Abstract
Background
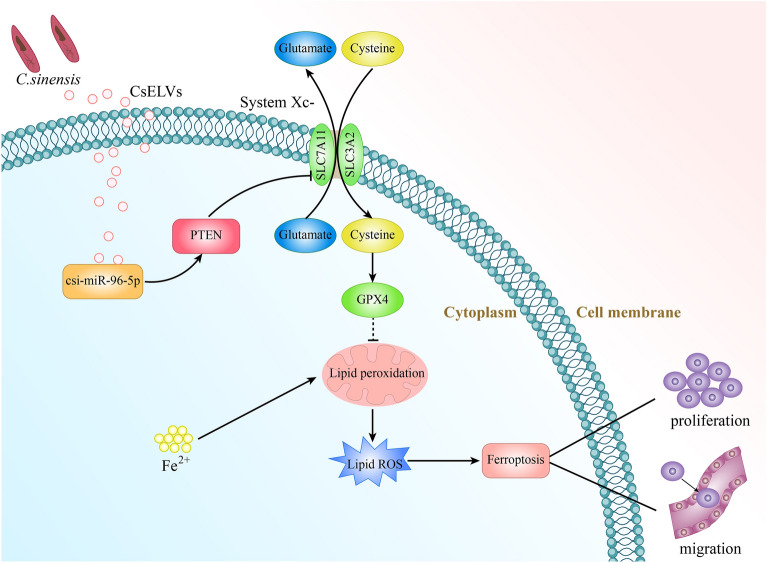
Clonorchis sinensis (CS) is classified as a group 1 carcinogen and can cause intrahepatic cholangiocarcinoma (ICC). CS extracellular vesicles (CsEVs) play important roles in mediating communication between parasitic helminths and humans. Ferroptosis is a novel cell death mechanism that is mainly induced by lipid peroxidation and iron overload. However, the role of CsEVs in the regulation of ferroptosis in ICC remains unclear. This study aimed to explore the role of CS-secreted miR-96-5p (csi-miR-96-5p) delivered by CsEVs in ICC progression and ferroptosis.
Methods
Tissue samples were collected from ICC patients with CS infection (CS-ICC) or without CS infection (NC-ICC). The levels of csi-miR-96-5p and PTEN gene were determined by quantitative polymerase chain reaction (qPCR) and western blotting, and survival analysis was performed. CsEVs were isolated and identified by ultracentrifugation and transmission electron microscopy. Lentiviruses were used to establish stable cell lines with csi-miR-96-5p mimic expression, PTEN overexpression (PTEN-EXO) and PTEN CRISPR/Cas9-based knockout (PTEN-KO) and their respective negative controls. Cell proliferation was assessed by performing Cell Counting Kit-8 assays in vitro and in a tumor xenograft model in vivo, and cell migration was assessed by performing Transwell assays. Erastin is used to induce ferroptosis. Ferroptosis levels were evaluated using biomarkers.
Results
High csi-miR-96-5p and low PTEN expression was observed in CS-ICC tissues and was associated with poor overall survival. csi-miR-96-5p was highly enriched in CsEVs and was taken up by ICC cells. csi-miR-96-5p mimics or PTEN-KO significantly promoted the growth and migration of ICC cells in vitro and in vivo, whereas PTEN-EXO exerted the opposite effect. Mechanistically, csi-miR-96-5p mimics or PTEN-KO inhibited erastin-induced ferroptosis, including reducing the accumulation of Fe2+, lipid reactive oxygen species, and malondialdehyde, increasing the GSH/GSSG ratio and levels of SLC7A11 and GPX4, whereas PTEN-EXOs exerted the opposite effect.
Conclusions
csi-miR-96-5p delivered by CsEVs reduced ferroptosis by regulating the expression of the PTEN/SLC7A11/GPX4 axis, thereby promoting ICC proliferation and migration. For the first time to our knowledge, we found that CS miRNAs could promote tumor development through ferroptosis.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13071-023-06075-7.
Keywords: Clonorchis sinensis, Extracellular vesicle, miRNA, Cholangiocarcinoma, Ferroptosis
Background
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver cancer, accounting for 10–20% of all primary liver cancers. Its incidence and mortality rates have increased in recent years [1]. ICC is characterized by insidious onset, difficulty in early diagnosis, high malignancy rate, rapid progression, and poor overall prognosis [2]. Multiple factors can lead to ICC occurrence, among which liver fluke infection (Clonorchis sinensis, CS) is defined as a group 1 carcinogen [3]. Recent clinical studies [4–6] indicate that CS infection not only promotes the occurrence of cholangiocarcinoma but also increases its malignant progression.
Extracellular vesicles (EVs) are all structures that cells release into their environment. Considering that these “cellular trash cans” have no biological functions, EVs are now recognized as an important medium for intercellular communication by transferring molecular signals, including proteins, nucleic acids, and lipids [7, 8]. EVs play multiple roles in maintaining normal physiological functions and are involved in the pathological environment, particularly in tumor progression [9, 10]. Recent studies have shown that parasite-derived EVs, especially the abundant miRNA cargo in EVs, can be taken up by host cells and notably affect the disease phenotype [11–14].
As a new cancer treatment strategy, ferroptosis has not been reported between CS and host cells, but it may be critical for understanding liver fluke-host interactions. Ferroptosis is a type of programmed cell death first discovered in 2012 [15]. Unlike apoptosis, necrosis, and autophagy, ferroptosis is driven by iron-dependent lipid peroxidation. The disruption of iron and lipid metabolism induces reactive oxygen species (ROS) production and accumulation, leading to mitochondrial damage, membrane integrity breakdown, and ultimately cell death [16]. Alterations in the ferroptosis regulatory network can lead to the development of various diseases including cancer. Evidence suggests that ferroptosis is suppressed in cancer cells, allowing them to survive and progress [17]. Whether parasitic worms can cause or inhibit ferroptosis in host cells and the related molecular mechanisms is yet to be explored but is crucial for understanding the malignant progression of tumors caused by CS.
Studies have found that CS-secreted miR-96-5p (csi-miR-96-5p) is highly expressed in CS adult worms and CS-secreted EVs (CsEVs) [14, 18]. In the present study, we performed quantitative polymerase chain reaction (qPCR) to analyze the abundance of csi-miR-96-5p and western blotting (WB) to analyze the expression of PTEN in CS-ICC tissues and studied the molecular mechanism by which csi-miR-96-5p promotes ICC malignant transformation, providing new insights into the prevention and treatment of ICC.
Methods
Human tissue samples
The preoperative diagnosis of CS mainly relies on stool microscopy, enzyme-linked immunosorbent assay, or endoscopy. Fresh liver and intrahepatic bile duct specimens were collected from ICC patients with (CS-ICC group) and without (NC-ICC group) CS infection during partial hepatectomy at the First Hospital of Jilin University. Tumor tissues and adjacent normal tissues were preserved. Three patients without the disease who underwent hepatic segment resection because of trauma were also enrolled. The clinical and pathological characteristics were recorded. This study was approved by the Ethics Committee of the First Hospital of Jilin University (permit no. 19K041-001) and conducted in strict accordance with the Declaration of Helsinki. Informed consent was obtained from all the participants.
Animals
Six-week-old nude mice were purchased from the Animal Center of Jilin University and bred in a specific pathogen-free animal laboratory. All the animals were provided with food and water ad libitum. The animal study protocol was approved by the Animal Ethics Committee of the First Hospital of Jilin University (permit no. 20210526).
Cell culture
A human ICC cell line (HuCCT1 cells) and normal bile duct cells were purchased from the cell bank of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in the RPMI-1640 medium containing 10% fetal bovine serum (FBS) and 100 U/ml penicillin/streptomycin solution (Sigma) at 37 °C in a humidified incubator with 5% CO2.
miRNA transfection, gene knockdown, and gene overexpression
csi-miR-96-5p mimics and a negative control (Shanghai Genchem Co., Ltd.) were transfected into cells using Lipofectamine 2000 (Thermo Fisher Scientific Inc.). To create stable cell lines, recombinant lentivirus (LV) vectors containing csi-miR-96-5p mimics (Shanghai Genchem Co., Ltd.), a PTEN overexpression plasmid (PTEN-EXO) (Sino Biological, China), and a PTEN CRISPR/Cas9 based knockout (PTEN-KO) plasmid (Shanghai Genchem Co., Ltd.) were constructed. HuCCT1 cells were infected with the above recombinant LV vectors and selected in the presence of 1.5 μg/ml puromycin (Sigma) over 14 days. To explore the effects of csi-miR-96-5p and PTEN on ferroptosis, HuCCT1 cells were treated with the ferroptosis activator erastin (1 μM) (MCE, China).
CsEV isolation and identification
The method of CsEV separation is as described above [14]. Eight weeks after CS infection, Sprague-Dawley rats were killed under deep ether anesthesia, the liver was removed, and adult worms were collected from the bile canals. The worms were washed five times with phosphate-buffered saline (PBS) containing 1% penicillin/streptomycin at 37 °C and then incubated in the RPMI-1640 medium for 24 h. The supernatant from the first 2 h of culture was discarded as it contained many host components. CsEVs were isolated by differential centrifugation. The culture solution was pooled and centrifuged for 15 min at 3000×g and then at 10,000×g for 30 min to remove cellular debris, worm eggs, and sperm, after which it was filtered through a 0.22-µm filter (Steriflip; Millipore). Finally, the supernatant was centrifuged twice at 100,000×g for 90 min, and the cell pellets, which contained CsEVs, were resuspended in 50 µl PBS and stored at − 80 °C.
Anti-CD63 and anti-TSG101 protein markers of CsEVs were detected by WB. For transmission electron microscopy (TEM) characterization, CsEVs were placed on a mesh in thin layers, to which a 2% uranyl acetate aqueous solution was added, and the mesh was subsequently dried overnight. To verify whether CsEVs could be taken up by HuCCT1 cells, CsEVs were labeled with a PKH67 green fluorescent labeling kit (Sigma) and cultured with HuCCT1 cells for 24 h. The cells were then observed using a confocal microscope (FV1200, Olympus).
Dual-luciferase reporter activity assay
Dual-luciferase assay was performed according to standard protocol (Promega, USA). The luciferase gene was cloned downstream of the wild-type or mutant region of the PTEN and co-transfected into HuCCT1 cells with the csi-miR-96-5p mimics or negative control. Forty-eight hours after transfection, the cells were lysed and the firefly luciferase activity was measured using a dual-luciferase assay kit (Promega, USA) following the manufacturer’s instructions. Renilla luciferase was used as the internal reference.
RNA isolation, reverse transcription, and quantitative real-time PCR (qPCR)
For RNA isolation, Trizol reagent (Takara, China) and miRNA isolation kit (Tiangen, China) were used to extract total RNA and small RNA (20–200 nt) from cells, tumor samples, or CS according to the manufacturer's instructions, respectively. For RNA reverse transcription, mRNAs were reverse transcribed into cDNA using PrimeScript RT Master Mix reagent kit (Takara, China) according to the manufacturer’s instructions. miRNAs were reverse transcribed into cDNA using Reverse Transcriptase M-MLV kit (Takara, China) according to the manufacturer’s instructions. For qPCR, SYBR green assay kit (Takara, China) was used, and the procedures were performed as described previously [14]. GAPDH was used as internal control for mRNA, U6 was used as internal control for miRNA, and csi-miR-96-5p in three disease-free normal samples was normalized as control. The relative expression was calculated using the 2−ΔΔCT method. As csi-miR-96-5p and hsa-miR-96-5p had the same seed sequence, two set of species-specific primers were designed; all primers used for reverse transcription and qPCR are listed in Additional file 1: Table S1.
Western blot
Cells or tissues were lysed in radio immunoprecipitation assay (RIPA) buffer with protease inhibitor (Beyotime Biotechnology, China); the lysate was centrifuged at 15,000×g for 15 min at 4 °C. Then, proteins were heated at 95 °C for 5 min followed by fractionation in 10% SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked in 5% BSA in TBST buffer (50 mM Tris-HCl at pH 7.5, 150 mM NaCl and 0.05% Tween-20) for 2 h and then incubated with primary antibodies at 4 °C overnight. The primary antibodies were as follows: PTEN (1:1000, Abclonal, China), GPX4 (1:1000, Abcam), SLC7A11 (1:10,000, Abclonal, China), and GAPDH (1:50,000, Abclonal, China). After three washes with TBST, the membranes were incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Invitrogen, Waltham, MA, USA) and visualized using an electrochemiluminescence reagent (Beyotime Biotechnology, China).
Establishment of tumor xenografts in vivo
Male BALB/c nude mice (4 weeks) were obtained from the Central Laboratory of Animal Science of Jilin University. HuCCT1 cells (5 × 106) stably transfected with csi-miR-96-5 mimics or PTEN-KO and their empty vector were injected into the nude mice for in situ implantation. Tumor size was measured every week. After 4 weeks, the mice were killed, and the tumors were removed for weight measurement.
Cell proliferation assay
The Cell Counting Kit-8 (CCK-8) assay kit (MCE, China) was used to quantify the number of HuCCT1 cells. The cells were seeded in a 96-well plate. After overnight incubation at 37 °C and 5% CO2, 10 μl of the CCK-8 solution was added to the cells in each well, and the cells were incubated again for 3 h. Absorbance at 450 nm was measured at 0, 24, 48, 72, and 96 h.
Transwell migration assays
A 12-well plate was purchased from Corning, and assays were performed according to the manufacturer’s protocol. Briefly, the cells were resuspended with serum-free Dulbecco’s Modified Eagle Medium (DMEM) for a total of 100 μl (1 × 105 cells) and added to the upper chamber, and the lower chamber was supplemented with 500 μl DMEM containing 20% FBS. The cells were incubated for 72 h. The cells were rinsed with PBS three times, and the non-migrated cells in the upper chamber were removed using a cotton swab. The cells were fixed with 4% polyformaldehyde for 20 min, stained with 1% crystal violet for 15 min, rinsed with PBS three times, and allowed to dry. Five randomly selected areas were examined and photographed.
Malondialdehyde (MDA) and glutathione (GSH)/GSH disulfide (GSSG) ratio assays
Assay kits to measure MDA (Beyotime, China) and GSH/GSSG count (Jiancheng Bioengineering Institute, China) were purchased. The assays were conducted according to the manufacturer’s protocols. Briefly, cells samples (1 × 106 cells) were lysed by 0.1 ml lysate and then centrifuged at 12,000×g for 10 min; the supernatant was used for absorbance measurement. The OD value was measured at 532 nm or 412 nm.
Ferrous iron (Fe2+) assay
Assay kit to measure Fe2+ (Solarbio, China) was purchased. The assays were conducted according to the manufacturer’s protocols. Briefly, cell samples (1 × 106 cells) were lysed by 0.1 ml lysate and broken by ultrasound (200 W, last 3 s and interval 7 s, repeat 30 times) and then centrifuged at 8000×g for 10 min; the supernatant was used for absorbance measurement. The OD value was measured at 510 nm.
miRNA target prediction
miRNA target gene prediction and analysis were performed using TargetScan (http://www.targetscan.org/), PicTar (http://pictar.mdc-berlin.de/), and miRanda (http://www.microrna.org/). To find target genes associated with ferroptosis, the ferroptosis-associated FerrDb database was searched [19].
Statistical analysis
All data were processed using IBM SPSS 20.0. Count data were compared by performing the chi-square test. The Kaplan-Meier method was used for survival analysis, and the log-rank test was used to compare differences between groups. P < 0.05 was considered statistically significant.
Results
Samples from humans
We collected data from 46 ICC patients with CS infection (CS-ICC group) and 46 ICC patients without CS infection (NC-ICC group). Another three patients who underwent hepatic segment resection because of trauma were also enrolled as controls. The basic and pathological characteristics of the patients are shown in Table 1. CS infection increased serum total bilirubin, tumor TNM stage, and tumor differentiation degree in patients with cholangiocarcinoma (P < 0.05).
Table 1.
Clinical and pathological characteristics of ICC patients with or without Clonorchis sinensis infection
| Characteristics | CS-ICC (n = 46) | NC-ICC (n = 46) | P-value |
|---|---|---|---|
| Age, years | 65.1 ± 9.5 | 66.3 ± 8.2 | 0.72 |
| Sex, male | 22 | 21 | 0.85 |
| BMI, kg/m2 | 22.9 ± 2.8 | 22.3 ± 2.5 | 0.78 |
| Length of stenosis, mm | 25.7 ± 6.4 | 23.0 ± 7.9 | 0.33 |
| Total bilirubin, mmol/l | 260.1 ± 76.9 | 150.3 ± 69.8 | < 0.05 |
| Tumor location | 0.68 | ||
| Left IBD | 26 | 24 | |
| Right IBD | 20 | 22 | |
| Cancer stage by AJCC, 7th | < 0.05 | ||
| I–II | 14 | 24 | |
| III–IV | 32 | 22 | |
| Tumor differentiation | < 0.05 | ||
| Well/moderate | 18 | 26 | |
| Poorly | 28 | 20 | |
Data are expressed as n or mean ± SD
ICC, intrahepatic cholangiocarcinoma; CS-ICC, ICC with Clonorchis sinensis infection; NC-ICC, ICC without Clonorchis sinensis infection; BMI, body mass index; IBD, intrahepatic bile duct; n, number of patients
Identification and validation of the abundant presence of csi-miR-96-5p in CS-ICC patients
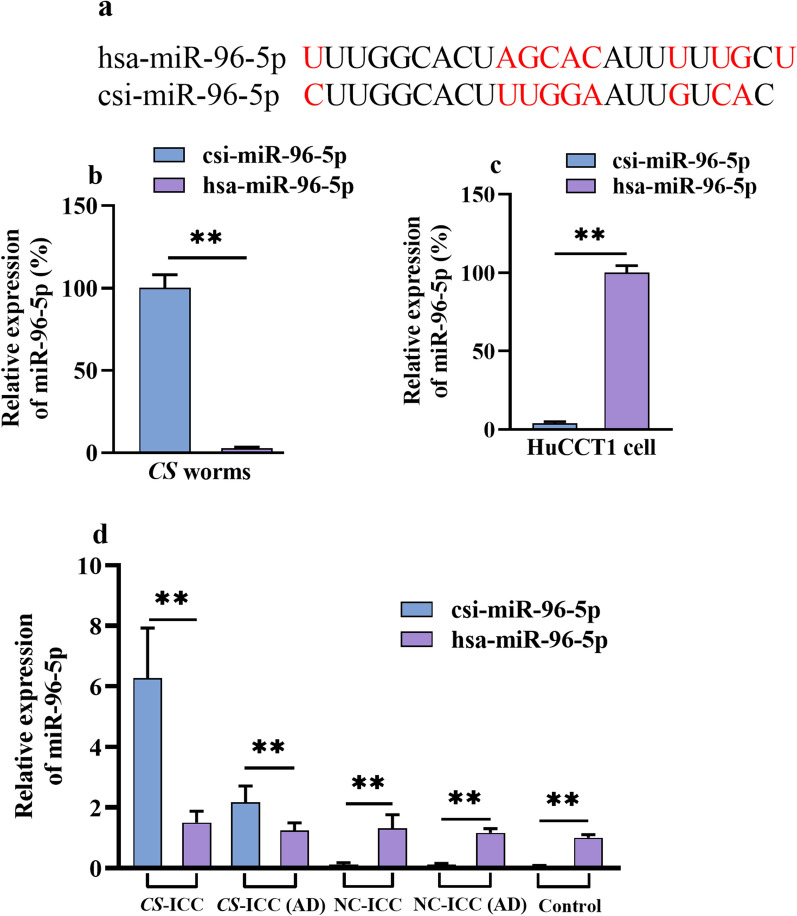
The specificity of the two sets of primers designed for human (hsa-miR-96-5p) and CS worms (csi-miR-96-5p) was examined first (Fig. 1a). Total small RNA from CS worms and HuCCT1 cells were extracted and used for reverse transcription. The results showed the CS set of primers successfully generated the csi-miR-96-5p from samples of CS worms but not HuCCT1 cells, whereas the human set of primers successfully generated the hsa-miR-96-5p from samples of HuCCT1 cells but not CS worms (Fig. 1b, c). Therefore, the above results proved that the two set of primers can effectively distinguish between each other and have strong specificity.
Fig. 1.
Detection of csi-miR-96-5p in CS-infected patients with ICC. a Design and validation of cross-species primers for csi-miR-96-5p and hsa-miR-96-5p. b, c Verifying the specificity of csi-miR-96-5p and hsa-miR-96-5p primers. Total RNA samples for CS worms and HuCCT1 cells were 500 ng. The same qPCR conditions were used for the two groups. c Expression of csi-miR-96-5p and hsa-miR-96-5p in patients with ICC. The control group refers to disease-free tissues from patients who underwent hepatectomy because of trauma. Data are presented as the mean ± SD from three replicates, *P < 0.05, **P < 0.01. ICC, intrahepatic cholangiocarcinoma; CS, Clonorchis sinensis; NC, negative control without CS infection; AD, tumor-adjacent normal tissue; SD, standard deviation; qPCR, quantitative polymerase chain reaction
Next, we investigated the expression of csi-miR-96-5p and hsa-miR-96-5p in CS-ICC patients and NC-ICC patients. The results showed that the level of csi-miR-96-5p in CS-ICC tumors (6.27 ± 1.65) was prominently higher than that in adjacent normal tissues (2.17 ± 0.54, P < 0.05) and was also significantly higher than that of hsa-miR-96-5p in CS-ICC tumors (1.51 ± 0.38) and adjacent normal tissues (1.24 ± 0.25), indicating the abundant presence of csi-miR-96-5p in patients with CS-ICC (Fig. 1c).
Prediction and identification of the PTEN gene as the target of csi-miR-96-5p
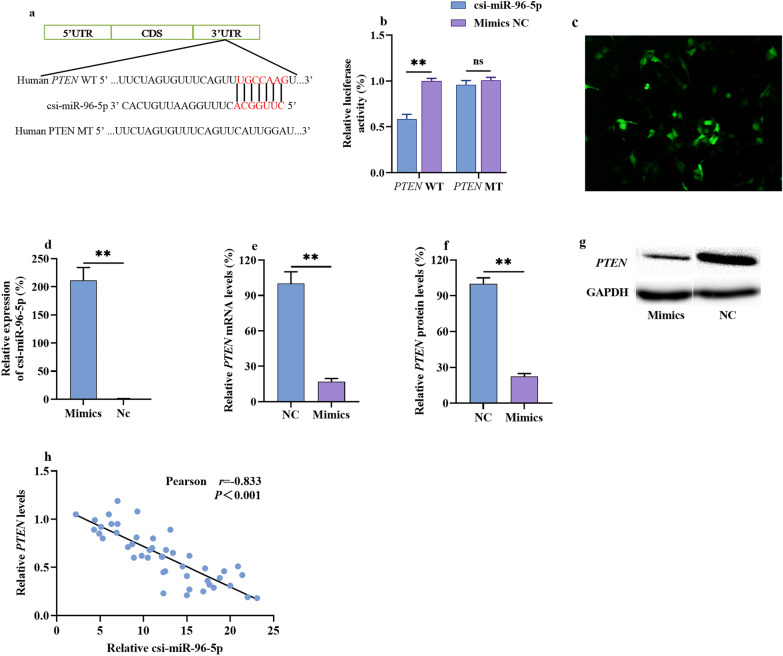
To further analyze the targets of csi-miR-96-5p, the miRDB, PicTar, and TargetScan databases were used to predict the downstream genes. Finally, twenty-six target genes of csi-miR-96-5p were mached, and by comparing the genes with the ferroptosis-associated FerrDb database, PTEN was identified as the single target associated with ferroptosis (Fig. 2a). Previous study had found that PTEN could potentially affect tumor survival, migration, and proliferation in humans [20]. The luciferase assay showed that the cells transfected with csi-miR-96-5p mimics significantly inhibited luciferase activity, which was shown by those transfected with the recombinant plasmid incorporating in the 3′ untranslated region of wild-type PTEN but not by mutant PTEN (Fig. 2b). By performing qPCR and WB, we analyzed PTEN expression in the HuCCT1 cells transfected with the csi-miR-96-5p mimics. The results showed that csi-miR-96-5p was successfully overexpressed (Fig. 2c, d) and PTEN mRNA and protein levels were significantly reduced (Fig. 2e–g). To further verify this regulatory relationship, we analyzed the expression of csi-miR-96-5p and PTEN in patients with ICC. All patients with CS-ICC were divided into high and low csi-miR-96-5p expression groups based on the median value. In the CS-ICC group, a high level of csi-miR-96-5p was associated with a lower PTEN level, and Pearson’s correlation test showed that PTEN was negatively correlated with csi-miR-96-5p (r = − 0.833) (Fig. 2h). Therefore, there is ample evidence suggesting that csi-miR-96-5p directly inhibits PTEN expression.
Fig. 2.
Prediction and validation of PTEN as a csi-miR-96-5p target gene. a The putative binding region of csi-miR-96-5p for PTEN. b The luciferase reporter gene method was used to determine the PTEN gene reporter activity. c, d csi-miR-96-5p mimics were successfully transfected into HuCCT1 cells. e–g PTEN mRNA and protein levels after transfection with csi-miR-96-5p mimics or NC mimics were detected using qPCR and WB, respectively. h Pearson correlation analysis of csi-miR-96-5p and PTEN levels in tissues from patients with CS-ICC. Data are presented as the mean ± SD from three replicates, *P < 0.05, **P < 0.01. ICC, intrahepatic cholangiocarcinoma; CS, Clonorchis sinensis; SD, standard deviation; ns, no statistical difference; qPCR, quantitative polymerase chain reaction
csi-miR-96-5p/PTEN axis was associated with a dismal overall survival (OS)
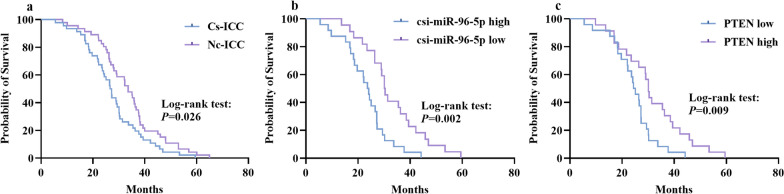
We performed a survival analysis based on long-term postoperative follow-up. The Kaplan-Meier curves showed that the OS of the CS-ICC group was prominently lower than that of the NC-ICC group, with a median OS of 26.9 months and 33.6 months, respectively (P < 0.05) (Fig. 3a). In the CS-ICC group, the Kaplan-Meier curves showed that patients with high levels of csi-miR-96-5p had shorter OS than patients with low levels of csi-miR-96-5p (P < 0.05) (Fig. 3b). A low level of PTEN was also associated with shorter OS (P < 0.05) (Fig. 3c).
Fig. 3.
Kaplan-Meier curves for OS of patients with ICC based on csi-miR-96-5p and PTEN expression. Patients were divided into high and low csi-miR-96-5p and PTEN expression groups according to the average value. a Kaplan-Meier analysis of OS between the CS-ICC and NC-ICC groups. b Kaplan-Meier analysis of OS in CS-ICC patients with high or low csi-miR-96-5p expression. c Kaplan-Meier analysis of OS in CS-ICC patients with high or low PTEN expression. OS, overall survival; ICC, intrahepatic cholangiocarcinoma; CS, Clonorchis sinensis
csi-miR-96-5p was delivered into ICC cells by CsEVs
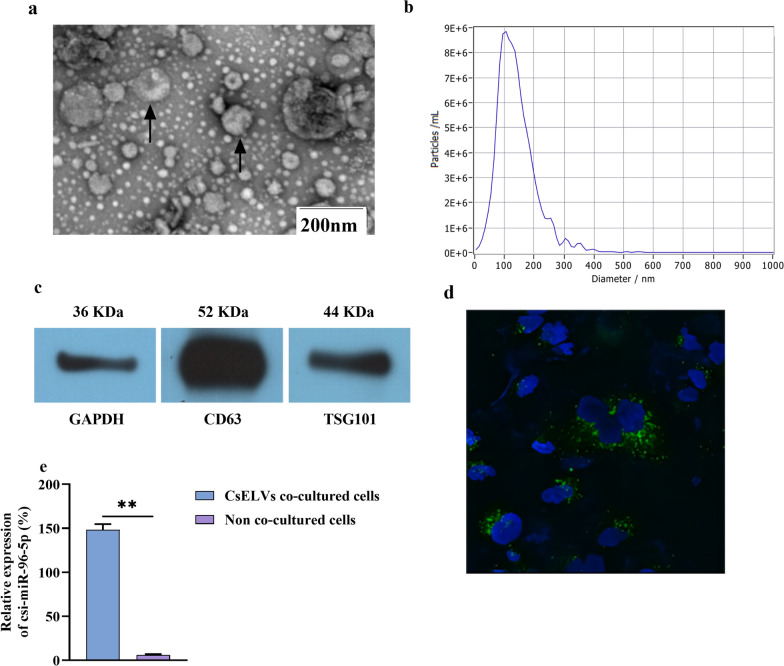
To verify whether csi-miR-96-5p in host tissues was delivered by CsEVs, we isolated CsEVs. The culture medium of adult worms in the RPMI-1640 medium at 37 °C for 24 h was collected. CsEVs were isolated from the excretory-secretory products (ESPs) by ultracentrifugation. Under TEM, vesicle morphological characteristics were observed and displayed as a “double-layer saucer,” which is consistent with the typical EV morphology (Fig. 4a). Nanoparticle tracking analysis indicated that the median particle size of the extracted exosomes was 112.1 nm and that the CsEV concentration was 1.1 × 1012 particles/ml (Fig. 4b). WB showed that characteristic EV proteins such as CD63 and TSG101 were present in the CsEVs (Fig. 4c).
Fig. 4.
Extraction and validation of csi-miR-96-5p in CsEVs. a Transmission electron microscopy analysis of CsEVs. b Nanoparticle tracking analysis of the sizes of CsEVs. c Western blot analysis of characteristic CsEV proteins (CD63 and TSG101). d CsEVs labeled with PKH-67 can be taken up by HuCCT1 cells. e qPCR analysis of the level of csi-miR-96-5p in cells treated with CsEVs. CsEVs, Clonorchis sinensis secreted extracellular vesicles; qPCR, quantitative polymerase chain reaction. *P < 0.05, **P < 0.01
To verify whether CsEVs could be taken up by host cells, the isolated CsEVs (50 uM) were stained with PKH67 (Sigma) and further co-cultured with HuCCT1 for 24 h. Green fluorescence staining was observed in HuCCT1 cells, and qRT-PCR showed the presence of csi-miR-96-5p in HuCCT1 cells, confirming that CsEVs can act as carriers to transport miRNAs into recipient cells (Fig. 4d, e).
csi-miR-96-5p promoted ICC proliferation and migration
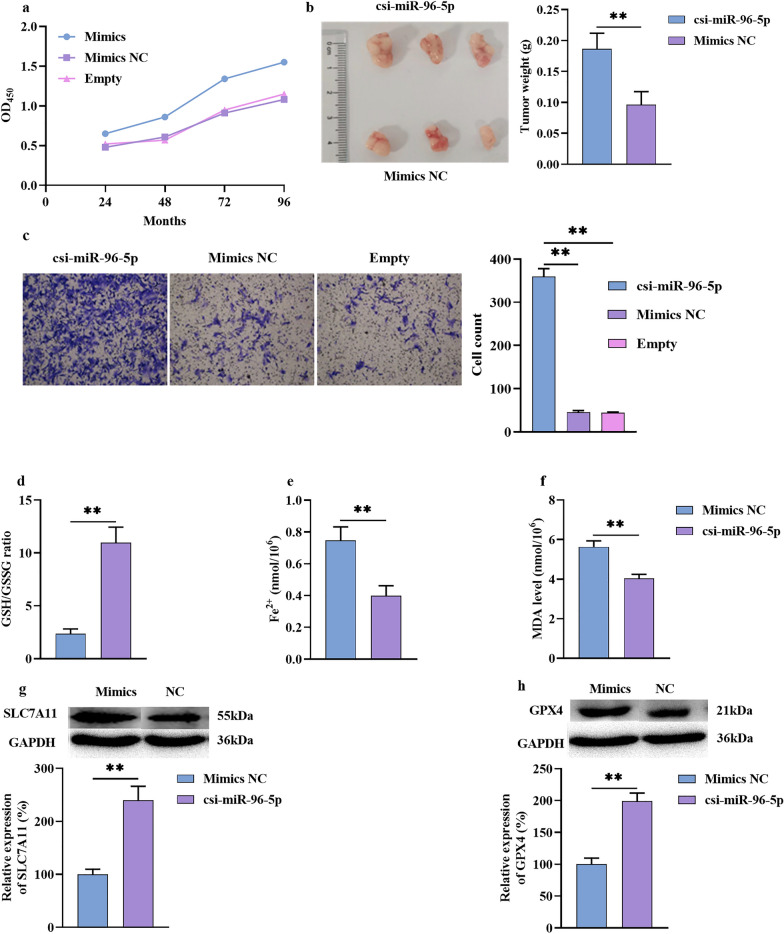
To explore the role of csi-miR-96-5p in ICC progression, csi-miR-96-5p mimics or control miRNA mimics were transfected into HuCCT1 cells. These mimics significantly promoted the proliferation of HuCCT1 cells, as determined by the CCK-8 assay in vitro, and the tumor size in xenograft tumor models in vivo (Fig. 5a, b). Furthermore, compared with NC, transfection with the miRNA mimics led to higher migration tendencies (Fig. 5c). These results suggest that csi-miR-96-5p promotes the proliferation and migration of ICC cells.
Fig. 5.
csi-miR-96-5p promoted ICC proliferation and migration via a ferroptosis mechanism. a Cell proliferation was measured by CCK-8 assay in vitro. b Cell proliferation was measured by tumor xenograft in vivo. c Cell migration was measured by transwell assay. d Cell GSH/GSSG radio assay. e Cell Fe2+ assay. f Cell MDA assay. g, h Cell WB assay of SLC7A11 and GPX4. ICC, intrahepatic cholangiocarcinoma; WB, western blotting; GSH, glutathione; GSSG, GSH disulfide; MDA, malondialdehyde. *P < 0.05, **P < 0.01
csi-miR-96-5p positively regulates ICC progression via ferroptosis
To determine whether csi-miR-96-5p promoted ICC progression by regulating ferroptosis, miRNA mimics or control miRNA was transfected into HuCCT1 cells; then, the cells were cultured in the presence of the ferroptosis activator erastin (1 μM). Several ferroptosis markers and molecules were assayed to explore the potential mechanisms of action. Cells transfected with the csi-miR-96-5p mimics exhibited significantly fewer ferroptosis-related abnormalities, including a remarkably higher GSH/GSSG ratio (Fig. 5d) and decreased accumulation of Fe2+ and toxic active aldehydes (MDA) (Fig. 5e, f). The expression of ferroptosis regulatory proteins was confirmed by WB and qPCR. The results showed that the csi-miR-96-5p mimics positively upregulated the expression of SLC7A11 and GPX4 (Fig. 5g, h). These data show that the abundant presence of csi-miR-96-5p promotes ICC cell progression in vitro and in vivo by inhibiting ferroptosis.
PTEN inhibited ICC proliferation and migration
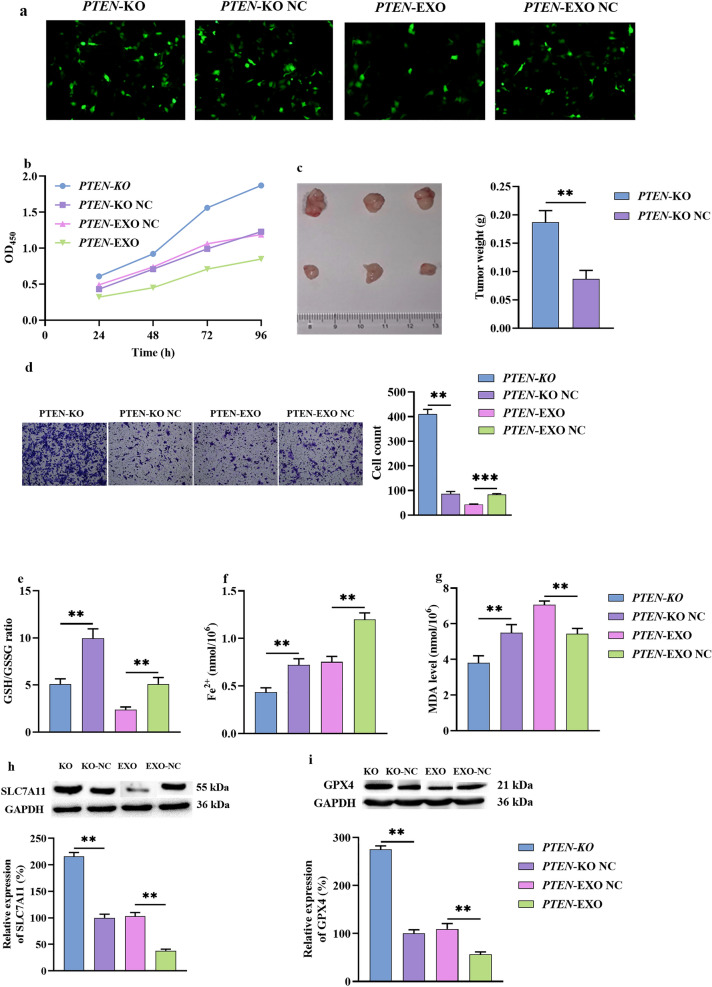
To explore the molecular mechanism of PTEN in ICC, LV vectors were used to construct HuCCT1 cells for stably expressing PTEN (PTEN-EXO) or CRISPR/Cas9-based (PTEN-KO), and the empty vector (PTEN-EXO NC) and NC LV (PTEN-KO NC) were used as controls. PTEN overexpression and silencing in HUCCT1 cells were confirmed (Fig. 6a). PTEN-EXOs caused a prominent decrease in cell growth in vitro as determined by CCK-8 and in vivo as determined using xenograft tumor models (Fig. 6b, c). Furthermore, the PTEN-EXOs exhibited lower migration tendencies (Fig. 6d). In contrast, the PTEN-KOs exhibited increased proliferation and migration tendencies. These results showed that PTEN could play a role as a tumor suppressor gene in ICC in vitro and in vivo.
Fig. 6.
PTEN inhibited ICC proliferation and migration via a ferroptosis mechanism. a Stable PTEN overexpression (PTEN-EXO) or CRISPR/Cas9-based knockout (PTEN-KO) HuCCT1 cell lines were established; the scale shown is 100 um. b Cell proliferation was measured by CCK-8 assay in vitro. c Cell proliferation was measured by tumor xenograft in vivo. d Cell migration was measured by transwell assay. e Cell GSH/GSSG radio assay. f Cell Fe2+ assay. g Cell MDA assay. h, i Cell WB assay of SLC7A11 and GPX4. ICC, intrahepatic cholangiocarcinoma; WB, western blotting; GSH, glutathione; GSSG, GSH disulfide; MDA, malondialdehyde. *P < 0.05, **P < 0.01
PTEN inhibited tumor progression via a ferroptosis-promoting mechanism
To explore whether PTEN inhibited ICC progression by regulating ferroptosis, stable PTEN-EXO and PTEN-KO HuCCT1 cell lines were constructed; then, the cells were cultured in the presence of erastin (1 μM). Compared with PTEN-EXO NC cells, PTEN-EXO cells showed more ferroptosis-related abnormalities, including a lower GSH/GSSG ratio and higher accumulation of MDA and Fe2+ (Fig. 6e–g). The detection of ferroptosis markers indicated that PTEN-EXOs substantially downregulated the expression of SLC7A11T and GPX4 (Fig. 6h, i). In contrast, PTEN-KO cells exhibited the opposite ferroptotic mechanism. These results indicated that PTEN could promote ferroptosis by regulating related molecules.
PTEN rescues the effect of csi-miR-96-5p on ICC
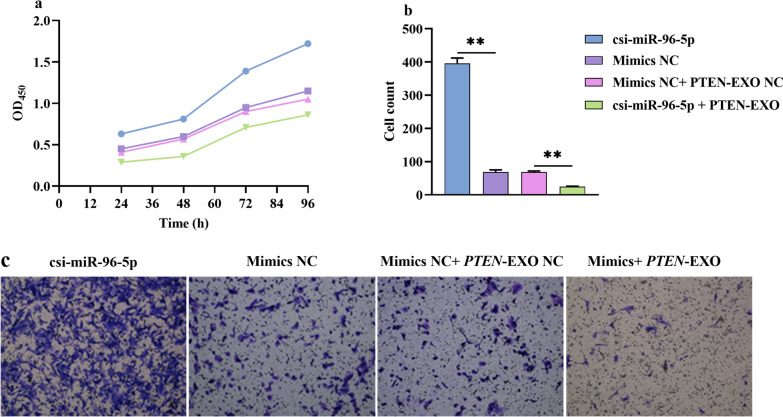
To determine the top-down relationship between the csi-miR-96-5p/PTEN axis and ICC, we designed a functional co-transfection rescue experiment. We cotransfected csi-miR-96-5p mimics and PTEN-EXOs into the cells. These results indicated that PTEN-EXOs rescued the tumor-promoting effect of csi-miR-96-5p, thereby inhibiting ICC cell proliferation and migration (Fig. 7a, b).
Fig. 7.
Co-transfect functional rescue experiment of csi-miR-96-5p and PTEN-EXO. a Cell proliferation was measured by CCK-8 assay in vitro. b Transwell assay of migration. CCK-8, cell counting kit-8; PTEN-EXO, PTEN overexpression. *P < 0.05, **P < 0.01
Discussion
Adult CS live in the human hepatobiliary duct, and their excretions, metabolites, and mechanical stimulation can damage the human bile duct epithelium and submucosal blood vessels, causing bile duct dilatation, epithelial hyperplasia, cholecystitis, gallstones, and ICC. Both clinical epidemiological studies and clinicopathological analyses suggest that CS infection is closely associated with ICC incidence [21–24]. Studies have reported that CS excretions are involved in the carcinogenesis of the bile duct epithelium; however, these studies did not clarify whether this carcinogenic effect was caused by ESPs or CsEVs [25]. EVs secreted by helminths have been shown to interactively enter host cells [12, 14]. Some studies have confirmed the roles of helminth miRNAs in host pathogenesis and related molecular mechanisms [26, 27]. hsa-miR-96-5p is a carcinogenic factor that promotes the viability, proliferation, and migration of various cancer cells, such as hepatocellular carcinoma, lung adenocarcinoma, and breast cancer. Multiple signaling-related genes, such as PTEN, caspase-9, and BNIP3/FAK, are involved in the molecular mechanism [28–30]. Our study identified a conserved miR-96-5p from CS, csi-miR-96-5p, which has the same seed sequence as hsa-miR-96-5p, despite differences in the remaining miRNA sequences between the species. We demonstrated that csi-miR-96-5p was present in host ICC cells and was delivered by CsEVs. Transfection with csi-miR-96-5p mimics promoted ICC cell proliferation and migration. In addition, csi-miR-96-5p promoted tumor growth in a xenograft animal model of ICC. The analysis of the molecular mechanism showed that csi-miR-96-5p played a role in regulating cell proliferation and invasion by targeting the PTEN gene.
PTEN is a tumor suppressor gene identified after the discovery of the p53 gene [31]. Many studies have shown that PTEN plays an important role in cell growth, apoptosis, adhesion, migration, and infiltration and is closely associated with the tumorigenesis process [32]. In recent years, some highly expressed ectopic miRNAs have been found to directly downregulate PTEN expression in the liver and biliary system and play cancer-promoting roles [33]. The results of this study showed that PTEN expression in CS-ICC tumor tissues was lower than that in normal tissues and that the level of csi-miR-96-5p was negatively correlated with PTEN expression. Low levels of csi-miR-96-5p and high levels of SLC7A11 were associated with increased TNM stage, lymph node metastasis, deeper muscle infiltration, and increased Ki-67 expression. These results indicate that the csi-miR-96-5p/PTEN axis promotes ICC progression in patients, in vitro and in vivo.
Ferroptosis is a newly identified type of programmed cell death mainly caused by iron overload and the accumulation of intracellular lipid ROS. GSH/GPX4 is a classical ferroptosis pathway. The key executor of this pathway is System Xc- (also known as the cystine/glutamate anti-transporter), which is composed of the catalytic subunit SLC7A11 and the chaperone subunit SL3A2. SLC7A11 exhibits important transport activities and is highly specific to cysteine and glutamate [34]. System Xc- takes up cystine at a ratio of 1:1 and exchanges glutamic acid. Cystine transported into the cell is rapidly reduced to cysteine, which participates in GSH synthesis. As an important antioxidant and free radical scavenger, GSH can inhibit ferroptosis. SLC7A11 is highly expressed in various malignant tumors, and tumor cells can maintain high levels of GSH by upregulating SLC7A11 expression to counteract oxidative stress caused by increased metabolic rate, a process that is positively correlated with cancer progression because of its antioxidant function [35]. Our study showed that PTEN loss by csi-miR-96-5p upregulated SLC7A11 in ICC cell lines and patient tumor samples, promoting cystine input and GSH production, thereby protecting cells from ferroptosis. A recent study also demonstrated that PTEN loss desensitized cells to ferroptosis induced by erastin and cystine deprivation. In this mechanism, the loss of PTEN activated AKT kinase to inhibit GSK3b and increases the transcription of NF-E2 P45-associated factor 2 (NRF2) and one of its known target genes encoding SLC7A11 [36].
Our study demonstrated that csi-miR-96-5p delivered by CsEVs and PTEN regulated ICC development by modulating ferroptosis. This study has several advantages. First, we are the first to our knowldge to explore and demonstrate ferroptosis in parasitic helminth-host interactions, which is essential for refining our understanding of the effect of parasitic worm-host interactions and providing targeted treatments for cancer. Second, we collected sufficient clinical samples and confirmed the reliability of the csi-miR-96-5p/PTEN/SLC7A11/GPX4 axis through in vitro and in vivo cell and animal experiments. Third, we up- and downregulated PTEN to fully verify multiple possible pathways of ferroptosis and performed functional rescue experiments to verify the role of the csi-miR-96-5p/PTEN axis.
However, in the present study, therapy targeting csi-miR-96-5p or PTEN has not been tested in clinical trials, and more clinical studies are needed to explore the feasibility of targeting this axis.
Conclusions
The results of this study demonstrate that csi-miR-96-5p delivered by CsEVs is abundant in patients with CS-ICC and downregulates PTEN, thus promoting the proliferation and migration of ICC through the SLC7A11/GPX4 ferroptosis axis. We are the first to our knowledge to find that CS miRNAs can promote ICC tumor development through ferroptosis.
Supplementary Information
Additional file 1: Table S1. Sequences of primers used for reverse transcription and qPCR.
Acknowledgements
None.
Abbreviations
- CS
Clonorchis sinensis
- EVs
Extracellular vesicles
- ICC
Intrahepatic cholangiocarcinoma
- CS-ICC
ICC with Clonorchis sinensis infection
- NC-ICC
ICC without Clonorchis sinensis infection
- qPCR
Quantitative polymerase chain reaction
Author contributions
LJW, JGY, and JXZ conceived and designed the study. LJW, YXW, and JXZ performed the experiments, analyzed the data, and wrote the manuscript. YXW and KL collected the specimens and clinical data. LJW and JXZ contributed materials and analytical tools. JGY, KL, and JXZ critically revised the manuscript. All the authors have read and approved the final version of this manuscript.
Funding
This study was supported by the Science and Technology Development Program of Jilin Province (Grant No. 20200201584JC).
Availability of data and materials
All datasets are available within the paper.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the First Hospital of Jilin University (permit no. 19K041-001). The animal study protocol was approved by the Animal Ethics Committee of First Hospital of Jilin University (permit no. 20210526). Informed consent was obtained from all the participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1186/s13071-024-06408-0
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/18/2024
This article has been retracted. Please see the Retraction Notice for more detail: 10.1186/s13071-024-06408-0
References
- 1.Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215–1229. doi: 10.1053/j.gastro.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kodali S, Shetty A, Shekhar S, Victor DW, Ghobrial RM. Management of intrahepatic cholangiocarcinoma. J Clin Med. 2021;10:2368. doi: 10.3390/jcm10112368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi D, Lim JH, Lee KT, Lee JK, Choi SH, Heo JS, et al. Cholangiocarcinoma and Clonorchis sinensis infection: a case-control study in Korea. J Hepatol. 2006;44:1066–1073. doi: 10.1016/j.jhep.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 4.Qian MB, Li HM, Jiang ZH, Yang YC, Lu MF, Wei K, et al. Severe hepatobiliary morbidity is associated with Clonorchis sinensis infection: The evidence from a cross-sectional community study. PLoS Negl Trop Dis. 2021;15:e9116. doi: 10.1371/journal.pntd.0009116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li W, Dong H, Huang Y, Chen T, Kong X, Sun H, et al. Clonorchis sinensis co-infection could affect the disease state and treatment response of HBV patients. PLoS Negl Trop Dis. 2016;10:e4806. doi: 10.1371/journal.pntd.0004806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang JI, Lee K, Kim D, Yang JI, Park JK, Choi K, et al. Clinical characteristics of Clonorchis sinensis-associated cholangiocarcinoma: a large-scale, single-center study. Front Med (Lausanne) 2021;8:675207. doi: 10.3389/fmed.2021.675207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thebaud B, Stewart DJ. Exosomes: cell garbage can, therapeutic carrier, or trojan horse? Circulation. 2012;126:2553–2555. doi: 10.1161/CIRCULATIONAHA.112.146738. [DOI] [PubMed] [Google Scholar]
- 8.Record M, Carayon K, Poirot M, Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta. 2014;1841:108–120. doi: 10.1016/j.bbalip.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Andaloussi S, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 11.de la Torre-Escudero E, Bennett A, Clarke A, Brennan GP, Robinson MW. Extracellular vesicle biogenesis in helminths: more than one route to the surface? Trends Parasitol. 2016;32:921–929. doi: 10.1016/j.pt.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Sheng ZA, Wu CL, Wang DY, Zhong SH, Yang X, Rao GS, et al. Proteomic analysis of exosome-like vesicles from Fasciola gigantica adult worm provides support for new vaccine targets against fascioliasis. Parasit Vectors. 2023;10:16–62. doi: 10.1186/s13071-023-05659-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J, Zhu L, Wang J, Qiu L, Chen Y, Davis RE, et al. Schistosoma japonicum extracellular vesicle miRNA cargo regulates host macrophage functions facilitating parasitism. PLoS Pathog. 2019;4:e1007817. doi: 10.1371/journal.ppat.1007817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan C, Zhou QY, Wu J, Xu N, Du Y, Li J, et al. Csi-let-7a-5p delivered by extracellular vesicles from a liver fluke activates M1-like macrophages and exacerbates biliary injuries. Proc Natl Acad Sci U S A. 2021;118:e2102206118. doi: 10.1073/pnas.2102206118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shan C, Liang Y, Wang K, Li P. Noncoding RNAs in cancer ferroptosis: from biology to clinical opportunity. Biomed Pharmacother. 2023;165:115053. doi: 10.1016/j.biopha.2023.115053. [DOI] [PubMed] [Google Scholar]
- 17.Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31:107–125. doi: 10.1038/s41422-020-00441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Duan S, Li X, Ding J, Zuo L, Sun B, et al. Differences in the secretory exosomes of Clonorchis sinensis adults at different incubation times. Acta Trop. 2022;10:106604. doi: 10.1016/j.actatropica.2022.106604. [DOI] [PubMed] [Google Scholar]
- 19.Zhou N, Yuan X, Du Q, Zhang Z, Shi X, Bao J, et al. FerrDb V2: update of the manually curated database of ferroptosis regulators and ferroptosis-disease associations. Nucleic Acids Res. 2023;6:D571–D582. doi: 10.1093/nar/gkac935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee YR, Chen M, Pandolfi PP. The functions and regulation of the PTEN tumor suppressor: new modes and prospects. Nat Rev Mol Cell Biol. 2018;19:547–562. doi: 10.1038/s41580-018-0015-0. [DOI] [PubMed] [Google Scholar]
- 21.Qian MB, Chen YD, Liang S, Yang GJ, Zhou XN. The global epidemiology of clonorchiasis and its relation with cholangiocarcinoma. Infect Dis Poverty. 2012;1:4. doi: 10.1186/2049-9957-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fürst T, Keiser J, Utzinger J. Global burden of human food-borne trematodiasis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:210–221. doi: 10.1016/S1473-3099(11)70294-8. [DOI] [PubMed] [Google Scholar]
- 23.Li YK, Zhao JF, Yang CL, Zhan GH, Zhang J, Qin SD, et al. Effects of Clonorchis sinensis combined with Hepatitis B virus infection on the prognosis of patients with Hepatocellular Carcinoma following Hepatectomy. PLoS Negl Trop Dis. 2023;17:e0011012. doi: 10.1371/journal.pntd.0011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uddin MH, Choi MH, Kim WH, Jang JJ, Hong ST. Involvement of PSMD10, CDK4, and Tumor suppressors in development of intrahepatic cholangiocarcinoma of syrian golden hamsters induced by Clonorchis sinensis and N-nitrosodimethylamine. PLoS Negl Trop Dis. 2015;9:e0004008. doi: 10.1371/journal.pntd.0004008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi Y, Yu K, Liang A, Huang Y, Ou F, Wei H, et al. Identification and analysis of the tegument protein and excretory-secretory products of the carcinogenic liver fluke Clonorchis sinensis. Front Microbiol. 2020;11:555730. doi: 10.3389/fmicb.2020.555730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng J, Feng Y, Rinaldi G, Yonglitthipagon P, Easley SE, Laha T, et al. The miRNAome of Opisthorchis viverrini induced intrahepatic cholangiocarcinoma. Genom Data. 2014;2:274–279. doi: 10.1016/j.gdata.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu C, Zhu S, Wang J, Lin Y, Ma L, Zhu L, et al. Schistosoma japonicum MiRNA-7-5p inhibits the growth and migration of hepatoma cells via cross-species regulation of S-phase kinase-associated protein 2. Front Oncol. 2019;9:175. doi: 10.3389/fonc.2019.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vahabi M, Pulito C, Sacconi A, Donzelli S, D'Andrea M, Manciocco V, et al. miR-96-5p targets PTEN expression affecting radio-chemosensitivity of HNSCC cells. J Exp Clin Cancer Res. 2019;38:141. doi: 10.1186/s13046-019-1119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwai N, Yasui K, Tomie A, Gen Y, Terasaki K, Kitaichi T, et al. Oncogenic miR-96-5p inhibits apoptosis by targeting the caspase-9 gene in hepatocellular carcinoma. Int J Oncol. 2018;53:237–245. doi: 10.3892/ijo.2018.4369. [DOI] [PubMed] [Google Scholar]
- 30.Wu P, Cao Y, Zhao R, Wang Y. miR-96-5p regulates wound healing by targeting BNIP3/FAK pathway. J Cell Biochem. 2019;120:12904–12911. doi: 10.1002/jcb.28561. [DOI] [PubMed] [Google Scholar]
- 31.Álvarez-Garcia V, Tawil Y, Wise HM, Leslie NR. Mechanisms of PTEN loss in cancer: it's all about diversity. Semin Cancer Biol. 2019;59:66–79. doi: 10.1016/j.semcancer.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Costa C, Wang Y, Ly A, Hosono Y, Murchie E, Walmsley CS, et al. PTEN loss mediates clinical cross-resistance to CDK4/6 and PI3Kα Inhibitors in breast cancer. Cancer Discov. 2020;10:72–85. doi: 10.1158/2159-8290.CD-18-0830. [DOI] [PubMed] [Google Scholar]
- 33.Mouw JK, Yui Y, Damiano L, Bainer RO, Lakins JN, Acerbi I, et al. Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nat Med. 2014;20:360–367. doi: 10.1038/nm.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paul BD, Sbodio JI, Snyder SH. Cysteine metabolism in neuronal redox homeostasis. Trends Pharmacol Sci. 2018;39:513–524. doi: 10.1016/j.tips.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koppula P, Zhuang L, Gan B. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. 2021;12:599–620. doi: 10.1007/s13238-020-00789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cahuzac KM, Lubin A, Bosch K, Stokes N, Shoenfeld SM, Zhou R, et al. AKT activation because of PTEN loss upregulates xCT via GSK3beta/NRF2, leading to inhibition of ferroptosis in PTEN-mutant tumor cells. Cell Rep. 2023;42:112536. doi: 10.1016/j.celrep.2023.112536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Sequences of primers used for reverse transcription and qPCR.
Data Availability Statement
All datasets are available within the paper.