Abstract
A 9-y-old Mangalarga Marchador gelding was referred to a veterinary hospital because of a swelling on the upper right side of the neck. Ultrasound examination revealed a multilocular structure adjacent to the thyroid gland with low echogenic content suggestive of fluid. The mass was removed surgically. Histologically, the cystic cavities in the surgical sample were filled with abundant eosinophilic secreta and lined by cuboidal, segmentally ciliated, columnar epithelium with interspersed goblet cells. Segmental crowding of the multilayered lining of the cyst was noted. Immunohistochemistry suggested the presence of both C cells and follicular cells, given the positivity of the immunomarkers calcitonin and TTF-1, respectively. The histogenesis of ultimobranchial cysts is uncertain. Based on clinical, histopathologic, and immunohistochemical identification, the cystic structure in this case is compatible with an ultimobranchial body cyst.
Keywords: branchial arches, histopathology, horses, thyroid gland, ultimobranchial cyst
Branchial cysts are congenital malformations that occur during the differentiation of branchial arches and are characterized by hollow areas with fluid accumulation and cell desquamation. 5 Branchial cysts are usually painless, with slow progressive growth, are located in the cervical region, and are more commonly diagnosed in humans in young people than adults.2,4,6,14 When this malformation is associated with the fifth branchial arch, it is called an ultimobranchial body cyst (UBC).10,19
A UBC was removed surgically and diagnosed histologically in a 1-y-old horse but was not further characterized immunophenotypically. 15 Remnants of the ultimobranchial body (microscopic organs formed during embryologic development) have been identified in thyroids of slaughterhouse horses using immunohistochemistry (IHC) 21 ; similar studies have been reported in other species, including cattle, 7 dogs, cats, 12 goats, 16 and humans. Although histologic evaluation provides good diagnostic clues, mainly to exclude other causes of neck masses, the definitive diagnosis of UBC is confirmed by immunohistochemical identification of C cells.7,21,22 We retrieved no cases of UBC diagnosed by IHC in horses in a search of Google Scholar, PubMed, CAB Direct, Web of Science, and Scopus, suggesting that no such descriptions of this condition have been reported in horses. We evaluated the clinical, histopathologic, and immunohistochemical features of an ultimobranchial cyst in an adult horse.
A 9-y-old, 397-kg, Mangalarga Marchador gelding was referred to the Veterinary Teaching Hospital of the School of Veterinary Medicine and Animal Science of the University of São Paulo, Brazil because of a swelling of 45-d duration in the right cranial cervical region. The owner reported that the onset was spontaneous, followed by a considerable increase in size over the following 2 wk. However, he did not observe any change in the behavior of the horse, or any similar previous condition in the animal.
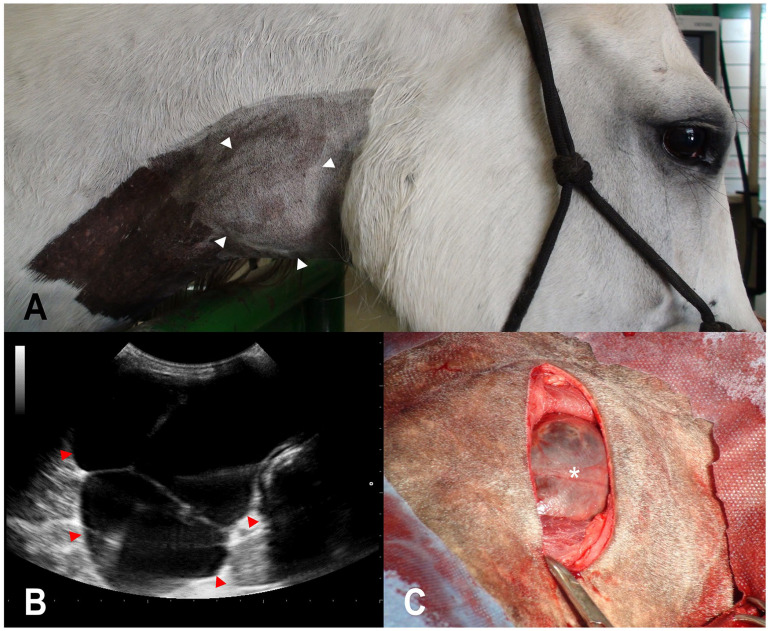
On physical examination, vital parameters were within normal limits. A swelling of ~10-cm diameter was identified in the ventral third of the most cranial cervical region on the right side (Fig. 1A). The swelling was fluctuant, with no pain or increase in local temperature. Ultrasound examination revealed a multilocular structure formed by thin septa with accumulation of predominantly anechoic content and areas of low echogenicity (Fig. 1B). The mass was adjacent to the thyroid gland, which was difficult to visualize. No changes were observed on the contralateral side of the neck. Endoscopy showed slight sagittal and left displacement of the first tracheal rings. Odorless brown liquid (500 mL) was obtained on puncture, and cytology revealed rare spindle-shaped cells. After 2 d, fluid accumulation was noted again. The hemogram and the biochemistry panel values were within RIs.
Figure 1.
An ultimobranchial cyst in a 9-y-old horse. A. Swelling on the right side of the neck (outlined by arrowheads). B. An ultrasonographic image of the multilocular septate cystic structure with low echogenic content (outlined by arrowheads). C. Surgical access to the cyst (asterisk), on the right side of the neck.
The mass was removed surgically under inhalation an-esthesia in the left lateral decubitus position, and a right hemithyroidectomy was performed (Fig. 1C). The mass was a 10 × 5 × 5-cm brown cystic structure with a multilocular irregular surface; after incision, brown-red filamentous friable material was seen adhered to the inner surface of the cysts (Fig. 2A).
A tissue sample was fixed in 10% neutral-buffered formalin and processed routinely; 5-µm sections were stained with H&E and examined by light microscopy. Histologic analysis revealed cysts lined by cuboidal epithelium with stretches of hyperplastic and sometimes ciliated columnar epithelium with interspersed goblet cells (Fig. 2B), surrounded by fibrovascular stroma and a thick fibrous capsule. In the lumen, a large amount of eosinophilic fibrillar hyaline material was observed. Within the stroma were hemorrhage and cholesterol clefts, as well as macrophages, lymphocytes, and plasma cells. The clinical and histopathologic findings were consistent with the diagnosis of an ultimobranchial cyst.
To confirm the diagnosis, 3-μm tissue sections were subjected to antigen retrieval (citric acid solution) followed by overnight incubation with a rabbit anti-human calcitonin polyclonal antibody (1:2,500 dilution; Dako); incubation with rabbit anti-thyroglobulin polyclonal antibody (1:50,000 dilution; Dako); and incubation with mouse anti-thyroid transcription factor 1 (TTF-1) monoclonal antibody (1:50 dilution; Cell marque, Rocklin). Signals were amplified (Novolink polymer detection system; Leica) and visualized with diaminobenzidine chromogen (MilliporeSigma). Sections were counterstained with Harris hematoxylin. Negative controls were tissue sections in which primary antibodies were replaced by non-immune serum of the species in which antibodies had been raised.
Immunohistochemically, we found positivity in the cytoplasm of the lining cells for calcitonin (Fig. 2C) and somatostatin (Fig. 2D), but despite pre-primary blocking, the marking was nonspecific and was also positive in macrophage cytoplasm, epithelial cells, and fibrocytes. The positive control used for calcitonin and somatostatin antibodies was a human medullary thyroid carcinoma (Fig. 2E, 2F); cross-reactivity in horses was confirmed. Lining cells were also positive for TTF-1 (Fig. 3A), but negative for anti-thyroglobulin (Fig. 3B). The positive control for TTF-1 and anti-thyroglobulin antibodies was thyroid tissue from a euthyroid horse (Fig. 3C, 3D).
Figure 2.
An ultimobranchial body cyst in a 9-y-old horse. A. The surgical specimen after sectioning shows the capsule and trabeculae. B. Cyst lined by cuboidal cells (asterisk), sometimes by ciliated columnar epithelium (arrowhead and in the inset), interspersed with goblet cells (arrow) H&E. C. Positive calcitonin reactivity in cell cytoplasm. IHC. D. Positive somatostatin reactivity in cell cytoplasm. IHC. E. Calcitonin positive control. IHC. F. Somatostatin positive control. IHC.
Figure 3.
Immunohistochemistry (IHC) of an ultimobranchial cyst in a 9-y-old horse. A. Positive TTF-1 reactivity in cell nucleus and cytoplasm. IHC. B. Negative anti-thyroglobulin reactivity for the cuboidal cells. IHC. C. TTF-1 positive control. IHC. D. Thyroglobulin positive control. IHC.
The surgical wound healed adequately, and no new cysts formed. The horse was discharged 13 d after surgery. Serum concentrations of total T3 and total T4 were measured before the procedure and after 60 d. Total T3 concentrations were within RIs in both evaluations. However, the total T4 concentration was decreased in the first evaluation and within the RI in the second evaluation. Two years after the procedure, the owner reported that the horse was healthy, and the owner was satisfied with the result.
Congenital cystic lesions in the cervical region are well-known in humans. Such lesions are described as malformations during the development of the branchial apparatus and have similar counterparts in other mammals.5,10 These cysts have been reported commonly in both foals and adult horses.2,6,13,15 The clinical presentation and the histopathologic findings in our case are very similar to those in a report in which a UBC was diagnosed histologically. 15
Although histopathology provides good evidence of cyst origin, immunoidentification of C cells is important to confirm the diagnosis of UBC. 11 The histogenesis of ultimobranchial cysts is uncertain, 11 both in humans and in dogs; the cysts may arise from the branchial cleft or from squamous metaplasia. 8
In our case, positivity for both TTF-1 and calcitonin in the immunohistochemical test was observed, demonstrating the presence of C cells in the examined cyst tissue. TTF-1 mRNA has been identified in C cells in rats and dogs 20 ; however, this staining has rarely been observed in thyroid C cells in canine tissue. 17 IHC (AE1/AE31 pancytokeratin antigen expression) has been used to diagnose 3 of the 8 cases of branchial remnant cysts in horses, 13 and, as in our report, thyroglobulin was negative, contributing to the diagnosis. We should highlight that our positive thyroglobulin control did not have labeling as strong as expected, probably because of the long storage time of the sample used, although this did not affect identification of the tissue. Given the scarcity of information on UBC in horses, interpreting the IHC results needs to be done in light of the histologic and clinical findings. Based on our clinical, histopathologic, and immunohistochemical findings, our case is consistent with an ultimobranchial body cyst. In horses, differential diagnoses for neck swellings include strangles abscess (Streptococcus equi), hematoma, follicular cell adenoma, C-cell adenoma or adenocarcinoma, mast cell tumor, or medullary thyroid carcinoma. 9
Although surgical removal of cervical cysts has been strongly recommended in horses, 1 primarily for esthetic reasons, complications, such as dysphagia and esophageal obstruction, have been reported. 13 The possible development of neoplasms from remnants of the UBC 21 reinforces the value of surgery. In our case, although the cyst was large and was in a region with many critical structures, no complications were noted post-surgery. Other treatment options include sclerotherapy and marsupialization; however, complications and longer convalescence are more likely.3,18
Acknowledgments
We thank Natalia Coelho Couto de Azevedo Fernandes and Juliana Mariotti Guerra from the Pathology Center of Adolfo Lutz Institute (São Paulo, SP, Brazil) and Luiz Augusto Santana Silva from Patho-DxVet Diagnóstico Anatomopatológico for their collaboration in the performance and interpretation of the immunohistochemical assays, and the Laboratory of Histology, Department of Pathology, School of Veterinary Medicine and Animal Science, University of São Paulo (USP) for processing tissues for histologic examination. We also thank the editor and reviewers for their valuable suggestions.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Our study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES)–Finance Code 001.
ORCID iD: Anderson F. Souza  https://orcid.org/0000-0001-8066-4787
https://orcid.org/0000-0001-8066-4787
Contributor Information
Anderson F. Souza, Departamento de Cirurgia, Faculdade de Medicina Veterinária e Zootecnia, Universidade de São Paulo, São Paulo, Brazil.
Maria E. Volpato, Departamento de Cirurgia, Faculdade de Medicina Veterinária e Zootecnia, Universidade de São Paulo, São Paulo, Brazil
Stefano C. F. Hagen, Departamento de Cirurgia, Faculdade de Medicina Veterinária e Zootecnia, Universidade de São Paulo, São Paulo, Brazil
Celina S. Takenaka, Departamento de Patologia, Faculdade de Medicina Veterinária e Zootecnia, Universidade de São Paulo, São Paulo, Brazil
Paulo C. Maiorka, Departamento de Patologia, Faculdade de Medicina Veterinária e Zootecnia, Universidade de São Paulo, São Paulo, Brazil
Rodrigo A. Ressio, Centro de Patologia, Instituto Adolfo Lutz, São Paulo, Brazil
Luis C. L. C. Silva, Departamento de Cirurgia, Faculdade de Medicina Veterinária e Zootecnia, Universidade de São Paulo, São Paulo, Brazil
Raquel Y. A. Baccarin, Departamento de Clínica Médica, Faculdade de Medicina Veterinária e Zootecnia, Universidade de São Paulo, São Paulo, Brazil
References
- 1. Beard WL. Surgical removal of cystic structures of the cranial cervical region of the horse. Equine Vet Educ 2010;22:170–171. [Google Scholar]
- 2. David F, et al. Congenital branchial apparatus malformation in a Haflinger colt. Vet Surg 2008;37:3–11. [DOI] [PubMed] [Google Scholar]
- 3. de Estrada J, Schumacher J. Treatment of an 18-year-old mare for bilateral, branchial remnant cysts. Equine Vet Educ 2013;25:129–133. [Google Scholar]
- 4. Field JR, et al. Ablation of a congenital neck mass in a foal. Can Vet J 1990;31:643–644. [PMC free article] [PubMed] [Google Scholar]
- 5. Grevellec A, Tucker AS. The pharyngeal pouches and clefts: development, evolution, structure and derivatives. Semin Cell Dev Biol 2010;21:325–332. [DOI] [PubMed] [Google Scholar]
- 6. Hance SR, et al. Branchial cyst in a filly. Equine Vet J 1992;24:329–331. [DOI] [PubMed] [Google Scholar]
- 7. Harmon BG, Kelley LC. Immunohistochemistry of ultimobranchial thyroid carcinomas in seven slaughtered cows and one bull. J Vet Diagn Invest 2001;13:101–105. [DOI] [PubMed] [Google Scholar]
- 8. Kameda Y. Follicular cell lineage in persistent ultimobranchial remnants of mammals. Cell Tissue Res 2019;376:1–18. [DOI] [PubMed] [Google Scholar]
- 9. Knottenbelt DC, et al. Endocrine and neuroendocrine neoplasms. In: Knottenbelt DC, et al. Clinical Equine Oncology. Elsevier, 2015:376–392. [Google Scholar]
- 10. LaRiviere CA, Waldhausen JHT. Congenital cervical cysts, sinuses, and fistulae in pediatric surgery. Surg Clin North Am 2012;92:583–597. [DOI] [PubMed] [Google Scholar]
- 11. Miyazaki M, et al. Branchial cleft-like cysts in Hashimoto’s thyroiditis: a case report and literature review. Pathol Int 2016;66:297–301. [DOI] [PubMed] [Google Scholar]
- 12. Nelson LL, et al. Pharyngeal pouch and cleft remnants in the dog and cat: a case series and review. J Am Anim Hosp Assoc 2012;48:105–112. [DOI] [PubMed] [Google Scholar]
- 13. Nolen-Walston RD, et al. Branchial remnant cysts of mature and juvenile horses. Equine Vet J 2009;41:918–923. [DOI] [PubMed] [Google Scholar]
- 14. Oliver A, Nolen-Walston R. A diagnostic approach to congenital neck masses in foals. Equine Vet Educ 2022;34:231–234. [Google Scholar]
- 15. Østergaard S, et al. An ultimobranchial thyroid cyst in a horse. Equine Vet Educ 2014;26:244–247. [Google Scholar]
- 16. Poobitha S, et al. Ultimobranchial cysts in the thyroid of goats. J Entomol Zool Stud 2020;8:1794–1797. [Google Scholar]
- 17. Ramos-Vara JA, et al. Immunohistochemical detection of thyroid transcription factor-1, thyroglobulin, and calcitonin in canine normal, hyperplastic, and neoplastic thyroid gland. Vet Pathol 2002;39:480–487. [DOI] [PubMed] [Google Scholar]
- 18. Rinnovati R, et al. Marsupialization and sclerotherapy with povidone iodine and ethanol of a branchial remnant cyst in an Arabian filly. J Equine Sci 2018;29:43–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sira J, Makura ZGG. Differential diagnosis of cystic neck lesions. Ann Otol Rhinol Laryngol 2011;120:409–413. [DOI] [PubMed] [Google Scholar]
- 20. Suzuki K, et al. Thyroid transcription factor 1 is calcium modulated and coordinately regulates genes involved in calcium homeostasis in C cells. Mol Cell Biol 1998;18:7410–7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tateyama S, et al. The ultimobranchial remnant and its hyperplasia or adenoma in equine thyroid gland. Nihon Juigaku Zasshi 1988;50:714–722. [DOI] [PubMed] [Google Scholar]
- 22. Vázquez-Román V, et al. Immunohistochemical profiling of the ultimobranchial remnants in the rat postnatal thyroid gland. J Morphol 2017;278:1114–1124. [DOI] [PubMed] [Google Scholar]