Abstract
Immunophenotyping of canine large-cell lymphoma (LCL) for B-cell and T-cell surface antigens is commonly performed to better predict the clinical outcome. Expression of surface antigen CD3 is associated with T-cell malignancies; surface antigen CD20 is expressed on B cells. However, a small subset of canine LCLs expresses both CD3 and CD20 (CD3+/CD20+); this form of lymphoma remains poorly defined at the molecular level. In a retrospective study, we aimed to better characterize immunophenotypic properties and antigen receptor clonality of CD3+/CD20+ LCL. We selected formalin-fixed, paraffin-embedded tissues from 10 cases of CD3+/CD20+ LCL and breed-matched controls of peripheral large T-cell lymphoma (PTCL) and diffuse large B-cell lymphoma (DLBCL). Using PCR for antigen receptor rearrangement (PARR), we identified monoclonal T-cell receptor gamma (TCRγ) rearrangements in all CD3+/CD20+ cases. Three of 10 cases had monoclonal rearrangements in the immunoglobulin heavy chain (IgH), supportive of cross-lineage rearrangement. There was no significant difference in the frequency of antigen receptor rearrangement between CD3+/CD20+ and PTCL cases. In comparison with DLBCL, CD3+/CD20+ LCL had TCRγ rearrangement more frequently and IgH rearrangement less frequently, respectively. Immunolabeling of the B-cell marker PAX5 occurred less frequently in all CD3+/CD20+ LCL cases compared to the DLBCL controls. Immunolabeling for BCL-2 was robust, regardless of immunophenotype. Nuclear Ki67 positivity was variable in CD3+/CD20+ cases, indicating a heterogeneity in proliferation. Overall, cases of canine CD3+/CD20+ LCL had properties similar to PTCL, suggesting a similar histogenesis of these 2 subsets.
Keywords: BCL-2, canine, CD3, CD20, DLBCL, large-cell lymphoma, PARR, PAX5, PTCL
Canine non-Hodgkin lymphoma refers to a diverse group of hematologic neoplasms, with aggressive, large-cell lymphomas (LCL) representing the most prevalent subset. 30 Diagnostic recommendations provided by the World Health Organization assume a specific cellular origin for these cancers. 28 Specifically, most lymphomas arise from either CD3+ T lymphocytes or CD20+ B lymphocytes. Diffuse large B-cell lymphoma (DLBCL) and peripheral large T-cell lymphoma (PTCL) remain the most common subtypes in dogs. 30 Immunophenotyping is clinically important because high-grade T-cell lymphomas have the shortest survival times, and T-cell neoplasms have been reported to have decreased response rates to chemotherapy.14,27 Therefore, immunohistochemistry (IHC) for B- (CD20 and PAX5) and T-lymphocyte (CD3) antigens is often recommended in the diagnostic workup of lymphoma.
Although immunophenotyping allows for either a B- or T-cell classification for most LCL cases, a small subset of canine lymphomas expresses both CD3 and CD20. Conversely, null LCL has been reported in dogs and lacks expression of both CD3 and CD20. 19 The CD3+/CD20+ phenotype has been described in a small number of case reports spanning a few different types of lymphoma, including enteropathy-associated T-cell lymphoma, anaplastic lymphoma, and cutaneous epitheliotropic T-cell lymphoma.2,9,15 –17,26 Despite these publications, understanding of CD3+/CD20+ LCL in dogs remains extremely limited.
We compared 10 CD3+/CD20+ LCLs, which affected mostly peripheral lymph nodes, to breed-matched cases of DLBCL and PTCL. More specifically, we determined clonality in all cases as well as provided immunolabeling properties for paired box gene 5 (PAX5), B-cell lymphoma 2 (BCL-2), and Ki67. We found that CD3+/CD20+ lymphomas are similar to PTCL with respect to antigen receptor rearrangement and PAX5 expression. Ki67 nuclear positivity was variable in our case series, indicating a difference in the proliferative activity of CD3+/CD20+ LCL.
Materials and methods
Case selection
We selected formalin-fixed, paraffin-embedded (FFPE) tissues retrospectively from the Michigan State University Veterinary Diagnostic Laboratory (IACUC exempt; MSU-VDL, Lansing, MI, USA). Cases of LCL, as determined by a board-certified anatomic pathologist (B.K. Harrington), that immunolabeled positive for both CD3 and CD20 met the criteria for inclusion in the study. DLBCL and PTCL case controls were selected based on breed similarity to the CD3+/CD20+ LCL group. Seven of 10 cases had exact breed matches. For the 3 unmatched patients, we selected Labrador Retriever controls for the Newfoundland and Shi Tzu controls for the Dachshund based on reports on the genetic similarities between these breeds,3,18 and a mixed-breed control for the Beagle (Tables 1, 2; Fig. 1).
Table 1.
Demographics of biopsy and autopsy submissions of canine lymphoma cases at the Michigan State University Veterinary Diagnostic Laboratory.
| Case | Breed | Sex | Age, y | Submission type |
|---|---|---|---|---|
| CD3+/CD20+ LCL cases | ||||
| 1 | Labrador Retriever | CM | 6.5 | Biopsy (not specified) |
| 2 | Dachshund | F | 10 | Biopsy (not specified) |
| 3 | Mixed | F | 5 | Biopsy (punch) |
| 4 | American Pit Bull Terrier | CM | 7 | Biopsy (excisional) |
| 5 | Newfoundland | CM | 6 | Biopsy (not specified) |
| 6 | Belgian Shepherd | CM | 12 | Autopsy (whole mass) |
| 7 | Beagle | CM | 8 | Autopsy (whole mass) |
| 8 | American Pit Bull Terrier | SF | 7 | Biopsy (wedge) |
| 9 | Mixed | CM | 4 | Biopsy (punch) |
| 10 | Dachshund | CM | 9 | Biopsy (not specified) |
| CD3+/CD20– PTCL cases | ||||
| 11 | Labrador Retriever | CM | 12 | Biopsy (not specified) |
| 12 | Shih Tzu | SF | 8 | Autopsy (whole mass) |
| 13 | Mixed | SF | 7 | Autopsy (whole mass) |
| 14 | American Pit Bull Terrier | CM | 10 | Autopsy (whole mass) |
| 15 | Labrador Retriever | F | 11 | Autopsy (whole mass) |
| 16 | Belgian Shepherd | SF | 6 | Biopsy (excisional) |
| 17 | Mixed | CM | 11 | Autopsy (whole mass) |
| 18 | American Pit Bull Terrier | M | 5 | Biopsy (not specified) |
| 19 | Mixed | CM | 7 | Biopsy (excisional) |
| 20 | Dachshund | CM | 8 | Biopsy (not specified) |
| CD3–/CD20+ DLBCL cases | ||||
| 21 | Labrador Retriever | SF | 7 | Autopsy (whole mass) |
| 22 | Shih Tzu | SF | 10 | Autopsy (whole mass) |
| 23 | Mixed | F | 6 | Autopsy (whole mass) |
| 24 | American Pit Bull Terrier | SF | 4 | Biopsy (not specified) |
| 25 | Labrador Retriever | SF | 11 | Biopsy (excisional) |
| 26 | Belgian Shepherd | CM | 8 | Autopsy (whole mass) |
| 27 | Beagle | M | 8 | Biopsy (excisional) |
| 28 | American Pit Bull Terrier | SF | 7 | Biopsy (excisional) |
| 29 | Mixed | CM | 5 | Biopsy (incisional) |
| 30 | Dachshund | CM | 2 | Biopsy (excisional) |
CM = castrated male; DLBCL = diffuse large B-cell lymphoma; F = female; LCL = large-cell lymphoma; M = male; PTCL = peripheral large T-cell lymphoma; SF = spayed female.
Table 2.
Nodal and/or extranodal involvement of biopsy and autopsy submissions of canine lymphoma cases at the Michigan State University Veterinary Diagnostic Laboratory.
| Case | Location | Multicentric involvement | Additional details |
|---|---|---|---|
| CD3+/CD20+ LCL cases | |||
| 1 | LN (not specified) | + | Gen. lymphadenopathy |
| 2 | LN (not specified) | + | Gen. lymphadenopathy |
| 3 | Popliteal LN | + | Gen. lymphadenopathy |
| 4 | Submandibular LN | – | Submandibular LN only |
| 5 | Popliteal LN | + | Gen. lymphadenopathy |
| 6 | Liver mass | + | Liver, small intestines |
| 7 | Heart mass | – | Cardiac tissue only |
| 8 | Popliteal, prescapular LN | + | Gen. lymphadenopathy |
| 9 | Submandibular LN | + | Gen. lymphadenopathy |
| 10 | Submandibular LN | + | Multiple masses |
| CD3+/CD20– PTCL cases | |||
| 11 | Submandibular LN | – | Submandibular LN only |
| 12 | Mass near duodenum | + | Intestines, heart |
| 13 | Axillary LN | + | Spleen, LN |
| 14 | Mesenteric LN | + | Disseminated |
| 15 | Mediastinal LN | – | Mediastinal LN only |
| 16 | Ureter, vena cava | + | Ureter, intestines |
| 17 | LN (not specified) | + | Heart, liver, spleen |
| 18 | Mesenteric LN | + | LN, spleen |
| 19 | Mesenteric mass | + | Mesentery, liver |
| 20 | Submandibular, popliteal LN | + | Gen. lymphadenopathy |
| CD3–/CD20+ DLBCL cases | |||
| 21 | Submandibular LN | + | Gen. lymphadenopathy |
| 22 | Submandibular, popliteal LN | + | Gen. lymphadenopathy |
| 23 | LN (multiple) | + | Gen. lymphadenopathy |
| 24 | Multiple LNs | + | Gen. lymphadenopathy |
| 25 | Soft palate mass | – | Soft palate mass only |
| 26 | Submandibular, popliteal LN | + | Gen. lymphadenopathy |
| 27 | Submandibular LN | – | Submandibular LN only |
| 28 | Prescapular LN | + | Gen. lymphadenopathy |
| 29 | Cervical LN | + | Gen. lymphadenopathy |
| 30 | Rectal MALT | – | Rectal MALT only |
DLBCL = diffuse large B-cell lymphoma; Gen. lymphadenopathy = generalized lymphadenopathy; LCL = large-cell lymphoma; LN = lymph node; MALT = mucosal-associated lymphoid tissue; PTCL = peripheral large T-cell lymphoma.
Figure 1.
Representative H&E stains and CD3 and CD20 immunolabeling images from CD3+/CD20+ large-cell lymphoma (LCL) and control cases. Case 10, CD3+/CD20+ LCL: A = H&E; B = CD3; C = CD20. Case 20, peripheral large T-cell lymphoma: D = H&E; E = CD3; F = CD20. Case 28, diffuse large B-cell lymphoma: G = H&E; H = CD3; I = CD20. Bars = 50 µm.
Parr
DNA was extracted from FFPE tissues (QIAamp DNA FFPE tissue kit; Qiagen) according to the manufacturer’s instructions. IgH and TCRγ primer sets were designed based on previous publications (Table 3).4,11 The negative reaction control was performed by using the DNA FFPE extraction kit with nuclease-free water. In the positive control condition, previously documented DNA from a canine lymphoma sample unrelated to our study was used. PCR was performed (Type-it mutation PCR kit; Qiagen) with 20–100 ng of DNA from each case. For amplification of the TCRγ CDR3 region, a forward and reverse primer “cocktail” of the 6 listed sets in equal parts was made as described previously. 11 PCR was conducted in a T100 thermocycler (Bio-Rad). Denaturing, annealing, and extension were allowed to proceed for 35 cycles. The annealing temperature for the TCRγ region was 64°C for 90 s; B-cell amplification was at 60°C for 30 s. The subsequent PCR product was then detected, separated by capillary electrophoresis, and analyzed (QIAxcel advanced instrument; Qiagen) using a high-resolution pre-cast gel cartridge. DNA intensity peaks were obtained (QIAxcel ScreenGel software; Qiagen).
Table 3.
Sequences of primers used in PCR for antigen receptor rearrangements to determine clonality in either the IgH or TCRγ gene in canine lymphoma cases.
| Target | Forward | Reverse |
|---|---|---|
| IgH Major CB1/CB3 | 5′-CAGCCTGAGAGCCGAGGACAC-3′ | 5′-TGAGGAGACGGTGACCAGGGT-3′ |
| IgH Minor CB2/CB3 | 5′-CAGCCTGAGAGCCGAGGACAC-3′ | 5′-TGAGGACACAAAGAGTGAGG-3′ |
| TCRγ | ||
| V2/6a/Ja | 5′-GAAGGCGTGTACTACTGCGCTG-3′ | 5′-TTGTGCCAGGACCAAACACTTT-3′ |
| V2/6b/Jb | 5′-GAGGGCGTGTACTACTGTGCTG-3′ | 5′-GGGGAGTTACTATGAGCTTAGTTCCTT-3′ |
| V3a/Jc | 5′-TGTTAAGGAAACAAGATGAGGCCA-3′ | 5′-GAGGAGTTACTATAAGCCTAGTACCTTCTG-3′ |
| V3b/J2-1 | 5′-TCTTAAGGAAACAACATGAGGCTGTG-3′ | 5′-GAGGAGTTACTATAAACCTGTTAACTTCTG-3′ |
| V7a/J5-1 | 5′-AAGTAAAAATGCTCTTACTTCCACTTCAAC-3′ | 5′-GGGGAGTTACTATGAGATTAGTTCCTTCTG-3′ |
| TV7b/J6-2 | 5′-GTAAAAATGCCGTTACTTCCACATCAACTT-3′ | 5′-GTGTGTCAGGACCCATCACTTTGTT-3′ |
IgH = immunoglobulin heavy chain; TCRγ = T-cell receptor gamma.
Immunohistochemistry
All tissues were fixed in 10% neutral-buffered formalin for 12–24 h and processed at the MSU-VDL. Slides were deparaffinized and re-hydrated using standard laboratory methods. IHC was conducted with the following parameters, as described previously5,12,24,25 (Table 4). Antigen retrieval was performed with CC1 (Cell conditioning I, Discovery Ultra; Ventana) at 95°C for 4 min, or ER1 (Bond epitope retrieval solution I; Leica) at 95°C for 20 min. A Bond-Max automated IHC stainer was used for CD3, CD20, and Ki67 with the Bond polymer detection system (Vision Biosystems; Leica) and 3,3′-diaminobenzidine (DAB) as the chromogen. The Discovery Ultra automated staining system was used for BCL-2 and PAX5 with the Discovery UltraMap alkaline phosphatase system and Discovery Red (Roche). Sections of canine lymph node tissue were used as a positive control for all antibodies. The antibody used for BCL-2 has been established in primary colorectal follicular canine lymphoma, marginal zone lymphoma, and mantle cell lymphoma.20,25
Table 4.
Primary antibody source, dilution, antigen retrieval method, and detection method employed in immunohistochemistry of canine lymphoma cases.
| Primary antibody | Host | Source | Catalog | Dilution (primary) | Antigen retrieval | Detection |
|---|---|---|---|---|---|---|
| BCL-2 | Mouse | Leica Biosystems | NCL-BCL-2-486 | 1:100 | CC1 | UltraMap Red |
| CD3 | Rabbit | Agilent | A0452 | 1:200 | ER1 | DAB |
| CD20 | Rabbit | Fisher Scientific | RB9013P | 1:200 | ER1 | DAB |
| PAX5 | Rabbit | Roche | 760-4270 | RTU | CC1 | UltraMap Red |
| Ki67 | Mouse | Agilent | GE020 | 1:50 | CCI | DAB |
CC1 = cell conditioning solution 1 (Tris-EDTA–based buffer, pH 7.8); ER1 = epitope retrieval solution 1; RTU = ready-to-use.
Ki67 quantitation
Nuclear Ki67 positivity was quantified using the QuPath (v.0.4.3) positive cell detection feature. 1 Positive cells were determined using the optical density sum and standard settings assuming default DAB and hematoxylin staining parameters.
Statistical analysis
Fisher exact tests were performed in Prism v.9.1.2 (GraphPad) between groups for comparisons involving positive or negative variables. P ≤ 0.0125 was considered statistically significant by using an alpha value of 0.05 and a Bonferroni adjustment with 4 comparisons (0.05/4). For analysis of Ki67 positivity, groups were examined using a one-way ANOVA and a Tukey honestly significant difference test, with p ≤ 0.05 being considered statistically significant.
Results
Antigen receptor clonality in CD3+/CD20+ LCL
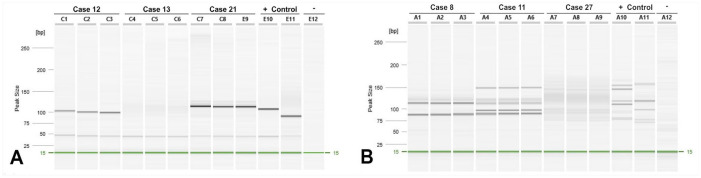
To ascertain antigen receptor clonality for each case, we performed PARR using primers specific to TCRγ and IgH genes (Fig. 2). Monoclonal TCRγ rearrangements occurred in all CD3+/CD20+ cases. Three of 10 cases also had rearrangements in the IgH, supportive of cross-lineage rearrangement (Table 5). Interestingly, 5 cases of PTCL and 2 cases of DLBCL also had cross-lineage rearrangement. CD3+/CD20+ LCLs had TCRγ rearrangement more frequently and IgH rearrangement less frequently than DLBCL cases (p = 0.0007 and 0.003, respectively). We found no statistical difference comparing the CD3+/CD20+ LCL and PTCL groups (Table 6), suggesting that CD3+/CD20+ LCLs resemble PTCLs with respect to antigen receptor clonality.
Figure 2.
Antigen receptor clonality in CD3+/CD20+ canine large-cell lymphoma. A. Representative images from the amplification of immunoglobulin heavy chain (IgH). C1–8 and E10–12 correspond to the lane identification in the QIAxcel advanced capillary electrophoresis cartridge. B. Representative images from the amplification of T-cell receptor gamma (TCRγ). A1–A12 correspond to the lane identification in the QIAxcel advanced capillary electrophoresis cartridge.
Table 5.
Frequency of antigen receptor rearrangements in CD3+/CD20+ large-cell lymphoma (LCL), diffuse large B-cell lymphoma (DLBCL), and peripheral large T-cell lymphoma (PTCL) cases.
| Group | TCRγ | IgH | Cross lineage | Total |
|---|---|---|---|---|
| CD3+/CD20+ LCL | 7 | 0 | 3 | 10 |
| PTCL* | 2 | 2 | 5 | 10 |
| DLBCL | 0 | 8 | 2 | 10 |
Neither immunoglobulin heavy chain (IgH) nor T-cell receptor gamma (TCRγ) were amplified for one case of PTCL.
Table 6.
Summary of Fisher exact tests comparing CD3+/CD20+ large-cell lymphoma (LCL), diffuse large B-cell lymphoma (DLBCL ), and peripheral large T-cell lymphoma (PTCL) for TCRγ or IgH rearrangements (*p ≤0.0125). All samples were run in triplicate with the appropriate positive and negative controls.
| Comparison | Variable | p |
|---|---|---|
| CD3+/CD20+ LCL vs. PTCL | TCRγ | p = 0.210 |
| CD3+/CD20+ LCL vs. DLBCL | TCRγ | p = 0.007* |
| CD3+/CD20+ LCL vs. PTCL | IgH | p = 0.179 |
| CD3+/CD20+ LCL vs. DLBCL | IgH | p = 0.003* |
IgH = immunoglobulin heavy chain; TCRγ = T-cell receptor gamma.
Immunophenotyping of CD3+/CD20+ LCL
To further characterize our CD3+/CD20+ LCL cases, we performed IHC for PAX5 and BCL-2 (Fig. 3). PAX5 is a nuclear transcription factor that regulates the differentiation and development of B cells and is, therefore, used commonly as a B-cell marker. 29 In all CD3+/CD20+ cases, we observed negative PAX5 immunolabeling. This was unlike our DLBCL group, which immunolabeled for PAX5 in 90% of cases. PAX5 was only positive in 10% of PTCL cases. We observed no statistical difference in the PAX5 immunolabeling between CD3+/CD20+ LCLs and PTCLs (p = 0.999). PAX5 immunolabeling occurred more frequently in DLBCLs compared to our CD3+/CD20+ LCL group (p = 0.0001; Table 7). Our PAX5 immunolabeling data support the idea that CD3+/CD20+ LCLs have histogenesis similar to PTCL cases.
Figure 3.
Expression of PAX5 and BCL-2 in CD3+/CD20+ canine large-cell lymphoma (LCL). Representative images of immunolabeling of PAX5 and BCL-2. Case 10, CD3+/CD20+ LCL: A = PAX5; B = BCL-2. Case 28, peripheral large T-cell lymphoma: C = PAX5; D = BCL-2. Case 11, diffuse large B-cell lymphoma: E = PAX5; F = BCL-2. Bars = 50 µm.
Table 7.
Summary of Fisher exact tests comparing PAX5 or BCL-2 immunolabeling in CD3+/CD20+ large-cell lymphoma (LCL), diffuse large B-cell lymphoma (DLBCL), or peripheral large T-cell lymphoma (PTCL) where indicated (*p ≤0.0125).
| Comparison | Variable | p |
|---|---|---|
| CD3+/CD20+ LCL vs. DLBCL | PAX5 | p = 0.0001* |
| CD3+/CD20+ LCL vs. PTCL | PAX5 | p = 0.999 |
| CD3+/CD20+ LCL vs. DLBCL | BCL-2 | p = 0.999 |
| CD3+/CD20+ LCL vs. PTCL | BCL-2 | p = 0.999 |
Next, we immunolabeled for BCL-2, an anti-apoptotic protein commonly overexpressed and therapeutically targeted in numerous cancer types. 7 BCL-2 has also been reported to be expressed ubiquitously in canine DLBCLs. 6 Moreover, a high BCL-2:BAX ratio has been associated more commonly with T-cell lymphomas, which may explain the poor clinical outcome in T-cell lymphomas. 13 Robust immunolabeling for BCL-2 occurred in all cases, and no difference was observed among groups (p = 0.999; Table 7).
High Ki67 positivity has been associated with a shorter survival time in dogs with DLBCL treated with chemotherapy. 23 Differences in proliferation markers, such as Ki67, and the apoptotic activity of the neoplastic cells, can account for variations in the proliferative activity of tumors and subsequent clinical outcome. 21 Therefore, we were interested in the Ki67 immunolabeling index of our CD3+/CD20+ LCL cases (Fig. 4A–D). Nuclear positivity was variable in both the PTCL and CD3+/CD20+ subsets (range: 4–99%), suggesting variability in the proliferation of these tumors. However, the average Ki67 immunolabeling index for the CD3+/CD20+ group was significantly different and higher than that of the DLBCL group (p = 0.04; Fig. 4E, Table 8).
Figure 4.
Ki67 immunolabeling index in CD3+/CD20+ canine large-cell lymphoma (LCL). Representative images of immunolabeling of Ki67 from CD3+/CD20+ lymphoma: A = case 1; CD3+/CD20+ LCL; B = case 4, CD3+/CD20+ LCL; C = case 11, peripheral large T-cell lymphoma; D = case 28, diffuse large B-cell lymphoma. Bars = 50 µm. E. Quantitation of % Ki67 positivity. Results were compared using a one-way ANOVA and a Tukey HSD (*p ≤ 0.05). The box-and-whisker plot indicates the minimum, first quartile, median, x– (“x”), third quartile, and maximum of each dataset.
Table 8.
Average Ki67 index and variation in CD3+/CD20+ large-cell lymphoma (LCL), diffuse large B-cell lymphoma (DLBCL), and peripheral large T-cell lymphoma (PTCL).
| Group | Average Ki67 index, % | SD, % |
|---|---|---|
| CD3+/CD20+ LCL | 53.4 | 27.1 |
| PTCL | 27.2 | 30.7 |
| DLBCL | 23.3 | 15.0 |
Discussion
The frequency with which the TCRγ and IgH were rearranged differed statistically between CD3+/CD20+ LCL cases and DLBCL cases. However, there was no statistical difference in antigen receptor rearrangement frequency between CD3+/CD20+ LCL cases and PTCL cases. Furthermore, PAX5 immunolabeling patterns were similar between PTCL and CD3+/CD20+ cases but differed statistically between DLBCL and CD3+/CD20+ cases. The Ki67 immunolabeling index was highly variable within all groups, but a significant difference was noted between the CD3+/CD20+ LCL and DLBCL groups. Overall, our data highlight similarities between PTCL and CD3+/CD20+ LCL, suggesting that CD3+/CD20+ LCL cases have histogenesis similar to PTCL.
Cross-lineage rearrangement occurred frequently in our study: in 7 of our control cases and 3 CD3+/CD20+ LCL cases. V(D)J recombination in both the T-cell receptor and IgH genes has been reported in dogs, 16 although with a lower frequency than we observed. However, the true frequency with which cross-lineage rearrangement occurs in canine lymphoma is not known, given that a large-scale study has not yet been reported. Additionally, PARR is often assessed in only one antigen receptor for many diagnostic cases, further hindering our ability to retrospectively assess the frequency with which this phenomenon occurs. Other investigators have developed an improved primer set for canine PARR. 8 The sensitivity of the IgH primer set that we used is reported to be ~70%, which should be considered when interpreting our results. 8 Overall, the frequency of cross-lineage rearrangement, in addition to occasional aberrant expression of PAX5 in PTCL, bring into question the accuracy and adequacy of immunophenotyping with a single surface marker for canine lymphoma cases.
Inhibitors of the anti-apoptotic protein BCL-2 are under investigation in a wide variety of human cancers and have shown success in some forms of non-Hodgkin lymphoma. 22 BCL-2 expression levels are not prognostic in determining a response to CHOP chemotherapy, but BH3 mimetics have had in vitro efficacy activity against canine T-cell neoplastic cells.6,10 We observed no differences among groups in the immunolabeling of the therapeutic target BCL-2 and noted that BCL-2 had robust expression regardless of lymphoma subtype. Our data support the exploration of BCL-2 inhibitors in canine LCL patients regardless of immunophenotype.
Limitations of our study include the small sample size and retrospective approach. Despite these shortcomings, we performed a robust statistical analysis, identifying clear similarities and differences among lymphoma subtypes. Future studies will be directed at determining prognostic differences among these patients and evaluating additional prognostic markers and therapeutic targets.
Acknowledgments
We thank all of the individuals in the MSU-VDL histology laboratory for their assistance with our project. Our work was presented as a student poster at the 2021 American College of Veterinary Pathologists Annual Meeting (Virtual: Oct 30–Nov 2, 2021).
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Morris Animal Foundation Veterinary Student Scholar Award.
ORCID iD: Cory M. Howard  https://orcid.org/0000-0002-2582-8977
https://orcid.org/0000-0002-2582-8977
Contributor Information
Cory M. Howard, Department of Pathobiology and Diagnostic Investigation, Michigan State University College of Veterinary Medicine, East Lansing, MI, USA Michigan State University Veterinary Diagnostic Laboratory, Lansing, MI, USA.
Steffanie Anderson, Michigan State University Veterinary Diagnostic Laboratory, Lansing, MI, USA.
Bonnie Harrington, Charles River Laboratories, Mattawan, MI, USA.
References
- 1. Bankhead P, et al. QuPath: open source software for digital pathology image analysis. Sci Rep 2017;7:16878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brachelente C, et al. CD3 and CD20 coexpression in a case of canine cutaneous epitheliotropic T-cell lymphoma (mycosis fungoides). Vet Pathol 2016;53:563–566. [DOI] [PubMed] [Google Scholar]
- 3. Brown EA, et al. FGF4 retrogene on CFA12 is responsible for chondrodystrophy and intervertebral disc disease in dogs. Proc Natl Acad Sci U S A 2017;114:11476–11481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burnett RC, et al. Diagnosis of canine lymphoid neoplasia using clonal rearrangements of antigen receptor genes. Vet Pathol 2003;40:32–41. [DOI] [PubMed] [Google Scholar]
- 5. Carrasco V, et al. Distinguishing intestinal lymphoma from inflammatory bowel disease in canine duodenal endoscopic biopsy samples. Vet Pathol 2015;52:668–675. [DOI] [PubMed] [Google Scholar]
- 6. Curran KM, et al. BCL2 and MYC are expressed at high levels in canine diffuse large B-cell lymphoma but are not predictive for outcome in dogs treated with CHOP chemotherapy. Vet Comp Oncol 2017;15:1269–1279. [DOI] [PubMed] [Google Scholar]
- 7. Delbridge ARD, et al. Thirty years of BCL-2: translating cell death discoveries into novel cancer therapies. Nat Rev Cancer 2016;16:99–109. [DOI] [PubMed] [Google Scholar]
- 8. Ehrhart EJ, et al. Polymerase chain reaction for antigen receptor rearrangement: benchmarking performance of a lymphoid clonality assay in diverse canine sample types. J Vet Intern Med 2019;33:1392–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ewing TS, et al. Prevalence of CD20+ cutaneous epitheliotropic T-cell lymphoma in dogs: a retrospective analysis of 24 cases (2011–2018) in the USA. Vet Dermatol 2019;30:51-e14. [DOI] [PubMed] [Google Scholar]
- 10. Jegatheeson S, et al. Sensitivity of canine hematological cancers to BH3 mimetics. J Vet Intern Med 2023;37:236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keller SM, Moore PF. A novel clonality assay for the assessment of canine T cell proliferations. Vet Immunol Immunopathol 2012;145:410–419. [DOI] [PubMed] [Google Scholar]
- 12. Kiupel M, et al. Prognostic factors for treated canine malignant lymphoma. Vet Pathol 1999;36:292–300. [DOI] [PubMed] [Google Scholar]
- 13. Meichner K, et al. Expression of apoptosis-regulating proteins Bcl-2 and Bax in lymph node aspirates from dogs with lymphoma. J Vet Intern Med 2016;30:819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moore AS. Treatment of T cell lymphoma in dogs. Vet Rec 2016;179:277. [DOI] [PubMed] [Google Scholar]
- 15. Nakagun S, et al. CD3 and CD20 co-expression in a case of canine peripheral T-cell lymphoma with prominent cardiac and peripheral nerve involvement. J Vet Diagn Invest 2018;30:779–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nicoletti A, et al. CD3-CD20–positive nodal lymphoma with cross-lineage rearrangement in a dog. J Vet Diagn Invest 2020;32:964–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Noland EL, Kiupel M. Coexpression of CD3 and CD20 in canine enteropathy-associated T-cell lymphoma. Vet Pathol 2018;55:241–244. [DOI] [PubMed] [Google Scholar]
- 18. Parker HG, et al. Genomic analyses reveal the influence of geographic origin, migration, and hybridization on modern dog breed development. Cell Rep 2017;19:697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pittaway R, et al. Diagnosis of anaplastic large-cell lymphoma in a dog using CD30 immunohistochemistry. J Vet Diagn Invest 2018;30:455–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Richardson MA, et al. Primary colorectal follicular lymphoma in 3 dogs. Vet Pathol 2019;56:404–408. [DOI] [PubMed] [Google Scholar]
- 21. Riondato F, et al. Variation of apoptotic and proliferative activity among lymphoma subtypes in dogs: a flow cytometric study. Res Vet Sci 2021;135:324–328. [DOI] [PubMed] [Google Scholar]
- 22. Roberts AW. Therapeutic development and current uses of BCL-2 inhibition. Hematology Am Soc Hematol Educ Program 2020;2020:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sierra Matiz OR, et al. Prognostic significance of Ki67 and its correlation with mitotic index in dogs with diffuse large B-cell lymphoma treated with 19-week CHOP-based protocol. J Vet Diagn Invest 2018;30:263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stein L, et al. Immunophenotypic characterization of canine nodal T-zone lymphoma. Vet Pathol 2021;58:288–292. [DOI] [PubMed] [Google Scholar]
- 25. Stein L, et al. Immunophenotypic characterization of canine splenic follicular-derived B-cell lymphoma. Vet Pathol 2019;56:350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Valente PCLG, et al. Co-expression of T- and B-cell markers in a canine intestinal lymphoma: a case report. Animals (Basel) 2022;12:3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Valli VE, et al. Canine lymphomas: association of classification type, disease stage, tumor subtype, mitotic rate, and treatment with survival. Vet Pathol 2013;50:738–748. [DOI] [PubMed] [Google Scholar]
- 28. Valli VE, et al. Classification of canine malignant lymphomas according to the World Health Organization criteria. Vet Pathol 2011;48:198–211. [DOI] [PubMed] [Google Scholar]
- 29. Willmann M, et al. Pax5 immunostaining in paraffin-embedded sections of canine non-Hodgkin lymphoma: a novel canine pan pre-B- and B-cell marker. Vet Immunol Immunopathol 2009;128:359–365. [DOI] [PubMed] [Google Scholar]
- 30. Zandvliet M. Canine lymphoma: a review. Vet Q 2016;36:76–104. [DOI] [PubMed] [Google Scholar]