Abstract
Bronchiectasis is irreversible bronchial dilation that can be congenital or acquired secondary to chronic airway obstruction. Feline bronchiectasis is rare and, to our knowledge, has not been reported previously in a non-domestic felid. An ~10-y-old female jungle cat (Felis chaus) was presented for evaluation of an abdominal mass and suspected pulmonary metastasis. The animal died during exploratory laparotomy and was submitted for postmortem examination. Gross examination revealed consolidation of the left caudal lung lobe and hila of the cranial lung lobes. Elsewhere in the lungs were several pale-yellow pleural foci of endogenous lipid pneumonia. On cut section, there was severe distension of bronchi with abundant white mucoid fluid. The remaining lung lobes were multifocally expanded by marginal emphysema. Histologically, ectatic bronchi, bronchioles, and fewer alveoli contained degenerate neutrophils, fibrin, and mucin (suppurative bronchopneumonia) with rare gram-negative bacteria. Aerobic culture yielded low growth of Proteus mirabilis and Escherichia coli. There was chronic bronchitis, marked by moderate bronchial gland hyperplasia, lymphoplasmacytic inflammation, and lymphoid hyperplasia. The palpated abdominal mass was a uterine endometrial polyp, which was considered an incidental, but novel, finding. Chronic bronchitis and bronchopneumonia should be considered as a cause of bronchiectasis and a differential diagnosis for respiratory disease in non-domestic felids.
Keywords: Escherichia coli, exotic animal, Felidae, lung, Proteus, respiratory disease, zoo animal
The jungle cat (Felis chaus) is the largest member in the genus Felis and is widely distributed in rural areas throughout the Eastern Hemisphere, including Africa, India, China, the Middle East, and Southwest Asia. The jungle cat is larger and heavier than the domestic cat (Felis catus), and is characterized by a tall body, tufted ears, long legs, and a short tail. 7 Jungle cats are classified as of “least concern” by the International Union for Conservation of Nature; however, habitat destruction, environmental pollution, and illegal hunting remain threats to jungle cats in the wild. 7 In the United States, most states ban private ownership of dangerous exotic animals and big cats as pets, although a few states, including North Carolina, do not have laws banning the keeping of exotic cats as pets.1,20
Peer-reviewed literature documenting diseases of the jungle cat is scant; reports are limited to documentation of parasites and neoplasia. Several gastrointestinal parasites have been recovered from wild jungle cats, including Toxocara cati, Alaria alata, and Mesocestoides lineatus 26 ; 2 jungle cats from the Udawalawe National Park in Sri Lanka had hepatomegaly secondary to infection with the zoonotic nematode Calodium hepaticum. 9 Concurrent thyroid adenocarcinoma, gastric adenocarcinoma, renal adenoma, and Sertoli cell tu-mor have been reported in a single aged captive jungle cat. 22
Bronchiectasis is permanent severe airway dilation that results from abnormal and irreversible destruction of elastic and muscular components in bronchi. 6 Bronchiectasis is rare in domestic cats and typically occurs secondary to chronic bronchitis or bronchiolitis, bronchopneumonia, neoplasia, emphysema, and endogenous lipid pneumonia. 19 Here, we describe a case of bronchiectasis subsequent to chronic bronchitis and bacterial pneumonia in a jungle cat.
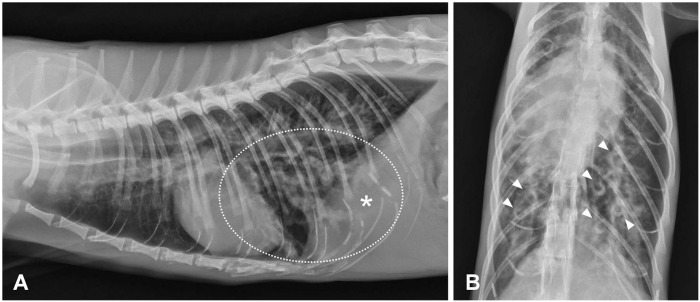
An ~10-y-old female jungle cat with an unknown clinical history was obtained by a zoologic facility in North Carolina from a private owner. On physical examination, which was performed as a part of standard clinical workup upon animal entry to the facility, an abdominal mass was palpated. Two-view thoracic and abdominal radiographs revealed a severe bronchial pattern in the caudal lung lobes (Fig. 1A). Within affected lobes, bronchi were markedly distended and tortuous, with luminal soft tissue opacity and markedly thickened and prominent bronchial walls (Fig. 1B). At the time of clinical workup, there were no observed clinical respiratory signs, and there was concern that these pulmonary changes could represent metastases from the palpated abdominal mass; exploratory laparotomy was pursued. The animal died while under anesthesia and was subsequently submitted for a cosmetic postmortem examination.
Figure 1.
Severe bronchial pattern with bronchiectasis in the caudal lung lobes in thoracic radiographs of a jungle cat. A. Bronchiectasis (circled) within the caudal lung lobes. * Denoted area corresponds to similarly annotated left caudal lung lobe shown in Fig. 2A. B. On dorsoventral view, bronchial walls are markedly thickened (“donuts”, arrowheads).
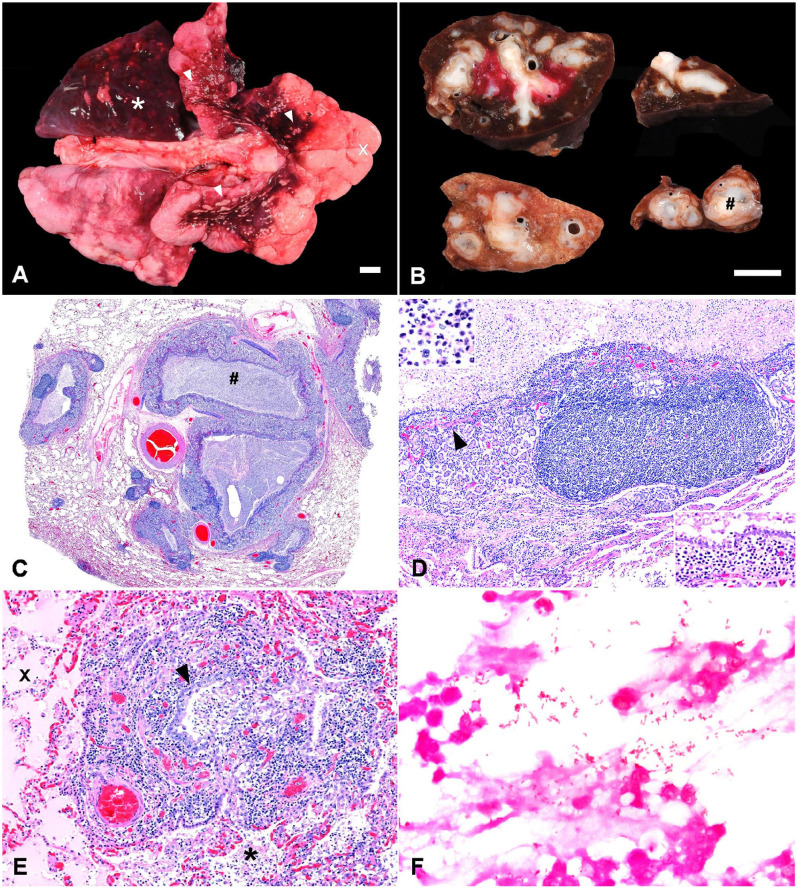
On postmortem examination, which was performed ~19 h after death, the lungs were diffusely mottled pale-pink to dark-red; the left caudal lobe and hilar regions of the cranial lobes were heavy, dark-red, and somewhat firm (Fig. 2A). Pinpoint to 5-mm diameter, irregularly marginated, flat, pale-tan to yellow foci were scattered across the pleural surface of the hilar regions of the cranial lobes (Fig. 2A). The remaining lung lobes had multifocal-to-coalescing pale-pink foci expanded by air-trapping, which was most severe at the margins of the lung lobes (Fig. 2A). On cut section of the left caudal lung lobe and hilar portions of cranial lung lobes, bronchi were severely distended, with a cross-sectional diameter of 0.5–1.5 cm. Affected bronchi had markedly thickened walls, and contained abundant, white to occasionally bright-yellow, opaque, viscous, mucoid material (Fig. 2B). Scant similar fluid was present in the trachea.
Figure 2.
Gross and histologic features of bronchiectasis, chronic bronchitis, and bronchopneumonia in a jungle cat. A. The left caudal lobe (*) and hilar regions of the cranial lobes are consolidated, dark-red, and heavy with several flat, pale-yellow, pleural foci of endogenous lipid pneumonia (arrowheads). The remaining lung lobes are emphysematous (x). Bar = 1 cm. B. On cut section of the formalin-fixed caudal lung lobe, dilated bronchi and bronchioles contained abundant white viscous mucoid material (#) and had markedly thickened walls. Bar = 1 cm. C. Histologically, bronchial dilation and wall thickening, with filling of the lumina with abundant exudate (#). H&E. D. The wall of a bronchus is thickened by hyperplastic submucosal glands, hyperplastic bronchial-associated lymphoid tissue, and mixed inflammatory cells predominated by lymphocytes and plasma cells (inset, lower right). Degenerate neutrophils and debris fill the lumen (inset, upper left). Smooth muscle bundles in the lamina propria are markedly reduced in frequency and size (arrowhead). H&E. E. Suppurative inflammation within a thickened inflamed terminal bronchiole (arrowhead) extends into surrounding alveoli (*) along with fibrin and edema (x). H&E. F. Uncommonly, gram-negative bacteria are present within suppurative luminal exudate. Gram stain.
Samples of thoracic and abdominal viscera were fixed in 10% neutral-buffered formalin and processed routinely, with H&E staining for microscopic evaluation; sections of lung were also evaluated with Gram staining. Additionally, samples of the consolidated lung with dilated bronchi were collected aseptically for aerobic bacterial culture.
Histologic evaluation of the lung revealed severe ectasia of bronchi and bronchioles (Fig. 2C). Affected bronchi contained abundant luminal inflammatory exudate composed of degenerate neutrophils with admixed nuclear and cellular debris, eosinophilic fibrillary material (interpreted as fibrin), and amphophilic homogeneous fluid (interpreted as mucin; Fig. 2D). Bronchial walls were also expanded greatly by a combination of markedly hyperplastic submucosal glands, hyperplastic bronchial-associated lymphoid tissue (BALT), and a mixture of plasma cells, lymphocytes, and macrophages with fewer degenerate neutrophils (Fig. 2D). Within the lamina propria subjacent to the bronchial wall mucosa, smooth muscle bundles were markedly reduced in both frequency and size; smooth muscle fibers were uniformly atrophic and isolated by inflammation into individual fibers or small clusters of 2–5 fibers. Bronchioles had similar lumi-nal exudate and BALT hyperplasia. Neutrophilic luminal inflammation extended from terminal bronchioles into surrounding alveoli, where it was accompanied by eosinophilic proteinaceous fibrillary to homogeneous material (interpreted as fibrin and edema fluid; Fig. 2E). Uncommonly, short bacilli were identified within the luminal inflammatory exudate in affected airways; the organisms were gram-negative (Fig. 2F), and bacterial culture of lung tissue yielded low growth of Escherichia coli and Proteus mirabilis. Additionally, within subpleural alveoli in tissue adjacent to affected bronchi, there was accumulation of hypertrophied alveolar macrophages with numerous discrete, clear, intracytoplasmic vacuoles (interpreted as endogenous lipid pneumonia). In lung tissue away from affected bronchi, alveoli were moderately to markedly distended multifocally by clear space (interpreted as emphysema).
Based on the radiographic, gross, and histopathologic findings, we diagnosed chronic bronchitis and bronchopneumonia with severe bronchiectasis. In the absence of other significant findings in this patient, respiratory compromise under anesthesia and secondary to these lesions was the suspected cause of death. Furthermore, E. coli and P. mirabilis were isolated from affected lung, which was consistent with the gram-negative bacteria identified within inflammatory exudate in affected bronchi. Although considered, postmortem contamination was thought to be less likely given the excellent postmortem condition of the animal and the gram-negative bacteria identified histologically. Using Google, PubMed, and North Carolina State University and The Ohio State University library databases with search terms of “jungle cat,” “bronchiectasis,” “bronchitis,” “bronchopneumonia,” and “lung,” we retrieved no cases similar to the one that we are reporting here, suggesting that neither this pulmonary condition nor other pulmonary diseases have been reported in jungle cats.
Bronchiectasis can be secondary to congenital processes, such as ciliary dyskinesia, bronchial dysplasia, cystic fibrosis, and alpha-1 antitrypsin deficiency.6,19 Alternatively, bronchiectasis can be acquired secondary to obstructive diseases such as asthma, chronic bronchitis, neoplasia, and/or pulmonary masses (e.g., granulomas secondary to infectious agents, including parasites), foreign bodies, or chronic suppurative pneumonia.6,19 As noted, feline bronchiectasis is typically acquired secondary to chronic inflammatory conditions, emphysema, or neoplasia. 19 However, bronchiectasis is rare in domestic cats and is typically detected in a single lung lobe, with caudal lobes more often affected. 19 Cats with bronchiectasis may be asymptomatic or can display coughing or dyspnea; aged cats are overrepresented. 19 Bacteria associated with feline bronchiectasis include Enterobacter, Fusobacterium, and Streptococcus spp. 19 Bronchiectasis is more common in dogs and humans than in cats, and is also reported in a variety of large animals (e.g., cattle, horses).5,6,13,19,23,27
The pulmonary lesions identified in this jungle cat have many similarities to those described in domestic cats with bronchiectasis. First, as with domestic cats, the left caudal lung lobe was most severely affected in our case. 19 Furthermore, our histologic findings of concurrent chronic bronchitis and bronchiolitis, suppurative bronchopneumonia, endogenous lipid pneumonia, and emphysema are all reported in cases of bronchiectasis in domestic cats. 19 However, contrary to reports in domestic cats, we isolated low growth of P. mirabilis and E. coli, agents that have not been reported previously in association with bronchiectasis in domestic cats. 19 Interestingly, E. coli infection has been reported in cases of bronchiectasis in humans and dogs.5,8,28 P. mirabilis has not been reported in cases of bronchiectasis in other domestic species and has been implicated only rarely in pulmonary infections in humans.3,10 Eosinophilic inflammation was absent in our case, suggesting that hypersensitivity or parasitism were not significant contributing factors to bronchiectasis.
Bronchiectasis is more often reported in dogs than cats and can be seen in conjunction with a variety of infectious and inflammatory respiratory diseases, including bronchitis, pneumonia, and eosinophilic bronchopneumopathy.5,8 In contrast to cats, the right cranial lung lobe is more often affected in dogs. 5 Aspiration pneumonia, interstitial pneumonia, and pulmonary foreign bodies have all been identified in conjunction with bronchiectasis in dogs. 5 Isolation of E. coli, Pseudomonas aeruginosa, and Mycoplasma spp. is frequently reported in canine cases of bronchiectasis.5,8 Eosinophilic bronchopneumopathy is a canine-specific condition that is associated with bronchiectasis and chronic irritation, with eosinophilic inflammation, epithelial desquamation, hyperplasia of the mucus glands, and airway obstruction.8,16
In human medicine, diseases associated with bronchiectasis include infectious bronchopneumonia, immunologic defects, primary ciliary dyskinesia, and exaggerated immune responses, such as allergic bronchopulmonary aspergillosis, which can be seen in patients with asthma or cystic fibrosis.24,27 Bronchiectasis typically affects lower lung lobes in humans, which is analogous to the caudal lobe distribution seen in cats. 14 Radiographic, gross, and histologic features are similar to those seen in our case, and include thickening of the bronchial walls with secondary mucosal ulceration and peribronchial fibrosis, impaction of dilated airways with thick mucoid-to-mucopurulent fluid, and formation of cysts arising from the bronchial wall. Haemophilus influenzae, Streptococcus pneumoniae, Moraxella catarrhalis, Mycobacterium spp., and P. aeruginosa are important bacteria found in cases of human bronchiectasis.12,17,27
Although the lack of apparent clinical respiratory signs and historical data preclude a full understanding of the pathogenesis of bronchiectasis in our case, based on the concurrent pulmonary lesions, a few hypotheses can be made. In humans, the “vicious cycle” hypothesis of the pathogenesis of bronchiectasis postulates that an initial event, most likely bacterial infection, triggers impaired mucociliary clearance, leading to chronic infection and inflammation that perpetuate progressive respiratory disease.11,12 Alternatively, it is possible that an underlying inflammatory airway disease, such as chronic bronchitis, contributes to chronic inflammation and mucociliary clearance impairment, leading to opportunistic infection. In our case, lesions of both chronic bacterial bronchopneumonia and chronic bronchitis were present. Therefore, either pathogenesis—both of which have been proposed in domestic cats—may be possible.21,25 In either scenario, chronic bronchitis and bacterial bronchopneumonia cause structural lung damage and trigger activation of the immune system, release of neutrophils and macrophages, and production of mucin.10,27 Over time, this process leads to bronchial dilation, thickening of the bronchial wall, and obstruction of the bronchial lumen with mucin and inflammatory exudate, ultimately leading to bronchiectasis. 11 In non-domestic felids, chronic bronchitis and bronchopneumonia should be considered as a cause of bronchiectasis and a differential diagnosis for respiratory disease or, as in our case, unexpected death.
Radiographs had a severe bronchial pattern and bronchial changes suggestive of bronchiectasis in our case, supporting the use of 2-view chest radiographs in the diagnosis of this condition in non-domestic felids. Characteristic radiographic features of bronchiectasis include bronchial wall thickening (prominent “ring signs,” “donuts,” or “tram tracks”), bronchial dilation, and tubular opacities reflecting mucus and/or exudate accumulated within bronchial lumens—all of which were present in our case.8,18 Several different subtypes of radiographic patterns can be seen in patients with bronchiectasis, including cylindrical, saccular, cystic, and varicose bronchiectasis. 18 Beyond traditional radiographs, computed tomography, bronchoscopy, and bronchography are useful diagnostic tools for the workup of bronchiectasis in both human and veterinary medicine.2,8,27
In addition to the pulmonary lesions, expanding and filling the lumen of the distal right uterine horn was a 6 × 3.5 × 3 cm, smooth, soft, ovoid, pedunculated mass that was focally adherent to the uterine wall. On cut section, the mass was homogeneously pale-tan to white (Fig. 3A). Histologic evaluation of the uterine mass revealed expansion of the endometrium by a pedunculated, expansile, well-demarcated mass comprised of well-differentiated endometrial stroma with numerous entrapped islands of endometrial epithelium (Fig. 3B). The epithelium was comprised of a single layer of cuboidal or columnar epithelium; multifocally, this epithelium transitioned to polygonal squamous epithelial cells, with piling of the epithelium up to 5 cell layers thick. There was multifocal necrosis of individual epithelial cells with sloughing into the uterine lumen of cellular and nuclear debris. Within both the stromal population and epithelial population, anisocytosis and anisokaryosis were mild, and mitotic figures were not apparent. Collectively, these findings were consistent with an endometrial stromal polyp with squamous metaplasia (Fig. 3).
Figure 3.
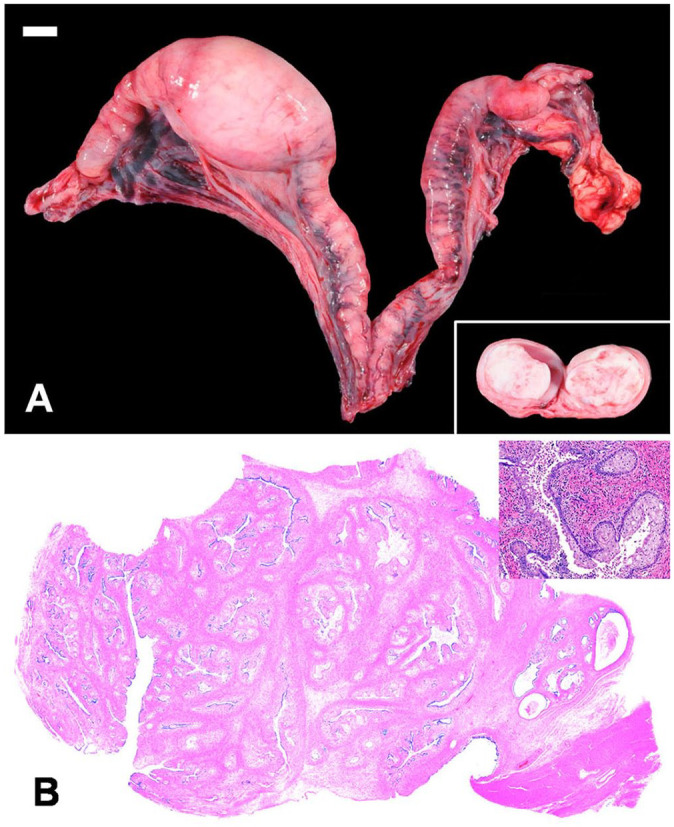
Gross and histologic features of a uterine endometrial polyp in a jungle cat. A. The right uterine horn is focally expanded by a firm, tan, pedunculated, intraluminal mass that is homogeneous on cut surface (inset). Bar = 1 cm. B. Histologically, the mass is comprised of a pedunculated exophytic mass of proliferating endometrial mucosa, stroma, and smooth muscle with multifocal squamous metaplasia (inset).
The exophytic uterine mass was considered incidental to the death of this animal. Endometrial polyps are uncommon benign uterine masses reported in domestic cats and are typically composed of a core of stromal tissue with overlying and entrapped endometrial glands.4,15 Although an incidental finding in our case, to our knowledge, endometrial polyps have not been reported previously in jungle cats. We retrieved no similar cases as the one described using Google, PubMed, and North Carolina State University and The Ohio State University library databases with search terms of “jungle cat,” “uterus,” “neoplasia,” and “endometrial polyp,” suggesting that this condition has not been reported in this species.
Acknowledgments
We acknowledge the expertise and contribution of the staff of the NCSU-CVM Histology Laboratory.
Footnotes
The authors declared no conflicts of interest regarding the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Megan E. Schreeg  https://orcid.org/0000-0001-5649-8854
https://orcid.org/0000-0001-5649-8854
Contributor Information
Danyue Kang, College of Veterinary Medicine, The Ohio State University, Columbus, OH, USA.
Mandy Womble, College of Veterinary Medicine, North Carolina State University, Raleigh, NC, USA.
John M. Cullen, College of Veterinary Medicine, North Carolina State University, Raleigh, NC, USA
Tara M. Harrison, College of Veterinary Medicine, North Carolina State University, Raleigh, NC, USA
Christopher Premanandan, College of Veterinary Medicine, The Ohio State University, Columbus, OH, USA.
Megan E. Schreeg, College of Veterinary Medicine, The Ohio State University, Columbus, OH, USA; College of Veterinary Medicine, North Carolina State University, Raleigh, NC, USA.
References
- 1. Big Cat Rescue. State laws exotic cats. 2022. [cited 2023 Mar 30]. https://bigcatrescue.org/state-laws-exotic-cats/
- 2. Cannon MS, et al. Quantitative and qualitative computed tomographic characteristics of bronchiectasis in 12 dogs. Vet Radiol Ultrasound 2013;54:351–357. [DOI] [PubMed] [Google Scholar]
- 3. Furman AC, et al. Lung abscess in patients with AIDS. Clin Infect Dis 1996;22:81–85. [DOI] [PubMed] [Google Scholar]
- 4. Gelberg HB, McEntee K. Hyperplastic endometrial polyps in the dog and cat. Vet Pathol 1984;21:570–573. [DOI] [PubMed] [Google Scholar]
- 5. Hawkins EC, et al. Demographic, clinical, and radiographic features of bronchiectasis in dogs: 316 cases (1988–2000). J Am Vet Med Assoc 2003;223:1628–1635. [DOI] [PubMed] [Google Scholar]
- 6. Husain AN. The lung. In: Kumar V, et al., eds. Robbins and Cotran Pathologic Basis of Disease. 9th ed. Saunders, 2015:669–726. [Google Scholar]
- 7. International Union for Conservation of Nature. Jungle cat: Felis chaus. The IUCN Red List of Threatened Species. 2021. [cited 2023 March 30]. https://www.iucnredlist.org/species/8540/200639312
- 8. Johnson LR, et al. Bronchoscopy, imaging, and concurrent diseases in dogs with bronchiectasis: (2003–2014). J Vet Intern Med 2016;30:247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karawita AC, et al. Calodium hepaticum in jungle cats (Felis chaus) in Sri Lanka. J Wildl Dis 2016;52:971–972. [DOI] [PubMed] [Google Scholar]
- 10. Katilov O, et al. Clinical management and outcome of childhood lung abscess (LA): a 15-year experience. Eur Respir J 2011;38(Suppl 55):1156. Abstract. [Google Scholar]
- 11. King P. Pathogenesis of bronchiectasis. Paediatr Respir Rev 2011;12:104–110. [DOI] [PubMed] [Google Scholar]
- 12. King PT. The role of the immune response in the pathogenesis of bronchiectasis. Biomed Res Int 2018;2018:6802637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lavoie J-P, et al. Bronchiectasis in three adult horses with heaves. J Vet Intern Med 2004;18:757–760. [DOI] [PubMed] [Google Scholar]
- 14. Litzky LA, Green LK. Emphysema and diseases of large airways. In: Zander DS, Farver CF, eds. Pulmonary Pathology. 2nd ed. (Foundations in Diagnostic Pathology series). Elsevier, 2018:409–426. [Google Scholar]
- 15. Madarame H, et al. Endometrial polyps in a cat. J Toxicol Pathol 2003;16:109–112. [Google Scholar]
- 16. Meler E, et al. Diffuse cylindrical bronchiectasis due to eosinophilic bronchopneumopathy in a dog. Can Vet J 2010;51:753–756. [PMC free article] [PubMed] [Google Scholar]
- 17. Menéndez R, Sibila O. Pathophysiology, immunology, and histopathology of bronchiectasis. In: Chalmers J, et al., eds. Bronchiectasis: The EMBARC Manual. Springer, 2018:51–64. [Google Scholar]
- 18. Norris CR. Bronchiectasis. In: King LG, ed. Textbook of Res-piratory Disease in Dogs and Cats. Saunders, 2004:376–379. [Google Scholar]
- 19. Norris CR, Samii VF. Clinical, radiographic, and pathologic features of bronchiectasis in cats: 12 cases (1987–1999). J Am Vet Med Assoc 2000;216:530–534. [DOI] [PubMed] [Google Scholar]
- 20. North Carolina Department of Agriculture and Consumer Se-rvices, Veterinary Division. Import-export requirements. 2019. March 26. [cited 2023 Mar 30]. https://www.ncagr.gov/vet/Livestock/ImpExp.htm
- 21. Reinero CR, et al. Perspectives in veterinary medicine: description and classification of bronchiolar disorders in cats. J Vet Intern Med 2019;33:1201–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sagartz JW, et al. Multiple neoplasia in a captive jungle cat (Felis chaus)—thyroid adenocarcinoma, gastric adenocarcinoma, renal adenoma, and Sertoli cell tumor. J Wildl Dis 1972;8:375–380. [DOI] [PubMed] [Google Scholar]
- 23. Scott P. Ultrasonographic findings in adult cattle with chronic suppurative pneumonia. In Pract 2013;35:460–469. [Google Scholar]
- 24. Shoemark A, et al. Aetiology in adult patients with bronchiectasis. Respir Med 2007;101:1163–1170. [DOI] [PubMed] [Google Scholar]
- 25. Sykes JE. Bacterial bronchopneumonia and pyothorax. In: Sykes JE, ed. Canine and Feline Infectious Diseases. Saunders, 2014:847–858. [Google Scholar]
- 26. Tabaripour SR, et al. Endoparasites of jungle cats (Felis ch-aus) and their pathologic lesions. Iran J Parasitol 2018;13:480–485. [PMC free article] [PubMed] [Google Scholar]
- 27. Tino G. Bronchiectasis. In: Grippi MA, et al., eds. Fishman’s Pulmonary Diseases and Disorders. 6th ed. McGraw-Hill, 2023:1–14. [Google Scholar]
- 28. Wang JY, et al. Recurrent infections and chronic colonization by an Escherichia coli clone in the respiratory tract of a patient with severe cystic bronchiectasis. J Clin Microbiol 2000;38:2766–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]