Abstract
Background
Many diets promoted specifically for multiple sclerosis have been suggested to improve disease activity. Dairy and gluten are two components for which the recommendations vary between these diets. Existing research into the association between these dietary components and disease activity has been conflicting.
Objective
To explore the relationship between dairy and gluten intake and disease activity in multiple sclerosis over a 2-year period, using no evidence of disease activity (NEDA) 3 status.
Methods
186 participants’ dairy and gluten intake was retrospectively estimated over 2 years using a dairy and gluten dietary screener. Estimated dairy and gluten intake was compared to disease activity, indicated by no evidence of disease activity 3 status, and quality of life, assessed by the Multiple Sclerosis International Quality of Life (MusiQoL) questionnaire.
Results
No significant association was found between mean estimated dairy or gluten intake and NEDA 3 status (p = 0.15 and 0.60, respectively). Furthermore, there was no significant relationship between dairy or gluten intake and MusiQoL) scores (p = 0.11 and 0.51, respectively).
Conclusion
Whilst we cannot rule out modest benefits due to our small sample size, we found that neither dairy nor gluten intake was associated with disease activity or quality of life in this study.
Keywords: Multiple sclerosis, diet, dairy, gluten, disease activity, no evidence of disease activity 3, quality of life, Multiple Sclerosis International Quality of Life
Introduction
Disease-modifying therapies are well established in the treatment of multiple sclerosis (MS), however, there is little evidence of the impact of lifestyle changes. Preliminary theories have arisen regarding certain lifestyle changes, including diets promoted specifically for MS (hereafter referred to as ‘MS diets’). While people with MS (hereafter referred to as ‘pwMS’) show interest in exploring dietary modifications, MS diets have complex guidelines that may impair long-term adherence.1,2 There are limited prospective trials investigating the efficacy of three commonly recommended diets, the Swank, overcoming MS (OMS), and Wahls diets.3–6 Dairy and gluten are two components for which the recommendations vary between these diets; the Wahls diet restricts both dairy and gluten intake, whereas the Swank and OMS diets restrict dairy but not gluten. These dietary exclusions were initially recommended based on studies that suggested a correlation between cow's milk consumption and MS prevalence as well as a correlation between coeliac disease and MS prevalence.7,8 It was later suggested that the cow's milk protein, butyrophilin, is a molecular mimic of myelin oligodendrocyte glycoprotein that can induce an inflammatory response in T cells in vitro and in the rat model of MS, experimental autoimmune encephalitis. 9 Since then, large studies have explored the relationship between dairy and MS with conflicting results.10,11 The Health Outcomes in a Sample of People with MS (HOLISM) study examined the diets of 2047 pwMS. 10 They reported that dairy consumers had higher relapse rates, recent disease activity, and lower quality of life. In contrast, another larger cross-sectional study utilising the North American Research Committee on MS registry found that higher intakes of dairy were associated with lower levels of disability. 11 Both studies used self-reporting tools to assess relapses and level of disability.
In recent decades, there has been a simultaneous rise in the prevalence of autoimmune diseases and the consumption of gluten-containing foods.12,13 Several case reports and anecdotal evidence suggested a benefit of a gluten-free diet on MS. 14 This prompted prospective cohort studies that investigated the role of gluten in the pathogenesis of MS, although the evidence remains controversial. One study by Johnson et al. 15 found that there was no link between overall gluten intake and self-reported MS symptoms. Conversely, Rodrigo et al. 16 showed that when compared to a regular diet, a gluten-free diet improved Expanded Disability Status Scale (EDSS) score and lesion activity on magnetic resonance imaging (MRI) but had no effect on annualised relapse rate. A 2019 systematic review evaluating 49 studies concluded that there was insufficient high-quality evidence to determine whether gluten plays a role in MS. 14
No evidence of disease activity (NEDA-3) status is a standardised MS-specific outcome composed of three parameters measured over time: Absence of clinical relapses, no disability progression (as assessed by the EDSS), and no new MRI lesions. 17 NEDA-3 is more sensitive in measuring disease activity in MS compared to using individual measures of disease activity. 18 Despite this, NEDA-3 has not previously been used as an outcome measure in studies investigating the relationship between dairy or gluten intake and MS.
Further research on the involvement of dairy and gluten in MS is necessary to establish more concise and effective dietary recommendations for pwMS. The primary aim of this study was to explore the relationship between dairy and gluten intake, using a dietary screener, and MS disease activity, indicated by NEDA-3 status over a 2-year period. The secondary objective was to investigate the relationship between dairy and gluten intake and quality of life in pwMS, using the Multiple Sclerosis International Quality of Life (MusiQoL) questionnaire.
Material and methods
Study design
This is a retrospective, cross-sectional study of pwMS registered on an MS database in the Hunter New England Local Health District, Australia. Recruitment was conducted from February to September 2020. Initially, during this period, a dairy and gluten dietary screener (Web Appendix A) was distributed to participants in outpatient clinics but later moved to distribution online and via post due to the coronavirus disease of 2019 (COVID-19) restrictions. The dietary screener asked participants to recall their dairy and gluten intake over the previous two years. Clinical records were used to assess disease activity via NEDA-3 status over the same 2-year period. Participants’ dairy and gluten intake was then correlated with disease activity.
Study sample
The Hunter New England Local Health District Human Research Ethics Committee approved this study (ETH2019/12349). All participants gave written informed consent at the time of completion of the dietary screener. Inclusion criteria were people aged over 18 with any type of MS, as diagnosed by the McDonald Criteria for clinically definite MS. 19 Participants were excluded if they were diagnosed during the 2-year period prior to completing the dietary screener, had Coeliac disease (autoimmune gluten intolerance), did not complete the survey or had insufficient clinical data to assess NEDA-3 status.
Primary dependent variables (dairy and gluten intake)
Because simple, but validated tools for quantifying dairy and gluten intake were not available, a dairy and gluten dietary screener (Web Appendix A) was designed in collaboration with an academic dietitian with expertise in dietary assessment and gastrointestinal research. This dietary screener retrospectively measured participants’ intake of dairy- and gluten-containing foods based on the diet they followed for the longest period over the last 2 years. Participants were asked about the frequency and quantity per sitting that they consumed. A key was developed that allocated a numerical value to each dairy- and gluten-containing food in the dietary screener. For gluten, the numerical value represented the foods’ gluten content, estimated using the Osborne calculation. 20 For dairy, the numerical value represented the servings of dairy in each food, based on serving sizes from the Australian Dietary Guidelines. 21 These numerical values were then multiplied by a factor, depending on their frequency of intake (as indicated by the participant), to generate an overall value representing the estimated dairy and/or gluten intake for each participant. Although not otherwise validated, the dietary screener included questions about all major foods in Australian supply known to contain dairy and gluten and used appropriate portion sizes to increase the accuracy of responses. Major foods and portion sizes were selected based on the Australian Dietary Guidelines summary, which outlines the major food groups and portion sizes (available at eatforhealth.gov.au).
Secondary dependent variable (MusiQoL)
The secondary outcome was the MusiQoL questionnaire, a reliable tool for measuring the quality of life of pwMS, consisting of 31 questions across nine domains. 22 It has been validated by multiple large studies and has been widely used in existing research looking at diet and quality of life in MS. 23 The MusiQoL questionnaire response was recorded at the time of completion of the dietary screener.
Primary independent (factor) variable
The primary factor was NEDA-3, a standardised binary tool used to indicate either the presence or absence of disease activity in MS (Table 1). 17 NEDA-3 status was retrospectively calculated using clinical data from neurologist assessments during a 2-year period. Positive NEDA-3 status indicates freedom from disease activity, and is achieved by satisfying the following three criteria; no clinical relapses, no disability progression, and no new MRI lesions. 17 Clinical relapse was defined as new or recurrent symptoms lasting more than 24 h, not preceded by fever, and after a 30-day period of clinical stability or improvement. 24 Disability progression was assessed via the EDSS, an MS-specific tool derived from a neurological assessment of eight functional systems. 17 New MRI lesions were defined as any new or enlarging brain or spinal cord lesions on MRI.
Table 1.
NEDA-3 criteria.
| NEDA-3 component | Criteria for positive NEDA-3 status |
|---|---|
| Clinical relapse | No clinical relapses |
| Disability progression | No confirmed EDSS disability progression sustained for 6+ months |
| |
| |
| |
| MRI lesions | No new or enlarging brain or spinal cord lesions on MRI |
EDSS: Expanded Disability Status Scale; MRI: magnetic resonance imaging; NEDA-3: no evidence of disease activity 3.
Adapted from: Beadnall H, Wang C, Van Hecke W, Ribbens A, Billiet T, Barnett M. Comparing longitudinal brain atrophy measurement techniques in a real-world multiple sclerosis clinical practice cohort: Towards clinical integration? Therapeutic Advances in Neurological Disorders. 2019;12:175628641882346. Table 1, NEDA 3 and NEDA 4 definitions; p. 5.
Statistical analysis
Statistical package for social sciences version 27 was used for all statistical analyses. 25 Initially, an a priori sensitivity analysis was conducted to assess the differences between relapsing-remitting MS and other MS subtypes in terms of the dependent variables. The differences in the statistical estimates of the main outcome variables were negligible and not statistically significant (p > 0.1). For this reason, we decided to include all MS subtypes in the main analysis to increase study power. For each dependent variable (estimated dairy intake, estimated gluten intake, and MusiQoL score), a general linear model univariate analysis of variance was generated. NEDA-3 status was included as the main factor variable. For each variable, estimated marginal means and standard errors were calculated and adjusted for the covariates: age, sex, disease duration, number of EDSS and MRI assessments during the study period, and treatment. The significance of the difference in the mean of the dependent variables between NEDA-3 groups was assessed based on an alpha threshold of 0.05. Pearson's correlation analysis was performed to assess the linear relationship among quantitative variables. Multi-factor regression was used to assess the conditional effect of diet variables on NEDA-3 status (logistic) and MusiQol (linear), including covariates. For this regression model, NEDA-3 and MusiQoL are the dependent variables and estimated dairy and gluten intake are independent variables.
Results
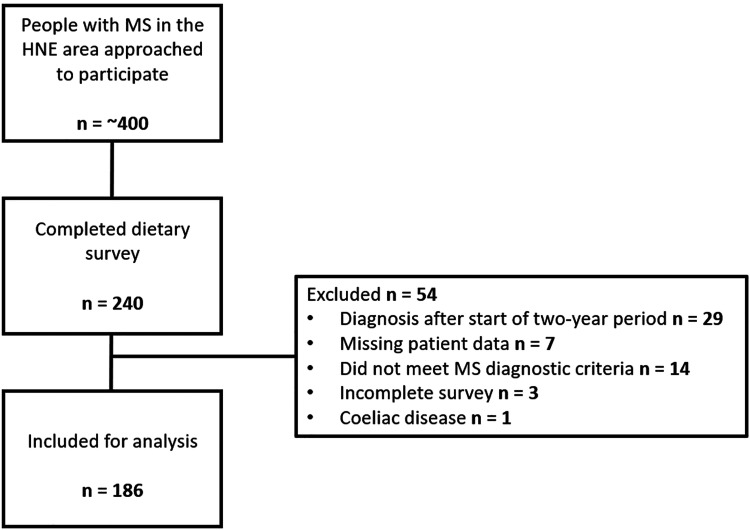
In this study, 240 pwMS were recruited from the Hunter New England Local Health District, Australia. Of these, 54 were excluded, leaving 186 participants for analysis (Figure 1).
Figure 1.
Flow diagram of study participants. Study flow demonstrating the inclusion and exclusion criteria at each step. The top square shows the number of people with MS in the HNE area who were approached to participate in the study. The following square shows how many completed the dietary survey. The right-hand square indicates the number of participants that were excluded and the reason. Note: Missing data refers to insufficient data available on the local database to assess their NEDA-3 status, incomplete survey refers to surveys where the participants had inadequately completed the dietary screener. The bottom square indicates the number of participants included for final analysis. HNE: Hunter New England; MS: multiple sclerosis; NEDA-3: no evidence of disease activity 3.
Participant demographics
Participant demographics are shown in Table 2. There was a statistically significant difference in mean age between the positive and negative NEDA-3 groups (51 ± 12.7) and 46 ± 13.0) years respectively, p = .003).
Table 2.
Participant characteristics.
| Overall | NEDA-3 positive | NEDA-3 negative | |
|---|---|---|---|
| Participants—number (%) | 186 | 87 (47) | 99 (53) |
| Sex—number (%) | |||
| Male | 35 | 15 (17) | 20 (20) |
| Female | 151 | 72 (83) | 79 (80) |
| Age | |||
| Mean (±SD) | 48 (±13.0) | 51 (±12.7) | 46 (±13.0) |
| Range | 21–76 | 22–73 | 21–76 |
| Type of MS—number (%) | |||
| Relapsing remitting | 159 | 73 (84) | 86 (87) |
| Secondary progressive | 23 | 13 (15) | 10 (10) |
| Primary progressive | 3 | 1 (1) | 2 (2) |
| Progressive relapsing | 1 | 0 (0) | 1 (1) |
| Time since symptom onset of MS—years | |||
| Mean (±SD) | 15 (±10.2) | 17 (±10.9) | 14 (±9.4) |
| Range | 3–56 | 3–56 | 2–42 |
| Treatment—number (%) | |||
| No treatment | 21 | 8 (9) | 13 (13) |
| Injectable | 19 | 16 (18) | 3 (3) |
| Oral | 74 | 34 (39) | 40 (40) |
| Monoclonal | 72 | 29 (33) | 43 (43) |
| Participants on diets—number (%) | |||
| MS specific diet | 20 | 8 (9) | 12 (12) |
| Dairy-free diet | 13 | 4 (5) | 9 (9) |
| Gluten-free diet | 8 | 4 (5) | 4 (4) |
| Both dairy- and gluten-free diet | 2 | 1 (1) | 1 (1) |
Percentages to the nearest whole. Standard deviations to the nearest decimal place. NEDA-3: no evidence of disease activity 3; MS: multiple sclerosis.
Disease activity indicated by NEDA-3 status
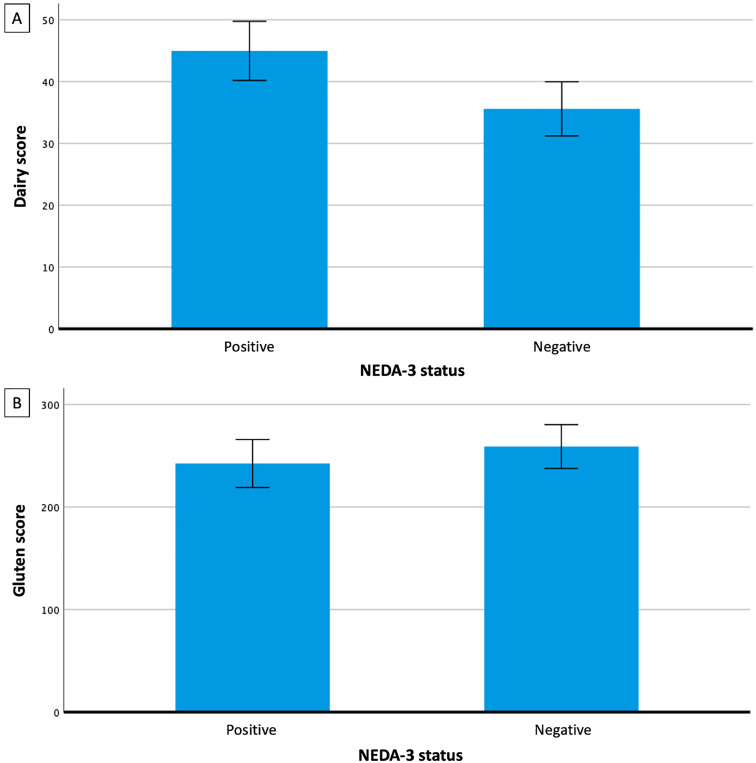
The distribution of participants’ quantitative dairy and gluten intake was found to be positively skewed, with a mean dairy value (representative of estimated intake) of 37.8 (± 37.9) and a mean gluten value (representative of estimated intake) of 219.6 (± 181.9). The mean estimated dairy and gluten intake values were compared with NEDA-3 status over a 2-year period. The NEDA-3 negative group had a 21% lower mean estimated dairy intake value and a 7% higher mean estimated gluten intake value compared to the NEDA-3 positive group (Figure 2); however, these differences were not statistically significant (p = 0.15, F = 2.1 and p = 0.60, F = 0.27 respectively). Dairy and gluten intake were also analysed against each individual component of NEDA-3. There was no statistically significant relationship between estimated dairy intake and clinical relapse, EDSS, or MRI (p = 0.15, 0.47, and 0.11 respectively), nor between estimated gluten intake and clinical relapse, EDSS, or MRI (p = 0.64, 0.22, and 0.47 respectively). A multi-factor regression model did not indicate any conjoint associations among these variables with NEDA-3 (p > 0.05 for all variables). There was a statistically significantly greater mean number of EDSS assessments and MRIs performed in participants in the NEDA-3 negative group compared to the NEDA-3 positive group (p = 0.015 and 0.007, respectively), and results were adjusted for this.
Figure 2.
The association between dairy and gluten intake and MS disease activity. Bar graph showing calculated means for dairy (A) and gluten (B) scores in each of NEDA-3 positive and negative groups. Error bars show SEM. NEDA-3: no evidence of disease activity 3; SEM: standard error of the mean.
Quality of life measured by MusiQoL questionnaire
Participants’ MusiQoL scores were analysed against their estimated dairy and gluten intake. No statistically significant association was found between estimated dairy or gluten intake and MusiQoL scores (p = 0.11 and 0.51, respectively). A multi-factor regression model did not indicate any conjoint associations among these variables with MusiQoL (p > 0.05 for all variables).
Post hoc power analysis
Recruitment was stopped at 240 participants due to the unforeseen limitations of the COVID-19 pandemic. Based on the resultant sample size (∼90 subjects in each NEDA-3 group), and using the pooled standard deviation observed for dairy and gluten intake values, this study had 80% power to detect a mean difference of at least 30% between outcome groups as being statistically significant (p < 0.05). These differences were not reached for either estimated dairy or gluten intake (21% and 7% respectively).
Discussion
Dairy intake and disease activity in pwMS
We found no statistically significant association between estimated dairy intake and NEDA-3 status in this study. Whilst we could not exclude more modest benefits, we conclude that dairy intake has no large impact on disease activity. This suggests that restricting dairy, as recommended by some MS diets, may not be of major benefit to pwMS.
Our findings support Zhang et al., 26 who reported that the intake of dairy products was not significantly associated with the risk of MS. They analysed dairy intake in a large pre-existing cohort using biennial food frequency questionnaires over approximately 15 years. However, in contrast to our study, they looked at the impact of dairy intake on MS disease incidence, rather than disease activity in pwMS. A more recent study by Munger et al. 27 utilised the same cohort and dataset to further look at the risk of MS specifically in adolescent females. They reported that total dairy intake was not related to MS risk in this group, but that consumption of whole milk at least three times per day was associated with a 47% increased risk of MS in adolescent women. 27 It would be of interest to further investigate the association between specific dairy products and disease activity, as well as the mechanism for this possible difference.
In contrast to our findings, a large cross-sectional study by Hadgkiss et al. (2015) found that participants with increasing MS disease activity and moderate or major disability were more likely to consume dairy. 28 However, all participant data, including relapse rate, was self-reported, and the participants’ diagnosis of MS was unable to be verified due to the online recruitment of participants through websites and social media. Our study, although smaller, ensured that accurate participant data was extracted from an online clinical database, and only included people with a confirmed diagnosis of MS according to the McDonald criteria.
Most recently, a cross-sectional study by Marck et al. 29 investigated the association between diet and health outcomes in a sample of 1490 Australians with MS. They found no association between dairy intake and disability, quality of life, depression, or anxiety, but they did note that exclusion of dairy was associated with fewer reported visual symptoms. 29 However, they measured disability and MS symptoms using self-reporting tools, which are vulnerable to recall and reporting bias and may be inexact. In contrast, our study uses NEDA-3, an objective measure of disease activity, improving accuracy and greatly reducing the risk of bias.
Gluten intake and disease activity in pwMS
Our results showed no statistically significant association between estimated gluten intake and NEDA-3 status in this study. Whilst we could not exclude more modest benefits, we conclude that gluten intake does not have a large impact on disease activity. Therefore, strict limitations of gluten, as recommended by some MS diets, may not be of major benefit to pwMS. In fact, pursuing a gluten-free diet without careful consideration for other nutrients has been associated with inadequate micronutrient intake, weight gain, and an increased risk of cardiovascular conditions.30–32 Furthermore, gluten-free substitutes pose a significant financial burden, with gluten-free bakery items being 267% more expensive than their gluten-containing equivalents, on average. 33
Our findings are supported by Johnson et al., 15 who found no association between overall gluten intake and self-reported MS symptoms in 75 pwMS. Additionally, to investigate total gluten intake, they also analysed the relationship between different gluten-containing foods and MS symptoms and found that a higher intake of bread was statistically significantly correlated with increased fatigue, muscle spasms, and depression. This warrants further research into the relationship between specific gluten-containing foods and disease activity and symptoms in MS.
A prospective study by Rodrigo et al. (2014) with 72 participants showed that when compared to a regular diet, a gluten-free diet improved EDSS and lesion activity on MRI but had no effect on the annual relapse rate. 16 EDSS, MRI, and relapse rate were each evaluated individually in this study, whereas our study used NEDA-3, which provides greater accuracy than the individual measurements alone. Interestingly, the regular diet group in Rodrigo et al.'s study was formed retrospectively from participants who were not compliant with the gluten-free diet they had been asked to follow during the first six months of the study. Therefore, up to 50% of participants enrolled in the study were unable to remain adherent to the diet. This demonstrates that diets with strict guidelines are difficult to follow and highlights the importance of having sufficient evidence around gluten-free diets before making a recommendation to pwMS.
A systematic review by Thomsen et al. 14 qualitatively evaluated 49 publications looking at gluten and MS. They found that most studies had a considerable risk of bias, and many reported that improvements in disease activity or disability could not be entirely attributed to the gluten-free diets. Many also used self-reported measures of disability, creating recall and reporting bias. Therefore, they concluded that there was still insufficient evidence as to whether gluten plays a role in MS.
Dairy and gluten intake and quality-of-life in pwMS
There was no statistically significant association between either estimated dairy or gluten intake and MusiQoL scores in our study, suggesting that neither dairy nor gluten intake has a large effect on the quality of life in MS.
Supporting our findings, a large study by Hadgkiss et al. 28 analysed data from 2087 participants recruited online and found that the consumption of dairy was not conclusively linked with health-related quality of life. Gluten intake was not examined, however better quality of life scores was more likely among people consuming a generally healthy diet. 28 On the contrary, a small randomised controlled trial by Irish et al. (2017) examined the relationship between a modified Palaeolithic diet (excludes both dairy and gluten) and quality of life, finding statistically significant improvement in quality of life in participants following the diet. 4 It is important to note that disease characteristics and other factors such as exercise and socioeconomic status can also significantly contribute to quality of life, making it difficult to assess the isolated impact of dietary factors alone.
Strengths and limitations
Our study is limited by the use of a retrospective dietary screener, which has the potential to be inexact and is highly susceptible to recall bias.34,35 Furthermore, our dietary screener has not been validated. However, it was designed in close consultation with a dietitian and focuses specifically on dairy and gluten, ensuring all major foods in Australian supply known to contain dairy and gluten were included. Most other studies in this field draw conclusions about participants’ dairy and/or gluten intake from questionnaires that assess overall diet quality, whereas our dietary screener is targeted to facilitate more accurate quantification of dairy and gluten intake specifically. There is also a risk of reverse causality, as worsening disease activity may have influenced participants’ diets in our study. Prospective, controlled, interventional trials are required to establish a temporal sequence between dairy and gluten and disease activity. Additionally, very few of our study participants actually followed a dairy-free diet (n = 13), gluten-free diet (n = 8), or both a dairy- and gluten-free diet (n = 2), and this limited sample size may have contributed to the potential null significance.
A major strength of our study is the use of NEDA-3, an objective, standardised tool for measuring disease activity in MS. NEDA-3 provides greater accuracy than singular measurements of disease activity or the self-reported MS symptoms used by existing studies in the field. 18 Its three parameters were obtained from the clinical assessment of participants by a neurologist during serial clinic visits. Furthermore, the participant characteristics of our study are representative of the wider Australian population with MS. 36
Participants with all MS subtypes were included for analysis to maximise power, given the relatively small sample size. MS subtypes were not included as covariates in the association models; therefore, we were unable to account for all types of relationships between subtypes and main outcome variables. However, there were only 27 participants with progressive MS (15%), and the sensitivity analysis (including subgroup analysis) showed that the differences were negligible and not statistically significant (p > 0.1).
Conclusion
This study excludes a large effect of dairy and gluten intake on NEDA-3 status in a group of pwMS. Detection of more modest effects will require larger sample sizes, however, it remains to be determined whether such effects will translate to clinically important differences in disease activity. Therefore, recommending a healthy, balanced diet for pwMS may be the best approach (encouraging low-fat dairy and wholegrain products in accordance with the Australian Dietary Guidelines). Whilst our findings contribute to the existing research in this field, the overall evidence remains contradictory, highlighting the need for further, large, prospective, interventional studies on the role of dietary interventions in MS.
Supplemental Material
Supplemental material, sj-pdf-1-mso-10.1177_20552173231218107 for Dairy and gluten in disease activity in multiple sclerosis by Isabel A Temperley, Alexandra N Seldon, Madeline AW Reckord, Claudia A Yarad, Farihah T Islam, Kerith Duncanson, Rodney A Lea, Jeannette Lechner-Scott and Vicki E Maltby in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Authors’ contributions
Amanda Lydon was involved in the conceptualisation. Analysis support was provided by Patrick Lochrin. Data collection and participant recruitment was supported by Jacob Cliff, Amouri Strydom and the clinical staff in the Neurology Department at John Hunter Hospital, Newcastle, Australia
Footnotes
Declaration of conflicting interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Vicki E. Maltby has received honoraria for presentations from Biogen and Merck Healthcare Pty Ltd. She received research funding from Merck KGgA and Biogen. Jeannette Lechner-Scott has accepted travel compensation from Novartis, Biogen and Merck. Her institution receives the honoraria for talks and advisory board commitment as well as research grants from Biogen Idec, Sanofi Genzyme, Merck, Roche, TEVA and Novartis. All other authors report no disclosures.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the University of Newcastle, Australia's Joint Medical Program® (JMP). There was no specific grant number for this project. This funding was used to assist with data collection.
ORCID iDs: Isabel A Temperley https://orcid.org/0000-0001-7079-5708
Alexandra N Seldon https://orcid.org/0000-0002-5514-2335
Vicki E Maltby https://orcid.org/0000-0002-3785-4742
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Kerith Duncanson, Centre of Research Excellence in Digestive Health, University of Newcastle, Callaghan, NSW, Australia.
Rodney A Lea, Hunter Medical Research Institute, New Lambton Heights, NSW, Australia; Centre for Genomics and Personalised Health, School of Biomedical Sciences, Queensland University of Technology, Brisbane, QLD, Australia.
References
- 1.Barnard E, Brown CR, Weiland TJ, et al. Understanding barriers, enablers, and long-term adherence to a health behavior intervention in people with multiple sclerosis. Disabil Rehabil 2020; 42: 822–832. [DOI] [PubMed] [Google Scholar]
- 2.Dunn M, Bhargava P, Kalb R. Your patients with multiple sclerosis have set wellness as a high priority— and the national multiple sclerosis society is responding. Touch Neurology 2015; 11: 80–86. [Google Scholar]
- 3.Swank RL, Goodwin J. Review of MS patient survival on a Swank low saturated fat diet. Nutrition 2003; 19: 161–162. [DOI] [PubMed] [Google Scholar]
- 4.Irish AK, Erickson CM, Wahls TL, et al. Randomized control trial evaluation of a modified paleolithic dietary intervention in the treatment of relapsing-remitting multiple sclerosis: a pilot study. Degener Neurol Neuromuscul Dis 2017; 7: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wahls T, Scott MO, Alshare Z, et al. Dietary approaches to treat MS-related fatigue: comparing the modified paleolithic (Wahls elimination) and low saturated fat (Swank) diets on perceived fatigue in persons with relapsing-remitting multiple sclerosis: study protocol for a randomized controlled trial. Trials 2018; 19: 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu M, Jelinek G, Simpson-Yap S, et al. Self-reported ongoing adherence to diet is associated with lower depression, fatigue, and disability, in people with multiple sclerosis. Front Nutr 2023; 10: 979380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shatin R. Multiple Sclerosis and Geography. New interpretation of epidemiological observations. Neurology 1964; 14: 338–344. [DOI] [PubMed] [Google Scholar]
- 8.Malosse D, Perron H, Sasco Aet al. et al. Correlation between milk and dairy product consumption and multiple sclerosis prevalence: a worldwide study. Neuroepidemiology 1992; 11: 304–312. [DOI] [PubMed] [Google Scholar]
- 9.Stefferl A, Schubart A, Storch M, et al. Butyrophilin, a milk protein, modulates the encephalitogenic T cell response to myelin oligodendrocyte glycoprotein in experimental autoimmune encephalomyelitis. J Immunol 2000; 165: 2859–2865. [DOI] [PubMed] [Google Scholar]
- 10.Weiland TJ, De Livera AM, Brown CR, et al. Health outcomes and lifestyle in a sample of people with multiple sclerosis (HOLISM): longitudinal and validation cohorts. Front Neurol. 2018;9:1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzgerald KC, Tyry T, Salter A, et al. Diet quality is associated with disability and symptom severity in multiple sclerosis. Neurology 2018; 90: e1–e11. [DOI] [PubMed] [Google Scholar]
- 12.Lerner A, Shoenfeld Y, Matthias T. Adverse effects of gluten ingestion and advantages of gluten withdrawal in nonceliac autoimmune disease. Nutr Rev 2017; 75: 1046–1058. [DOI] [PubMed] [Google Scholar]
- 13.Miller FW. The increasing prevalence of autoimmunity and autoimmune diseases: an urgent call to action for improved understanding, diagnosis, treatment, and prevention. Curr Opin Immunol 2023; 80: 102266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomsen HL, Jessen EB, Passali Met al. et al. The role of gluten in multiple sclerosis: A systematic review. Mult Scler Relat Disord 2019; 27: 156–163. [DOI] [PubMed] [Google Scholar]
- 15.Johnson S, Seaborn C, Deery Ket al. et al. The effects of gluten and dairy intake on multiple sclerosis symptoms. J Acad Nutr Diet 2012; 112: A39. [Google Scholar]
- 16.Rodrigo L, Hernandez-Lahoz C, Fuentes D, et al. Randomised clinical trial comparing the efficacy of a gluten-free diet versus a regular diet in a series of relapsing-remitting multiple sclerosis patients. Int J Neurol Neurother 2014; 1: 4. [Google Scholar]
- 17.Giovannoni G, Tomic D, Bright JRet al. et al. No evident disease activity": The use of combined assessments in the management of patients with multiple sclerosis. Mult Scler 2017; 23: 1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu G, Beadnall HN, Barton J, et al. The evolution of “No evidence of disease activity” in multiple sclerosis. Mult Scler Relat Disord 2018; 20: 231–238. [DOI] [PubMed] [Google Scholar]
- 19.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2017; 17: 162–170. [DOI] [PubMed] [Google Scholar]
- 20.Assor E, Davies-Shaw J, Marcon MAet al. et al. Estimation of Dietary Gluten Content using Total Protein in Relation to Gold Standard Testing in a Variety of Foods. J Nutr Food Sci 2014; 4: 2. [Google Scholar]
- 21.Australian Dietary Guidelines https://www.eatforhealth.gov.au/: NHMRC; 2013
- 22.Jamroz-Wisniewska A, Stelmasiak Z, Bartosik-Psujek H. Validation analysis of the Polish version of the multiple sclerosis international quality of life questionnaire (MusiQoL). Neurol Neurochir Pol 2011; 45: 235–244. [DOI] [PubMed] [Google Scholar]
- 23.Simeoni M, Auquier P, Fernandez O, et al. Validation of the multiple sclerosis international quality of life questionnaire. Mult Scler 2008; 14: 219–230. [DOI] [PubMed] [Google Scholar]
- 24.Pandit L. No evidence of disease activity (NEDA) in multiple sclerosis—shifting the goal posts. Ann Indian Acad Neurol 2019; 22: 261–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.IBM. IBM SPSS Statistics for Windows version 27.0. Armonk NY: IBM Corp., 2020. [Google Scholar]
- 26.Zhang SM, Willett WC, Hernan MA, et al. Dietary fat in relation to risk of multiple sclerosis among two large cohorts of women. Am J Epidemiol 2000; 152: 1056–1064. [DOI] [PubMed] [Google Scholar]
- 27.Munger KL, Chitnis T, Frazier AL, et al. Dietary intake of vitamin D during adolescence and risk of multiple sclerosis. J Neurol 2011; 258: 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hadgkiss EJ, Jelinek GA, Weiland TJ, et al. The association of diet with quality of life, disability, and relapse rate in an international sample of people with multiple sclerosis. Nutr Neurosci 2015; 18: 125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marck CH, Probst Y, Chen J, et al. Dietary patterns and associations with health outcomes in Australian people with multiple sclerosis. Eur J Clin Nutr 2021; 75: 1506–1514. [DOI] [PubMed] [Google Scholar]
- 30.Lebwohl B, Cao Y, Zong G, et al. Long term gluten consumption in adults without celiac disease and risk of coronary heart disease: prospective cohort study. Br Med J 2017; 357: 8–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valletta E, Fornaro M, Cipolli M, et al. Celiac disease and obesity: need for nutritional follow-up after diagnosis. Eur J Clin Nutr 2010; 64: 1371–1372. [DOI] [PubMed] [Google Scholar]
- 32.Vici G, Belli L, Biondi Met al. et al. Gluten free diet and nutrient deficiencies: a review. Clin Nutr 2016; 35: 1236–1241. [DOI] [PubMed] [Google Scholar]
- 33.Jones AL. The gluten-free diet: fad or necessity? Diabetes Spectr 2017; 30: 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jager KJ, Tripepi G, Chesnaye NC, et al. Where to look for the most frequent biases? Nephrology (Carlton) 2020; 25: 435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ventura AK, Loken E, Mitchell DC, et al. Understanding reporting bias in the dietary recall data of 11-year-old girls. Obesity (Silver Spring) 2006; 14: 1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Australia MS. Key facts and figures about Multiple Sclerosis. https://www.msaustralia.org.au/wp-content/uploads/2021/09/msa-key-facts-figures_2020-2.pdf.2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-mso-10.1177_20552173231218107 for Dairy and gluten in disease activity in multiple sclerosis by Isabel A Temperley, Alexandra N Seldon, Madeline AW Reckord, Claudia A Yarad, Farihah T Islam, Kerith Duncanson, Rodney A Lea, Jeannette Lechner-Scott and Vicki E Maltby in Multiple Sclerosis Journal – Experimental, Translational and Clinical