Abstract
Introduction
This study sought to compare medication efficacy in participants with medical comorbidities who smoke in the Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES) trial, a double-blind, triple-dummy, placebo- and active-controlled randomized controlled trial.
Aims and Methods
Participants were from the U.S. cohort of the main trial and randomized (1:1:1:1) to varenicline, bupropion, nicotine replacement therapy (NRT) patch, or placebo for 12 weeks with follow-up through week 24. Medical comorbidity data were derived from the baseline medical screening questionnaire and categorized into four subgroups (cardiac, respiratory, vascular, and diabetes). Within each comorbidity, generalized linear mixed models were used to assess the association between treatment and continuous abstinence rates from weeks 9–12 to 9–24. Similar models were used to test the effect of number of comorbidities on abstinence.
Results
Varenicline resulted in the highest week 12 abstinence rates across all pharmacotherapies and compared to placebo in all comorbidity subgroups: Cardiac (40.0% vs. 3.6%; odds ratios [OR] = 23.3 [5.1–107.1]), respiratory (24.7% vs. 12.8%; OR = 2.2 [1.3–3.8]), vascular (29.1% vs. 10.4%; OR = 3.6 [2.3–5.7]), and diabetes (30.9% vs. 8.3%; OR = 6.5 [2.3–19.0]). This was maintained at week 24 for those with cardiac (23.3% vs. 1.8%; OR = 21.7 [2.7–178.2]), vascular (18.9% vs. 7.1%; OR = 3.1 [1.8–5.3]), and diabetes (20.6% vs. 4.2%; OR = 8.4 [2.1–33.7]) comorbidities. Treatment contrasts within some comorbidity subgroups revealed superior efficacy of varenicline over other pharmacotherapies. All pharmacotherapies increased the odds of abstinence regardless of number of comorbidities.
Conclusions
Varenicline is the most efficacious option for patients with manageable cardiac, respiratory, vascular, and diabetes conditions to quit smoking, supporting recent clinical practice guidelines that recommend varenicline as first-line pharmacotherapy. Bupropion and NRT demonstrated efficacy for some comorbidity subgroups.
Implications
This secondary analysis of the EAGLES trial demonstrated that varenicline is the most efficacious option for patients with cardiac, respiratory, vascular, and diabetes diagnoses to quit smoking. This demonstration of varenicline efficacy among individuals with comorbid medical conditions supports recent clinical practice guidelines that recommend varenicline as a first-line pharmacotherapy for smoking cessation.
Introduction
Cigarette smoking remains the leading cause of morbidity and mortality with more than 16 million people in the U.S. living with a smoking-related disease.1 Smoking causes cancer, cardiovascular diseases, respiratory diseases, and diabetes.1 Furthermore, conditions that are not necessarily caused by smoking (eg, psychiatric comorbidities, HIV) are still highly correlated with smoking and the associated health impacts for these populations are compounded.2 Medical and psychiatric comorbidities present a complex issue for the treatment of smoking as well as treatment of the comorbidities. Many individuals with comorbidities may be interested in quitting smoking, but the comorbid disease may impact motivation to quit, treatment response, and ability to quit smoking successfully.2
Clinical practice guidelines for treating tobacco use indicate that combination of behavioral and pharmacological approaches are most effective and can be offered to any person who smokes, including those with medical comorbidities (assuming no contraindications for pharmacological agents).3–6 First-line pharmacotherapy options include varenicline and nicotine replacement therapy (NRT), which are routinely used in clinical practice to treat people who smoke with and without comorbidities. However, choice of pharmacotherapy may need to be tailored because of contraindications, comorbidities, and for specific populations.2,7 Available smoking cessation pharmacotherapies may have differential effects across comorbid populations, and research has yet to elucidate these effects. Exploring the efficacy of pharmacotherapies for medically complex patients has important implications for tobacco treatment clinical strategies and broader population generalizability.
The Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES) trial was a large, multi-site, placebo-controlled, randomized controlled trial examining the safety and efficacy of varenicline, bupropion, and NRT, for smoking cessation among individuals with (N = 4116) and without (N = 4028) psychiatric comorbidities.8 This trial found that varenicline was more effective than placebo, NRT, and bupropion for smoking abstinence, whereas bupropion and nicotine patches were more effective than placebo. Importantly, this study demonstrated no significant increase in neuropsychiatric adverse events among those taking varenicline or bupropion relative to nicotine patches or placebo.8 A secondary analysis of the EAGLES trial also demonstrated the cardiovascular safety of varenicline.9 The EAGLES trial presents a unique opportunity to evaluate efficacy of these tobacco treatment pharmacotherapies among those who have medical and psychiatric comorbidities. The present study is a secondary analysis of the trial comparing (1) the efficacy of varenicline, bupropion, and NRT for people who smoke who are diagnosed with medical comorbidities and (2) the efficacy of varenicline, bupropion, and NRT for people who smoke with increasing numbers of medical comorbidities irrespective of type.
Methods
Design
This study is a secondary analysis of the EAGLES trial (ClinicalTrials.gov NCT01456936), a double-blind, triple-dummy, placebo-controlled, and active-controlled (NRT; 21 mg per day with taper), randomized (1:1:1:1) clinical trial of varenicline (1 mg twice daily) and bupropion (150 mg twice daily).8 Enrolled participants were asked to set a quit date for 1 week following randomization into treatment arm and initiation of medication use. Participants were also given brief (≤10 minutes) smoking cessation counseling at the onset of treatment and at each clinic visit. Study medications were administered for 12 weeks with a nontreatment follow-up through week 24. The protocol and consent documents were approved by the institutional review boards or ethics committees at each site. This secondary analysis was performed from October 2021 to June 2022. The data from U.S. sites were available from the primary trial sponsor (Pfizer) for the present analysis.
Participants
U.S. participants (N = 4207) in the primary EAGLES trial were individuals who smoked an average of ten or more cigarettes per day during the previous year (confirmed by exhaled breath carbon monoxide >10 ppm at screening), aged 18–75 years, with and without prespecified psychiatric diagnoses, and who were motivated to stop smoking by accepting a set quit date as part of their participation in the trial. The primary trial excluded individuals who had severe medical comorbidities. A complete description of the inclusion and exclusion criteria can be found in the appendix of the primary publication.8 Of relevance to the present study, participants were excluded if they had: (1) severe chronic obstructive pulmonary disease, (2) clinically significant cardiovascular disease or cerebrovascular disease in the past 2 months, and (3) other severe acute or chronic medical or psychiatric condition or laboratory abnormality that may increase the risk associated with study participation or interfere with the interpretation of study results. Despite excluding individuals with severe respiratory and cardiovascular disease, participants were still enrolled who had manageable medical conditions.
Outcomes
The primary outcomes were continuous cigarette abstinence rate for weeks 9 to 12 and 9 to 24. Abstinence was defined as self-reported cigarette abstinence throughout the specified period, biochemically confirmed by exhaled carbon monoxide less than 10 ppm. Missing self-reported data was classified as smoking.
Variables of Interest
Medical comorbidity variables were derived from the data on the medical screening questionnaire at baseline. Any present medical conditions endorsed by participants were recorded using their respective MedDRA Lowest Level Term code.10 Using the Surgeon General’s Report1 as a guide for identifying conditions caused by smoking, six representative comorbidity classes were selected using the system organ class or high-level term MedDRA codes: Neoplasms; metabolism and nutrition disorders (diabetes mellitus type II only); respiratory, thoracic, and mediastinal disorders (eg, asthma); cardiac disorders (eg, atrial fibrillation, congestive heart failure); vascular disorders (eg, hypertension); and immune system disorders (rheumatoid arthritis and HIV only).
Psychiatric comorbidity was defined in the primary trial per the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR),11 and included endorsement of any one of the following: History of suicidal ideation or behavior, history of a psychiatric diagnosis (defined as the primary diagnosis: None, mood disorder, anxiety disorder, psychotic disorder, or borderline personality disorder), or alcohol or substance abuse disorder via structured clinical interview. Because the parent study specifically aimed to evaluate differences in psychiatric comorbidity, this variable was included as a covariate in the present analysis.
Additional variables included biological sex, age, race, cigarettes per day in the month prior to enrollment, cigarette dependence (Fagerström Test for Cigarette Dependence),12 age of starting smoking, length of time as smoker, lifetime serious quit attempts (yes or no and number), and discontinuation from the study drug. A new variable, number of comorbidities, was calculated from the medical data as described above. Each medical comorbidity listed in the baseline medical assessment was totaled for each participant and categorized (0, 1, 2, 3, 4, or ≥5).
Statistical Analysis
Generalized linear mixed-effects models were utilized to determine the efficacy of varenicline, bupropion, and NRT on abstinence within each medical comorbidity group. We assessed linear contrast to estimate odds ratios (OR) and 95% confidence intervals (CI) for specific treatment contrasts; specifically, varenicline versus NRT, varenicline versus bupropion, NRT versus bupropion, and each versus placebo on abstinence outcomes (weeks 9 to 12 [end of treatment] and weeks 9 to 24 [follow-up]). Models included terms for treatment group, cohort (non-psychiatric cohort and psychiatric cohort), sex (male and female), age (continuous values from 18 to 75), and race (white and nonwhite black, Asian, Hispanic, or Other). Of note, models were run that included a treatment X psychiatric cohort interaction term, as well as models that included a discontinuation of study drug term; however, these terms did not significantly change outcomes reported from the more parsimonious models and therefore are not reported.
Additional models assessed number of comorbidities (0 [reference], 1, 2, 3, 4, or ≥5), on end-of-treatment and follow-up abstinence outcomes for varenicline versus other treatments, with cohort, sex, age, and race as covariates. All statistical analyses were performed with SAS 9.4.
Results
Trial findings were reported following the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for RCTs and a diagram is presented in Supplementary Figure 1. The present analysis includes 4151 (98.7%) individuals from the U.S. sites who had complete medical history data at the baseline screening assessment. Of those in this final analytic sample, n = 2359 (56.8%) were classified into the psychiatric cohort and n = 1792 (43.2%) were in the non-psychiatric cohort.
Comorbidities
Individuals with severe medical and psychiatric comorbidities were excluded from the primary study. As such, although cancer, rheumatoid arthritis, and HIV were included in the initial assessment of self-reported medical comorbidities at baseline, the case counts were small and too low for inclusion in the planned analyses (Ns = 77, 34, and 24, respectively). Of the 4151 participants evaluated in the present study, 252 had documented cardiac conditions (6.07%), 817 had respiratory conditions (19.68%), 1,211 had vascular conditions (29.17%), and 282 had diabetes (6.79%). Individuals could have been in multiple comorbidity categories. In the overall sample, 2317 (55%) reported zero medical comorbidities, 1,148 (27.66%) reported 1, 434 (10.46%) reported 2, 152 (3.66%) reported 3, 56 (1.35%) reported 4, and 44 (1.05%) had 5 or more comorbidities. Participant demographics by medical condition are presented in Supplementary Table 1.
Cigarette Abstinence
Abstinence rates by treatment arm and condition are presented in Figure 1 (week 12) and Figure 2 (week 24), and the full models are presented in Table 1 (week 12) and Table 2 (week 24). Varenicline demonstrated superior efficacy in nearly every comparison across disease groups.
Figure 1.
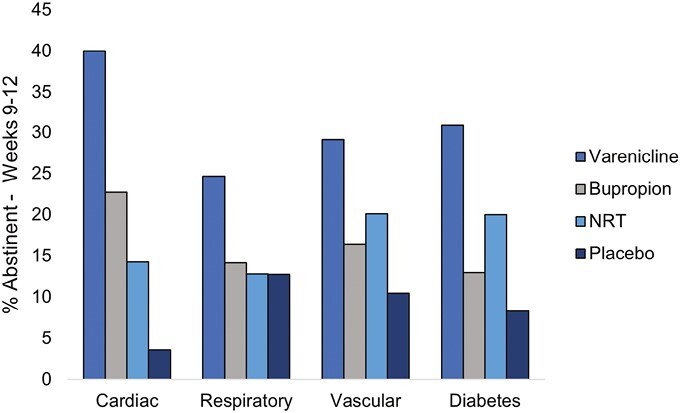
Week 12 continuous abstinence rates by medical comorbidity and treatment group.
Figure 2.
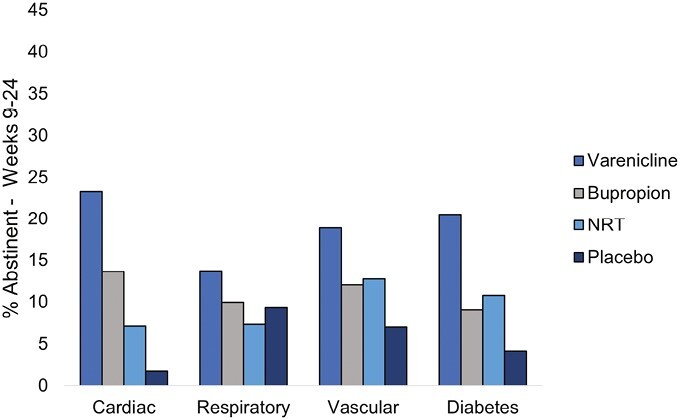
Week 24 continuous abstinence rates by medical comorbidity and treatment group.
Table 1.
Twelve-Week Abstinence by Treatment
| Cardiac N = 252 |
Respiratory N = 817 |
Vascular N = 1211 |
Diabetes N = 282 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abstinence rates by treatment | Treatment n | Abstinent n = 51 (20.24%) |
Treatment n | Abstinent n = 133 (16.28%) |
Treatment n | Abstinent n = 231 (19.08%) | Treatment n | Abstinent n = 50 (17.73%) |
|||||
| Varenicline | 60 | 24 (40.00%) | 219 | 54 (24.66%) | 302 | 88 (29.14%) | 68 | 21 (30.88%) | |||||
| Bupropion | 66 | 15 (22.73%) | 191 | 27 (14.14%) | 299 | 49 (16.39%) | 77 | 10 (12.99%) | |||||
| NRT | 70 | 10 (14.29%) | 203 | 26 (12.81%) | 313 | 63 (20.13%) | 65 | 13 (20.00%) | |||||
| Placebo | 56 | 2 (3.57%) | 204 | 26 (12.75%) | 297 | 31 (10.44%) | 72 | 6 (8.33%) | |||||
| Full model–treatment effect | Type III F (DF) | Type III F (DF) | Type III F (DF) | Type III F (DF) | |||||||||
| 6.98 (3, 244) | 5.23 (3, 809) | 11.36 (3, 1203) | 4.84 (3, 274) | ||||||||||
| Individual treatment estimates (treatment | reference) |
β | Type III F | OR (95%CI) | β | Type III F | OR (95%CI) | β | Type III F | OR (95%CI) | β | Type III F | OR (95%CI) | |
| Varenicline | Bupropion | 0.86 | 4.68 |
2.38
(1.08 to 5.25) |
0.70 | 7.23 |
2.01
(1.21 to 3.37) |
0.75 | 13.75 |
2.12
(1.42 to 3.16) |
1.20 | 7.37 |
3.35
(1.39 to 8.06) |
| NRT | 1.31 | 8.06 |
3.69
(1.48 to 9.18) |
0.84 | 10.01 |
2.31
(1.37 to 3.89) |
0.49 | 6.48 |
1.63
(1.11 to 2.37) |
0.71 | 2.60 | 2.04 (0.85 to 4.90) |
|
| Placebo | 3.15 | 16.69 |
23.29
(5.06 to 107.08) |
0.81 | 9.42 |
2.24
(1.34 to 3.76) |
1.28 | 30.86 |
3.61
(2.29 to 5.67) |
1.88 | 12.21 |
6.54
(2.26 to 18.95) |
|
| Bupropion | NRT | 0.29 | 0.36 | 1.33 (0.52 to 3.44) |
0.19 | 0.41 | 1.21 (0.66 to 2.20) |
−0.24 | 1.29 | 0.79 (0.52 to 1.19) |
−0.58 | 1.49 | 0.56 (0.22 to 3.78) |
| Placebo | 2.21 | 7.82 |
9.05
(1.91 to 42.97) |
0.11 | 0.13 | 1.12 (0.62 to 2.00) |
0.48 | 3.65 | 1.61 (0.98 to 2.62) |
0.52 | 0.85 | 1.68 (0.55 to 5.13) |
|
| NRT | Placebo | 2.82 | 7.10 |
16.84
(2.07 to 137.31) |
-0.04 | 0.02 | 0.96 (0.53 to 1.74) |
0.73 | 9.37 |
2.08
(1.30 to 3.33) |
1.17 | 4.00 |
3.23
(1.01 to 10.27) |
% abstinent represent column %s. Full models included age, race, sex, and psychiatric cohort. Italics results indicate p < .05. Additional models were run with treatment*cohort interaction term, which did not produce significant findings and thus were not reported.
Table 2.
Twenty-Four-week Abstinence by Treatment
| Cardiac N = 252 |
Respiratory N = 817 |
Vascular N = 1211 |
Diabetes N = 282 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abstinence rates by treatment | Treatment n | Abstinent n = 29 (11.51%) |
Treatment n | Abstinent n = 83 (10.16%) |
Treatment n | Abstinent n = 154 (12.72%) |
Treatment n | Abstinent n = 31 (10.99%) |
|||||
| Varenicline | 60 | 14 (23.33%) | 219 | 30 (13.70%) | 302 | 57 (18.87) | 68 | 14 (20.59%) | |||||
| Bupropion | 66 | 9 (13.64%) | 191 | 19 (9.95%) | 299 | 36 (12.04%) | 77 | 7 (9.09%) | |||||
| NRT | 70 | 5 (7.14%) | 203 | 15 (7.39%) | 313 | 40 (12.78%) | 65 | 7 (10.77%) | |||||
| Placebo | 56 | 1 (1.79%) | 204 | 19 (9.31%) | 297 | 21 (7.07%) | 72 | 3 (4.17%) | |||||
| Full model–treatment effect | Type III F (DF) | Type III F (DF) | Type III F (DF) | Type III F (DF) | |||||||||
| 3.84 (3, 244) | 1.71 (3, 809) | 5.98 (3, 1203) | 3.76 (3, 274) | ||||||||||
| Individual treatment estimates (treatment | reference) |
β | Type III F | OR (95%CI) | β | Type III F | OR (95%CI) | β | Type III F | OR (95%CI) | β | Type III F | OR (95%CI) | |
| Varenicline | Bupropion | 0.73 | 2.34 | 2.08 (0.81 to 5.38) |
0.38 | 1.46 | 1.46 (0.78 to 2.70) |
0.55 | 5.56 |
1.73
(1.09 to 2.74) |
1.09 | 4.50 |
2.99
(1.08 to 8.32) |
| NRT | 1.35 | 5.38 |
3.86
(1.21 to 12.26) |
0.72 | 4.63 |
2.06
(1.06 to 3.98) |
0.45 | 4.04 |
1.58
(1.01 to 2.46) |
0.86 | 2.69 | 2.38 (0.84 to 6.77) |
|
| Placebo | 3.08 | 8.39 |
21.74
(2.65 to 178.23) |
0.44 | 1.98 | 1.55 (0.84 to 2.89) |
1.12 | 17.00 |
3.08
(1.80 to 5.25) |
2.13 | 9.28 |
8.43
(2.11 to 33.65) |
|
| Bupropion | NRT | 0.36 | 0.35 | 1.44 (0.43 to 4.89) |
0.44 | 1.43 | 1.55 (0.75 to 3.21) |
−0.07 | 0.07 | 0.94 (0.58 to 1.52) |
−0.23 | 0.16 | 0.79 (0.25 to 2.52) |
| Placebo | 2.34 | 4.59 |
10.40
(1.19 to 90.36) |
0.06 | 0.04 | 1.07 (0.54 to 2.11) |
0.54 | 3.38 | 1.71 (0.96 to 3.03) |
0.62 | 0.70 | 1.86 (0.43 to 8.13) |
|
| NRT | Placebo | 2.24 | 3.12 | 9.36 (0.76 to 114.93) |
−0.32 | 0.77 | 0.72 (0.35 to 1.49) |
0.62 | 4.76 |
1.87
(1.06 to 3.27) |
1.28 | 2.60 | 3.62 (0.75 to 17.52) |
% abstinent represent column %s. Full models included age, race, sex, and psychiatric cohort. Italics results indicate p < .05. Additional models were run with treatment*cohort interaction term, which did not produce significant findings and thus were not reported.
Cardiac Conditions
For individuals with cardiac conditions, those in the varenicline arm were more likely to be abstinent (40.0%) compared to those in the bupropion (22.7%; odds ratio [OR] = 2.4, 95% confidence interval [CI] [1.1, 5.3]), NRT (14.3%; OR = 3.7, 95% CI [1.5, 9.2]), and placebo (3.6%; OR = 23.3, 95% CI [5.1, 107.1]) arms at week 12. At week 24, those in the varenicline arm were more likely to be abstinent (23.3%) compared to those in the NRT (7.1%; OR = 3.9, 95% CI [1.2, 12.3]) and placebo (1.8%; OR = 21.7, 95% CI [2.7, 178.2]) arms. Furthermore, at week 12, those randomized to bupropion were more likely to be abstinent compared to placebo (OR = 9.1, 95% CI [1.9, 43.0]), and those randomized to NRT were more likely to be abstinent compared to placebo (OR = 16.8, 95% CI [2.1, 137.3]). At week 24, bupropion continued to produce higher abstinence rates compared to placebo (OR = 10.4, 95% CI [1.2, 90.4]).
Respiratory Conditions
For individuals with respiratory conditions, those in the varenicline arm were more likely to be abstinent (24.7%) compared to those in the bupropion (14.1%; OR = 2.0, 95% CI [1.2, 3.4]), NRT (12.8%; OR = 2.3, 95% CI [1.4, 3.9]), and placebo (12.8%; OR = 2.2, 95% CI [1.3, 3.8]) arms at week 12. At week 24, those in the varenicline arm were more likely to be abstinent (13.7%) compared to those in the NRT (7.4%; OR = 2.1, 95% CI [1.1, 4.0]) arms but not compared to placebo.
Vascular Conditions
For individuals with vascular conditions, those in the varenicline arm were more likely to be abstinent (29.1%) compared to those in the bupropion (16.4%; OR = 2.1, 95% CI [1.4, 3.2]), NRT (20.1%; OR = 1.6, 95% CI [1.1, 2.4]) and placebo (10.4%; OR = 3.6, 95% CI [2.3, 5.7]) arms at week 12. At week 24, those in the varenicline arm were more likely to be abstinent (18.9%) compared to those in bupropion (12.0%; OR = 1.7, 95% CI [1.1, 2.7]), NRT (12.8%; OR = 1.6, 95% CI [1.0, 2.5]) and placebo (7.1%; OR = 3.1, 95% CI [1.8, 5.3]) arms. Furthermore, at week 12, those randomized to NRT were more likely to be abstinent compared to placebo (OR = 2.1, 95% CI [1.3, 3.3]) which was maintained at week 24 (OR = 1.9, 95% CI [1.1, 3.3]).
Diabetes
For individuals with diabetes, those in the varenicline arm were more likely to be abstinent (30.9%) compared to those in the bupropion (13.0%; OR = 3.4, 95% CI [1.4, 8.1]) and placebo (8.3%; OR = 6.5, 95% CI [2.3, 19.0]) arms at week 12. At week 24, those in the varenicline arm were more likely to be abstinent (20.6%) compared to those in bupropion (9.1%; OR = 3.0, 95% CI [1.1, 8.3]) and placebo (4.12%; OR = 8.4, 95% CI [2.1, 33.7]) arms. Furthermore, at week 12, those randomized to NRT were more likely to be abstinent compared to placebo (OR = 3.2, 95% CI [1.0, 10.3]).
Number of Comorbidities
The effect of treatment and number of medical comorbidities on abstinence rates is presented in Table 3. The total number of health comorbidities did not affect abstinence outcomes at week 12 (F5, 4137 = 0.80) or week 24 (F5, 4137 = 1.89). All pharmacotherapies increased the odds of abstinence, relative to placebo, regardless of number of comorbidities.
Table 3.
Effect of Treatment and Number of Medical Health Conditions on Abstinence Rates
| Twelve-week | ||||
|---|---|---|---|---|
| Full model effects | Type III F (DF) | |||
| Treatment | 33.97 (3, 4141) | |||
| # of Health Conditions | 0.80 (5, 4137) | |||
|
Individual Treatment Estimates
(treatment | reference) |
β | Type III F | OR (95%CI) | |
| Varenicline | Bupropion | 0.58 | 28.13 | 1.79 (1.44 to 2.22) |
| NRT | 0.55 | 25.67 | 1.74 (1.40 to 2.15) | |
| Placebo | 1.27 | 97.21 | 3.58 (2.78-4.62) | |
| Bupropion | NRT | −0.02 | 0.03 | 0.98 (0.77 to 1.23) |
| Placebo | 0.69 | 25.95 | 2.01 (1.53 to 2.63) | |
| NRT | Placebo | 0.70 | 26.85 | 2.02 (1.54 to 2.64) |
| Twenty-four-week | ||||
| Full model effects | Type III F (DF) | |||
| Treatment | 15.11 (3, 4141) | |||
| # of Health Conditions | 1.89 (5, 4137) | |||
|
Individual Treatment Estimates
(treatment | reference) |
β | Type III F | OR (95%CI) | |
| Varenicline | Bupropion | 0.38 | 8.67 | 1.47 (1.14 to 1.89) |
| NRT | 0.47 | 12.87 | 1.61 (1.24 to 2.08) | |
| Placebo | 1.01 | 43.68 | 2.74 (2.03 to 3.69) | |
| Bupropion | NRT | 0.10 | 0.53 | 1.11 (0.84 to 1.46) |
| Placebo | 0.63 | 15.42 | 1.88 (1.37 to 2.57) | |
| NRT | Placebo | 0.52 | 10.55 | 1.69 (1.23 to 2.32) |
Full model included age, race, sex, and psychiatric cohort. Health condition categories included 0, 1, 2, 3, 4, ≥5 (0 as reference). Italics results indicate p < .01.
Discussion
This secondary analysis of the EAGLES trial demonstrated that varenicline is the most efficacious option for patients with manageable cardiac, respiratory, vascular, and diabetes conditions to quit smoking. Superior efficacy was observed for varenicline compared to bupropion and placebo for week 12 abstinence across all comorbid disease categories, and superior efficacy compared to NRT across 3 conditions. At week 24, abstinence rates for varenicline were higher compared to placebo in the cardiac, vascular, and diabetes groups. Bupropion demonstrated superior efficacy compared to placebo among those with cardiac conditions at weeks 12 and 24. NRT demonstrated superior efficacy compared to placebo among cardiac, vascular, and diabetes groups at week 12, and this effect was maintained for those with vascular conditions at week 24. A study being conducted simultaneously found similar outcomes in the international EAGLES dataset, lending support to the consistency and reproducibility of these findings.13 The present study included individuals with past or present alcohol dependence and used age, race, and sex as covariates.
This demonstration of varenicline efficacy among individuals with manageable comorbid medical conditions supports recent clinical practice guidelines that recommend varenicline as a first-line pharmacotherapy for smoking cessation.4,6 In light of potential side effects and contraindications to varenicline, the finding that bupropion and NRT increase quit rates compared to placebo at week 24 in certain comorbidity groups is promising as an alternative. As the number of comorbidities increases, any of the pharmacotherapy options increases the odds of abstinence, demonstrating that medically complex individuals benefit from smoking cessation pharmacotherapy. Consistent with the primary publication findings, abstinence rates tended to decrease across all study arms by week 24. Some comorbidity subgroups had abstinence rates for specific pharmacotherapies that were greater than placebo at week 12, but those trends did not maintain at week 24. This finding may reflect the general trend of relapse over time, but may also highlight comorbidity-specific challenges with maintaining abstinence (eg, high medical stress, comorbidity symptom burden, etc.). Relapse prevention remains an important issue among all individuals who smoke, especially those with comorbidities who may require additional cessation support.
The differential effects of these medications are relevant for potential tailored treatment approaches, especially if varenicline is not tolerated. Individuals with medical and ipsychiatric comorbidities may be taking other pharmacological agents that are contraindicated with varenicline, or use of varenicline may result in elevated liver enzymes that would warrant discontinuation. Some individuals have also reported allergic reactions (ie, skin rash) when taking varenicline, although these allergies are relatively rare. In these instances, selecting bupropion or NRT may be preferable and these data may help to guide those clinical choices.
In general, the individuals selected for participation in the EAGLES trial were healthy sample because of the parent trial exclusion criteria. The results of the present publication should be replicated among those with more significant comorbidities. Participants in the parent trial were mostly white (82%) and results should be replicated with more diverse samples, especially given that there are clear racial disparities among many comorbid medical conditions. Of note, for those receiving NRT, only patches were given. Dual NRT or other combination therapy (eg, varenicline plus NRT) may have different efficacy profiles in these subgroups. Furthermore, the study was not powered to assess differences in comorbid conditions. Small sample sizes mean high variability in estimates as seen by large CI (eg, in the cardiac condition when placebo was used as the reference), and intent-to-treat analyses have inherent limitations.14
Patients with medical comorbidities face an urgent need to quit smoking for the management of their condition as well as their long-term health. Coupled with the existing neuropsychiatric and cardiovascular safety data,8,9 the present study is not only practice confirming for providers already prescribing varenicline but will likely be practice changing for those who are unsure of best treatment options for their medically complex patients. Treating individuals with comorbidities who smoke should be a regular part of the clinical care plan for the comorbid condition. For example, managing hypertension in the clinic should include referral to tobacco treatment behavioral support programs and prescription of varenicline just as a provider would prescribe antihypertensives and diet modifications. Beyond replicating the present study’s findings, future research should leverage implementation science approaches to optimize tobacco treatment delivery in the context of medical comorbidities.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Acknowledgment
This publication is based on research using data from Pfizer that has been made available through Vivli, Inc. Vivli has not contributed to or approved, and is not in any way responsible for, the contents of this publication.
Contributor Information
Alana M Rojewski, Department of Public Health Sciences, Medical University of South Carolina, Charleston SC, USA; Hollings Cancer Center, Medical University of South Carolina, Charleston, SC, USA.
Amanda M Palmer, Department of Public Health Sciences, Medical University of South Carolina, Charleston SC, USA.
Nathaniel L Baker, Department of Public Health Sciences, Medical University of South Carolina, Charleston SC, USA.
Benjamin A Toll, Department of Public Health Sciences, Medical University of South Carolina, Charleston SC, USA; Hollings Cancer Center, Medical University of South Carolina, Charleston, SC, USA.
Funding
This study has been funded by National Cancer Institute grant K07CA214839 (Rojewski) and National Heart, Lung, and Blood Institute grant NIH-T32-HL144470 (Palmer).
Declaration of Interest
Dr. Toll testifies on behalf of plaintiffs who have filed litigation against the tobacco industry.
Author Contributions
Alana Rojewski (Conceptualization [Lead], Supervision [Lead], Writing – original draft [Equal], Writing – review & editing [Equal]), Amanda Palmer (Conceptualization [Supporting], Data curation [Lead], Formal analysis [Lead], Writing – original draft [Equal], Writing – review & editing [Equal]), Nathaniel Baker (Formal analysis [Supporting], Writing – review & editing [Equal]), and Benjamin Toll (Conceptualization [Supporting], Writing – review & editing [Equal]).
Data Availability
The authors of the present manuscript obtained the data through request from Vivli Inc., a third party responsible for managing the study sponsor (Pfizer) data. We are unable to make data-sharing decisions. The code used in this secondary analysis are available by request to the authors.
References
- 1. US Department of Health and Human Services. The Health Consequences of Smoking-50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 2. Rojewski AM, Baldassarri S, Cooperman NA, et al. ; Comorbidities Workgroup of the Society for Research on Nicotine and Tobacco (SRNT) Treatment Network. Exploring issues of comorbid conditions in people who smoke. Nicotine Tob Res. 2016;18(8):1684–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fiore MC, Jaen CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Rockville, MD: U.S. Department of Health and Human Services, U.S. Public Health Service; 2008. [Google Scholar]
- 4. Leone FT, Zhang Y, Evers-Casey S, et al. Initiating pharmacologic treatment in tobacco-dependent adults. an official american thoracic society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(2):e5–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shields PG, Herbst RS, Arenberg D, et al. Smoking cessation, version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(11):1430–1468. [DOI] [PubMed] [Google Scholar]
- 6. Barua RS, Rigotti NA, Benowitz NL, et al. 2018 ACC expert consensus decision pathway on tobacco cessation treatment: a report of the american college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol. 2018;72(25):3332–3365. [DOI] [PubMed] [Google Scholar]
- 7. Rigotti NA. Pharmacotherapy for smoking cessation in adults. In: Aronson MD, Kathuria H, eds. UpToDate. 2023: https://www.uptodate.com/contents/pharmacotherapy-for-smoking-cessation-in-adults.
- 8. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507–2520. [DOI] [PubMed] [Google Scholar]
- 9. Benowitz NL, Pipe A, West R, et al. Cardiovascular safety of varenicline, bupropion, and nicotine patch in smokers: a randomized clinical trial. JAMA Intern Med. 2018;178(5):622–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mozzicato P. MedDRA. Pharmaceut Med. 2009;23(2):65–75. [Google Scholar]
- 11. American Psychological Association. Diagnostic and Statistical Manual for Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 12. Fagerström K. Determinants of tobacco use and renaming the FTND to the fagerström test for cigarette dependence. Nicotine Tob Res. 2011;14(1):75–78. [DOI] [PubMed] [Google Scholar]
- 13. Tønnesen P, Lawrence D, Tonstad S.. Medication-assisted quit rates in participants with smoking-related diseases in EAGLES: Post hoc analyses of a double-blind, randomized, placebo-controlled clinical trial. Tob Induc Dis. 2022;20(May):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gupta SK. Intention-to-treat concept: areview. Perspect Clin Res. 2011;2(3):109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors of the present manuscript obtained the data through request from Vivli Inc., a third party responsible for managing the study sponsor (Pfizer) data. We are unable to make data-sharing decisions. The code used in this secondary analysis are available by request to the authors.


