ABSTRACT
Teredinibacter turnerae is an intracellular bacterial symbiont that resides in the gills of shipworms, wood-eating bivalve mollusks. This bacterium produces a catechol siderophore, turnerbactin, required for the survival of this bacterium under iron-limiting conditions. The turnerbactin biosynthetic genes are contained in one of the secondary metabolite clusters conserved among T. turnerae strains. However, Fe(III)-turnerbactin uptake mechanisms are largely unknown. Here, we show that the first gene of the cluster, fttA, a homolog of Fe(III)-siderophore TonB-dependent outer membrane receptor genes, is indispensable for iron uptake via the endogenous siderophore, turnerbactin, as well as by an exogenous siderophore, amphi-enterobactin, ubiquitously produced by marine vibrios. Furthermore, three TonB clusters containing four tonB genes were identified, and two of these genes, tonB1b and tonB2, functioned not only for iron transport but also for carbohydrate utilization when cellulose was a sole carbon source. Gene expression analysis revealed that none of the tonB genes and other genes in those clusters were clearly regulated by iron concentration while turnerbactin biosynthesis and uptake genes were upregulated under iron-limiting conditions, highlighting the importance of tonB genes even in iron-rich conditions, possibly for utilization of carbohydrates derived from cellulose.
IMPORTANCE
This study highlights diversity in iron acquisition and regulation in bacteria. The mechanisms of iron acquisition and its regulation in Teredinibacter turnerae, as well as its connection to cellulose utilization, a hallmark phenotype of T. turnerae, expand the paradigm of bacterial iron acquisition. Two of the four TonB genes identified in T. turnerae exhibit functional redundancy and play a crucial role in siderophore-mediated iron transport. Unlike typical TonB genes in bacteria, none of the TonB genes in T. turnerae are clearly iron regulated. This unusual regulation could be explained by another important finding in this study, namely, that the two TonB genes involved in iron transport are also essential for cellulose utilization as a carbon source, leading to the expression of TonB genes even under iron-rich conditions.
KEYWORDS: iron, outer membrane, endosymbionts, symbiosis, Teredinidae, siderophore, cellulose
INTRODUCTION
Iron is an essential nutrient for almost all living organisms including bacteria. However, available free iron is extremely limited in the marine environment due to its insolubility in the presence of oxygen and in the host due to iron chelation by host iron-binding proteins; thus, the amount of available free iron is much lower than the amount that bacteria require for their proliferation. Therefore, bacteria have evolved active transport systems to sequester sufficient amounts of iron to survive and prosper in those environments (1). One of these systems is siderophore-mediated iron transport. Siderophores are small-molecule iron-chelating compounds synthesized by a nonribosomal peptide synthetase (NRPS) system or NRPS-independent pathway. The structure of siderophores described in this study is shown in Fig. S1. Siderophores exported to external environments form stable complexes with ferric iron, and in Gram-negative bacteria, Fe(III)-siderophore complexes are transported to the bacterial cytosol via specific outer membrane receptors across the outer membrane and ABC- or MSF-type siderophore transporters across inner membranes (2–7). Gram-negative bacteria require TonB complexes typically composed of TonB, ExbB, and ExbD, that locate in the inner membrane, to transduce energy derived from proton motive force to the Fe(III)-siderophore-specific outer membrane receptors for their activity (8–10). Although essential, an excess amount of iron is toxic due to its radical potential; therefore, the expression of genes required for iron transport is tightly regulated by the concentration of iron to maintain a suitable cellular iron concentration (11). It has also been demonstrated in many bacteria that iron not only influences the expression of iron metabolism genes but also acts as a signal that regulates the expression of genes that affect bacterial adaptation to environmental and/or host conditions (11–13).
Shipworms of the family Teredinidae are marine bivalve mollusks; most of which bore wood and consume wood as a nutrient source (14, 15). To utilize wood as a nutrient, insoluble lignocellulose needs to be broken down into soluble forms of carbohydrate. This enzymatic activity relies on symbiotic gammaproteobacteria that reside in bacteriocytes in the gills (16–19). Teredinibacter turnerae is the first bacterial symbiont isolated from shipworms. This bacterium produces cellulolytic enzymes and fixes atmospheric nitrogen that could contribute to shipworm metabolism in woody environments where the amount of nitrogen is restricted (20–23). T. turnerae T7901 carries many secondary metabolite gene clusters, and production of bioactive compounds has been reported (24–27). One of the secondary metabolite gene clusters, Region 7, carries the genes that are responsible for the biosynthesis of siderophore turnerbactin (25). Sequencing and metagenomic analysis revealed that the Region 7 cluster and its relatives were found to fall within the gene cluster family GCF_8, members of which occur in all T. turnerae strains sequenced as well as other shipworm symbiotic bacteria, indicating the importance of this cluster for the physiology of shipworm symbiotic bacteria (28). The tnbF gene encoding a nonribosomal peptide synthetase in this cluster was shown to be essential for the biosynthesis of turnerbactin and survival of this bacterium under iron-limiting conditions (25). Turnerbactin was detected in the shipworm, Lyrodus pedicellatus, harboring T. turnerae, suggesting the potential importance of turnerbactin in the symbiotic state. T. turnerae might have elevated iron requirements due to the need to synthesize iron-rich nitrogenase (25). It has been reported that T. turnerae carries two TonB gene clusters, TonB2 and TonB3, that resemble clusters found in marine vibrios although the function of those genes is yet to be characterized (29). In this work, we show the essential role of the fttA gene encoding the Fe(III)-turnerbactin outer membrane receptor for iron acquisition in T. turnerae. Additionally, two of four tonB genes in the genome were indispensable for growth under iron-limiting conditions. These tonB genes were further found to be necessary for the efficient growth of T. turnerae when cellulose was used as a sole carbon source. Furthermore, we report that tonB genes in T. turnerae T7901 are not clearly regulated by iron as compared with other iron transport-related genes, suggesting that T. turnerae requires TonB genes even under iron-rich condition to utilize carbohydrate(s) originating from cellulose.
MATERIALS AND METHODS
Strains, plasmids, and growth media
Bacterial strains and plasmids used in this study are listed in Table S1 while PCR primers are listed in Table S2. The whole-genome sequence of Teredinibacter turnerae T7901 was previously determined (NCBI accession number: NC_012997) (30). T. turnerae strains were cultured at 30°C in a modified chemically defined shipworm basal medium (SBM) containing NaCl (17.94gm/L), NH4Cl (250mg/L), Na2SO4 (3.01gm/L), NaHCO3 (0.147gm/L), Na2CO3 (10.5mg/L), KCl (0.5gm/L), KBr (73.5mg/L), H3BO3 (22.36mg/L), SrCl2·6H2O (18mg/L), KH2PO4 (15.24mg/L), C6H8O7 (2.75mg/L), NaF (2.25mg/L), Na2MoO4·2H2O (2.4mg/L), MnCl2·4H2O (1.81mg/L), ZnSO4·7H2O (0.22mg/L), CuSO4·5H2O (0.079mg/L), Co(NO3)2·6H2O (0.049mg/L), HEPES (4.77gm/L, pH = 8.0), and appropriate amounts of carbon sources. MgCl2·6H2O, CaCl2·2H2O, and ferric ammonium citrate (FAC) were supplemented in the medium. Sucrose (0.5%), cellulose (Sigmacell 101; 0.2%), and carboxymethylcellulose (0.5%) were used as carbon sources, and agar (1%) was added to prepare solid media. Under our standard growth conditions, which include 50µM of MgCl2·6H2O and 10µM of CaCl2·2H2O, cell aggregation was observed. However, we found that by a reducing the concentration of MgCl2·6H2O (0.05µM) and CaCl2·2H2O (0.5µM) in the SBM medium, referred to as low-SBM (L-SBM), T. turnerae grew without aggregation. Escherichia coli strains were cultured in Luria-Bertani broth or agar. Thymidine at 0.3 mM (f/c) was supplemented for the growth of E. coli π3813. When required, antibiotics were supplemented in the growth medium at the following concentration: ampicillin (Amp) at 100 µg/mL for E. coli, kanamycin (Km) at 50 µg/mL for E. coli, and T. turnerae and carbenicillin (Carb) at 100 µg/mL for T. turnerae.
Construction of plasmids
The plasmid pHN31(pDM4-Km), used for mutant construction, was constructed as follows. The Km resistance cassette from pBBR1MCS-2 (31) was PCR amplified using Km-F-EcoRV and Km-R-EcoRV primers and ligated into T-vectors. After confirming the nucleotide sequences, the Km cassette was cloned into the EcoRV site of pDM4 (32), generating pHN31.
To express genes in T. turnerae, we used the pHN33(pPROBE-tacP-GenP) plasmid constructed as follows. pMMB208 was digested with ScaI and AgeI, and the DNA fragment containing lacI, the tac promoter, and a multiple-cloning site was ligated into the corresponding restriction enzyme sites of pPROBE'-gfp[ASV] (33), generating pHN32. The DNA fragment containing the gentamicin resistance gene promoter from pBBR1MCS-3 (31) was PCR amplified using primers, GenP-F-HindIII and GenP-R-SalI, and cloned into T-vector. After confirmation of the nucleotide sequence, the plasmid was digested by HindIII and SalI, and the promoter sequence was ligated in the corresponding restriction enzyme sites of pHN32 plasmid, generating pHN33. pPROBE-gfp[ASV] was a gift from Steven Lindow (Addgene plasmid # 40166 ; http://n2t.net/addgene:40166 ; RRID:Addgene_40166).
Mutant construction and complementation
DNA fragments of upstream and downstream regions of the target genes to be mutated were combined by splicing by overhang extension PCR with modification as described before (34, 35), and the PCR-amplified fragments were ligated into pGEM-T easy (Promega). After sequence confirmation, the deletion fragments were ligated into the corresponding restriction enzyme sites of pHN31. The plasmids thus obtained were transformed into E. coli strains S17-1λpir or π3813 (thymidine auxotroph) and conjugated into T. turnerae T7901. When E. coli π3813 was used, thymidine (f/c 0.3 mM) was supplemented to the growth medium, and E. coli π3813 that carries pEVS104 (36) was used as a conjugation helper strain. To counterselect E. coli, first recombinants were selected by plating exconjugants on SBM-cellulose plates (for S17-1λpir conjugation) with Km (50 µg/mL) or SBM-sucrose without thymidine plates (for π3813 conjugation) supplemented with Km (50 µg/mL). First recombinants thus obtained were grown in liquid medium without antibiotics, streaked on SBM containing 15% sucrose, and incubated until colonies were formed. The deletion mutants were obtained by screening the colonies that were sensitive to Km, by colony PCR using primers. To complement mutants, DNA fragments that contain wild-type genes and their potential ribosomal binding sites were PCR amplified and cloned into T-vectors. After sequence confirmation, the DNA fragments were cloned into pHN33, and the plasmid was conjugated into T. turnerae as described above.
RNA extraction
All glassware was soaked in a 10% hydrochloric acid bath and then rinsed with milliQ water, to remove iron. T. turnerae T7901, and its derivatives were grown in iron-limiting (L-SBM-sucrose with 0.1 µM FAC) and iron-rich (L-SBM-sucrose with 10 µM FAC) conditions until exponential phase (OD600 0.2–0.3), and cell pellets were resuspended in TRIzol Reagent (Invitrogen), and the samples were kept in a −80°C freezer until being processed. Total RNAs were extracted by the TRIzol-RNeasy hybrid protocol (37). During RNA extraction, contaminated DNA was digested by treating three times with an RNase-Free DNase Set (Qiagen), and the absence of DNA contamination in extracted RNA was confirmed by PCR.
Quantitative RT-PCR
cDNA was synthesized from total RNA (1 µg) as a template using Superscript III Reverse Transcriptase and random hexamer primers (Invitrogen), and quantitative PCR was performed by StepOnePlus Fast Real-Time PCR System (Applied Biosystems) using Power SYBR Green PCR Master Mix (Applied Biosystems). The fold change of gene expression in two different conditions was measured by calculating ∆∆Ct values as described in reference (38).
RESULTS
Characterization of the Fe-turnerbactin outer membrane receptor gene, fttA
One of the secondary metabolite clusters, Region 7 of T. turnerae T7901, contains nonribosomal peptide synthetase genes (Fig. 1), and the major NRPS gene, tnbF, was shown to be essential for the siderophore turnerbactin production (25). The first gene of Region 7, TERTU_RS18025 (old locus tag, TERTU_4055), was annotated as a homolog of the TonB-dependent outer membrane receptor (TBDR) gene, CCD03052, from Azospirillum brasilense Sp245 (25). Further comparison of the predicted amino acid sequence of TERTU_RS18025 (named fttA in this study) with known Fe(III)-siderophore outer membrane receptors revealed that FttA shows similarity to E. coli fepA (27% identity/44% similarity in amino acid level) and Vibrio anguillarum fetA (30% identity/48% similarity in amino acid level), suggesting its potential role as a Fe(III)-turnerbactin uptake receptor. Although the TBDRs play an essential role for the iron uptake in bacteria, there are cases in which Fe(III)-siderophores can be transported via multiple TBDRs encoded by genes that reside in different chromosomal loci (4, 39–45). To investigate the role of the fttA gene, we constructed an in-frame fttA deletion mutant, and the growth of the fttA mutant was compared with that of the wild-type strain and turnerbactin biosynthetic mutant (∆tnbF), under iron-rich and limiting conditions. As shown in Fig. 2, the ∆fttA mutant did not grow under the iron-limiting condition as compared with the wild-type strain while this mutant still grew well in the iron-rich growth condition. The growth of the ∆fttA mutant under the iron-limiting condition was recovered when the fttA gene was expressed in trans in the fttA mutant confirming that the growth defect was due to the deletion of the fttA gene (Fig. 3). These results indicate that the fttA gene is essential for the growth of T. turnerae under iron-limiting conditions.
Fig 1.
Gene clusters involved in siderophore-mediated iron transport. (A) The turnerbactin biosynthesis and transport cluster. (B) Three tonB clusters. The figure was modified from a gene cluster map constructed by Gene Graphics (46). Green arrows indicate genes already characterized or predicted to be responsible for siderophore biosynthesis. Orange arrows indicate genes annotated to be involved in Fe(III)-siderophore uptake, and of those, TonB-homologs are shown in red color.
Fig 2.
Involvement of fttA and TonB genes in iron transport and carbohydrate derived from cellulose. (A) Growth of T. turnerae mutants under different growth conditions. Sucrose (0.5%) or carboxymethyl cellulose (0.5%) were added as a sole carbon source in the SBM agar plates, and FAC (1 µM) and EDDA (10 µM) and FAC (10 µM) were supplemented in the SBM medium to obtain iron-limiting and rich conditions, respectively. T. turnerae strains were streaked on the plates, and the pictures were taken after 7 days incubation at 30°C. FAC, ferric ammonium citrate; EDDA, ethylenediamine-N,N′-bis(2-hydroxyphenylacetic acid). (B) Growth response of tonB deletions to iron restriction and carboxymethyl cellulose combined. “wt” indicates the presence of wild-type tonB genes while “∆” shows the absence of the tonB gene (the in-frame gene deletion). The strains that grew under iron-limiting conditions when sucrose was used as a carbon source or when carboxymethyl cellulose was used as a carbon source under iron-rich conditions were highlighted as green and shown as “+” while the strains that did not grow were highlighted as red and shown as “–.”
Fig 3.
Complementation of fttA and tonB mutants. Sucrose or carboxymethyl cellulose was added as a sole carbon source in the SBM agar plates. FAC (1 µM) and EDDA (10 µM) and FAC (10 µM) were added in the SBM medium to obtain iron-limiting and rich conditions, respectively. T. turnerae strains were streaked on the plates, and the pictures were taken after 7 days of incubation at 30°C. FAC, ferric ammonium citrate; EDDA, ethylenediamine-N,N′-bis(2-hydroxyphenylacetic acid; pHN45, plasmid expression vector; tonBs-, ∆tonB1ab∆tonB2∆tonB3.
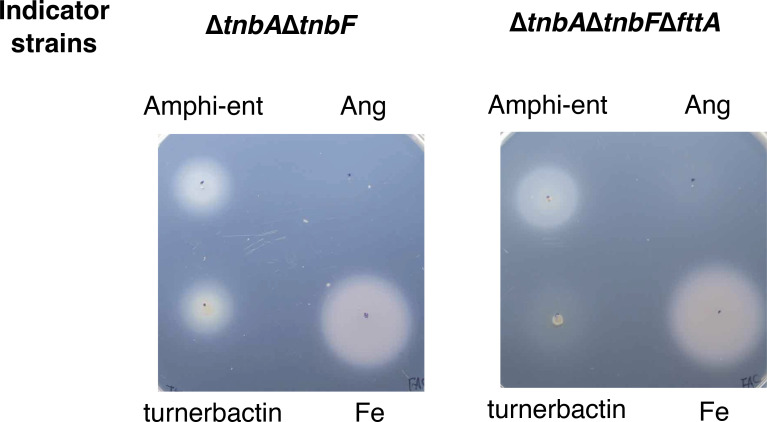
To further investigate whether the growth deficiency of the ∆fttA mutant is due to the failure of Fe(III)-turnerbactin uptake, we performed a bioassay (siderophore cross-feeding assay). We first constructed a turnerbactin production-deficient strain, ∆tnbA∆tnbF. The tnbA gene was also mutated to eliminate the 2,3-dihydroxybenzoate-2,3-dehydrogenase (2,3-DHBA) production since 2,3-DHBA also acts as an iron chelator (47). The fttA gene was mutated in the ∆tnbA∆tnbF background. Supplementation of the iron chelator, ethylenediamine-di-(o-hydroxyphenyl acetic acid) (EDDA), into growth medium led to the failure of the growth of the turnerbactin production-deficient strains, ∆tnbA∆tnbF and ∆tnbA∆tnbF∆fttA (Fig. 4). This growth defect of ∆tnbA∆tnbF was overcome when the wild-type strain producing turnerbactin was spotted on the agar plate containing ∆tnbA∆tnbF (see the growth halo around the spot). However, the ∆tnbA∆tnbF∆fttA strain in which the fttA gene was deleted didn’t recover its growth in the presence of the wild-type strain spot while spotting ferric ammonium citrate was able to recover its growth. These results indicate that the fttA gene is essential for the uptake of turnerbactin produced by the wild-type strain. Furthermore, to test whether T. turnerae T7901 can utilize an exogenous siderophore produced by marine bacterium Vibrio campbellii, we used extracts obtained from wild-type V. campbellii that produces amphi-enterobactin and anguibactin and its derivatives, an amphi-enterobactin producer and an anguibactin producer (34). Extracts rather than cultures were used because V. campbellii strains cannot grow on SBM medium. The growth of T. turnerae was recovered when amphi-enterobactin was provided by the indicator strain while anguibactin was not able to compensate for the growth defect under iron-limiting conditions. These results indicate that T. turnerae can take up amphi-enterobactin but not anguibactin produced by the marine pathogenic bacterium V. campbellii.
Fig 4.
Bioassay to test the involvement of FttA in transport of endogenous and exogenous siderophores. ∆tnbA∆tnbF produces neither turnerbactin nor its precursor due to the mutation in tnbF and tnbA, respectively, and therefore cannot grow under iron-limiting growth condition generated by supplementing an iron chelator, ethylenediamine-N,N′-bis(2-hydroxyphenylacetic acid. T. turnerae strains were grown under nonaggregation conditions. T7901, 5 µL of T. turnerae T7901 culture (containing turnerbactin); Amphi-ent, extracts containing amphi-enterobactin obtained from V. campbellii HY01∆angR (34); Ang, extracts containing anguibactin prepared from V. campbellii HY01∆aebF (34); Fe, 5 µL of ferric ammonium citrate. Pictures were taken after 7 days of incubation at 30°C.
Identification of TonB clusters in T. turnerae T7901
The presence of the TonB2 and TonB3 clusters in T. turnerae was briefly described before, and those are similar to the TonB2 and TonB3 clusters of marine vibrios such as Vibrio vulnificus (29), but the function of those tonB genes has not been elucidated yet. By sequence similarity searching of protein sequences annotated in T. turnerae T7901 with well-characterized TonB genes from E. coli K-12 and marine bacteria including V. vulnificus, V. cholerae, V. anguillarum, and Aeromonas hydrophila, we identified two more tonB gene homologs in a cluster (named here TonB1 cluster) in addition to TonB2 and TonB3 clusters. Interestingly, the TonB1 cluster carries two TonB genes, tonB1a and tonB1b, located next to each other and an exbD gene homolog (exbD1), but an exbB homolog was not found in this cluster (Fig. 1). The TonB1 clusters in vibrios (consisting of tonB1, exbB1, and exbD1) are located linked to the heme/hemoglobin transport cluster (48–52). However, there is no heme cluster near the TonB1 system in T. turnerae. Prediction of transmembrane helices with TMHMM server version 2 (https://services.healthtech.dtu.dk/service.php?TMHMM-2.0) (53) indicated that TonB1b, TonB2, and TonB3 harbor one transmembrane domain typically found in classical TonB proteins while TonB1a is an unusual TonB protein that carries an extended N-terminal domain predicted to carry four transmembrane domains that can be found in a small number of bacteria (54).
Two TonB genes are essential for the growth of T. turnerae under iron-limiting growth conditions
To understand which TonB gene(s) facilitate the growth of T. turnerae under specified conditions, single- and multiple-tonB gene mutants were constructed. Since tonB1a and tonB1b genes are co-located, both tonB1a and tonB1b were deleted together, generating the ∆tonB1ab mutant. The growth of those strains was compared under iron-rich and limiting growth conditions. As shown in Fig. 2, single mutants that lack tonB gene(s) in each TonB cluster such as ∆tonB1ab, ∆tonB2, and ∆tonB3 as well as the double-tonB gene mutants in the TonB1 and TonB3 cluster (∆tonB1ab∆tonB3) and in the TonB2 and TonB3 cluster (tonB2∆tonB3) showed growth under both iron-rich and limiting growth conditions. On the other hand, the tonB gene mutants in both the TonB1 and TonB2 cluster, ∆tonB1ab∆tonB2, and the quadruple-tonB gene mutant in which all tonB genes were eliminated, ∆tonB1ab∆tonB2∆tonB3, did not grow under iron-limiting conditions. Similar results were observed in the turnerbactin biosynthetic-deficient mutant ∆tnbF and the ferric-turnerbactin transport-deficient ∆fttA mutant. These results indicate that the tonB genes in both the TonB1 and TonB2 cluster are involved in the iron transport in T. turnerae T7901.
We further performed complementation experiments to confirm that the growth defect of some of mutants was not due to polar effects and/or secondary mutations and also to understand which tonB1 genes (tonB1a or tonB1b) are responsible for the growth of T. turnerae T7901 under iron-limiting conditions. tonB genes with their ribosomal binding sites were cloned in the expression vector pHN33 and conjugated into the ∆tonB1ab∆tonB2∆tonB3 mutant. The expression of all four TonB genes was confirmed by reverse transcriptase PCR (RT-PCR) (Fig. S2). As shown in Fig. 3, the growth of the quadruple-tonB mutant under iron-limiting growth conditions was recovered only when tonB1b or tonB2 genes are expressed in trans in the quadruple-tonB mutant. All strains grew well under an iron-rich growth condition. From these results, we conclude that out of four tonB genes, tonB1b and tonB2 are responsible for the growth of T. turnerae T7901 under iron-limiting conditions, and tonB1a and tonB3 are not responsible for iron uptake under this growth condition.
Involvement of TonB genes in the growth of T. turnerae T7901 cellulose as a carbon source
During the course of mutant construction in TonB genes, it was very hard to obtain the ∆tonB1ab∆tonB2 mutant. We realized that this ∆tonB1ab∆tonB2 mutant does not grow when cellulose is used as a sole carbon source in the growth medium. This mutant did not show a growth defect on sucrose plates. Since supplementation of cellulose and carboxymethyl cellulose (cellulose derivative) into the growth medium resulted in the same consequences, we decided to use carboxymethyl cellulose due to its solubility in growth medium. We further tested the growth of all single- and multiple-tonB gene mutants on SBM medium supplemented with either sucrose or carboxymethyl cellulose as a carbon source, and we found that the mutants missing tonB genes in the both TonB1 and TonB2 clusters (∆tonB1ab∆tonB2) and the strain that lacks all tonB genes (∆tonB1ab∆tonB2∆tonB3) showed a dramatic growth defect when carboxymethyl cellulose was used as a sole carbon source (Fig. 2). The rest of the mutants tested grew on both sucrose and cellulose media. The growth defect in the quadruple-TonB mutant, ∆tonB1ab∆tonB2∆tonB3, was recovered when the tonB1b or tonB2 genes were expressed in trans in the mutant while tonB1a and tonB3 were not able to compensate the growth defect on cellulose plates (Fig. 3). These results demonstrate that tonB1b and tonB2 are involved in carbohydrate utilization when cellulose is provided as a sole carbon source. Turnerbactin biosynthesis- (∆tnbF) and transport- (∆fttA) deficient mutants did not show growth defects on cellulose plates; therefore, the growth defect appears to be independent of turnerbactin production and utilization.
Iron regulation of turnerbactin biosynthesis and transport genes
It has been proposed that Region 7 consists of two iron-regulated transcriptional units and both operons might be regulated by the ferric uptake regulator since two possible Fur binding sites (Fur boxes) were identified in the upstream regions of fttA and tnbC (25). We performed quantitative RT-PCR (qRT-PCR) analysis to test whether genes in Region 7 are actually iron-regulated. Our results clearly showed that three representative genes such as tnbA, tnbF, and fttA are upregulated under iron-limiting growth conditions (Fig. 5). We also performed a Fur titration assay to test whether the E. coli ferric uptake regulator (Fur) binds to these putative TonB boxes. The result in Fig. S3 shows that E. coli Fur can bind to two Fur boxes as compared with two negative controls, indicating that Fur is involved in the upregulation of those genes under iron-limiting conditions.
Fig 5.
Regulation of iron transport-related genes in T. turnerae T7901. Expression of genes between iron-limiting and iron-rich growth conditions was compared by qRT-PCR. The data represent the mean value of at least three biological replicates with error bars that are the standard error of the mean.
Iron regulation of TonB genes
In many bacteria, TonB genes are typically regulated by iron to control internal iron concentration (51, 52, 55–63). To test iron regulation of TonB genes in T. turnerae, we performed qRT-PCR using primers to amplify each TonB gene (Fig. 5). The results indicate that relative expression levels of all TonB genes were not changed as much as those of tnbA and tnbF which were dramatically increased under iron-limiting conditions as compared with iron-rich conditions.
DISCUSSION
One of the compounds Teredinibacter turnerae produces is the siderophore turnerbactin that is used to acquire iron which is an essential metal for their growth in iron-limiting environments. It has been suggested that turnerbactin might be used to compete for iron with casually associated environmental bacteria to survive under iron-limiting conditions which are typically found in marine environments and inside hosts (25). Turnerbactin-related genes were found in the secondary metabolite cluster, Region 7, located within GCF_8 (identified by metagenomics), and the tnbF gene is essential for turnerbactin biosynthesis (25, 28). However, the transport mechanism of Fe(III)-turnerbactin was not characterized yet. To transport Fe(III)-siderophore across the outer membrane, Gram-negative bacteria require the TonB system that typically consists of TonB, ExbB, and ExbD that transduce proton motive force generated in the inner membrane to outer membrane receptors, resulting in conformational change in the outer membrane receptors (64). The TonB system was originally found and has been extensively characterized in E. coli (65). E. coli and many other bacteria carry a single set of the TonB system, but after finding two TonB systems in V. cholerae (50), multiple TonB systems have been identified and characterized in a number of bacteria, including many Vibrio species (two or three systems) (51, 52, 66–68), Aeromonas hydrophila (three systems) (69–71), Pseudomonas aeruginosa (three systems) (63, 72, 73), Acinetobacter baumannii (three systems) (74, 75), and Bacteroides fragilis (six systems) (76). In those examples, some TonB systems are functionally independent while others show functional redundancy, for transport for particular substances such as siderophores and other nutrients, or physiological activities.
The aim of this study is to explain the Fe(III)-turnerbactin uptake mechanism. The fttA gene located in Region 7 is a homolog of Fe(III)-siderophore TonB-dependent outer membrane receptors. qRT-PCR analysis showed that the fttA gene and two turnerbactin biosynthetic genes, tnbA and tnbF, are clearly upregulated under iron-limiting growth conditions. Iron regulation of genes in Region 7 was further analyzed by RNA sequencing (RNA-seq), and all annotated iron transport-related genes in Region 7, fttA to TERTU_RS18085, were upregulated under iron-limiting conditions (Table S3). Furthermore, the Fur titration assay (FURTA) showed that the E. coli ferric uptake regulator, Fur, can bind to the potential promoter regions previously identified and located upstream of fttA and tnbC whereas the upstream region of TERTU_RS18075 showed a negative result. Taken together, the iron regulation of Region 7 is caused by at least two distinct promoters in a Fur-dependent manner, as proposed before (25). We constructed an in-frame deletion mutant of fttA and showed that the fttA gene is responsible for Fe(III)-turnerbactin transport and indispensable for growth under iron-limiting conditions while the fttA mutant grew well under iron-rich growth conditions, demonstrating that FttA is the sole TBDR involved in Fe(III)-turnerbactin uptake. We also tested the ability of T. turnerae to transport xenosiderophores, amphi-enterobactin and anguibactin, from Vibrio campbellii. Our results showed that T. turnerae can utilize Fe(III)-amphi-enterobactin or its hydrolyzed derivatives as an iron source and it was independent of fttA, whereas Fe(III)-anguibactin failed to enhance the growth of T. turnerae under iron-limiting conditions. Amphi-enterobactin is produced by both V. campbellii and Vibrio harveyi that are members of the Harveyi clade ubiquitously found in marine environments, and some strains are causative agents of vibriosis that affect marine vertebrates and invertebrates. On the other hand, anguibactin is produced by V. campbellii but not by V. harveyi (34, 77). Our findings indicate that T. turnerae possess the ability to “steal” iron from the siderophore or its derivatives commonly found in different species rather than from the species-specific siderophore, and this might provide an advantage to T. turnerae to survive in marine environments where amphi-enterobactin is available. It is still unknown what gene(s) is encoding the outer membrane receptor for Fe(III)-amphi-enterobactin since fttA was not required for Fe(III)-amphi-enterobactin utilization. In the T. turnerae T7901 genome, 38 genes were annotated to encode TonB-dependent outer membrane receptors, and our RNA-seq result indicated that six genes in addition to fttA were upregulated (logFC > 1) under iron-limiting growth conditions (Table S4), and one or some of them might be responsible for Fe(III)-amphi-enterobactin utilization.
By searching in the genome of T. turnerae, we identified four TonB genes that are located in three TonB clusters. The tonB1 cluster of T. turnerae is a unique tonB cluster that contains two tonB genes, tonB1a and tonB1b, and exbD1 but lacks exbB typically found in TonB clusters. TonB1a carries a N-terminal extension as compared with conventional TonB proteins, and this type of TonB protein was identified by bioinformatic analysis, but the function is still unknown (54). The gene organization of TonB2 and TonB3 clusters resembles marine vibrios and contains homologs of ttpB, ttpC, exbB, exbD, tonB, and ttpD in which ttpB, ttpC, and ttpD are specifically found in vibrios and some marine bacteria (29, 78). In vibrios, the TonB2 cluster is involved in iron transport while the function of the TonB3 cluster is still unknown (79, 80). The similarity of TonB2 and TonB3 systems, especially the presence of ttpB, ttpC, and ttpD, to those of vibrios indicates that the tonB2 system could provide benefits to adapt in coastal waters where both T. turnerae and vibrios live. Conversely, the TonB1 cluster of T. turnerae did not show similarity to that of vibrios. Vibrio TonB1 systems are linked to gene clusters that are responsible for hemin/hemoglobin uptake and are involved in hemin/hemoglobin uptake (48–50, 52, 81–83). We speculate that T. tunerae did not evolve a similar TonB1 cluster possibly due to the absence of a heme/hemoglobin cluster, and T. turnerae does not encounter environments in which hemin and/or hemoglobin is available during their life cycle, due to the absence of hemoglobin in bivalves such as the shipworm hosts.
In most bacteria, TonB genes are normally upregulated in iron-limiting conditions (actually repressed under iron-rich conditions) because excess amounts of iron are toxic to bacteria because it leads to Fenton reaction causing the overproduction of reactive oxygen species in the presence of oxygen. Interestingly, qRT-PCR results showed that none of TonB genes as well as other genes in tonB clusters are clearly regulated under iron-limiting conditions and the expression pattern of TonB genes was further confirmed by RNA-seq, supporting the result of qRT-PCR and also suggesting that the regulation occurs at a cluster level. It has been reported that tonB3 genes in V. vulnificus and A. hydrophila are not iron regulated (69, 79). However, neither of the tonB3 genes in those bacteria are involved in iron transport. It is of interest that all T. turnerae TonB genes are not clearly iron regulated even though tonB1b and tonB2 genes are involved in Fe(III)-turnerbactin utilization. These results indicated that T. turnerae might still need tonB genes expressed even in iron-rich conditions.
One of the unusual features of T. turnerae is its ability to degrade lignocellulose from wood and utilize its derivatives as a carbon source (19, 21). It has been reported that some bacteria use TBDRs to take up plant-derived carbohydrates and mono- and polysaccharides. Xanthomonas campestris pv. campestris (Xcc) use TBDR to take up sucrose, and the comparative genomic and gene expression analysis suggested that Xcc as well as some marine bacteria possibly take up plant carbohydrates via TBDRs (84). Caulobacter crescentus uses the TonB1 system to transport maltose and maltodextrins (85, 86). We showed that two of the tonB genes, tonB1b and tonB2, are involved in carbohydrate utilization derived from cellulose in T. turnerae whereas mutations in other tonB genes (tonB1a and tonB3) did not affect the growth. These results indicate that the same set of tonB1b and tonB2 is functional not only for Fe(III)-turnerbactin uptake but also cellulose utilization. Further studies are required to identify TBDRs involved in the uptake of cellulose-derived carbohydrates and what carbohydrate(s) are transported across the outer membrane. Some TBDRs are located close to genes potentially involved in hemicellulose degradation (data not shown). It is worth noting that the experiments were performed under iron-rich conditions; therefore, the lack of growth was not due to iron limitation. These results indicate that tonB1b and tonB2 are functional even under iron-rich conditions. All tonB genes are expressed in both iron-rich and iron-limiting growth conditions (Table S3). This dual role of TonB1b and TonB2 for iron and carbohydrate uptake could explain why T. turnerae does not clearly regulate those genes depending on iron concentrations although TonB genes are downregulated under iron-rich conditions in most bacteria.
We still have much to understand regarding the acquisition of cellulose-derived carbon sources by shipworm bacterial symbionts. The shipworm symbiosis presents a unique system where bacterial symbionts reside in vesicles of bacteriocytes in the gland of Deshayes within the gills of shipworms (87), while cellulose degradation takes place in a separate, nearly bacteria-free organ known as the caecum. Cellulolytic enzymes produced by gill bacterial symbionts and the host are utilized in this process. In this model, under in vivo conditions, it is highly likely that symbiotic bacteria do not have direct contact with cellulose. Therefore, the mechanism by which shipworm bacterial symbionts in the gill acquire carbon sources after their enzymes remotely digest cellulose in a distant organ remains a mystery. Previous studies have reported that cellulolytic enzymes produced by gill symbionts are transported to the mouth area and reach the caecum along with ingested wood particles (88, 89). The transport of cellulolytic enzymes from the gill symbiont to the mouth area occurs through the ducts of Deshayes, although the exact mechanism is still unknown (88). One possibility is that shipworm symbionts might acquire cellulose derivatives through systems such as the ducts of Deshayes from the caecum and/or the mouth. Alternatively, symbiotic bacteria may obtain carbon sources from the host cytosol through host metabolism, where cellulose-derived carbon sources are absorbed in the caecum.
Shipworm symbiotic bacteria might also have direct contact with cellulose in the environment, although this remains undiscovered. Previous studies have shown that in one shipworm species, Bankia setacea, bacterial symbionts are acquired through vertical transmission directly from parents (90). However, a recent report demonstrated that juveniles of other species such as Lyrodus pedicellatus and/or Teredo bartschi are initially free of bacterial symbionts and acquire them through horizontal transmission from the environment (91). T. turnerae has been isolated from these species, and the genome sequence of T. turnerae does not exhibit features typically observed in obligate intracellular symbionts, such as reduced genome size, GC content, and loss of genes involved in the core metabolism. Taken together, there is a possibility that T. turnerae survives in the marine environment and might need to acquire carbon sources by directly contacting cellulose in free-living conditions.
In summary, the fttA gene, a homolog of Fe(III)-siderophore TBDR genes, is indispensable for the survival of T. turnerae under iron-limiting growth conditions because it is essential for Fe(III)-turnerbactin utilization as an iron source. FttA appears to be essential for the transport of Fe(III)-turnerbactin across the outer membrane, and Fe(III)-amphi-enterobactin produced by other marine bacteria can be utilized as an iron source without FttA. Two out of four tonB genes, tonB1b and tonB2, show functional redundancy for the survival of T. turnerae under iron-limiting conditions as well as the growth of T. turnerae when cellulose was supplied as a sole carbon source. Since tonB genes are known to energize TBDRs to substrate import across the outer membrane, those findings indicate that carbohydrates derived from cellulose are likely transported by TBDRs. All of the genes in Region 7 encompassing fttA to TERTU_RS18085 were repressed under iron-rich conditions to avoid intracellular excess iron whereas the expression of the tonB genes remained under iron-rich conditions, indicating the importance of tonB genes even under iron-rich conditions possibly for the utilization of cellulose as a carbon source.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health Grant 1U01TW008163.
We would like to thank Drs. Aaron Puri (University of Utah), Daniel Distel (Northeastern University), and Alison Butler (University of California, Santa Barbara) for the valuable comments.
Contributor Information
Margo G. Haygood, Email: mhaygood@ucsd.edu.
Isaac Cann, University of Illinois Urbana-Champaign, Urbana, Illinois, USA.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/aem.00744-23.
Supplemental tables and figures, experimental details.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Crichton RR. 2016. Iron metabolism: from molecular mechanisms to clinical consequences. Fourth edition. ed. John Wiley & Sons. [Google Scholar]
- 2. Crosa JH, Walsh CT. 2002. Genetics and assembly line enzymology of siderophore biosynthesis in bacteria. Microbiol Mol Biol Rev 66:223–249. doi: 10.1128/MMBR.66.2.223-249.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cuív PO, Clarke P, Lynch D, O’Connell M. 2004. Identification of rhtX and fptX, novel genes encoding proteins that show homology and function in the utilization of the siderophores rhizobactin 1021 by Sinorhizobium meliloti and pyochelin by Pseudomonas aeruginosa, respectively. J Bacteriol 186:2996–3005. doi: 10.1128/JB.186.10.2996-3005.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hannauer M, Barda Y, Mislin GLA, Shanzer A, Schalk IJ. 2010. The ferrichrome uptake pathway in Pseudomonas aeruginosa involves an iron release mechanism with acylation of the siderophore and recycling of the modified desferrichrome. J Bacteriol 192:1212–1220. doi: 10.1128/JB.01539-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raymond KN, Dertz EA. 2004. Biochemical and physical properties of Siderophores, p 1–17. In Crosa JH, Mey AR, Payne SM (ed), Iron transport in bacteria. John Wiley & Sons, Inc. [Google Scholar]
- 6. Reimmann C. 2012. Inner-membrane transporters for the siderophores pyochelin in Pseudomonas aeruginosa and enantio-pyochelin in Pseudomonas fluorescens display different enantioselectivities. Microbiology (Reading) 158:1317–1324. doi: 10.1099/mic.0.057430-0 [DOI] [PubMed] [Google Scholar]
- 7. Winkelmann G. 2004. Ecology of Siderophores, p 435–450. In Crosa JH, Mey AR, Payne SM (ed), Iron transport in bacteria. John Wiley & Sons, Inc. [Google Scholar]
- 8. Braun V. 1995. Energy-coupled transport and signal transduction through the gram-negative outer membrane via TonB-ExbB-ExbD-dependent receptor proteins. FEMS Microbiol Rev 16:295–307. doi: 10.1111/j.1574-6976.1995.tb00177.x [DOI] [PubMed] [Google Scholar]
- 9. Noinaj N, Guillier M, Barnard TJ, Buchanan SK. 2010. Tonb-dependent transporters: regulation, structure, and function. Annu Rev Microbiol 64:43–60. doi: 10.1146/annurev.micro.112408.134247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Postle K, Kadner RJ. 2003. Touch and go: tying Tonb to transport. Mol Microbiol 49:869–882. doi: 10.1046/j.1365-2958.2003.03629.x [DOI] [PubMed] [Google Scholar]
- 11. Andrews SC, Robinson AK, Rodríguez-Quiñones F. 2003. Bacterial iron homeostasis. FEMS Microbiol Rev 27:215–237. doi: 10.1016/S0168-6445(03)00055-X [DOI] [PubMed] [Google Scholar]
- 12. Crosa JH. 1997. Signal Transduction and transcriptional and posttranscriptional control of iron-regulated genes in bacteria. Microbiol Mol Biol Rev 61:319–336. doi: 10.1128/mmbr.61.3.319-336.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fleischhacker AS, Kiley PJ. 2011. Iron-containing transcription factors and their roles as sensors. Curr Opin Chem Biol 15:335–341. doi: 10.1016/j.cbpa.2011.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Distel DL, Amin M, Burgoyne A, Linton E, Mamangkey G, Morrill W, Nove J, Wood N, Yang J. 2011. Molecular phylogeny of Pholadoidea Lamarck, 1809 supports a single origin for xylotrophy (wood feeding) and xylotrophic bacterial endosymbiosis in Bivalvia. Mol Phylogenet Evol 61:245–254. doi: 10.1016/j.ympev.2011.05.019 [DOI] [PubMed] [Google Scholar]
- 15. Turner RD. 1966. A survey and illustrated catalogue of the Teredinidae (Mollusca: Bivalvia). Museum of Comparative Zoology, Harvard University, Cambridge. doi: 10.5962/bhl.title.67017 [DOI] [Google Scholar]
- 16. Distel DL, Beaudoin DJ, Morrill W. 2002. Coexistence of multiple proteobacterial endosymbionts in the gills of the wood-boring Bivalve Lyrodus pedicellatus . Appl Environ Microbiol 68:6292–6299. doi: 10.1128/AEM.68.12.6292-6299.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ekborg NA, Morrill W, Burgoyne AM, Li L, Distel DL. 2007. Celab, a multifunctional cellulase encoded by Teredinibacter turnerae T7902t, a culturable symbiont isolated from the wood-boring marine bivalve Lyrodus pedicellatus. Appl Environ Microbiol 73:7785–7788. doi: 10.1128/AEM.00876-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Luyten YA, Thompson JR, Morrill W, Polz MF, Distel DL. 2006. Extensive variation in intracellular symbiont community composition among members of a single population of the wood-boring bivalve Lyrodus pedicellatus. Appl Environ Microbiol 72:412–417. doi: 10.1128/AEM.72.1.412-417.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Waterbury JB, Calloway CB, Turner RD. 1983. A cellulolytic nitrogen-fixing bacterium cultured from the gland of deshayes in shipworms (bivalvia: teredinidae). Science 221:1401–1403. doi: 10.1126/science.221.4618.1401 [DOI] [PubMed] [Google Scholar]
- 20. Altamia MA, Wood N, Fung JM, Dedrick S, Linton EW, Concepcion GP, Haygood MG, Distel DL. 2014. Genetic differentiation among isolates of Teredinibacter turnerae, a widely occurring intracellular endosymbiont of shipworms. Mol Ecol 23:1418–1432. doi: 10.1111/mec.12667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Distel DL, Morrill W, MacLaren-Toussaint N, Franks D, Waterbury J. 2002. Teredinibacter turnerae gen nov. sp. nov., a dinitrogen-fixing, cellulolytic, endosymbiotic gamma-proteobacterium isolated from the gills of wood-boring molluscs (Bivalvia: Teredinidae). Int J Syst Evol Microbiol 52:2261–2269. doi: 10.1099/00207713-52-6-2261 [DOI] [PubMed] [Google Scholar]
- 22. Lechene CP, Luyten Y, McMahon G, Distel DL. 2007. Quantitative imaging of nitrogen fixation by individual bacteria within animal cells. Science 317:1563–1566. doi: 10.1126/science.1145557 [DOI] [PubMed] [Google Scholar]
- 23. O’Connor RM, Fung JM, Sharp KH, Benner JS, McClung C, Cushing S, Lamkin ER, Fomenkov AI, Henrissat B, Londer YY, Scholz MB, Posfai J, Malfatti S, Tringe SG, Woyke T, Malmstrom RR, Coleman-Derr D, Altamia MA, Dedrick S, Kaluziak ST, Haygood MG, Distel DL. 2014. Gill bacteria enable a novel digestive strategy in a wood-feeding mollusk. Proc Natl Acad Sci U S A 111:E5096–104. doi: 10.1073/pnas.1413110111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Elshahawi SI, Trindade-Silva AE, Hanora A, Han AW, Flores MS, Vizzoni V, Schrago CG, Soares CA, Concepcion GP, Distel DL, Schmidt EW, Haygood MG. 2013. Boronated tartrolon antibiotic produced by symbiotic cellulose-degrading bacteria in shipworm gills. Proc Natl Acad Sci U S A 110:E295–304. doi: 10.1073/pnas.1213892110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Han AW, Sandy M, Fishman B, Trindade-Silva AE, Soares CAG, Distel DL, Butler A, Haygood MG. 2013. Turnerbactin, a novel triscatecholate siderophore from the shipworm endosymbiont Teredinibacter turnerae T7901. PLoS One 8:e76151. doi: 10.1371/journal.pone.0076151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miller BW, Lim AL, Lin Z, Bailey J, Aoyagi KL, Fisher MA, Barrows LR, Manoil C, Schmidt EW, Haygood MG. 2021. Shipworm symbiosis ecology-guided discovery of an antibiotic that kills colistin-resistant acinetobacter. Cell Chem Biol 28:1628–1637. doi: 10.1016/j.chembiol.2021.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miller BW, Schmidt EW, Concepcion GP, Haygood MG. 2022. Halogenated metal-binding compounds from shipworm symbionts. J Nat Prod 85:479–484. doi: 10.1021/acs.jnatprod.1c01049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Altamia MA, Lin Z, Trindade-Silva AE, Uy ID, Shipway JR, Wilke DV, Concepcion GP, Distel DL, Schmidt EW, Haygood MG. 2020. Secondary metabolism in the gill microbiota of shipworms (Teredinidae) as revealed by comparison of metagenomes and nearly complete symbiont genomes. mSystems 5:e00261-20. doi: 10.1128/mSystems.00261-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kuehl CJ, Crosa JH. 2010. The TonB energy transduction systems in Vibrio species. Future Microbiol 5:1403–1412. doi: 10.2217/fmb.10.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang JC, Madupu R, Durkin AS, Ekborg NA, Pedamallu CS, Hostetler JB, Radune D, Toms BS, Henrissat B, Coutinho PM, Schwarz S, Field L, Trindade-Silva AE, Soares CAG, Elshahawi S, Hanora A, Schmidt EW, Haygood MG, Posfai J, Benner J, Madinger C, Nove J, Anton B, Chaudhary K, Foster J, Holman A, Kumar S, Lessard PA, Luyten YA, Slatko B, Wood N, Wu B, Teplitski M, Mougous JD, Ward N, Eisen JA, Badger JH, Distel DL, Ahmed N. 2009. The complete genome of Teredinibacter turnerae T7901: an intracellular endosymbiont of marine wood-boring bivalves (shipworms). PLoS ONE 4:e6085. doi: 10.1371/journal.pone.0006085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM, Peterson KM. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176. doi: 10.1016/0378-1119(95)00584-1 [DOI] [PubMed] [Google Scholar]
- 32. Milton DL, O’Toole R, Horstedt P, Wolf-Watz H. 1996. Flagellin A is essential for the virulence of Vibrio anguillarum. J Bacteriol 178:1310–1319. doi: 10.1128/jb.178.5.1310-1319.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miller WG, Leveau JH, Lindow SE. 2000. Improved gfp and inaZ broad-host-range promoter-probe vectors. Mol Plant Microbe Interact 13:1243–1250. doi: 10.1094/MPMI.2000.13.11.1243 [DOI] [PubMed] [Google Scholar]
- 34. Naka H, Reitz ZL, Jelowicki AM, Butler A, Haygood MG. 2018. Amphi-enterobactin commonly produced among Vibrio campbellii and Vibrio harveyi strains can be taken up by a novel outer membrane protein FapA that also can transport canonical Fe(III)-enterobactin. J Biol Inorg Chem 23:1009–1022. doi: 10.1007/s00775-018-1601-5 [DOI] [PubMed] [Google Scholar]
- 35. Senanayake SD, Brian DA. 1995. Precise large deletions by the PCR-based overlap extension method. Mol Biotechnol 4:13–15. doi: 10.1007/BF02907467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stabb EV, Ruby EG. 2002. Rp4-based plasmids for conjugation between Escherichia coli and members of the vibrionaceae. Methods Enzymol 358:413–426. doi: 10.1016/s0076-6879(02)58106-4 [DOI] [PubMed] [Google Scholar]
- 37. Lopez JA, Bohuski E. 2007. Total RNA extraction with TRIZOL reagent and purification with QIAGEN Rneasy mini kit. DGC. Indiana University, Bloomington, IN. [Google Scholar]
- 38. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 39. Ghysels B, Dieu BTM, Beatson SA, Pirnay JP, Ochsner UA, Vasil ML, Cornelis P. 2004. FpvB, an alternative type I ferripyoverdine receptor of Pseudomonas aeruginosa. Microbiology (Reading) 150:1671–1680. doi: 10.1099/mic.0.27035-0 [DOI] [PubMed] [Google Scholar]
- 40. Mey AR, Wyckoff EE, Oglesby AG, Rab E, Taylor RK, Payne SM. 2002. Identification of the Vibrio cholerae enterobactin receptors VctA and IrgA: IrgA is not required for virulence. Infect Immun 70:3419–3426. doi: 10.1128/IAI.70.7.3419-3426.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Naka H, Crosa JH. 2012. Identification and characterization of a novel outer membrane protein receptor FetA for ferric enterobactin transport in Vibrio anguillarum 775 (pJM1). Biometals 25:125–133. doi: 10.1007/s10534-011-9488-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Payne SM, Mey AR, Wyckoff EE. 2016. Vibrio iron transport: evolutionary adaptation to life in multiple environments. Microbiol Mol Biol Rev 80:69–90. doi: 10.1128/MMBR.00046-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Poole K, Neshat S, Krebes K, Heinrichs DE. 1993. Cloning and nucleotide sequence analysis of the ferripyoverdine receptor gene fpvA of Pseudomonas aeruginosa. J Bacteriol 175:4597–4604. doi: 10.1128/jb.175.15.4597-4604.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rabsch W, Voigt W, Reissbrodt R, Tsolis RM, Bäumler AJ. 1999. Salmonella typhimurium iron and FepA proteins mediate uptake of enterobactin but differ in their specificity for other siderophores. J Bacteriol 181:3610–3612. doi: 10.1128/JB.181.11.3610-3612.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wyckoff EE, Allred BE, Raymond KN, Payne SM. 2015. Catechol siderophore transport by Vibrio cholerae. J Bacteriol 197:2840–2849. doi: 10.1128/JB.00417-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Harrison KJ, Crécy-Lagard V de, Zallot R. 2018. Gene graphics: a genomic neighborhood data visualization web application. Bioinformatics 34:1406–1408. doi: 10.1093/bioinformatics/btx793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bellaire BH, Elzer PH, Baldwin CL, Roop RM. 2003. Production of the siderophore 2,3-dihydroxybenzoic acid is required for wild-type growth of Brucella abortus in the presence of erythritol under low-iron conditions in vitro . Infect Immun 71:2927–2932. doi: 10.1128/IAI.71.5.2927-2932.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kustusch RJ, Kuehl CJ, Crosa JH. 2012. The ttpC gene is contained in two of three TonB systems in the human pathogen Vibrio vulnificus, but only one is active in iron transport and virulence. J Bacteriol 194:3250–3259. doi: 10.1128/JB.00155-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. O’Malley SM, Mouton SL, Occhino DA, Deanda MT, Rashidi JR, Fuson KL, Rashidi CE, Mora MY, Payne SM, Henderson DP. 1999. Comparison of the heme iron utilization systems of pathogenic vibrios. J Bacteriol 181:3594–3598. doi: 10.1128/JB.181.11.3594-3598.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Occhino DA, Wyckoff EE, Henderson DP, Wrona TJ, Payne SM. 1998. Vibrio cholerae iron transport: haem transport genes are linked to one of two sets of tonB, exbB, exbD genes. Mol Microbiol 29:1493–1507. doi: 10.1046/j.1365-2958.1998.01034.x [DOI] [PubMed] [Google Scholar]
- 51. Stork M, Di Lorenzo M, Mouriño S, Osorio CR, Lemos ML, Crosa JH. 2004. Two tonB systems function in iron transport in Vibrio anguillarum, but only one is essential for virulence. Infect Immun 72:7326–7329. doi: 10.1128/IAI.72.12.7326-7329.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang Q, Liu Q, Cao X, Yang M, Zhang Y. 2008. Characterization of two TonB systems in marine fish pathogen Vibrio alginolyticus: their roles in iron utilization and virulence. Arch Microbiol 190:595–603. doi: 10.1007/s00203-008-0407-1 [DOI] [PubMed] [Google Scholar]
- 53. Krogh A, Larsson B, von Heijne G, Sonnhammer EL. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305:567–580. doi: 10.1006/jmbi.2000.4315 [DOI] [PubMed] [Google Scholar]
- 54. Chu BCH, Peacock RS, Vogel HJ. 2007. Bioinformatic analysis of the TonB protein family. Biometals 20:467–483. doi: 10.1007/s10534-006-9049-4 [DOI] [PubMed] [Google Scholar]
- 55. Beddek AJ, Sheehan BJ, Bossé JT, Rycroft AN, Kroll JS, Langford PR. 2004. Two TonB systems in Actinobacillus pleuropneumoniae: their roles in iron acquisition and virulence. Infect Immun 72:701–708. doi: 10.1128/IAI.72.2.701-708.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bender KS, Yen H-CB, Hemme CL, Yang Z, He Z, He Q, Zhou J, Huang KH, Alm EJ, Hazen TC, Arkin AP, Wall JD. 2007. Analysis of a ferric uptake regulator (Fur) mutant of Desulfovibrio vulgaris Hildenborough. Appl Environ Microbiol 73:5389–5400. doi: 10.1128/AEM.00276-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bjarnason J, Southward CM, Surette MG. 2003. Genomic profiling of iron-responsive genes in Salmonella enterica serovar typhimurium by high-throughput screening of a random promoter library. J Bacteriol 185:4973–4982. doi: 10.1128/JB.185.16.4973-4982.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bosch M, Garrido E, Llagostera M, Pérez de Rozas AM, Badiola I, Barbé J. 2002. Pasteurella multocida exbB, exbD and tonB genes are physically linked but independently transcribed. FEMS Microbiol Lett 210:201–208. doi: 10.1111/j.1574-6968.2002.tb11181.x [DOI] [PubMed] [Google Scholar]
- 59. Mey AR, Wyckoff EE, Kanukurthy V, Fisher CR, Payne SM. 2005. Iron and fur regulation in Vibrio cholerae and the role of fur in virulence . Infect Immun 73:8167–8178. doi: 10.1128/IAI.73.12.8167-8178.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ochsner UA, Wilderman PJ, Vasil AI, Vasil ML. 2002. Genechip expression analysis of the iron starvation response in Pseudomonas aeruginosa: identification of novel pyoverdine biosynthesis genes. Mol Microbiol 45:1277–1287. doi: 10.1046/j.1365-2958.2002.03084.x [DOI] [PubMed] [Google Scholar]
- 61. Osorio CR, Lemos ML, Braun V. 2004. Identification of fur regulated genes in the bacterial fish pathogen Photobacterium damselae ssp. piscicida using the fur titration assay. Biometals 17:725–733. doi: 10.1007/s10534-004-1652-7 [DOI] [PubMed] [Google Scholar]
- 62. Young GM, Postle K. 1994. Repression of tonB transcription during anaerobic growth requires fur binding at the promoter and a second factor binding upstream. Mol Microbiol 11:943–954. doi: 10.1111/j.1365-2958.1994.tb00373.x [DOI] [PubMed] [Google Scholar]
- 63. Zhao Q, Poole K. 2000. A second tonB gene in Pseudomonas aeruginosa is linked to the exbB and exbD genes. FEMS Microbiol Lett 184:127–132. doi: 10.1111/j.1574-6968.2000.tb09002.x [DOI] [PubMed] [Google Scholar]
- 64. Ratliff AC, Buchanan SK, Celia H. 2022. The ton motor. Front Microbiol 13:852955. doi: 10.3389/fmicb.2022.852955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Postle K, Larsen RA. 2007. TonB-dependent energy transduction between outer and cytoplasmic membranes. Biometals 20:453–465. doi: 10.1007/s10534-006-9071-6 [DOI] [PubMed] [Google Scholar]
- 66. Alice AF, Naka H, Crosa JH. 2008. Global gene expression as a function of the iron status of the bacterial cell: influence of differentially expressed genes in the virulence of the human pathogen Vibrio vulnificus. Infect Immun 76:4019–4037. doi: 10.1128/IAI.00208-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Seliger SS, Mey AR, Valle AM, Payne SM. 2001. The two TonB systems of Vibrio cholerae: redundant and specific functions. Mol Microbiol 39:801–812. doi: 10.1046/j.1365-2958.2001.02273.x [DOI] [PubMed] [Google Scholar]
- 68. Tanabe T, Funahashi T, Shiuchi K, Okajima N, Nakao H, Miyamoto K, Tsujibo H, Yamamoto S. 2012. Characterization of Vibrio parahaemolyticus genes encoding the systems for utilization of enterobactin as a xenosiderophore. Microbiology (Reading) 158:2039–2049. doi: 10.1099/mic.0.059568-0 [DOI] [PubMed] [Google Scholar]
- 69. Dong Y, Geng J, Liu J, Pang M, Awan F, Lu C, Liu Y. 2019. Roles of three TonB systems in the iron utilization and virulence of the Aeromonas hydrophila Chinese epidemic strain NJ-35. Appl Microbiol Biotechnol 103:4203–4215. doi: 10.1007/s00253-019-09757-4 [DOI] [PubMed] [Google Scholar]
- 70. Dong Y, Liu J, Pang M, Du H, Wang N, Awan F, Lu C, Liu Y. 2016. Catecholamine-stimulated growth of Aeromonas hydrophila requires the TonB2 energy transduction system but is independent of the Amonabactin siderophore. Front Cell Infect Microbiol 6:183. doi: 10.3389/fcimb.2016.00183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dong Y, Xu M, Wan X, Zhao D, Geng J, Huang H, Jiang M, Lu C, Liu Y. 2023. TonB systems are required for Aeromonas hydrophila motility by controlling the secretion of flagellin. Microbes and Infection 25:105038. doi: 10.1016/j.micinf.2022.105038 [DOI] [PubMed] [Google Scholar]
- 72. Huang B, Ru K, Yuan Z, Whitchurch CB, Mattick JS. 2004. TonB3 is required for normal twitching motility and extracellular assembly of type IV Pili. J Bacteriol 186:4387–4389. doi: 10.1128/JB.186.13.4387-4389.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Poole K, Zhao Q, Neshat S, Heinrichs DE, Dean CR. 1996. The Pseudomonas aeruginosa tonB gene encodes a novel TonB protein. Microbiology (Reading) 142 (Pt 6):1449–1458. doi: 10.1099/13500872-142-6-1449 [DOI] [PubMed] [Google Scholar]
- 74. Runci F, Gentile V, Frangipani E, Rampioni G, Leoni L, Lucidi M, Visaggio D, Harris G, Chen W, Stahl J, Averhoff B, Visca P. 2019. Contribution of active iron uptake to Acinetobacter baumannii pathogenicity. Infect Immun 87:e00755-18. doi: 10.1128/IAI.00755-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zimbler DL, Arivett BA, Beckett AC, Menke SM, Actis LA. 2013. Functional features of TonB energy transduction systems of Acinetobacter baumannii. Infect Immun 81:3382–3394. doi: 10.1128/IAI.00540-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Parker AC, Seals NL, Baccanale CL, Rocha ER. 2022. Analysis of six tonB gene homologs in Bacteroides fragilis revealed that TonB3 is essential for survival in experimental intestinal colonization and intra-abdominal infection. Infect Immun 90:e0046921. doi: 10.1128/IAI.00469-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zane HK, Naka H, Rosconi F, Sandy M, Haygood MG, Butler A. 2014. Biosynthesis of amphi-enterobactin siderophores by Vibrio harveyi BAA-1116: identification of a bifunctional nonribosomal peptide synthetase condensation domain. J Am Chem Soc 136:5615–5618. doi: 10.1021/ja5019942 [DOI] [PubMed] [Google Scholar]
- 78. Barnes AD, Pfeifer HJ, Zbylicki BR, Roberts EK, Rudd JC, Manzo MA, Phillips EA, Berry MM, Kenton RJ. 2020. Two novel proteins, TtpB2 and TtpD2, are essential for iron transport in the TonB2 system of Vibrio vulnificus. Microbiologyopen 9:e00947. doi: 10.1002/mbo3.947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Alice AF, Crosa JH. 2012. The TonB3 system in the human pathogen Vibrio vulnificus is under the control of the global regulators Lrp and cyclic AMP receptor protein. J Bacteriol 194:1897–1911. doi: 10.1128/JB.06614-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Duong-Nu TM, Jeong K, Hong SH, Nguyen HV, Ngo VH, Min JJ, Lee SE, Rhee JH. 2016. All three TonB systems are required for Vibrio vulnificus CMCP6 tissue Invasiveness by controlling flagellum expression. Infect Immun 84:254–265. doi: 10.1128/IAI.00821-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lemos ML, Osorio CR. 2007. Heme, an iron supply for vibrios pathogenic for fish. Biometals 20:615–626. doi: 10.1007/s10534-006-9053-8 [DOI] [PubMed] [Google Scholar]
- 82. Mouriño S, Osorio CR, Lemos ML. 2004. Characterization of heme uptake cluster genes in the fish pathogen Vibrio anguillarum . J Bacteriol 186:6159–6167. doi: 10.1128/JB.186.18.6159-6167.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Septer AN, Wang Y, Ruby EG, Stabb EV, Dunn AK. 2011. The haem-uptake gene cluster in vibrio fischeri is regulated by fur and contributes to symbiotic colonization. Environ Microbiol 13:2855–2864. doi: 10.1111/j.1462-2920.2011.02558.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Blanvillain S, Meyer D, Boulanger A, Lautier M, Guynet C, Denancé N, Vasse J, Lauber E, Arlat M, Redfield R. 2007. Plant carbohydrate scavenging through tonB-dependent receptors: a feature shared by phytopathogenic and aquatic bacteria. PLoS ONE 2:e224. doi: 10.1371/journal.pone.0000224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lohmiller S, Hantke K, Patzer SI, Braun V. 2008. TonB-dependent maltose transport by Caulobacter crescentus. Microbiology (Reading) 154:1748–1754. doi: 10.1099/mic.0.2008/017350-0 [DOI] [PubMed] [Google Scholar]
- 86. Neugebauer H, Herrmann C, Kammer W, Schwarz G, Nordheim A, Braun V. 2005. ExbBD-dependent transport of maltodextrins through the novel mala protein across the outer membrane of Caulobacter crescentus. J Bacteriol 187:8300–8311. doi: 10.1128/JB.187.24.8300-8311.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Distel DL, DeLong EF, Waterbury JB. 1991. Phylogenetic characterization and in situ localization of the bacterial symbiont of shipworms (Teredinidae: Bivalvia) by using 16S rRNA sequence analysis and oligodeoxynucleotide probe hybridization. Appl Environ Microbiol 57:2376–2382. doi: 10.1128/aem.57.8.2376-2382.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Altamia MA, Distel DL. 2022. Transport of symbiont-encoded cellulases from the gill to the gut of shipworms via the enigmatic ducts of Deshayes: a 174-year mystery solved. Proc Biol Sci 289:20221478. doi: 10.1098/rspb.2022.1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pesante G, Sabbadin F, Elias L, Steele-King C, Shipway JR, Dowle AA, Li Y, Busse-Wicher M, Dupree P, Besser K, Cragg SM, Bruce NC, McQueen-Mason SJ. 2021. Characterisation of the enzyme transport path between shipworms and their bacterial symbionts. BMC Biol 19:233. doi: 10.1186/s12915-021-01162-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sipe AR, Wilbur AE, Cary SC. 2000. Bacterial symbiont transmission in the wood-boring shipworm Bankia setacea (Bivalvia: Teredinidae). Appl Environ Microbiol 66:1685–1691. doi: 10.1128/AEM.66.4.1685-1691.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Speare L, Distel DL. 2022. Cultivation and fluorescent in situ hybridization suggest that some shipworm species acquire endosymbiotic bacteria through indirect horizontal transmission. Microbiology. doi: 10.1101/2022.11.13.516348 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental tables and figures, experimental details.