Abstract
The presence of two systems in Escherichia coli for gluconate transport and phosphorylation is puzzling. The main system, GntI, is well characterized, while the subsidiary system, GntII, is poorly understood. Genomic sequence analysis of the region known to contain genes of the GntII system led to a hypothesis which was tested biochemically and confirmed: the GntII system encodes a pathway for catabolism of l-idonic acid in which d-gluconate is an intermediate. The genes have been named accordingly: the idnK gene, encoding a thermosensitive gluconate kinase, is monocistronic and transcribed divergently from the idnD-idnO-idnT-idnR operon, which encodes l-idonate 5-dehydrogenase, 5-keto-d-gluconate 5-reductase, an l-idonate transporter, and an l-idonate regulatory protein, respectively. The metabolic sequence is as follows: IdnT allows uptake of l-idonate; IdnD catalyzes a reversible oxidation of l-idonate to form 5-ketogluconate; IdnO catalyzes a reversible reduction of 5-ketogluconate to form d-gluconate; IdnK catalyzes an ATP-dependent phosphorylation of d-gluconate to form 6-phosphogluconate, which is metabolized further via the Entner-Doudoroff pathway; and IdnR appears to act as a positive regulator of the IdnR regulon, with l-idonate or 5-ketogluconate serving as the true inducer of the pathway. The l-idonate 5-dehydrogenase and 5-keto-d-gluconate 5-reductase reactions were characterized both chemically and biochemically by using crude cell extracts, and it was firmly established that these two enzymes allow for the redox-coupled interconversion of l-idonate and d-gluconate via the intermediate 5-ketogluconate. E. coli K-12 strains are able to utilize l-idonate as the sole carbon and energy source, and as predicted, the ability of idnD, idnK, idnR, and edd mutants to grow on l-idonate is altered.
In Escherichia coli, the Entner-Doudoroff (ED) pathway serves as a metabolic “funnel” receiving intermediates formed by catabolism of several sugar acids (17). Hexuronic acids undergo rearrangement in the inducible Ashwell pathways (1) to form 2-keto-3-deoxygluconate, which is then phosphorylated to produce 2-keto-3-deoxy-6-phosphogluconate (KDPG). KDPG is cleaved by KDPG aldolase, encoded by eda, providing for entry of carbon into glycolysis. The other enzyme of the ED pathway is 6-phosphogluconate dehydratase, encoded by edd, which is induced only for catabolism of gluconate and also forms KDPG, the key intermediate of the ED pathway (7). Long considered to be of more significance than is readily obvious (9), the finding that eda and edd eda double mutants are unable to colonize the mouse large intestine underscores the possible ecological importance of ED metabolism (32). The implication from these colonization studies is that colonic mucus, which contains several sugar acids, may serve as an important source of nutrients for E. coli in the gut.
Also participating in gluconate catabolism are several gluconate transporters and two gluconate kinases which appear, based upon their regulation, to comprise two distinct systems (2, 13). The GntI (main) system consists of gntT, gntU, and gntK, which code for high- and low-affinity gluconate transporters and a thermoresistant gluconate kinase, respectively (23–25, 33). Expression of the GntR regulon, that is, GntI together with the edd-eda operon, is negatively controlled by the gntR gene product. The GntII (subsidiary) system is comprised of a thermosensitive gluconate kinase and a gluconate transporter which function for gluconate catabolism in the absence of the GntI system (2, 11, 13, 22). It appears that the subsidiary gluconate transporter, which has an apparent Km for gluconate of 60 μM (23), is encoded by a gene (idnT) which is adjacent to the gene encoding the thermosensitive gluconokinase (idnK) at 96.8 min.
The DNA sequence of the GntII system genes, located at 4492 kb on the genome, was revealed by the E. coli Genome Project (5, 6). If the GntII system had evolved as a subsidiary pathway for gluconate catabolism, one would expect it to contain only a gluconate transporter and gluconate kinase. However, in addition to the divergent idnK and idnT genes, this region also encodes two “dehydrogenase-like” enzymes. The similarity of idnO to gno of Gluconobacter oxydans, which encodes d-gluconate:NADP 5-oxidoreductase (GNO) (15), led to the testing of ketogluconates as enzyme substrates for the two newly identified dehydrogenases. A process of deductive reasoning and biochemical experiments led to the conclusion that the GntII system in fact comprises a novel metabolic pathway for catabolism of l-idonic acid, in which gluconate is a key intermediate. Accordingly, the genes involved in l-idonate metabolism have been given the designation idn (see Table 1 for gene nomenclature).
TABLE 1.
Gene and enzyme nomenclaturea
| Gene designation
|
Gene product | % Identity of proteinb | |
|---|---|---|---|
| Previous | New (acces- sion no.) | ||
| gntV | idnK (P39208) | d-Gluconate kinase | 45 (GntKc) |
| yjgV | idnD (P39346) | l-Idonate 5-dehydrogenase | 30.6 (sheep DHSOd) |
| yjgU | idnO (P39345) | 5-Keto-d-gluconate 5-reductase | 56 (GNOe) |
| gntW | idnT (P39344) | l-Idonate transporter | 61 (GntTf) |
| yjgS | idnR (P39343) | l-Idonate regulator | 46 (GntRg) |
All accession numbers are Swiss-Prot database accession numbers.
Percent identity of the amino acid sequence of the Idn protein to that of the protein shown in parentheses.
E. coli gluconate kinase encoded by gntK (P46859).
Sheep sorbitol dehydrogenase encoded by sorD (P07846).
G. oxydans gluconate:NADP 5-oxidoreductase encoded by gno (P50199).
E. coli gluconate transporter encoded by gntT (P39835).
E. coli gluconate regulator encoded by gntR (P46860).
(Part of this work has been presented previously [3].)
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The E. coli strains and plasmids used for this study are listed in Table 2. E. coli strains were routinely grown at 37°C in Luria broth (LB) (19) or M63 minimal medium (28) with or without added carbohydrate (0.15%). Cell growth was measured spectrophotometrically with a Spectronic 601 spectrometer (Milton Roy Co.). Ampicillin (50 mg/liter), tetracycline (10 mg/liter), and kanamycin (25 mg/liter) were included in growth media where appropriate.
TABLE 2.
Strains and plasmids used in this study
| Strain or plasmid | Relevant genotype, phenotype, or characteristic | Source or reference |
|---|---|---|
| Strains | ||
| W1485 | K-12 wild type | CGSCa |
| DH5α | lacZΔM15 recA | BRLb |
| NP202 | Δ(gntR gntK gntU) | This study |
| NP250 | ΔidnR | This study |
| CB350 | ΔidnD recD | This study |
| TUG287 | F−gntK gntV his trp | 33 |
| DPB271 | recD | 24 |
| P90C | ara Δ(pro-lac) thi | 30 |
| BM129 | Δ(edd) | This study |
| M15 | recA+uvr+ F−mlt gal ara lac (pREP4) | Qiagen, Inc. |
| Plasmids | ||
| pCB95 | idnO | This study |
| pCB96 | idnD | This study |
| pCB98 | idnT | This study |
| pCU102 | idnT in pQE30 (idnT controlled expression) | This study |
| pNP204 | idnK idnD idnO idnT idnR′ | |
| pUC18 | bla lacZ′c | This study |
| pUC19 | bla lacZ′ | This study |
| pBR322 | Tcrbla | |
| pQE30 | Expression vector; His6 affinity tag | Qiagen, Inc. |
CGSC, E. coli Genetic Stock Collection.
BRL, Bethesda Research Laboratories.
Incomplete genes.
Construction of mutants.
To construct the idnR mutant, E. coli NP250, two DNA fragments were generated by PCR from E. coli W1485 chromosomal DNA. First, a 4.2-kb BamHI-KpnI fragment, using PCR primers 5′GCGGATCCGCGTAGCGATATCCTGTAAA3′ and 5′GCGGTACCCTTATGAGCTGCGTAAGCTG3′, was cloned into pUC19 to produce pNP204. Next, a 2.7-kb HindIII-BamHI fragment, using PCR primers 5′GCAAGCTTGGAGCAAAATCTTCCAGCCG3′ and 5′GCGGATCCTAGAATCCGTCACCTCTGAG3′, was ligated into pNP204, generating a subclone of the idn genes containing a 230-bp deletion within the idnR gene. Then a Kanr gene cassette was cloned into the BamHI site within the idnR′ gene. The resulting plasmid was digested with KpnI and PvuII, and the fragment was purified by electroelution from an agarose gel and transformed by electroporation into E. coli DPB271. Kanr transformants were analyzed by PCR. The idnR mutation was then transduced into the wild-type background of E. coli W1485 by using the phage P1vir (21). The idnD and gntRKU mutants were generated in a similar way. For the gntRKU mutant, E. coli NP202, a 1.3-kb StuI-BglII fragment on pTC221 (33) was replaced by the Kanr gene fragment by deleting the entire gntK gene and portions of the gntU and gntR genes. For the idnD mutant, E. coli CB350, the Kanr gene was cloned into EcoRI site of the idnD gene on pNP204. Last, the edd mutant, E. coli BM129, was constructed by using a Tcr insertion in the NcoI site of pTC199 (8).
Plasmids.
DNA restriction digestion, ligation, transformation, and other DNA manipulations were carried out by standard methods (28). Construction of pNP204 was described above. The idnO gene was subcloned from pNP204 as a 1.6-kb EcoRI-NruI fragment cloned into the EcoRI and SmaI sites of pUC18 to construct pCB95. The idnD gene was subcloned from pNP204 as a 1.7-kb HindIII-SspI fragment cloned into the HindIII and SmaI sites of pUC19 to construct pCB96. The idnT gene was subcloned from pNP204 as a 1.6-kb SspI-SspI fragment cloned into the SmaI site of pUC18 to construct pCB98. The idnT gene was PCR amplified from pCB98 by using primers immediately adjacent to the idnT structural gene (5′GCGAATTCGCTGCTTTTCTGGCACTA3′ and 5′GCGGATCCGCATAACTTCTCCCAACGTC3′), and the PCR fragment was cloned into the EcoRI and BamHI sites of pQE30 to construct pCU102. All plasmid constructions were confirmed by DNA sequence analysis (29).
Enzyme assays.
Cells were harvested in mid-logarithmic phase, washed three times with 100 mM Tris-HCl buffer (pH 7.0), and then resuspended in 500 μl of the same buffer to a final A550 of 1.0. Cell suspensions were lysed by sonic oscillation for 30 s (three bursts of 10 s each, with 60 s on ice between bursts) by using a Fisher Sonic Dismembrator model 300. Crude extracts were centrifuged (13,000 × g for 20 min at 4°C) in order to minimize nonspecific NAD(P)H oxidase activity. 5-Ketogluconate (5KG) reduction was assayed by mixing 50 μl of cell extract with 950 μl of assay buffer containing 100 mM Tris-HCl (pH 6.5), 150 μM NADPH or NADH, and 300 mM potassium 5KG. Similarly, l-idonate and d-gluconate oxidations were assayed in a buffer containing 100 mM Tris-HCl (pH 8.0), 500 μM NAD, and 300 mM sodium d-gluconate or sodium l-idonate. Other enzyme substrates were added to final concentrations of 300 mM in the same assay buffers. All enzyme reactions were conducted at 25°C and monitored spectrophotometrically at 340 nm by using a Lambda-12 UV-visible light spectrometer (Perkin-Elmer). Protein concentrations were determined by the method of Lowry et al. (18).
Idonate uptake.
E. coli M15(pCU102) was used to measure gluconate uptake. Expression of idnT was induced by the addition of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) to a culture growing in mid-logarithmic phase on LB containing 0.4% glucose, which leads to catabolite repression of the native gluconate transporters (25, 33). Uptake experiments were started by mixing 100 μl of sodium [6-14C]gluconate (200 μM, 5.6 μCi/μmol) and 100 μl of the cell suspension which had been preincubated for 3 min with 2 mM competing sugar, as described previously (33). Assays were conducted in triplicate. Radioactivity was measured with a Packard Tri-Carb 2100TR liquid scintillation counter.
Purification of l-idonate.
A large-scale reaction mixture containing 100 mM Tris-HCl (pH 7.0), 50 mM potassium 5KG, 100 mM NADH, and 2.5 ml of crude cell extract (described above) was mixed continuously in an Oak-Ridge tube at room temperature for 4 h. Protein was denatured by being boiled for 5 min and was removed by centrifugation at 10,000 × g for 10 min, and the supernatant was filtered by using a 0.2-μm-pore-size Acrodisc filter (Gelman Sciences).
Low-pressure liquid chromatography (LPLC) was carried out at room temperature using a BioLogic system (Bio-Rad, Hercules, Calif.) with a formate-based AGX1 anion-exchange resin (Econo column [1 by 5 cm] equipped with a flow adapter; Bio-Rad). The sample was applied to the column, washed with 1 mM formic acid, and eluted with a linear gradient of 1 to 200 mM formic acid. Fractions were collected (Bio-Rad model 2110) and analyzed by high-pressure liquid chromatography (HPLC).
Thin-layer chromatography (TLC) of the appropriate LPLC fractions applied to glass plates (10 by 20 cm) coated with silica gel was accomplished essentially as described previously (10). Control samples were detected by spraying with 1% AgNO3 in acetone, followed by air drying, fixation with 0.5 N NaOH in methanol, and baking at 100°C for 3 to 5 min. The Rf value of the sample was calculated for the control TLC plate, and the sample was scraped off of an identical plate (without detection) and then eluted from the silica gel with 4 volumes of double-distilled H2O.
HPLC was performed by using a DX-500 system (Dionex, Sunnyvale, Calif.) equipped with a GP40 microbore gradient pump, an ED40 electrochemical detector, a Dell Optiplex XL 590 computer running PeakNet 4.11A software, and a 2-mm-diameter PA-10 anion-exchange column. Isocratic chromatography with 450 mM NaOH allowed excellent separation of the various hexonates analyzed in this study.
NMR analysis.
1H nuclear magnetic resonance (NMR) spectra were recorded at 500 MHz on a Bruker DMX-500 instrument at room temperature. Samples were dissolved in D2O and referenced to DOH Δ = 4.78 ppm.
Chemicals and enzymes.
Restriction enzymes and DNA-modifying enzymes were obtained from Bethesda Research Laboratories, Inc. (Gaithersburg, Md.). The T7 Sequenase version 2.0 kit was acquired from Amersham Life Science (Arlington Heights, Ill.). Radioactive sodium [6-14C]gluconate was purchased from American Radiolabeled Chemicals (St. Louis, Mo.). TLC plates and biochemicals were obtained from Sigma Chemical Corp. (St. Louis, Mo.). The sodium l-idonate was a generous gift from Robert Lazurus (Genentech).
RESULTS
Homology searches and organization of the GntII (idn) genes.
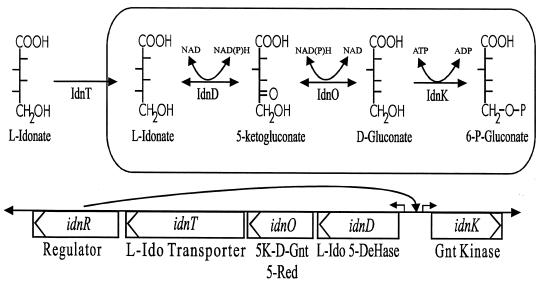
The arrangement of the idn genes is shown in Fig. 1 (6). The biochemical evidence presented below proves that these genes encode the enzymes of a pathway for l-idonate catabolism, and hence the genes have been given the designation idn (Table 1). Each of the peptide sequences deduced from the idn structural genes was used as a query against the peptide sequence database (BLASTP), and the data are summarized in Table 1. IdnK is 45% identical to GntK (33); IdnD is 31% identical to mammalian sorbitol dehydrogenase (14); IdnO is 56% identical to Gno from G. oxydans (15); IdnT is 61% identical to GntT (25), a match which was the highest among a total of seven gluconate permease orthologs in E. coli (23); and the helix-turn-helix protein IdnR is 46% identical to GntR, strongly suggesting that IdnR is involved in regulation of idnK and the idnDOTR operon.
FIG. 1.
Enzymes and genes of l-idonate catabolism. Abbreviations: 6-P-Gluconate, 6-phosphogluconate; L-Ido, l-idonate; 5K-D-Gnt 5-Red, 5-keto-d-gluconate 5-reductase; L-Ido 5-DeHase, l-idonate 5-dehydrogenase; Gnt, gluconate.
Biochemical analysis of IdnO and IdnD.
The general approach used to determine the biochemical functions of the Idn proteins involved subcloning of the corresponding gene, overproduction of the gene product, and assay of the enzyme activity in crude cell extracts. The individual gene fragments were designed to contain the structural gene only, including the ribosome binding site. The similarity of IdnO to GNO from G. oxydans (15) led to the testing of 2-ketogluconate and 5KG as substrates for a crude extract prepared from E. coli DH5α(pCB95), which was constructed to specifically overexpress IdnO. The biochemical data are summarized in Table 3. IdnO is able to reduce 5KG with either NADH or NADPH as cofactor; no other compounds which were tested could be reduced by IdnO. The reduction of 5KG by IdnO is reversible, as evidenced by oxidation of d-gluconate (and, to a 10-fold-lesser extent, 6-phosphogluconate) using NADP as a cofactor; no other sugar acids are oxidized, nor does NAD serve as a cofactor. The reactions catalyzed by IdnO were confirmed by HPLC analysis: d-gluconate is formed by reduction of 5KG (Fig. 2, chromatogram A), and 5KG is formed by oxidation of d-gluconate (data not shown). In the crude extract, IdnO shows an apparent Km of 2 mM for gluconate and an apparent Km of 0.5 mM for 5KG. While these Km values are high, the kinetic data clearly indicate that the IdnO-catalyzed reaction is saturatable. Thus, IdnO encodes a specific 5-keto-d-gluconate 5-reductase. Since the d-gluconate formed by reduction of 5KG would be phosphorylated by IdnK to form 6-phosphogluconate, which would be metabolized via the ED pathway, it seemed logical that 5KG is an intermediate of the pathway leading to 6-phosphogluconate. This consideration led to the hypothesis that IdnD is involved in 5KG formation, functioning upstream in the pathway. Since IdnD showed the highest similarity to sorbitol dehydrogenase (l-iditol:2-dehydrogenase), it was anticipated that IdnD would catalyze the oxidation of 5KG at carbon number two to form 2,5-diketogluconate. Surprisingly, 5KG is not oxidized by a crude extract of E. coli DH5α(pCB96), which specifically overexpresses IdnD, but rather IdnD catalyzes the reduction of 5KG, using either NADH or NADPH as a cofactor. Furthermore, the reduction of 5KG is highly specific, as IdnD fails to reduce d-glucose, d-galactonate, d-galacturonate, d-glucuronate, d-sorbose, d-sorbitol, and 2-ketogluconate; nor does IdnD oxidize any of these sugars. At the time, the identity of the product formed by reduction of 5KG by IdnD was not clear.
TABLE 3.
Biochemical characterization of IdnO and IdnD activitiesa
| Substrateb | Sp act (nmol/min/mg)c
|
Approx. Kmd
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IdnO
|
IdnD
|
IdnO | IdnD | |||||||
| NAD | NADP | NADH | NADPH | NAD | NADP | NADH | NADPH | |||
| d-Gluconate | 107.0 | 1.6 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 2 mM | |
| d-Gluconate-6-phosphate | 10.2 | 0.4 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | ||
| 5KG | <0.01 | <0.01 | 4,000.0 | 3,340.0 | <0.01 | <0.01 | 48.0 | 36.0 | 500 μM | 1 mM |
| l-Idonate | <0.01 | <0.01 | <0.01 | <0.01 | 17.4 | <0.01 | <0.01 | <0.01 | 25 mM | |
E. coli DH5α cells harboring plasmid pCB95 (IdnO) or pCB96 (IdnD) were induced by the addition of 1 mM IPTG for 2 h.
Substrate concentrations of 300 mM.
Lower than detectable activity was measured with d-glucose, d-galactonate, d-galacturonate, d-glucuronate, d-sorbose, d-sorbitol, and 2-ketogluconate.
Approximate Km values were determined with crude cell extracts.
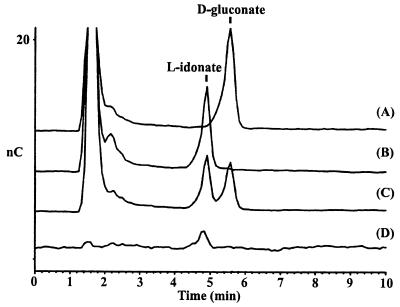
FIG. 2.
HPLC analysis of substrate conversion. Conversion from 5KG to d-gluconate by 5-keto-d-gluconate 5-reductase (A), from 5KG to l-idonate by l-idonate 5-dehydrogenase (B), and from 5KG to both l-idonate and d-gluconate by 5-keto-d-gluconate 5-reductase and l-idonate 5-dehydrogenase (C) are shown. Authentic l-idonate standard (D) is also shown.
Production, purification, and analysis of the IdnD substrate.
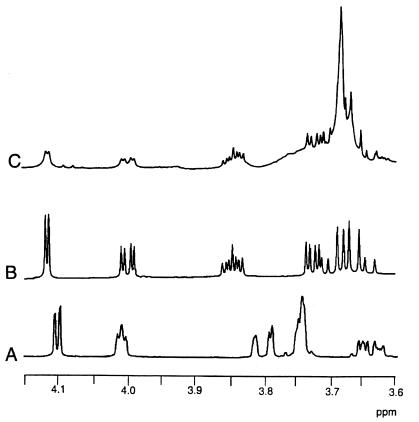
Conditions were optimized, as described in Materials and Methods, for synthesis of the 5KG reduction product by using the crude cell extract of E. coli DH5α(pCB96) containing IdnD. HPLC analysis showed that the resulting reaction contained an unidentified compound (Fig. 2, chromatogram B). Furthermore, this unidentified compound is distinct from d-gluconate yet possesses similar chemical properties, as indicated by the similar retention time on the anion-exchange column. An authentic standard of l-idonate was obtained and shown to cochromatograph with the unidentified compound (Fig. 2, chromatogram D). Thus, the results of HPLC analysis strongly suggested that IdnD catalyzes the reduction of 5KG to form l-idonate. The reaction mixture also contains significant amounts of 5KG (data not shown). Therefore, it was necessary to purify the l-idonate from the reaction mixture in order to prove its identity by NMR analysis. The putative l-idonate formed by IdnD was purified by anion-exchange chromatography and preparative TLC. This preparation was analyzed by proton NMR, and the putative l-idonate was shown to have the same spectrum as the authentic l-idonate standard, a spectrum which is significantly different from that of d-gluconate (Fig. 3). These data confirm that the product formed by reduction of 5KG by IdnD is indeed l-idonate. Furthermore, IdnD is able to specifically oxidize l-idonate with NAD as a cofactor to form 5KG (Table 3). Thus, IdnD is a novel enzyme, l-idonate 5-dehydrogenase; IdnD and IdnO catalyze consecutive metabolic steps which allow for conversion of l-idonate to d-gluconate, with 5-KG as an intermediate. The pathway for l-idonate catabolism is shown in Fig. 1.
FIG. 3.
Partial 1H NMR spectra of sodium d-gluconate (A), sodium l-idonate (B), and an unknown compound (C) isolated from reaction of l-idonate 5-dehydrogenase with 5KG and NADH, indicating that the unknown compound is l-idonate. Spectra were recorded at 500 MHz in D2O.
Physiology of l-idonate growth, transport, and induction.
As predicted, the presence of the GntII pathway in E. coli allows growth on l-idonate as the sole source of carbon and energy (Fig. 4). The generation time of wild-type E. coli W1485 on minimal medium containing l-idonate is approximately 1.4 h, compared to generation times of approximately 1.0 h on gluconate and glucose. Another prediction for growth on l-idonate is that the ED pathway is used for metabolism of the d-gluconate formed from l-idonate catabolism, and indeed an edd mutant grows more slowly than the wild type on l-idonate (Fig. 4), as it also does on gluconate, which is known to be metabolized in edd mutants via the pentose phosphate pathway (36). The reason for the extended lag phase of the edd mutant on l-idonate, but not on d-gluconate, is not understood at present. Furthermore, E. coli TUG287, a gntK idnK double mutant, and E. coli CB350, an idnD deletion strain, are both unable to grow on minimal medium containing l-idonate, highlighting the essential nature of the idnK and idnD genes for l-idonate catabolism (data not shown). As expected, E. coli NP202, a gntRKU deletion strain with the wild-type idn genes, grows well on l-idonate. Interestingly, E. coli NP202 is also able to grow on d-gluconate and at the same rate as the wild type, strongly suggesting that d-gluconate is able to induce idnK under certain conditions. Accordingly, growth of E. coli NP202 on rich medium containing gluconate results in a fourfold induction of gluconate kinase activity (data not shown).
FIG. 4.
Growth of E. coli on l-idonate, d-gluconate, and d-glucose. E. coli W1485 (open circles), E. coli BM129 (edd mutant; open squares), and E. coli NP250 (idnR mutant; closed circles) grown on M63 minimal medium containing l-idonate, d-gluconate, and d-glucose are shown.
Presumably, l-idonate is transported into the cell by IdnT, which was recently proven to function as a d-gluconate transporter with an apparent Km of 60 μM for gluconate (23). Since it is not possible to confirm directly whether IdnT transports l-idonate, because radioactive l-idonate is not available, competition of unlabeled l-idonate for uptake of radioactive gluconate was measured instead. The data shown in Table 4 indicate that a 20-fold excess of unlabeled l-idonate is able to inhibit uptake of radioactive d-gluconate to about the same extent as the control value determined for a 20-fold excess of unlabeled d-gluconate. l-Iduronic acid and d-glucose did not inhibit gluconate transport, while 5-keto-d-gluconic acid had a modest effect. d-Glyceric acid, which has the same anomeric configuration as l-idonate, also had an effect. These data indicate that IdnT functions for the transport of both l-idonate and d-gluconate.
TABLE 4.
Inhibition of gluconate transport (IdnT) by alternative substrates
| Competing sugara | Rate (nmol/min/mg) | % of wild-type rate |
|---|---|---|
| None (control) | 2.13 | 100 |
| d-Gluconic acid | 0.30 | 14 |
| l-Idonic acid | 0.42 | 14 |
| d-Glucose | 3.58 | 168 |
| 5-Keto-d-gluconic acid | 1.71 | 80 |
| d-Glyceric acid | 1.40 | 66 |
| l-Iduronic acid | 2.09 | 98 |
Cells preincubated in 2 mM competing sugar prior to uptake of 200 μM [14C]gluconate.
Another prediction is that l-idonate or an intermediate of its catabolism serves to induce the enzymes of the l-idonate pathway. Conditions which lead to induction of IdnO and IdnK in E. coli W1485 were tested (Table 5). In rich medium, there is a 15-fold induction of IdnO by l-idonate, an 80-fold induction of IdnO by 5KG, and a 4-fold induction of IdnK by 5KG. Similarly, there is a 15-fold induction of IdnO on minimal medium containing l-idonate and a 20-fold induction of IdnO on minimal medium containing 5KG. These data suggest that 5KG rather than l-idonate is the true inducer. However, 5KG is a very poor growth substrate; E. coli W1485 has a generation time of more than 7 h when growing on minimal medium containing 5KG (not shown). Perhaps the reason for the slow growth on 5KG is poor uptake, as suggested by its modest inhibition of gluconate transport via IdnT as described above. Since induction of IdnO by l-idonate or 5KG is reduced twofold by growth on glucose, it seems likely that the idn genes are catabolite repressed (Table 5). Last, an idnR deletion mutant, E. coli NP250, is unable to grow on l-idonate but grows normally on glucose and gluconate (Fig. 4). This result suggests that IdnR is a positive regulator of the idn regulon.
TABLE 5.
Induction of IdnO and GntK activities in E. coli W1485 cellsa
| Carbohydrate (0.2%) | IdnO sp actb
|
IdnK sp act,b LB | |
|---|---|---|---|
| LB | M63c | ||
| None | 10.0 | NTd | <0.01 |
| d-Glucose | 16.0 | 6.2 | NT |
| d-Gluconate | 12.5 | 8.0 | 1.3 |
| 5KG | 825.0 | 135.0 | 5.7 |
| 5KG + d-glucose | NT | 78.0 | NT |
| l-Idonate | 158.0 | 99.0 | NT |
| l-Idonate + d-glucose | NT | 69.3 | NT |
Cells all harvested during log phase.
Expressed as nanomoles per minute per milligram of total cell protein.
M63 minimal medium.
NT, not tested.
DISCUSSION
The experiments described in this paper outline a general strategy for deducing the biochemical functions of unidentified gene products based upon clues from genomic sequences. Proof for the physiological operation of a presumptive metabolic pathway can be provided by biochemical analysis of the pathway enzymes, chemical analysis of the pathway intermediates, and growth experiments involving mutants with lesions in specific steps of the pathway. This strategy was specifically applied to the GntII system of E. coli, which has been shown in this study to code for a previously unknown pathway for catabolism of and growth on l-idonate.
For many years, the physiology and regulation of the subsidiary gluconate pathway (GntII system) were mysteries. Genetic evidence for the presence of two systems for gluconate transport and phosphorylation was first provided by Bächi and Kornberg (2). Gluconate-fermenting pseudorevertants of E. coli HfrG6ΔMD2, a GntI deletion mutant (bioH-asd) which cannot grow on gluconate, were selected after extended incubation on minimal medium containing gluconate (11–13, 22). The pseudorevertant strains were found to have induced a thermosensitive gluconate kinase and an alternative gluconate transporter when growing on gluconate. A secondary mutation of idnK (gntV) eliminated the subsidiary gluconate kinase activity as well as the ability of the pseudorevertants to grow on gluconate (11, 13). A mutation affecting the subsidiary gluconate transporter was designated gntS (2), but it was never proven whether the gntS locus is a structural or regulatory gene, although it has been suggested that the gntS product positively controls expression of idnK (gntV) (2, 11, 13). Recent evidence apparently confirms the location of gntS upstream of fbp in the 95-min region and further supports the conclusion that gntS is a regulatory locus (12). However, examination of the genomic sequence in the 95-min region does not provide any further insights into the nature of gntS. It is now believed that the subsidiary gluconate transporter is encoded by idnT (gntW), which is adjacent to the proven location of idnK at 96.8 min (12, 23). The nature and role of gntS remain obscure.
It was never established during the previous studies how the GntII system is regulated. The GntII system was thought to have evolved as a subsidiary pathway for gluconate catabolism (12). If so, the GntII system would need contain only a gluconate transporter and gluconate kinase. However, analysis of the genomic sequence containing the idnK and idnT genes indicated that this region also contains two genes that encode “dehydrogenase-like” enzymes, idnD and idnO, which are part of an operon with idnT and idnR, and have been suggested to also be constituents of the GntII system (35). The similarity of idnO to gno of G. oxydans, which encodes GNO (15), led us to overexpress, and thereby prove, that IdnO can interconvert 5KG and d-gluconate. Therefore, IdnO is a 5-keto-d-gluconate 5-reductase. The other dehydrogenase, IdnD, can reduce 5KG but cannot oxidize it. Therefore, IdnD is an l-idonate 5-dehydrogenase. Furthermore, the activities of both IdnD and IdnO are highly specific. The product formed by the IdnD-dependent reduction of 5KG was purified and proven by NMR analysis to be l-idonic acid. Thus, it was shown that l-idonate is converted via the two consecutive oxidation and reduction steps to d-gluconate, which is in turn phosphorylated by IdnK to form 6-phosphogluconate, an intermediate of central carbon metabolism.
The in vivo sequence of l-idonate catabolism was confirmed by the growth properties of specific pathway mutants: wild-type E. coli is able to grow very well on l-idonate, an idnD deletion mutant is unable to grow on l-idonate, an idnK mutant (also defective in gntK) is unable to grow on l-idonate, and an edd mutation significantly slows growth on l-idonate to the same extent as growth on d-gluconate is affected (the pentose phosphate pathway plays a backup role for 6-phosphogluconate metabolism in edd mutants [36]). l-Idonate is the transport substrate for IdnT, as indicated by strong competitive inhibition of d-gluconate uptake. In summary, these data prove that l-idonate is the true substrate of the GntII system and is catabolized via a pathway involving d-gluconate as an intermediate (Fig. 1).
The natural occurrence of l-idonate is apparently limited to its involvement as an intermediate in catabolism of 2,5-diketogluconate by Erwinia sp. (34) and G. oxydans (27), as well as tartaric acid formation from ascorbic acid in grapes (20, 26). Interestingly, l-idonate 5-dehydrogenase is considered to be an undesirable activity in recombinant bacteria specifically engineered to produce 2-keto-l-gulonate, a precursor of ascorbic acid biosynthesis (16). In addition to E. coli, the only other organism reported to grow on l-idonate is Erwinia sp. strain ATCC 39140 (34), and it will be interesting to find out whether other microorganisms can grow on l-idonate. The l-idonate catabolic pathway may not be unique to E. coli and Erwinia sp. strain ATCC 39140, since enzymes of ketogluconate metabolism are also present in several bacteria, including G. oxydans (15, 27), Chromobacterium (4), and Corynebacterium sp. (31). However, there are currently no orthologs of E. coli idnD present in the databases.
Genomic analysis indicates that the idnK gene is monocistronic and is transcribed divergently from an operon containing the idnD-idnO-idnT-idnR genes (6). It stands to reason that a molecular genetic analysis of the 217 bp of intervening sequence between the idnK and idnD genes will be very interesting. The fact that the GntII system encodes a pathway for catabolism of l-idonate leads to several predictions concerning regulation of the idn genes. First, l-idonate (or the intermediate, 5KG) should be the inducer for the pathway. This was confirmed biochemically by induction of IdnO and IdnK activities in cells grown on l-idonate. Second, the ED pathway should be induced for efficient catabolism of the 6-phosphogluconate formed from l-idonate. Third, the idnR product should regulate the idn regulon. The fact that an idnR deletion mutant cannot grow on l-idonate suggests that IdnR positively regulates the idn regulon. Last, since gluconate is an intermediate of the l-idonate pathway, there is likely to be cross talk with the gnt regulon (GntI). The inducer generated by the l-idonate pathway should not induce the GntI system, which is unnecessary for l-idonate catabolism, but a signal is still necessary to induce the ED pathway for growth on l-idonate; gluconate is most likely this inducer. This suggests an additional role for the idnR product: repression of the gntKU and gntT genes when growing on l-idonate. The presence of a highly conserved GntR binding site within the idnK-idnD intragenic region supports the hypothesis of cross talk from the gnt regulon (24, 25).
The results presented in this paper answer many of the longstanding questions concerning the GntII system (2, 13, 22, 37). It is now clear that the genes of the GntII system encode a pathway for catabolism of l-idonate in which d-gluconate is an intermediate. There are certain conditions under which d-gluconate can induce the l-idonate (GntII) pathway, but the natural inducer of the idn genes is apparently l-idonate. With the new understanding provided by the current study, mechanisms for induction of the GntII system by d-gluconate can now be proposed. As mentioned above, the GntI deletion strain E. coli HfrG6ΔMD2 is able to grow on gluconate by apparently acquiring a mutation which renders the subsidiary gluconate transporter and gluconate kinase inducible by d-gluconate. In stark contrast, it was shown that E. coli NP202, a gntK deletion mutant, is able to grow on d-gluconate. The absence of the gluconate-inducible gluconate kinase (GntK) in E. coli NP202 would result in intracellular accumulation of d-gluconate when cells are first exposed to d-gluconate, since this strain carries a wild-type copy of gntT. Under these conditions, the inducer of idnK could be formed from d-gluconate by reversal of the IdnO- and/or IdnD-catalyzed reactions to generate 5KG or l-idonate. Alternatively, it could be that d-gluconate is itself the inducer of the idn genes but that IdnR requires a higher concentration of d-gluconate for induction than does GntR. It has been previously noted that mutation of gntR does not affect regulation of the GntII genes (12), nor does gluconate induce idnK in the wild-type strain, but it remains possible that the gntR mutation plays a role in allowing induction of idnK in the particular case of E. coli NP202, which contains a gntRKU deletion. Nevertheless, the best explanation for induction of the idn genes by d-gluconate in E. coli NP202 involves formation of the inducer of the idn regulon. As for the pseudorevertants of E. coli HfrG6ΔMD2, it seems quite possible that the gntS locus is actually a mutation of idnR which leads to induction of the idn genes by altering the normal regulatory properties of IdnR.
ACKNOWLEDGMENTS
We thank Fred Blattner and Guy Plunkett for providing error-free data prior to publication, and we also thank Guy Plunkett for numerous helpful discussions. Thanks to Bob Lazurus for providing sodium l-idonate.
Work on this project is supported by grants from the DOE (DE-FG02-95ER20178) and NSF (MCB-9723593).
REFERENCES
- 1.Ashwell G. Enzymes of glucuronic and galacturonic acid metabolism in bacteria. Methods Enzymol. 1962;5:190–208. [Google Scholar]
- 2.Bächi B, Kornberg H L. Genes involved in the uptake and catabolism of gluconate by Escherichia coli. J Gen Microbiol. 1975;90:321–335. doi: 10.1099/00221287-90-2-321. [DOI] [PubMed] [Google Scholar]
- 3.Bausch C, Peekhaus N, Blais T, Conway T. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Characterization of the gluconate subsidiary system (GntII) in Escherichia coli, abstr. K-75; p. 354. [Google Scholar]
- 4.Bernaerts M, DeLey J. 2,5-Diketogluconate formation by Chromobacterium. Antonie Leeuwenhoek. 1971;37:185–195. doi: 10.1007/BF02218480. [DOI] [PubMed] [Google Scholar]
- 5.Blattner F R, Plunkett III G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 6.Burland V, Plunkett III G, Sofia H J, Daniels D L, Blattner F R. Analysis of the Escherichia coli genome VI: DNA sequence of the region from 92.8 through 100 minutes. Nucleic Acids Res. 1995;23:2105–2119. doi: 10.1093/nar/23.12.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conway T. The Entner-Doudoroff pathway: history, physiology, and molecular biology. FEMS Microbiol Rev. 1992;103:1–27. doi: 10.1111/j.1574-6968.1992.tb05822.x. [DOI] [PubMed] [Google Scholar]
- 8.Egan S, Fliege R, Tong S, Shibata A, Wolf R E, Jr, Conway T. Molecular characterization of the Entner-Doudoroff pathway in Escherichia coli: sequence analysis and localization of promoters for the edd-eda operon. J Bacteriol. 1992;174:4638–4646. doi: 10.1128/jb.174.14.4638-4646.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraenkel D G. Glycolysis. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 189–198. [Google Scholar]
- 10.Fried B. Carbohydrates. In: Sherma B F A J, editor. Thin-layer chromatography: techniques and applications. M. New York, N.Y: Dekker; 1982. pp. 255–256. [Google Scholar]
- 11.Isturiz T, Vitelli-Flores J, Mardeni J. El metabolismo del gluconato en E. coli. Estudio de una mutante delecionada en la region bioH-asd del mapa cromosomico. Acta Cient Venez. 1979;30:391–395. [PubMed] [Google Scholar]
- 12.Isturiz T, Celaya J. The metabolism of gluconate in Escherichia coli. The subsidiary system and the nature of the gntS gene. J Basic Microbiol. 1997;37:105–114. doi: 10.1002/jobm.3620370205. [DOI] [PubMed] [Google Scholar]
- 13.Istúriz T, Palmero E, Vitelli-Flores J. Mutations affecting gluconate catabolism in Escherichia coli. Genetic mapping of the locus for the thermosensitive gluconokinase. J Gen Microbiol. 1986;132:3209–3212. doi: 10.1099/00221287-132-11-3209. [DOI] [PubMed] [Google Scholar]
- 14.Jeffery J, Cederlund E, Jornvall H. Sorbitol dehydrogenase. The primary structure of the sheep-liver enzyme. Eur J Biochem. 1984;140:7–16. doi: 10.1111/j.1432-1033.1984.tb08059.x. [DOI] [PubMed] [Google Scholar]
- 15.Klasen R, Bringer-Meyer S, Sahm H. Biochemical characterization and sequence analysis of the gluconate:5-oxidoreductase gene from Gluconobacter oxydans. J Bacteriol. 1995;177:2637–2643. doi: 10.1128/jb.177.10.2637-2643.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazarus R A, Stafford R K, Seymour J L, Dennis M S, Lazarus M G, Hughes E J L, Miller H I, Marks C B, Anderson S. Presented at the 6th International Symposium on Genetics of Industrial Microorganisms, Strasbourg, France. 1990. [Google Scholar]
- 17.Lin E C C. Sugars, polyols, and carboxylates. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 307–342. [Google Scholar]
- 18.Lowry O H, Rosebrough N J, Farr A L, Randall F J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 19.Luria S E, Delbruck M. Mutations of bacteria from virus sensitivity to virus resistance. Genetics. 1943;28:491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malipiero V, Ruffner H P, Rast D M. Ascorbic to tartaric acid conversion in grapevines. J Plant Physiol. 1987;129:33–40. [Google Scholar]
- 21.Miller J H. A short course in bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. Preparation and use of P1vir lysates; pp. 268–272. [Google Scholar]
- 22.Nagel De Zwaig R, Zwaig N, Isturiz T, Sanchez R S. Mutations affecting gluconate metabolism in Escherichia coli. J Bacteriol. 1973;114:463–468. doi: 10.1128/jb.114.2.463-468.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peekhaus N, Tong S, Reizer J R, Saier M, Murray E, Conway T. Characterization of a novel transporter family that includes multiple Escherichia coli gluconate transporters and their homologues. FEMS Microbiol Lett. 1997;147:233–238. doi: 10.1111/j.1574-6968.1997.tb10247.x. [DOI] [PubMed] [Google Scholar]
- 24.Peekhaus N, Conway T. Positive and negative transcriptional regulation of the Escherichia coli gluconate regulon gene gntT by GntR and the cyclic AMP (cAMP)-cAMP receptor protein complex. J Bacteriol. 1998;180:1777–1785. doi: 10.1128/jb.180.7.1777-1785.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porco A, Peekhaus N, Bausch C, Tong S, Isturiz T. Molecular genetic characterization of the Escherichia coli gntT gene of GntI, the main system for gluconate metabolism. J Bacteriol. 1997;179:1584–1590. doi: 10.1128/jb.179.5.1584-1590.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saito K, Kasai Z. Conversion of L-ascorbic acid to L-idonic acid, L-idono-gammalactone, and 2-keto-L-idonic acid in slices of immature grapes. Plant Cell Physiol. 1982;23:499–507. [Google Scholar]
- 27.Saito Y, Ishii Y, Hayashi H, Imao Y, Akashi T, Yoshikawa K, Noguchi Y, Soeda S, Yoshida M, Niwa M, Hosoda J, Shimomura K. Cloning of genes coding for l-sorbose and l-sorbosone dehydrogenases from Gluconobacter oxydans and microbial production of 2-keto-l-gulonate, a precursor of l-ascorbic acid, in a recombinant G. oxydans strain. Appl Environ Microbiol. 1997;63:454–460. doi: 10.1128/aem.63.2.454-460.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simons R W, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 31.Sonoyama T, Kageyama B, Yagi S, Mitsushima K. Biochemical aspects of 2-keto-L-gulonate accumulation from 2,5-diketo-D-gluconate by Corynebacterium sp. and its mutants. Agric Biol Chem. 1987;51:3039–3047. [Google Scholar]
- 32.Sweeney N J, Laux D C, Cohen P S. Escherichia coli F-18 and E. coli K-12 eda mutants do not colonize the streptomycin-treated mouse large intestine. Infect Immun. 1996;64:3504–3511. doi: 10.1128/iai.64.9.3504-3511.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tong S, Porco A, Isturiz T, Conway T. Cloning and molecular genetic characterization of the Escherichia coli gntR, gntK, and gntU genes of GntI, the main system for gluconate metabolism. J Bacteriol. 1996;178:3260–3269. doi: 10.1128/jb.178.11.3260-3269.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Truesdell S J, Sims J C, Boerman P A, Seymour J L, Lazarus R A. Pathways for metabolism of keto-aldonic acids in an Erwinia sp. J Bacteriol. 1991;173:6651–6656. doi: 10.1128/jb.173.21.6651-6656.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamada M, Kawai T, Izu H. Analysis of the Escherichia coli gntT and gntU genes and comparison of the products with their homologues. Eur J Biochem. 1996;60:1548–1550. doi: 10.1271/bbb.60.1548. [DOI] [PubMed] [Google Scholar]
- 36.Zablotny R, Fraenkel D G. Glucose and gluconate metabolism in a mutant of Escherichia coli lacking gluconate-6-phosphate dehydrase. J Bacteriol. 1967;93:1579–1581. doi: 10.1128/jb.93.5.1579-1581.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zwaig N, Nagel de Zwaig R, Isturiz T, Wecksler M. Regulatory mutation affecting the gluconate system in Escherichia coli. J Bacteriol. 1973;114:469–473. doi: 10.1128/jb.114.2.469-473.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]