Abstract
INTRODUCTION:
Non-Hispanic Black, compared to non-Hispanic White, older adults are at increased risk for dementia. This may be partly due to greater exposure to psychosocial stressors, such as discrimination; however, few studies have examined this association.
METHODS:
We examined the association of perceived discrimination (e.g., everyday, lifetime, and discrimination burden) with dementia risk in 1,583 Black adults co-enrolled in the Atherosclerosis Risk in Communities Study (ARIC) and the Jackson Heart Study (JHS). Perceived discrimination (defined continuously and using tertiles) was assessed at JHS Exam 1 (2000–2004; age±SD:66.2±5.5) and related to dementia risk through ARIC visit 6 (2017) using covariate-adjusted Cox proportional hazards models.
RESULTS:
Associations of perceived everyday, lifetime, and burden of discrimination with dementia risk were not supported in age-adjusted models or demographic- and cardiovascular health-adjusted models. Results were similar across sex, income, and education.
DISCUSSION:
In this sample, associations between perceived discrimination and dementia risk were not supported.
Keywords: dementia risk, discrimination, macro level factors, older Black adults
1. BACKGROUND
Black, compared to non-Hispanic White, older adults have higher rates of Alzheimer’s disease (AD) and all-cause dementia1,2. The elevated dementia risk among Black older adults may be due to modifiable factors such as reduced access to health care, greater prevalence of cardiovascular illness, as well as psychosocial factors, such as discrimination and stress, which are found to be more prevalent among Black individuals3–6. Discrimination is defined as unfair treatment based upon group membership, and perceived discrimination reflects the subjective experience of discrimination7. The burden of discrimination represents a major source of both acute and chronic stress that has been shown to contribute to poor health-related outcomes in Black individuals8–11. In fact, prior studies report that prevalence rates of perceived discrimination for Black individuals are as high as 57% in some contexts12–14. While perceived discrimination has been associated with outcomes such as hypertension and cardiovascular disease in Black adults8–10, important risk factors for dementia, less is known about how discrimination directly relates to dementia risk in older adults15.
Chronic stress may lead to prolonged exposure to elevated stress hormones and circulating inflammatory mediators (e.g., interleukin-4 [IL-4], interleukin-6 [IL-6] and C-reactive protein [CRP]) that may, over time, have deleterious effects on the structure and function of brain regions important for memory and other cognitive processes among all ethnic groups16–18. In support of this hypothesis, psychosocial stressors, including discrimination, have been found to be associated with poorer performance on measures of memory as well as greater cognitive decline in Black adults11,19. Several studies have examined the association of discrimination with cognitive function and cognitive decline, and the results of these analyses have been largely mixed3,5,6,20. To the best of our knowledge only one study has examined the relationship between discrimination and dementia risk. One such study in Brazilian Black and White adults found that informant report of discrimination was cross-sectionally associated with higher odds of prevalent dementia21. However, to our knowledge, no studies have examined whether perceived discrimination is associated with prospective incident dementia risk among Black older adults within the U.S.
Complicating the understanding of how perceived discrimination may relate to dementia risk is the finding that many of the factors that may influence exposure to discrimination can also influence cognition. For example, studies have demonstrated that Black men, as well as Black individuals with a higher income and educational attainment, report higher levels of experienced discrimination, compared, respectively, to women and those of lower income and educational attainment7,22. It is therefore possible that some of the protective sociodemographic factors associated with cognitive reserve (e.g., the brain’s capacity to use pre-existing cognitive processing approaches to cope with damage23) and reduced dementia risk in the general population may also expose Black individuals to greater discrimination.
Understanding how perceived discrimination is associated with dementia risk may provide further insight into how psychosocial factors contribute to the disproportionate burden of dementia among Black older adults. The present study examined the association of perceived levels of everyday and lifetime discrimination with 17-year dementia risk using data from Black older adults co-enrolled in the Atherosclerosis Risk in Communities (ARIC) Study and the Jackson Heart Study (JHS). To the authors’ knowledge, this is the first study to examine whether perceived discrimination is associated with incident dementia risk among older Black adults in the United States. We hypothesize that perceived everyday and lifetime discrimination are positively associated with the risk of dementia among older adults. Given the sex difference in dementia risk (greater risk among women24), and prior studies showing that higher income and educational attainment are associated with reduced risk for dementia25,26, we hypothesized that male sex, higher income level, and a greater level of education may buffer the effects of discrimination on dementia risk. To test this hypothesis, we examined whether sex (male/female), income, and education, moderate the association between discrimination and incident dementia.
2. METHODS
2.1. Participants
Participants in the current study were co-enrolled in the ARIC Study and the JHS. ARIC and JHS are ongoing longitudinal, community-based cohort studies. Between 1987–1989, 15,792 mostly Black and White adults aged 45–64 years were recruited to be a part of the ARIC study from four different sites across the United States (Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi; and Minneapolis suburbs, Minnesota)27. Participants completed subsequent clinic visits every three years until visit 4. Participants then returned for visit 5 in 2011–2013 and visit 6 in 2016–2017. ARIC participants completed cognitive assessments at visits 2, 4, 5, and 6, as described below (Figure 1). A subset of the ARIC participants, enrolled at the Jackson, MS site, were also enrolled in the JHS. Between 2000 and 2004, 5,306 non-institutionalized Black adults aged 21–94 were recruited to be a part of the JHS from the Hinds, Madison, and Rankin counties in the Jackson, MS metropolitan area. After the first exam for the JHS (2000–2004), participants returned for subsequent in-person visits; visit 2 (2005–2008) and visit 3 (2009–2013; Figure 1). Detailed information about the JHS and ARIC study have been previously published27,28. As indicated in Figure 2, participants included in this study 1) were older Black adults co-enrolled in the JHS and ARIC study; 2) had information available for one or more measures of discrimination; and 3) were dementia-free at baseline. All participants provided written informed consent at each study visit. For participants who were judged to lack capacity, proxies (usually next of kin or other family members) provided consent. Institutional review boards at each of the participating centers (ARIC: The Johns Hopkins University, Wake Forest University, University of Mississippi Medical Center, and University of Minnesota; JHS: Jackson State University, Tougaloo College, and the University of Mississippi Medical Center) approved the study.
Figure 1.
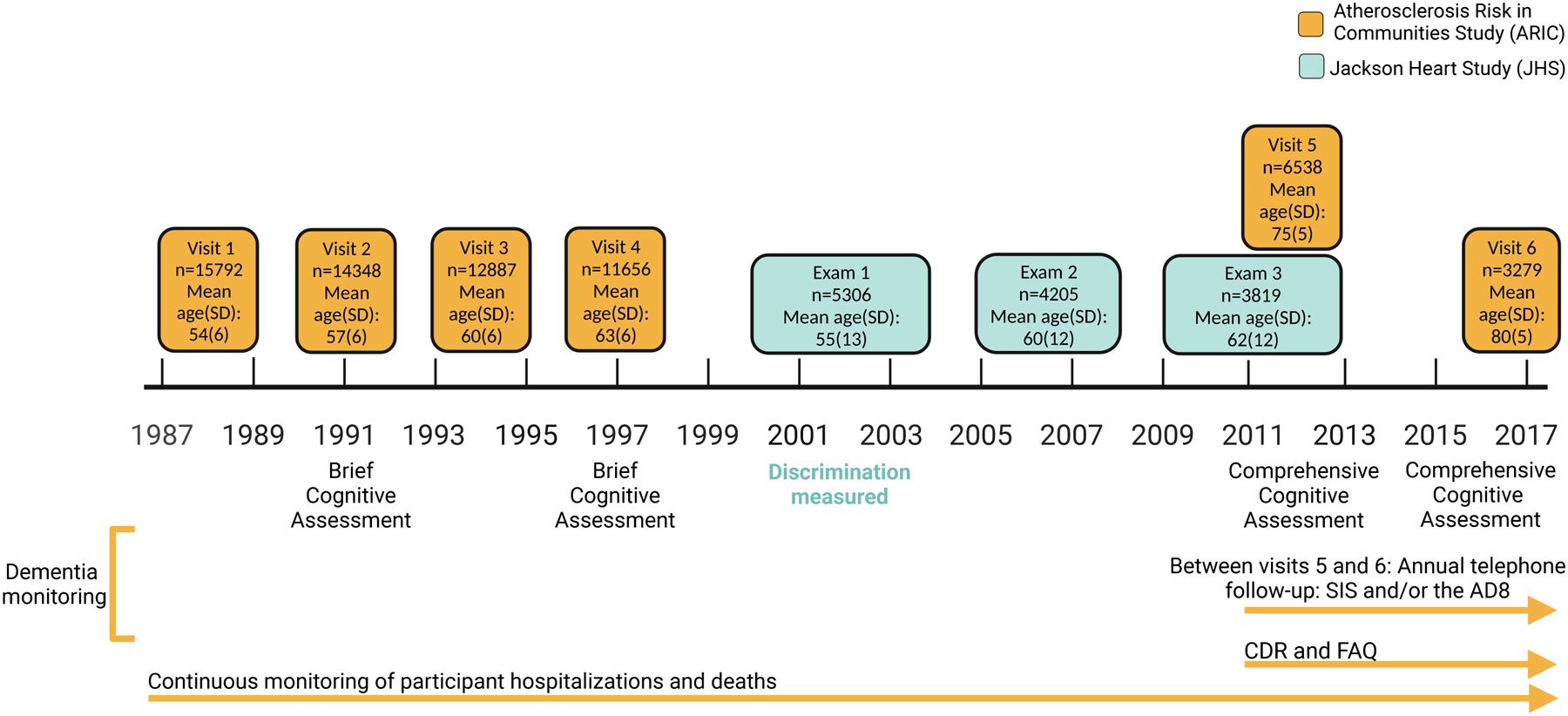
Study timeline. Measures of discrimination were assessed during Exam 1 of the JHS. Following the assessment of discrimination, participants completed comprehensive cognitive testing during visits 5 and 6 of the ARIC study. Prior to ARIC visit 5, dementia was ascertained using the International Classification of Diseases, Ninth Revision (ICD-9) and diagnostic codes from death certificates. At ARIC visit 5, participants with suspected dementia received a modified version of the Clinical Dementia Rating scale (CDR), and their informant received a Functional Activities Questionnaire (FAQ). For participants who did not attend visit 5, dementia was diagnosed using the CDR and FAQ measures, the Telephone Interview for Cognitive Status–Modified (TICSm), and ICD-9 hospital discharge diagnosis codes or diagnostic codes from death certificates. Between visits 5 and 6, participants were administered the Six-item screener (SIS) and the Ascertain Dementia 8-item Informant Questionnaire (AD8) annually. Throughout the ARIC study, continuous hospital surveillance was also used to captured ICD-9 and hospital discharge diagnosis codes or diagnostic codes from death certificates to identify dementia. Brief cognitive assessment (visits 2 [1990–1992] and 4 [1996–1998]): Digit symbol substitution, Delayed word recall, Semantic fluency. Comprehensive cognitive assessment (visit 5 [2011–2013]): Mini-Mental State Examination (MMSE), Digit Span backward, Digit symbol substitution, Trail making part A, Delayed word recall, Logical Memory II, Word fluency test (FAS), Animal fluency, Boston naming test, Clock reading. Comprehensive cognitive assessment (visit 6 [2016–2017]): Mini-Mental State Examination (MMSE), Digit symbol substitution, Delayed word recall, Logical Memory I and II, Incidental learning, Word fluency test (FAS), Animal naming, Boston naming test, Trail Making parts A and B.
Figure 2.
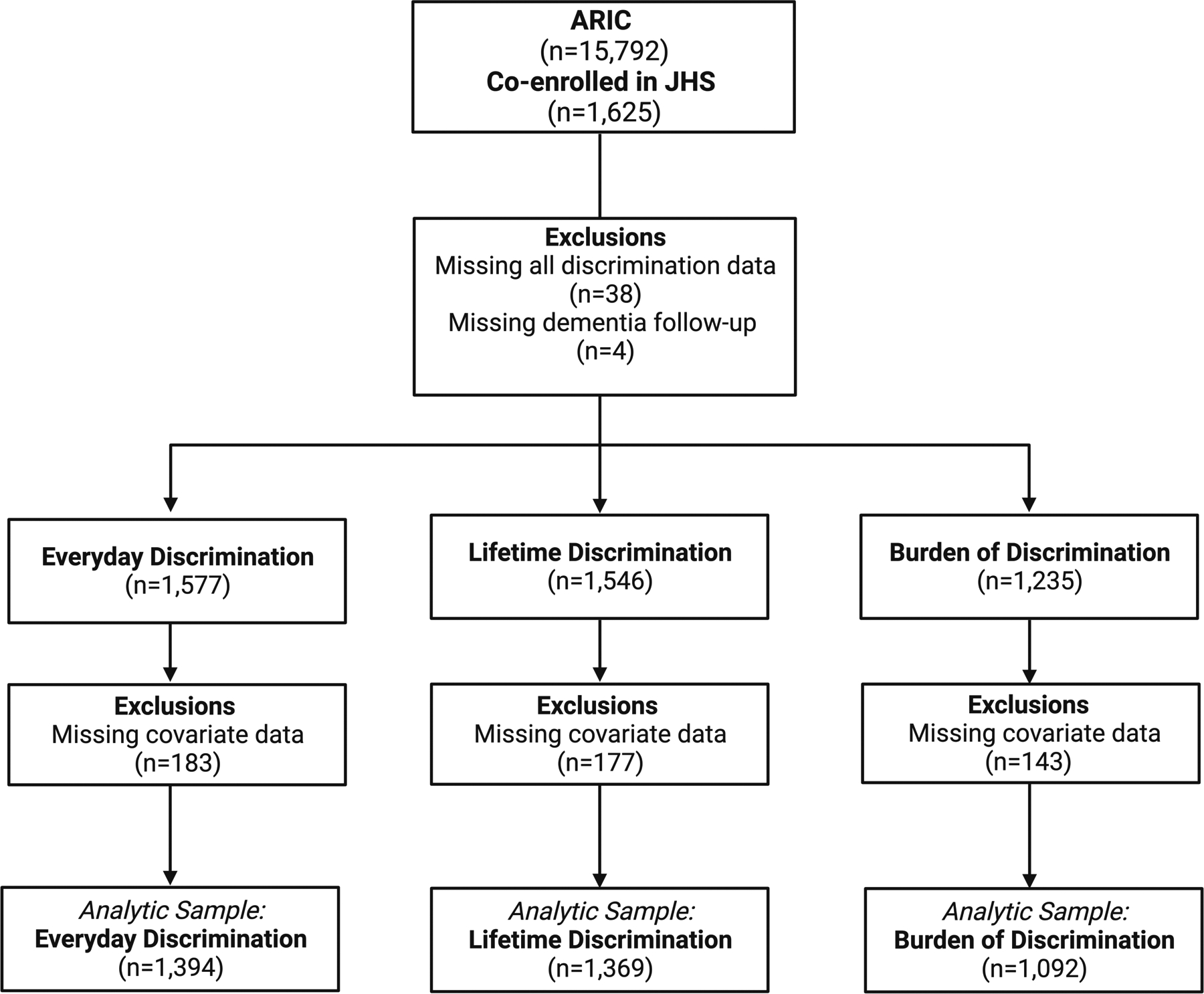
Participant Exclusions. Participant exclusion flowchart. ARIC: Atherosclerosis Risk in Communities; JHS: Jackson Heart Study. Analytic sample reflects the number of participants used for each respective analysis in the present study.
2.2. Discrimination measures
Three different types of discrimination measures were assessed during Exam 1 of the JHS (Figure 1). Everyday discrimination was assessed using the 9-item Everyday Discrimination Scale29,30. Participants rated to what extent they had day-to-day experiences of unfair treatment on a scale from 1 (never) to 7 (several times a day). Item examples include “People are afraid of you,” and “You are treated with less courtesy.” Lifetime discrimination was assessed using a dichotomous scale (yes[1]/no[0])10,31. Participants were asked whether they had experienced unfair treatment over their lifetime at school, in getting a job, at work, in getting housing, in getting resources or money, in getting medical care, on the street or public place, in getting services, and in any other way. The nine items were summed to reflect a total lifetime discrimination score (range 0–9), with higher scores reflecting greater lifetime discrimination. Of participants who reported lifetime discrimination, burden of lifetime discrimination (described as burden of discrimination throughout the text) was assessed using three items where participants rated the extent to which the events of discrimination were stressful, interfered with their lives, and how difficult events made their lives9,32. For analysis, each discrimination measure was divided into four categories (no discrimination, and three tertiles divided among participants with some discrimination). Participants who reported no discrimination were included as the reference group.
2.3. Dementia ascertainment
Using ARIC surveillance methods and data from in-person ARIC assessments, dementia was identified from the time of discrimination assessment (JHS Exam 1) through ARIC visit 6 using previously described methods (Figure 1)33,34. At ARIC visits 5 and 6, dementia classification was based on a comprehensive cognitive and functional exam, using criteria of the National Institute on Aging-Alzheimer’s Association (NIA-AA) workgroups and the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5)35,36. Algorithmic diagnoses were then confirmed by expert clinical adjudicators, which included both a physician and a neuropsychologist. Between JHS exam 1 and ARIC visit 5, dementia diagnosis was ascertained using the International Classification of Diseases, Ninth Revision (ICD-9) hospital discharge codes, diagnostic codes from death certificates, and retrospectively from informant interview (CDR) administered at visit 5. For participants who did not attend visit 5 but responded to the annual telephone follow-up interview conducted during that time, dementia was also diagnosed using the Telephone Interview for Cognitive Status–Modified (TICSm)37. Between ARIC visits 5 and 6, the Ascertain Dementia 8-item Questionnaire (AD8) and Six-item screener (SIS) were issued annually via phone to determine cognitive status. For participants who did not attend visit 6, dementia was diagnosed using the AD8 and SIS, ICD-9 hospital discharge diagnosis codes, and diagnostic codes from death certificates. For participants who were diagnosed with dementia at visits 5 or 6, inter-visit cognitive/functional/informant assessments and hospital discharge codes were used to approximate the date of dementia onset.
2.4. Covariate assessment
All covariates were assessed during the baseline JHS examination (2000–2004). Age in years, sex (male/female), educational attainment (less than high school; high school graduate, vocational training, general equivalency diploma (GED); or beyond high school), income (based on family size, U.S. Census poverty levels, and year of baseline clinic visit), and cigarette smoking (yes/no), were self-reported by participants. Income was classified as poor (income <poverty level), lower‐middle (income 1 to 1.5 times the poverty level), upper‐middle (income >1.5 but <3.5 times the poverty level), and affluent (income ≥3.5 times the poverty level)38,39. Presence of diabetes (classified based on fasting glucose level ≥126mg/dL, nonfasting glucose level ≥200mg/dL, self-report of physician-diagnosed diabetes, or use of diabetes medications) was recorded. Prevalent coronary heart disease (yes/no) and prevalent stroke (yes/no) before ARIC visit 1 were both self-reported, with incident events from ARIC after visit 1 adjudicated by expert review. Incident coronary heart disease was ascertained by the ARIC Morbidity and Mortality Classification Committee using hospitalization records, follow-up telephone calls, and/or death certificates40. Presence of hypertension (yes/no) was based on systolic blood pressure ≥140mmHg, diastolic blood pressure ≥90mmHg, or use of antihypertensive medications. Physical activity was categorized as poor (0 minutes of moderate or vigorous physical activity per week), intermediate (<150 minutes of moderate, <75 minutes of vigorous, or <150 minutes of moderate and vigorous physical activity per week), and ideal (≥150 minutes of moderate, ≥75 minutes of vigorous, or ≥150 minutes of moderate and vigorous physical activity per week) physical activity41,42. Depression was assessed using the 20-item Center for Epidemiological Studies Depression (CESD)43 measure completed during Exam 1.
2.5. Statistical analyses
One-way analysis of variance (ANOVA) and chi-square tests were used to examine differences in participant characteristics across categories of discrimination. To assess whether discrimination was associated with the hazard of incident dementia, a time-to-event analysis, including Kaplan Meier and Cox proportional hazards models, was completed to estimate dementia hazard ratios from the time of discrimination assessment (JHS exam 1) through ARIC visit 6 (final date of follow-up: December 31, 2017). Separate models were constructed for each independent variable: everyday discrimination, lifetime discrimination, and burden of discrimination. Participants who reported no discrimination were considered the reference group. We examined three covariate models. Model 1 adjusted for age; model 2 adjusted for age, sex, education, and income status; and model 3 adjusted for age, sex, education, income status, and baseline hypertension, diabetes, stroke, coronary heart disease, and cigarette smoking status. Sensitivity analyses were also conducted examining the effect of adjusting for baseline depression symptoms. Using separate models, we also examined sex, education, and income as potential effect modifiers using multiplicative interaction terms. Stata version 14 (StataCorp) was used for all statistical analyses. A significance threshold of p<0.05 was used for the present study.
3. RESULTS
3.1. Participant characteristics
A total of 1,583 participants with non-missing dementia follow-up and any form of discrimination (e.g., everyday, lifetime, burden of) data were co-enrolled in the ARIC and JHS study (mean age±SD=66.2±5.5 years; 66.7% women; Table 1; Figure 2). In total, 1,191 (76%) of 1,577 participants reported experiencing at least some everyday discrimination (Mean±SD: 1.9±0.9); 1,245 (81%) of 1,546 participants reported experiencing at least some lifetime discrimination (Mean±SD: 2.6±2.0); 1,134 (92%) of 1,235 participants reported experiencing stress from lifetime discrimination (burden of discrimination; Mean±SD: 2.4±0.8). Participant characteristics are stratified by categories of everyday discrimination (Supplemental Table 1), lifetime discrimination (Supplemental Table 2) and burden of discrimination (Supplemental Table 3). Participants who reported higher levels of everyday discrimination were more likely to be younger, report higher symptoms of depression, and have higher education and income than those who reported lower levels/no everyday discrimination (Supplemental Table 1). Participants who reported higher levels of lifetime discrimination were more likely to be younger, male, report higher levels of weekly physical activity, and have higher education and income than those who reported lower levels/no lifetime discrimination (Supplemental Table 2). Participants who reported experiencing higher burden of discrimination were more likely be male, report higher symptoms of depression, and have lower income than those who reported lower levels/no burden of discrimination (Supplemental Table 3). A total of 381 (24.1%) of the 1,583 participants included in this study developed dementia over the median 13.6 (Range: 0.1 to 17.0) years of follow-up. The dementia incidence rate was 20.4 dementia cases per 1000 person-years during follow-up.
Table 1.
Baseline participant characteristics
| Full sample | |
|---|---|
| n | 1583 |
| Age in Years, mean (SD) | 66.2 (5.5) |
| Male Sex | 525 (33.2%) |
| Education Attainment | |
| Less than high school | 511 (32.5%) |
| High school/ General Education Diploma | 353 (22.5%) |
| Attended college, vocational, trade school | 708 (45.0%) |
| Income Status | |
| Poor | 219 (15.4%) |
| Lower-middle | 454 (32.0%) |
| Upper-middle | 398 (28.1%) |
| Affluent | 347 (24.5%) |
| Risk Factors | |
| Cigarette Use (ever) | 567 (35.9%) |
| Hypertension | 1177 (74.4%) |
| Diabetes | 516 (32.8%) |
| Coronary heart disease | 177 (11.2%) |
| Stroke | 111 (7.0%) |
Note. Unless otherwise indicated, data are presented as frequency (percentage) or mean (standard deviation)
Abbreviations: SD: standard deviation
3.2. Discrimination and incident dementia
In a model that adjusted for age, we found no support for the relationship between measures of perceived everyday discrimination, or perceived lifetime discrimination, and dementia risk (Figures 3 and 4). Among participants who reported experiencing lifetime discrimination, we found no support for the relationship between burden of discrimination and dementia risk. Specifically, participants who reported experiencing any form of discrimination – ranked across tertiles based on severity – were not supported as having greater risk for dementia, compared to participants who reported experiencing no discrimination of any form. In models adjusting for age, sex, education, and income status we found no support for the association between any measure of perceived discrimination and dementia risk. Finally, after further adjusting for demographic characteristics and cardiovascular risk factors, we again found no significant association between any form of perceived discrimination and dementia risk (Figures 3 and 4). When we examined each form of discrimination as continuous variables, we similarly found no support for the relationship between everyday discrimination (HR = 1.10, 95% CI: 0.98–1.24), lifetime discrimination (HR = 0.99, 95% CI: 0.93, 1.04), burden of discrimination (HR = 1.01, 95% CI: 0.86, 1.18) and dementia risk after adjusting for demographic characteristics and cardiovascular risk factors. We note that dementia risk factors included in our models as covariates (i.e., age, sex, education, income, diabetes, and history of stroke) were consistently associated with dementia risk (Supplementary Tables 4–6). Adjusting for baseline depressive symptoms did not meaningfully change the results.
Figure 3.
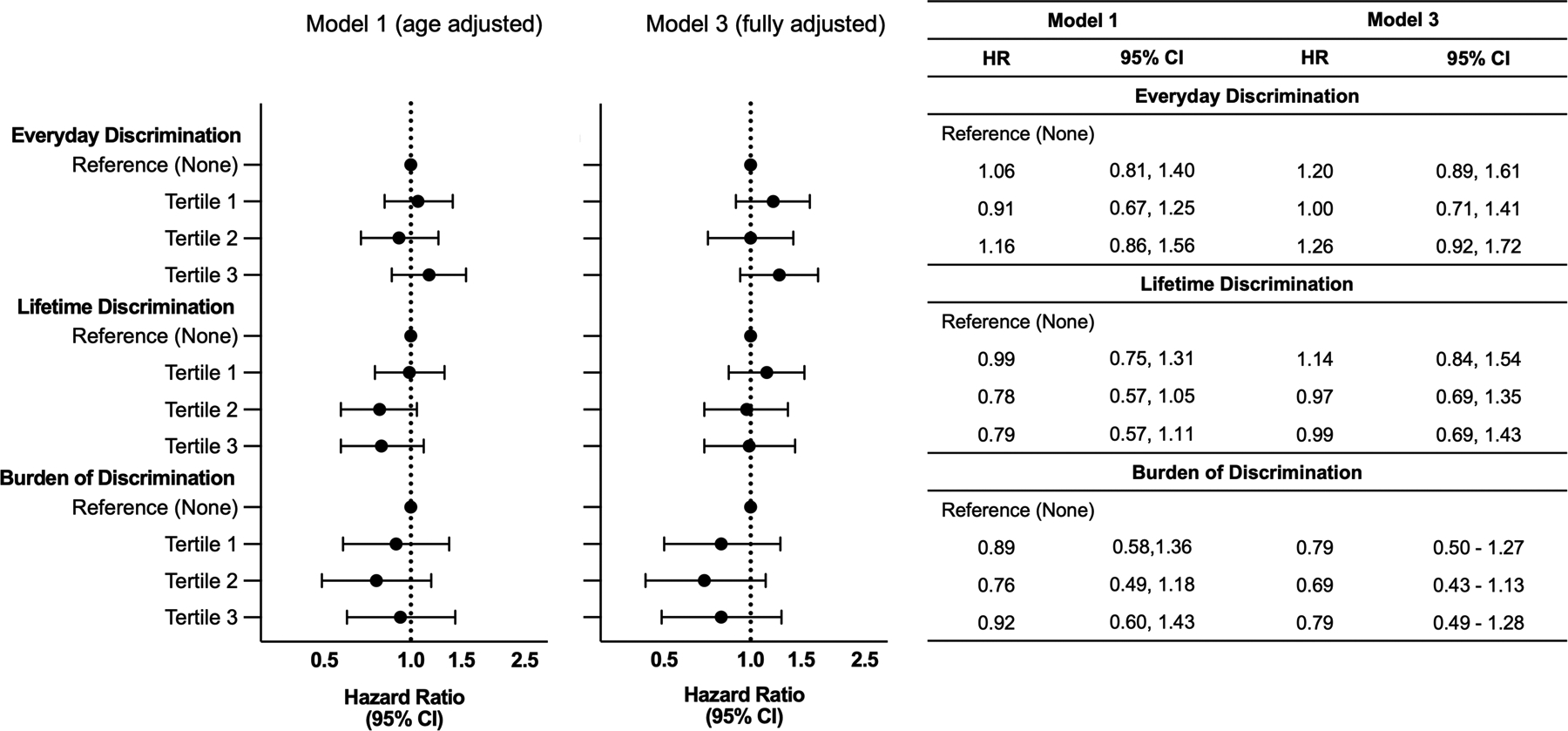
Association between Discrimination and Incident Dementia. Hazard ratio (HR) and 95% confidence interval (CI) for the association of discrimination with incident dementia. Participants who reported no discrimination were used as a reference group. Participants who reported discrimination levels greater than “None” were divided into ascending tertiles based on level of reported discrimination. Model 1was adjusted for age. Model 3 was adjusted for age, sex, education, income status, hypertension, diabetes, stroke, coronary heart disease status/history, and cigarette smoking status. Sample sizes for Models 1 and 3 are as follows: Everyday discrimination (n = 1,394); Lifetime discrimination (n = 1,369); Burden of Discrimination (n = 1,092). Results for model 2, which adjusted for age, sex, education, and income status, are presented in the Supplementary Tables 4–6.
Figure 4.
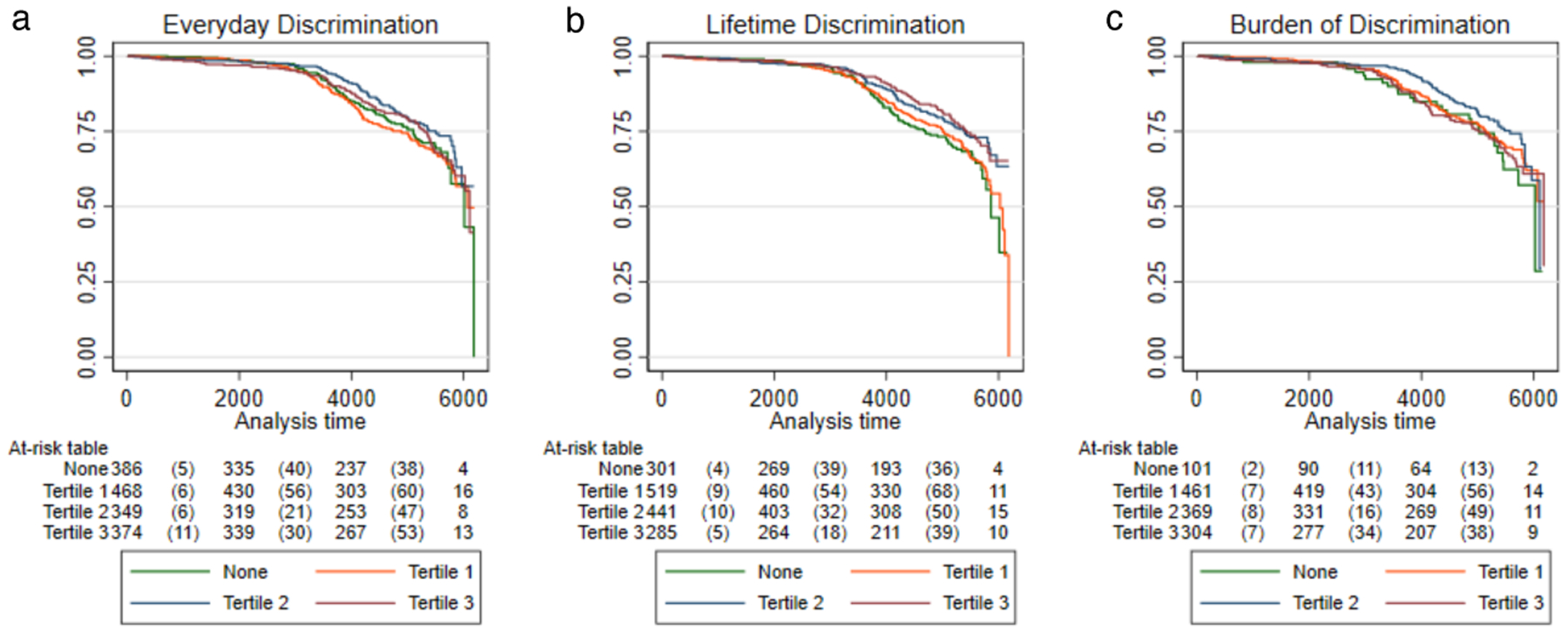
Kaplan-Meier Curves for Time-to-Dementia Onset by Discrimination. Figure depicts the Kaplan-Meier curves for time to dementia onset by a) everyday discrimination, b) lifetime discrimination, and c) burden of discrimination. For all analyses, participants who reported no discrimination were used as the reference group. Participants who reported discrimination levels greater than “None” were divided into ascending tertiles based on level of reported discrimination.
Our examination of interaction terms found no support for a moderating effect of income and education on the relationship between any form of discrimination and dementia risk. However, our assessment of effect modification by sex did find that lifetime discrimination was more strongly associated with dementia risk among women compared to men (p-interaction for 1st tertile=0.02). However, the association between lifetime discrimination and dementia risk was not statistically significant for men or women in sex stratified analyses (Supplementary Table 7).
3.3. Post hoc analysis: discrimination and incident mortality
Post hoc analyses were completed to determine whether the lack of a significant association between any form of discrimination and incident dementia may reflect increased mortality rates, as previous studies have found a positive association between perceived everyday and lifetime discrimination and risk of death44,45. In the current study, the incident mortality rate was 29.7 deaths per 1000 person-years. Time-to-event analyses suggest that greater perceived discrimination of any form was not associated with increased mortality (Supplemental Table 8).
4. DISCUSSION
The present study examined the association between discrimination and 17-year dementia risk in Black older adults co-enrolled in two community-based cohorts. Perceived discrimination, as defined by measures of everyday discrimination, lifetime discrimination, and burden of discrimination, was not associated with dementia risk. Though we did not find support for the relationship between perceived discrimination and dementia risk, known dementia risk factors, including age, diabetes, and history of stroke were associated with higher dementia risk in the current study. Interestingly, although education and income level tended to be higher in groups who reported experiencing more everyday and lifetime discrimination46,47, these characteristics were associated with decreased dementia risk, consistent with protective effects of high education and high income, as demonstrated in prior studies25,26,48. To determine whether the relationship between discrimination and dementia differed across demographic strata, we examined sex, income, and education as effect modifiers. While our results suggest that lifetime discrimination may be more strongly associated with dementia risk in women than in men, the sex-specific associations were not statistically significant in subgroup analyses. Moreover, income and education did not appear to modify the relationship between discrimination and dementia risk.
Although perceived discrimination has been associated with adverse health outcomes, studies examining how perceived everyday and lifetime discrimination relate to dementia in Black older adults are scarce. One study found that informant-reported experience of discrimination was positively associated with the odds of dementia in a Brazilian sample of Black and White participants21. While this Brazilian study offers some evidence for a discrimination-dementia relationship, this study has numerous limitations (e.g., use of informant report, retrospective assessment, cross-sectional design) that limit the generalizability of the results. To the best of our knowledge, no study to date has examined the relationship between perceived discrimination and incident dementia. However, several previous studies have examined the association between perceived (everyday/lifetime) discrimination and cognitive outcomes among Black participants. These studies have produced mixed results. For instance, Zahodne and colleagues found no association between everyday or lifetime discrimination and global cognition in Black and Hispanic older adults5. On the other hand, several other studies found associations between discrimination and cognitive outcomes, including subjective reports of cognitive function. Specifically, greater discrimination has been associated with poorer subjective cognitive functioning and performance-based tests of memory, as well as memory decline among Black adults3,6,19,20.
In light of documented relationships between discrimination and negative health outcomes, including poor cognition, the lack of association between discrimination and dementia risk is quite surprising. We observed a non-significant increase in dementia risk associated with everyday discrimination, yet lifetime and burden of discrimination showed non-significant associations in the opposite direction. Although speculative, we suspect that one reason for the absence of supported associations between perceived discrimination and increased dementia risk is the link between discrimination and protective factors in the present study. For instance, in comparison to those reporting low discrimination, participants who reported high discrimination were younger, more likely to be male, and more likely to report high income and education, all of which are factors associated with decreased risk for dementia7,22,25,26,48. It is possible that Black adults with high education and occupational attainment are exposed to situations where the likelihood of discrimination (race-based or otherwise) is particularly high. It is also possible that Black older adults with more education and income are better able to identify examples of discrimination in their own life46,47. Further, one study demonstrated that higher income was associated with greater mastery (i.e., feelings of control over personal circumstances) and a decreased association between perceived discrimination and adverse health outcomes compared to those with lower income49. This study aligns with prior work that shows that those below the poverty threshold and with less than a high school education appear to have the worst health outcomes, and additionally shows that coping skills mediate the relationship between discrimination and adverse health outcomes49,50. Factors that correlate positively with exposure to discrimination, through their positive effects on brain health (i.e., cognitive reserve, coping style), may outweigh potential negative effects of perceived discrimination on dementia risk.
There are several alternative explanations for the non-supported associations between perceived discrimination and dementia risk. Although unlikely, it is possible that discrimination, while associated with other adverse health outcomes and lower cognition, is simply not a risk factor for the pathological processes that underly the most common forms of dementia. Some studies have examined the association between perceived discrimination and neurologic features associated with dementia. Specifically, prior studies have demonstrated that discrimination is associated with greater endothelial plasma proteins (ET-1)51, and both cross-sectional and longitudinal brain characteristics such as smaller hippocampal volume and increased white matter hyperintensities among Black adults52, but is not associated with stroke in older adults after adjusting for covariates53. Future studies should continue to examine associations among perceived discrimination and dementia endophenotypes such as brain structure and CSF/plasma biomarkers. Another possibility is that an association between greater discrimination and early mortality functioned as a competing risk that prevented observation of an existing discrimination-dementia relationship. However, the association between perceived discrimination and incident mortality was not supported in the present study. Finally, it is possible that discrimination that is not perceived still has adverse effects on health. For instance, prior studies suggest that systemic factors (e.g., unequal access to resources) and not necessarily individual experiences of discrimination may contribute to adverse health outcomes including dementia54–56. Researchers should aim to account for systemic factors when examining whether experiences of discrimination are associated with dementia.
Participant characteristics and study geography should also be considered in the interpretation of the results. While participants in the present study reported perceived everyday and lifetime discrimination scores that were comparable to one study57, they reported lower perceived everyday discrimination than in other studies18,49,58. Further, the reduced rates of discrimination reported in the present study may reflect participant age, as the present study included only older Black adults and prior studies have shown that younger Black adults report greater discrimination compared to older Black adults59. Additionally, the reduced rates of discrimination reported in the current study may also reflect the ethnic makeup of participants’ region. Mississippi, the location of the JHS, has the highest percentage of Black residents60 of all U.S. states. It is possible that given the large population of Black residents, participants in the present study do not experience discrimination that results in adverse neurocognitive outcomes. Supporting this hypothesis, one study, which found that Hispanic participants reported less discrimination than non-Hispanic whites, also found that Hispanic participants lived in an ethnically homogenous region of the United States5.
The present study has several strengths including a large sample size, prospective study design, an extended follow-up period for dementia assessment, dementia adjudication, and an in-depth measurement of distinct forms of discrimination. However, the present study is not without limitations. First, lifetime discrimination required participants to retrospectively estimate the discrimination experienced throughout their lives. Retrospective data collection and potential limitations inherent in the discrimination questionnaires – which, for example, did not specify a discrimination source – may have limited our ability to detect associations. Next, while the present study is among the largest including Black adults, the subgroup moderation analyses may have been underpowered to detect effects. Finally, data from the present study was collected at a single site in the southeastern United States, and thus may not be generalizable to other regions within or outside the United States. For example, for Black individuals living in communities in which they are a clear minority, the level and form of discrimination perceived everyday or throughout the lifetime may be quite different. Therefore, similar studies – particularly multi-site studies – are needed to fully understand whether discrimination may influence dementia risk.
4.1. Conclusion
Despite limitations, results from the present study suggest that while sociodemographic factors and cardiovascular health are associated with dementia risk, perceived discrimination may not be a substantial dementia risk factor in this population of Black older adults. Provided that this study examined Black participants living in the Southern United States, future studies should aim to investigate associations between discrimination and dementia risk in other regions of the United States, as this relationship may be influenced by local demographics.
Supplementary Material
Research in Context.
Systematic Review: The authors reviewed the literature using PubMed. Non-Hispanic Black adults have higher rates of dementia compared to non-Hispanic White adults. Psychosocial factors may play a role in these reported differing rates of dementia. Several studies have found an association between greater discrimination and poorer cognition in Black older adults. However, to the best of our knowledge, only one study has examined the association between discrimination and dementia risk, showing that individuals diagnosed with dementia had greater levels of perceived discrimination. However, the abovementioned study was limited by use of informant report and a retrospective, cross-sectional design.
Interpretation: In a large cohort of Black adults in the southeastern region of the U.S. with 17-years of follow-up, the association between perceived discrimination and dementia risk was not supported.
Future Directions: Future prospective cohort studies are needed, particularly those representative of different geographic regions across the U.S., as results may vary by community-level factors.
Highlights.
In Black older adults perceived discrimination not associated with dementia risk
Younger age and greater education linked to greater perceived discrimination
Older age and less education among factors associated with dementia risk
Factors increasing exposure to discrimination (education) are also neuroprotective
Acknowledgements:
The authors thank the staff and participants of the ARIC study and JHS for their important contributions.
Funding Sources:
The Jackson Heart Study (JHS) is supported and conducted in collaboration with Jackson State University (HHSN268201800013I), Tougaloo College (HHSN268201800014I), the Mississippi State Department of Health (HHSN268201800015I) and the University of Mississippi Medical Center (HHSN268201800010I, HHSN268201800011I and HHSN268201800012I) contracts from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute on Minority Health and Health Disparities (NIMHD). The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (75N92022D00001, 75N92022D00002, 75N92022D00003, 75N92022D00004, 75N92022D00005). The ARIC Neurocognitive Study is supported by U01HL096812, U01HL096814, U01HL096899, U01HL096902, and U01HL096917 from the NIH (NHLBI, NINDS, NIA and NIDCD).
HED, JC, and KAW are supported by the National Institute on Aging Intramural Research Program. This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging. JAD is supported by the National Institutes of Health (Grant number: 3K01AG054693). RFG was supported by the NINDS Intramural Research Program.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
Disclaimer statement: The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Consent statement: All participants provided written informed consent at each study visit. For participants who were judged to lack capacity, proxies (usually next of kin or other family members) provided consent.
REFERENCES
- 1.Steenland K, Goldstein FC, Levey A, Wharton W. A meta-analysis of Alzheimer’s disease incidence and prevalence comparing African-Americans and Caucasians. Journal of Alzheimer’s Disease. 2016;50(1):71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matthews KA, Xu W, Gaglioti AH, et al. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged≥ 65 years. Alzheimer’s & Dementia. 2019;15(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes LL, Lewis T, Begeny C, Yu L, Bennett D, Wilson R. Perceived discrimination and cognition in older African Americans. Journal of the International Neuropsychological Society. 2012;18(5):856–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottesman RF, Fornage M, Knopman DS, Mosley TH. Brain aging in African-Americans: the atherosclerosis risk in communities (ARIC) experience. Current Alzheimer Research. 2015;12(7):607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zahodne LB, Sharifian N, Kraal AZ, et al. Socioeconomic and psychosocial mechanisms underlying racial/ethnic disparities in cognition among older adults. Neuropsychology. 2021;35(3):265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zahodne LB, Sol K, Kraal Z. Psychosocial pathways to racial/ethnic inequalities in late-life memory trajectories. The Journals of Gerontology: Series B. 2019;74(3):409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banks KH, Kohn-Wood LP, Spencer M. An examination of the African American experience of everyday discrimination and symptoms of psychological distress. Community mental health journal. 2006;42(6):555–570. [DOI] [PubMed] [Google Scholar]
- 8.Lewis TT, Williams DR, Tamene M, Clark CR. Self-reported experiences of discrimination and cardiovascular disease. Current cardiovascular risk reports. 2014;8(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sims M, Diez-Roux AV, Dudley A, et al. Perceived discrimination and hypertension among African Americans in the Jackson Heart Study. American journal of public health. 2012;102(S2):S258–S265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krieger N. Racial and gender discrimination: risk factors for high blood pressure? Social science & medicine. 1990;30(12):1273–1281. [DOI] [PubMed] [Google Scholar]
- 11.Turner AD, James BD, Capuano AW, Aggarwal NT, Barnes LL. Perceived stress and cognitive decline in different cognitive domains in a cohort of older African Americans. The American Journal of Geriatric Psychiatry. 2017;25(1):25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris KM, Halpern CT, Smolen A, Haberstick BC. The national longitudinal study of adolescent health (Add Health) twin data. Twin Research and Human Genetics. 2006;9(6):988–997. [DOI] [PubMed] [Google Scholar]
- 13.Lee RT, Perez AD, Boykin CM, Mendoza-Denton R. On the prevalence of racial discrimination in the United States. PloS one. 2019;14(1):e0210698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bleich SN, Findling MG, Casey LS, et al. Discrimination in the United States: experiences of black Americans. Health services research. 2019;54:1399–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berger M, Sarnyai Z. “More than skin deep”: stress neurobiology and mental health consequences of racial discrimination. Stress. 2015;18(1):1–10. [DOI] [PubMed] [Google Scholar]
- 16.Cuevas AG, Ong AD, Carvalho K, et al. Discrimination and systemic inflammation: A critical review and synthesis. Brain, behavior, and immunity. 2020;89:465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature reviews neuroscience. 2009;10(6):434–445. [DOI] [PubMed] [Google Scholar]
- 18.Ong AD, Williams DR. Lifetime discrimination, global sleep quality, and inflammation burden in a multiethnic sample of middle-aged adults. Cultural Diversity and Ethnic Minority Psychology. 2019;25(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thames AD, Hinkin CH, Byrd DA, et al. Effects of stereotype threat, perceived discrimination, and examiner race on neuropsychological performance: simple as black and white? Journal of the International Neuropsychological Society. 2013;19(5):583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coogan P, Schon K, Li S, Cozier Y, Bethea T, Rosenberg L. Experiences of racism and subjective cognitive function in African American women. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring 2020;12(1):e12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farfel JM, Barnes LL, Capuano A, Sampaio MCdM, Wilson RS, Bennett DA. Informant-Reported Discrimination, Dementia, and Cognitive Impairment in Older Brazilians. Journal of Alzheimer’s Disease. 2021;84(3):973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borrell LN, Kiefe CI, Williams DR, Diez-Roux AV, Gordon-Larsen P. Self-reported health, perceived racial discrimination, and skin color in African Americans in the CARDIA study. Social science & medicine. 2006;63(6):1415–1427. [DOI] [PubMed] [Google Scholar]
- 23.Stern Y Cognitive reserve. Neuropsychologia. 2009;47(10):2015–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beam CR, Kaneshiro C, Jang JY, Reynolds CA, Pedersen NL, Gatz M. Differences between women and men in incidence rates of dementia and Alzheimer’s disease. Journal of Alzheimer’s Disease. 2018;64(4):1077–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deckers K, Cadar D, van Boxtel MP, Verhey FR, Steptoe A, Köhler S. Modifiable risk factors explain socioeconomic inequalities in dementia risk: evidence from a population-based prospective cohort study. Journal of Alzheimer’s disease. 2019;71(2):549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petersen JD, Wehberg S, Packness A, et al. Association of socioeconomic status with dementia diagnosis among older adults in Denmark. JAMA network open. 2021;4(5):e2110432–e2110432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright JD, Folsom AR, Coresh J, et al. The ARIC (atherosclerosis risk in communities) study: JACC focus seminar 3/8. Journal of the American College of Cardiology. 2021;77(23):2939–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sempos CT, Bild DE, Manolio TA. Overview of the Jackson Heart Study: a study of cardiovascular diseases in African American men and women. The American journal of the medical sciences. 1999;317(3):142–146. [DOI] [PubMed] [Google Scholar]
- 29.Williams DR, Neighbors HW, Jackson JS. Racial/ethnic discrimination and health: Findings from community studies. American journal of public health. 2003;93(2):200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams DR, Yu Y, Jackson JS, Anderson NB. Racial differences in physical and mental health: Socio-economic status, stress and discrimination. Journal of health psychology. 1997;2(3):335–351. [DOI] [PubMed] [Google Scholar]
- 31.Krieger N, Sidney S. Racial discrimination and blood pressure: the CARDIA Study of young black and white adults. American journal of public health. 1996;86(10):1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sims M, Wyatt SB, Gutierrez ML, Taylor HA, Williams DR. Development and psychometric testing of a multidimensional instrument of perceived discrimination among African Americans in the Jackson Heart Study. Ethnicity & disease. 2009;19(1):56. [PMC free article] [PubMed] [Google Scholar]
- 33.Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: the atherosclerosis risk in communities neurocognitive study. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring. 2016;2:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiayaspathi A, Chen LY, Selvin E, et al. Relation of Diabetes Mellitus to Incident Dementia in Patients With Atrial Fibrillation (from the Atherosclerosis Risk in Communities Study). The American Journal of Cardiology. 2022;165:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, D.C.: American Psychiatric Publishing; 2013. [Google Scholar]
- 36.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia. 2011;7(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alonso A, Mosley T, Gottesman RF, Catellier D, Sharrett AR, Coresh J. Risk of dementia hospitalisation associated with cardiovascular risk factors in midlife and older age: the Atherosclerosis Risk in Communities (ARIC) study. Journal of Neurology, Neurosurgery & Psychiatry. 2009;80(11):1194–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sims M, Diez-Roux AV, Gebreab SY, et al. Perceived discrimination is associated with health behaviours among African-Americans in the Jackson Heart Study. J Epidemiol Community Health. 2016;70(2):187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang SY, Tan AS, Claggett B, et al. Longitudinal associations between income changes and incident cardiovascular disease: the Atherosclerosis risk in Communities study. JAMA cardiology. 2019;4(12):1203–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gutierrez A, Norby FL, Maheshwari A, et al. Association of abnormal P‐wave indices with dementia and cognitive decline over 25 years: ARIC‐NCS (The Atherosclerosis Risk in Communities Neurocognitive Study). Journal of the American Heart Association. 2019;8(24):e014553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaffey AE, Cavanagh CE, Rosman L, et al. Depressive symptoms and incident heart failure in the Jackson Heart Study: differential risk among black men and women. Journal of the American Heart Association. 2022;11(5):e022514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. [DOI] [PubMed] [Google Scholar]
- 43.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied psychological measurement. 1977;1(3):385–401. [Google Scholar]
- 44.Obaoye JO, Dawson AZ, Thakkar M, Williams JS, Egede LE. Understanding the relationship between perceived discrimination and mortality in United States adults. Aging & Mental Health. 2022:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barnes LL, De Leon CFM, Lewis TT, Bienias JL, Wilson RS, Evans DA. Perceived discrimination and mortality in a population-based study of older adults. American journal of public health. 2008;98(7):1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stepanikova I, Oates GR. Perceived discrimination and privilege in health care: the role of socioeconomic status and race. American journal of preventive medicine. 2017;52(1):S86–S94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barnes LL, De Leon CFM, Wilson RS, Bienias JL, Bennett DA, Evans DA. Racial differences in perceived discrimination in a community population of older blacks and whites. Journal of aging and health. 2004;16(3):315–337. [DOI] [PubMed] [Google Scholar]
- 48.Valenzuela MJ, Sachdev P. Brain reserve and dementia: a systematic review. Psychological medicine. 2006;36(4):441–454. [DOI] [PubMed] [Google Scholar]
- 49.Miller B, Rote SM, Keith VM. Coping with racial discrimination: Assessing the vulnerability of African Americans and the mediated moderation of psychosocial resources. Society and Mental Health. 2013;3(2):133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Braveman P, Egerter S, Williams DR. The social determinants of health: coming of age. Annual review of public health. 2011;32:381–398. [DOI] [PubMed] [Google Scholar]
- 51.Cooper DC, Mills PJ, Bardwell WA, Ziegler MG, Dimsdale JE. The effects of ethnic discrimination and socioeconomic status on endothelin-1 among blacks and whites. American journal of hypertension. 2009;22(7):698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zahodne LB, Sharifian N, Kraal AZ, et al. Longitudinal associations between racial discrimination and hippocampal and white matter hyperintensity volumes among older Black adults. Social Science & Medicine. 2023;316:114789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dunlay SM, Lippmann SJ, Greiner MA, et al. Perceived discrimination and cardiovascular outcomes in older African Americans: insights from the Jackson Heart Study. Elsevier; 2017:699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Troxel WM, Haas A, Dubowitz T, et al. Sleep Disturbances, Changes in Sleep, and Cognitive Function in Low-Income African Americans. J Alzheimers Dis. 2022;(Preprint):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pohl DJ, Seblova D, Avila JF, et al. Relationship between residential segregation, later-life cognition, and incident dementia across race/ethnicity. Int J Environ Res Public Health. 2021;18(21):11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams DR, Jackson PB. Social sources of racial disparities in health. Health Aff (Millwood). 2005;24(2):325–334. [DOI] [PubMed] [Google Scholar]
- 57.Tamura K, Orstad SL, Cromley EK, et al. The Mediating role of perceived discrimination and stress in the associations between neighborhood social environment and TV Viewing among Jackson Heart Study participants. SSM-population health. 2021;13:100760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Campbell J, Walker R, Garacci E, Dawson A, Williams J, Egede L. Relationship between adverse childhood experiences and perceived discrimination in adulthood. Journal of affective disorders. 2020;277:999–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kessler RC, Mickelson KD, Williams DR. The prevalence, distribution, and mental health correlates of perceived discrimination in the United States. Journal of health and social behavior. 1999:208–230. [PubMed] [Google Scholar]
- 60.U.S. Census Bureau. Race and Ethnicity in the United States. 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


