Abstract
Background:
Cyclin-Dependent Kinase 4/6 inhibitors (CDK4/6i) combined with Endocrine Therapy (ET) are the standard treatment for patients with Hormone Receptor-positive/HER2-negative advanced breast cancer (HR+/HER2− aBC).
Objectives:
While CDK4/6i are known to reduce several peripheral blood cells, such as neutrophils, lymphocytes and platelets, the impact of these modulations on clinical outcomes is unknown.
Design:
A multicenter, retrospective-prospective Italian study.
Methods:
We investigated the association between baseline peripheral blood cells, or their early modifications (i.e. 2 weeks after treatment initiation), and the progression-free survival (PFS) of HR+/HER2− aBC patients treated with ETs plus CDK4/6i. Random Forest models were used to select covariates associated with patient PFS among a large list of patient- and tumor-related variables.
Results:
We evaluated 638 HR+/HER2− aBC patients treated with ET plus CDK4/6i at six Italian Institutions between January 2017 and May 2021. High baseline lymphocyte counts were independently associated with longer PFS [median PFS (mPFS) 20.1 versus 13.2 months in high versus low lymphocyte patients, respectively; adjusted Hazard Ratio (aHR): 0.78; 95% confidence interval (CI): 0.66–0.92; p = 0.0144]. Moreover, patients experiencing a lower early reduction of lymphocyte counts had significantly longer PFS when compared to patients undergoing higher lymphocyte decrease (mPFS 18.1 versus 14.5 months; aHR: 0.82; 95% CI: 0.73–0.93; p = 0.0037). Patients with high baseline lymphocytes and undergoing a lower reduction, or even an increase, of lymphocyte counts during CDK4/6i therapy experienced the longest PFS, while patients with lower baseline lymphocytes and undergoing a higher decrease of lymphocytes had the lowest PFS (mPFS 21.4 versus 11 months, respectively).
Conclusion:
Baseline and on-treatment modifications of peripheral blood lymphocytes have independent prognostic value in HR+/HER2− aBC patients. This study supports the implementation of clinical strategies to boost antitumor immunity in patients with HR+/HER2− aBC treated with ETs plus CDK4/6i.
Keywords: advanced breast cancer, CDK4/6 inhibitors, endocrine therapy, lymphocytes, prognostic biomarkers
Introduction
The Cyclin-Dependent Kinase 4/6 inhibitors (CDK4/6i) palbociclib, ribociclib and abemaciclib have revolutionized the treatment of Hormone Receptor-positive (HR+), Human Epidermal growth factor Receptor 2 (HER2)-negative advanced (unresectable, locally advanced or metastatic) breast cancer (aBC). Based on results of the randomized phase III trials PALOMA-2, PALOMA-3, MONALEESA-2, MONALEESA-3, MONALEESA-7, MONARCH-2 and MONARCH-3, CDK4/6i have become the standard-of-care treatment for patients with both endocrine-sensitive and endocrine-resistant HR+/HER2− aBC in combination with aromatase inhibitors (AIs) or with the selective estrogen receptor degrader fulvestrant, respectively.1–12 Indeed, when compared to endocrine therapies (ETs) alone, the combination of CDK4/6i and ETs resulted in clinically and statistically significant improvement of patient progression-free survival (PFS)1–4,7 and overall survival (OS).5,6,8–11
The most acknowledged antitumor mechanism of CDK4/6i consists in cancer cell-autonomous (direct) effects, which are mediated by the inhibition of the retinoblastoma protein (RB1) phosphorylation, leading to impaired E2F transcriptional activity and reduced cyclin A and cyclin E expression. 13 These biological modulations result in the inhibition of the G1-to-S transition of the cell cycle, finally causing HR+/HER2− BC cell cycle arrest in G1 phase. 13
However, recent preclinical and clinical evidence indicates that CDK4/6i-induced enhancement of antitumor immunity could play a crucial role in affecting the antitumor effects of this class of drugs. For instance, Goel et al. showed that CDK4/6i can promote cancer cell antigen presentation to antitumor lymphocytes, and that they inhibit the proliferation and immunosuppressive activity of regulatory T cells (Tregs) in murine and human HR+/HER2− BC. 14 In detail, CDK4/6i treatment resulted in E2F inhibition in tumor cells, finally leading to an increased production of type III interferon (IFN) and to enhanced tumor antigen processing and presentation as a result of a global activation of IFN-driven transcriptional programs. Moreover, CDK4/6i promoted tumor infiltration by CD3+ T cells and a concomitant reduction of CD4+FOXP3+ Tregs in murine BC models, paralleled by a reduction of peripheral blood Tregs. 14
One of the most common toxicities associated with CDK4/6i treatment consists of the reduction of total white blood cells (leukopenia) and neutrophil counts (neutropenia), which mainly results from the inhibition of CDK6 in the precursors of these cells in the bone marrow. 15 These hematologic toxicities are more commonly observed with palbociclib and ribociclib than with abemaciclib, which is approximately 13 times more selective against CDK4 as compared to CDK6. 16 Differently from chemotherapy-induced leuko-neutropenia, CDK4/6i-induced leukopenia and neutropenia are not associated with a significantly increased risk of developing severe infections, probably due to the fact that, unlike cytotoxic agents, CDK4/6i do not cause irreversible damage and apoptotic cell death in white blood cell precursors in the bone marrow.1–3,5–8,17 Instead, CDK4/6i-induced leuko-neutropenia could even favor their antitumor activity by relieving neutrophil- and myeloid cell-mediated immunosuppression and by boosting lymphocyte activation and cytotoxicity. 18
Here, we conducted a multicenter, observational study to investigate the potential impact of baseline and on-treatment changes of peripheral blood cells implicated in systemic inflammation and immunity (i.e. total leukocytes, neutrophils, monocytes, platelets and lymphocytes) and their composite scores (i.e. the neutrophil-to-lymphocyte ratio or NLR, the platelet-to-lymphocyte ratio or PLR, the monocyte-to-lymphocyte ratio or MLR, and the Pan-Immune-Inflammation Value or PIV) on the PFS of HR+/HER2− aBC patients treated with CDK4/6i plus ETs.
Methods
Patient population and enrollment criteria
This was an observational, retrospective-prospective, multicenter study conducted in six Italian Cancer Centers [Fondazione IRCCS Istituto Nazionale dei Tumori di Milano (coordinating center); Istituto Oncologico Veneto di Padova; Istituto Europeo di Oncologia, IRCCS – IEO di Milano; Humanitas Clinical and Research Center – IRCCS di Milano; ASST di Cremona; Spedali Civili di Brescia].
Data were collected through an electronic database. Main enrollment criteria were: (i) age ⩾18 years; (ii) histologically/cytologically confirmed diagnosis of HR+/HER2− aBC; (iii) treatment with palbociclib, ribociclib or abemaciclib in combination with ETs (AIs or fulvestrant) as any line of therapy for advanced disease; (iv) pre-, peri- or post-menopausal women [pre- and peri-menopausal patients also received concomitant treatment with a luteinizing hormone releasing hormone (LHRH) analog (i.e. goserelin, triptorelin or leuprolide)]; (v) availability of at least one measurement of blood cell counts at baseline, 2 weeks after treatment initiation, or 12 weeks after treatment initiation; (vi) at least 3 months of follow up at the date of data collection. All patients were followed up until death, loss of contact, or time of data lock (31 May 2021). Written informed consent was obtained from all patients who were alive at the time of study conduction. The study was carried out in accordance with the Good Clinical Practice guidelines and the Declaration of Helsinki.
Study objectives and statistical plan
The objective of this study was to evaluate the association between baseline immune cell crude counts [neutrophils (N_t0), lymphocytes (L_t0), platelets (PLT_t0), monocytes (M_t0)], or their on-treatment modifications (T1: 2 weeks after treatment initiation, and T2: at the time of first radiological disease assessment, that is, approximately 12 weeks after treatment initiation), and the PFS of women with HR+/HER2− aBC treated with CDK4/6i in combination with ET.
To assess the prognostic role of immune cell modulations during CDK4/6i-based therapy, we evaluated the deltas (d1), as defined as absolute cell count difference at T1 as compared to T0 [neutrophil count d1 (N_d1), lymphocyte count d1 (L_d1), platelet count d1 (PLT_d1), monocyte count d1 (M_d1)], or their ratios (r1), here defined as immune cell counts at T1 divided by the same count at T0 [neutrophil count r1 (N_r1), lymphocyte count r1 (L_r1), platelet count r1 (PLT_r1), monocyte count r1 (M_r1)]. We also explored the potential prognostic role of immunological variables in terms of modulation after three treatment cycles (T2) as compared to T0 assessments (here included as delta d2, or ratio, r2).
We also evaluated the association between baseline and on-treatment modulation of NLR (NLR_t0; NLR_d1; NLR_r1; NLR_d2; NLR_r2), PLR (PLR_t0; PLR_d1; PLR_r1; PLR_d2; PLR_r2), MLR (MLR_t0; MLR_d1; MLR_r1; MLR_d2; MLR_r2), or PIV (PIV_t0; PIV_d1; PIV_r1; PIV_d2; PIV_r2), and patient PFS. The NLR was calculated as the ratio between neutrophil and lymphocyte counts; the PLR was calculated as the ratio between platelet and lymphocyte counts; the MLR was calculated as the ratio between monocyte and lymphocyte counts; the PIV was calculated as: (neutrophil count × platelet count × monocyte count)/lymphocyte count.
PFS was defined as the time between the initiation of ET + CDK4/6i therapy and the detection of clinical/radiological disease progression (according to RECIST v1.1 criteria) or patient death from any cause, whichever occurred first. 19 OS was calculated as the time between treatment initiation and patient death from any cause. 19 Patients without an event were censored at the date of last follow up.
Statistical analyses
Standard descriptive statistics were used to describe clinical and biological patients’ characteristics. Spearman’s rank correlation was used to evaluate the correlation between the line of therapy for advanced disease and the line of ET for advanced disease. Unpaired t-test was used to compare peripheral blood immune cell counts, or their combined scores, at baseline, at T1 and T2. Median patient follow-up was calculated with the reverse Kaplan–Meier estimator. 20 Survival curves for PFS or OS were obtained with the Kaplan–Meier method. Optimal cut-points for classifying L_t0 and L_r1 as ‘high’ versus ‘low’ were calculated through the maximally selected rank statistic method. 21 A two-step strategy was used to perform multivariable analysis, as detailed below.
Random forest models
In the first step of multivariable analyses, the impact of several covariates on patient PFS was modeled by using a Random Forest approach. 22 Random Forest method was used with the purpose of (a) detecting and excluding, on the basis of minimal depth statistic, prognostically irrelevant covariates; (b) detecting interactions among covariates and nonlinear effects of continuous predictors; (c) estimating, through error rate estimation, the global predictive performance of the model. The variables selected through Random Forest Models were chosen to be evaluated in multivariable Cox regression models. Depending on the type of analysis, we used either original or imputed data.
Cox regression models
Cox regression modeling was used in the second step of our statistical analyses, with the proportional hazard assumption checked by testing and plotting Schoenfeld residuals. Means of restricted cubic splines were used to handle nonlinear effects for all continuous variables. Results of Cox models were summarized using Hazard Ratios, along with the corresponding 95% confidence intervals (CIs) and Wald p values, while overall model performance was assessed in terms of discrimination with the bootstrap-adjusted Harrell c-index. In Cox models, the HR for continuous variables was reported as the HR related to the inter-quartile range (IQR; interval between the 75th and 25th quantiles). Furthermore, to quantify the effect of high/low values of L_t0 and L_r1 in terms of PFS, we estimated adjusted survival PFS curves. 23 Given the presence of missing data, Cox model analyses were performed both with single and after 10-fold multiple imputations. The main study analyses were repeated in the subset of patients receiving CDK4/6i-based therapy in the first-line treatment setting.
Statistical analyses were performed with SAS (version 9.4, SAS Institute) and R software (version 4.1.2, R Foundation for Statistical Computing). The conventional 5% two-sided threshold was set for statistical significance.
Results
Study cohort
We evaluated a total number of 656 HR+/HER2− aBC patients treated with ET plus CDK4/6i between January 2017 and May 2021. Of these, 13 patients were excluded because they received CDK4/6i monotherapy, 5 patients were excluded because of the lack of at least one measurement of blood cell counts at baseline, at 2 weeks or at 12 weeks after treatment initiation. Finally, 638 patients fulfilling all the enrollment criteria, and who initiated treatment with CDK4/6i plus ET, were enrolled. The study flow diagram is shown in Supplemental Figure 1. In detail, 168 patients were enrolled at Fondazione IRCCS Istituto Nazionale dei Tumori of Milan (coordinating center); 179 patients at Istituto Oncologico Veneto of Padua; 77 patients at Istituto Europeo di Oncologia, IRCCS – IEO of Milan; 53 patients at Humanitas Clinical and Research Center – IRCCS of Milan; 58 patients at ASST of Cremona; 103 patients at Spedali Civili of Brescia.
Baseline patient- and tumor-related characteristics are summarized in Table 1. Median patient age was 61 years (IQR: 52–70 years). The majority of patients were post-menopausal (n = 530; 83.1%), had an Eastern cooperative oncology group performance status (ECOG PS) of 0 (n = 464, 72.8%) and were treated with Palbociclib-based therapy (n = 529; 82.9%). Approximately half of enrolled patients received CDK4/6i-based therapy in the first-line setting (n = 335; 52.5%) and had visceral disease (n = 334; 52.4%).
Table 1.
Patient characteristics.
| N = 638 | |
|---|---|
| Characteristic | N (%) |
| ECOG PS | |
| 0 | 464 (72.8) |
| 1 | 153 (23.9) |
| 2 | 21 (3.3) |
| NA | – |
| Line of ET + CDK4/6i treatment | |
| 1 | 335 (52.5) |
| 2 | 158 (24.8) |
| ⩾3 | 145 (22.7) |
| NA | – |
| Type of CDK4/6i | |
| Palbociclib | 529 (82.9) |
| Ribociclib | 74 (11.6) |
| Abemaciclib | 35 (5.5) |
| NA | – |
| CDK4/6i dose reduction | |
| Yes | 266 (41.7) |
| No | 372 (58.3) |
| NA | – |
| Type of ET | |
| AI | 259 (40.6) |
| Fulvestrant | 379 (59.4) |
| NA | – |
| Postmenopausal status | |
| Yes | 530 (83.1) |
| No | 108 (16.9) |
| NA | |
| No. metastatic sites | |
| 1–2 | 458 (71.8) |
| >2 | 180 (28.2) |
| NA | – |
| Visceral disease | 334 (52.4) |
| NA | 1 |
| Bone metastases | 444 (69.6) |
| NA | |
| Liver metastases | 188 (29.5) |
| NA | 1 |
| Lung metastases | 200 (31.3) |
| NA | – |
| Brain metastases | 18 (2.8) |
| NA | – |
| Skin/soft tissue metastases | 86 (13.5) |
| NA | – |
| LN metastases | 303 (47.5) |
| NA | – |
| Age | |
| Median (IQR) | 61 (52–70) |
| NA | – |
| DFI (years) | |
| Median (IQR) | 4.8 (0.8–10.4) |
| NA | 24 |
| ERα in primary tumor | |
| Median (IQR) | 90 (80–95) |
| NA | 47 |
| Progesterone receptor in primary tumor | |
| Median (IQR) | 47.6 (5–85) |
| NA | 54 |
| Ki-67 in primary tumor | |
| Median (IQR) | 24 (13;31) |
| NA | 168 |
Data are presented as absolute numbers (N) and their percentage within the indicated category (%), unless otherwise specified.
AI, aromatase inhibitor; CDK4/6i, Cyclin-Dependent Kinase 4/6 inhibitor; DFI, disease free interval; ECOG PS, eastern cooperative oncology group performance status; ER: estrogen receptor; ET, endocrine therapy; IQR, inter-quartile range; LHRH, luteinizing hormone-releasing hormone; LN, lymph node; N, number; NA, not available.
At data cut-off and analysis (31 May 2021), 358 patients (56.1%) had experienced disease progression, and 166 patients (26.0%) had died. With a median follow-up time of 25 months (95% CI: 15.2–35.0), median PFS in the whole study cohort was 18.1 months (95% CI: 15.1–22.3). Median PFS was 26.1 months (95% CI: 23.4–30.8) in patients receiving CDK4/6i treatment as a first-line therapy, while median PFS was 11.9 months (95% CI: 9.9–13.9) in patients receiving CDK/4/6i as subsequent lines of therapy (Supplemental Figure 2). These data are consistent with results of phase III clinical trials1–3,5–8,12 and with data from previously published real-world cohorts24–27 of patients treated with ET plus CDK4/6i.
CDK4/6i-induced modulation of peripheral blood immune cells
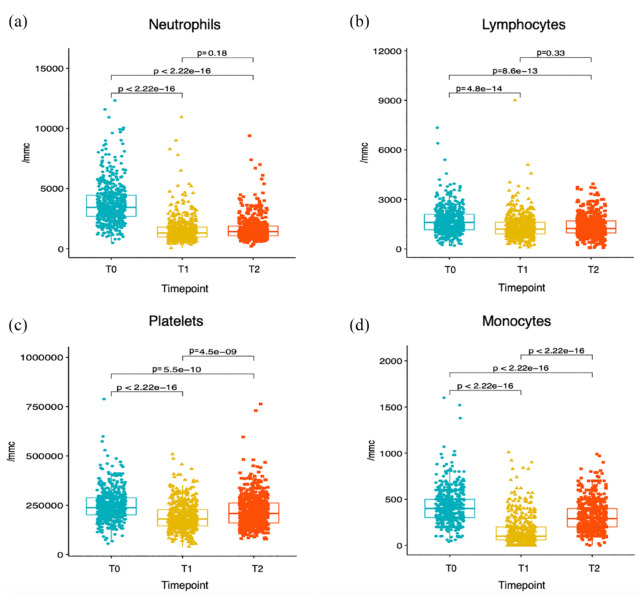
The modulation of peripheral blood cell counts during CDK4/6i therapy was evaluated by comparing immune cell counts at T0 (baseline), at T1 (2 weeks after treatment initiation) and at T2 (at the time of first radiological disease assessment, that is, approximately 12 weeks after treatment initiation). On-treatment modulation of these parameters is reported in Figure 1. Neutrophil, lymphocyte, platelet and monocyte counts were all significantly and precociously reduced after treatment initiation (T1 versus T0). At T2, there was a partial recovery of monocyte and platelet counts when compared to their values at T1, while neutrophil and lymphocyte counts were not significantly different at T2 as compared to T1. Immune cell scores also underwent significant modifications; in detail, NLR was significantly reduced at T1, and it remained low at T2; conversely, the MLR and the PIV, which were significantly reduced at T1, were partially restored at T2, which reflects the partial restoration of monocyte and platelet counts at this timepoint. Finally, the PLR did not undergo a significant early reduction, while it was minimally increased after 12 weeks of CDK4/6i-based therapy (Supplemental Figure 3).
Figure 1.
CDK4/6i-induced modulation of peripheral blood cell counts. Boxplots depicting the kinetics of the indicated peripheral blood cell counts at three different timepoints, namely T0 (baseline, i.e. before CDK4/6i treatment initiation), T1 (~2 weeks after CDK4/6i treatment initiation) and T2 (~12 weeks after CDK4/6i treatment initiation). For each of the indicated comparisons, the p value of the unpaired t-test is reported.
CDK4/6i, Cyclin-Dependent Kinase 4/6 inhibitor.
Selection of potentially prognostic clinical and immunological parameters
We used Random Forest models to screen the potential prognostic role of 28 patient- and tumor-related variables. In a first model exploring the role of several clinical variables, and in which missing data were imputed, the following variables emerged as associated with patient PFS: line of therapy for advanced disease, ECOG PS, presence of liver metastases, line of ET for advanced disease, number of metastatic sites, disease-free interval (DFI), percentage of Ki-67-positive cells in primary tumor specimens, patient age, and presence of ERα expression in primary tumor specimens (Supplemental Figure 4). The line of therapy for advanced disease and the line of ET for advanced disease were positively correlated (Spearman’s rank correlation coefficient, ρ = 0.83, p < 0.001), thus indicating that these two variables provide redundant information. Therefore, in subsequent multivariable Cox regression models we only included the line of therapy for advanced disease, while we excluded the line of ET for advanced disease.
Then, we fitted several Random Forest models, with or without imputation of missing data, in which we assessed the potentially prognostic role of previously selected clinical variables, along with peripheral blood immune cells and composite immune scores, evaluated as baseline values (T0), or in terms of their modulation during CDK4/6i-based therapy at T1 or T2 [deltas (d1, d2) and ratios (r1, r2)]. Error rate estimations of these Random Forest Models are summarized in Supplemental Table 1. Among these models, the one with the lowest error rate estimation, that is, the one associated with the best predictive performance in terms of patient PFS, was the model that included the T1–T0 ratio (r1) of immune cells (error rate: 29.2%). Therefore, we selected N_r1, L_r1, PLT_r1 and M_r1 for subsequent evaluation in multivariable Cox regression models. In these models we also included baseline raw immune cell counts (N_t0, L_t0, PLT_t0 and M_t0) in light of their established prognostic role.28,29 Although composite immune scores (NLR, PLR, MLR, PIV) did not improve the predictive performance of Random Forest models when compared to T0 and r1 crude count ratios (Supplemental Table 1), we also evaluated these parameters in multivariable Cox models due to their established prognostic role.30–36
Association between baseline and on-treatment immune parameters with patient PFS
We fitted several multivariable Cox models to investigate the prognostic role of each immune-related variable, as evaluated at T0 and as r1, after adjustment for previously selected clinical covariates (Supplemental Figure 4). In these models, we also explored the impact of nonlinear effects of individual immune cell subsets, as well as of the interaction between t0 and r1 immune cell counts. A summary of results of these models is shown in Supplemental Table 2. Of note, lymphocyte counts and the NLR were the only immune parameters to be independently associated with patient PFS when evaluated both at T0 and as r1.
As for lymphocytes, the higher the baseline lymphocyte counts (L_t0), the lower the risk of disease progression [adjusted HR as a continuous variable (aHR): 0.78; 95% CI: 0.66–0.92; p = 0.0144] (Table 2). Regarding on-treatment lymphocyte modulation, the lower the reduction of blood lymphocytes during the treatment (i.e. the higher L_r1), the lower the risk of disease progression (aHR as a continuous variable: 0.82; 95% CI: 0.73–0.93; p = 0.0037) (Table 2). We did not find an interaction between L_t0 and L_r1 in affecting PFS (p = 0.8465). Similar results were obtained when we fitted the model after multiple data imputation (Supplemental Table 3). Higher baseline L_t0 and L_r1 were also associated with independently better OS (aHR for L_t0: 0.67; 95% CI: 0.52–0.88; p = 0.0052; aHR for L_r1: 0.71; 95% CI: 0.59–0.85; p = 0.0006) (Supplemental Table 4).
Table 2.
Multivariable Cox regression model including L_t0 and L_r1.
| Variables | Type of variable | HR | 95% CI | p Value |
|---|---|---|---|---|
| Age | Continuous | 0.72 | 0.52–0.99 | 0.0005 |
| ER primary | Continuous | 0.77 | 0.62–0.96 | 0.0499 |
| Ki–67 primary | Continuous | 1.10 | 0.95–1.28 | 0.1958 |
| No. metastatic sites | Continuous | 1.41 | 1.12–1.76 | 0.0029 |
| DFI | Continuous | 0.92 | 0.74–1.23 | 0.0042 |
| Line of treatment | Continuous | 1.19 | 1.09–1.29 | 0.0001 |
| ECOG PS | ||||
| 1 versus 0 | 1.63 | 1.27–2.09 | <0.0001 | |
| 2 versus 0 | 3.52 | 2.00–6.18 | ||
| Presence of liver metastases | Yes versus No | 1.54 | 1.20–1.99 | 0.0009 |
| L_t0 | Continuous | 0.78 | 0.66–0.92 | 0.0144 |
| L_r1 | Continuous | 0.82 | 0.73–0.93 | 0.0037 |
The p value is indicated in bold numbers when statistically significant (<0.05).
DFI, disease free interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status; ER, estrogen receptor; L_t0, lymphocyte count at T0; L_r1, lymphocyte T1–T0 ratio.
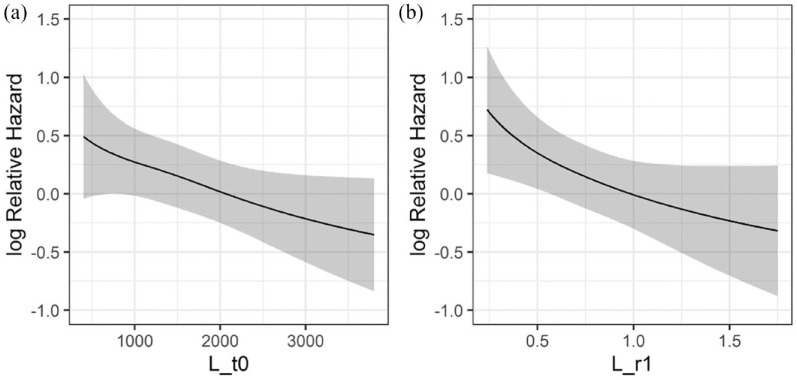
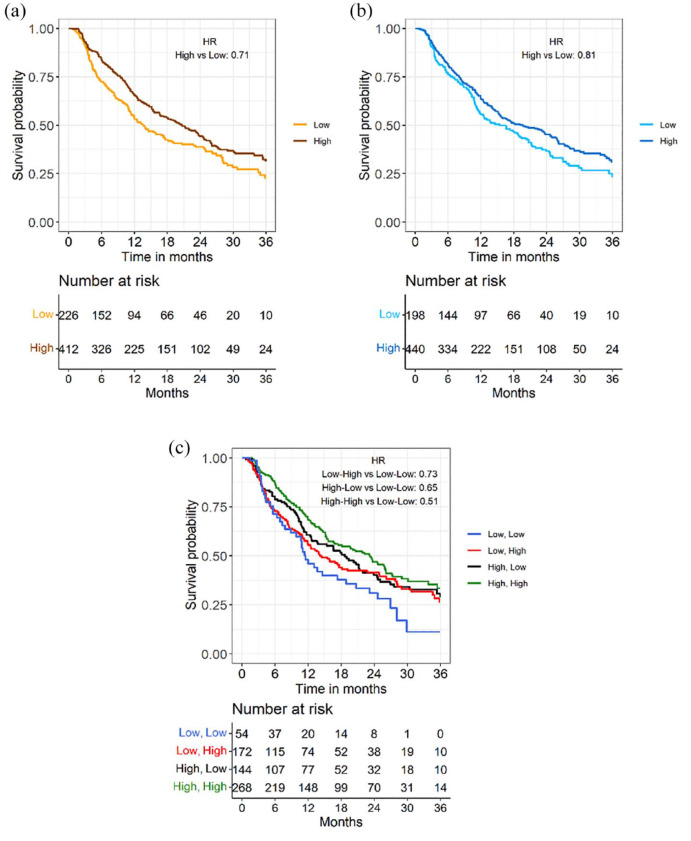
The continuous relationship between lymphocyte counts at T0 or r1 and the log Relative Hazard is shown in Figure 2, while Supplemental Figure 5 shows the continuous relationship between neutrophils, platelets or monocytes and the log Relative Hazard. We also plotted adjusted PFS curves for L_t0 and L_r1 after dichotomizing L_t0 and L_r1 according to cut-offs calculated through the maximally selected rank statistic method (cut-off for L_t0 = 1376.1; cut-off for L_r1 = 0.711). 37 Patients with higher baseline lymphocytes had better PFS when compared to patients with lower baseline lymphocyte counts (median PFS 20.1 months, 95% CI: 16.8–23.6, versus 13.2 months, 95% CI: 11.7–16.6, for patients with high versus low baseline lymphocyte counts, respectively) [Figure 3(a)]. In addition, patients undergoing a lower decrease, or even an increase of peripheral blood lymphocyte counts during CDK4/6i-based therapy had longer PFS when compared to patients undergoing higher lymphocyte decrease (median PFS 18.1 months, 95% CI: 13.3–23.3, versus 14.5 months, 95% CI: 10.8–19.8) [Figure 3(b)]. When we combined baseline and on-treatment lymphocyte assessments, patients with high L_t0 and high L_r1 had the longest PFS (median PFS: 21.4 months, 95% CI: 17.4–25.8), while patients with low L_t0 and low L_r1 had the worst PFS outcomes (median PFS: 11 months, 95% CI: 7.8–17.3); finally, patients with high L_t0 and low L_r1 and low L_t0 and high L_r1 had intermediate PFS outcomes (median PFS 17.6 months, 95% CI: 11.6–22.8, and median PFS 13.8 months, 11.3–18.4, respectively) [Figure 3(c)]. Therefore, combining baseline and on-treatment modulation of lymphocyte counts helps in discriminating patients with better and worse survival outcomes.
Figure 2.
Continuous relationship between peripheral blood lymphocytes and the risk of disease progression. Curves showing the continuous relationship between baseline lymphocyte counts (L_t0), or (a) T1–T0 lymphocyte ratios (L_r1), and (b) log relative Hazard for disease progression.
Figure 3.
Impact of baseline lymphocyte counts and their early modulation on patients’ PFS. Adjusted PFS curves according to baseline lymphocyte counts (high versus low). (a) Adjusted PFS curves according to T1–T0 lymphocyte ratio (high versus low). (b) Adjusted PFS curves according to both baseline lymphocyte counts (high versus low) and T1–T0 lymphocyte ratio at T1, or r1 (high versus low). (c) The cut-off values to divide patients with high versus low lymphocyte counts at baseline or high versus low lymphocyte ratios were calculated with maximally rank selected statistics method.
PFS, progression free survival.
Among composite immune scores, higher NLR was the only variable to be significantly and independently associated with worse patient PFS both at t0 (NLR_t0: HR: 1.21; 95% CI: 1.05–1.40; p = 0.034) and as r1 (NLR_r1: HR: 1.36; 95% CI: 1.16–159; p = 0.0007) (Supplemental Table 2). These data are also reflected by the monotonic relationship between NLR_t0 or NLR_r1 and the risk of disease progression (Supplemental Figure 5). On the other hand, MLR, PLR and PIV did not show a monotonic relationship with the log relative hazard of disease progression (Supplemental Figure 5), in line with the lack of an association between these variables and clinical outcomes (Supplemental Table 2).
Baseline NLR (NLR_t0) has consistently emerged as an independent prognostic variable in several series of patients with different advanced malignancies, including HR+/HER2− aBC.30–32,38,39 For this reason, we fitted another Cox regression model to investigate the independent role of L_r1, which accounts for the modulation of lymphocytes during the treatment, and NLR_t0; in this model, we did not include L_t0 because its prognostic role is already accounted for by NLR_t0 assessment. Of note, higher L_r1 maintained an independent and statistically significant association with better PFS (HR: 0.86; 95% CI: 0.76–0.97, p = 0.0225), while NLR_t0 was not associated with the risk of disease progression (HR: 1.11; 95% CI: 0.97–1.28; p = 0.3110) (Supplemental Table 5). These results indicate that the modulation of lymphocyte counts during ET + CDK4/6i treatment is a better predictor of PFS than baseline NLR in patients with HR+/HER2− aBC.
Association between early lymphocyte modulation and patient PFS in the first-line setting
Since the majority of HR+/HER2− aBC patients nowadays receive CDK4/6i in the first-line treatment setting, we performed a subgroup analysis, in which we evaluated the association between L_t0 and L_r1 and the PFS of patients treated with CDK4/6i in the first-line setting (n = 335; 52.5%). As shown in Supplemental Table 6, multivariable analysis revealed an independent and statistically significant association between higher L_r1 and a lower risk of disease progression; on the other hand, L_t0 was not associated with patient PFS. In this model, a higher DFI was associated with better PFS, while a higher number of metastatic sites, as well as higher ECOG PS, were associated with significantly worse PFS. We also adjusted the prognostic role of L_r1 for NLR_t0 in this patient subset (Supplemental Table 7). Of note, L_r1 was independently associated with patient PFS (HR: 0.83; 95% CI: 0.71–0.99; p = 0.0325), while the NLR_t0 was not [HR: 1.08; 95% CI: 0.86–1.35 (p = 0.5085)]. Together, these data confirm and reinforce data previously observed in the whole patient cohort, that is, that the precocious modulation of peripheral blood lymphocytes has prognostic significance in HR+/HER2− aBC patients treated with first-line CDK4/6i-based therapy.
Discussion
We showed that baseline peripheral blood lymphocyte counts and their early modulation are independently associated with the PFS and OS of HR+/HER2− aBC patients treated with ET plus CDK4/6i as any line of treatment for advanced disease. We also found that precocious modulation of lymphocytes is associated with PFS of HR+/HER2− aBC patients treated with CDK4/6i therapy in the first-line setting.
CDK4/6i-based therapy resulted in a significant reduction of peripheral blood neutrophil, platelet and lymphocyte counts (see Figure 1), thus confirming data previously reported in phase II and phase III clinical trials.1–3,5,6,8 In particular, the reduction of total white blood cell and neutrophil counts is a well acknowledged adverse event (AE) of palbociclib, ribociclib and, less commonly, of abemaciclib-based therapy, and it is frequently associated with drug dose reduction.1–3,5,6,8,15 Regarding other hematological toxicities, any-grade thrombocytopenia was reported in around 9.3–53.2% of patients treated with CDK4/6i in phase III trials, with only around 1% of patients undergoing grade 3 or grade 4 thrombocytopenia.1,2,7–10 Moreover, grade 3/4 lymphopenia was reported in approximately 30% of patients enrolled in the phase II PALOMA-1 trial, 40 but not in the original publications of the phase III trials PALOMA 2 and PALOMA 3.1,2 In a long-term, pooled safety analysis of PALOMA 1, 2 and 3 trials, any-grade 5-year lymphopenia occurred in 2.8% of patients, and 0.9% of patients experienced grade 3/4 lymphopenia. 41 In the MONALEESA-2 trial, the incidence of grade 3 and grade 4 lymphopenia was 7% and 0.9%, respectively. 3 As for abemaciclib, any grade lymphopenia occurred in 52.7–62.9% of patients enrolled in MONARCH-2 and MONARCH-3 trials, with grade 3/4 lymphopenia being reported in 7.3–12% and 0.2–1.3% of patients, respectively.7,8 In the MONARCH-2 trial, 1.4% of abemaciclib-related dose reductions were attributed to the occurrence of lymphopenia. 42 Finally, the impact of CDK4/6i-based therapy on peripheral blood monocyte counts has not been reported in the PALOMA, MONALEESA and MONARCH trials.1–3,5,6,8 Therefore, our analysis is the first study to show that CDK4/6i cause a significant reduction of peripheral blood monocyte counts.
The main aim of our study was to investigate the association between CDK4/6i-induced modulation of peripheral blood immune cell subsets and the PFS of patients with HR+/HER2− aBC. By combining Random Forest and multivariable Cox regression models, we found that baseline and on-treatment lymphocyte counts are consistently associated with clinical outcomes. In particular, patients undergoing a lower decrease, or even an increase of lymphocyte counts during CDK4/6i therapy, had significantly better PFS and OS when compared to patients experiencing a higher decrease of peripheral blood lymphocytes. Other immune parameters explored in our analyses did not show an independent association with patient PFS, with the only exception of high NLR, which was associated with worse PFS when evaluated as NLR_t0 and as NLR_r1. However, the prognostic role of NLR likely reflects the prognostic role of lymphocytes; indeed, when the association between L_r1 and NLR_t0 with PFS was independently evaluated in the same multivariable Cox model, only the early modulation of lymphocytes maintained a significant association with patient PFS.
Since we found that baseline lymphocyte counts and their early on-treatment modulation are stronger predictors of patient PFS in multivariable models, also when adjusted for NLR, results of our study are in part in contrast with a large body of accumulating evidence in other clinical contexts, where composite immune cell parameters, namely NLR, PLR, MLR and PIV, were shown to be better predictors of clinical outcomes than crude immune cell counts in patients with both limited-stage and advanced malignancies, including breast cancer.30–36,43 These results may reflect an especially important role of blood lymphocytes, both at baseline and during CDK4/6i therapy, in affecting the clinical outcomes of HR+/HER2− aBC patients treated with ET + CDK4/6i as any line of therapy.
Consistent with our findings, recent preclinical evidence revealed that CDK4/6i activate specific subsets of cytotoxic and memory T cells that could contribute to the antitumor effects of this class of drugs.18,44,45 For instance, Deng et al. showed that CDK6 but not CDK4 inhibition results in an increased expression of Nuclear Factor of Activated T cells (NFAT) family proteins, which modulate the activation of tumor infiltrating lymphocytes. 18 In addition, intratumor levels of the Th1 chemokines CXCL9 and CXCL10, which modulate the intratumor trafficking of effector T cells, were increased after CDK4/6i-based therapy, while CD11+ myeloid-derived suppressor cells and their products, namely IL-6, IL-10 and IL-23, were reduced. Notably, Tregs were more susceptible to CDK 4/6 inhibition than other T cells, probably as a result of higher expression of CDK6. 18 In the work by Schaer et al. 44 abemaciclib monotherapy was associated with increased intra-tumor immune inflammation in tumor-bearing mice, and the combination of abemaciclib with anti-PD-L1 therapy led to tumor regressions and enhanced T cell immune response and antigen presentation. 44 In another work, CDK4/6 promoted cancer cell senescence through the activation of a senescence-associated secretory phenotype, finally resulting in enhanced tumor immune-cell infiltration. 45 Finally, in a recent biomarker analysis conducted in a subset of patients enrolled in the PALOMA-2 trial, lower expression of the immune checkpoint PD-1 in basal tumor specimens was associated with a relatively higher benefit from palbociclib, thus suggesting that palbociclib might stimulate intratumor immunity in HR+/HER2− aBCs with lower basal lymphocyte infiltration and/or with less efficient antitumor immunity (i.e. those with low PD-1 expression). 46
Based on the immunosuppressive effects of neutrophils and monocytes in cancer patients, 47 we expected that CDK4/6i-induced reduction of peripheral blood neutrophils and monocytes could be associated with better patient PFS. Our data show that this hypothesis was not correct. Indeed, while the precocious reduction of neutrophil counts during CDK4/6i therapy showed a trend toward an association with better patient PFS, CDK4/6i-induced reduction of monocyte and platelet counts was even associated with a trend toward worse PFS (Supplemental Figure 5–7). On the other hand, the finding that the modulation of blood lymphocytes was the only immunological variable to be consistently and independently associated with patient PFS indicates that lymphocytes might play an especially important role in affecting the long-term clinical outcomes in HR+/HER2− aBC patients treated with CDK4/6i-based therapy.
Consistent with recently published works,48,49 we found that CDK4/6i dose reduction was not associated with worse patient PFS (Supplemental Figure 4). However, in our study we made no distinction between early (first 3 months of treatment) and late dose reductions, whereas in the studies published by Kristensen et al. 48 and Roncato et al., 49 a precocious reduction of CDK4/6i dosage was associated with worse clinical outcomes. Therefore, we cannot exclude that we may have failed to capture an effect of dose reduction on patient PFS because of this specific limitation of our study. However, we would like to highlight the fact that lymphopenia is very rarely a cause of CDK4/6i dose reduction. Therefore, the main study results regarding the prognostic significance of early reduction of blood lymphocytes are unlikely to be affected by precocious reduction of CDK4/6i dosage.
Along with preclinical data supporting a role of cytotoxic lymphocytes in mediating the antitumor effects of CDK4/6i, 18 results of our study pave the way for evaluating CDK4/6i in combination with immune checkpoint inhibitors (ICIs) in patients with limited-stage or advanced HR+/HER2− BC. Scirocchi et al. 50 recently found that CDK4/6i + ET treatment downregulates immune suppressive subpopulations, such as Tregs, M-MDSCs, and PMN-MDSCs, in HR+/HER2− aBC patients. Strikingly, patients responding to the treatment showed a higher decrease in Tregs when compared to non-responding patients, and CDK4/6i treatment resulted in an increase in CD4+ T cells and anti-tumor CD137+ CD8+ T cells. 50
In this respect, only few clinical data from prospective trials are available. In the multicenter, non-randomized, open-label, multi-cohort, phase Ib trial NCT02779751, which enrolled 28 endocrine-resistant HR+/HER2− aBC patients treated with abemaciclib plus pembrolizumab after receiving 1 or 2 prior lines of chemotherapy for advanced disease, tumor overall response rate was 29%, and disease control rate was 82%; moreover, median PFS and OS were 8.9 months and 26.3 months, respectively. 51 Another phase I/II trial, namely NCT02778685, enrolled previously untreated HR+/HER2− aBC patients, who received a combination of palbociclib, letrozole and pembrolizumab; of 16 patients, 31% achieved complete response, 25% had partial response (PR) and 31% reported stable disease (SD) as best responses according to RECIST 1.1 criteria. Median PFS was 25.2 months and median OS was 36.9 months. 52 In the recently presented phase II study PACE (NCT03147287), which randomized HR+/HER2− aBC patients progressing to ET plus CDK4/6i to receive fulvestrant alone, fulvestrant plus palbociclib, or fulvestrant plus palbociclib plus avelumab, patients receiving the triple treatment experienced better median PFS (8.1 months) when compared to patients treated with fulvestrant alone (4.8 months), or with fulvestrant plus palbociclib (4.6 months). 53
In the early-stage setting, the ImmunoADAPT (NCT03573648) trial is randomizing stage II–III HR+/HER2− BC to receive ET plus avelumab with or without palbociclib. However, the CheckMate 7A8 phase II study, which evaluated the combination of nivolumab, palbociclib and anastrozole in patients with early HR+/HER2− BC, was closed after the safety run-in due to the increased hepatic toxicities of the experimental triplet treatment. 54 These contradictory findings impose to deeply investigate the safety of combining ET plus CDK4/6i and ICIs in specific clinical contexts.
The following are major strengths of our study: (i) this was the first, relatively large multicenter study to show that baseline lymphocytes and their early modulation during CDK4/6i therapy are associated with PFS and OS in HR+/HER2− aBC patients; (ii) the multicenter nature of the study and the large sample size make our data robust, with PFS data observed in the whole study cohort being consistent with results of phase III trials investigating CDk4/6i and with data from real world cohorts1–12; (iii) the main study findings were confirmed by different multivariable models including clinical and biological variables; (iv) the independent prognostic role of CDK4/6i-induced early lymphocyte modulation was confirmed in the subset of patients treated in the first-line setting.
This study also has several limitations. Firstly, is its retrospective design, with some original data missing at the time of data analysis; however, Random Forest Models fitted with either original or imputed data confirmed the main study findings, thus partially obviating to this limitation (see Supplemental Table 1). In addition, the main Cox models were fitted after both single and multiple imputation to increase the robustness of our results (see Supplemental Table 3). Secondly, the majority of patients enrolled in our study received palbociclib, thus not allowing us to generalize our findings to ribociclib or abemaciclib. In this respect, the ongoing PALMARES-2 study, a large, real-world Italian study involving 23 centers, will clarify if the findings of the current study are also confirmed in larger cohorts of patients treated with ribociclib or abemaciclib. Thirdly, the study results were not validated in an independent study cohort. The ongoing PALMARES-2 study will be instrumental also in this respect. Finally, we did not perform a centralized evaluation of peripheral blood cells; however, blood cell count assessment relies on fully standardized methodologies across laboratories in Italian Cancer Centers. Therefore, we tend to exclude that the study findings may have been influenced by the lack of centralized evaluation of peripheral blood cells.
Conclusion
In conclusion, higher baseline blood lymphocyte counts, or a lower early reduction of lymphocyte counts during CDK4/6i therapy, are independently associated with better PFS and OS in HR+/HER2− aBC patients treated with CDK4/6i plus ET. Among patients receiving CDK4/6i in the first-line treatment setting for advanced disease – that is, the vast majority of HR+/HER2− aBC patients treated with CDK4/6i-based therapy nowadays – a lower early reduction of lymphocyte counts was the only immunological variable to be associated with significantly better PFS. Although results of our study need to be validated in prospective studies, early modifications of lymphocyte counts during CDK4/6i and ET treatment could become a novel and easily assessable prognostic biomarker in the ever-growing population of patients with HR+/HER2− aBC treated with CDK4/6i. Due to the relevance of lymphocytes in affecting the antitumor efficacy of CDK4/6i, experimental combination strategies aimed at boosting antitumor immunity in HR+/HER2− aBC patients, such as ICIs, should be explored to improve the clinical efficacy of CDK4/6i.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359231204857 for Peripheral blood lymphocytes predict clinical outcomes in hormone receptor-positive HER2-negative advanced breast cancer patients treated with CDK4/6 inhibitors by Emma Zattarin, Luigi Mariani, Alice Menichetti, Rita Leporati, Leonardo Provenzano, Francesca Ligorio, Giovanni Fucà, Riccardo Lobefaro, Luca Lalli, Andrea Vingiani, Federico Nichetti, Gaia Griguolo, Marianna Sirico, Ottavia Bernocchi, Antonio Marra, Chiara Corti, Paola Zagami, Elisa Agostinetto, Flavia Jacobs, Pierluigi Di Mauro, Daniele Presti, Caterina Sposetti, Carlo Alberto Giorgi, Valentina Guarneri, Rebecca Pedersini, Agnese Losurdo, Daniele Generali, Giuseppe Curigliano, Giancarlo Pruneri, Filippo de Braud, Maria Vittoria Dieci and Claudio Vernieri in Therapeutic Advances in Medical Oncology
Acknowledgments
We would like to thank AIRC, ‘Associazione Italiana per la Ricerca sul Cancro’ (MFAG 2019. Id. 22977. PI: Claudio Vernieri; AIRC IG 2021. PI: Giancarlo Pruneri; 5 per Mille 2019 – ID. 22759 program-G.L. Valentina Guarneri) and the Scientific Directorate of Fondazione IRCCS Istituto Nazionale dei Tumori for funding our research. We would also like to thank Salvatore Lo Vullo and Angela Ficchí for fruitful scientific discussions.
Footnotes
ORCID iDs: Emma Zattarin  https://orcid.org/0000-0002-3445-9245
https://orcid.org/0000-0002-3445-9245
Rita Leporati  https://orcid.org/0000-0001-6671-9809
https://orcid.org/0000-0001-6671-9809
Flavia Jacobs  https://orcid.org/0000-0001-9126-8325
https://orcid.org/0000-0001-9126-8325
Caterina Sposetti  https://orcid.org/0000-0003-3211-8516
https://orcid.org/0000-0003-3211-8516
Claudio Vernieri  https://orcid.org/0000-0003-1577-8176
https://orcid.org/0000-0003-1577-8176
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Emma Zattarin, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Luigi Mariani, Unit of Clinical Epidemiology and Trial Organization, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Alice Menichetti, Oncology 2, Istituto Oncologico Veneto IOV – IRCCS, Padova, Italy.
Rita Leporati, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Leonardo Provenzano, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Francesca Ligorio, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy; IFOM ETS, the AIRC Institute of Molecular Oncology, Milan, Italy.
Giovanni Fucà, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Riccardo Lobefaro, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Luca Lalli, Unit of Clinical Epidemiology and Trial Organization, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Andrea Vingiani, Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy; Pathology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Federico Nichetti, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy; Computational Oncology, Molecular Diagnostics Program, National Center for Tumor Diseases (NCT) and German Cancer Research Center (DKFZ), Heidelberg, Germany.
Gaia Griguolo, Oncology 2, Istituto Oncologico Veneto IOV – IRCCS, Padova, Italy; Department of Surgery, Oncology and Gastroenterology-DiSCOG, University of Padova, Padova, Italy.
Marianna Sirico, Department of Medical Oncology, IRCCS Istituto Romagnolo per lo Studio dei Tumori (IRST) “Dino Amadori”, Meldola, Italy.
Ottavia Bernocchi, Farmacia Ospedaliera ASST Cremona, Cremona, Lombardia, Italy.
Antonio Marra, Division of Early Drug Development for Innovative Therapies, IEO, European Institute of Oncology IRCCS, Milan, Italy; Breast Medicine Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Chiara Corti, Division of Early Drug Development for Innovative Therapies, IEO, European Institute of Oncology IRCCS, Milan, Italy.
Paola Zagami, Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy; Division of Early Drug Development for Innovative Therapies, IEO, European Institute of Oncology IRCCS, Milan, Italy.
Elisa Agostinetto, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy; Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy; Institut Jules Bordet and l’Université Libre de Bruxelles, Bruxelles, Belgium.
Flavia Jacobs, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy; Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy.
Pierluigi Di Mauro, Medical Oncology Unit, ASST Spedali Civili, Brescia, Italy.
Daniele Presti, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Caterina Sposetti, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Carlo Alberto Giorgi, Oncology 2, Istituto Oncologico Veneto IOV – IRCCS, Padova, Italy.
Valentina Guarneri, Oncology 2, Istituto Oncologico Veneto IOV – IRCCS, Padova, Italy; Department of Surgery, Oncology and Gastroenterology-DiSCOG, University of Padova, Padova, Italy.
Rebecca Pedersini, Medical Oncology Unit, ASST Spedali Civili, Brescia, Italy.
Agnese Losurdo, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy; Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy.
Daniele Generali, Breast Cancer Unit & Translational Research Unit, ASST Cremona, Cremona, Italy; Department of Medical, Surgery and Health Sciences, University of Trieste, Trieste, Italy.
Giuseppe Curigliano, Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy; Division of Early Drug Development for Innovative Therapies, IEO, European Institute of Oncology IRCCS, Milan, Italy.
Giancarlo Pruneri, Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy; Pathology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Filippo de Braud, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy; Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy.
Maria Vittoria Dieci, Oncology 2, Istituto Oncologico Veneto IOV – IRCCS, Padova, Italy; Department of Surgery, Oncology and Gastroenterology-DiSCOG, University of Padova, Padova, Italy.
Claudio Vernieri, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Via Venezian 1, Milan 20133, Italy IFOM ETS, the AIRC Institute of Molecular Oncology, Milan, Italy.
Declarations
Ethics approval and consent to participate: The study protocol was approved by the Ethics Committee of the coordinating center (internal registration number: INT 138/20), and, subsequently, by Ethics Committees of each participating site. Enrolled patients signed an informed consent for inclusion in the study and for the use of clinical data for research purposes.
Consent for publication: Not applicable.
Author contributions: Emma Zattarin: Data curation; Formal analysis; Writing – original draft.
Luigi Mariani: Formal analysis.
Alice Menichetti: Data curation; Writing – review & editing.
Rita Leporati: Data curation; Writing – review & editing.
Leonardo Provenzano: Writing – review & editing.
Francesca Ligorio: Writing – review & editing.
Giovanni Fucà: Writing – review & editing.
Riccardo Lobefaro: Writing – review & editing.
Luca Lalli: Writing – review & editing.
Andrea Vingiani: Writing – review & editing.
Federico Nichetti: Writing – review & editing.
Gaia Griguolo: Data curation; Writing – review & editing.
Marianna Sirico: Data curation; Writing – review & editing.
Ottavia Bernocchi: Data curation; Writing – review & editing.
Antonio Marra: Data curation; Writing – review & editing.
Chiara Corti: Data curation; Writing – review & editing.
Paola Zagami: Data curation; Writing – review & editing.
Elisa Agostinetto: Data curation; Writing – review & editing.
Flavia Jacobs: Data curation; Writing – review & editing.
Pierluigi Di Mauro: Data curation; Writing – review & editing.
Daniele Presti: Writing – review & editing.
Caterina Sposetti: Writing – review & editing.
Carlo Alberto Giorgi: Writing – review & editing.
Valentina Guarneri: Data curation; Writing – review & editing.
Rebecca Pedersini: Data curation; Writing – review & editing.
Agnese Losurdo: Data curation; Writing – review & editing.
Daniele Generali: Data curation; Writing – review & editing.
Giuseppe Curigliano: Data curation; Writing – review & editing.
Giancarlo Pruneri: Writing – review & editing.
Filippo de Braud: Supervision; Writing – review & editing.
Maria Vittoria Dieci: Supervision; Writing – review & editing.
Claudio Vernieri: Conceptualization; Investigation; Supervision; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: AIRC, ‘Associazione Italiana per la Ricerca sul Cancro’ (MFAG 2019. Id. 22977. PI: Claudio Vernieri; IG 2021. PI: Giancarlo Pruneri; 5 per Mille 2019 – ID. 22759 program-G.L. Valentina Guarneri) and the Scientific Directorate of Fondazione IRCCS Istituto Nazionale dei Tumori.
Competing interests: Dr. GG reports personal fees from Novartis, Eli Lilly. Dr. EA reports consultancy fees or honoraria from Eli Lilly, Sandoz; support to attend medical conferences from Roche, Novartis, Eli Lilly, Genetic, Istituto Gentili. Prof. VG reports personal fees from Eli Lilly, Novartis, Roche, and MSD outside the submitted work. Prof. DG reports consultancy fees or honoraria from Eli Lilly, Novartis, Pfizer, Roche, Istituto Gentili, Eisai. Prof. GC reports funding from Astra Zeneca, Daichii Sankyo, Merck; consulting fees from BMS, Roche, Pfizer, Novartis, Lilly, Astra Zeneca, Daichii Sankyo, Merck, Seagen, Ellipsis; honoraria from Pfizer, Lilly; support for attending meetings from Roche, Pfizer. Prof. GP reports personal fees from Roche Foundation One, Bayer, Novartis, and personal fees from Lilly outside the submitted work. Prof. FdeB reports an advisory role at Roche, EMD Serono, NMS Nerviano Medical Science, Sanofi, MSD, Novartis, Incyte, BMS, Menarini; speaker role for BMS, Healthcare Research & Pharmacoepidemiology, Merck Group, ACCMED, Nadirex, MSD, Pfizer, Servier, Sanofi, Roche, AMGEN, Incyte, Dephaforum; Principal Investigator for Novartis, F.Hoffmann-LaRoche Ltd, BMS, Ignyta Operating INC, Merck Sharp & Dohme Spa, Kymab, Pfizer, Tesaro, MSD, MedImmune LCC, Exelixis Inc., LOXO Oncology Incorporated, DAICHI SANKIO Dev. Limited, Basilea Pharmaceutica International AG, Janssen-Cilag International NV, Merck KGAA. Prof. MVD reports personal fees from Eli Lilly, MSD, Exact Sciences, Novartis, Pfizer, Seagen, Dr. CV reports an advisory role for Novartis, Eli Lilly, Pfizer and Daiichi Sankyo; honoraria as a speaker: Eli Lilly, Novartis, Istituto Gentili, Roche, Pfizer; research grants: Roche. All other authors report no competing interests.
Availability of data and materials: The datasets and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. New Engl J Med 2016; 375: 1925–1936. [DOI] [PubMed] [Google Scholar]
- 2. Turner NC, Slamon DJ, Ro J, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. New Engl J Med 2018; 379: 1926–1936. [DOI] [PubMed] [Google Scholar]
- 3. Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib for HR-Positive, Advanced Breast Cancer. N Engl J Med 2017; 376: 289. [DOI] [PubMed] [Google Scholar]
- 4. Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol 2018; 29: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 5. Im SA, Lu YS, Bardia A, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. New Engl J Med 2019; 381: 307–316. [DOI] [PubMed] [Google Scholar]
- 6. Slamon DJ, Neven P, Chia S, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. New Engl J Med 2020; 382: 514–524. [DOI] [PubMed] [Google Scholar]
- 7. Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017; 35: 3638–3646. [DOI] [PubMed] [Google Scholar]
- 8. Sledge Gw, Jr, Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol 2020; 6: 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Slamon DJ, Neven P, Chia S, et al. Corrigendum to ‘Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall survival’. Ann Oncol 2021; 32: 1015–1024. [DOI] [PubMed] [Google Scholar]
- 10. Hortobagyi GN, Stemmer SM, Burris HA, et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. New Engl J Med 2022; 386: 942–950. [DOI] [PubMed] [Google Scholar]
- 11. Lu YS, Im SA, Colleoni M, et al. Updated overall survival of ribociclib plus endocrine therapy versus endocrine therapy alone in pre- and perimenopausal patients with HR+/HER2- advanced breast cancer in MONALEESA-7: a phase III randomized clinical trial. Clin Cancer Res 2022; 28: 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cristofanilli M, Rugo HS, Im SA, et al. Overall survival (OS) with palbociclib (PAL) + fulvestrant (FUL) in women with hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2–) advanced breast cancer (ABC): updated analyses from PALOMA-3. J Clin Oncol 2021; 39: 1000–1000. [Google Scholar]
- 13. Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol 2006; 24: 1770–1783. [DOI] [PubMed] [Google Scholar]
- 14. Goel S, DeCristo MJ, Watt AC, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 2017; 548: 471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scheicher R, Hoelbl-Kovacic A, Bellutti F, et al. CDK6 as a key regulator of hematopoietic and leukemic stem cell activation. Blood 2015; 125: 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen P, Lee NV, Hu W, et al. Spectrum and degree of CDK drug interactions predicts clinical performance. Mol Cancer Ther 2016; 15: 2273–2281. [DOI] [PubMed] [Google Scholar]
- 17. Hu W, Sung T, Jessen BA, et al. Mechanistic investigation of bone marrow suppression associated with palbociclib and its differentiation from cytotoxic chemotherapies. Clin Cancer Res 2016; 22: 2000–2008. [DOI] [PubMed] [Google Scholar]
- 18. Deng J, Wang ES, Jenkins RW, et al. CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Discov 2018; 8: 216–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gourgou-Bourgade S, Cameron D, Poortmans P, et al. Guidelines for time-to-event end point definitions in breast cancer trials: results of the DATECAN initiative (definition for the assessment of time-to-event endpoints in CANcer trials). Ann Oncol 2015; 26: 2505–2506. [DOI] [PubMed] [Google Scholar]
- 20. Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996; 17: 343–346. [DOI] [PubMed] [Google Scholar]
- 21. Lausen B, Schumacher M. Maximally selected rank statistics. Biometrics 1992; 48: 73–85. [Google Scholar]
- 22. Ishwaran H, Kogalur UB. Consistency of random survival forests. Stat Probab Lett 2010; 80: 1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Therneau TM, Crowson CS, Atkinson EJ. Adjusted survival curves. 2015. https://cran.r-project.org/web/packages/survival/vignettes/adjcurve.pdf2015
- 24. Porte B, Carton M, Lerebours F, et al. Real life efficacy of palbociclib and endocrine therapy in HR positive, HER2 negative advanced breast cancer. Breast 2020; 54: 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Agrawal C, Goyal P, Agarwal A, et al. Multicentric real world evidence with palbociclib in hormone positive HER2 negative metastatic breast cancer in Indian population. Sci Rep 2021; 11: 16236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Singer CF, Egle D, Greil R, et al. 195P REACH AUT: Efficacy and safety of first-line (1L) ribociclib (RIB) + endocrine therapy (ET) in HR+, HER2- metastatic breast cancer (MBC) from a real-world (RW) study: 3rd interim analysis. Ann Oncol 2022; 33: S217. [Google Scholar]
- 27. Cuyun Carter G, Sheffield KM, Gossai A, et al. Real-world treatment patterns and outcomes of abemaciclib for the treatment of HR+, HER2- metastatic breast cancer. Curr Med Res Opin 2021; 37: 1179–1187. [DOI] [PubMed] [Google Scholar]
- 28. Swierczak A, Mouchemore KA, Hamilton JA, et al. Neutrophils: important contributors to tumor progression and metastasis. Cancer Metastasis Rev 2015; 34: 735–751. [DOI] [PubMed] [Google Scholar]
- 29. Giannakeas V, Kotsopoulos J, Brooks JD, et al. Platelet count and survival after cancer. Cancers 2022; 14: 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dell’Aquila E, Cremolini C, Zeppola T, et al. Prognostic and predictive role of neutrophil/lymphocytes ratio in metastatic colorectal cancer: a retrospective analysis of the TRIBE study by GONO. Ann Oncol 2018; 29: 924–930. [DOI] [PubMed] [Google Scholar]
- 31. Ethier JL, Desautels D, Templeton A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res 2017; 19: 2–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014; 106: dju124. [DOI] [PubMed] [Google Scholar]
- 33. Fucà G, Guarini V, Antoniotti C, et al. The pan-immune-inflammation value is a new prognostic biomarker in metastatic colorectal cancer: results from a pooled-analysis of the valentino and TRIBE first-line trials. Br J Cancer 2020; 123: 403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ligorio F, Fucà G, Zattarin E, et al. The pan-immune-inflammation-value predicts the survival of patients with human epidermal growth factor receptor 2 (HER2)-positive advanced breast cancer treated with first-line taxane-trastuzumab-pertuzumab. Cancers (Basel) 2021; 13: 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ding N, Pang Z, Shen H, et al. The prognostic value of PLR in lung cancer, a meta-analysis based on results from a large consecutive cohort. Sci Rep 2016; 6: 34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tiainen S, Rilla K, Hämäläinen K, et al. The prognostic and predictive role of the neutrophil-to-lymphocyte ratio and the monocyte-to-lymphocyte ratio in early breast cancer, especially in the HER2+ subtype. Breast Cancer Res Treat 2021; 185: 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gu XB, Tian T, Tian XJ, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in non-small cell lung cancer: a meta-analysis. Sci Rep 2015; 5: 12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ferrucci PF, Ascierto PA, Pigozzo J, et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol 2016; 27: 732–738. [DOI] [PubMed] [Google Scholar]
- 39. Corbeau I, Jacot W, Guiu S. Neutrophil to lymphocyte ratio as prognostic and predictive factor in breast cancer patients: a systematic review. Cancers 2020; 12: 958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015; 16: 25–35. [DOI] [PubMed] [Google Scholar]
- 41. Finn RS, Rugo HS, Gelmon KA, et al. Long-term pooled safety analysis of palbociclib in combination with endocrine therapy for hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: updated analysis with up to 5 years of follow-up. Oncologist 2021; 26: e749–e755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Neven P, Rugo HS, Tolaney SM, et al. Abemaciclib plus fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in premenopausal women: subgroup analysis from the MONARCH 2 trial. Breast Cancer Res 2021; 23: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fucà G, Beninato T, Bini M, et al. The pan-immune-inflammation value in patients with metastatic melanoma receiving first-line therapy. Target Oncol 2021; 16: 529–536. [DOI] [PubMed] [Google Scholar]
- 44. Schaer DA, Beckmann RP, Dempsey JA, et al. The CDK4/6 inhibitor abemaciclib induces a T cell inflamed tumor microenvironment and enhances the efficacy of PD-L1 checkpoint blockade. Cell Rep 2018; 22: 2978–2994. [DOI] [PubMed] [Google Scholar]
- 45. Coppé JP, Desprez PY, Krtolica A, et al. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 2010; 5: 99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Finn RS, Liu Y, Zhu Z, et al. Biomarker analyses of response to cyclin-dependent kinase 4/6 inhibition and endocrine therapy in women with treatment-naïve metastatic breast cancer. Clin Cancer Res 2020; 26: 110–121. [DOI] [PubMed] [Google Scholar]
- 47. DeNardo DG, Brennan DJ, Rexhepaj E, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov 2011; 1: 54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kristensen KB, Thomsen IMN, Berg T, et al. Dose modifications of ribociclib and endocrine therapy for treatment of ER+ HER2- metastatic breast cancer. Breast Cancer Res Treat 2021; 188: 799–809. [DOI] [PubMed] [Google Scholar]
- 49. Roncato R, Peruzzi E, Gerratana L, et al. Clinical impact of body mass index on palbociclib treatment outcomes and effect on exposure. Biomed Pharmacother 2023; 164: 114906. [DOI] [PubMed] [Google Scholar]
- 50. Scirocchi F, Scagnoli S, Botticelli A, et al. Immune effects of CDK4/6 inhibitors in patients with HR+//HER2- metastatic breast cancer: relief from immunosuppression is associated with clinical response. EBioMedicine 2022; 79: 104010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rugo HS, Kabos P, Beck JT, et al. A phase Ib study of abemaciclib in combination with pembrolizumab for patients with hormone receptor positive (HR+), human epidermal growth factor receptor 2 negative (HER2-) locally advanced or metastatic breast cancer (MBC) (NCT02779751): Interim results. J Clin Oncol 2020; 38: 1051–1051. [Google Scholar]
- 52. Yuan Y, Lee JS, Yost SE, et al. Phase I/II trial of palbociclib, pembrolizumab and letrozole in patients with hormone receptor-positive metastatic breast cancer. Eur J Cancer 2021; 154: 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mayer EL, Ren Y, Wagle N, et al. Palbociclib after CDK4/6i and endocrine therapy (PACE): a randomized phase II study of fulvestrant, palbociclib, and avelumab for endocrine pre-treated ER+/HER2- metastatic breast cancer. Paper presented at 2022 San Antonio Breast Cancer Symposium, 6–10 December 2022, San Antonio, TX. Abstract GS3-06. [Google Scholar]
- 54. Tolaney S, Jerusalem G, Prat A. 92MO neoadjuvant nivolumab (NIVO) + palbociclib (PALBO) + anastrozole (ANA) for estrogen receptor-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2−) primary breast cancer (BC): CheckMate 7A8. Ann Oncol 2022; 33: S165–S166. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359231204857 for Peripheral blood lymphocytes predict clinical outcomes in hormone receptor-positive HER2-negative advanced breast cancer patients treated with CDK4/6 inhibitors by Emma Zattarin, Luigi Mariani, Alice Menichetti, Rita Leporati, Leonardo Provenzano, Francesca Ligorio, Giovanni Fucà, Riccardo Lobefaro, Luca Lalli, Andrea Vingiani, Federico Nichetti, Gaia Griguolo, Marianna Sirico, Ottavia Bernocchi, Antonio Marra, Chiara Corti, Paola Zagami, Elisa Agostinetto, Flavia Jacobs, Pierluigi Di Mauro, Daniele Presti, Caterina Sposetti, Carlo Alberto Giorgi, Valentina Guarneri, Rebecca Pedersini, Agnese Losurdo, Daniele Generali, Giuseppe Curigliano, Giancarlo Pruneri, Filippo de Braud, Maria Vittoria Dieci and Claudio Vernieri in Therapeutic Advances in Medical Oncology