests.
References
- 1.Harcourt EM, Kietrys AM & Kool ET Chemical and structural effects of base modifications in messenger RNA. Nature 541, 339–346 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boccaletto P. et al. MODOMICS: A database of RNA modification pathways. 2017 update. Nucleic Acids Res. (2018) doi: 10.1093/nar/gkx1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dominissini D. et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485, 201–206 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Meyer KD et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 149, 1635–1646 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi H, Wei J. & He C. Where, When, and How: Context-Dependent Functions of RNA Methylation Writers, Readers, and Erasers. Molecular Cell (2019) doi: 10.1016/j.molcel.2019.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia G. et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol 7, 885–7 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng G. et al. ALKBH5 Is a Mammalian RNA Demethylase that Impacts RNA Metabolism and Mouse Fertility. Mol. Cell 49, 18–29 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng X, Su R, Stanford S. & Chen J. Critical Enzymatic Functions of FTO in Obesity and Cancer. Frontiers in Endocrinology (2018) doi: 10.3389/fendo.2018.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geula S. et al. Stem cells. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science (80-. ). 347, 1002–6 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Meyer KD et al. 5′ UTR m6A Promotes Cap-Independent Translation. Cell 163, 999–1010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou J. et al. Dynamic m6A mRNA methylation directs translational control of heat shock response. Nature 526, 591–594 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu N. et al. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature 518, 560–564 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yue Y, Liu J. & He C. RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes and Development vol. 29 1343–1355 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu Y, Dominissini D, Rechavi G. & He C. Gene expression regulation mediated through reversible m(6)A RNA methylation. Nat. Rev. Genet 15, 293–306 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Li Z. et al. FTO Plays an Oncogenic Role in Acute Myeloid Leukemia as a N6-Methyladenosine RNA Demethylase. Cancer Cell 31, 127–141 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su R. et al. R-2HG Exhibits Anti-tumor Activity by Targeting FTO/m6A/MYC/CEBPA Signaling. Cell 172, 90–105 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vu LP et al. The N 6 -methyladenosine (m 6 A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat. Med 23, 1369–1376 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Y. et al. Small-Molecule Targeting of Oncogenic FTO Demethylase in Acute Myeloid Leukemia. Cancer Cell (2019) doi: 10.1016/j.ccell.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su R. et al. Targeting FTO Suppresses Cancer Stem Cell Maintenance and Immune Evasion. Cancer Cell (2020) doi: 10.1016/j.ccell.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui Q. et al. m6A RNA Methylation Regulates the Self-Renewal and Tumorigenesis of Glioblastoma Stem Cells. Cell Rep. 18, 2622–2634 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang S. et al. m6A mRNA demethylase FTO regulates melanoma tumorigenicity and response to anti-PD-1 blockade. Nat. Commun (2019) doi: 10.1038/s41467-019-10669-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhuang C. et al. N6-methyladenosine demethylase FTO suppresses clear cell renal cell carcinoma through a novel FTO-PGC-1α signalling axis. J. Cell. Mol. Med 23, 2163–2173 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rong ZX et al. Downregulation of fat mass and obesity associated (FTO) promotes the progression of intrahepatic cholangiocarcinoma. Front. Oncol (2019) doi: 10.3389/fonc.2019.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wen L, Pan X, Yu Y. & Yang B. Down-regulation of FTO promotes proliferation and migration, and protects bladder cancer cells from cisplatin-induced cytotoxicity. BMC Urol. (2020) doi: 10.1186/s12894-020-00612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niu Y. et al. RNA N6-methyladenosine demethylase FTO promotes breast tumor progression through inhibiting BNIP3. Mol. Cancer 18, 46 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu L, Wu D, Ning J, Liu W. & Zhang D. Changes of N6-methyladenosine modulators promote breast cancer progression. BMC Cancer 19, 326 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Y. et al. The FTO/miR-181b-3p/ARL5B signaling pathway regulates cell migration and invasion in breast cancer. Cancer Commun. (2020) doi: 10.1002/cac2.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peitzsch C, Tyutyunnykova A, Pantel K. & Dubrovska A. Cancer stem cells: The root of tumor recurrence and metastases. Semin. Cancer Biol. 44, 10–24 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Moro M. et al. Establishment of patient derived xenografts as functional testing of lung cancer aggressiveness. Sci. Rep. 7, 6689 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Y. et al. Meclofenamic acid selectively inhibits FTO demethylation of m6A over ALKBH5. Nucleic Acids Res. 43, 373–384 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gil-Rendo A. et al. Association between [18F]fluorodeoxyglucose uptake and prognostic parameters in breast cancer. Br. J. Surg. 96, 166–170 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Groheux D, Espié M, Giacchetti S. & Hindié E. Performance of FDG PET/CT in the Clinical Management of Breast Cancer. Radiology 266, 388–405 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Angeloni V, Tiberio P, Appierto V. & Daidone MG Implications of stemness-related signaling pathways in breast cancer response to therapy. Semin. Cancer Biol. 31, 43–51 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Velloso FJ et al. The crossroads of breast cancer progression: Insights into the modulation of major signaling pathways. Onco. Targets. Ther. 10, 5491–5524 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou S. et al. FTO regulates the chemo-radiotherapy resistance of cervical squamous cell carcinoma (CSCC) by targeting β-catenin through mRNA demethylation. Mol. Carcinog. 57, 590–597 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Hu B. et al. Epigenetic Activation of WNT5A Drives Glioblastoma Stem Cell Differentiation and Invasive Growth. Cell 167, 1281–1295 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mikels AJ & Nusse R. Purified Wnt5a protein activates or inhibits β-catenin-TCF signaling depending on receptor context. PLoS Biol. (2006) doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hung TH et al. Wnt5A regulates ABCB1 expression in multidrug-resistant cancer cells through activation of the non-canonical PKA/β-catenin pathway. Oncotarget (2014) doi: 10.18632/oncotarget.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veeman MT, Axelrod JD & Moon RT A second canon: Functions and mechanisms of β-catenin-independent Wnt signaling. Dev. Cell 5, 367–377 (2003). [DOI] [PubMed] [Google Scholar]
- 40.Desmedt C. et al. Genomic Characterization of Primary Invasive Lobular Breast Cancer. in Journal of Clinical Oncology (2016). doi: 10.1200/JCO.2015.64.0334. [DOI] [PubMed] [Google Scholar]
- 41.Bartosovic M. et al. N6-methyladenosine demethylase FTO targets pre-mRNAs and regulates alternative splicing and 3′-end processing. Nucleic Acids Res. 45, 11356–11370 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ji Z, Lee JY, Pan Z, Jiang B. & Tian B. Progressive lengthening of 3’ untranslated regions of mRNAs by alternative polyadenylation during mouse embryonic development. Proc. Natl. Acad. Sci. 106, 7028–7033 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonsalves FC et al. An RNAi-based chemical genetic screen identifies three small-molecule inhibitors of the Wnt/wingless signaling pathway. Proc. Natl. Acad. Sci. 108, 5954–5963 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan A, Dang Y, Chen G. & Mo Z. Overexpression of the fat mass and obesity associated gene (FTO) in breast cancer and its clinical implications. Int. J. Clin. Exp. Pathol. 8, 13405–13410 (2015). [PMC free article] [PubMed] [Google Scholar]
- 45.Pastushenko I. et al. Identification of the tumour transition states occurring during EMT. Nature 556, 463–468 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y. & Weinberg RA Epithelial-to-mesenchymal transition in cancer: complexity and opportunities. Front. Med. 12, 361–373 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin X. et al. RNA m6A methylation regulates the epithelial mesenchymal transition of cancer cells and translation of Snail. Nat. Commun. 10, 2065 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Rao VK et al. Phosphorylation of Tet3 by cdk5 is critical for robust activation of BRN2 during neuronal differentiation. Nucleic Acids Res. (2020) doi: 10.1093/nar/gkz1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi FT et al. Ten-eleven translocation 1 (Tet1) is regulated by o-linked n-acetylglucosamine transferase (ogt) for target gene repression in mouse embryonic stem cells. J. Biol. Chem. (2013) doi: 10.1074/jbc.M113.460386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blaschke K. et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature (2013) doi: 10.1038/nature12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Losman JA & Kaelin WG What a difference a hydroxyl makes: Mutant IDH, (R)-2-hydroxyglutarate, and cancer. Genes and Development (2013) doi: 10.1101/gad.217406.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beerling E. et al. Plasticity between Epithelial and Mesenchymal States Unlinks EMT from Metastasis-Enhancing Stem Cell Capacity. Cell Rep. (2016) doi: 10.1016/j.celrep.2016.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu J. et al. Landscape and Regulation of m6A and m6Am Methylome across Human and Mouse Tissues. Mol. Cell (2020) doi: 10.1016/j.molcel.2019.09.032. [DOI] [PubMed] [Google Scholar]
- 54.Clevers H. & Nusse R. Wnt/β-catenin signaling and disease. Cell (2012) doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 55.Pai SG et al. Wnt/beta-catenin pathway: modulating anticancer immune response. J. Hematol. Oncol. 10, 101 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mukherjee N. & Panda CK Wnt/β-Catenin Signaling Pathway as Chemotherapeutic Target in Breast Cancer: An Update on Pros and Cons. Clinical Breast Cancer (2020) doi: 10.1016/j.clbc.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 57.Tian TV et al. Identification of novel TMPRSS2:ERG mechanisms in prostate cancer metastasis: Involvement of MMP9 and PLXNA2. Oncogene 33, 2204–2214 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Yuan BF Liquid chromatography-mass spectrometry for analysis of RNA adenosine methylation. in Methods in Molecular Biology (2017). doi: 10.1007/978-1-4939-6807-7_3. [DOI] [PubMed] [Google Scholar]
- 59.Ross R, Cao X, Yu N. & Limbach PA Sequence mapping of transfer RNA chemical modifications by liquid chromatography tandem mass spectrometry. Methods 107, 73–78 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lánczky A. et al. miRpower: a web-tool to validate survival-associated miRNAs utilizing expression data from 2178 breast cancer patients. Breast Cancer Res. Treat. (2016) doi: 10.1007/s10549-016-4013-7. [DOI] [PubMed] [Google Scholar]
- 61.Curtis C. et al. The genomic and transcriptomic architecture of 2, 000 breast tumours reveals novel subgroups. Nature 486, 346–352 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Varambally S. et al. Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell (2005) doi: 10.1016/j.ccr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 63.Rokavec M, Kaller M, Horst D. & Hermeking H. Pan-cancer EMT-signature identifies RBM47 down-regulation during colorectal cancer progression. Sci. Rep. (2017) doi: 10.1038/s41598-017-04234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen S. et al. AfterQC: Automatic filtering, trimming, error removing and quality control for fastq data. BMC Bioinformatics (2017) doi: 10.1186/s12859-017-1469-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bolger AM, Lohse M. & Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics (2014) doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dobin A. et al. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Love MI, Huber W. & Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. (2014) doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anders S, Pyl PT & Huber W. HTSeq-A Python framework to work with high-throughput sequencing data. Bioinformatics (2015) doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paulovich A. et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. (2005) doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee JY, Yeh I, Park JY & Tian B. PolyA_DB 2: mRNA polyadenylation sites in vertebrate genes. Nucleic Acids Res. (2007) doi: 10.1093/nar/gkl870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quinlan AR & Hall IM BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics (2010) doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bailey TL et al. MEME Suite: Tools for motif discovery and searching. Nucleic Acids Res. (2009) doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Veeman MT, Slusarski DC, Kaykas A, Louie SH & Moon RT Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr. Biol. 13, 680–5 (2003). [DOI] [PubMed] [Google Scholar]
- 75.Metzger-Filho O. et al. Genomic grade adds prognostic value in invasive lobular carcinoma. Ann. Oncol. (2013) doi: 10.1093/annonc/mds280. [DOI] [PubMed] [Google Scholar]
- 76.Grembergen O. Van et al. Portraying breast cancers with long noncoding RNAs. (2016) doi: 10.1126/sciadv.1600220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Paired-end RNA sequencing analyses. For DeSeq2-derived data, two-sided paired Wald test and Benjamini-Hochberg adjustment were used to provide the “pvalue” and “padj”, respectively. For GSEA-derived data, two-sided false discovery rate (FDR) was used to provide the adjusted “FDR q val”. For ROAR-derived data, two-sided paired Fisher test was used to provide p values.
Supplementary Table 2. Differential m6A enrichment analysis in SKBR3 breast cancer cells. For Ingenuity-derived data, right-tailed Fisher’s exact test was used.
Supplementary Table 3. m6A enrichment analysis in human breast cancer biopsies. For MACS2-derived peaks, Poisson test with Benjamini-Hochberg adjustment was used to provide q values.
Supplementary Table 4. Differential m6A enrichment analysis in PC3 prostate cancer cells. For Ingenuity-derived data, right-tailed Fisher’s exact test was used.
Supplementary Table 5. List of primers and antibodies.
Supplementary Table 6. Beta-catenin IHC staining in human breast cancer biopsies.
Data Availability Statement
Sequencing data that support the findings of this study have been deposited in the Gene Expression Omnibus (GEO) repository under accession number GSE128582 (http://ncbi.nlm.nih.gov/geo). Human cancer data (bladder urothelial carcinoma, breast invasive carcinoma, cervical squamous cell carcinoma and endocervical adenocarcinoma, glioblastoma multiforme, kidney chromophobe, lung adenocarcinoma and lung squamous cell carcinoma, prostate adenocarcinoma, thyroid carcinoma, and uterine corpus endometrial carcinoma) were derived from the TCGA Research Network: http://cancergenome.nih.gov/. RNA-seq data from TCGA were obtained as normalised FPKM counts from GDC (https://portal.gdc.cancer.gov/repository) and PAM50 subtype information was extracted from UCSC Xena (https://xenabrowser.net). METABRIC expression and clinical data61 were downloaded through the European Genome-Phenome Archive (EGA) (http://www.ebi.ac.uk/ega/,accessionnumberEGAS00000000083 ). Previously published microarray data that were re-analysed here are available under accession code GSE9195, GSE10780, GSE10810, GSE12276, GSE19615, GSE20711, and GSE21653. Reannotation and processing of Affymetrix Human Genome U133 Plus 2.0 array data was performed as previously detailed76.
The hg19 reference transcriptome (version 85) was obtained from the ensembl portal (ensembl.org). Hallmark gene sets were obtained from MsigDB database (https://www.gsea-msigdb.org/gsea/msigdb). Human polyadenylation sites were obtained from PolyA_DB2 (http://polya.umdnj.edu/PolyA_DB2).
The data underlying Figs 1b, 1d–h, 2c–e, 3a–b, 3d, 3g, 4c, 4e, 5e, 5g, 5j–k, 6e, 7a, 7c, 8b–d and Extended Data Figs 1a–c, 1e–g, 2c–d, 2g, 3a–b, 3d–e, 4c, 4e, 4g–h, 5h, 5n, 6a–b, 7a–c, 7e–f are provided as Source Data files. For motility assays related to Figs 2a–b, 3c, 3e, 7b and Extended Data Figs 2a–b, 2d–e, 3c, 3f, 4d, independent repeats have been deposited on the Figshare repository, under the following DOI: 10.6084/m9.figshare.14602932, 10.6084/m9.figshare.14602944, 10.6084/m9.figshare.14602953, 10.6084/m9.figshare.14602965, 10.6084/m9.figshare.14602971 and 10.6084/m9.figshare.14602986). All other data supporting the findings of this study are available from the corresponding author on reasonable request.