Abstract
Bacillus subtilis was found to possess one detectable superoxide dismutase (Sod) in both vegetative cells and spores. The Sod activity in vegetative cells was maximal at stationary phase. Manganese was necessary to sustain Sod activity at stationary phase, but paraquat, a superoxide generator, did not induce the expression of Sod. The specific activity of purified Sod was approximately 2,600 U/mg of protein, and the enzyme was a homodimer protein with a molecular mass of approximately 25,000 per monomer. The gene encoding Sod, designated sodA, was cloned by the combination of several PCR methods and the Southern hybridization method. DNA sequence analysis revealed the presence of one open reading frame consisting of 606 bp. Several putative promoter sites were located in the upstream region of sodA. The deduced amino acid sequence showed high homology with other bacterial manganese Sods. Conserved regions in bacterial manganese Sod could also be seen. The phenotype of double mutant Escherichia coli sodA sodB, which could not grow in minimal medium without supplemental amino acids, was complemented by the expression of B. subtilis sodA.
Aerobic organisms preferentially utilize oxygen as a terminal electron acceptor in the respiratory chain and effectively obtain ATP for their proliferation in aerobiosis. As a consequence of the partial reduction of oxygen, active oxygen species, such as superoxide anion (O2−), hydrogen peroxide (H2O2), and hydroxyl radical (·OH), are often formed (32). These reactive oxygens are implicated in cellular damage, including DNA strand breakage, protein inactivation, and membrane lipid peroxidation (29, 32). Superoxide dismutase (Sod, EC 1.15.1.1), which occurs in almost all aerobic organisms and in some anaerobic organisms, catalyzes the conversion of superoxide radical to oxygen and hydrogen peroxide and plays an important role in cellular defense against oxidative stress (20). Three kinds of Sod with respect to its metal cofactor, the copper-zinc type (Cu,Zn-Sod), the manganese type (Mn-Sod), and the iron type (Fe-Sod), have been identified so far. In Escherichia coli, these three types of Sods are known to exist in the cytoplasm and periplasm. Mn-Sod, encoded by sodA (53, 54), located in the cytoplasm or interacting with DNA, has been suggested to defend DNA and proteins from oxidation (28, 52). Fe-Sod, encoded by sodB (13, 46), is present near the inner membrane and is suggested to protect membrane lipids (52) as well as cytoplasmic proteins (28) from oxidation. Cu,Zn-Sod, encoded by sodC (4, 30), has been suggested to work in the periplasm to remove exogenous superoxide. In addition, it was revealed that a double mutant of E. coli lacking both sodA and sodB was extremely sensitive to oxidative stress and could not grow aerobically in minimal medium (12). Single mutants lacking either sodA or sodB grew in a manner similar to the wild-type strain, although they were more sensitive to oxidants than the wild-type strain (12). It seemed therefore that the difference in the localization of each Sod was due to its structural property and was important for effective protection against oxidative stress and/or oxidants (28).
Whereas the properties of Sods in E. coli have been investigated in detail, not much is known about Sods in gram-positive bacteria, although some of their Sod genes have been cloned (8, 9, 15, 22, 23, 41, 44). Bacilli are strict aerobes and produce spores that are highly resistant to a variety of stresses, including heat, UV light, and oxidants (51). Since some of these bacilli often contaminate and spoil foods, pharmaceuticals, and other environments, oxidants such as hydrogen peroxide and peracetic acid have been employed as effective disinfectants. Therefore, we are interested in the characteristics of oxidative stress in bacilli, since it is presumed that bacilli may possess a potent oxidative-stress defense system in spores as well as vegetative cells. Most of the studies on this subject have been confined to the response to hydrogen peroxide (6, 25). Very recently, Casillas-Martinez and Setlow constructed a Sod-deficient strain of B. subtilis and indicated that this mutant was sensitive to paraquat but not to heat or to hydrogen peroxide (14). We therefore investigated and characterized in this study the gene and protein of B. subtilis Sod, which plays a key role in the response to superoxide in this bacterium.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacillus subtilis 168 trpC2 (11) was used in the present study and also served as a chromosomal DNA donor. Escherichia coli JM109 (recA1 endA1 gyrA96 thi hsdR17 supE44 relA1 Δ(lac-proAB)/F[traD36 proAB+ lacIq lacZΔM15]) (47) was used as a host strain for the gene cloning of B. subtilis sodA. E. coli IM301 (sodA), IM302 (sodB), and IM303 (sodA sodB) from laboratory stock, which were constructed from E. coli MM294 (endA1 hsdR17 supE44 thi) by homologous recombination methods (26), were also used as host strains for the expression and cloning of the complete sodA gene. A high-copy-number plasmid, pUC19 (56), and a low-copy-number plasmid, pMW118 (Nippon Gene), were used as vectors for cloning and nucleotide sequencing.
Growth conditions.
B. subtilis 168 was basically grown aerobically at 37°C in Luria-Bertani (LB) broth (39) or nutrient broth (NB), if necessary, supplemented with MgCl2 or FeSO4 at a concentration of 0.1 mM. Spizizen salts (1) supplemented with 0.5 g of glucose and 20 mg of tryptophan per liter was used as a minimal medium in some of the experiments. Schaeffer’s sporulation medium contained 0.1 mM MnCl2 · 4H2O, 1 mM Ca(NO3)2 · 4H2O, 2 mM MgSO4 · 7H2O, 1 μM FeSO4 · 7H2O, and 27 mM KCl (48). E. coli strains were cultivated in LB broth. When necessary, ampicillin was added to the growth medium at 50 μg/ml to maintain a plasmid.
The cell growth was monitored by the optical density of culture at 650 nm. The spores were counted as follows. A portion of the culture was heat treated at 80°C for 15 min. After the sample was diluted and plated on nutrient agar, the plates were incubated at 37°C for 1 day, and the resultant colonies were considered to be the number of spores.
Cloning of sodA. (i) Amplification of a portion of sodA by PCR.
PCR was carried out with ELONGase enzyme mix (Gibco BRL) as a thermostable DNA polymerase with 30 cycles of the following three-step cycle (30 s at 94°C, 30 s at 57°C, and 1 min at 74°C) and a final step of 10 min at 74°C. Two primers were constructed, SOD1, 5′-TA(T/C)GA(T/C)GCTCTAGA(A/G)CC(N)CA-3′ (from Tyr12 to His18), and SOD2, 5′-TA(N)GCATGCTCCCA(N)AC(A/G)TC-3′ (from Tyr170 to Asp164), which contained XbaI and SphI restriction sites, respectively, indicated by underlines (see Fig. 4 and 5). These primers were designed on the basis of highly conserved regions of amino acid sequences in bacterial Mn-Sods. The amplified DNA fragment was digested with XbaI and SphI and cloned into XbaI-SphI sites in pUC19, and the resultant plasmid was designated probe pUS. Its sequence was analyzed with the ALFred sequencer system (Pharmacia Biotech).
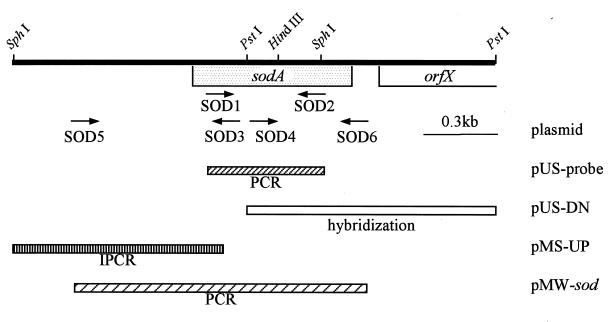
FIG. 4.
The restriction map around the sodA region of B. subtilis chromosome DNA and sodA cloning strategy. Arrows indicate constructed oligonucleotide primers (SOD1 to SOD6) for DNA cloning and sequencing. Boxes below the restriction map indicate cloning regions.
FIG. 5.
Nucleotide sequence of sodA. The sodA-coding region starts at nt 701 and ends at nt 1309. The amino acid sequence is given below the nucleotide sequence. The ribosome-binding site (RBS) is shown by bold letters, and a putative transcriptional terminator forming a stem-and-loop structure is shown with arrows. Double-underlined amino acid sequence is coincident with the N-terminal amino acid sequence obtained from the purified protein, and the first amino acid residue (in parentheses), methionine, is released for maturation.
(ii) Cloning of the complete sodA gene.
DNA cloning was carried out by colony hybridization (2). A DNA labeling and detection kit purchased from Boehringer Mannheim was used for labeling probes and selecting positive clones. Using the PCR-amplified fragment described above as a probe, we attempted to select a sodA recombinant plasmid which included the whole sodA gene (Fig. 4) from the chromosomal libraries, which were digested by BamHI, EcoRI, or XbaI, inserted into pUC19 or pMW118 by colony hybridization. This attempt eventually failed. Then the sodA region was divided into two fragments at the PstI site, and the resultant two fragments were used to obtain clones by colony hybridization with the same probe. The 966-bp genomic fragment including the sodA downstream region was cloned into pUC19 and designated pUS-DN, whereas the cloning of the sodA upstream region including the sodA promoter region and translation-initiating site was unsuccessful.
Then the nucleotide sequence of the promoter region was determined. Two primers, SOD3, 5′-TGTTTCCGTCGACCGCTT-3′, and SOD4, 5′-GAGGCGAGTCGACTGGCGCG-3′, including SalI sites, indicated by underlines, were constructed, corresponding to the two sequences of nucleotides [nt] 839 to 822 and 981 to 1000, respectively (see Fig. 4 and 5). After the chromosomal DNA of B. subtilis was digested by SphI, resultant fragments were self-ligated by T4 DNA ligase (DNA concentration, 10 μg/ml in the reaction solution) and then digested by PstI. An approximately 1-kb fragment of the promoter region of sodA, which was amplified by inverse PCR with the above-mentioned two primers, was digested by SphI and SalI and cloned into the SphI-SalI region of pMW118, designated pMS-UP, and its sequence was then determined. The inverse PCR conditions were the same as the PCR conditions, described above, except for the template DNA concentration of 1 mg/ml.
The complete sodA was amplified by PCR with the following two primers: SOD5, 5′-CCGCAGTAGAATTCGCAGCA-3′, and SOD6, 5′-TGTTTACGTCGACATCGCCT-3′, corresponding to nt 215 to 234 and 1458 to 1439, respectively (see Fig. 4 and 5), involving the EcoRI and SalI sites indicated by underlines. The amplified sodA fragment was cloned into a low-copy-number plasmid, pMW118, creating pMW-sod.
Preparation of crude extracts of vegetative cells and spores.
Cells washed twice with PBE (50 mM potassium phosphate buffer [KPB] containing 0.1 mM EDTA, pH 7.8) were resuspended in fresh PBE containing 0.1 mg of lysozyme (but 2 mg for Sod purification) and 3 μg of DNase I per ml. The suspension was then incubated for 30 min at 37°C. After centrifugation at 20,000 × g for 30 min at 4°C, the resultant supernatant was recovered as the vegetative cell extract. The pellet was resuspended in PBE containing 5 mg of lysozyme per ml, and the suspension was incubated for 30 min at 37°C to destruct vegetative cells completely. The pellet containing spores was collected by centrifugation at 4,000 × g for 10 min at 4°C, washed 10 times with distilled water, and then suspended in 1 ml of fresh PBE. The spore suspension was disrupted by cell homogenizer (B. Braun), with five cycles of a 30-s treatment. After centrifugation at 6,000 × g for 20 min at 4°C, the resultant supernatant was recovered as the spore extract. The crude extracts of vegetative cells and spores obtained were stored at −80°C.
Purification of Sod from vegetative cell extract.
A 3-liter culture of B. subtilis 168 grown for 25 h was harvested by centrifugation. The crude extract from vegetative cells, prepared as described above, was fractionated by 70 to 90% saturated ammonium sulfate. After the extract was stirred for 30 min at 25°C, it was centrifuged at 25,000 × g for 15 min at 4°C. The resultant precipitate was collected by centrifugation, resuspended in 4 ml of PBE, and then dialyzed against fresh PBE for 12 h. The precipitate that formed during dialysis was removed, and the supernatant was applied onto a DEAE Sepharose CL-6B (Pharmacia Biotech) column equilibrated with PBE. Proteins were eluted with a linear gradient of 0 to 0.5 M NaCl equilibrated in PBE, and the fractions containing Sod activity were collected and dialyzed against 50 mM KPB (pH 7.8). This sample obtained was applied onto a Mono Q HR 5/5 (Pharmacia Biotech) column equilibrated with 50 mM KPB (pH 7.8). After Sod was eluted with a linear NaCl gradient, its activity-containing fractions were collected and dialyzed similarly.
The molecular mass of the native protein was estimated with a Superdex 200 HR 10/30 (Pharmacia Biotech) gel filtration column equilibrated with 50 mM KPB (pH 7.8) containing 150 mM NaCl. A gel filtration standard kit (Bio-Rad) was used as a molecular mass marker. The molecular mass of monomer protein was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Enzyme activity and protein analyses.
Sod activity was determined by two methods. The first method, a qualitative method, involved a native PAGE gel (7.5% [wt/vol] acrylamide), by which Sod activity on a native gel was visualized with nitroblue tetrazolium (3, 18). The second, quantitative method, was based upon the inhibition of cytochrome c reduction by superoxide generated from xanthine-xanthine oxidase reaction (38). Enzyme purity was determined by SDS-PAGE (12% [wt/vol]) (33), in which proteins were visualized with Coomassie brilliant blue R250 stain. Protein concentration was determined by bicinchoninic acid protein assay reagent (Pierce), with bovine serum albumin as a standard protein. The N-terminal sequence of purified Sod was determined with a model 476A protein sequencer (Applied Biosystems).
Nucleotide sequence accession number.
The sequence of sodA has been deposited in DDBJ under accession no. D86856.
RESULTS
Sod activity in B. subtilis.
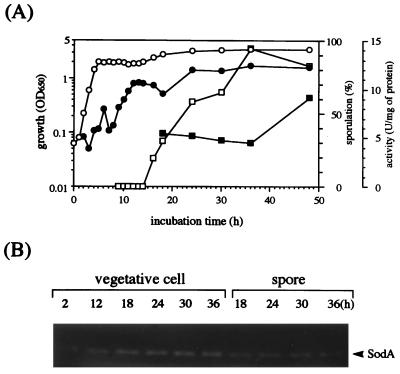
When B. subtilis cells were grown in Schaeffer’s sporulation medium, the Sod activity in vegetative cells increased gradually over the logarithmic and stationary phases (Fig. 1A). A similar pattern of activity was observed with cells grown in minimal medium, Spizizen salts medium, although the activity was lower (data not shown). Interestingly, a substantial level of Sod activity was also observed in spores. Since there is a possibility that the activity obtained in spores is due to contamination from vegetative cells, catalase activity in both extracts was assayed by the catalase activity stain on a native polyacrylamide gel. It has already been reported that the types of catalase present in vegetative cells and spores are different (7, 34, 35). We could confirm this for the vegetative cell and spore extracts obtained in this study (37), indicating no substantial contamination of Sod activity from vegetative cells in the spore.
FIG. 1.
Sod activity in B. subtilis 168 during cell growth and sporulation periods. Cells were cultivated in Schaeffer’s sporulation medium containing potassium chloride, magnesium sulfate, calcium nitrate, and manganese chloride under aerobic conditions. Cell growth (○) and sporulation efficiency (□) were measured as described in Materials and Methods. (A) Sod activities in the vegetative cell (•) and spore extracts (▪) are indicated. OD650, optical density at 650 nm. (B) Sod activities in both extracts obtained after cultivation for times (in hours) indicated over each lane were visualized by the activity stain on a native polyacrylamide gel.
Although three types of Sod have been reported in E. coli and are presumed to be located at different sites of cells (4, 28, 52), in B. subtilis, only one activity band with the same migration on a native polyacrylamide gel could be seen in both vegetative cells and spores (Fig. 1B). No secreting type of Sod could be detected (data not shown).
Inducibility of Sod activity in vegetative cells.
We observed that the manganese salt supplementation improved the increased doubling time and the decreased cell yield in the minimal medium but not in rich media, LB broth, NB, or Schaeffer’s sporulation medium (data not shown). The Sod activity of cells grown in LB broth was increased by manganese supplementation and reached a relatively high level at stationary phase (Fig. 2). On the other hand, the Sod activity in cells grown in LB medium supplemented with ferrous salt or in LB medium without metals was slightly increased in the exponential phase but slightly decreased in the stationary phase. Although the Mn-Sod activity in E. coli has been reported to be repressed by the addition of ferric salt to the culture (27), this effect was not observed with B. subtilis (Fig. 2). The Sod activity in the exponential phase increased transiently at 4 h. This increase was reproducible in four independent experiments, although the reason for it remains unclear. Furthermore, the intracellular Sod activity in E. coli is known to be induced by exposing cells to 50 μM paraquat (45, 55), but no such inducible effect of the oxidant was observed in B. subtilis cells grown with or without manganese ion (data not shown).
FIG. 2.
Effect of added metal ions on Sod activity in B. subtilis 168 cells. Cells were grown in LB broth without metal ions (□, ▪) and with manganese ion (○, •) or ferrous ion (▵, ▴). Open symbols, growth; closed symbols, Sod activity; OD650, optical density at 650 nm.
Purification and characterization of Sod.
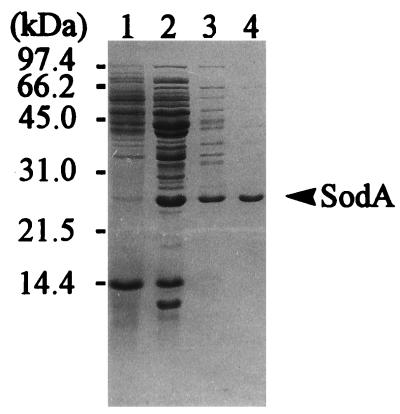
In this experiment, the activity of only one Sod protein was detected on the native gel. We purified the Sod from the vegetative cells cultivated in manganese-supplemented NB when the Sod activity was maximal for 36 h. The results of a series of purification processes are summarized in Table 1 and Fig. 3. The specific activity of purified Sod was about 2,600 U/mg of protein at 25°C. The molecular mass of this protein was estimated to be approximately 45 kDa by gel filtration and approximately 25 kDa when the protein was visualized as a single band by SDS-PAGE, indicating that Sod is a homodimer. The heat stability of this Sod was examined at several temperatures. Approximately 80% of the Sod activity remained after heating at 60°C for 30 min, but the activity was lost after 80°C for 30 min. These characteristics of the B. subtilis Sod were similar to those of other bacterial Sods (20).
TABLE 1.
Purification of B. subtilis SodA
| Fraction | Protein concn (mg/ml) | Total protein (mg) | Sp act (U/mg) | Total U | Fold purification | Enzyme yield (%) |
|---|---|---|---|---|---|---|
| Crude extract | 5.70 | 358 | 14.7 | 5,260 | 1 | 100 |
| Ammonium sulfate (70–90% saturated) | 3.00 | 8.10 | 196 | 1,590 | 13.3 | 30.2 |
| DEAE Sepharose CL-6B | 0.128 | 1.02 | 1,120 | 1,150 | 76.2 | 21.9 |
| Mono Q | 0.112 | 0.152 | 2,580 | 392 | 176 | 7.5 |
FIG. 3.
Protein patterns in each step of SodA purification as visualized by Coomassie brilliant blue stain on SDS-polyacrylamide gel. Lane 1, crude extract of B. subtilis 168 cells grown in manganese-containing NB; lane 2, ammonium sulfate precipitation; lane 3, DEAE Sepharose CL-6B column chromatography; lane 4, Mono Q column chromatography. The arrow indicates the band of SodA protein, and the numbers at the left indicate the molecular masses of standard proteins.
The N-terminal amino acid sequence was determined with a protein sequencer to be AYELPELPYAYDALEPHIDK, being highly homologous to other bacterial Mn-Sods in the DDBJ database.
sodA gene cloning.
We attempted to clone the sodA gene by the colony hybridization method described in Materials and Methods and by the shut gun cloning method, using the Sod-deficient strain E. coli IM303 as a host, which required some amino acids because of the Sod deficiency. Because the complete gene fragment could not be obtained directly in one step, a partial fragment of the sodA open reading frame region was cloned by PCR with two primers, SOD1 and SOD2, which corresponded to conserved regions of bacterial Sods’ amino acid sequences (Fig. 4 and 5; also see Fig. 6), and then sequenced. As a result, the amino acid sequence predicted from the nucleotide sequence obtained was similar to those of the corresponding parts of several bacterial Mn-Sods, especially to that of the enzyme of Bacillus caldotenax (Fig. 5, nt 745 to 1206) (15). On the basis of this nucleotide sequence, the whole sodA region was cloned, and its nucleotide sequence was determined by the procedure described in Materials and Methods. We succeeded in cloning the PstI fragment, including the 3′-downstream region of sodA, by colony hybridization (Fig. 4), but not the fragment including the 5′-upstream region of sodA. Then, the sequence of this PstI fragment including the 3′-downstream region of sodA was determined (Fig. 5). Furthermore, to determine the nucleotide sequence of the 5′-upstream region, the chromosomal DNA was digested by SphI, and the digested fragment was self-ligated by T4 DNA ligase and then digested by PstI. With primers SOD3 and SOD4 and this DNA fragment as the template, the 5′-upstream region of sodA was amplified by inverse PCR (Fig. 4). The nucleotide sequence of the amplified DNA region was then determined directly (Fig. 5). Consequently, we successfully determined the complete nucleotide sequence of sodA and the sequence of its upstream regulatory region. Finally, we cloned the complete sodA gene by amplification with PCR with two primers, SOD5 and SOD6, into a low-copy-number plasmid, pMW118 (Fig. 4). The nucleotide sequence of this amplified fragment was identical to that of sodA in the chromosome. The resultant recombinant plasmid was designated pMW-sod.
FIG. 6.
Comparison of the amino acid sequence of B. subtilis Sod with those of other bacterial Sods. Amino acid sequences of Sods of B. caldotenax (accession no. X62682), B. stearothermophilus (accession no. M81914), and E. coli (accession no. X03951) from DDBJ, EMBL, and GenBank nucleotide sequence databases are shown. Dashes indicate identical amino acid residues. Four metal-binding sites deduced from the three-dimensional structural analysis of B. stearothermophilus Mn-Sod are indicated with asterisks above the amino acid sequence of B. subtilis.
Nucleotide sequence of sodA.
The complete nucleotide sequence of sodA is shown in Fig. 5, together with the amino acid sequence. The open reading frame consisted of 606 nt (202 amino acids), and the N-terminal amino acid sequence deduced from the nucleotide sequence was consistent with the sequence determined from the purified protein except for the methionine in the first residue. The first methionine was presumed to be removed for maturation, as has been done previously for other bacterial Sod proteins (8, 43, 53). The molecular mass predicted from the sequence was 22,358, which was close to that of purified Sod as calculated by SDS-PAGE. These results verify that this gene encodes the Sod purified here. Moreover, we analyzed the nucleotide sequence of the regulatory region near sodA. A putative ribosome-binding site (31) localized at 14 to 8 bp upstream from the translation-starting site. Six putative promoters whose nucleotide sequences were similar to the consensus sequence of the respective promoters identified in B. subtilis (24) were found in the upstream region of the putative ribosome-binding site (Fig. 5). These promoters were predicted to be recognized by independent sigma factors ςA, ςB, ςC, ςD, ςF, and ςK. In addition, the presence of three secondary structures was predicted in the region close to sodA. One of these structures was located in the sodA downstream region and was suggested to be a transcriptional terminator (Fig. 5), and another two sites, IR1 and IR2, were located upstream of the sodA-coding region (Fig. 5). IR1 overlapped with the −35 region of the putative promoter recognized by ςA, and IR2 overlapped with the ribosome-binding site. It is suggested that these two regions are the binding sites for any possible transcriptional or translational regulatory factor involving sodA expression, as discussed later.
A comparison of the deduced amino acid sequence of Sod with other bacterial Sods’ amino acid sequences with the Blast program indicated that the deduced amino acid sequence of Sod was highly homologous to those of Mn-Sods from Bacillus stearothermophilus (8) and B. caldotenax (15), with 80% identity for both (Fig. 6). Four metal-binding sites, which have been proposed in the Mn-Sod molecule from B. stearothermophilus by the analysis of a three-dimensional structure and are presumed to function as an element of the active site of Sod (43), were completely conserved.
Expression of the cloned sodA in E. coli.
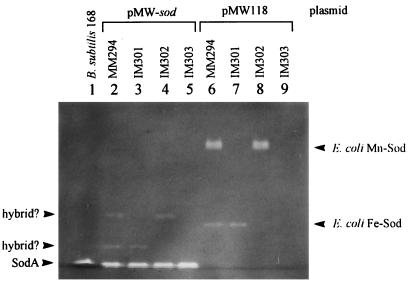
The Sod activity in E. coli IM303 (sodA sodB) bearing pMW-sod was analyzed by native PAGE followed by activity stain. The band of Sod produced in this strain migrated to the same position as that of Sod produced in B. subtilis 168. It is known that intracellular Sod-deficient mutants of E. coli cannot grow aerobically in minimal medium but can normally grow aerobically in rich medium and anaerobically in minimal medium (12). Such a defect in the growth of the Sod-deficient mutant was complemented by the introduction of a B. subtilis sodA-bearing plasmid into the E. coli cell (data not shown). Furthermore, when Sod activity was examined in E. coli IM301 (sodA), IM302 (sodB), and their wild-type strain MM294, each bearing B. subtilis sodA (Fig. 7), some new bands with weak Sod activity appeared, but they did not coincide with the Sod of B. subtilis or with Mn-Sod, Fe-Sod, and the hybrid Sod proteins of E. coli. These weak Sod activities were presumed to indicate hybrid Sod proteins constructed from the Sod monomer of B. subtilis and the counter monomer of Mn-Sod or Fe-Sod of E. coli, since these Sod proteins on the native polyacrylamide gel migrated at the intermediate positions between B. subtilis Sod and Mn-Sod or Fe-Sod of E. coli. These results suggested that the three-dimensional structure of B. subtilis Sod is highly similar to those of Mn-Sod and Fe-Sod of E. coli.
FIG. 7.
Expression of B. subtilis sodA in intracellular Sod-deficient strains of E. coli. The presence of Sod activity in E. coli strains bearing a sodA recombinant plasmid, pMW-sod, or pMW118 was visualized by the Sod activity stain on a native polyacrylamide gel.
DISCUSSION
We determined in this study that only one detectable Sod, designated SodA, was present in B. subtilis. Nucleotide sequence analysis indicated that sodA possessed several putative promoters, including a ςF-recognizing promoter (Fig. 5), which works in forespore (24). Kobayashi and his coworkers have determined the nucleotide sequence of a gene corresponding to sodA reported in this study, yqgD, in the B. subtilis genome project (accession no. D84432 in DDBJ). Comparison of these two sequence data indicated two differences: 666G→T in the upstream point of sodA and 1299AAGCA1303→ACGA in the internal region of the sodA gene, located just before the stop codon. The latter mismatch leads the misdeduced amino acid sequence. The findings of a high similarity of the putative amino acid sequence of B. subtilis SodA to those of Mn-Sod from B. caldotenax and B. stearothermophilus, the presence of four conserved metal-binding sites, and the inducibility of manganese for Sod activity strongly suggested that the purified Sod of B. subtilis is manganese associated.
It is interesting that the B. subtilis Sod was present both in vegetative cells and in spores. Sod might have some role in spore resistance and/or in resistance to oxidative stress possibly generated during the sporulation and/or germination period(s). It should be noted that Sod activity could not be induced with paraquat added to the culture. In Escherichia coli, Sod activity was induced by oxidative stress (50) and by a shift from an anaerobic to an aerobic milieu (36). Under oxidative stress, no inducibility of Sod activity in B. subtilis may be attributed to the aerobic property of this strain.
It remains unclear whether the observed inducible effect of manganese on Sod activity during the stationary phase is at the transcriptional or the posttranslational level, although in E. coli, both types of regulation for the expression of the Mn-Sod gene (sodA) are known to exist (5, 17, 27, 45, 49). Recently, in E. coli sodA expression was induced by the addition of MnCl2 at the transcriptional level with the involvement of Fur (49). Based upon this evidence relating to E. coli sodA expression, the expression of sodA in B. subtilis might be regulated by Fur, which is affected by the manganese salt concentration. Although it remains unclear whether manganese concentration is one of the elements regulating Sod activity in B. subtilis, it is suggested that manganese has an important role not only for the activity itself but also for regulating Sod.
The presence of several putative promoters, including those recognized by ςA, ςB, and ςF (24), upstream of sodA suggests that the sodA expression may be complicated. Since its activity was relatively high through the stationary phase and in spores, the Sod protein present during the sporulation period might be expressed from the ςF promoter, whereas the protein produced at late exponential phase might be expressed from ςB. It remains unclear which promoters are active and why so many promoters are possibly involved in the expression of Sod in B. subtilis. It is known that three different catalases, KatA, KatE, and KatX, are present in B. subtilis, depending upon the phase of cell development (6). KatA (7, 34) is expressed in vegetative cells during the whole exponential phase, while KatE (19) is expressed during the late exponential and stationary phases, and KatX (21) exists in spores. In the case of substantially the Sod alone, therefore, the presence of several promoters for sodA expression may be required to produce functional Sod in all phases of growth and in spores.
The presence of two inverted-repeat sequences, IR1 and IR2, at the upstream region of sodA implies that they may be regulatory elements, such as a recognition site for a possible repressor protein. In E. coli sodA, such an inverted-repeat sequence has also been found on the promoter of sodA (17, 40), and this site is known to be the binding site for Fur, which senses intracellular iron concentration (42). We compared the sequences of these two inverted repeats, IR1 and IR2, with that of the Fur-binding site in E. coli and with other regulatory regions of a variety of genes. Partial similarity was found between IR1 of B. subtilis sodA and the Fur box in E. coli as well as the Per box in B. subtilis, both of which were already known to be similar (16). In B. subtilis, it is known that the Per box is an oxidative stress-regulatory element and present in the upstream regions of katA (10), ahpCF (10), and mrgA (16). The expression of these genes has been reported to be repressed by the addition of iron salt or manganese salt, while it can be induced with hydrogen peroxide. The results obtained in this study, indicating that neither the repressive effect of iron and manganese nor the inducible effect of hydrogen peroxide was observed (although the addition of manganese salt increased SodA activity at stationary phase), suggested that IR1 could not function as the Per box. The possibility that the function of the Per box could not be observed in our study because of the complication of sodA expression cannot be ruled out, however. The other inverted-repeat sequence, IR2, had no homology with the other inverted-repeat sequence surveyed, and its function remains to be examined.
At present, we are analyzing the transcriptional starting site by primer extension and investigating the regulatory mechanism of sodA expression. Furthermore, we have constructed a sodA-deficient mutant by homologous recombination and are investigating its characteristics. These studies may clarify the detailed functions of SodA in B. subtilis cells and spores exposed to oxidative stress and contribute to the understanding of the properties and mechanisms of bactericidal oxidants.
ACKNOWLEDGMENTS
We thank Tadayuki Imanaka for providing Sod-deficient mutants of E. coli and Yasuji Oshima for encouragement in this study.
This work was supported by a research grant from Kansai University.
REFERENCES
- 1.Anagnostopoulos C, Spizizen J. Requirements for transformation in Bacillus subtilis. J Bacteriol. 1961;81:741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, Albright L M, Coen D M, Varki A, Janssen K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 3.Beachamp C, Fridovich I. Superoxide dismutase improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 4.Benov L T, Fridovich I. Escherichia coli expresses a copper- and zinc-containing superoxide dismutase. J Biol Chem. 1994;269:25310–25314. [PubMed] [Google Scholar]
- 5.Beyer W F, Jr, Fridovich I. In vivo competition between iron and manganese for occupancy of the active site region of the manganese-superoxide dismutase of Escherichia coli. J Biol Chem. 1991;266:303–308. [PubMed] [Google Scholar]
- 6.Bol D K, Yasbin R E. Characterization of an inducible oxidative stress system in Bacillus subtilis. J Bacteriol. 1990;172:3503–3506. doi: 10.1128/jb.172.6.3503-3506.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bol D K, Yasbin R E. The isolation, cloning and identification of a vegetative catalase gene from Bacillus subtilis. Gene. 1991;109:31–37. doi: 10.1016/0378-1119(91)90585-y. [DOI] [PubMed] [Google Scholar]
- 8.Bowler C, Kaer L V, Camp W V, Montagu M V, Inze D, Dhaese P. Characterization of the Bacillus stearothermophilus manganese superoxide dismutase gene and its ability to complement copper/zinc superoxide dismutase deficiency in Saccharomyces cerevisiae. J Bacteriol. 1990;172:1539–1546. doi: 10.1128/jb.172.3.1539-1546.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brehm K, Haas A, Goebel W, Kreft J. A gene encoding a superoxide dismutase of the facultative intracellular bacterium Listeria monocytogenes. Gene. 1992;118:121–125. doi: 10.1016/0378-1119(92)90258-q. [DOI] [PubMed] [Google Scholar]
- 10.Bsat N, Chen L, Helmann J D. Mutation of the Bacillus subtilis alkyl hydroperoxide reductase (ahpCF) operon reveals compensatory interactions among hydrogen peroxide stress genes. J Bacteriol. 1996;178:6579–6586. doi: 10.1128/jb.178.22.6579-6586.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burkhold P R, Giles N H. Induced biochemical mutant in Bacillus subtilis. Am J Bot. 1947;33:345–348. [PubMed] [Google Scholar]
- 12.Carlioz A, Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986;5:623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlioz A, Ludwig M L, Stallings W C, Fee J A, Steinman H M, Touati D. Iron superoxide dismutase. Nucleotide sequence of the gene from Escherichia coli K12 and correlations with crystal structures. J Biol Chem. 1988;263:1555–1562. [PubMed] [Google Scholar]
- 14.Casillas-Martinez L, Setlow P. Alkyl hydroperoxide reductase, catalase, MrgA, and superoxide dismutase are not involved in resistance of Bacillus subtilis spores to heat or oxidizing agents. J Bacteriol. 1997;179:7420–7425. doi: 10.1128/jb.179.23.7420-7425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chambers S P, Brehm J K, Michael N P, Atkinson T, Minton N P. Physical characterisation and over-expression of the Bacillus caldotenax superoxide dismutase gene. FEMS Microbiol Lett. 1992;70:277–284. doi: 10.1016/0378-1097(92)90710-6. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, James L P, Helmann J D. Metalloregulation in Bacillus subtilis: isolation and characterization of two genes differentially repressed by metal ions. J Bacteriol. 1993;175:5428–5437. doi: 10.1128/jb.175.17.5428-5437.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Compan I, Touati D. Interaction of six global transcription regulators in expression of manganese superoxide dismutase in Escherichia coli K-12. J Bacteriol. 1993;175:1687–1696. doi: 10.1128/jb.175.6.1687-1696.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis B J. Disc electrophoresis. II. Method and application to human serum protein. Ann N Y Acad Sci. 1964;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- 19.Engelmann S, Lindner C, Hecker M. Cloning, nucleotide sequence, and regulation of katE encoding a sigma B-dependent catalase in Bacillus subtilis. J Bacteriol. 1995;177:5598–5605. doi: 10.1128/jb.177.19.5598-5605.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 21.Fujita, Y. 1997. (Fukuyama University). Personal communication.
- 22.Gaillot O, Poyart C, Berche P, Trieu-Cuot P. Molecular characterization and expression analysis of the superoxide dismutase gene from Streptococcus agalactiae. Gene. 1997;204:213–218. doi: 10.1016/s0378-1119(97)00548-9. [DOI] [PubMed] [Google Scholar]
- 23.Haas A, Goebel W. Cloning of a superoxide dismutase gene from Listeria ivanovii by functional complementation in Escherichia coli and characterization of the gene product. Mol Gen Genet. 1992;231:313–322. doi: 10.1007/BF00279805. [DOI] [PubMed] [Google Scholar]
- 24.Haldenwang W G. The sigma factors of Bacillus subtilis. Microbiol Rev. 1995;59:1–30. doi: 10.1128/mr.59.1.1-30.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartford O M, Dowds B C A. Isolation and characterization of a hydrogen peroxide resistant mutant of Bacillus subtilis. Microbiology. 1994;140:297–304. doi: 10.1099/13500872-140-2-297. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton C M, Aldea M, Washburn B K, Babitzke P, Kushner S R. New method for generating deletions and gene replacements in Escherichia coli. J Bacteriol. 1989;171:4617–4622. doi: 10.1128/jb.171.9.4617-4622.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hassan H M, Schrum L W. Roles of manganese and iron in the regulation of the biosynthesis of manganese-superoxide dismutase in Escherichia coli. FEMS Microbiol Rev. 1994;14:315–323. doi: 10.1111/j.1574-6976.1994.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 28.Hopkin K A, Papazian M A, Steinman H M. Functional differences between manganese and iron superoxide dismutases in Escherichia coli K-12. J Biol Chem. 1992;267:24253–24258. [PubMed] [Google Scholar]
- 29.Imlay J A, Linn S. DNA damage and oxygen radical toxicity. Science. 1988;240:1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- 30.Imlay K R, Imlay J A. Cloning and analysis of sodC, encoding the copper-zinc superoxide dismutase of Escherichia coli. J Bacteriol. 1996;178:2564–2571. doi: 10.1128/jb.178.9.2564-2571.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983;47:1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krieg N, Hoffman P S. Microaerophily and oxygen toxicity. Annu Rev Microbiol. 1986;40:107–130. doi: 10.1146/annurev.mi.40.100186.000543. [DOI] [PubMed] [Google Scholar]
- 33.Laemmli M K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 34.Loewen P C, Switala J. Multiple catalases in Bacillus subtilis. J Bacteriol. 1987;169:3601–3607. doi: 10.1128/jb.169.8.3601-3607.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loewen P C. Genetic mapping of katB, a locus that affects catalase 2 levels in Bacillus subtilis. Can J Microbiol. 1989;35:807–810. doi: 10.1139/m89-134. [DOI] [PubMed] [Google Scholar]
- 36.Matsumura Y, Takagi M, Imanaka T. Regulation of Escherichia coli superoxide dismutase genes (sodA and sodB) by oxygen. Biotechnol Lett. 1993;15:229–234. [Google Scholar]
- 37.Matsumura, Y., M. Mizu, and T. Tsuchido. Unpublished data.
- 38.McCord J M, Fridovich I. Superoxide dismutase and peroxidase: a positive activity stain applicable to polyacrylamide gel electropherograms. Arch Biochem Biophys. 1977;183:511–515. doi: 10.1016/0003-9861(77)90386-1. [DOI] [PubMed] [Google Scholar]
- 39.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 23–56. [Google Scholar]
- 40.Naik S M, Hassan H M. Use of site-directed mutagenesis to identify an upstream regulatory sequence of sodA gene of Escherichia coli K-12. Proc Natl Acad Sci USA. 1990;87:2618–2622. doi: 10.1073/pnas.87.7.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakayama K. Nucleotide sequence of Streptococcus mutans superoxide dismutase gene and isolation of insertion mutants. J Bacteriol. 1992;174:4928–4934. doi: 10.1128/jb.174.15.4928-4934.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niederhoffer E C, Naranjo C M, Bradley K L, Fee J A. Control of Escherichia coli superoxide dismutase (sodA and sodB) genes by the ferric uptake regulation (fur) locus. J Bacteriol. 1990;172:1930–1938. doi: 10.1128/jb.172.4.1930-1938.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parker M W, Blake C C. Crystal structure of manganese superoxide dismutase from Bacillus stearothermophilus at 2.4 A resolution. J Mol Biol. 1988;199:649–661. doi: 10.1016/0022-2836(88)90308-7. [DOI] [PubMed] [Google Scholar]
- 44.Poyart C, Berche P, Trieu-Cuot P. Characterization of superoxide dismutase genes from gram-positive bacteria by polymerase chain reaction using degenerate primers. FEMS Microbiol Lett. 1995;131:41–45. doi: 10.1016/0378-1097(95)00232-t. [DOI] [PubMed] [Google Scholar]
- 45.Privalle C T, Fridovich I. Transcriptional and maturational effects of manganese and iron on the biosynthesis of manganese-superoxide dismutase in Escherichia coli. J Biol Chem. 1992;267:9140–9145. [PubMed] [Google Scholar]
- 46.Sakamoto H, Touati D. Cloning of the iron superoxide dismutase gene (sodB) in Escherichia coli K-12. J Bacteriol. 1984;159:418–420. doi: 10.1128/jb.159.1.418-420.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sambrook J G, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 48.Schaeffer P, Millet J, Aubert J. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci USA. 1965;54:701–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schrum L W, Hassan M H. Transcriptional activation of Mn-superoxide dismutase gene (sodA) of Escherichia coli by MnCl2. Biochim Biophys Acta. 1993;1216:186–190. doi: 10.1016/0167-4781(93)90143-2. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz C E, Krall J, Norton L, McKay K, Kay D, Lynch R E. Catalase and superoxide dismutase in Escherichia coli. J Biol Chem. 1983;258:6277–6281. [PubMed] [Google Scholar]
- 51.Setlow P. Mechanisms for the prevention of damage to DNA in spores of Bacillus species. Annu Rev Microbiol. 1995;49:29–54. doi: 10.1146/annurev.mi.49.100195.000333. [DOI] [PubMed] [Google Scholar]
- 52.Steinman H M, Weinstein L, Brenowitz M. The manganese superoxide dismutase of Escherichia coli K-12 associates with DNA. J Biol Chem. 1994;269:28629–28634. [PubMed] [Google Scholar]
- 53.Takeda Y, Avila H. Structure and gene expression of the E. coli Mn-superoxide dismutase gene. Nucleic Acids Res. 1986;14:4577–4589. doi: 10.1093/nar/14.11.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Touati D. Cloning and mapping of the manganese superoxide dismutase gene (sodA) of Escherichia coli K-12. J Bacteriol. 1983;155:1078–1087. doi: 10.1128/jb.155.3.1078-1087.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Touati D. Transcriptional and posttranscriptional regulation of manganese superoxide dismutase biosynthesis in Escherichia coli, studied with operon and protein fusions. J Bacteriol. 1988;170:2511–2520. doi: 10.1128/jb.170.6.2511-2520.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yanisch P C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequence of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]