Abstract
Intramuscular injection of long-acting cabotegravir and rilpivirine is a novel, long-acting antiretroviral therapy (ART) combination approved for use as a fully suppressive regimen for people living with HIV. Long-acting cabotegravir with rilpivirine ART has reduced required dosing frequency from once daily to once every month or every 2 months injections. This new era of long-acting ART, which includes other antiretrovirals and formulations in various stages of clinical development, holds tremendous promise to change the standard of HIV treatment. Although long-acting ART has high potential to be revolutionary in the landscape of HIV care, prevention, and treatment cascade, more data are needed to substantiate its efficacy and cost-effectiveness among patients at risk of non-adherence and across age groups, pregnancy, and post partum. Advocacy efforts and policy changes to optimise a sustained, high-quality, equitable reach of long-acting ART, especially in low-income and middle-income countries where most people living with HIV reside, are needed to realise the full benefits of long-acting ART.
Introduction
Although there are several highly effective options for once-daily oral antiretroviral therapy (ART) for people living with HIV, many individuals struggle with adherence because of complex behavioural, cognitive, and structural barriers, including the characteristics of HIV therapies and drug delivery.1,2 Long-acting ART offers a novel and promising therapeutic approach that could provide a much-needed alternative strategy both to treat and to prevent HIV. Long-acting ART can benefit patients who struggle with adherence because of side-effects, pill fatigue, pill aversion, or stigma. For example, injections could help to mitigate privacy concerns by reducing the risk of unintended HIV disclosure outside of a clinical setting and the associated stigma that can occur with obtaining and taking daily oral antiretrovirals.3
One long-acting ART regimen is available for HIV treatment: separate intramuscular injections of long-acting cabotegravir, an integrase strand transfer inhibitor, and long-acting rilpivirine (a non-nucleoside reverse transcriptase inhibitor).4 The combination of long-acting cabotegravir plus long-acting rilpivirine is approved for the treatment of non-pregnant adolescents and adults with viral suppression below the limits of HIV RNA detection in plasma. Long-acting cabotegravir is also approved for HIV prevention as pre-exposure prophylaxis (PrEP). Several other long-acting antiretrovirals are available or in development for both treatment and prevention (table 1). The monoclonal antibody ibalizumab-uiyk (administered as an intravenous infusion or intravenous push every two weeks) and the HIV capsid inhibitor lenacapavir (administered subcutaneously every six months after an oral lead-in period) are both approved in some high-income settings. Both ibalizumab-uiyk and lenacapavir must be used in combination with other oral antiretrovirals to form an optimised regimen for heavily treatment-experienced individuals with multidrug-resistant HIV. Lenacapavir is also being studied in clinical trials of initial ART, in combination with other antiretrovirals, and in HIV prevention trials as PrEP. Islatravir, a nucleoside reverse transcriptase translocation inhibitor, will be studied in combination with lenacapavir, both as once-weekly oral formulations. Intravenously administered broadly neutralising antibodies are also in phase 1 and 2 clinical trials as potential long-acting options for both HIV treatment and PrEP.
Table 1:
Long-acting antiretrovirals in development or in clinical use
| Formulation | Development stage | Approved dose | |
|---|---|---|---|
| Entry inhibitors | |||
| Albuvirtide | Intravenous infusion | Approved for marketing in China | Loading dose of 320 mg per day for 3 days and maintenance dose of 320 mg per week; dosed in combination with optimised background ART |
| Combinectin (GSK3732394) | Subcutaneous injection | Phase 1 | .. |
| Broadly neutralising antibodies * | |||
| Ibalizumab-uiyk | Intravenous infusion or intravenous push | Approved for marketing by regulatory authorities in high-income countries | Loading dose of 2000 mg and maintenance dose 800 mg twice per week; dosed in combination with optimised background ART |
| Leronlimab (PRO 140) | Subcutaneous injection | Phase 2 and 3 | .. |
| VRC01 and VRC01LS | Intravenous infusion or subcutaneous injection | Phase 2 and 3 | .. |
| VRC07 and VRC07-523LS | Subcutaneous injection | Phase 2/3 | .. |
| Semzuvolimab (UB-421) | Intravenous infusion | Phase 2 and 3 | .. |
| Elipovimab (GS-9722) | Intravenous infusion | Phase 1 | .. |
| Nucleoside reverse transcriptase translocation inhibitor | |||
| Islatravir (MK-8591) | Oral and implant | Phase 2 | .. |
| Non-nucleoside reverse transcriptase inhibitors | |||
| Rilpivirine† | Intramuscular injection | Approved for marketing by regulatory authorities in high-income countries | Once every month regimen: loading dose of 900 mg and maintenance dose of 600 mg monthly; once every 2 months regimen: loading dose of 900 mg per month for 2 months and maintenance dose 900 mg once every 2 months; dosed with cabotegravir |
| Integrase strand transfer inhibitor | |||
| Cabotegravir† | Intramuscular injection | Approved for marketing by regulatory authorities in high-income countries | Once per month regimen with rilpivirine: loading dose of 600 mg and maintenance dose of 400 mg once per month; once every 2 months regimen with rilpivirrine or alone as PrEP: loading dose of 600 mg per month for 2 months and maintenance dose of 600 mg once every 2 months |
| Capsid inhibitors | |||
| Lenacapavir (GS-6207) | Subcutaneous injection and oral | Approved for marketing by regulatory authorities in high-income countries | Oral initiation of 600 mg on day 1 and day 2, 300 mg on day 8; subcutaneous maintenance of 927 mg beginning day 15 and then once every 6 months |
PrEP=pre-exposure prophylaxis.
Rapidly expanding research field with various broadly neutralising antibodies in preclinical phases.
Optional oral lead-in before initiation of long-acting antiretrovirals.
Long-acting drugs for the treatment and prevention of other conditions have shown improved adherence compared with treatments that require more frequent administration, leading to better outcomes and retention in care.5 However, more data are needed about how long-acting ART might help people with HIV who are at risk for non-adherence outside the structure of clinical trials. Here, we outline the potential advantages and disadvantages of long-acting ART, with an emphasis on the first long-acting ART regimen containing cabotegravir and rilpivirine (panel). We also discuss the current evidence and identify gaps in knowledge to support the use of long-acting ART in pregnant women, adolescents, and children.
Clinical overview of long-acting ART from an adherence perspective
Phase 3 clinical trials of once every month or once every two months intramuscular administration of long-acting cabotegravir and rilpivirine ART showed non-inferiority to daily oral three-drug ART and sustained viral suppression for 124 weeks in patients who were virologically suppressed at the time of their first injections (table 2).7,9,11,12 In clinical trials, any dose given during the 7 day period before or after the planned injection visit was considered an on-time injection. In a pooled analysis of monthly long-acting cabotegravir and rilpivirine through week 48 in the ATLAS and FLAIR trials, high adherence to this dosing window was observed, with 98% of participants receiving the injection within 7 days of the scheduled visit.13 A strategy examining long-acting cabotegravir and rilpivirine given every 2 months (ATLAS-2M trial) also showed high rates of virological suppression at 96 weeks.8 A pooled analysis of FLAIR, ATLAS, and ATLAS-2M showed that virological failure occurred in only 1·4% of individuals.14 Although a high rate of adherence is typical in clinical trials, outcomes from the real-world implementation data of long-acting ART in a more diverse patient population are needed.
Table 2:
Phase 3 trials of cabotegravir and rilpivirine for the treatment of HIV6
| Participant characteristics | Regimens | Primary endpoint* difference (95% CI) | Final published data* difference | |
|---|---|---|---|---|
| ATLAS7,8 | Virologically suppressed, ART-experienced adults with HIV | Oral PI, NRTI, or INSTI with a two-NRTI backbone vs oral lead-in of cabotegravir 30 mg/day plus rilpivirine 25 mg/day for 4 weeks, followed by 600 mg of long-acting cabotegravir intramuscularly plus 900 mg long-acting rilpivirine intramuscularly once on week 4, followed by 400 mg long-acting cabotegravir intramuscularly plus 600 mg long-acting rilpivirine intramuscularly every 4 weeks beginning at week 8 | Week 48: 0·6% (−1·2 to 2·5) | Week 96: 100% (23 of 23) in long-acting arm and 97% (28 of 29) in switch arm had HIV-1 RNA <50 copies per mL† |
| ATLAS-2M9,10 | Virologically suppressed (HIV-1 RNA <50 copies per mL) participants who completed the ATLAS trial | Long-acting cabotegravir 400 mg intramuscularly plus long-acting rilpivirine 600 mg intramuscularly every 4 weeks vs long-acting cabotegravir 600 mg intramuscularly plus long-acting rilpivirine 900 mg intramuscularly every 8 weeks | Week 48: 0·8% (−0·6 to 2·2) | Week 124: 1·0% (95% CI −0·6 to 2·4) |
| FLAIR11,12 | ART-naive adults with HIV | All participants inducted on oral dolutegravir, abacavir, and lamivudine daily for 20 weeks then randomly assigned to continue or switch to oral lead-in of cabotegravir 30 mg daily plus rilpivirine 25 mg daily for 4 weeks followed by long-acting cabotegravir 600 mg intramuscularly plus long-acting rilpivirine 900 mg intramuscularly once at week 4 followed by long-acting cabotegravir 400 mg intramuscularly plus long-acting rilpivirine 600 mg intramuscularly every 4 weeks beginning at week 8 | Week 48: −0·4% (−2·8 to 2·1) | Week 124: 450 (82%) of 551 had HIV-1 RNA <50 copies per mL‡ |
INSTI=integrase strand transfer inhibitor. NRTI=nucleoside or nucleotide reverse transcriptase inhibitor. NNRTI=non-nucleoside reverse transcriptase inhibitor. PI=protease inhibitor. All trials were phase 3, randomised, multicentre, open-label, non-inferiority trials.
Endpoint was HIV-1 RNA of 50 copies per mL or less, unless indicated.
52 participants continued long-acting therapy (long-acting arm) or were randomised to oral ART but who chose to switch to long-acting therapy (switch arm); these participants were included in the week 96 analysis for which the primary endpoint was the proportion of patients with HIV-1 RNA <50 copies per mL.
At week 100, participants originally assigned to the oral therapy comparator group were offered the option to switch to long-acting cabotegravir with rilpivirine; 111 chose a direct-to-inject option without an oral lead-in whereas 121 opted for the oral lead-in, 110 in the direct-to-inject group (99%; 95% CI 97–100%) and 113 in the oral lead-in group (93%; 95% CI 89–98%) had an HIV-1 RNA of less than 50 copies per mL at week 124.
Outside of clinical trials, four reports are available to describe the implementation of monthly long-acting ART in clinical settings. 35 patients accessed long-acting cabotegravir and rilpivirine ART in a compassionate use programme in Europe, North America, and South Korea. 28 (80%) participants were not virologically suppressed at the start of the trial, and 15 (43%) were enrolled due to oral antiretroviral adherence barriers, including difficulty with swallowing pills, pill fatigue, and stigma.15 After a median of 10 months (range 1–47 months) of follow-up, 22 (63%) of 35 participants were virologically suppressed (HIV RNA <50 copies per mL). Of the 13 participants who were not suppressed, three (23%) had not been enrolled in the programme for long enough to achieve viral suppression and seven (54%) switched therapy because of virological failure. Participant characteristics that predisposed individuals to virological failure included high BMI and pre-existing non-nucleoside reverse transcriptase inhibitor or integrase strand transfer inhibitor resistance mutations. The proportion of people who received on-time injections was not reported.
Outcomes were reported from 39 patients in San Francisco (CA, USA) who initiated long-acting cabotegravir with rilpivirine ART, including 15 participants with detectable HIV RNA at initiation (mean log10 viral load 4·68, SD 1·16).16 Of these 15 participants, 12 (80%; 95% CI 55–93) achieved viral suppression after six (range 3–11) once-monthly injections, and the remaining three had a 2-log decline in plasma HIV RNA after a median of 22 days from initiation of long-acting ART. Of the 24 patients who were already virologically suppressed at long-acting ART initiation, all patients (100%; 95% CI 86–100) remained suppressed after six (range 2–8) once-monthly injections. On-time injection visits were reported for 35 patients (89%; 95% CI 73–94). In an updated analysis, of 41 patients who started on long-acting cabotegravir and rilpivirine with viraemia (mean log10 viral load 4·22, SD 1·33), 98% achieved virological suppression by a median of 41 days.17 In the larger US-based OPERA cohort, 383 patients received their first injection of long-acting cabotegravir and rilpivirine between January, 2021, and February, 2022.18 Of those, 273 (71%) had recorded virological outcomes by October, 2022. Of 252 who started with virological suppression, 249 (99%) remained virally suppressed after switching to long-acting ART; of the 21 patients who started without virological suppression, 19 (90%) achieved virological suppression.
Collins and colleagues19 reported outcomes for their first 15 patients after a median of six (quartile 1 3·5, quartile 3 7) once-monthly injections of long-acting cabotegravir and rilpivirine in Atlanta (GA, USA).19 14 patients remained virologically suppressed (HIV RNA <200 copies per mL) 3 months after long-acting ART initiation, but one patient with data at 6 months had an HIV RNA concentration of 750 copies per mL, despite no missed or delayed injections, and subsequently switched back to oral ART. Another patient, who initiated long-acting cabotegravir and rilpivirine with a viral load of 767 copies per mL resulting from difficulty with oral therapy (ie, severe malabsorption), did not suppress on long-acting ART after 6 months (HIV RNA 689 copies per mL) despite no delayed or missed injections.
Data on injections of long-acting cabotegravir and rilpivirine given every 2 months as a switch strategy are available from a phase 3b, open-label, implementation-effectiveness trial in 18 clinical centres in Europe (CARISEL)20 and a German multicentre cohort study (CARLOS).21 Among 430 participants in CARISEL, 87% (95% CI 83·2–89·8%) maintained virological suppression, 0·7% had virological failure (HIV RNA ≥50 copies per mL; CI 95% 0·1–2%), and 13% had no virological data available up to month 12.20 Of 2376 injections, most (99%) occurred before or within the 7-day dosing window, 1% occurred after the dosing window, and only 22 injections were missed. Among 200 patients included in a CARLOS analysis, 89·5% were virologically suppressed after 6 months on long-acting ART.21 Of 633 injection visits, 18 (2·8%) occurred late (>7 days after scheduled injection).
Although long-acting ART addresses several challenges associated with daily oral pill regimens (including the daily reminder of living with HIV, potential unintended disclosure of HIV status, stigma of being seen taking medication, side-effects, or challenges related to daily ingestion of tablets or pills) new challenges could emerge regarding retention in care, adherence to more frequent injection visits, or anxiety around injections. On the basis of phase 3 trials and emerging data from the implementation of long-acting cabotegravir and rilpivirine ART, adherence to the injection visits is generally high. The currently approved product labelling requires Z-track intragluteal injections in a health-care setting. Frequent visits, even if monthly or less frequently, to the clinic during typical business hours remain challenging for some patients and, importantly, could limit access to long-acting ART for patients who have caregiving responsibilities, inflexible work schedules, long commutes, or other transportation barriers. Clinical monitoring systems to follow up delayed or missed injections visits should be established to help identify patients who need to be engaged, retained, or re-engaged in care. Evidence indicates that similar pharmacokinetic results can be achieved by administering long-acting cabotegravir and long-acting rilpivirine in the lateral thigh, an approach that could allow self-administration or expand the number of locations at which injections could be administered.22 Although self-administered long-acting ART could be beneficial for global implementation, it also eliminates the adherence monitoring benefits of long-acting ART administration during clinic visits and thus warrants further implementation research to maximise adherence to self-administered long-acting ART.
Low-income and middle-income countries must have access to long-acting ART, which involves supply, affordability, and appropriate infrastructure (eg, storage and distribution). With access, adherence can be monitored objectively, but systems will need to be developed and refined to allow this.19 Although WHO has not yet endorsed long-acting cabotegravir and rilpivirine ART for low-income and middle-income countries, we believe that the expansion of this regimen into these regions could help to address stigma and some barriers to adherence. ViiV Healthcare and the Medicines Patent Pool signed a new voluntary licensing agreement for patents relating to long-acting cabotegravir23 that allows it to be produced by generic manufacturers for less than US$3·00 per vial.24
Emergence of drug resistance
Parenteral long-acting ART offers adherence advantages over daily oral ART; however, drug resistance can become problematic even if doses are taken on schedule. Treatment failure with new resistance-associated mutations occurred in only 1–1·5% of participants who received the long-acting cabotegravir and rilpivirine ART, with a latest pooled virological failure rate of 1·4% across 152 weeks across three phase 3 clinical trials.14 All six participants who had confirmed virological failure in the ATLAS and FLAIR studies had new phenotypic resistance to cabotegravir, rilpivirine, or both. Two participants with confirmed virological failure during the first 48 weeks of the two studies developed resistance to rilpivirine alone, one to cabotegravir alone, and three to both drugs, although the fold-change in sensitivity was moderate (2–4 to 9·4-fold).7,11
There were 13 cases of confirmed virological failure (1·25%) among 1039 adults who received long-acting cabotegravir–rilpivirine ART as part of phase 2 and 3 clinical trials.25 The presence of rilpivirine-resistance mutations in proviral DNA, HIV-1 subtype A6 or A1, high BMI, and lower week 8 rilpivirine trough concentrations were all significantly associated with risk of unsuccessful treatment.25 Although nearly all participants received their week-4 or week-8 injections on time, nine of 13 who developed confirmed virological failure had plasma concentrations of cabotegravir and rilpivirine that were less than the median, and seven had week-8 plasma trough concentrations of both drugs in the lowest quartile.25 We theorise that in patients with BMIs over 30, associations with unsuccessful treatment might result from difficulties with intramuscular administration of the cabotegravir and rilpivirine nanosuspensions. Drug administration to the muscle could be improved with longer needles (≥2 inches, instead of the 1·5 inch needle provided with the drug product) to yield the desired intramuscular depot. However, pharmacokinetic analyses suggest that although cabotegravir concentrations were lower in patients with high BMI, rilpivirine concentrations did not differ by BMI, potentially because other factors (including lipophilicity) influenced absorption from the depot compartment.26,27 Finally, although low week-8 cabotegravir concentrations were associated with high BMI, concentrations of cabotegravir and rilpivirine were overlapping for the high and low BMI groups by week-16 and beyond,25 suggesting that risk of confirmed virological failure related to low drug concentrations might be higher during the first 4 months of treatment.
In prevention trials with injectable long-acting cabotegravir PrEP, incident HIV in women receiving long-acting cabotegravir PrEP in the HIV Prevention Trials Network (HPTN) 084 study were exceedingly rare (three of 1614, 0·2%); cases occurred only in the setting of low or undetectable cabotegravir plasma concentrations and without detectable cabotegravir resistance.28 In participants who acquired HIV despite adherence to all injection visits in the HPTN 083 study, HIV resistance to integrase strand transfer inhibitors occurred in five cabotegravir recipients who had genotyping data available.29 All five participants received at least one cabotegravir injection after they acquired HIV but before the virus was detected, and three participants retained phenotypic susceptibility to dolutegravir and bictegravir.29 There was no consistent profile of cabotegravir-resistance mutations among the five cases. Because treatment with long-acting cabotegravir as PrEP seems partly to suppress replication of HIV resistant to integrase strand transfer inhibitors and can delay diagnosis, individuals at risk are given the recommendation to monitor HIV-RNA at each injection visit.30 Unfortunately, this strategy could be cost-prohibitive in low-income and middle-income countries.
Treatment-emergent resistance to long-acting lenacapavir has developed during trials in patients with virological failure on ART and drug resistance before addition of long-acting lenacapavir. Identified drug-resistant mutations exhibit reduced replication capacity in vitro.31 In the phase 3 CAPELLA trial in heavily treatment-experienced patients, new lenacapavir resistance was detected in eight (11%) of 72 patients receiving long-acting lenacapavir plus an optimised background regimen.32 In all cases, this turned out to be a consequence of unintended lenacapavir monotherapy resulting from inadequate sensitivity of the patients’ virus to the background regimen.32 Monotherapy with broadly neutralising antibodies to HIV is strongly associated with emergence of resistance, which can result from a virus with pre-existing resistance or mutations in the HIV envelope that can rapidly evolve with incomplete viral suppression.33 To effectively treat and prevent HIV, broadly neutralising antibodies will likely have to be administered in combination or with a long-acting antiretroviral small molecule.
Managing the clinical challenges of the pharmacokinetic tail
The prevailing hypothesis is that the emergence of new drug resistance in patients being treated with ART or a single-drug oral regimen for PrEP is a consequence, at least in part, of suboptimal concentrations of one or more drugs within the regimen, which leads to virological failure or breakthrough infection. As a long-acting formulation is designed to sustain effective concentrations for several months after injection, on-time drug administration at the end of each dosing interval is required to avoid the long pharmacokinetic tail with subtherapeutic concentrations (figure).34 Cabotegravir concentrations were detected a median of 43·7 after the last injection in male participants and 67·3 weeks in female participants. In the HIV treatment and prevention setting, the greatest impediment to successful use of long-acting antiretroviral has been management of this tail. The half-life of long-acting rilpivirine exceeds that of long-acting cabotegravir (13–28 weeks vs 6–12 weeks, respectively);4 therefore, a missed dose of the combination regimen can result in prolonged rilpivirine monotherapy.
Figure: Individual participant log-linear regression curves of plasma cabotegravir concentrations using time between the maximum measured concentration and the last quantifiable concentration after the last injection by sex assigned at birth.
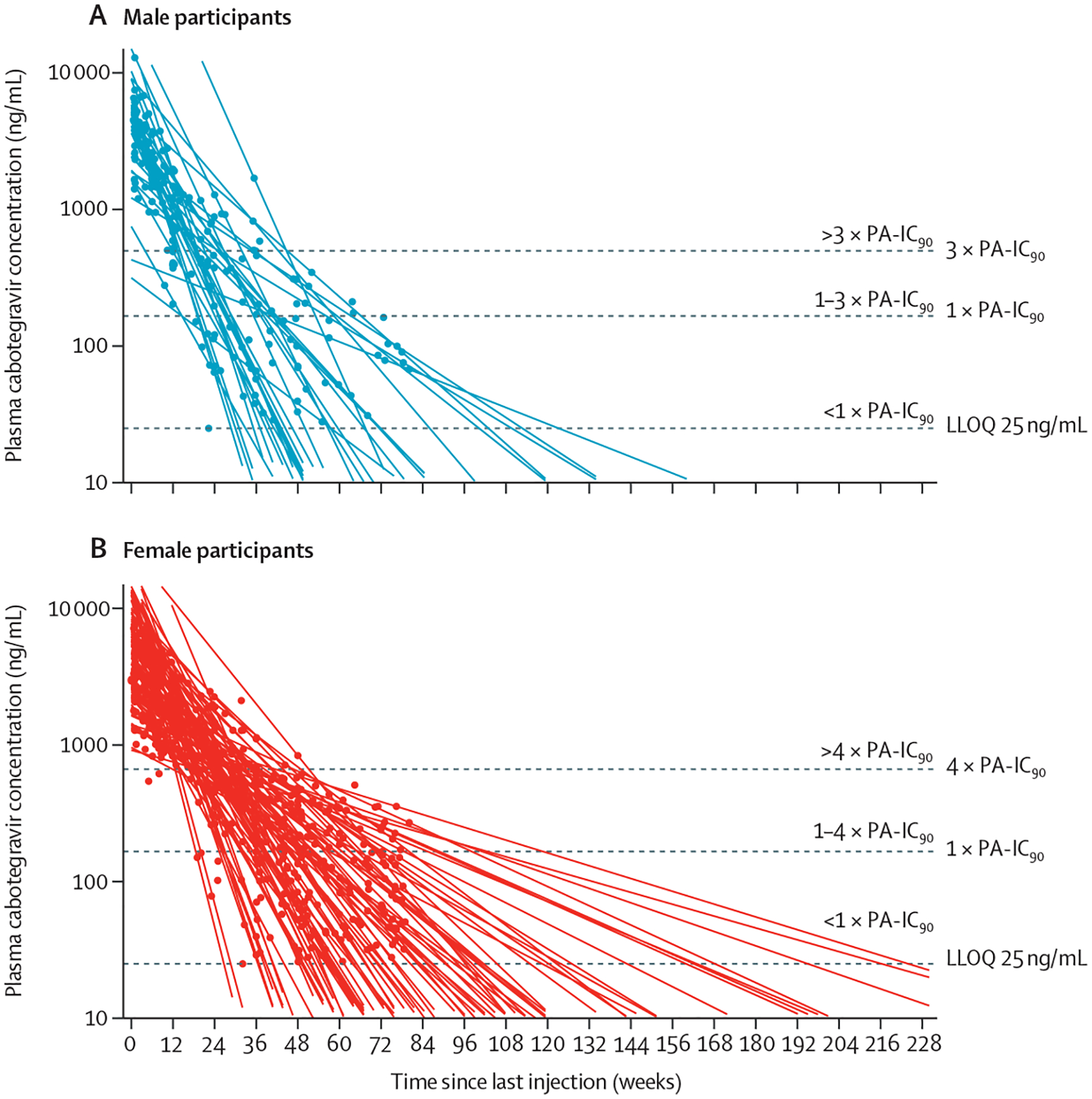
Male participants (A). Female participants (B). Curves were fitted for each individual extrapolated to the intersection with LLOQ and all extended beyond the observed concentrations. Dots represent individual participants’ values based on days elapsed since the last injection. Horizontal dashed lines show estimates of protection based on the simian-human immunodeficiency virus challenge model indicating proportion of rectal or vaginal challenges protected for males and females, respectively. LLOQ=lower limit of quantification. PA-IC₉₀=protein-binding-adjusted 90% inhibitory concentration. Reproduced from Landovitz and colleagues,34 by permission of the authors.
When long-acting cabotegravir and rilpivirine ART is administered every 8 weeks rather than every 4 weeks, the number of patients who had unsuccessful treatment with resistance and subsequent drug resistance increased, but these differences are not statistically significant. In the ATLAS-2M study, after 96 weeks of treatment with long-acting cabotegravir and rilpivirine, only two (>1%) of 523 participants randomised to the 4-week arm had confirmed virological failure, compared with nine (2%) of 522 in the 8-week arm.10 Patients receiving long-acting cabotegravir and rilpivirine ART or long-acting cabotegravir for PrEP who must miss a scheduled injection by more than 7 days should receive daily oral therapy to bridge treatment until the long-acting therapy can be resumed. When discontinuing long-acting cabotegravir and rilpivirine ART or long-acting cabotegravir for PrEP in patients at ongoing risk for HIV exposure, patients should switch to oral therapy within 7 days of the next scheduled dose.4,35 Due to the slow elimination of cabotegravir, oral therapy must continue for at least one year.4,35 To achieve sustained viral suppression and mitigate the risk of resistance due to slow elimination of these drugs, effort should be made to ensure patients are retained in care. For patients who are lost to follow-up while receiving long-acting therapy, additional clinical assessments are required before reinitiating ART, long-acting ART, or PrEP.1,4,35
Treatment in pregnancy and post partum
ART adherence for women and other people with HIV during pregnancy and post partum is crucial to both promote maternal health and prevent HIV transmission, yet studies have shown a substantial proportion of pregnant and post partum women with HIV across different income-level countries face challenges with maintaining optimal ART adherence.36–38 Suboptimal ART adherence is associated with rebound viraemia, leading to enhanced risk of perinatal HIV transmission during pregnancy and delivery or through breastfeeding.39 The few randomised trials evaluating the effects of behavioural or SMS interactive interventions on post-partum retention and ART adherence among women on lifelong ART have shown no benefit.40–42 Although antenatal care and ART integration, family-centred approaches, and the use of lay health-care providers were effective in increasing service uptake and retention of mothers and their infants in HIV programmes, these data were limited by the absence of control groups.43 Long-acting ART has the potential to improve ART adherence and outcomes substantially and sustainably, especially among people with HIV who struggle with adherence during pregnancy and post partum.44 Current and future long-acting antiretroviral options approved by the US Food and Drug Administration offer alternatives to once or twice daily administration of oral ART that could prevent barriers related to pill burden, daily adherence, and inadvertent HIV status disclosure and associated stigma in this vulnerable population.44–47 Pill burden and pill aversion are especially relevant in pregnancy, as nausea and vomiting can further complicate tolerance of oral regimens.
To date, there are minimal data from pregnant or post partum people with HIV initiating or continuing long-acting ART. Prospective evaluation in pregnancy is crucial given that physiological changes during pregnancy can effect both pharmacokinetics and pharmacodynamics, with potential alterations in drug absorption, distribution, metabolism, and elimination.48,49 Data on pregnancy outcomes and pharmacokinetics of the long-acting cabotegravir and rilpivirine tail in pregnancy are available from a small number of women with HIV who became pregnant while participating in clinical trials and were required to discontinue long-acting cabotegravir and rilpivirine ART and initiate an alternative oral ART regimen.50 However, pharmacokinetic data in women or others who continue or initiate long-acting ART during pregnancy are not available. There are no human data on placental transfer or secretion into breastmilk among individuals initiating or continuing long-acting cabotegravir and rilpivirine in pregnancy or post partum. The only available data are from an ex vivo human cotyledon perfusion model, which indicated lower placental transfer of cabotegravir compared with other integrase strand transfer inhibitor drugs.51
There are pharmacokinetic data for orally administered rilpivirine during pregnancy that show the concentration to be 20–50% lower in the second and third trimesters than in non-pregnant people. This lower rilpivirine concentration in pregnancy has been attributed to lower albumin concentrations during pregnancy and pregnancy-related cytochrome P450 3A4 enzyme induction; these data led to US perinatal guidelines recommending increased monitoring of HIV RNA if rilpivirine is used in pregnancy but no empirical dose adjustment. Prospective pharmacokinetic research is needed to evaluate the degree to which long-acting rilpivirine is affected by pregnancy-related physiological changes.52–57
Published pharmacokinetic and safety data on long-acting cabotegravir and long-acting rilpivirine in pregnancy are limited to four abstracts. Two phase 3 clinical trials describe rilpivirine pharmacokinetics in four pregnancies with early drug exposures. Specifically, these trials describe rilpivirine pharmacokinetics in women receiving injections of long-acting cabotegravir and long-acting rilpivirine who became pregnant while on long-acting cabotegravir and rilpivirine and were subsequently switched to oral ART upon diagnosis of pregnancy.56,57 A third abstract update from the phase 3 trial reported on cabotegravir and rilpivirine pharmacokinetic tails after discontinuation in seven women after pregnancy was recognised.50 The fourth abstract describes safety and pharmacokinetics among 26 pregnancies with early drug exposure to long-acting cabotegravir for HIV PrEP in HPTN 084; the women were subsequently switched to oral PrEP once pregnancy was recognised.58 In the phase 3 treatment trial, serial measurement of the cabotegravir tail concentrations during pregnancy showed rates of decline in seven participants with normal bodyweight and pharmacokinetic data available that were similar to non-pregnant people, and population pharmacokinetic modelling predicted that cabotegravir concentrations would remain therapeutic throughout pregnancy.50 In one participant, a more rapid than expected decline in cabotegravir concentrations was documented in the third trimester, possibly attributable to altered absorption due to low BMI.56 Similarly, data on long-acting cabotegravir exposure during early pregnancy in HPTN 084 found that the apparent terminal phase half-life in pregnant participants was comparable to non-pregnant people.59 Long-acting rilpivirine tail concentrations in the phase 3 clinical trials were also similar between pregnant and non-pregnant individuals.60 None of the above studies noted safety signals related to early pregnancy exposure.
Children and adolescents living with HIV
HIV RNA suppression, a surrogate biomarker of adherence to highly active combination ART, occurs in a lower proportion of children and adolescents with HIV than adults.61 Reasons for this observed difference include the scarcity of choice of paediatric formulations to accommodate dosing needs of growing infants and children and the complex psychosocial factors affecting adherence to treatment. Although the increased availability of palatable, simple, once-daily oral regimens has improved virological suppression, substantial adherence challenges remain.62 As children transition to adolescents, they confront several new challenges, including being informed of their HIV status, disclosure to peers and potential partners, treatment fatigue, unwanted side-effects (eg, weight gain), and internal and external HIV stigma. These factors are in addition to the psychological developmental changes that occur during adolescence and affect the successful transition to independent self-care that typifies adult care.62 The availability of long-acting ART has the potential to address many of these adherence challenges by allowing children and adolescents living with HIV to age into adulthood without the burden of adhering to complex daily oral treatment regimens.
Developing long-acting ART for children and adolescents is not without challenges. Changes in the weight and size of children and age-related changes in metabolic enzymes require pharmacokinetic evaluations and dose adjustments over time. WHO weight bands for antiretroviral drugs are a widely accepted standard to simplify dosing in children and adolescents. In the two studies of children and adolescents that evaluated long-acting cabotegravir and long-acting rilpivirine, population pharmacokinetic modelling was used to successfully predict the initial dose for evaluation across weight bands.63,64 However, these standard weight-bands do not consider the rapid changes in body composition that occur during adolescence, thus limiting the usefulness of the models. The site and maximum volume of injections can vary with age of the child, size of needles, and method of administration. Intramuscular injections in children generally have lower administration volumes than adults because the paediatric dose is also lower and, due to lower muscle bulk in the gluteal muscles in children, are often administered in the lateral thigh.65,66 Changes in absorption from the administration site and changes in metabolism during ageing, along with hepatic metabolic maturity, can affect the dose and dosing frequency. Apart from the pharmacokinetic differences, acceptability and preference requirements need to be evaluated for children and adolescents and their caregivers.
One cross-sectional study evaluated attitudes toward long-acting ART in 303 adolescents and young adults with HIV aged 13–24 years.67 Overall, 266 participants (88%) reported a willingness to try long-acting ART, with higher interest indicated by individuals with HIV RNA of more than 1000 copies per mL than by virologically suppressed participants. Reducing the pain of administration and increasing the duration between doses are likely to be key considerations when developing long-acting formulations for this population.45
Long-acting cabotegravir and long-acting rilpivirine injections are in phase 2 studies in adolescents and children. Interim data showed that these formulations were well tolerated and achieved the target pharmacokinetic exposure, and the regimens were found to be acceptable to adolescents and their parents or caregivers.68,69 These data were used to support FDA approval for switching to long-acting cabotegravir and rilpivirine ART in virally suppressed adolescents younger than 12 years who weigh more than 35 kg.70 A separate study will evaluate the acceptability, safety, and dosing of long-acting cabotegravir–rilpivirine in children aged 2–12 years. Lessons learned from these studies will advance our understanding of long-acting formulations use in the paediatric population.
Implementation considerations
Whether long-acting ART will change population health outcomes depends not only on the properties of the medications but also on the perceived acceptability, appropriateness, and feasibility among health systems, providers, patients, and communities. Even though long-acting ART can be used in patients who are already virologically suppressed to improve quality of life, the question for public health is whether long-acting ART will be used to improve population health and reduce HIV transmission through HIV RNA suppression. The one available long-acting ART combination is only approved for individuals who are virally suppressed, and clinical trials included a 1 month oral lead-in of cabotegravir and rilpivirine to assure tolerability before long-acting administration. The oral lead-in presents additional barriers for individuals who have pill-aversion or other barriers to oral medications. Fortunately, due to high tolerability of the regimen in clinical trials, the current product labelling for both ART and PrEP allows for optional use of the oral lead-in for patients and providers, decided through shared decision making.4,35
Given that many patients who are not virally suppressed also have social and structural barriers71 (eg, housing insecurity), systemic racism, and mental health comorbidities, strategies to use long-acting ART must also address determinants of the inverse care law—that the populations who could most benefit from an intervention are the least likely to receive it.72 Injectable and depot psychotropic medications offer a cautionary tale.73 Hailed as revolutionary for management of psychotic disorders, these formulations have not been as widely used as anticipated and have had little effect on population mental health outcomes. Furthermore, implementation research has identified problems with delivery (eg, offices not properly equipped to give injections) and the perception that injectables are for patients who are not adherent, leading to stigmatisation (ie, offering long-acting ART to only virally suppressed patients could unintentionally stigmatise unsuppressed patients).73 The use of long-acting ART must avoid the same stigma.
Conclusion and future directions
Although long-acting ART has great potential to change the landscape of HIV care, prevention, and treatment, additional data are needed to test their effectiveness in HIV patients at risk of non-adherence and in patients with high BMIs. Several potential delivery methods for long-acting formulations in adults, adolescents, and children are in various stages of development. These include intramuscular injections, subcutaneous injections or implants, oral extended-release or long-acting formulations, and transcutaneous microarray patches. Such new formulations could permit home-based community administration or, potentially, self-administration of the drugs, which would reduce frequency of clinic visits and clinic burden. However, the trade-off is removal of observed long-acting ART dosing, which could lead to missed doses and a subsequent prolonged pharmacokinetic tail. Despite the pros and cons, current and future long-acting ART must be evaluated across age groups and during pregnancy and post partum and be made accessible to all patients with HIV, especially those in low-income and middle-income countries, to have a substantial effect on HIV prevention, care, treatment cascade, and, ultimately, the course of the HIV pandemic.74 Affordability and large-scale implementation of long-acting ART (when compared with oral therapy) is a major hurdle to universal access. Initial modelling suggests that long-acting ART will be most cost-effective in low-income and middle-income countries if it is made available to individuals with suboptimal adherence to ART.75 Health–economic evaluations will be important to determine how best to implement long-acting ART in resource-constrained environments. Implementation science must be prioritised to ensure systems, organisations, providers, and communities are poised to optimise reach, quality, access, and sustainability. It is only by addressing these hurdles that long-acting ART will fulfil its promise as a groundbreaking advance in the landscape of HIV prevention and care.
Key messages.
Long-acting antiretroviral therapy will become a standard treatment option for people living with HIV; four long-acting antiretrovirals are currently available in some countries, and many more are in development
Virological failure with drug resistance is a risk in individuals who switch to long-acting ART and are not retained in care or discontinue long-acting products without initiating alternative therapy; this is due to the long pharmacokinetic tail of declining drug concentrations after product discontinuation
Although infrequent, confirmed virological failure with drug resistance mutations occurred during clinical trials in patients with on-time injections of long-acting ART
Patients will require ongoing monitoring in real-world settings as long-acting ART becomes more widely implemented, but early demonstration studies of injectable long-acting ART show high levels of adherence to injection schedules and continued viral suppression
If there is a need for virological suppression with oral formulations of cabotegravir plus rilpivirine prior to initiation of long-acting injectable formulations, this will be a barrier to realising the potential benefits of long-acting ART
Clinical trials are needed to examine the efficacy of long-acting ART among people who are challenged by adherence to once-daily oral ART
Further data, including from implementation research, are urgently needed to ensure the optimal effectiveness of long-acting ART in special populations of individuals with HIV, including children, adolescents, and pregnant people.
Ensuring equitable access to novel, long-acting therapies worldwide must be a priority for clinicians, manufacturers, and policy makers to maximise the effect of these drugs on the HIV pandemic
Search strategy and selection criteria.
We searched PubMed using MeSH. We focused on terms about antiretroviral treatment (MeSH terms: “anti-retroviral agents”, “antiretroviral therapy”, “highly active”, and “anti-HIV agents”, including subheadings on therapeutic use, therapy, and pharmacology) for HIV (MeSH terms: “HIV infections” and “HIV”) in adults, adolescents, children, and pregnant people. We added terms about adherence (MeSH terms: “medication adherence”, “treatment adherence and compliance”, and “patient compliance”) and drug resistance. We reviewed full-length articles, including abstracts, that discussed long-acting antiretroviral treatment for HIV that were published in English from the start of PubMed records until Oct 30, 2022. Authors also recommended specific articles to review. The final reference list was generated on the basis of originality of the articles and relevance to the broad scope of this Review.
Panel: Advantages and challenges of administering long-acting antiretrovirals compared with daily antiretroviral therapy.
Advantages
No absorption complications associated with oral dosing (eg, food or drug interactions)
Less frequent dosing
Potential for improved adherence in individuals with adherence barriers to oral therapy
Less stigma
Less opportunity for breaching health privacy; decreased potential for disclosure is specific to disclosure to people outside of the clinical care setting
Objective monitoring of adherence
Improvement in wellbeing and quality of life
Challenges
Cold chain storage might be required for intramuscular or intravenous injections (eg, rilpivirine)
Current products require health-care provider administration
Injection volume could limit the ability to dose (eg, infants and children)
An oral lead-in dosing period might be required
Management of drug–drug interactions might be more difficult due to the prolonged pharmokinetic tail and difficulty with long-acting dose adjustment
Absence of pharmacokinetic data in specific populations (eg, growing infants and children, pregnant people, people with high BMIs)
Incomplete adherence could lead to a subtherapeutic pharmacokinetic tail, risking the development of drug resistance
Affordability of these drugs in low-income and middle-income countries, especially when compared with current oral therapy
Need for monitoring systems and outreach for on-time injections
Acknowledgments
We thank Charles W Flexner (Divisions of Infectious Diseases and Clinical Pharmacology, Johns Hopkins School of Medicine, Baltimore, MD, USA) and Prof Gary Maartens (Division of Clinical Pharmacology, Department of Medicine, University of Cape Town, South Africa) for critical review and advice. JBN is supported by the US National Institutes of Health (NIH), National Institutes of Allergy and Infectious Diseases (NIAID) and Fogarty International Center (FIC) grant numbers NIH/FIC 1R25TW011217–01; NIH/FIC 1D43TW010937–01A1; NIH/FIC D43TW011827–01A1; NIH/FIC 1R21TW011706–0; and NIH/NIAID 5U01AI096299–13. KKS is supported by NIH/NICHD grant number R01HD085887. ED is supported by NIH/FIC grant number R21 TW 012185–01; and NIH/NIAID grant number R01AI152119–01. MG is supported by NIH/NIAID grant number 2R01AI098472. JWM is supported by US NIH/NIAID grant numbers UM1 AI106701, UM1 AI126603, UM1 AI164556 and UM1 AI164565, and NCI Contract 75N91019D00024.
Declaration of interests
RKS reports investigator-sponsored research awards from Gilead Science and ViiV Healthcare; and awards managed by MedStar Health Research Institute. KKS reports investigator-initiated research funding paid to her institution from Organon. LMM reports funding from ViiV Healthcare provided to the Elizabeth Glaser Pediatric AIDS Foundation for a birth outcomes surveillance project in Eswatini; and serving as a paid consultant to WHO on the safety of antiretroviral drugs in pregnancy. JWM is a consultant to Gilead Sciences and a grant recipient from Gilead Sciences to the University of Pittsburgh. All other authors declare no competing interests.
References
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/archive/AdultandAdolescentGL_2021_08_16.pdf (accessed Oct 31, 2022).
- 2.Altice F, Evuarherhe O, Shina S, Carter G, Beaubrun AC. Adherence to HIV treatment regimens: systematic literature review and meta-analysis. Patient Prefer Adherence 2019; 13: 475–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerrigan D, Mantsios A, Gorgolas M, et al. Experiences with long acting injectable ART: a qualitative study among PLHIV participating in a Phase II study of cabotegravir + rilpivirine (LATTE-2) in the United States and Spain. PLoS One 2018; 13: e0190487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration. CABENUVA (cabotegravir extended-release injectable suspension; rilpivirine extended-release injectable suspension), co-packaged for intramuscular use. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/212888s005s006lbl.pdf (accessed Oct 18, 2022).
- 5.Scarsi KK, Swindells S. The promise of improved adherence with long-acting antiretroviral therapy: what are the data? J Int Assoc Provid AIDS Care 2021; 20: 23259582211009011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bares SH, Scarsi KK. A new paradigm for antiretroviral delivery: long-acting cabotegravir and rilpivirine for the treatment and prevention of HIV. Curr Opin HIV AIDS 2022; 17: 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swindells S, Andrade-Villanueva J-F, Richmond GJ, et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med 2020; 382: 1112–23. [DOI] [PubMed] [Google Scholar]
- 8.Swindells S, Lutz T, Van Zyl L, et al. Week 96 extension results of a phase 3 study evaluating long-acting cabotegravir with rilpivirine for HIV-1 treatment. AIDS 2022; 36: 185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Overton E, Richmond G, Rizzardini G, et al. Long-acting cabotegravir + rilpivirine every 2 months: ATLAS-2M Week 152 results. Conference on Retroviruses and Opportunisitic Infections; Feb 12–16, 2022. [Google Scholar]
- 10.Jaeger H, Overton ET, Richmond G, et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2M), 96-week results: a randomised, multicentre, open-label, phase 3b, non-inferiority study. Lancet HIV 2021; 8: e679–89. [DOI] [PubMed] [Google Scholar]
- 11.Orkin C, Arasteh K, Górgolas Hernández-Mora M, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med 2020; 382: 1124–35. [DOI] [PubMed] [Google Scholar]
- 12.Orkin C, Bernal Morell E, Tan DHS, et al. Initiation of long-acting cabotegravir plus rilpivirine as direct-to-injection or with an oral lead-in in adults with HIV-1 infection: week 124 results of the open-label phase 3 FLAIR study. Lancet HIV 2021; 8: e668–78. [DOI] [PubMed] [Google Scholar]
- 13.Teichner P, Cutrell A, D’Amico R, et al. 884. Patient adherence to long-acting injectable cabotegravir + rilpivirine through 48 weeks of maintenance therapy in the phase 3 ATLAS and FLAIR studies. Open Forum Infect Dis 2019; 6 (suppl 2): s20. [Google Scholar]
- 14.Orkin C, Schapiro J, Perno C, et al. Expanded multivariable models to assist patient selection for long-acting cabotegravir + rilpivirine treatment: clinical utility of a combination of patient, drug concentration, and viral factors associated with virologic failure over 152 weeks. HIV Glasgow; Oct 23–26, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Amico R, Cenoz Gomis S, Moodley R, et al. Compassionate use of long-acting cabotegravir plus rilpivirine for people living with HIV-1 in need of parenteral antiretroviral therapy. HIV Med 2022; 24: 202–11. [DOI] [PubMed] [Google Scholar]
- 16.Christopoulos KA, Grochowski J, Mayorga-Munoz F, et al. First demonstration project of long-acting injectable antiretroviral therapy for persons with and without detectable HIV viremia in an urban HIV clinic. Clin Infect Dis 2023; 76: 645–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gandhi M, Salazar J, Hickey MD, et al. High virologic suppression rates on long-acting ART in a safety-net clinic population. Conference on Retroviruses and Opportunistic Infections; Feb 19–22, 2023. [Google Scholar]
- 18.Sension M Real-world use of long-acting cabotegravir + rilpivirine in the US: effectiveness in the first year. ID Week; Oct 19–23, 2022. [Google Scholar]
- 19.Collins LF, Corbin-Johnson D, Asrat M, et al. Early experience implementing long-acting injectable cabotegravir/rilpivirine for Human Immunodeficiency Virus-1 treatment at a Ryan White-funded clinic in the US south. Open Forum Infect Dis 2022; 9: ofac455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Wit S, Rami A, Bonnet F, et al. CARISEL: a hybrid type III implementation effectiveness study of implementation of cabotegravir + rilpivirine long-acting (CAB + RPV LA) in European health care settings; key clinical and implementation outcomes by implementation arm. ID Week; Oct 19–23, 2022. [Google Scholar]
- 21.Borch J, Scherzer J, Jonsson-Odenbuttel C, et al. 6-month outcomes of every 2 months long-acting cabotegravir and rilpivirine in a real-world setting—effectiveness, adherence to injections, and patient-reported outcomes of people living with HIV in the German CARLOS cohort. HIV Glasgow; Oct 23–26, 2022. [Google Scholar]
- 22.Han K, Shaik J, Crauwels H, et al. Pharmacokinetics and tolerability of cabotegravir and rilpivirine long-acting intramuscular injections to the vastus lateralis (lateral thigh) muscles of healthy adult participants. 24th International AIDS Conference; July 29–Aug 2, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medicines Patent Pool. Cabotegravir long-acting (LA) for HIV pre-exposure prophylaxis (PrEP). 2022. https://medicinespatentpool.org/licence-post/cabotegravir-long-acting-la-for-hiv-pre-exposure-prophylaxis-prep (accessed Nov 14, 2022).
- 24.Medicins Sans Frontiers. ViiV will not license new game-changing long-acting HIV prevention drug to generic manufacturers. March 4, 2022. https://msfaccess.org/viiv-will-not-license-new-game-changing-long-acting-hiv-prevention-drug-generic-manufacturers (accessed Nov 14, 2022).
- 25.Cutrell AG, Schapiro JM, Perno CF, et al. Exploring predictors of HIV-1 virologic failure to long-acting cabotegravir and rilpivirine: a multivariable analysis. AIDS 2021; 35: 1333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han K, Baker M, Lovern M, et al. Population pharmacokinetics of cabotegravir following administration of oral tablet and long-acting intramuscular injection in adult HIV-1-infected and uninfected subjects. Br J Clin Pharmacol 2022; 88: 4607–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neyens M, Crauwels HM, Perez-Ruixo JJ, Rossenu S. Population pharmacokinetics of the rilpivirine long-acting formulation after intramuscular dosing in healthy subjects and people living with HIV. J Antimicrob Chemother 2021; 76: 3255–62. [DOI] [PubMed] [Google Scholar]
- 28.Eshleman SH, Fogel JM, Piwowar-Manning E, et al. Characterization of human immunodeficiency virus (HIV) infections in women who received injectable cabotegravir or tenofovir disoproxil fumarate/emtricitabine for HIV prevention: HPTN 084. J Infect Dis 2022; 225: 1741–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landovitz RJ, Donnell D, Clement ME, et al. Cabotegravir for HIV prevention in cisgender men and transgender women. N Engl J Med 2021; 385: 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marzinke MA, Grinsztejn B, Fogel JM, et al. Characterization of human immunodeficiency virus (HIV) infection in cisgender men and transgender women who have sex with men receiving injectable cabotegravir for hiv prevention: HPTN 083. J Infect Dis 2021; 224: 1581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Margot N, Vanderveen L, Naik V, et al. Phenotypic resistance to lenacapavir and monotherapy efficacy in a proof-of-concept clinical study. J Antimicrob Chemother 2022; 77: 989–95. [DOI] [PubMed] [Google Scholar]
- 32.Margot NA, Naik V, VanderVeen L, et al. Resistance analyses in highly treatment-experienced people with human immunodeficiency virus (HIV) treated with the novel capsid HIV inhibitor lenacapavir. J Infect Dis 2022; 226: 1985–91. [DOI] [PubMed] [Google Scholar]
- 33.Bar KJ, Sneller MC, Harrison LJ, et al. Effect of HIV antibody VRC01 on viral rebound after treatment interruption. N Engl J Med 2016; 375: 2037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Landovitz RJ, Li S, Eron JJ Jr, et al. Tail-phase safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in HIV-uninfected adults: a secondary analysis of the HPTN 077 trial. Lancet HIV 2020; 7: e472–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.US Food and Drug Administration. APRETUDE (cabotegravir extended-release injectable suspension), for intramuscular use. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215499s000lbl.pdf (accessed Oct 18, 2022).
- 36.Nachega JB, Uthman OA, Anderson J, et al. Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries: a systematic review and meta-analysis. AIDS 2012; 26: 2039–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matthews LT, Orrell C, Bwana MB, et al. Adherence to HIV antiretroviral therapy among pregnant and postpartum women during the Option B+ era: 12-month cohort study in urban South Africa and rural Uganda. J Int AIDS Soc 2020; 23: e25586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abuogi LL, Castillo-Mancilla J, Hampanda K, et al. Tenofovir diphosphate in dried blood spots in pregnant and postpartum women with HIV in Kenya: a novel approach to measuring peripartum adherence. J Acquir Immune Defic Syndr 2022; 89: 310–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Landes M, van Lettow M, van Oosterhout JJ, et al. Early post-partum viremia predicts long-term non-suppression of viral load in HIV-positive women on ART in Malawi: implications for the elimination of infant transmission. PLoS One 2021; 16: e0248559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Odeny TA, Hughes JP, Bukusi EA, et al. Text messaging for maternal and infant retention in prevention of mother-to-child HIV transmission services: a pragmatic stepped-wedge cluster-randomized trial in Kenya. PLoS Med 2019; 16: e1002924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kinuthia J, Ronen K, Unger JA, et al. SMS messaging to improve retention and viral suppression in prevention of mother-to-child HIV transmission (PMTCT) programs in Kenya: a 3-arm randomized clinical trial. PLoS Med 2021; 18: e1003650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abuogi LL, Onono M, Odeny TA, et al. Effects of behavioural interventions on postpartum retention and adherence among women with HIV on lifelong ART: the results of a cluster randomized trial in Kenya (the MOTIVATE trial). J Int AIDS Soc 2022; 25: e25852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vrazo AC, Firth J, Amzel A, Sedillo R, Ryan J, Phelps BR. Interventions to significantly improve service uptake and retention of HIV-positive pregnant women and HIV-exposed infants along the prevention of mother-to-child transmission continuum of care: systematic review. Trop Med Int Health 2018; 23: 136–48. [DOI] [PubMed] [Google Scholar]
- 44.Venkatesan P Long-acting injectable ART for HIV: a (cautious) step forward. Lancet Microbe 2022; 3: e94. [DOI] [PubMed] [Google Scholar]
- 45.Nachman S, Townsend CL, Abrams EJ, et al. Long-acting or extended-release antiretroviral products for HIV treatment and prevention in infants, children, adolescents, and pregnant and breastfeeding women: knowledge gaps and research priorities. Lancet HIV 2019; 6: e552–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Omonaiye O, Kusljic S, Nicholson P, Manias E. Medication adherence in pregnant women with human immunodeficiency virus receiving antiretroviral therapy in sub-Saharan Africa: a systematic review. BMC Public Health 2018; 18: 805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thoueille P, Choong E, Cavassini M, Buclin T, Decosterd LA. Long-acting antiretrovirals: a new era for the management and prevention of HIV infection. J Antimicrob Chemother 2022; 77: 290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Costantine MM. Physiologic and pharmacokinetic changes in pregnancy. Front Pharmacol 2014; 5: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol 2015; 39: 512–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel P, Ford SL, Baker M, et al. 885. Pregnancy outcomes and pharmacokinetics in pregnant women living with HIV exposed to long-acting cabotegravir and rilpivirine in clinical trials. Open Forum Infect Dis 2021; 8 (suppl 1): s534. [DOI] [PubMed] [Google Scholar]
- 51.Pencolé L, Lê MP, Bouchet-Crivat F, Duro D, Peytavin G, Mandelbrot L. Placental transfer of the integrase strand inhibitors cabotegravir and bictegravir in the ex-vivo human cotyledon perfusion model. AIDS 2020; 34: 2145–49. [DOI] [PubMed] [Google Scholar]
- 52.Schalkwijk S, Colbers A, Konopnicki D, et al. Lowered rilpivirine exposure during the third trimester of pregnancy in human immunodeficiency virus type 1-infected women. Clin Infect Dis 2017; 65: 1335–41. [DOI] [PubMed] [Google Scholar]
- 53.Panel on Treatment of HIV During Pregnancy and Prevention of Perinatal Transmission. Recommendations for the use of antiretroviral drugs during pregnancy and interventions to reduce perinatal HIV transmission in the United States. 2022. https://clinicalinfo.hiv.gov/en/guidelines/perinatal/ (accessed March 29, 2023).
- 54.Jeong H Altered drug metabolism during pregnancy: hormonal regulation of drug-metabolizing enzymes. Expert Opin Drug Metab Toxicol 2010; 6: 689–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tran AH, Best BM, Stek A, et al. Pharmacokinetics of rilpivirine in HIV-infected pregnant women. J Acquir Immune Defic Syndr 2016; 72: 289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Osiyemi O, Yasin S, Zorrilla C, et al. Pharmacokinetics, antiviral activity, and safety of rilpivirine in pregnant women with HIV-1 infection: results of a phase 3b, multicenter, open-label study. Infect Dis Ther 2018; 7: 147–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eke AC, Chakhtoura N, Kashuba A, et al. Rilpivirine plasma and cervicovaginal concentrations in women during pregnancy and postpartum. J Acquir Immune Defic Syndr 2018; 78: 308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel P, Thiagarajah S, Ford S, et al. Cabotegravir pharmacokinetic tail in pregnancy and neonatal outcomes. Conference on Retroviruses and Opportunistic Infections; March 8–11; 2020. [Google Scholar]
- 59.Delany-Moretlwe S, Hughes J, Guo X, et al. Evaluation of CAB-LA safety and PK in pregnant women in the blinded phase of HPTN 084. Conference on Retroviruses and Opportunistic Infections; March 8–11, 2022. [Google Scholar]
- 60.Crauwels H, Rice D, Neyens M, et al. Rilpivirine long-acting pharmacokinetic tail and pregnancy. International Workshop on HIC & Women; April 26–28, 2021 (abstr 14). [Google Scholar]
- 61.Nachega JB, Hislop M, Nguyen H, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. J Acquir Immune Defic Syndr 2009; 51: 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arpadi SM, Shiau S, De Gusmao EP, Violari A. Routine viral load monitoring in HIV-infected infants and children in low- and middle-income countries: challenges and opportunities. J Int AIDS Soc 2017; 20 (suppl 7): e25001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han WM, Law MG, Egger M, et al. Global estimates of viral suppression in children and adolescents and adults on antiretroviral therapy adjusted for missing viral load measurements: a multiregional, retrospective cohort study in 31 countries. Lancet HIV 2021; 8: e766–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.WHO. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. 2021. https://www.who.int/publications-detail-redirect/9789240031593 (accessed Oct 31, 2022). [PubMed]
- 65.Shaw FE Jr, Guess HA, Roets JM, et al. Effect of anatomic injection site, age and smoking on the immune response to hepatitis B vaccination. Vaccine 1989; 7: 425–30. [DOI] [PubMed] [Google Scholar]
- 66.Bergeson PS, Singer SA, Kaplan AM. Intramuscular injections in children. Pediatrics 1982; 70: 944–48. [PubMed] [Google Scholar]
- 67.Weld ED, Rana MS, Dallas RH, et al. Interest of youth living with HIV in long-acting antiretrovirals. J Acquir Immune Defic Syndr 2019; 80: 190–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moore C, Capparelli E, Calabrese K, et al. Safety and PK of long-acting cabotegravir and rilpivirine in adolescents. Conference on Retroviruses and Opportunistic Infections; Feb 12–16, 2022. [Google Scholar]
- 69.Lowenthal E, Chapman J, Calabrese K, et al. Adolescent and parent experiences with long-acting injectables in the MOCHA study. Conference on Retroviruses and Opportunistic Infections; Feb 12–16, 2022. [Google Scholar]
- 70.Johnson & Johnson. US FDA approves CABENUVA (cabotegravir and rilpivirine) for adolescents, expanding the indication of the first and only complete long-acting injectable hiv regimen. March 29, 2022. https://www.jnj.com/u-s-fda-approves-cabenuvacabotegravir-and-rilpivirine-for-adolescents-expanding-the-indication-of-the-first-and-only-complete-long-acting-injectable-hiv-regimen (accessed Oct 31, 2022).
- 71.Kimmel AD, Masiano SP, Bono RS, et al. Structural barriers to comprehensive, coordinated HIV care: geographic accessibility in the US South. AIDS Care 2018; 30: 1459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hart JT. The inverse care law. Lancet 1971; 1: 405–12. [DOI] [PubMed] [Google Scholar]
- 73.Bosanac P, Castle DJ. Why are long-acting injectable antipsychotics still underused? BJPsych Adv 2015; 21: 98–105. [Google Scholar]
- 74.Chandiwana NC, Serenata CM, Owen A, et al. Impact of long-acting therapies on the global HIV epidemic. AIDS 2021; 35 (suppl 2): s137–43. [DOI] [PubMed] [Google Scholar]
- 75.Phillips AN, Bansi-Matharu L, Cambiano V, et al. The potential role of long-acting injectable cabotegravir-rilpivirine in the treatment of HIV in sub-Saharan Africa: a modelling analysis. Lancet Glob Health 2021; 9: e620–27. [DOI] [PMC free article] [PubMed] [Google Scholar]


