Three maize transcription factors interact and regulate a dynamic gene network that inhibits cellular development and promotes storage compound accumulation at grain filling.
Abstract
NAKED ENDOSPERM1 (NKD1), NKD2, and OPAQUE2 (O2) are transcription factors important for cell patterning and nutrient storage in maize (Zea mays) endosperm. To study the complex regulatory interrelationships among these 3 factors in coregulating gene networks, we developed a set of nkd1, nkd2, and o2 homozygous lines, including all combinations of mutant and wild-type genes. Among the 8 genotypes tested, we observed diverse phenotypes and gene interactions affecting cell patterning, starch content, and storage proteins. From ∼8 to ∼16 d after pollination, maize endosperm undergoes a transition from cellular development to nutrient accumulation for grain filling. Gene network analysis showed that NKD1, NKD2, and O2 dynamically regulate a hierarchical gene network during this period, directing cellular development early and then transitioning to constrain cellular development while promoting the biosynthesis and storage of starch, proteins, and lipids. Genetic interactions regulating this network are also dynamic. The assay for transposase-accessible chromatin using sequencing (ATAC-seq) showed that O2 influences the global regulatory landscape, decreasing NKD1 and NKD2 target site accessibility, while NKD1 and NKD2 increase O2 target site accessibility. In summary, interactions of NKD1, NKD2, and O2 dynamically affect the hierarchical gene network and regulatory landscape during the transition from cellular development to grain filling in maize endosperm.
IN A NUTSHELL.
Background: The endosperm of cereal plants such as rice (Oryza sativa) and maize (Zea mays) serves as a storage tissue for nutrients, supplying energy for germination and the initial growth of seedlings. It is also a source for human food, livestock feed, and industrial applications. The endosperm develops in 2 stages, a cellular development phase characterized by extensive cell division and cell differentiation, and a second phase, grain filling, in which these cells accumulate storage materials, particularly starches and proteins. Three key transcription factors, NAKED ENDOSPERM1 (NKD1), NKD2, and OPAQUE2 (O2), play important roles in maize endosperm cellular development and storage material accumulation. The mutants of these factors showed several common defects in endosperm grain filling, and this suggests that they may have some related functions. However, we know little about how the 3 factors interactively regulate downstream genes and the regulatory landscapes that modulate endosperm development.
Question: How do NKD1, NKD2, and O2 coregulate gene networks and the regulatory landscape associated with endosperm development and how do they interact to control seed phenotype?
Findings: We developed a set of homozygous lines consisting of nkd1, nkd2, and o2 mutants in all single, double, and triple mutant combinations, as well as wild-type. We identified synthetic phenotypes suggesting that the 3 factors interactively affect endosperm development. We found that the 3 factors may constrain hormone responses, cell wall organization, as well as other cellular developmental processes, and promote starch metabolism, lipid storage, and storage protein accumulation during the transition from cellular development to storage compound accumulation. These processes are regulated through a dynamic, hierarchical gene network, and the 3 factors function as the central regulators. We also reported potential direct target genes of NKD1 and NKD2 along with their chromatin accessibility status and differentially expressed genes between wild-type and each mutant.
Next steps: In the future, we plan to test some key direct targets of NKD1 and NKD2 by developing mutant lines to further explore how they work together to regulate endosperm development.
Introduction
Cereal endosperm is a nutrient storage tissue that provides energy for germination and early seedling development and is important for human food, livestock feed, and industrial commodity. Following double fertilization, the triploid endosperm undergoes coenocytic development (∼1 to 3 d after pollination, DAP) and cellularization (∼3 to 4 DAP) before entering the proliferation and differentiation stage (starting at ∼4 DAP), where cells differentiate into various cell types with specific locations, cellular characteristics, and biological functions (Olsen 2004; Sabelli and Larkins 2009b; Leroux et al. 2014).
In maize (Zea mays), major cell types include starchy endosperm (SE), basal endosperm transfer layer (BETL), aleurone (AL), and embryo surrounding region (ESR), which become morphologically identifiable at around 4 to 5 DAP, followed by further differentiation of other cell types, such as subaleurone (SA) and conducting zone (CZ) (Leroux et al. 2014). In SE, mitotic activity peaks around 8 DAP, after which the cells undergo a transition to endoreduplication (Sabelli and Larkins 2009a; Sabelli and Larkins 2009b). Soon after, SE enters grain filling where cells accumulate starch and storage proteins, resulting in a massive increase of kernel weight (Sabelli and Larkins 2009a, 2009b; Dante et al. 2014). AL is a single, outermost layer of endosperm that plays an important role during germination to digest starch and storage proteins in SE for seedling growth (Hoecker et al. 1999; Becraft and Yi 2011). In contrast to SE, after ∼10 DAP, AL cells form thickened walls, accumulate protein and lipid but not starch, and do not undergo endoreduplication nor programed cell death (Burton and Fincher 2014). BETL contains multiple layers of transfer cells located at the basal region of endosperm adjacent to the maternal placento–chalazal region. The cells feature cell wall ingrowths that increase the surface area of cell membrane to facilitate transport of nutrient compounds into the endosperm (Zheng and Wang 2010). ESR cells are small, densely cytoplasmic cells surrounding the embryo at early stages and are believed to function in communication between the embryo and endosperm (Schel et al. 1984; Cosségal et al. 2007; Leroux et al. 2014).
From ∼8 to ∼16 DAP is an important transition from cellular development to nutrient accumulation for grain filling. Among the many factors involved in regulating these processes, NAKED ENDOSPERM1 (NKD1), NKD2, and OPAQUE2 (O2) are central. NKD1 and NKD2 are duplicate INDETERMINATE DOMAIN (IDD) transcription factors (TFs) encoded by nkd1 (Zm00001d002654) and nkd2 (Zm00001d026113), while O2 is a BASIC LEUCINE ZIPPER (bZIP) domain TF encoded by o2 (Zm00001d018971) (Schmidt et al. 1987, 1990; Yi et al. 2015; Jiao et al. 2017).
Double mutants of nkd1 nkd2 have pleiotropic effects on endosperm development. They disrupt AL cell patterning and cause multiple layers of peripheral cells with compromised cell identity, indicating that NKD1 and NKD2 function to limit the number of AL cell layers and promote AL cell differentiation (Yi et al. 2015). Additionally, NKD1 and NKD2 are important factors in grain filling. In SE, they positively regulate storage protein genes, and the nkd1 nkd2 double mutant showed reduction in total starch abundance, altered starch branching patterns, and irregular starch granules (Gontarek et al. 2016).
Mutations in o2 cause increased endosperm lysine content and opaque kernel phenotype due to reduction of storage protein content (Mertz et al. 1964; Hartings et al. 1989; Schmidt et al. 1992). O2 binds to a GENERAL CONTROL OF NITROGEN4-like motif (or O2-box) at promoters of target genes (Schmidt et al. 1990, 1992). Chromatin immunoprecipitation sequencing (ChIP-seq) analysis showed that O2 direct target genes are mainly involved in nutrient reservoir activity (genes encoding zein storage proteins) (Li et al. 2015; Zhan et al. 2018). In addition, O2 forms protein complexes with other TFs, such as PROLAMIN-BOX BINDING FACTOR1, OPAQUE2 HETERODIMERIZING PROTEIN1 (OHP1) and OHP2, ZmbZIP22, and ZmMADS47, to coordinately regulate zein production, starch biosynthesis, and nutrient metabolism (Dai et al. 2021). Additionally, O2 is involved in AL and SE cell fate specification and differentiation, abiotic response, and endoreduplication (Kowles and Phillips 1985; Zhan et al. 2018). These studies suggest that O2 is a key regulator involved in multiple gene networks and metabolic pathways during grain filling.
NKD1, NKD2, and O2 have close relationships among one another. The nkd1 or nkd2 single mutants showed increased expression of nkd2 or nkd1, respectively (Yi et al. 2015), and a dual-luciferase reporter assay showed that NKD2 may directly repress the expression of nkd1 (Gontarek et al. 2016). This feedback regulation may help limit NKD TF levels. In addition, NKD1 and NKD2 can form homodimers or heterodimers (Gontarek et al. 2016), although the biological significance of this is unknown. NKD1 and NKD2 can also transactivate expression of the o2 gene and in nkd1 nkd2 double mutants, and o2 is downregulated (Gontarek et al. 2016). O2 also transactivates nkd2, and O2 and NKD2 may coactivate floury2, bzip17, and tryptophan aminotransferase related3 genes, which are involved in storage protein biosynthesis (Zhan et al. 2018). Further, there is a strong overlap of differentially expressed genes (DEGs) between the nkd1 nkd2 double mutant and o2 mutant and also of putative target genes between NKD2 and O2 (Gontarek et al. 2016; Zhan et al. 2018).
In this study, we sought to explore how NKD1, NKD2, and O2 coregulate gene networks associated with endosperm development and how they interact to control seed phenotype. A set of homozygous lines was developed consisting of nkd1, nkd2, and o2 mutants in all combinations of single, double, and triple mutants as well as wild-type (WT). They manifested diverse kernel phenotypes that suggested that NKD1, NKD2, and O2 interactively affect endosperm development. RNAseq was performed on developing endosperm tissues from 8 (cellular development, early onset of storage product accumulation) to 16 DAP (extensive storage compound accumulation). We constructed a hierarchical regulatory network through weighted gene coexpression network analysis (WGCNA) and NKD1 and NKD2 DNA affinity purification sequencing (DAP-seq) analysis, as well as assay for transposase-accessible chromatin using sequencing (ATAC-seq) analysis. We found NKD1, NKD2, and O2 function dynamically and interactively to regulate gene networks that play important roles in the grain-filling transition, to constrain the cell proliferation and differentiation processes and promote nutrient biosynthesis and storage.
Results
Genetic interactions among nkd1, nkd2, and o2 genes during grain filling
To investigate combinatory effects of nkd1, nkd2 and o2 genes, homozygous mutant combinations were developed in a W22 inbred background. The o2 mutants have normal size kernels with reduced translucency (Mertz et al. 1964), and the nkd1 nkd2 double mutant shows small, wrinkled kernels with reduced translucency (Yi et al. 2015; Gontarek et al. 2016), whereas the nkd1 nkd2 o2 triple mutant manifested a highly shrunken and wrinkled phenotype (Fig. 1A; Supplemental Fig. S1A). In addition, nkd1 o2 double mutant kernels have an indentation below the crown region not seen in any other genotypes (Supplemental Fig. S1, A to C and Table S1). These phenotypes suggest that the nkd1, nkd2, and o2 genes interact during grain development.
Figure 1.
The defective phenotypes of the nkd1 nkd2 o2 triple mutant. A) Kernel phenotypes of WT, o2, nkd1 nkd2, and nkd1 nkd2 o2. Black background: kernel surface view; white background: kernel opaqueness. Scale bar = 10 mm. B) Light micrographs of WT and nkd1 nkd2 o2. Scale bar = 10 μm. C) TEM images of AL and SA in WT and nkd1 nkd2 o2. Rows 1 and 3, low magnification. Scale bars = 5 μm. Rows 2 and 4, high magnification. Scale bars 1 μm. D) TEM images of starch (Row 1) and protein bodies (Row 2) in SE. Scale bar = 5 μm (Row 1) and 0.5 μm (Row 2). Aggregated starch granules are encircled. E) Starch content among the 8 genotypes. Values are the means of 4 biological replicates from 4 independent ears of each genotype, each replicate a pool of 3 endosperms. The letters above the bars represent significance levels (nonoverlapping letters indicate significant differences at P < 0.05) by pairwise t-test. Error bars represent Ses. F) Zein profiles among the 8 genotypes. Numbers following the lower case letters at the bottom of each column (a1, a2, …, a14) represent different HPLC peaks indicating different isoforms of the corresponding types of zeins. The letters in the heatmap grid represent significance levels (P < 0.05) by pairwise t-test. The colors in the heatmap grid represent relative amount of corresponding zein isoforms in arbitrary units (A.U.) based on the means of 3 biological replicates from 3 independent ears of each genotype, each replicate a pool of 3 endosperms. G) Relative nuclei number of the corresponding ploidy between WT and nkd1 nkd2 o2. Values are means of 4 biological replicates, from 2 independent ears of each genotype, 2 replicates per ear, each replicate a pool of 3 endosperms. * P < 0.05 and **P < 0.01, respectively, by pairwise t-test. Error bars represent Sd.
Light and transmission electron microscopy (TEM) were used to study the endosperm phenotypes at the cellular level, and various phenotypic alternations were observed in AL, SA, and SE (Fig. 1, B to D; Supplemental Figs. S2 to S4). Compared with WT, nkd1 nkd2 and the nkd1 nkd2 o2 triple mutant have multiple layers of poorly differentiated AL cells containing multiple large vacuoles, fewer lipid bodies, and irregular cell wall orientation (Fig. 1C; Supplemental Fig. S2). WT has 1 to 2 layers of SA cells containing partial features of AL, such as lipid bodies, and partial features of SE, such as starch grains and small developing protein bodies (Fig. 1C), whereas nkd1 nkd2 and nkd1 nkd2 o2 have 2 to 5 layers of highly vacuolated SA cells lacking protein bodies (Fig. 1C). In the triple mutant SE, starch granules were much fewer than WT (Fig. 1D; Supplemental Figs. S2 to S4). Interestingly, aggregate starch granules were present in all samples of the triple mutant endosperm. We measured total starch content in mature dry kernels, and compared with WT, the starch contents were significantly decreased in o2, nkd1 nkd2 (Hasjim et al. 2009; Gontarek et al. 2016), and nkd1 o2 mutants, whereas the triple mutant content was less than 30% of WT and was significantly lower than all other genotypes (Fig. 1E).
While TEM of WT protein bodies shows a core of light-staining α- and δ-zeins surrounded by dark-staining β- and γ-zeins (Lending and Larkins 1989), the protein bodies of o2 single mutant showed light spots randomly distributed in dark-staining background (Supplemental Fig. S4A). Protein bodies of other mutants showed variable sizes and internal staining (Supplemental Fig. S4). HPLC zein profiling of 16 DAP endosperms of all genotypes containing o2 mutants showed a significant decrease of most α-zein isoforms (Fig. 1F). The nkd1 nkd2 double mutants showed greater dark-staining areas with reduced, variably sized light staining areas, compared with WT (Supplemental Fig. S4A), and zein profiling showed a significant decrease in levels of 3 α-zein isoforms (a4, a5, and a12) (Fig. 1F). Protein body morphology of the triple mutant is similar to nkd1 o2, but the zein profiles show that levels of most isoforms in all classes of zeins are significantly lower than any other genotype (Fig. 1, D and F; Supplemental Fig. S4A). These data indicate that the nkd1 and nkd2 mutants enhance the effect of o2 on storage protein accumulation.
To test the influence of NKD1, NKD2, and O2 on endoreduplication, nuclei from 16 DAP endosperm of all 8 genotypes were FACS-sorted by ploidy. The o2 mutant increased ploidy levels in endosperm cells compared with WT (Supplemental Fig. S5F; Kowles and Phillips 1985). The nkd1 mutant, alone or in combination with nkd2, o2, or both, tended to decrease the proportion of lower ploidy nuclei, particularly 6C, and increase the proportion of nuclei 24C and 48C (Fig. 1G; Supplemental Fig. S5, A, C, and D). These data suggest that NKD1, NKD2, and O2 function to constrain endoreduplication.
The nkd1, nkd2, and o2 genes function in the transition from cellular development to nutrient accumulation
To investigate the regulatory relationship among the 3 TFs, a transcriptomic analysis was performed on endosperm tissue from each genotype at 8, 12, and 16 DAP. Among all 96 samples (8 genotypes × 3 timepoints × 4 biological replicates), an average of 48,924,625 reads per sample were aligned to B73 reference genome (Zm-B73-REFERENCE-GRAMENE-4.0) (Jiao et al. 2017) with a median alignment rate of 84.2%. Principal component analysis (PCA) of the 96 transcriptomes showed major effects due to developmental time, with secondary effects due to genotype (Fig. 2A). Eight DAP transcriptomes were well separated from later samples indicating a dramatic change of transcriptomes between 8 and 12 DAP, likely reflecting the major change in activity as the endosperm transitions from early cell proliferation and differentiation to grain-filling stages.
Figure 2.
Transcriptomic relationships between genotypes from 8 to 16 DAP. A) PCA of the 8 genotypes at 8, 12, and 16 DAP in 4 biological replicates (n1 = nkd1, n2 = nkd2, n1o2_12 represents nkd1 o2 double mutant at 12 DAP). The arrow highlights the transcriptome of the nkd1 nkd2 o2 triple mutant at 16 DAP, which is close to the o2 single/double/triple mutants at 12 DAP. B) to L). Temporal change of the eigengenes (MEs) of the 8 genotypes at 3 timepoints within each of the 11 coexpression modules (M1 to M11). Enriched gene ontology (GO) terms are shown for each respective module with the FDR in parentheses.
Genotype had a greater impact at 12 or 16 DAP than at 8 DAP. Strikingly, genotypes containing mutant o2 genes were distinct from O2+genotypes (containing a homozygous WT O2 allele, regardless of the genotypes at other loci) at 12 and 16 DAP reflecting the key function of O2 in grain filling. Double mutant nkd1 nkd2 genotypes consistently separated from single mutant and Nkd1+ Nkd2+ (WT Nkd1 and Nkd2 alleles) as expected due to their redundancy. Triple mutant nkd1 nkd2 o2 transcriptomes appeared to cluster with earlier timepoints, possibly reflecting a developmental retardation of the triple mutant endosperm.
To study the effects of these TFs on gene expression patterns, gene readcounts of each mutant were compared against WT at each timepoint using DESeq2 (Love et al. 2014). DEGs were called when log2-fold change (log2FC) was >1 or <−1 and false discovery rate (FDR) <0.05. A total of 14,099 genes were differentially expressed in at least one mutant genotype for at least one timepoint. At 8, 12, and 16 DAP, a total of 6,235, 8,406, and 10,510 DEGs, respectively, were called from at least one mutant genotype, indicating an increasing impact of the 3 TFs on the transcriptome over time (Supplemental Fig. S6 and Data Set 1).
To investigate the gene expression network in which the 3 key TFs participate, WGCNA (Langfelder and Horvath 2008) was performed using log2-transformed value of transcripts per million reads (TPM) of the 14,099 DEGs. A total of 12,458 genes were assigned into 11 coexpression modules (M1 to M11) correlated with nkd1, 2, and/or o2 expression levels. The sizes of these modules range from 100 (M5) to 3,501 (M4) genes (Supplemental Table S2 and Data Set 2). Six of the 11 modules correlated with 2 or more of the TFs suggesting substantial overlap in the genes and processes they regulate. GO enrichment analysis was performed to study the biological processes associated with each module. Every module, except M5 and M8, was enriched (FDR < 0.05) for several GO terms, and the top 1 or 2 informative and significantly enriched terms are shown in Fig. 2 and Supplemental Data Set 3. Cell wall development and hormone pathways are notably prevalent.
To explore the temporal dynamics of this network, we analyzed the behavior of each genotype over time. Module eigengenes (MEs) are defined as the first principal component of the expression matrix of the corresponding module and reflect a general expression level of the genes in that module (Langfelder and Horvath 2007). Eigengene values were calculated for each genotype at each timepoint, within every module (Fig. 2, B to L). In general, most mutants behaved similar to one another within most modules. A notable exception is the nkd1 nkd2 o2 triple mutant, which increased over time relative to other genotypes in modules M2, M9, M10, and M11 (Fig. 2, C, J, K, and L). Intriguingly, these modules are involved in cell wall and epidermal development, suggesting that as development progresses, the 3 TFs may cooperate to restrict cellular development.
NKD1, NKD2, and O2 coregulate different sets of coexpression modules at each timepoint
The coexpression network above was generated using all DEGs across DAPs (pooled timepoint WGCNA). MEs of most modules did not show substantial separation among genotypes at 8 DAP (Fig. 2), likely because later timepoints had more DEGs which skewed the analysis. Also, gene expression is dynamic, so we hypothesize that coexpression patterns may change over time. To test this hypothesis and to unveil expression network information that may have been missed by pooled timepoint WGCNA, we performed WGCNA using DEGs of each DAP individually (individual timepoint WGCNA).
There were 5, 9, and 8 coexpression modules identified at 8, 12, and 16 DAP, respectively, and each module correlates with at least 1 of the 3 TFs (Fig. 3A). The size of modules varied across timepoints, ranging from 148 (M8-3) to 3855 (M8-4) genes, from 96 (M12-5) to 3112 (M12-8) genes, and from 132 (M16-5) to 3676 genes (M16-7) at 8, 12, and 16 DAP, respectively (Supplemental Data Sets 4 to 6). As anticipated, modules were identified at 8 DAP that were not called in pooled timepoint WGCNA, which supports the hypothesis of dynamic coexpression networks. These contained additional functional information; e.g. M8-1, negatively correlated with nkd1 and nkd2, may regulate auxin signaling, auxin transport, and lipid metabolism, as well as cell wall organization. M8-2, positively correlated with nkd1, may regulate starch and lipid metabolism (Fig. 3, A and B; Supplemental Data Set 7).
Figure 3.
Coexpression modules correlated with nkd1, 2, and o2 at individual timepoints and dynamic relationships of modules between different timepoints. A) The correlation of nkd1, nkd2, and o2 gene expression with the ME of corresponding coexpression modules at different DAPs (e.g. M12-5 represents the Module #5 at 12 DAP). The heatmap indicates positive (upregulation) or negative (downregulation) correlations between the 3 key TFs and the corresponding modules. *P < 0.05, **P < 0.01, and ***P < 0.001, respectively, by Pearson correlation test. B) Enriched gene ontology (GO) terms of the corresponding coexpression modules. The heatmap indicates –logFDR of the GO terms. C) The chord diagram shows the dynamic change of coexpression modules over time. The size of the arcs is proportional to the number of genes in the corresponding modules. The chord connecting 2 arcs represents genes expressed at both timepoints in the corresponding modules. D) The split and convergence of the modules over time. The main enriched GO terms are listed in the boxes corresponding to the modules. The FDR of each GO term is shown in parentheses. Lines connecting boxes represent genes overlapping between the 2 modules. The GO term(s) of the overlapping genes are listed above or below the line.
Modules at 12 and 16 DAP were generally similar to those called by pooled timepoint and had many similarities to each other in TF correlations and functions. For instance, both M12-8 and M16-2 are negatively correlated with nkd1, 2, and o2 and are associated with auxin signaling, cell wall organization, and plant epidermal cells; both M12-3 and M16-3 are positively correlated with o2, and both associated with nutrient reservoir activity (Supplemental Data Set 7). These patterns are consistent with many modules called by pooled timepoint WGCNA (such as M1, M7, M9, and M10) (Fig. 2, B, H, J and K); however, some modules were identified specific to 12 DAP, such as M12-4, 5, and 6 associated with stress response (Fig. 3B).
Several modules at different timepoints had similar TF correlations and enriched GO terms; hence, we traced module gene members over time to reveal the dynamic relationships among modules. The result showed that modules split and converge from one timepoint to the next (Fig. 3, C and D). For example, 12.9% and 25.9% of genes in M12-8 were shared with M8-1 and M8-4, respectively, 47.1% and 14.3% of genes in M16-2 were shared with M12-8 and M8-4, respectively, and 22.4% and 19.8% of genes in M16-7 were shared with M12-2 and M12-8, respectively. The similarities in gene membership reflect functional similarities among modules at different timepoints as seen in enriched GO term similarities of these modules (Fig. 3, B and D). For example, genes involved in sucrose metabolic processes in M8-2 and fructose response in M8-4 merge to M12-9 and may contribute to carbohydrate response and starch metabolic processes, whereas genes involved in lipid storage and other processes in plastids in M12-9 split into 2 modules at 16 DAP associated with more specific functions (Fig. 3D).
NKD1, 2, and O2 interact to regulate gene networks
Coexpression network analysis suggested that NKD1, NKD2, and O2 may interact to regulate gene networks; MEs of double or triple mutants showed different dynamic patterns compared with corresponding single mutants (Fig. 2), and some modules are correlated with more than 1 of the 3 key TFs (Fig. 3A). To further analyze the interactions of NKD1, 2, and O2 in regulating gene networks, a multifactorial analysis was performed on DEGs of each timepoint. If the log2FC value of a DEG in mutant TF1, given WT TF2, is significantly different from mutant TF1, given mutant TF2, this gene is considered to be affected by the interaction between TF1 and TF2.
Interactions were identified among all TF combinations, and the number of DEGs affected varied over time (Fig. 4A; Supplemental Data Set 8). NKD1–NKD2, NKD1–O2, and NKD2–O2 interactions affect greater numbers of genes at 8 DAP than 12 or 16 DAP, whereas the interaction among NKD1–NKD2–O2 influences gradually increasing numbers of genes from 8 to 16 DAP (Fig. 4A). The regulatory functions of each interaction are dynamic. For instance, the NKD1–NKD2 interaction most strongly impacts genes in hormone response, phenylpropanoid metabolism, and cell wall formation at 8 DAP, response to temperature and hydrogen peroxide at 12 DAP, and nucleosome assembly at 16 DAP (Fig. 4B). The effect of NKD1–NKD2–O2 interaction on nutrient reservoir activity gradually becomes stronger from 8 to 16 DAP and also affects response to temperature and hydrogen peroxide at 16 DAP (Fig. 4B).
Figure 4.
The effects of interaction between nkd1, nkd2, and o2 on DEGs and coexpression networks. A) Number of DEGs affected by corresponding interactions (e.g. nkd1-o2 represents the interaction effect of nkd1 and o2). B) Gene ontology (GO) terms affected by corresponding interactions (e.g. nkd1-o2 represents the interaction effect of nkd1 and o2). The heatmap represents –logFDR as shown on the scale (blue = 0, red = 10 or greater). C) Coexpression modules affected by corresponding interactions. The heatmap indicates the significance level, via hypergeometric test, of the overlap between the genes affected by corresponding interactions and the genes in the corresponding modules (e.g. M8-1 = module 8-1). Colors represent –logp as shown on the scale. D) The normalized relative gene expression by genotypes in selected modules. The uppercase letters represent significance levels (P < 0.05) by pairwise t-test of mean gene expression values of each genotype. The upper 2 graphs show overall suppression, while the lower 4 illustrate enhancement interactions between 2 individual factors. For example, D (M16-3) showed that the genes in this module were influenced by enhancement effect of nkd1–o2 interaction (expression in nkd1 o2 is significantly lower than the additive effect of nkd1 and o2). TPM, transcripts per million; M, module.
Interactions among the 3 key TFs were also dynamic in regulating coexpression modules. Hypergeometric tests between interaction-affected DEGs and coexpression modules showed that M8-1, 2, 4, 5; M12-4, 5, 8; and M16-1, 2, 3, 7 have significant overlap (−logp > 50) with at least one of the interaction pairs (Fig. 4C), indicating that these modules are likely to be influenced by interactions among the TFs. Interactions caused diverse effects on gene expression in different modules (Fig. 4D). For instance, genes in M8-1 were downregulated in o2 mutant compared with nkd1 nkd2 double mutant, whereas there was no significant difference between nkd1 nkd2 o2 triple mutant and nkd1 nkd2 (Fig. 4D). Hence, mutations of nkd1 and nkd2 may suppress the effect of mutation of o2 in this module. In genes of M16-3, no significant expression change was identified between WT and nkd1, whereas gene expression decreased more in nkd1 o2 double mutant than o2 single mutant (Fig. 4D), suggesting that nkd1 may enhance the effect of o2 in this module.
Functions of NKD1, 2, and O2 gene networks in endosperm tissue types
To explore how NKD1, NKD2, and O2 regulate gene expression in specific endosperm tissues, data from this study were compared with previously published genes expressed preferentially in specific tissue types, which were obtained by performing RNAseq on specific tissues isolated from 8 DAP endosperm using laser capture microscopy (Zhan et al. 2015). Hypergeometric tests were performed to test overlap between these tissue-specific endosperm genes and 8 DAP genes from our study that were: (i) differentially expressed in each mutant genotype, (ii) correlated with coexpression modules, or (iii) affected by interactions of the 3 key TFs. DEGs of nkd1 nkd2 have significant overlap with genes specific to every cell type except BETL, whereas o2 DEGs are only associated with BETL and CSE (Fig. 5). Coexpression modules M8-1 and M8-3 contain significant numbers of AL genes (Fig. 5). These modules are involved with lipids, hormones, and cell wall (Fig. 3B), functions of particular importance to AL. M8-1 is also enriched for CZ and ESR genes, while M8-2 (starch metabolism) overlaps with CSE genes. Various gene interactions affected genes expressed in every tissue except AL.
Figure 5.
Overlap of endosperm tissue-preferential genes with DEGs, coexpression modules, or genes affected by interactions at 8 DAP. The upper number in the grid represents the number of overlapping genes, and the number in the parentheses represents the corresponding P-value via hypergeometric test. n1, n2, o2, n1o2, n2o2, n1n2, and n1n2o2 represent DEGs, relative to WT, of nkd1, nkd2, o2, nkd1 o2, nkd2 o2, nkd1 nkd2, and nkd1 nkd2 o2. M8-1 to M8-5 represent coexpression modules 8-1 to 8-5. nkd1-nkd2, nkd1-o2, nkd2-o2, and nkd1 nkd2-o2 represent interaction effects of nkd1 with nkd2, nkd1 with o2, nkd2 with o2, and nkd1 nkd2 with o2, respectively. CSE, central SE.
NKD1, 2, and O2 regulate coexpression modules directly or indirectly through hub TFs
To further characterize regulatory functions, DNA affinity purification and sequencing (DAP-seq) was performed on NKD1 and NKD2 proteins produced in vitro. Motif enrichment analysis showed potential binding motifs of NKD1 and NKD2 similar to reported (Gontarek et al. 2016) with a TTTGTC core (Fig. 6A). There were 1,951 peaks called for NKD1 and 56,855 for NKD2, of which 93 and 1,692 peaks were located within 3 kb of annotated transcription start sites (TSSs) of 92 and 1,233 genes, respectively (Fig. 6B; Supplemental Data Sets 9 and 10). All NKD1 bound genes were contained among the NKD2 bound genes. The NKD2 consensus binding sequence, TTTGTC[CT]T, was found at 225,434 sites throughout the genome, of which 13.96% were located within 3 Kb of the annotated TSS. Among the bound sites, 28.76% were within 3 Kb of the annotated TSS for NKD1, and 21.82% for NKD2 (Supplemental Fig. S7, A to C). Among the sites flanking the annotated TSS, bound sites had a propensity to occur in 2 or more copies per gene, whereas the unbound sites mainly occurred as singlets (Supplemental Fig. S7, D and E).
Figure 6.
DAP-seq analysis of NKD1 and NKD2 and hierarchical network layout based on individual timepoint WGCNA and DAP-seq. A) Binding motif of NKD1 and NKD2. B) Venn diagram illustrating the number of NKD1 and NKD2 bound genes. C) Hypergeometric test of the NKD1, NKD2, and O2 bound genes overlapping with DEGs of the mutant genotypes at 8, 12, and 16 DAP. The upper number in the grid represents the number of overlapping genes, and the number in parentheses represents the corresponding P-value. D) Hierarchical gene network layout of selected coexpression modules generated via Cytoscape 3.7.2. The size of the circles is based on the tiers of the genes in the hierarchy. The heavy edges connect the TFs and their potential direct targets, and the light edges connect coexpressed genes in the corresponding modules that are not direct targets. E) Pull-down assay showed protein–protein interaction between IDDP10 and NKD1. IDDp10-6xHIS was used as bait, and NKD1 or NKD2 fused with GLUTATHIONE-S-TRANSFERASE (GST) were prey. In vitro translated proteins were mixed and applied to a 6xHIS affinity resin, followed by elution. Prey proteins were detected by immunoblot analysis using anti-GST antibodies. Lanes 1 to 3 show crude input, 4 to 6 show eluate. The IDDp10-6xHIS bait was able to bind to GST-NKD1 (lane 5, lower panel) but not to GST-NKD2 (lane 6), nor GST alone (lane 4). In the absence of bait protein, no prey proteins were retained by the resin (upper panel).
The NKD1 and NKD2 bound genes, together with published ChIP-seq O2 targets (Zhan et al. 2018), were incorporated with DEGs identified for mutant genotypes at each timepoint. The intersection between DAP-seq targets or published ChIP-seq O2 targets and DEGs were considered potential direct targets of NKD1, 2, or O2 regulation. O2 ChIP-seq targets had significant (P < 1e−6) overlap with o2 mutant DEGs across all 3 timepoints (Fig. 6C). NKD2 DAP-seq targets significantly overlapped (P < 1e−6) with DEGs of nkd1, o2, nkd1 o2, and nkd1 nkd2 o2 at 8 DAP, whereas at 12 and 16 DAP, only nkd1 nkd2 and nkd1 nkd2 o2 DEGs overlapped (Fig. 6C). NKD1 DAP-seq targets had low overlap due to the low number of peaks. Among the putative direct targets of NKD1 and NKD2, 25% to 50% were TFs, and the most significantly enriched GO term was “transcription factor activity” (GO:0003700) across all timepoints (Table 1; Supplemental Table S3).
Table 1.
GO terms of NKD1 or NKD2 potential direct targets at 8, 12, and 16 DAP
| TF | DAP | GO | Description | FDR |
|---|---|---|---|---|
| NKD1 | 8 | GO:0003700 | Transcription factor activity, sequence-specific DNA binding | 4.30E−02 |
| NKD1 | 8 | GO:0046983 | Protein dimerization activity | 4.30E−02 |
| NKD2 | 8 | GO:0003700 | Transcription factor activity, sequence-specific DNA binding | 1.80E−28 |
| NKD2 | 8 | GO:0046983 | Protein dimerization activity | 6.60E−13 |
| NKD2 | 8 | GO:0007389 | Pattern specification process | 6.30E−16 |
| NKD2 | 8 | GO:0009888 | Tissue development | 1.10E−08 |
| NKD2 | 8 | GO:0009755 | Hormone-mediated signaling pathway | 1.10E−08 |
| NKD2 | 8 | GO:0001708 | Cell fate specification | 1.50E−05 |
| NKD2 | 8 | GO:0019252 | Starch biosynthetic process | 7.10E−04 |
| NKD1 | 12 | GO:0044255 | Cellular lipid metabolic process | 2.50E−02 |
| NKD2 | 12 | GO:0003700 | Transcription factor activity, sequence-specific DNA binding | 7.50E−24 |
| NKD2 | 12 | GO:0046983 | Protein dimerization activity | 2.30E−05 |
| NKD2 | 12 | GO:0045735 | Nutrient reservoir activity | 1.40E−03 |
| NKD2 | 12 | GO:0009755 | Hormone-mediated signaling pathway | 3.00E−12 |
| NKD2 | 12 | GO:0048316 | Seed development | 7.30E−07 |
| NKD2 | 12 | GO:0008610 | Lipid biosynthetic process | 7.70E−06 |
| NKD2 | 12 | GO:0005984 | Disaccharide metabolic process | 1.20E−04 |
| NKD1 | 16 | GO:0044249 | Cellular biosynthetic process | 4.40E−02 |
| NKD2 | 16 | GO:0003700 | Transcription factor activity, sequence-specific DNA binding | 1.70E−29 |
| NKD2 | 16 | GO:0046983 | Protein dimerization activity | 1.80E−14 |
| NKD2 | 16 | GO:0045735 | Nutrient reservoir activity | 5.20E−03 |
| NKD2 | 16 | GO:0009755 | Hormone-mediated signaling pathway | 5.00E−11 |
| NKD2 | 16 | GO:0048316 | Seed development | 1.40E−07 |
| NKD2 | 16 | GO:0009311 | Oligosaccharide metabolic process | 2.10E−04 |
| NKD2 | 16 | GO:0034728 | Nucleosome organization | 2.20E−04 |
| NKD2 | 16 | GO:0008610 | Lipid biosynthetic process | 6.30E−03 |
To further investigate how O2, NKD1, and NKD2 coregulate gene expression, hub genes were called with module membership scores greater than 0.8, indicating significant connectivity within the module. Hub genes that were potential targets of NKD1 and NKD2 included genes encoding TFs, kinases, and protein processing-related factors (Table 2), suggesting that NKD1 and 2 may regulate coexpression modules in a hierarchical pattern. O2 target hub genes included the TF genes bzip17 and g-box binding factor1 (gbf1) indicating that O2 also functions hierarchically within this network. Genes for AUXIN RESPONSE FACTOR1 (ARF1) and ARF29 were among the potential NKD2 targets, and since published DAP-seq targets were available for these factors (Galli et al. 2018; Zhan et al. 2018), they were also incorporated to build a gene regulatory network with selected modules (Fig. 6D). At 8 DAP, NKD1 and NKD2 regulate M8-1 responsible for cell wall organization, auxin signaling, and transmembrane transport possibly through TFs PLANT AT-RICH SEQUENCE AND ZINC BINDING PROTEIN2 (PLATZ2), GROWTH REGULATING FACTOR TF (GRFTF3), and ARF29.
Table 2.
Potential NKD1, NKD2, and O2 modulated hub genes of selected modules
| Module | DAP | Hub gene id | Hub gene product | Description | Module membership score | Potential target of |
|---|---|---|---|---|---|---|
| M8-1 | 8 | Zm00001d029437 | PLATZ2 | PLATZ transcription factor family protein | 0.938873691 | NKD1, NKD2 |
| M8-1 | 8 | Zm00001d021362 | GRFTF3 | Homolog of growth-regulating factor 9 | 0.864321005 | NKD1, NKD2 |
| M8-1 | 8 | Zm00001d017908 | BHLH156 | Homolog of transcription factor BIM3 | 0.846721007 | NKD2 |
| M8-1 | 8 | Zm00001d026540 | ARF29 | Homolog of auxin response factor 5 | 0.800065026 | NKD2 |
| M8-2 | 8 | Zm00001d007382 | BHLH96 | Homolog of Transcription factor ICE1 | 0.824640415 | NKD2 |
| M12-2 | 12 | Zm00001d031522 | ARF1 | Auxin response factor 1 | 0.975753404 | NKD2 |
| M12-2 | 12 | Zm00001d024488 | MYB155 | Homolog of MYB domain protein 118 | 0.959466374 | NKD2 |
| M12-2 | 12 | Zm00001d051568 | TUBTF6 | Tubby-like F-box protein 6 | 0.952357799 | NKD2 |
| M12-2 | 12 | Zm00001d036416 | CDPK13 | Calcium-dependent protein kinase13 | 0.879817661 | NKD2 |
| M12-3 | 12 | Zm00001d018971 | O2 | Regulatory protein opaque-2 | 0.976845488 | NKD2 |
| M12-3 | 12 | Zm00001d046937 | BZIP17 | Basic leucine zipper 17 | 0.978356856 | O2 |
| M12-3 | 12 | Zm00001d039065 | GBF1 | G-box binding factor 1 | 0.947779763 | O2 |
| M12-3 | 12 | Zm00001d038288 | MYBR13 | Putative MYB DNA-binding domain superfamily protein | 0.963581808 | NKD2 |
| M12-5 | 12 | Zm00001d033987 | HSFTF17 | Homolog of heat stress transcription factor A-6b | 0.907691436 | NKD2 |
| M12-8 | 12 | Zm00001d021362 | GRFTF3 | Homolog of growth-regulating factor 9 | 0.903802679 | NKD1, NKD2 |
| M12-8 | 12 | Zm00001d029437 | PLATZ2 | PLATZ transcription factor family protein | 0.89337778 | NKD1, NKD2 |
| M12-8 | 12 | Zm00001d033174 | GAP1 | Golgi associated protein homolog | 0.93285082 | NKD2 |
| M12-8 | 12 | Zm00001d017726 | GLK11 | Homeodomain-like superfamily protein | 0.912192373 | NKD2 |
| M12-8 | 12 | Zm00001d007173 | ABI39 | Homolog of B3 domain-containing transcription repressor VAL2 | 0.899143178 | NKD2 |
| M12-8 | 12 | Zm00001d026540 | ARF29 | Homolog of auxin response factor 5 | 0.837692812 | NKD2 |
| M16-2 | 16 | Zm00001d017900 | DOF42 | Homolog of Dof zinc finger protein DOF5.4 | 0.943253299 | NKD1, NKD2 |
| M16-2 | 16 | Zm00001d008808 | MYBR24 | Putative MYB DNA-binding domain superfamily protein | 0.940741423 | NKD1, NKD2 |
| M16-2 | 16 | Zm00001d002234 | HB75 | Homeodomain leucine zipper family IV protein | 0.935349746 | NKD2 |
| M16-2 | 16 | Zm00001d020670 | HB11 | Homolog of homeobox-leucine zipper protein HAT3 | 0.92812669 | NKD2 |
| M16-3 | 16 | Zm00001d018971 | O2 | Regulatory protein opaque-2 | 0.923257678 | NKD2 |
| M16-3 | 16 | Zm00001d039065 | GBF1 | G-box binding factor 1 | 0.872074634 | O2 |
| M16-3 | 16 | Zm00001d046937 | BZIP17 | Basic leucine zipper 17 | 0.830433051 | O2 |
| M16-7 | 16 | Zm00001d031522 | ARF1 | Auxin response factor 1 | 0.988098645 | NKD2 |
| M16-7 | 16 | Zm00001d024488 | MYB155 | Homolog of MYB domain protein 118 | 0.963596519 | NKD2 |
While not identified as a potential target of NKD1 or NKD2, idd protein10 (iddp10) was called as a hub gene for M8-2, associated with starch metabolism. IDDP10 is closely related to NKD1 and 2 (Yi et al. 2015; Coelho et al. 2018) and can heterodimerize with NKD1 based on a pull-down assay (Fig. 6E), indicating NKD1 and IDDP10 may coregulate M8-2. At 12 DAP, NKD2 potential targets cyclin dependent kinase13 (cdpk13) and myb155 are hub genes for M12-2 that contribute to ABA response; ARF29 and GOLGI ASSOCIATED PROTEIN1 (GAP1) may regulate M12-8 associated with auxin signaling and cell wall organization, respectively. At 16 DAP, ARF1, a potential target of NKD2, may regulate M16-7 responsible for DNA replication and nucleosome assembly. Furthermore, as a direct target of NKD2, O2 itself is a hub gene regulating M12-3 and M16-3, involved in nutrient storage (Fig. 6D).
To further explore the architecture of the gene networks, DAP-seq was performed on select downstream TFs from key modules (Supplemental Fig. S8A). For example, GBF1 is a TF in modules M12-3 and M16-3 and is a direct target of O2 (Zhan et al. 2018). GO enrichment analysis of its DAP-seq targets suggested that GBF1 contributes to amino acid and protein production in both modules (Supplemental Fig. S8, A and B and Data Set 11). In M12-9, heat shock factor TF10 (hsftf10) is a potential direct target of NKD2 () and shares targets with NKD2, including chalcone flavone isomerase1 (chi1), chloroplast rna splicing protein2 (crs2), and oil yellow1 (oy1), functioning in the plastid (Supplemental Fig. S8, A and C and Data Set 12).
NKD1, NKD2, and O2 interactively affect endosperm chromatin accessibility
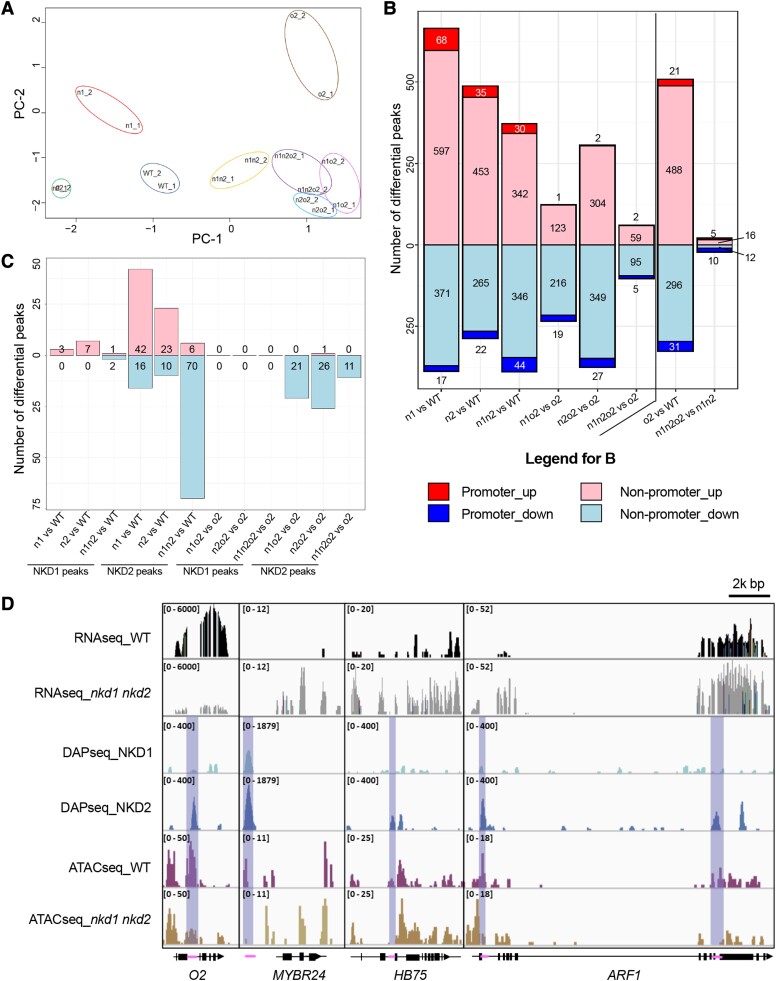
TF action is often apparent by alterations in chromatin accessibility (Xie and Liu 2019). To investigate how interactions among NKD1, NKD2, and O2 affect the endosperm regulatome, we performed ATAC-seq for 16 DAP endosperms of all 8 genotypes (2 biological replicates per genotype). Among 16 sequenced samples, 20,478 to 49,640 (mean: 34,766) peaks were called, representing potential regions of accessible chromatin. Distribution of the peaks on chromatin varies among samples: from 54.35% to 66.73% of peaks are located at distal intergenic regions (>3 kb upstream and >300 bp downstream of an annotated gene), while 6.7% to 12.1% are located in promoter regions (within 3 kb flanking an annotated TSS), and 4.62% to 8.88% are located within 1 kb flanking an annotated TSS (Supplemental Fig. S9 and Data Sets 13 to 20).
PCA showed that the regulatome of WT, nkd1, nkd2, o2 single mutants and nkd1 nkd2 double mutant are well separated from one another, whereas o2 double or triple mutants are clustered together (Fig. 7A). This suggests that NKD1, NKD2, and O2 interactively influence the endosperm regulatory landscape. To further explore this possibility, differential peak/accessibility analysis of nkd1, nkd2, and nkd1 nkd2 versus WT was compared with nkd1 o2, nkd2 o2, and nkd1 nkd2 o2 versus o2. Compared with WT, nkd1 and nkd2 single mutants have overall greater numbers of open regions (upregulated peaks) than closed regions (downregulated peaks), whereas nkd1 nkd2 double mutant has similar numbers of open and closed regions (Fig. 7B). In contrast, in o2-backgrounds, all nkd1, nkd2, and nkd1 nkd2 mutants have greater numbers of closed than open regions, suggesting that O2 may influence the ways NKD1 and NKD2 interact with chromatin. On the other hand, we also compared the differential peak/accessibility of o2 versus WT with nkd1 nkd2 o2 versus nkd1 nkd2. In the WT (or Nkd1+ Nkd2+) and nkd1 nkd2 mutant backgrounds, o2 has 836 and 43 differential peaks, respectively (Fig. 7B). This difference indicates that NKD1 and NKD2 also influence how O2 interacts with chromatin.
Figure 7.
Chromatin accessibility profiles among genotypes of 16 DAP maize endosperm. A) PCA of accessible peaks among 8 genotypes (in 2 biological replicates). B) Number of differential peaks categorized by more opening (up) or closing (down) peaks at promoter or nonpromoter regions. C) Number of overlapping peaks between the ATAC-seq and DAP-seq analysis. D) Tracks of RNAseq (WT and nkd1 nkd2), DAPseq (NKD1, NKD2), and ATAC-seq (WT and nkd1 nkd2) of selected potential direct targets of NKD1 and/or NKD2 listed at Table 2. Differential peaks are highlighted with shading, and the potential NKD1 and/or NKD2 binding regions represented with magenta bars on the gene models. Numbers in brackets represent scale range of corresponding tracks. O2 is downregulated in nkd1 nkd2 (log2FC = −1.20, adjusted P = 0.0022), and MYBR24, HB75, and ARF1 are upregulated in nkd1 nkd2 (log2FC = 2.75, 1.75 and 1.87; adjusted P = 0.017, 1.41E−11, and 1.70E−14, respectively).
To investigate chromatin regions that may be directly regulated by NKD1 or NKD2, DAP-seq data were compared with the differential peaks from ATAC seq, and peaks that overlapped between the 2 datasets were identified. Fewer overlapping peaks were identified for nkd mutants in an o2 mutant background than O2+ (Fig. 7C), which agrees with the above finding that O2 affects chromatin accessibility of NKD1 and NKD2. Interestingly, in the O2+ background, the number of open chromatin regions for NKD1 or NKD2 are greater in single mutants of nkd2 or nkd1, respectively, whereas when both nkd1 and nkd2 are mutated; there is a sharp increase of closed chromatin regions (Fig. 7C).
To examine how the differential chromatin accessibility could affect the expression of NKD1 or NKD2 potential target genes, we incorporated the tracks of WT and nkd1 nkd2 RNAseq and ATAC-seq, as well as NKD1 and 2 DAPseq peaks for selected hub genes from coexpression modules at 16 DAP (Fig. 7D; Table 2). For the o2 gene, RNAseq showed decreased readcounts in the nkd1 nkd2 mutant compared with WT. In intron 2 of o2, DAPseq identified a NKD2 binding site that is coincident with a region of chromatin that ATAC-seq showed had decreased accessibility in the nkd1 nkd2 mutant (Fig. 7D). This suggests that NKD2 may upregulate o2 by binding to the second intron. Similarly, there is a differentially accessible NKD2 binding site in intron 2 of homeobox TF75 (hb75), which shows elevated expression in nkd1 nkd2 mutant, suggesting NKD2 binding may downregulate hb75 (Fig. 7D). NKD1 and NKD2 may downregulate mybr24 by binding to a promoter element (Fig. 7D), while multiple NKD2 binding sites in the transcribed regions of arf1 may downregulate expression (Fig. 7D).
To elucidate the nature of increased accessibility regions, motif enrichment analysis was performed on potential NDK2 DAP-seq target regions overlapping with differential accessibility regions caused by nkd1, nkd2, or nkd1 nkd2 mutants. The result showed that binding motifs of IDD family TFs are most significantly enriched, followed by TFs of other families, including HMG, bZIP, bHLH, DOF, RING-type, and MADS (Supplemental Table S4).
Discussion
Synthetic phenotypes of nkd1, nkd2, and o2 mutants may be associated with disruption of grain filling processes
The nkd1 o2 double mutant and nkd1 nkd2 o2 triple mutant produced synthetic kernel phenotypes not observed in single or nkd1 nkd2 double mutants (Fig. 1; Supplemental Fig. S1). While the nkd1 single mutant did not produce a visible phenotype, nkd1 o2 double mutants have wrinkled kernels in addition to the opaque phenotype of o2. Similarly, the nkd2 o2 double mutant showed only the opaque phenotype of o2, while nkd1 nkd2 o2 showed extremely small, wrinkled kernels. These genetic interactions indicate that NKD1, NKD2, and O2 have related functions in kernel development. Wrinkled kernel phenotypes often result from disruption of grain filling processes (Tsai and Nelson 1966; James et al. 1995; Zhang et al. 2016, 2019a, 2019b; Yang et al. 2021). Protein bodies and starch granules normally form a matrix that produces the hard, vitreous endosperm and desirable physiochemical properties (Wu et al. 2010; Holding 2014). Defects in either proteins or starch may produce a loose unstable matrix that results in opaque, chalky, or shrunken endosperm (Wu et al. 2010). This is consistent with our phenotypic and transcriptomic analyses. Mutant combinations with wrinkled kernels caused decreased starch accumulation and defects in starch grain morphology (Fig. 1, D and E). They also caused smaller protein bodies and pronounced deficiencies in zein storage protein accumulation (Fig. 1, D and F; Supplemental Figs. S2 to S4). WGCNA indicated that regulating the accumulation of grain storage products is a major function of these 3 TFs. At 8 DAP, nkd1 is positively correlated with module M8-2 associated with starch metabolism (Fig. 3, A, B, and D), and at 16 DAP, nkd2 and o2 interact to regulate M16-3 associated with storage protein production (Fig. 4, B to D).
NKD1 and NKD2 paralogs have different interaction patterns with O2
As described, Nkd1 and Nkd2 genes mutually regulate each other in a feedback mechanism that partially compensates single mutants of either gene (Yi et al. 2015; Gontarek et al. 2016). The ATAC-seq analysis gave further support to this mutual compensation model. The single mutant of nkd1 has a greater number of opened chromatin regions for NKD2, while nkd2 enhances chromatin accessibility for NKD1 (Fig. 7C), whereas this is not observed in the nkd1 nkd2 double mutant. Increased open chromatin regions in nkd1 may enhance binding by NKD2 and vice versa, to further contribute to the genetic complementation for each gene by the other.
Although clearly paralogs, this study showed that NKD1 and NKD2 have divergent functions; they are correlated with different sets of coexpression modules (Fig. 3A) and have different interaction patterns with O2 (Fig. 4). The nkd2 o2 double mutant has a similar kernel phenotype as o2, whereas nkd1 o2 showed a wrinkled kernel phenotype (Supplemental Fig. S1). In addition, single mutants of either gene result in over a thousand DEGs, especially at 8 DAP (Supplemental Fig. S6). Thus, NKD1 and NKD2 have divergent functions in addition to redundancy. Two possible models include: (i) neofunctionalization, when one or both duplicated genes may gain new functions and (ii) subfunctionalization, when one or both duplicated genes may partially lose the function of their ancestral gene (Pickett and Meeks-Wagner 1995; Moore and Purugganan 2005).
NKD1, 2, and O2 affect each other's regulatory landscapes
We assayed the influence of these TFs on chromatin by comparing mutant with WT using ATAC-seq. Interestingly, the effects of NKD1 and/or NKD2 on chromatin accessibility were greater in the presence of a WT O2+ allele than if o2 was mutant. Further, in an O2+ background, nkd1 and nkd2 mutants showed increased numbers of accessible sites, compared with Nkd1+ or Nkd2+, whereas in an o2 mutant background, nkd1 and nkd2 mutants showed decreased numbers of accessible sites (Fig. 7). Notably, fewer potential direct target sites of NKD1 and NKD2 were available in o2 mutant than in O2+ WT. This indicates that O2 plays an important role in modeling the global NKD1 and NKD2 regulatory landscape. Conversely, the number of differentially accessible chromatin peaks of o2 mutant versus O2+ WT is nearly 20 times greater in the Nkd1+ Nkd2+ background than in the nkd1 nkd2 mutant background, indicating that NKDs also affect the O2 regulatory landscape.
The mode by which NKDs and O2 impact the regulatomes is unclear, but several scenarios can be envisaged. O2 directly promotes expression of the nkd2 gene; NKD2 negatively regulates expression of the nkd1 gene, while NKD1 and NKD2 positively regulate the expression of the o2 gene (Gontarek et al. 2016; Zhan et al. 2018). This dynamic regulatory circuit could contribute to variations in NKD1 and NKD2 activity between O2+ and o2 backgrounds, and O2 activity between Nkd1+ Nkd2+ and nkd1 nkd2 backgrounds. The regulatory landscapes of NKD1, NKD2, and O2 may also be affected indirectly due to increased endoreduplication in the o2 and nkd1 nkd2 mutant backgrounds, respectively (Supplemental Fig. S5) (Kowles and Phillips 1985; Sabelli 2012). Increased endoreduplication may cause increased chromatin condensation and disruption of global chromatin accessibility for TFs (van Zanten et al. 2011; Sabelli et al. 2013). Consistent with O2 functioning to restrict endoreduplication, we found that O2 was negatively correlated with coexpression module M16-7, responsible for nucleosome assembly and regulation of DNA replication (Fig. 3, A, B, and D). NKD1 and 2 may also genetically interact with O2 and coregulate the module M16-7 (Fig. 4, B to D). Furthermore, auxin has been implicated in promoting endoreduplication (Sabelli 2012), and ARF1 was identified as a potential direct target of NKD2 and a hub gene of M16-7 (Figs. 6D and 7G; Table 2).
NKD1, 2, and O2 function dynamically in the transition to grain filling
Beginning at about 8 DAP, maize endosperm undergoes a transition from lag phase, characterized by cell proliferation and differentiation, to grain filling with extensive storage compound accumulation (Wu et al. 2022). Our data indicate these 3 genes function dynamically throughout this transition. Mutants show defects in cell patterning and differentiation as well as impaired accumulation of starch, storage proteins, and lipids (Fig. 1; Supplemental Figs S2, S3, and S4) (Schmidt et al. 1992; Hasjim et al. 2009; Yi et al. 2015; Gontarek et al. 2016). WGCNA of WT aligned well with processes of endosperm development and the transition to grain filling, reflecting decreases in cell wall production-related processes and increases in nutrient reservoir activity from 8 to 16 DAP (Fig. 2), whereas various mutant combinations of nkd1, nkd2, and o2 manifested different temporal patterns in these processes.
Strikingly, at later stages, the nkd1 nkd2 o2 triple mutant had elevated cell wall production-related processes (Fig. 2, C, J, and L) and a dramatically delayed increase of nutrient accumulation (Fig. 2B), suggesting that the transition from lag phase to grain filling might be retarded. This was further supported by PCA showing that the triple mutant transcriptome at 16 DAP clustered with other o2 mutant genotypes at 12 DAP (Fig. 2A). The genetic interactions between nkd1, nkd2, and o2 also underwent a transition from cell wall organization, hormone response, and plant epidermis development at 8 DAP to nutrient reservoir activity, nucleosome assembly, and DNA replication at 16 DAP (Fig. 4B). In summary, these 3 TFs have key roles in both cellular development and grain filling, and they function and interact dynamically during the transition between these stages.
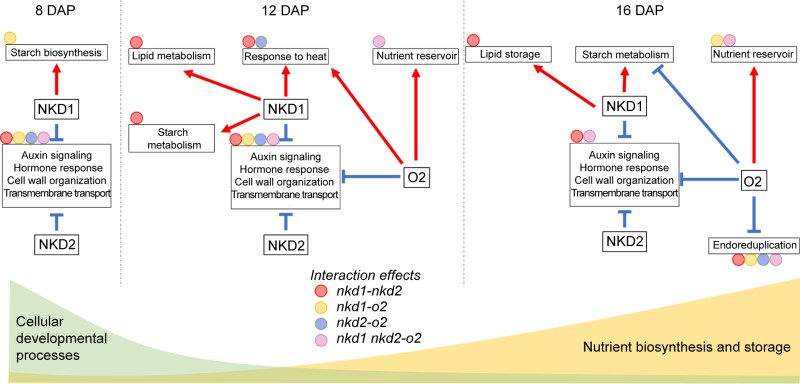
Working model of NKD1, 2, and O2 regulating the transition processes during the endosperm development
This study demonstrated that O2, NKD1, and NKD2 are key regulators that interact in a dynamic network that performs changing functions over developmental time, from primarily cellular development at 8 DAP to storage compound accumulation at 16 DAP. The coexpression modules undergo migration of genes from 8 to 16 DAP, resulting in merged or split modules from one timepoint to another, as the network undergoes temporal changes in developmental functions (Fig. 3, C and D).
Some of the key functions are summarized in Fig. 8. NKD1 and NKD2 function to restrict auxin signaling, cell wall organization, and other cellular developmental processes throughout endosperm development, which may help explain the mutant phenotype of extra cell layers (Yi et al. 2015). These functions are accomplished through modules M8-1, 12-8, and 16-2. Hub genes at 8 DAP include ARF29, PLATZ2, and GRFTF3, which are potential direct targets of NKD1, NKD2, or both. PLATZ2 encodes a plant AT-rich sequence and zinc-binding (PLATZ) protein. In rice (Oryza sativa), a PLATZ factor, SHORT GRAIN6 (SG6), influences grain size by regulating multiple cellular processes, including cell cycle and cell differentiation (Zhou and Xue 2020). GRFTF3 is a putative ortholog of Arabidopsis (Arabidopsis thaliana) GROWTH-REGULATING FACTOR9 (GRF9) (Table 2), which regulates cell growth (Omidbakhshfard et al. 2018).
Figure 8.
Models of NKD1, 2, and O2 regulating the transition processes in endosperm development. Processes are shown in boxes. Positive regulation is shown by red arrows, negative regulation by blue T-shaped lines, and interactions are represented by circles attached to the corresponding boxes.
By 12 DAP, additional hub genes include gap1, g2-like TF11 (glk11), and aba insensitive39 (abi39) (Fig. 6D; Table 2). GAP1 is a putative ortholog of the Arabidopsis REVERSIBLY GLYCOSYLATED POLYPEPTIDE1 (RGP1), which is a Golgi-associated protein involved in the cell wall biosynthesis (Welner et al. 2017). GLK11 is homologous to Arabidopsis ALTERED PHLOEM DEVELOPMENT (APL), associated with asymmetric cell divisions and differentiation of phloem (Bonke et al. 2003). ABI39 is homologous to Arabidopsis VP1/ABI3-LIKE2 (VAL2), a TF that maintains the embryonic state by interacting with PRC2 to modify chromatin (Suzuki et al. 2007; Yuan et al. 2021). Beginning at 12 DAP, O2 also participates in negatively regulating developmental processes via M12-8 and M16-2.
Functions in storage compound accumulation have been well documented for all 3 TFs (Fig. 1) (Yi et al. 2015; Gontarek et al. 2016; Zhang et al. 2016; Zhan et al. 2018; Deng et al. 2020). At 8 DAP, the module M8-2 is associated with early starch biosynthesis, and NKD1 and NKD2 potentially regulate M8-2 by modulating or interacting with the hub genes bhlh96 and iddp10 (Figs. 3A, 3B, and 6D; Table 2). While not a target of either NKD1 or NKD2 regulation, IDDP10 can physically interact with NKD1 (Fig. 6E) and has also been proposed to interact with NKD1 in leaf mesophyll cells (Coelho et al. 2018). Additionally, the Arabidopsis AtIDD5 is a positive regulator of STARCH SYNTHASE4 (SS4) and may control starch granule formation (Ingkasuwan et al. 2012).
From 8 to 16 DAP, the transition from cellular development to nutrient storage is associated with dynamic interactions of NKD1, 2, and O2, which constrain the cellular developmental processes, and promote biosynthesis and storage of starch, proteins, and lipids. By 12 DAP, synthesis and accumulation of storage compounds have greatly increased, and all 3 TFs interact to promote this activity. The effects of these interactions are apparent from the synthetic phenotypes of nkd1 o2 and nkd1 nkd2 o2 mutants with dramatic grain-filling deficiencies affecting both starch and protein accumulation (Fig. 1, A, D, E, and F; Supplemental Fig. S1). O2 positively regulates zein synthesis and accumulation via modules M12-3 and M16-3 (Figs. 3 and 6D), and NKD1 and NKD2 synergistically interact with O2 on these processes (Fig. 4, B to D). In addition, NKD1 and NKD2 interact to promote lipid metabolism via modules M12-9 and M16-6 (Figs. 3, 5C, 6B, and 6C). Finally, NKD1, 2, and O2 may interact to modulate endoreduplication via module M16-7 (Figs. 3, 4D, 5B, 5C, and 5D), leading to differential distributions of chromosomal ploidy among WT and mutants (Fig. 1G; Supplemental Fig. S5). These processes are briefly summarized in our working model illustrated in Fig. 8.
Materials and methods
Plant materials and growth conditions
All nkd1, nkd2, and o2 homozygous single, double, and triple mutants, as well as the WT maize (Z. mays), were derived from a cross between the nkd1-Ds nkd2-null (Ahern et al. 2009; Yi et al. 2015) double mutant and o2 single mutant, each in the W22 genetic background. Segregating lines were developed by self-pollinating the triple heterozygote (+/nkd1 +/nkd2 +/o2), from which homozygotes of each genotype were identified. All plant materials were collected from descendants of the same segregating ears grown in the greenhouse at Molecular Biology Building or in the field at Curtiss Research Farm, Iowa State University (Ames, IA). Materials may be requested from the Maize Cooperation Genetic Stock Center, Urbana, IL, USA.
RNA-seq and data analysis
Total RNA of 8, 12, and 16 DAP endosperms of nkd1, nkd2, and o2 homozygous single, double, and triple mutants, as well as the WT counterpart (4 biological replicates, from 4 independent ears of each genotype, each replicate contains a pool of 3 endosperms), were extracted following the published LiCl precipitation-based protocol (Li et al. 2014), except that GeneJET RNA cleanup and concentration kit (Thermo Fisher Scientific, Waltham, MA, USA) were used for DNaseI-treated RNA purification. RNA quality control was performed via AATI Fragment Analyzer (Agilent, Santa Clara, CA, USA) at the DNA Facility, Iowa State University. High-quality (RQN > 9.0) RNA samples were sent to the HSC core, University of Utah (Salt Lake City, UT) for library preparation with Illumina TruSeq RNA Library Prep Kit (Illumina, San Diego, CA, USA) and 150 bp paired end sequencing using NovaSeq 6000 with the S4 cell.
The raw read quality control was performed via FastQC (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). The raw reads were mapped to B73 RefGen_v4 (AGPv4) by HISAT2 (v2.1.0) (Kim et al. 2015; Jiao et al. 2017). The mapped transcripts in output files were sorted and assembled by SAMtools (v1.9) and StringTie (v1.3.6) (Pertea et al. 2016), respectively. Readcounts were calculated and analyzed by HTSeq (v0.11.1) and DEseq2 (v1.26.0) (Love et al. 2014), respectively. DEGs were identified using a generalized linear model-based approach with the cutoff criteria log2FC >1 or <−1 and FDR < 0.05.
Gene coexpression network analyses were performed via WGCNA (Langfelder and Horvath 2008) using the TPM (in log2-transformed values) generated from StringTie (Pertea et al. 2016) of all DEGs following the previously published procedures (Zhan et al. 2015). For the pooled timepoint WGCNA, soft-thresholding power was set to 10 to build a signed network with the scale-free topology. The merge threshold and minimum module size were set as 0.1 and 60, respectively. For the individual timepoint WGCNA, soft-thresholding power was set to 7, 9, and 7 for 8, 12, and 16 DAP, respectively. The merge threshold and minimum module size were set as 0.3 and 60, respectively. The module hub genes were called with the Module Membership (MM) score >0.8. The network was visualized by Cytoscape 3.7.2.
Transcriptomics data were analyzed to investigate effects of NKD1, NKD2, and O2 interactions on gene expression using generalized linear model analysis based on multifactorial experimental design via edgeR (v3.38.4). We included 2 factors (Gene A and Gene B) and their interaction in the generalized linear model, where each factor has 2 levels (WT and mutant). The interaction effect between 2 TFs GeneA and GeneB can be interpreted as the difference of gene expression fold change (FC) for downstream genes introduced by Gene A (mutant/WT) given Gene B mutant vs Gene B WT. We controlled FDR at 5% using the Benjamini–Hochberg (BH) procedure.
DAP-seq
DAP-seq genomic DNA libraries were generated as described previously (Bartlett et al. 2017). Briefly, 5 μg of phenol:chloroform:IAA extracted DNA from maize B73 14-d-old seedlings was diluted in EB (10 mm Tris-HCl pH 8.5) and sheared to 200 bp fragments using a Covaris S2 sonicator. DNA was purified using AmpureXP beads with a 2:1 bead to DNA ratio and then end-repaired using an End-It kit (Lucigen) according to the manufacturer's recommendations. Samples were cleaned using a Qiaquick PCR purification kit (Qiagen), A-tailed using Klenow (3′ to 5′ exo-), cleaned with Qiaquick and ligated overnight with a truncated Illumina Y-adapter. Final libraries were purified using AMPureXP beads with a 1:1 bead to DNA ratio and eluted in 30 μL of EB. DNA concentration was determined using the Qubit HS kit.
The pENTR/SD/D-TOPO constructs of GBF1 and HSFTH10 ORF were ordered from TFome stock center (Burdo et al. 2014) (https://abrc.osu.edu/stocks/766964). The entry clone constructs of NKD1 and 2 ORF were made by cloning of the coding sequences of NKD1 and NKD2 into the pDONR221 donor vector (Thermo Fisher Scientific, Waltham, MA, USA) through the BP reaction. The primers for NKD1 and NKD2 cloning are shown in Supplemental Table S5. ORF clones were recombined into the pIX-HALO vector (Bartlett et al. 2017) using LR Clonase II (Thermo Fisher Scientific, Waltham, MA, USA) and purified using a Qiagen miniprep kit. Protein expression was carried out using the TnT SP6 Wheat Germ Protein Expression System (Promega, Madison, WI, USA) using 1 μg of plasmid DNA according to the manufacturer's recommendations. Samples were incubated for 2 h at 30 °C. Fifty microliters of expression reaction was then diluted in 50 μL of wash buffer (1×PBS with 0.005% [v/v] NP40) containing 10 μL of MagneHaloTag beads (Promega, Madison, WI, USA). Samples were rotated for 1 h at room temperature and then washed 3 times with wash buffer. Beads were suspended in 50 μL wash buffer, and 1 μg of B73 genomic DNA library in 50 μL of EB was added. Samples were then rotated at room temperature for 1 h and washed 7 times with wash buffer. To elute DNA, 30 μL of EB was added, and samples were heated at 98 °C for 10 min. DNA was transferred to a new tube prior to 19 cycles of PCR enrichment and barcoding (Bartlett et al. 2017). DNA was sequenced on a NextSeq500 with 75 bp SE reads for GBF1 and HSFTH10 and on Novaseq with 150 bp PE for NKD1 and NKD2.
Mapping and peak calling of DAP-seq samples were performed as follows. DAP-seq reads were trimmed using Trimmomatic (Bolger et al. 2014) with the following parameters: ILLUMINACLIP:TruSeq3-SE:2:30:10 LEADING:3 TRAILING:3 SLIDINGWINDOW:4:15 MINLEN:50. Trimmed reads were mapped to the B73v4 reference genome using Bowtie2.3.3. Mapped reads were filtered for reads containing >MAPQ30 using SAMtools (SAMtools view -b -q 30) (Li et al. 2009). Peaks were called using GEM v2.5 (Guo et al. 2012) with default thresholds and the following parameters: –d Read_Distribution_default.txt –k_min 6 –k_max 20 –outNP. Background peaks were subtracted using a HALO-GST negative control sample and a list of blacklisted regions from Galli et al. (2018) with the B73v3 sites converted to B73v4 coordinates. Peak distribution was analyzed via ChIPseeker (1.36.0) using default settings (Yu et al. 2015).
Endosperm nucleus extraction and ATAC-seq
Frozen 16 DAP endosperms of nkd1, nkd2, and o2 single, double, and triple mutants, as well as the WT counterpart were collected. ATAC-seq analysis contained 2 biological replicates from 2 independent ears of each genotype, each replicate contained a pool of 3 endosperms. Endoreduplication analysis consisted of 4 biological replicates, from 2 independent ears of each genotype, 2 replicates per ear, each replicate contained a pool of three endosperms. Crude nuclei were extracted following the published protocol (Lu et al. 2017) with the following modifications: 0.2 g of endosperm were ground in liquid nitrogen followed by addition of 2 mL of prechilled lysis buffer (15 mm Tris-HCl pH7.5, 20 mm NaCl, 80 mm KCl, 0.5 mm spermine, 5 mm 2-ME, and 0.2% [v/v] TritonX-100) to resuspend the ground powder. Then, the resuspension was filtered 3 times through miracloth. The crude nuclei were stained with DAPI (from Millipore Sigma, St. Louis, MO, USA) (4 μL DAPI per ∼500 μL filtered resuspension) and sent to the Flow Cytometry Facility at Iowa State University (Ames, IA) for nuclear counting and sorting using BD FACSAria III flow cytometer (BD Biosciences; San Jose, CA, USA). The nuclei with ploidies of 3C, 6C, 12C, 24C, and 48C were counted for endoreduplication analysis, and at least 50,000 triploid nuclei (3C) were sorted for ATAC-Seq library construction. The library was constructed using the Active Motif ATAC-Seq Kit (Active Motif, Carlsbad, CA, USA) following the manufacturer's manual. The library was sequenced using NovaSeq 6000 SP flow cell sequencing platform (DNA Facility, Iowa State University, Ames, IA) at 2X50 bp paired-end in 50 million bp depth. The raw read quality control was performed via FastQC (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/), followed by the adapter trimming via Trimmomatic (v0.32). The Trimmomatic parameters were set as ILLUMINACLIP:Adapter2.fasta:2:30:10 LEADING:3 TRAILING:3 CROP:36 SLIDINGWINDOW:4:15 MINLEN:30, where the Adapter2.fasta file contained 2 adapter sequences: (i) CTGTCTCTTATACACATCT and (ii) AGATGTGTATAAGAGACAG. The trimmed reads were mapped to B73 RefGen_v4 (AGPv4) (Jiao et al. 2017) via Bowtie2 (v 2.4.2) (Langmead and Salzberg 2012) using a “–very-sensitive” set-up. The mapped reads in the output files were sorted, assembled, and filtered by SAMtools (v1.9) using default parameters, followed by the peak calling via MACS2 (Feng et al. 2012) using the parameters “-f BAMPE -g 2e9 –keep-dup all –nomodel –cutoff-analysis -q 1e-02 -n merged.”
Microscopy
Kernels of nkd1, nkd2, and o2 single, double, and triple mutants, as well as the WT counterpart, were harvested at 16 DAP and fixed for microscopy in 4% (v/v) formaldehyde/2% (v/v) glutaraldehyde in phosphate buffer, pH 7, and stored at 4 °C until processing. Kernels from ears of 3 plants of each of the 8 genotypes were cut in medial longitudinal hand sections, and then an abgerminal piece containing AL, SA, and SE was collected. Samples were washed in 50 mm sodium phosphate buffer pH 7 and then postfixed with buffered 2% osmium tetroxide at 4 °C overnight or 2 h at room temperature. After buffer washes, the kernels were dehydrated by ethanol series and embedded in Spurr's resin. Blocks were sectioned at 1 µm using a RMC Powertome (Boekeler Instruments, Tucson, AZ, USA), stained with 1% (w/v) Toluidine Blue O in 1% sodium borate, and viewed and imaged with a Zeiss Axiophot equipped with a Zeiss AxioCam MRC (Carl Zeiss, Oberkochen, Germany). Thin-sections were cut at 70 to 80 µm with a RMC Powertome, stained with Uranyless followed by lead citrate (both Electron Microscopy Sciences, Hatfield, PA, USA), and imaged with Hitachi HT7700 Scanning/Transmission Electron Microscope (Hitachi Instruments, Tokyo, Japan).
For protein body (PB) measurements, the diameter of PBs in electron micrographs at 1,000× magnification was measured with the line tool using Image Pro Premier software (Media Cybernetics, Rockville, MD, USA). Images of SE cells 7 or more cells internal from the pericarp were chosen, and the largest protein bodies in each image measured to avoid tangentially sectioned PBs. Three plants were analyzed per genotype, 3 to 7 images were analyzed per plant, with a range of 4 to 25 protein bodies measured per image, and a minimum sample size n = 171 per genotype.
Starch content analysis and zein profiling
Total starch from mature kernels of nkd1, 2, and o2 single, double, and triple mutants, as well as the WT counterpart (4 biological replicates from 4 independent ears of each genotype, each replicate contained a pool of 3 endosperms), were extracted following the published method (Dinges et al. 2001) with the following modifications: the endosperm starch was washed with chilled deionized water twice followed by chilled 80% ethanol (centrifuged 3,000 × g at 4 °C for 10 min after each liquid addition). The pellet was dissolved in 100% DMSO and boiled in a water bath for 1 h. The starch content was measured following the published procedures (Gontarek et al. 2016).
Zein proteins from 16 DAP endosperm of nkd1, nkd2, and o2 single, double, and triple mutants, as well as WT (3 biological replicates from 3 individual ears of each genotype, each replicate contained a pool of 3 endosperms), were extracted based on published procedures (Wilson 1991) with the following modifications. The frozen endosperms were ground into powder in liquid nitrogen. One hundred grams of the power was suspended in 1 mL of the extraction buffer (70% ethanol, 61 mm sodium acetate, and fresh 5% [v/v] 2-mercaptoethanol), followed by incubation with shaking (225 rpm at 37 °C) for 1 h and centrifugation at 15,870 g for 10 min. The supernatant was sent to the Protein Facility at Iowa State University (Ames, IA) for HPLC.
Pull-down assay
The pull-down assay was conducted using in vitro translated GST-tagged NKD1 and NKD2 proteins and 6X-Histidine (HIS)-tagged IDDP10 protein. The GST-tagged NKD1 and NKD2 entry constructs were previously made in the lab following published procedures (Gontarek et al. 2016). The 6X-HIS-tagged IDDP10 entry constructs were made by cloning the coding sequence of iddp10 into pET34b vectors (Novogene Co. Durham, NC, USA), through the In-Fusion cloning approach following the manufacturer's manual (Takara Bio, Kusatsu, Shiga, Japan) using the primers shown in Supplemental Table S5. Then, the GST-tagged NKD1 and NKD2, and 6X-HIS-tagged IDDP10 fragments were In-Fusion (Takara Bio, Kusatsu, Shiga, Japan) cloned into the expression vector pF3K WG (BYDV) Flexi (Promega, Madison, WI, USA) using the primers shown in Supplemental Table S5. The pF3K expression constructs were in vitro transcribed and translated via the TnT SP6 High-Yield Wheat Germ Protein Expression System following the manufacturer's instructions (Promega, Madison, WI, USA). The GST-tagged NKD1 and NKD2 proteins or GST-only proteins (negative control) were mixed with 6X-Histidine (HIS)-tagged IDDP10 protein in phosphate buffered saline with 0.15 mm phenylmethylsulfonyl fluoride (PMSF) and incubated for 1 h at room temperature. The mixture was then passed through HIS-Select HF Nickel Affinity Gel resin (Sigma-Aldrich, St. Louis, MO, USA), and washed by 6X-HIS wash buffer (50 mm phosphate buffer pH 7.0, 300 mm NaCl, and 1 mm imidazole), followed by elution with the 6X-HIS elution buffer (50 mm phosphate buffer pH 7.0, 300 mm NaCl, and 150 mm imidizole). The GST signal of the candidate binding protein partners of 6X-HIS-IDDP10 was detected by SDS-PAGE with immunoblotting using a GST antibody (dilution 1:1000) (MA4-004-HRP, Thermo Fisher Scientific, Waltham, MA, USA).
Gene ontology enrichment analysis
GO term enrichment analysis was performed at AgriGOv2 (http://systemsbiology.cau.edu.cn/agriGOv2/) with the Maize-GAMER (AGPv4) dataset used as the annotated background (Wimalanathan et al. 2018). The significant enrichment cutoff FDR was 0.05.
Statistical analyses
All DNA sequencing readcount-related data in this study were normalized to fit the generalized linear model (GLM), and Empirical Bayes shrinkage estimation was used for calculating dispersions and FCs, as well as the P-values and the FDR (adjusted P-values). In addition, the interaction effects of NKD1, NKD2, and O2 on downstream genes were investigated via multifactorial experimental design. Two factors (2 of the 3 key TFs) and 2 levels (WT and mutant) were fitted in GLM to calculate the FC, and the FDRs were calculated via the BH procedure. Pearson correlation test was used to analyze the correlation between the expression of the key TF genes (nkd1, nkd2, and o2) and corresponding coexpression MEs. Significance levels were set to 0.05, 0.01, and 0.001.
Pairwise t-tests were used to compare the starch content, zein protein quantity, the normalized relative gene expression in selected modules between genotypes, and the relative nuclei number of the corresponding ploidy between WT and mutants. Significance level was set to P < 0.05. All pairwise t-tests were performed by JMP Pro 16 with default settings (SAS Institute).
Chi-square test was used to test if the observed phenotypic ratio among WT, nkd1, o2, and nkd1 o2 agrees with the ratio of Mendel's law of segregation and to test if there is any nkd1–o2 interaction. The threshold for significance was set to P < 0.05. If the corresponding probability level >0.05, we accept the null hypothesis that the data fit the expected ratio.
Hypergeometric tests were used to statistically evaluate if 2 groups of genes were significantly related by calculating the number of overlapping genes relative to the total number of protein-coding genes in the maize B73 RefGen_v4 genome (Jiao et al. 2017). The tests were performed as described (Zhan et al. 2015) and visualized as heatmaps that were built by the R package gplots (https://github.com/talgalili/gplots). The P-values were transformed by −log10p.
The statistical data are provided in Supplemental Data Set 21.
Accession numbers
The nkd1 gene corresponds to Zm00001d002654 in genome assembly B73 RefGen_v4 or Zm00001eb073830 in B73 RefGen_v5, nkd2 corresponds to Zm00001d026113 or Zm00001eb428940, respectively, and o2 is Zm00001d018971 or Zm00001eb301570, respectively. Key hub gene accessions are shown in Table 2. The RNA-seq and ATAC-seq data of nkd1, nkd2, and o2 single, double, and triple mutants, as well as DAP-seq data of TFs have been deposited in Gene Expression Omnibus (GEO), with the accession numbers GSE174059 (RNA-seq), GSE214415 (ATAC-seq), and GSE214166 (DAP-seq), respectively.
Supplementary Material
Acknowledgments
The authors thank Gary Drews and the High-Throughput Genomics facility of the Huntsman Cancer Institute, University of Utah, for performing RNA sequencing and thank Geetu Tuteja (Iowa State University) and Robert Schmitz (University of Georgia) for ATAC-seq technical advice.
Contributor Information
Hao Wu, Genetics, Development and Cell Biology Department, Iowa State University, Ames, IA 50011, USA.
Mary Galli, Waksman Institute of Microbiology, Rutgers University, Piscataway, NJ 08901-8520, USA.
Carla J Spears, Department of Biology, Central Michigan University, Mount Pleasant, MI 48859, USA.
Junpeng Zhan, School of Plant Sciences, University of Arizona, Tucson, AZ 85721, USA.
Peng Liu, Department of Statistics, Iowa State University, Ames, IA 50011, USA.
Ramin Yadegari, School of Plant Sciences, University of Arizona, Tucson, AZ 85721, USA.
Joanne M Dannenhoffer, Department of Biology, Central Michigan University, Mount Pleasant, MI 48859, USA.
Andrea Gallavotti, Waksman Institute of Microbiology, Rutgers University, Piscataway, NJ 08901-8520, USA; Department of Plant Biology, Rutgers University, New Brunswick, NJ.
Philip W Becraft, Genetics, Development and Cell Biology Department, Iowa State University, Ames, IA 50011, USA; Department of Agronomy, Iowa State University, Ames, IA 50011, USA.
Author contributions
H.W. designed and performed research, analyzed and interpreted data, and wrote the paper. M.G. and A.G. performed DAP-seq analysis and contributed to writing. C.J.S. and J.M.D. performed microscopy, prepared figures, and contributed to writing. J.Z. and R.Y. contributed unpublished RNA-seq data and contributed to writing. P.L. provided statistical tools to analyze data and contributed to writing. P.W.B. contributed to project design, data interpretation, writing, and provided overall project oversight.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Kernel phenotypes of WT and nkd1, nkd2, and o2 mutant combinations.
Supplemental Figure S2. Light micrographs of AL, SA, and SE from 2 plants each of the 8 genotypes.
Supplemental Figure S3. Electron micrographs of AL, SA, and SE for select genotypes (scale bar = 10 μm).
Supplemental Figure S4. Protein body phenotype and size for selected genotypes.
Supplemental Figure S5. Interaction effects of nkd1, nkd2, and o2 on nuclear ploidy.
Supplemental Figure S6. Number of DEGs in each mutant compared to WT at 8, 12, and 16 DAP.
Supplemental Figure S7. The distribution of potential NKD consensus binding motifs.
Supplemental Figure S8. Binding motifs of GBF1, HSFTH10, and corresponding networks.
Supplemental Figure S9. Genomic distribution of ATAC-seq (assay for transposase accessible chromatin with sequencing) peaks in the 8 genotypes.
Supplemental Table S1. Kernel phenotype statistics on +/nkd1, +/o2 self-crossed segregating ears, and chi-square test for nkd1-o2 interaction.
Supplemental Table S2. Pooled timepoint WGCNA module size.
Supplemental Table S3. Number of potential NKD1 and NKD2 targets and percentage of targets annotated as TFs at 8, 12, and 16 DAP.
Supplemental Table S4. Motif enrichment analysis of differential peaks overlapping with NKD2 target regions.
Supplemental Table S5. Primers and adapters.
Supplemental Data Set 1. DEGs mutant vs. WT.
Supplemental Data Set 2. Module gene content for pooled timepoint WGCNA.
Supplemental Data Set 3. GO terms for pooled timepoint WGCNA modules.
Supplemental Data Set 4. Module gene content for individual timepoint WGCNA at 8 DAP.
Supplemental Data Set 5. Module gene content for individual timepoint WGCNA at 12 DAP.
Supplemental Data Set 6. Module gene content for individual timepoint WGCNA at 16 DAP.
Supplemental Data Set 7. GO terms for individual timepoint WGCNA modules.
Supplemental Data Set 8. Interactions among nkd1, nkd2, and o2 on gene expression.
Supplemental Data Set 9. NKD1-bound DNA sites by DAPseq.
Supplemental Data Set 10. NKD2-bound DNA sites by DAPseq.
Supplemental Data Set 11. GBF1-bound DNA sites by DAPseq.
Supplemental Data Set 12. HSFTF10-bound DNA sites by DAPseq.
Supplemental Data Set 13. Differentially accessible chromatin between nkd1 vs. WT by ATAC-seq.
Supplemental Data Set 14. Differentially accessible chromatin between nkd2 vs. WT by ATAC-seq.
Supplemental Data Set 15. Differentially accessible chromatin between nkd1 nkd2 vs. WT by ATAC-seq.
Supplemental Data Set 16 . Differentially accessible chromatin between nkd1 o2 vs. WT by ATAC-seq.
Supplemental Data Set 17. Differentially accessible chromatin between nkd2 o2 vs. WT by ATAC-seq.
Supplemental Data Set 18. Differentially accessible chromatin between nkd1 nkd2 o2 vs. o2 by ATAC-seq.
Supplemental Data Set 19. Differentially accessible chromatin between o2 vs. WT by ATAC-seq.
Supplemental Data Set 20. Differentially accessible chromatin between nkd1 nkd2 o2 vs. nkd1 nkd2 by ATAC-seq.
Supplemental Data Se 21. Statistical analyses.
Funding
This research was supported by the National Science Foundation awards IOS-1444568 to R.Y., J.M.D., and P.W.B., IOS-1916804 to A.G., and DBI-1531277 to Central Michigan University for the TEM and Hatch project IOW03649 to P.W.B. The DNA facility, microscopy facility, and proteomics facility at Iowa State University provided equipment and technical assistance.
Data availability
All sequencing data have been deposited in Gene Expression Omnibus (GEO), with the accession numbers GSE174059 (RNA-seq), GSE214415 (ATAC-seq), and GSE214166 (DAP-seq). All other data required to evaluate conclusions is included in the paper or in Supplemental Data.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
References
- Ahern KR, Deewatthanawong P, Schares J, Muszynski M, Weeks R, Vollbrecht E, Duvick J, Brendel VP, Brutnell TP. Regional mutagenesis using dissociation in maize. Methods. 2009:49(3):248–254. 10.1016/j.ymeth.2009.04.009 [DOI] [PubMed] [Google Scholar]
- Bartlett A, O'Malley RC, Huang SSC, Galli M, Nery JR, Gallavotti A, Ecker JR. Mapping genome-wide transcription-factor binding sites using DAP-seq. Nat Protoc. 2017:12(8):1659–1672. 10.1038/nprot.2017.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becraft PW, Yi G. Regulation of aleurone development in cereal grains. J Exp Bot. 2011:62(5):1669–1675. 10.1093/jxb/erq372 [DOI] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014:30(15):2114–2120. 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonke M, Thitamadee S, Mähönen AP, Hauser MT, Helariutta Y. APL regulates vascular tissue identity in Arabidopsis. Nature. 2003:426(6963):181–186. 10.1038/nature02100 [DOI] [PubMed] [Google Scholar]
- Burdo B, Gray J, Goetting-Minesky MP, Wittler B, Hunt M, Li T, Velliquette D, Thomas J, Gentzel I, dos Santos Brito M, et al. The maize TFome–development of a transcription factor open reading frame collection for functional genomics. Plant J. 2014:80(2):356–366. 10.1111/tpj.12623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton RA, Fincher GB. Evolution and development of cell walls in cereal grains. Front Plant Sci. 2014:5:456. 10.3389/fpls.2014.00456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho CP, Huang P, Lee DY, Brutnell TP. Making roots, shoots, and seeds: IDD gene family diversification in plants. Trends Plant Sci. 2018:23(1):66–78. 10.1016/j.tplants.2017.09.008 [DOI] [PubMed] [Google Scholar]
- Cosségal M, Vernoud V, Depège N, Rogowsky PM. The embryo surrounding region. In Endosperm. Berlin: (Heidelberg: ): Springer Berlin Heidelberg; 2007. p. 57–71. [Google Scholar]
- Dai D, Ma Z, Song R. Maize endosperm development. J Integr Plant Biol. 2021:63(4):613–627. 10.1111/jipb.13069 [DOI] [PubMed] [Google Scholar]
- Dante RA, Larkins BA, Sabelli PA. Cell cycle control and seed development. Front Plant Sci. 2014:5:493. 10.3389/fpls.2014.00493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Wang J, Zhang Z, Wu Y. Transactivation of Sus1 and Sus2 by Opaque2 is an essential supplement to sucrose synthase-mediated endosperm filling in maize. Plant Biotechnol J. 2020:18(9):1897–1907. 10.1111/pbi.13349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinges JR, Colleoni C, Myers AM, James MG. Molecular structure of three mutations at the maize sugary1 locus and their allele-specific phenotypic effects. Plant Physiol. 2001:125(3):1406–1418. 10.1104/pp.125.3.1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Liu T, Qin B, Zhang Y, Liu XS. Identifying ChIP-seq enrichment using MACS. Nat Protoc. 2012:7(9):1728–1740. 10.1038/nprot.2012.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli M, Khakhar A, Lu Z, Chen Z, Sen S, Joshi T, Nemhauser JL, Schmitz RJ, Gallavotti A. The DNA binding landscape of the maize AUXIN RESPONSE FACTOR family. Nat Commun. 2018:9(1):4526. 10.1038/s41467-018-06977-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gontarek BC, Neelakandan AK, Wu H, Becraft PW. NKD transcription factors are central regulators of maize endosperm development. Plant Cell. 2016:28(12):2916–2936. 10.1105/tpc.16.00609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Mahony S, Gifford DK. High resolution genome wide binding event finding and motif discovery reveals transcription factor spatial binding constraints. PLoS Comput Biol. 2012:8(8):e1002638. 10.1371/journal.pcbi.1002638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartings H, Maddaloni M, Lazzaroni N, Di Fonzo N, Motto M, Salamini F, Thompson R. The O2 gene which regulates zein deposition in maize endosperm encodes a protein with structural homologies to transcriptional activators. EMBO J. 1989:8(10):2795–2801. 10.1002/j.1460-2075.1989.tb08425.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasjim J, Srichuwong S, Scott MP, Jane JL. Kernel composition, starch structure, and enzyme digestibility of opaque-2 maize and quality protein maize. J Agric Food Chem. 2009:57(5):2049–2055. 10.1021/jf803406y [DOI] [PubMed] [Google Scholar]
- Hoecker U, Vasil IK, McCarty DR. Signaling from the embryo conditions Vp1-mediated repression of alpha-amylase genes in the aleurone of developing maize seeds. Plant J. 1999:19(4):371–377. 10.1046/j.1365-313X.1999.00521.x [DOI] [PubMed] [Google Scholar]
- Holding DR. Recent advances in the study of prolamin storage protein organization and function. Front Plant Sci. 2014:5:276. 10.3389/fpls.2014.00276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingkasuwan P, Netrphan S, Prasitwattanaseree S, Tanticharoen M, Bhumiratana S, Meechai A, Chaijaruwanich J, Takahashi H, Cheevadhanarak S. Inferring transcriptional gene regulation network of starch metabolism in Arabidopsis thaliana leaves using graphical Gaussian model. BMC Syst Biol. 2012:6(1):100. 10.1186/1752-0509-6-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MG, Robertson DS, Myers AM. Characterization of the maize gene sugary1, a determinant of starch composition in kernels. Plant Cell. 1995:7(4):417–429. 10.1105/tpc.7.4.417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Peluso P, Shi J, Liang T, Stitzer MC, Wang B, Campbell MS, Stein JC, Wei X, Chin CS, et al. Improved maize reference genome with single-molecule technologies. Nature. 2017:546(7659):524–527. 10.1038/nature22971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015:12(4):357–360. 10.1038/nmeth.3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowles RV, Phillips RL. DNA amplification patterns in maize endosperm nuclei during kernel development. Proc Natal Acad Sci U S A. 1985:82(20):7010–7014. 10.1073/pnas.82.20.7010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfelder P, Horvath S. Eigengene networks for studying the relationships between co-expression modules. BMC Syst Biol. 2007:1(1):54. 10.1186/1752-0509-1-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008:9(1):559. 10.1186/1471-2105-9-559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. Fast gapped-read alignment with bowtie 2. Nat Methods. 2012:9(4):357–359. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lending CR, Larkins BA. Changes in the zein composition of protein bodies during maize endosperm development. Plant Cell. 1989:1(10):1011–1023. 10.1105/tpc.1.10.1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux BM, Goodyke AJ, Schumacher KI, Abbott CP, Clore AM, Yadegari R, Larkins BA, Dannenhoffer JM. Maize early endosperm growth and development: from fertilization through cell type differentiation. Am J Bot. 2014:101(8):1259–1274. 10.3732/ajb.1400083 [DOI] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. The sequence alignment/map format and SAMtools. Bioinformatics. 2009:25(16):2078–2079. 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Qiao Z, Qi W, Wang Q, Yuan Y, Yang X, Tang Y, Mei B, Lv Y, Zhao H, et al. Genome-wide characterization of cis-acting DNA targets reveals the transcriptional regulatory framework of opaque2 in maize. Plant Cell. 2015:27(3):532–545. 10.1105/tpc.114.134858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Wang D, Yang R, Logan K, Chen H, Zhang S, Skaggs MI, Lloyd A, Burnett WJ, Laurie JD, et al. Temporal patterns of gene expression in developing maize endosperm identified through transcriptome sequencing. Proc Natal Acad Sci U S A. 2014:111(21):7582–7587. 10.1073/pnas.1406383111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014:15(12):550. 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Hofmeister BT, Vollmers C, DuBois RM, Schmitz RJ. Combining ATAC-seq with nuclei sorting for discovery of cis-regulatory regions in plant genomes. Nucleic Acids Res. 2017:45(6):e41. 10.1093/nar/gkw1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz ET, Bates LS, Nelson OE. Mutant gene that changes protein composition and increases lysine content of maize endosperm. Science. 1964:145(3629):279–280. 10.1126/science.145.3629.279 [DOI] [PubMed] [Google Scholar]
- Moore RC, Purugganan MD. The evolutionary dynamics of plant duplicate genes. Curr Opin Plant Biol. 2005:8(2):122–128. 10.1016/j.pbi.2004.12.001 [DOI] [PubMed] [Google Scholar]
- Olsen OA. Nuclear endosperm development in cereals and Arabidopsis thaliana. Plant Cell. 2004:16(suppl_1):S214–S227. 10.1105/tpc.017111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omidbakhshfard MA, Fujikura U, Olas JJ, Xue GP, Balazadeh S, Mueller-Roeber B. GROWTH-REGULATING FACTOR 9 negatively regulates Arabidopsis leaf growth by controlling ORG3 and restricting cell proliferation in leaf primordia. PLoS Genet. 2018:14(7):e1007484. 10.1371/journal.pgen.1007484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat Protoc. 2016:11(9):1650–1667. 10.1038/nprot.2016.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett FB, Meeks-Wagner DR. Seeing double: appreciating genetic redundancy. Plant Cell. 1995:7(9):1347–1356. 10.1105/tpc.7.9.1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabelli PA. Replicate and die for your own good: endoreduplication and cell death in the cereal endosperm. J Cereal Sci. 2012:56(1):9–20. 10.1016/j.jcs.2011.09.006 [DOI] [Google Scholar]
- Sabelli PA, Larkins BA. The contribution of cell cycle regulation to endosperm development. Sex Plant Reprod. 2009a:22(4):207–219. 10.1007/s00497-009-0105-4 [DOI] [PubMed] [Google Scholar]
- Sabelli PA, Larkins BA. The development of endosperm in grasses. Plant Physiol. 2009b:149(1):14–26. 10.1104/pp.108.129437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabelli PA, Liu Y, Dante RA, Lizarraga LE, Nguyen HN, Brown SW, Klingler JP, Yu J, LaBrant E, Layton TM, et al. Control of cell proliferation, endoreduplication, cell size, and cell death by the retinoblastoma-related pathway in maize endosperm. Proc Natal Acad Sci U S A. 2013:110(19):E1827–E1836. 10.1073/pnas.1304903110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schel JHN, Kieft H, van Lammeren AAMV. Interactions between embryo and endosperm during early developmental stages of maize caryopses (Zea mays). Can J Bot. 1984:62(12):2842–2853. 10.1139/b84-379 [DOI] [Google Scholar]
- Schmidt RJ, Burr FA, Aukerman MJ, Burr B. Maize regulatory gene opaque-2 encodes a protein with a “leucine-zipper” motif that binds to zein DNA. Proc Natl Acad Sci U S A. 1990:87(1):46–50. 10.1073/pnas.87.1.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RJ, Burr FA, Burr B. Transposon tagging and molecular analysis of the maize regulatory locus opaque-2. Science. 1987:238(4829):960–963. 10.1126/science.2823388 [DOI] [PubMed] [Google Scholar]
- Schmidt RJ, Ketudat M, Aukerman MJ, Hoschek G. Opaque-2 is a transcriptional activator that recognizes a specific target site in 22-kD zein genes. Plant cell. 1992:4(6):689–700. 10.1105/tpc.4.6.689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Wang HHY, McCarty DR. Repression of the LEAFY COTYLEDON 1/B3 regulatory network in plant embryo development by VP1/ABSCISIC ACID INSENSITIVE 3-LIKE B3 genes. Plant Physiol. 2007:143(2):902–911. 10.1104/pp.106.092320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CY, Nelson OE. Starch-deficient maize mutant lacking adenosine dephosphate glucose pyrophosphorylase activity. Science. 1966:151(3708):341–343. 10.1126/science.151.3708.341 [DOI] [PubMed] [Google Scholar]
- van Zanten M, Koini MA, Geyer R, Liu Y, Brambilla V, Bartels D, Koornneef M, Fransz P, Soppe WJ. Seed maturation in Arabidopsis thaliana is characterized by nuclear size reduction and increased chromatin condensation. Proc Natl Acad Sci U S A. 2011:108(50):20219–20224. 10.1073/pnas.1117726108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welner DH, Shin D, Tomaleri GP, DeGiovanni AM, Tsai AY-L, Tran HM, Hansen SF, Green DT, Scheller HV, Adams PD. Plant cell wall glycosyltransferases: high-throughput recombinant expression screening and general requirements for these challenging enzymes. PLoS One. 2017:12(6):e0177591. 10.1371/journal.pone.0177591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CM. Multiple zeins from maize endosperms characterized by reversed-phase high performance liquid chromatography. Plant Physiol. 1991:95(3):777–786. 10.1104/pp.95.3.777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimalanathan K, Friedberg I, Andorf CM, Lawrence-Dill CJ. Maize GO annotation-methods, evaluation, and review (maize-GAMER). Plant Direct. 2018:2(4):e00052. 10.1002/pld3.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Becraft PW, Dannenhoffer JM. Maize endosperm development: tissues, cells, molecular regulation and grain quality improvement. Front Plant Sci. 2022:13:852082. 10.3389/fpls.2022.852082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Holding DR, Messing J. Gamma-zeins are essential for endosperm modification in quality protein maize. Proc Natal Acad Sci U S A. 2010:107(29):12810–12815. 10.1073/pnas.1004721107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie JH, Liu HD. Study of database application for human open chromatin regions from ATAC-seq. Aip Conf Proc. 2019:2110:020056. 10.1063/1.5110850 [DOI] [Google Scholar]
- Yang T, Guo L, Ji C, Wang H, Wang J, Zheng X, Xiao Q, Wu Y. The B3 domain-containing transcription factor ZmABI19 coordinates expression of key factors required for maize seed development and grain filling. Plant Cell. 2021:33(1):104–128. 10.1093/plcell/koaa008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi G, Neelakandan AK, Gontarek BC, Vollbrecht E, Becraft PW. The naked endosperm genes encode duplicate INDETERMINATE domain transcription factors required for maize endosperm cell patterning and differentiation. Plant Physiol. 2015:167(2):443–456. 10.1104/pp.114.251413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Wang L, He Q. ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics. 2015:31(14):2382–2383. 10.1093/bioinformatics/btv145 [DOI] [PubMed] [Google Scholar]
- Yuan L, Song X, Zhang L, Yu Y, Liang Z, Lei Y, Ruan J, Tan B, Liu J, Li C. The transcriptional repressors VAL1 and VAL2 recruit PRC2 for genome-wide Polycomb silencing in Arabidopsis. Nucleic Acids Res. 2021:49(1):98–113. 10.1093/nar/gkaa1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan J, Li G, Ryu CH, Ma C, Zhang S, Lloyd A, Hunter BG, Larkins BA, Drews GN, Wang X, et al. Opaque-2 regulates a complex gene network associated with cell differentiation and storage functions of maize endosperm. Plant Cell. 2018:30(10):2425–2446. 10.1105/tpc.18.00392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan J, Thakare D, Ma C, Lloyd A, Nixon NM, Arakaki AM, Burnett WJ, Logan KO, Wang D, Wang X, et al. RNA Sequencing of Laser-capture microdissected compartments of the maize kernel identifies regulatory modules associated with endosperm cell differentiation. Plant Cell. 2015:27(3):513–531. 10.1105/tpc.114.135657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Dong J, Ji C, Wu Y, Messing J. NAC-type transcription factors regulate accumulation of starch and protein in maize seeds. Proc Natl Acad Sci U S A. 2019b:116(23):11223–11228. 10.1073/pnas.1904995116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Mogel KJH, Lor VS, Hirsch CN, De Vries B, Kaeppler HF, Tracy WF, Kaeppler SM. Maize sugary enhancer1 (se1) is a gene affecting endosperm starch metabolism. Proc Natl Acad Sci U S A. 2019a:116(41):20776–20785. 10.1073/pnas.1902747116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zheng X, Yang J, Messing J, Wu Y. Maize endosperm-specific transcription factors O2 and PBF network the regulation of protein and starch synthesis. Proc Natal Acad Sci U S A. 2016:113(39):10842–10847. 10.1073/pnas.1613721113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Wang Z. Current opinions on endosperm transfer cells in maize. Plant Cell Rep. 2010:29(9):935–942. 10.1007/s00299-010-0891-z [DOI] [PubMed] [Google Scholar]
- Zhou SR, Xue HW. The rice PLATZ protein SHORT GRAIN6 determines grain size by regulating spikelet hull cell division. J Integr Plant Biol. 2020:62(6):847–864. 10.1111/jipb.12851 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All sequencing data have been deposited in Gene Expression Omnibus (GEO), with the accession numbers GSE174059 (RNA-seq), GSE214415 (ATAC-seq), and GSE214166 (DAP-seq). All other data required to evaluate conclusions is included in the paper or in Supplemental Data.