Abstract
In a previous study, we observed that monoclonal antibodies raised against the hook protein FlgE of Campylobacter jejuni LIO 36, isolate 5226, bound exclusively to this strain. The aim of this study was to elucidate the molecular basis for these binding specificities. The hook protein-encoding gene flgE of C. jejuni was cloned in Escherichia coli and sequenced. The flgE genes of four additional C. jejuni strains were amplified by PCR and also sequenced. Comparison of the deduced amino acid sequences revealed a high degree of variability in the central parts of the FlgE proteins among the strains, including variable and hypervariable domains. These findings may indicate a selective pressure of C. jejuni hosts, forcing the bacteria to generate variations in surface-exposed antigenic determinants.
Campylobacter jejuni is a common cause of food-borne human gastroenteritis. Motility of the bacterium is required for successful colonization of the intestinal tract and is therefore considered a major virulence determinant (15, 22). Flagella, the locomotory organelles of bacteria, are composed of three structural units: the filament, the hook, and the basal structure. The basal structure is anchored in the outer and inner membranes, whereas the hook and filament are located on the cell surface. The flagellar filament of Campylobacter has been extensively studied with regard to its genetics and its biochemical and immunological properties. In particular, the unsheathed flagellum is an immunodominant antigen (23, 30) and undergoes phase and antigenic variation (3, 10, 19). The flagellin protein of C. jejuni carries a terminal sialic acid (5, 7). This is so far the only description of a sialyl modification of bacterial flagellin. Two flagellin genes, flaA and flaB, are present on the C. jejuni genome and are known to be differentially expressed (8, 24).
Much less is known about the hook, which connects the filament to the basal body and functions as a joint to transmit the rotation of the rod of the basal body to the filament. In C. jejuni, in addition to the flagellin protein, a 92-kDa surface protein has been identified as exhibiting serological heterogeneity, and it has been speculated that this protein comprises the flagellar hook subunit (13, 20, 30). Power et al. (27) investigated purified hook proteins of C. jejuni and Campylobacter coli strains, which have molecular masses of 92.5 to 94 kDa. Immunochemical analysis with hook-specific antisera showed that serospecific epitopes were immunodominant (27). In addition, in a very recent study, Kinsella et al. (16) reported the cloning of the flgE gene from C. coli.
In a previous study in our laboratory, monoclonal antibodies (MAbs) were raised against the purified hook of C. jejuni LIO 36, clinical isolate 5226 (6). All of these MAbs bound exclusively to that strain, from which the hook had been isolated and used for immunization of mice. None of the MAbs reacted with any other tested C. jejuni LIO 36 strain. Likewise, the MAbs did not bind to any tested C. jejuni strain of other LIO serotypes (6). The aim of this study was to elucidate the molecular basis of this binding specificity. Therefore, we attempted to clone and express the flgE gene, which encodes the flagellar hook protein of C. jejuni, in Escherichia coli. To further investigate the antigenic variability of the hook protein, we sequenced the PCR-amplified flgE genes of different C. jejuni strains.
Cloning and expression in E. coli of the flgE gene from C. jejuni.
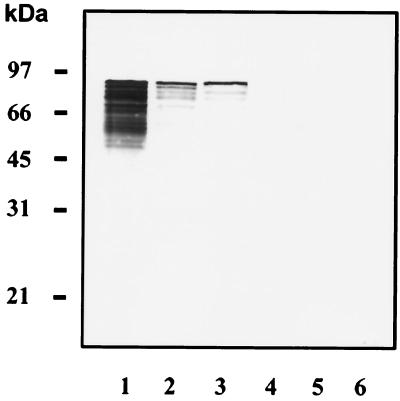
A genomic expression library was constructed by ligating chromosomal DNA fragments of 5 to 6 kb from C. jejuni LIO 36 (isolate 5226) into the expression vector pGEX-3X, which can be used to generate glutathione S-transferase fusion proteins. Screening of colonies for expression of the flgE gene was done with MAb 02B5 by colony blotting, performed essentially as described elsewhere (21). MAb 02B5 had been raised against the purified hook filament of C. jejuni LIO 36, isolate 5226 (6). The 02B5-reactive clone DH5α(pCAH32) was isolated and further analyzed. Western blot analysis revealed that a 92-kDa protein, identical to the size of the native C. jejuni hook protein, was synthesized in DH5α(pCAH32) (Fig. 1). Therefore, cloning of the full-length hook gene was assumed. As can be seen in Fig. 1, proteolytic degradation of the hook subunit protein occurs in C. jejuni as well as in the E. coli host strain.
FIG. 1.
Western blot analysis of native and recombinant C. jejuni hook protein. Bacterial cells (108) from fresh overnight cultures were loaded in each lane. By Ponceau S staining of nitrocellulose membranes, the presence of equal amounts of protein in all of the lanes was confirmed. Immunoblots were stained with MAb 02B5. Lanes: 1, E. coli DH5α(pCAH32) (Ptac promoter of pGEX-3X induced by IPTG [isopropyl-β-d-thiogalactopyranoside]); 2, E. coli DH5α(pCAH32) without IPTG; 3, C. jejuni LIO 36 (isolate 5226); 4, C. jejuni LIO 36 (isolate 9907); 5, C. jejuni LIO 36 (isolate 567); 6, C. jejuni LIO 36 (isolate 68).
Sequence analysis of plasmid pCAH32.
Sequence analysis of the 5.7-kb insert of plasmid pCAH32 revealed an open reading frame of 2,586 bp, which encoded an 862-amino-acid protein with a calculated molecular mass of 91,560 Da and an isoelectric point of 4.56. The calculated molecular mass corresponds well to the molecular mass of 92 kDa of the native and recombinant hook proteins as determined by Western blot analysis (Fig. 1).
Comparison of the C. jejuni flgE sequence with the 2,553-bp flgE gene of C. coli (16) revealed 78.5% identity. On the amino acid level, the identity was found to be 79% between the two species. The 2,154-bp flgE gene of the closely related species Helicobacter pylori (25) was found to exhibit 50.6% identity to the C. jejuni flgE sequence. The amino acid sequences of the FlgE proteins of C. jejuni and H. pylori showed 49.4% identity. The G+C content of the C. jejuni flgE coding sequence amounts to 38%. Six nucleotides upstream from the start codon is a potential Shine-Dalgarno sequence. Approximately 100 bases upstream from the start codon is a putative ς54 promoter sequence (GGAACAGAACTTGC) (4). A possible ς54 promoter has also been detected upstream of the H. pylori flgE gene (25), and for C. coli it has been shown that flgE, as well as flaB, is under the control of the alternative sigma factor ς54 (1, 16). The C. jejuni ς54 flgE promoter sequence is completely identical to that of C. coli (16).
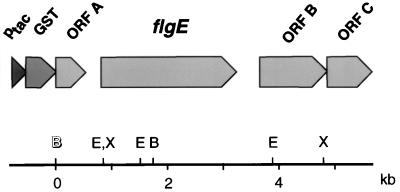
Preceding the 242-bp intergenic region upstream from flgE is an open reading frame (ORF A) with homology to the ruvC gene of E. coli (28). Downstream from the flgE gene are two further open reading frames, designated ORF B and ORF C. Following a 429-bp intergenic spacer downstream from flgE, which does not resemble any known transcriptional terminator sequence, is a 1,268-bp open reading frame (ORF B) oriented in the same direction of transcription. The deduced amino acid sequence of ORF B showed 43% identity to the 42.6-kDa methionine-gamma lyase megL of Pseudomonas putida (12). ORF C is incompletely present on plasmid pCAH32 and has homology to yeeE of E. coli, a hypothetical 38-kDa integral membrane protein of unknown function (2). A physical map of the 5.7-kb insert of pCAH32 is depicted in Fig. 2.
FIG. 2.
Physical map of the 5.7-kb insert of plasmid pCAH32. The location of the Ptac promoter and the glutathione S-transferase (GST) gene of the cloning vector pGEX-3X and the orientation of the insert are depicted. Restriction sites for the following endonucleases are noted: E, EcoRI; B, BamHI (open letter indicates cloning site); X, XmnI.
The N-terminal sequence of the C. jejuni FlgE protein determined in this study exhibited differences from the N-terminal sequence of C. jejuni VC74 serotype LIO 11, which has been defined by protein sequencing (27). Four of 17 amino acids (positions 6, 10, 11, and 15) were not identical.
Sequence determinations of flgE genes from different C. jejuni strains.
In order to elucidate the molecular basis for the binding characteristics of MAbs raised against the purified hook of C. jejuni LIO 36 (isolate 5226), we sequenced the flgE genes of different C. jejuni strains. All strains employed in this study are listed in Table 1. Using primers derived from the cloned C. jejuni flgE gene, we amplified the flgE gene of two further isolates of the LIO 36 serotype (isolates 2772 and 9907) as well as of one strain of the LIO 4 (isolate 5231) and LIO 7 (isolate 5232) serotypes, respectively. For variable regions, specific primers for PCR and sequencing were designed for each strain. The PCR fragments were purified and directly sequenced. Sequence data covering amino acid residues 102 to 736 were analyzed.
TABLE 1.
C. jejuni strains used in this study
| Serotype (isolate no.) | Source |
|---|---|
| LIO 36 (5226)a | W. Bär, Hannover, Germany |
| LIO 36 (9907) | J. Bockemühl, Hamburg, Germany |
| LIO 36 (567) | J. Bockemühl, Hamburg, Germany |
| LIO 36 (68) | J. Bockemühl, Hamburg, Germany |
| LIO 36 (2772) | J. P. Butzler, Brussels, Belgium |
| LIO 4 (5231) | W. Bär, Hannover, Germany |
| LIO 7 (5232) | W. Bär, Hannover, Germany |
Purified hook protein from this strain was used for immunization of mice in order to raise MAbs. Genomic DNA from this strain was employed to establish a C. jejuni expression library in E. coli.
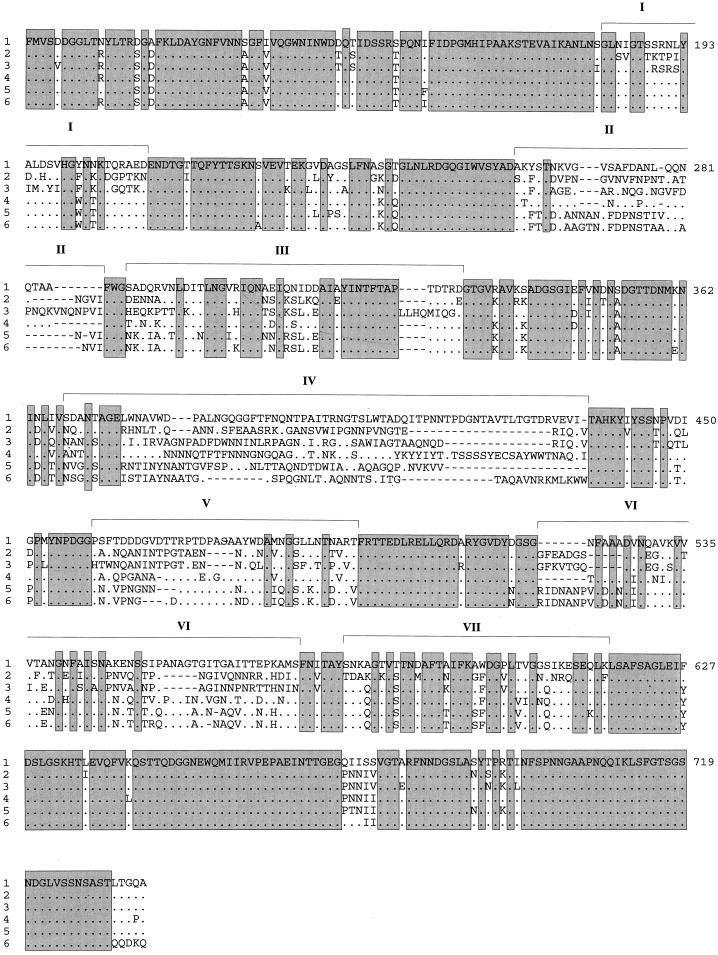
In Fig. 3, an alignment of the amino acid sequences as deduced from the nucleotide sequences of the five investigated C. jejuni strains, as well as of C. coli (16), is shown. Toward the N and C termini, there were highly conserved regions with only minor differences among all investigated Campylobacter strains. In contrast, in the central part of the hook protein, several regions with highly variable sequences were observed. In particular, seven distinct domains consisting of either semivariable (>20% sequence identity), variable (10 to 20% identity), or hypervariable stretches (<10% identity) were defined. These domains are separated by conserved sequences of various lengths. The variable domains (I through VII) are depicted in Fig. 3. Domains I, III, and VII are semivariable, with 31, 40, and 46% sequence identity, respectively. Variable domain VI exhibits 17% sequence identity. Among the hypervariable domains, II, IV, and V, domain IV comprises the largest block of completely variable amino acids. The sequence pattern of hypervariable domain IV indicates a mosaiclike arrangement.
FIG. 3.
Alignment of the amino acid sequences of FlgE hook proteins from different C. jejuni strains. 1, LIO 36 (isolate 5226 [sequence derived from the cloned flgE gene]); 2, LIO 36 (isolate 2772); 3, LIO 36 (isolate 9907); 4, LIO 4 (isolate 5231); 5, LIO 7 (isolate 5232); 6, C. coli. Identical amino acids are represented by dots. Dashes represent gaps introduced for optimal alignment. Boxed sequences indicate amino acids which are identical not only among C. jejuni strains but also to C. coli FlgE (16). Numbering of amino acids on the right refers to the cloned C. jejuni FlgE sequence.
Our data show that within the species C. jejuni, the hook protein exhibits immense differences in amino acid composition. Moreover, these differences were also observed among three strains belonging to one serotype (LIO 36) of C. jejuni. The majority of the amino acids that are conserved among C. jejuni strains are also conserved in C. coli (Fig. 3). The same holds true for the variable regions: in domains with high variability among C. jejuni strains, the C. coli sequence also differs from the C. jejuni sequences.
Moreover, apart from hypervariable domain IV, C. jejuni LIO 7 (isolate 5232) reveals an FlgE sequence that is generally nearly identical to that of C. coli. This finding might confirm that the division of these thermophilic campylobacters into the distinct species C. jejuni and C. coli is rather artificial.
Conclusions.
With a molecular mass in the range of 90 to 94 kDa, the hook protein of Campylobacter species is larger than that of any other bacterial hook known so far. The closely related species H. pylori and Helicobacter mustelae have FlgE proteins of 78 and 87 kDa, respectively (25), whereas the hook proteins from enterobacteria and spirochetes are considerably smaller, ranging from 42 to 50 kDa (2, 11, 14, 17, 18). The differences in size of hook subunit proteins from bacteria belonging to different genera have been discussed with regard to the requirement for motility in a viscous environment, such as the mucus in the gastrointestinal tract (26, 27). Nevertheless, the relation of hook protein size to hook function is still unclear.
The N-terminal region of the hook protein is required for its secretion, whereas the C-terminal region is necessary for assembly of the hook filament (16). The functional relevance of the N and C termini is reflected in their conserved amino acid composition. In contrast, the functional significance of the central part of the hook protein, whose sequence varies greatly among different species, is not known. In C. jejuni, the large central region of the hook protein exhibits hypervariability among strains of one species and even of one serotype. The variable and hypervariable domains are likely to be exposed to the surface, since immunoelectron microscopy reveals that the MAbs employed in our studies bind to the intact hook (6). The surface exposure of variable domains may reflect the need of C. jejuni to generate antigenic diversity. It is conceivable that C. jejuni responds to the selective pressure of its hosts by altering surface-exposed antigenic determinants. Variable structures may be of selective advantage especially in areas where C. jejuni is endemic and reinfections of hosts occur frequently. Host immunity acquired from prior infections is useless when novel antigens are exposed during reinfections. It remains unclear if there is any functional relevance of the variable region apart from the selective advantages it confers for antigenic diversity and immune escape. Conserved and variable domains are also features of the C. jejuni flaA and flaB genes, which encode flagellin. These two highly homologous genes are present in the Campylobacter genome, and variations at the loci encoding flagellin have been shown to occur via intragenomic and intergenomic recombination (9, 29). Horizontal gene transfer may be another mechanism of generating highly variable domains in the central part of the C. jejuni flgE gene.
Nucleotide sequence accession numbers.
The nucleotide sequence of the C. jejuni LIO 36, isolate 5226, flgE gene has been deposited in the EMBL database under accession no. AJ002074. The sequences of the PCR-amplified flgE genes from other C. jejuni strains have been deposited in the EMBL database under accession no. AJ224790 (LIO 36, isolate 2772), AJ224791 (LIO 36, isolate 9907), AJ224792 (LIO 4, isolate 5232), and AJ224793 (LIO 7, isolate 5231).
Acknowledgments
E.G.-C. was supported by a grant from the Deutscher Akademischer Austauschdienst.
REFERENCES
- 1.Alm R A, Guerry P, Trust T J. The Campylobacter ς54flaB flagellin promoter is subject to environmental regulation. J Bacteriol. 1993;175:4448–4455. doi: 10.1128/jb.175.14.4448-4455.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blattner F R, Plunkett G, Bloch C A, Perna N T, Burland V, Riley M, Colladovides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 3.Caldwell M B, Guerry P, Lee E C, Burans J P, Walker R I. Reversible expression of flagella in Campylobacter jejuni. Infect Immun. 1985;50:941–943. doi: 10.1128/iai.50.3.941-943.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coppard J R, Merrick M J. Cassette mutagenesis implicates a helix-turn-helix motif in promoter recognition by the novel RNA polymerase sigma factor sigma 54. Mol Microbiol. 1991;5:1309–1317. doi: 10.1111/j.1365-2958.1991.tb00777.x. [DOI] [PubMed] [Google Scholar]
- 5.Doig P, Kinsella N, Guerry P, Trust T J. Characterization of a post-translational modification of Campylobacter flagellin: identification of a sero-specific glycosyl moiety. Mol Microbiol. 1996;19:379–387. doi: 10.1046/j.1365-2958.1996.370890.x. [DOI] [PubMed] [Google Scholar]
- 6.Glenn-Calvo E, Bär W, Frosch M. Isolation and characterization of the flagellar hook of Campylobacter jejuni. FEMS Microbiol Lett. 1994;123:299–304. doi: 10.1016/0378-1097(94)90208-9. [DOI] [PubMed] [Google Scholar]
- 7.Guerry P, Doig P, Alm R A, Burr D H, Kinsella N, Trust T J. Identification and characterization of genes required for post-translational modification of Campylobacter coli VC167 flagellin. Mol Microbiol. 1996;19:369–378. doi: 10.1046/j.1365-2958.1996.369895.x. [DOI] [PubMed] [Google Scholar]
- 8.Guerry P, Logan S M, Thornton S, Trust T J. Genomic organization and expression of Campylobacter flagellin genes. J Bacteriol. 1990;172:1853–1860. doi: 10.1128/jb.172.4.1853-1860.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrington C S, Thomson-Carter F M, Carter P E. Evidence for recombination in the flagellin locus of Campylobacter jejuni: implications for the flagellin gene typing scheme. J Clin Microbiol. 1997;35:2386–2392. doi: 10.1128/jcm.35.9.2386-2392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris L A, Logan S M, Guerry P, Trust T J. Antigenic variation of Campylobacter flagella. J Bacteriol. 1987;169:5066–5071. doi: 10.1128/jb.169.11.5066-5071.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Homma M, DeRosier D J, Macnab R M. Flagellar hook and hook-associated proteins of Salmonella typhimurium and their relationship to other axial components of the flagellum. J Mol Biol. 1990;213:819–832. doi: 10.1016/S0022-2836(05)80266-9. [DOI] [PubMed] [Google Scholar]
- 12.Inoue H, Inagaki K, Sugimoto M, Esaki N, Soda K, Tanaka H. Structural analysis of the l-methionine gamma-lyase gene from Pseudomonas putida. J Biochem (Tokyo) 1995;117:1120–1125. doi: 10.1093/oxfordjournals.jbchem.a124816. [DOI] [PubMed] [Google Scholar]
- 13.Jin T, Penner J L. Role of the 92.5-kilodalton outer membrane protein of Campylobacter jejuni in serological reactions. J Clin Microbiol. 1988;26:2480–2483. doi: 10.1128/jcm.26.12.2480-2483.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jwang B, Dewing P, Fikrig E, Flavell R A. The hook protein of Borrelia burgdorferi, encoded by the flgE gene, is serologically recognized in Lyme disease. Clin Diagn Lab Immunol. 1995;2:609–615. doi: 10.1128/cdli.2.5.609-615.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ketley J M. Pathogenesis of enteric infection by Campylobacter. Microbiology. 1997;143:5–21. doi: 10.1099/00221287-143-1-5. [DOI] [PubMed] [Google Scholar]
- 16.Kinsella N, Guerry P, Cooney J, Trust T J. The flgE gene of Campylobacter coli is under the control of the alternative sigma factor ς54. J Bacteriol. 1997;179:4647–4653. doi: 10.1128/jb.179.15.4647-4653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Ruby J, Charon N, Kuramitsu H. Gene inactivation in the oral spirochete Treponema denticola: construction of an flgE mutant. J Bacteriol. 1996;178:3664–3667. doi: 10.1128/jb.178.12.3664-3667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Limberger R J, Slivienski L L, Samsonoff W A. Genetic and biochemical analysis of the flagellar hook of Treponema phagedenis. J Bacteriol. 1994;176:3631–3637. doi: 10.1128/jb.176.12.3631-3637.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Logan S M, Guerry P, Rollins D M, Burr D H, Trust T J. In vivo antigenic variation of Campylobacter flagellin. Infect Immun. 1989;57:2583–2585. doi: 10.1128/iai.57.8.2583-2585.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Logan S M, Trust T J. Molecular identification of surface protein antigens of Campylobacter jejuni. Infect Immun. 1983;42:675–682. doi: 10.1128/iai.42.2.675-682.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer T F, Mlawer N, So M. Pilus expression in Neisseria gonorrhoeae involves chromosomal rearrangement. Cell. 1982;30:45–52. doi: 10.1016/0092-8674(82)90010-1. [DOI] [PubMed] [Google Scholar]
- 22.Morooka T, Umeda A, Amako K. Motility as an intestinal colonization factor for Campylobacter jejuni. J Gen Microbiol. 1985;131:1973–1980. doi: 10.1099/00221287-131-8-1973. [DOI] [PubMed] [Google Scholar]
- 23.Nachamkin I, Yang X H. Human antibody response to Campylobacter jejuni flagellin protein and a synthetic N-terminal flagellin peptide. J Clin Microbiol. 1989;27:2195–2198. doi: 10.1128/jcm.27.10.2195-2198.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nuijten P J, van Asten F J, Gaastra W, van der Zeijst B A. Structural and functional analysis of two Campylobacter jejuni flagellin genes. J Biol Chem. 1990;265:17798–17804. [PubMed] [Google Scholar]
- 25.O’Toole P W, Kostrzynska M, Trust T J. Non-motile mutants of Helicobacter pylori and Helicobacter mustelae defective in flagellar hook production. Mol Microbiol. 1994;14:691–703. doi: 10.1111/j.1365-2958.1994.tb01307.x. [DOI] [PubMed] [Google Scholar]
- 26.Ottemann K M, Miller J F. Roles for motility in bacterial host interactions. Mol Microbiol. 1997;24:1109–1117. doi: 10.1046/j.1365-2958.1997.4281787.x. [DOI] [PubMed] [Google Scholar]
- 27.Power M E, Alm R A, Trust T J. Biochemical and antigenic properties of the Campylobacter flagellar hook protein. J Bacteriol. 1992;174:3874–3883. doi: 10.1128/jb.174.12.3874-3883.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahagi M, Iwasaki H, Nakata A, Shinagawa H. Molecular analysis of the Escherichia coli ruvC gene, which encodes a Holliday junction-specific endonuclease. J Bacteriol. 1991;173:5747–5753. doi: 10.1128/jb.173.18.5747-5753.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wassenaar T M, Fry B N, van der Zeijst B A. Variation of the flagellin gene locus of Campylobacter jejuni by recombination and horizontal gene transfer. Microbiology. 1995;141:95–101. doi: 10.1099/00221287-141-1-95. [DOI] [PubMed] [Google Scholar]
- 30.Wenman W M, Chai J, Louie T J, Goudreau C, Lior H, Newell D G, Pearson A D, Taylor D E. Antigenic analysis of Campylobacter flagellar protein and other proteins. J Clin Microbiol. 1985;21:108–112. doi: 10.1128/jcm.21.1.108-112.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]