Abstract
Purpose:
Little is known about the effects of exercise training (ET) on lexical characteristics during fluency task and its association with cerebellum functional connectivity. The purposes of this study were (1) to investigate whether ET alters response patterns during phonemic and semantic fluency tasks; (2) to assess the association between ET-related changes in cerebellum functional connectivity (FC) and lexical characteristics during fluency tasks.
Methods:
Thirty-five older adults (78.0±7.1 years; 17 mild cognitive impairment (MCI) and 18 healthy cognition (HC)) underwent a 12-week treadmill ET. Before and after ET, cardiorespiratory fitness tests, phonemic and semantic fluency tests, and resting-state fMRI scans were administered. We utilized a seed-based correlation analysis to measure cerebellum FC and linear regression to assess the association of residualized ET-induced Δcerebellum FC with Δtask performance.
Results:
Improved mean switches and frequency during the phonemic fluency task were observed following ET in all participants. There were significant associations between ET-induced increases in cerebellum FC and greater phonemic fluency task log frequency, increases in mean switches, and a reduction in the number of syllables in HC. Lastly, there was a significant interaction between group and cerebellar connectivity on phonemic fluency mean log frequency and number of syllables.
Conclusion:
A 12-week walking ET is related to enhanced phonemic fluency lexical characteristics in older adults with MCI and HC. The association between ET-induced increases in cerebellum FC and enhanced response patterns after ET suggests that cerebellum may play an important role in ET-related improvement in phonemic fluency performance in cognitively healthy older adults.
Keywords: aging, exercise training, functional connectivity, cerebellum, MCI, phonemic fluency
INTRODUCTION
Declining phonemic and semantic fluency is one of the most noticeable age-related cognitive deficits [1]. Difficulties in finding and remembering words during spontaneous speech are often exhibited as verbal fluency degeneration in older individuals [2]. The inability to complete phonological information and decline in the size of the pool of semantic knowledge engender ineffective communication subsequently leading to difficulties in receiving proper care [3]. Moreover, phonemic and semantic fluency dysfunction due to aging serves as a presymptomatic biomarker for Alzheimer’s disease (AD) transition and mild cognitive impairment (MCI) [1] which is a prodromal stage of AD, characterized by the presence of cognitive impairment while maintaining activities of independent daily living [4].
Widely used in neuropsychological test batteries, the phonemic fluency test is a word generation task that measures the verbal component of executive control [5]. The test assesses participant’s ability to select words in compliance with a predetermined word retrieval cue (e.g., F, A, S) [6]. The semantic fluency test scoring the total number of correct words produced within a given semantic category (e.g., animals) is also widely accepted in neuropsychological assessment as a measure of executive function and semantic memory [7]. These tests are straightforward to administer [8] and can evaluate phonological/semantic process and word searching strategy [9]. Hence, it is an useful tool to identify lexico-semantic impairments [10,11]. Older adults generally produce fewer words during the phonemic and semantic fluency tests [12,13]. Furthermore, older individuals with MCI performed poorer compared to their cognitively healthy counterparts in all categories of phonemic fluency tasks [14].
Emerging evidence suggests exercise training (ET) and higher cardiorespiratory fitness are associated with improved phonemic and semantic fluency performance in older adults. In a cross-sectional study, cognitively normal older individuals who frequently engage in intense physical activity demonstrated better phonemic fluency test performance [15]. Also, improved aerobic fitness in response to 12-weeks of spin ET was linked to enhanced semantic fluency task performance in sedentary older individuals [16]. In older adults with MCI, those completing 6-months of aerobic ET compared to a stretching intervention saw a significant increase in word generation during the phonemic fluency task [17]. Similarly in our prior investigation, improved phonemic fluency performance was found following a 12-week walking exercise regimen in older adults with healthy cognition (HC) and MCI [18]. In the present study, using the phonemic and semantic fluency performance data collected in the same sample, we investigated the effects of a 12-week walking ET intervention on the response patterns of the words produced during fluency tasks. Discerning the task response pattern is essential to understand how ET alters fluency task processing in older adults. These findings will add to the current body of research as no work to date has examined the ET-related changes in lexical characteristics during the phonemic and semantic fluency tasks. Our hypothesis was that both HC and MCI groups will demonstrate improved lexical characteristics during the phonemic and semantic fluency tasks after ET.
To understand the neural mechanism underpinning alterations in lexical characteristics during fluency task following ET, we further explored the cerebellum functional connectivity (FC). Based on the coherence of fMRI blood oxygenation level-dependent (BOLD) signals [19], resting-state FC analysis is a non-invasive approach to understand the patterns of functional interaction between spatially remote brain regions during a period of wakeful rest [20]. It has been reported that regular participation in exercise promotes increased communications between regions (i.e., elevated connectivity) in the aging brain [21]. Exercise-elicited adaptation in greater functional brain connectivity reflect enhanced ability to withstand to the age-related changes or increased recruitment of alternative networks required by the age-related changes in the brain [22].
Despite its well-established roles of motor control and coordination, evidence supports the notion that cerebellum is a part of cognitive functional networks via its anatomical connection to prefrontal and parietal cortices [23–25]. Through this anatomical substrate, the cerebellum receives and sends information to prefrontal and parietal cortices, engaging in higher cognition such as executive function, language, and working memory [26]. Specifically, cerebellar subregions including bilateral Crus II and Lobule VI, midline Lobule VII, and right Crus I were associated with language and Lobule VI, VIIB, and left Crus I were relevant to verbal working memory [27]. Studies have proposed the linkage between cerebellum and phonemic and semantic fluency task performance. For example, impaired verbal fluency task performance was found in those with cerebellar infarction [28]. According to neuroimaging studies, greater cerebellar gray matter density was related to better semantic fluency task performance [29] and consistent fMRI activation patterns have been identified in cerebellum during phonemic word generation task [30]. Importantly, greater functional synchronization between cerebellum and thalamus was found to be relevant to better phonemic fluency performance [31].
Earlier investigations demonstrated that ET resulted in greater brain derived neurotrophic factor (BDNF) expression in the rat cerebellum [32,33] and increased cerebellum gray matter volume in humans [34]. Despite this converging evidence of beneficial effects of ET on cerebellum, it has not been established whether ET-related changes in functional network of cerebellum is accompanied by alteration in fluency task performance. This is a critical knowledge gap since cerebellum is increasingly recognized as a brain area involved in fluency task performance. Furthermore, whether ET alters cognition through functional changes in the neural circuitry of cerebellum remains unknown. To address this gap in the literature, the second purpose of this study was to investigate the link between changes in cerebellum FC and fluency performance (both phonemic and semantic) following ET. We hypothesized that ET-related increase in cerebellum network connectivity would be associated with improved fluency task performance.
MATERIALS AND METHODS
Participants
Our study sample consisted of thirty-five older adults from the local community (17 MCI and 18 healthy older adults; ages 60–88) who were recruited by newspaper advertisements, in-person informational sessions at local retirement communities, and physician referrals. A standardized telephone interview was administered for those who volunteered for this study to determine preliminary eligibility (e.g., preclusive health conditions and MRI contraindications). Final eligibility was determined after participants underwent a neurological assessment. Qualified participants provided written consent form, obtained physician approval to participate in moderate-intensity exercise, and reviewed all study procedures. The present study was approved by the Institutional Review Board of the Medical College of Wisconsin and was conducted in compliance with the Declaration of Helsinki.
Inclusion and Exclusion Criteria
Older adults who are not physically active (i.e., engaging in moderate-intensity physical activity < 3 days/week for the past six months based on their self-report) were included to the study. We previously reported a complete list of eligibility criteria used in the present study [35]. Briefly, individuals were excluded if they reported neurological or cerebrovascular conditions (e.g., Parkinson’s disease, Huntington’s disease, multiple sclerosis, and epilepsy), untreated Axis I psychiatric disturbance meeting DSM-IV Axis I criteria, severe depressive symptoms, history of cardiovascular disease (e.g., stroke), MR contraindications (e.g., metal implants, pacemakers, and a history of claustrophobia), left-handedness (i.e., < 50 of laterality quotient [36]), history of ischemic attack, > 15 score on the Geriatric Depression Scale [37] or relatively impaired activities of daily living [38].
Cognitive Status Testing
A team of clinical investigators evaluated each participant’s cognitive status (i.e., HC or MCI). The core clinical criteria for the diagnosis of MCI by NIH-Alzheimer’s association workshop on MCI due to AD [39] was used to make this determination. MCI was defined as a subjective cognition-related concerns, impaired in at least one cognitive domain, preserved activities of daily living, and absence of dementia. Further examination by a neurologist was administered on individuals with probable MCI to exclude other possible causes of cognitive decline.
Phonemic and Semantic Fluency Tasks
Before and following a 12-week ET, participants completed the Controlled Oral Word Association Task (COWAT) and semantic fluency task as part of a larger neuropsychological battery (administered between 7 AM and 11 AM). The average day between the last day of ET intervention and reassessment was 5.3±3.9 days across participants. The COWAT is designed to examine phonemic fluency and participants were instructed to produce words that begin with given letters (e.g., F, A, S) within 60 seconds [40]. During the semantic fluency task, participants were instructed to generate as many words within the animal category as possible under the time constraint of 60 seconds [41]. The responses were transcribed by experimenter during the tests.
The total number of words produced during the tests were reported in our past work [18]. For the current analysis, we evaluated the lexical characteristics (e.g., average size of clusters, number of switches, word frequency, age of acquisition, and syllable length) for each unique word produced. Cluster size was calculated based on the number of words within a subcategory where two words were considered as a cluster size of one. For example, the animals were considered one cluster (e.g., dog, cat; pet). The cluster sizes were computed after a second word of the same subcategory if generated in sequence (e.g., cluster size = total of animals in a given cluster −1). The mean cluster size is the sum of all clusters sizes generated divided by the number of clusters [7]. Switching refers to the ability to shift efficiently to a new category when a subcategory is exhausted and was calculated by counting the number of transitions between clusters. For example, if a participant said dog, cat (pet); lady bug, worm (insect), one switching occurred as there was a changing from the clusters of pet to a cluster of insect [7]. Word frequency was calculated based on how frequently the word occurs out of 1 million words in American English [42]. Age of acquisition indicates the age at which the words are acquired and was obtained based on the age of acquisition scale [43]. Syllable length was determined by counting the number of syllables.
Cardiorespiratory Fitness Test
To assess cardiorespiratory fitness, all subjects underwent a modified submaximal stress test before and immediately after the 12-week exercise intervention. The test was performed on a General Electric (GE) motorized treadmill (Milwaukee, WI) using a modified Balke-Ware protocol (initial exercise speed = 3.2 km/h at 0° grade and grade increase 1°/min). Measures of ventilation, rate of oxygen (O2) consumption, rate of carbon dioxide (CO2) production, and the respiratory exchange ratio (RER; CO2 production/O2 consumption) were obtained using a calibrated metabolic measurement system (Parvo Medics, Salt Lake City, UT). The ratings of perceived exertion (RPE) scale [44] was used to monitor subjective effort every minute. Heart rate (HR) and blood pressure were measured every 2 min. The test was terminated upon reaching 85% of the participant’s maximal heart rate reserve (HRR) or upon observation of absolute exercise contraindications (e.g., diastolic blood pressure > 110 mmHg), or if the participant requests to stop the test. The highest (ml/kg/min) value during the test was determined as .
Exercise Intervention
Participants underwent a 12-week treadmill walking intervention under the supervision of a certified personal trainer or exercise physiologist. The frequency of the training was 4 sessions/week and the duration of each session was 30 min. The exercise session occurred either individually or in a group of two in local recreation centers. HR monitor (Polar Electro, Kempele, Finland) and RPE scale were used to assess exercise intensity during the training sessions. During the first 4 weeks, the exercise duration and frequency were gradually increased. During the remaining weeks (5–12), the exercise intensity was targeted at 50–60% of HRR. The treadmill speed and grade were modified each session based on HR and RPE to personalize the exercise intensity for each participant. The exercise session began with a 10-min warm-up and ended with a 10-min cool-down consisted of light walking and flexibility exercise.
MRI Acquisition
All MRI data were acquired on a 3.0 Tesla GE (Waukesha, WI) MR scanner. A high-resolution T1-weighted anatomical image was acquired for co-registration with the following sequence parameters: 3D Spoiled Gradient Recalled at steady state protocol (SPGR), field of view (FOV) = 240 mm, voxel size = 0.94 × 0.94 × 1.00 mm, number of excitations (NEX) = 1, slice thickness = 1 mm, repetition time (TR) = 9.6 ms, echo time (TE) = 3.9 ms, inversion recovery preparation time = 450 ms, flip angle = 12°, resolution = 256 × 224, and sequence duration = 6 min. The resting state BOLD data were acquired using the following sequence parameters: gradient echo planar images, FOV = 240 mm, NEX = 1 mm, slice thickness = 1.0 mm, TR/TE = 2000/25 ms, axial slices = 36, flip angle = 77°, resolution = 64 × 64, and sequence duration = 6 min. During the resting state scan, participants were instructed to remain still and to look at a fixation cross projected in the monitor.
MRI Data Processing
The T1-weighted anatomical image was processed using FreeSurfer’s (version 5.3.0) automated pipeline (recon-all) for removing non-brain tissue and generating surface models of the cortical and subcortical reconstructions based on tissue-specific intensities and atlas probabilities [45]. All estimated reconstructions were visually inspected for segmentation errors. To mitigate magnetization disequilibrium, the first three volumes of the functional image time-series were discarded using Analysis of Functional NeuroImage’s (AFNI, version 17.2.10) 3dTcat function [46]. The truncated time-series were realigned using Slice-Oriented Motion Correction (SLOMOCO) for slice-wise motion parameter estimation and correction [47]. Next, the motion-corrected functional volumes were aligned to FreeSurfer-processed anatomical images using AFNI’s Local Pearson Correlation cost function (align_epi_anat). The aligned anatomical and functional data were visually inspected for quality assurance before being further processed using AFNI’s single-subject preprocessing stream (proc.py). Functional data were despiked (using 3dDespike) and each volume of the time series were time-shifted to the beginning of the TR (using 3dTshift). TRs with excessive motion (outlier fraction > 10%) were censored. Non-linear transformation of each participant’s anatomical volume to the standard space (AFNI’s MNI152_T1_2009c template) was then performed (using 3dQwarp). Lastly, nuisance physiological artifacts were removed by eroding the motion derivatives and signals from ventricles and white matter estimated by Freesurfer segmentation. The resolution of the resulting transformation matrix had a grid spacing of 2 × 2 × 2 mm3 and was used in the subsequent connectivity analyses. The percentage of censored TRs between scans occurred at before (0.86±0.27%) and after ET (0.94±0.34%) did not significantly differ (p = 0.079), suggesting that the effects of head movement did not confound our fMRI data interpretation.
Cerebellum Functional Connectivity Analysis
We utilized a seed-based analysis to interrogate the cerebellum FC. Cerebellar seed regions were selected based on a meta-analysis studying the functional topography in the human cerebellum neuroimaging studies [48]. From this meta-analysis, the peak cerebellar coordinates of the clusters related to verbal and language function were identified including cluster 1 (MNI 37.9 −63.7 −29.7), cluster 2 (MNI 12.5 −86.1 −32.9), and cluster 3 (MNI −42 −58 −24). The peak coordinates from each cluster were further evaluated using Neurosynth [49] where we measured the number of studies that demonstrated seed-based network correlation of each coordinate. A total of 18 findings identified the association between the clusters and verbal/language function. Next, the cerebellar seed regions of interest were defined as spherical seed masks (10 mm in diameter) positioned on each of the cluster coordinate (Seed 1 MNI 37.9 −63.7 −29.7; Seed 2 MNI 12.5 −86.1 −32.9; Seed 3 MNI −42 −58 −24), respectively. The mean functional time-series were then extracted from each seed and cross-correlated with all voxels in the brain to isolate a FC brain map. These maps were created for each participant and each experimental time point (e.g., before and after ET). Lastly, using a Fisher’s r-to-z transformation, correlation coefficients were standardized for group-level analysis.
Gray Matter Voxel-Wise Group Analysis
For group-level analysis, the whole-brain anatomical mask containing only voxels defined as gray matter from FreeSurfer segmentation was created for each participant. White matter and ventricle segmentations were excluded from this mask. The gray matter mask was then merged across participants for a group-level intersection mask. Subject-level z-scored correlation maps were submitted to AFNI’s linear mixed model function (3dLME) to compute the main effects of time and group by time interaction. To correct for multiple comparisons, family-wise error rate (FWER) was controlled using AFNI’s 3dClustSim where an uncorrected voxel-wise threshold of p < 0.001 and a FWER-corrected alpha value of 0.05 engendered a cluster-size threshold of k ≥ 41 voxels (NN = 1).
A Priori Region of Interest FC Analysis
Using an anatomically defined region of interest, we also examined the effect of ET on the FC between cerebellum and inferior frontal gyrus (LIFG), based on previous evidence regarding the engagement of IFG in lexical decision and semantic fluency [40,50]. AFNI’s MNI reference dataset (MNI152_T1_2009c) was processed using FreeSurfer’s recon-all function [45]. Using the Desikan-Killiany aparc+aseg segmentation [51], a structurally defined LIFG and RIFG masks were created based on the resulting Freesurfer parcellation using AFNI’s voxel-by-voxel arithmetic computation function (3dcalc). Using this mask, the mean connectivity signals between LIFG and each cerebellar seed as well as RIFG and each cerebellar seed were extracted from participants’ parametric maps for before and after ET, respectively. The difference between before and after ET was determined using a paired t-test (p < 0.05).
Statistical Analysis
Before evaluating the baseline demographic characteristic of the participants, the Shapiro-Wilk test was performed to determine normality. The group differences (MCI vs HC) in the baseline demographic characteristics were analyzed using independent sample t-tests (or Wilcoxon signed-rank tests) and Fisher’s exact test (for categorical variables). Normality of fluency test results was also tested using the Shapiro-Wilk test. As we observed that mean frequency data for both phonemic and semantic fluency tasks were not normally distributed, log transformation was performed. Repeated measures of ANOVA were utilized to compute the main effects of time (i.e., after ET minus before ET) and group (HC vs MCI) × time (before vs after ET) interaction on the phonemic and semantic fluency test performances.
We used multivariable linear regression to investigate the association between ET-induced changes in fluency task performance (Δfluency performance) and cerebellar FC (ΔCerebellum-FC). Bivariate correlation tests revealed no significant correlations between demographic characteristics (age, sex, education) and variables of interest (Δfluency performance and ΔCerebellum-FC); thus, the correlation analyses were not adjusted. ET-related changes were computed by subtracting before from after ET values. Regression models included ΔCerebellum-FC as independent variables and Δfluency performance as a dependent variable. Subsequently, we conducted a post-hoc analysis to determine the interaction between group (MCI vs HC) and ΔCerebellum-FC on Δfluency performance. To accomplish this, among the results displaying significant associations between ΔCerebellum-FC and Δfluency performance, we performed ANCOVA, with group and ΔCerebellum-FC as independent variables, and Δfluency performance as a dependent variable. Lastly, group × ΔCerebellum-FC interaction term was modeled into the analysis. The statistical significance was determined using a two-tailed alpha = 0.05. All statistical tests were conducted using SPSS (v. 26.0).
RESULTS
Demographic Characteristic
Of the 407 older adults who responded to study recruitment and advertisement, 92 signed the informed consent and underwent neurological examination, 39 individuals started the exercise program, and 35 individuals (17 MCI and 18 HC) completed the entire study protocol. Detailed information on recruitment and enrollment has been reported in our prior work [35]. Overall, the participants averaged 78 years of age (71% female; 12 total apolipoprotein E epsilon 4 allele (APOE-ε4) carriers) and 16 years of education. There were no significant differences between MCI and HC in the baseline demographic characteristics including age, sex, education, percentage of APOE-ε4 carriers, and . The mean adherence rate to the exercise session was 96.1±5%. The mean intensity during the ET were HRR of 54.7±11.0% and RPE of 10.8±2.0 (assoicted with verbal anchor ‘Light’). Demographics, cardiorespiratory fitness, depression, activities of daily living, and cognitive data for all participants are illustrated in Table 1.
Table 1.
Demographic information for study participants.
| Total Sample (n = 35) | MCI (n = 17) | HC (n = 18) | Group Differences | ||
|---|---|---|---|---|---|
|
|
|||||
| Mean (SD) | Mean (SD) | Mean (SD) | p-value | ||
|
| |||||
| Demographics | |||||
| Age (years) | 78.0 (7.1) | 79.5 (6.8) | 76.5 (7.2) | 0.91 | |
| Female (n,%) | 25 (71.4%) | 10 (58.8%) | 15 (83.3%) | 0.15F | |
| Education (years) | 16.0 (2.6) | 15.6 (3.1) | 16.5 (2.0) | 0.25 | |
| APOE-ε4 Carriers | 12 | 6 | 6 | 0.59F | |
| Cardiorespiratory Fitness | |||||
| Baseline (ml/kg/min) | 19.2 (4.5) | 18.3 (3.7) | 20.1 (5.1) | 0.44 | |
| Depression | |||||
| Baseline GDS | 4.7 (3.3) | 5.8 (3.7) | 3.8 (2.7) | 0.07 | |
| Activities of Daily Living | |||||
| Bassline Lawton IADL | 4.7 (0.5) | 4.7 (0.5) | 4.7 (0.5) | 0.98 | |
Notes: MCI, Mild Cognitive Impairment; HC, Healthy Cognition; F, Fisher’s Exact Test; APOE-ε4, apolipoprotein E epsilon 4 allele; , peak rate of oxygen consumption; GDS, Geriatric Depression Scale; IADL, Instrumental Activities of Daily Living.
Cardiorespiratory Fitness and Task Performance
was significantly increased from before to after ET across participants (missing data 2 MCI and 1 HC) [F(1,30) = 9.735, p = 0.004, η2p = 0.245], confirming the success of our 12-week walking exercise intervention. In all participants, mean switches [F(1,33) = 4.679, p = 0.038, η2p = 0.124] (Figure 1; Panel A) and mean log frequency [F(1,33) = 6.407, p = 0.016, η2p = 0.163] (Figure 1; Panel B) during the phonemic fluency task were significantly increased in response to ET. Whereas none of the significant main effects of time were found in the semantic fluency task after completion of ET (Table 2). There were significant group by time interaction on the number of syllables during the phonemic fluency test [F(1,33) = 4.868, p = 0.034, η2p = 0.129] (Figure 1; Panel C), mean switches [F(1,30) = 5.036, p = 0.032, η2p = 0.144] (missing data 3 MCI; Figure 1; Panel D), and mean cluster size during the semantic fluency test [F(1,30) = 5.036, p = 0.032, η2p = 0.144] (missing data 3 MCI; Figure 1; Panel E). As we reported previously [52], there were significantly increased total number of words during the phonemic fluency task [F(1,30) = 10.986, p = 0.005, η2p = 0.423], but no changes were found in the semantic fluency task performance [F(1,30) = 0.136, p = 0.717, η2p = 0.009] across participants.
Fig 1.
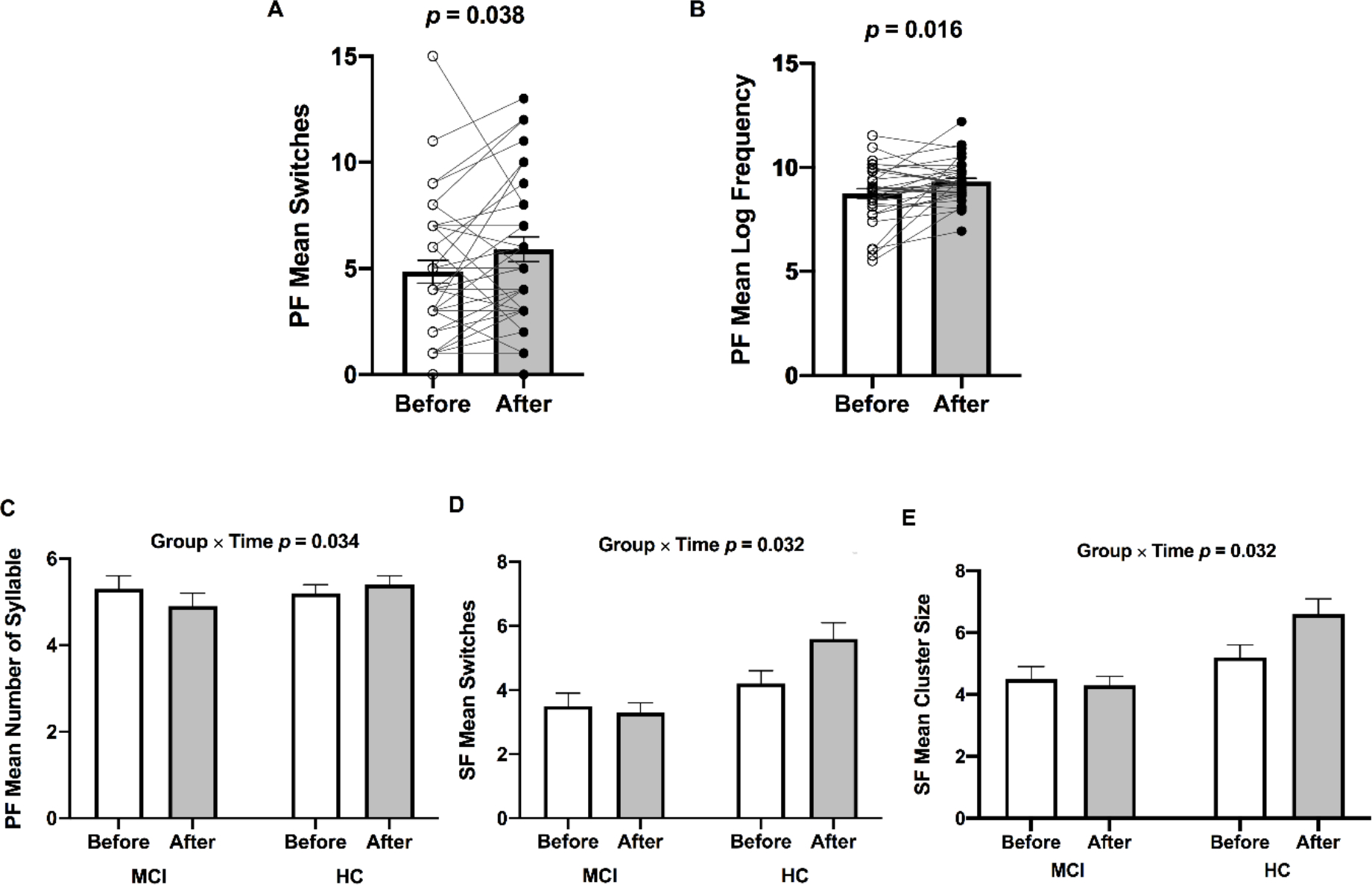
Bar graphs (±SEM) illustrate increased (A) mean switches and (B) mean log frequency during the phonemic fluency task following ET in all participants (n = 35). Significant Group (HC vs MCI) × Time (before vs after ET) interactions were found in the (C) phonemic fluency mean number of syllables, (D) semantic fluency mean switches, and (E) semantic fluency cluster size. p-values above bar graphs indicate the effects of time (Panels A and B) and Group by Time interaction (Panels C, D, and E), respectively. Notes: PF, phonemic fluency task; SF, semantic fluency task.
Table 2.
Cardiorespiratory fitness, phonemic fluency, and semantic fluency performance data.
| Total Sample (n = 35) | MCI (n = 17) | HC (n = 18) | Time | Group × Time | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Before | After | Before | After | Before | After | |||
|
|
||||||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | p-value (η2p) | p-value (η2p) | |
|
| ||||||||
| Cardiorespiratory Fitness | ||||||||
| (ml/kg/min) | 19.6 (4.5) | 21.4 (3.7) | 19.1 (4.1) | 21.5 (3.4) | 20.1(4.9) | 21.2 (4.1) | 0.004 (0.245) | 0.240 (0.046) |
| Phonemic Fluency | ||||||||
| Switches | 4.9 (3.2) | 5.9 (3.5) | 3.7 (2.5) | 5.2 (3.6) | 5.9 (3.4) | 6.6 (3.3) | 0.038 (0.124) | 0.360 (0.025) |
| Cluster Size | 7.6 (3.4) | 8.6 (4.0) | 6.3 (3.1) | 7.7 (4.3) | 8.9 (3.4) | 9.5 (4.0) | 0.060 (0.103) | 0.447 (0.018) |
| Log Frequency | 8.7 (1.4) | 9.3 (1.0) | 8.8 (1.2) | 9.4 (1.3) | 8.7 (1.6) | 9.3 (0.7) | 0.016 (0.163) | 0.901 (0.000) |
| Age of Acquisition | 19.9 (3.6) | 20.1 (2.7) | 20.1 (3.9) | 19.8 (3.3) | 19.9 (3.5) | 20.4 (2.1) | 0.763 (0.003) | 0.368 (0.025) |
| Number of Syllables | 5.2 (1.1) | 5.1 (1.0) | 5.2 (1.3) | 4.9 (1.0) | 5.2 (1.0) | 5.4 (0.9) | 0.696 (0.015) | 0.034 (0.129) |
| Semantic Fluency | ||||||||
| Switches | 3.9 (1.6) | 4.6 (2.2) | 3.5 (1.7) | 3.3 (1.9) | 4.2 (1.6) | 5.6 (1.9) | 0.115 (0.081) | 0.032 (0.144) |
| Cluster Size | 4.9 (1.6) | 5.6 (2.2) | 4.5 (1.7) | 4.3 (2.0) | 5.2 (1.6) | 6.6 (1.9) | 0.115 (0.081) | 0.032 (0.144) |
| Log Frequency | 2.7 (0.3) | 2.7 (0.3) | 2.8 (0.3) | 2.7 (0.4) | 2.6 (0.2) | 2.6 (0.1) | 0.330 (0.031) | 0.873 (0.001) |
| Age of Acquisition | 5.3 (0.7) | 5.2 (0.6) | 5.0 (0.7) | 5.0 (0.7) | 5.5 (0.6) | 5.4 (0.5) | 0.553 (0.012) | 0.751 (0.003) |
| Number of Syllables | 1.6 (0.3) | 1.6 (0.3) | 1.6 (0.2) | 1.6 (0.3) | 1.6 (0.2) | 1.7 (0.2) | 0.513 (0.014) | 0.427 (0.020) |
Notes: MCI, Mild Cognitive Impairment; HC, Healthy Cognition; , peak rate of oxygen consumption; p-values and effect size (η2p) reflect the Time and Group × Time effects from repeated measures ANOVA; Bold indicates p < 0.05.
Post-hoc within-group analyses revealed that none of the groups demonstrated significantly altered phonemic fluency mean number of syllables following ET (MCI p = 0.121, η2p = 0.144; HC p = 0.162, η2p = 0.111). Conversely, while HC group demonstrated significant improvements in semantic fluency mean switches and cluster size (both mean cluster size and switches p = 0.006, η2p = 0.372), there were no significant changes in MCI group (both mean cluster size and switches p = 0.711, η2p = 0.011) after ET. Cardiorespiratory fitness and fluency task performance data are presented in Table 2.
Voxel-Wise Cerebellum FC Analysis
Thirty-three participants’ data were included for the cerebellum FC analysis (missing data 1 MCI and 1 HC). Although there were no significant main effects of time and group by time interaction across participants, a significantly greater connectivity between the Seed 1 (MNI 37.9 −63.7 −29.7) and the right inferior parietal lobule (IPL; MNI 37 −55 47 [LPI], BA 40, 864 mm3) was found after ET in HC participants (Figure 2; Panel A). Also, there was an ET-related increased connectivity between the Seed 2 (MNI 12.5 −86.1 −32.9) and the bilateral precuneus (MNI −1 59 35 [LPI], BA 7, 512 mm3) in HC participants (Figure 2; Panel B). None of the effects were observed in MCI participants. Lastly, the Seed 3 (MNI −42 −58 −24) did not show any significant effects following ET.
Fig 2.
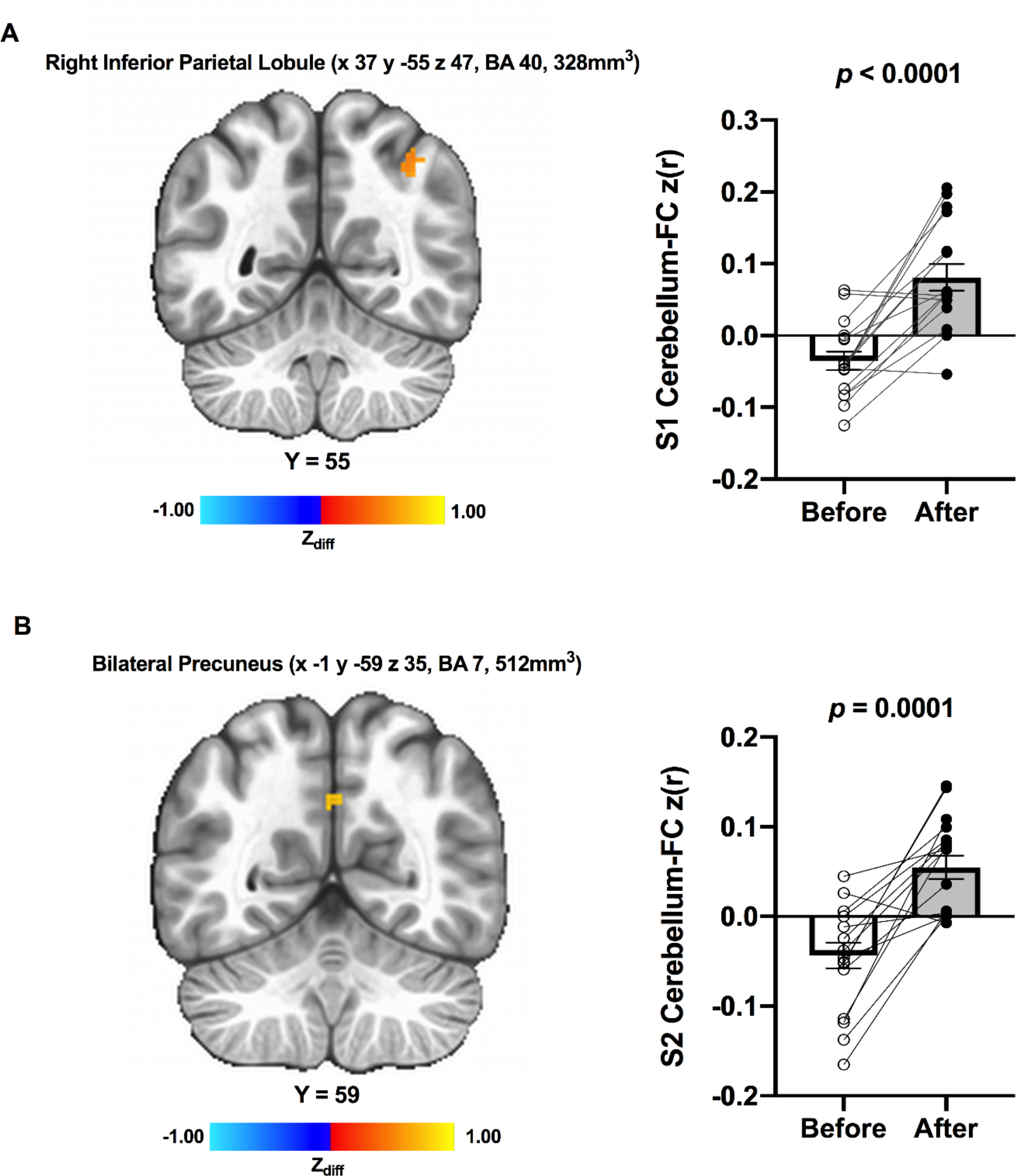
Resting-state functional cerebellum connectivity was increased from before to after ET among individuals with HC; (A) Increased connectivity between the cerebellum seed 1 (MNI 37.9 −63.7 −29.7) and region within the right inferior parietal lobule (t(16) = 5.612, p < 0.0001, Cohen’s d = 1.361); (B) Increased connectivity between the cerebellum seed 2 (MNI 12.5 −86.1 −32.9) and region within bilateral precuneus (t(16) = 5.059, p = 0.0001, Cohen’s d = 1.226). Adjacent bar graphs indicate the connectivity between cerebellar seeds and each region (±SEM) for before and after ET, respectively. p-values above bar graphs indicate statistical difference from before to after ET. Notes: S1, seed 1; S2, seed 2.
A Priori Voxel-Wise Cerebellum FC Analysis
As none of the significant results were found in the cerebellar seed 3, cerebellar seed 1 and 2 were used for this priori analysis. We did not observe significant connectivity pattern between cerebellum and LIFG/RIFG in the whole-brain analysis; thus, we further conducted a priori cerebellum-IFG connectivity analysis. In HC, there were no significant main effects of time on the changes in the connectivity between cerebellar Seed 1 and LIFG connectivity [t(16) = −0.281, p = 0.782, d = −0.068, 95% of d = −0.543, 0.408], cerebellar Seed 2 and LIFG [t(16) = 0.259, p = 0.798, d = 0.063, 95% of d = −0.413, 0.537], cerebellar Seed 1 and RIFG [t(16) = 0.318, p = 0.754, d = 0.077, 95% of d = −0.400, 0.522], and cerebellar Seed 2 and RIFG [t(16) = 1.625, p = 0.123, d = 0.394, 95% of d = −0.105, 0.882]. Similarly in MCI, none of significant main effects of time on the changes in the connectivity between cerebellar Seed 1 and LIFG [t(15) = 0.686, p = 0.503, d = 0.171, 95% of d = −0.325, 0.662], cerebellar Seed 2 and LIFG [t(15) = −0.780, p = 0.447, d = −0.195, 95% of d = −0.686, 0.302], cerebellar Seed 1 and RIFG [t(15) = 0.140, p = 0.889, d = 0.035, 95% of d = −0.455, 0.524] and cerebellar Seed 2 and RIFG [t(15) = −0.842, p = 0.412, d = −0.210, 95% of d = −0.702, 0.288].
Associations of Changes in Cerebellum FC and Fluency Performance
As the significant changes in cerebellum FC were observed only among HC, the association between ET-related changes in cerebellum FC and fluency performance was assessed only in HC individuals. 17 HC participants’ data were included for this analysis (1 missing fMRI data). Average z-scored connectivity within the Seed 1 and Seed 2, respectively, were extracted from subject-level correlation maps in order to quantify the ET-induced changes in cerebellum FC. Results revelated that variances in the Seed 1 ΔCerebellum-FC explained 38.1% of the phonemic fluency Δmean log frequency [R = 0.618, R2 = 0.381, F(1, 15) = 9.27, p = 0.0082] (Figure 3; Panel A), 41.2% of Δmean switches [R = 0.643, R2 = 0.412, F(1, 15) = 10.55, p = 0.0054] (Figure 3; Panel B), and 44.4% of Δmean cluster size [R = 0.666, R2 = 0.444, F(1, 15) = 11.99, p = 0.0035] (Figure 3; Panel C). In addition, variances in the Seed 2 ΔCerebellum-FC explained 28.9% of variances in the phonemic fluency Δmean number of syllable [R = 0.537, R2 = 0.289, F(1, 15) = 6.100, p = 0.026] (Figure 3; Panel D). None of the significant relationship between cerebellum FC and semantic fluency performance was found.
Fig 3.
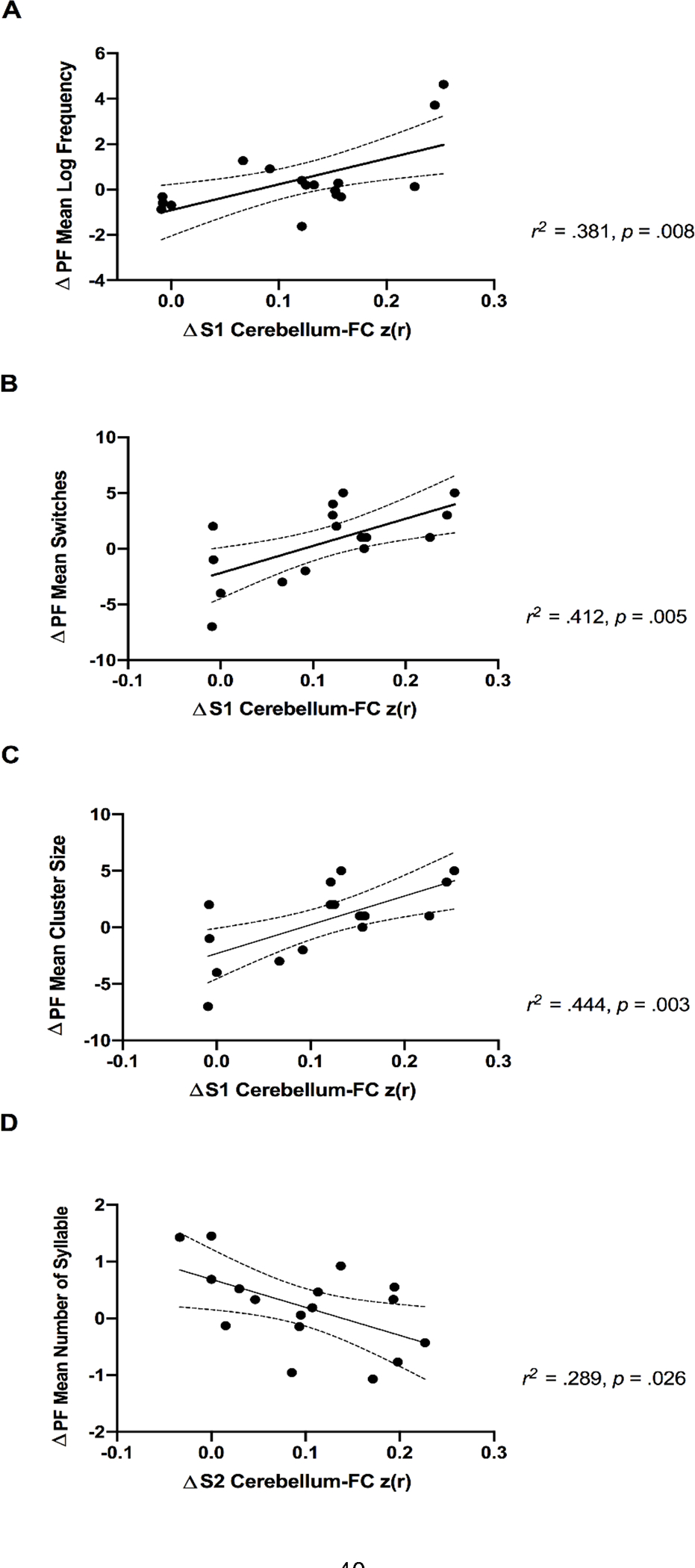
Positive associations of exercise-related increase in cerebellum-FC (seed 1 and right IPL) and lexical characteristics during the phonemic fluency task in cognitively healthy older adults (Panel A, B, and C). Panel D shows a negative association of exercise-related increase in cerebellum-FC (seed 2 and bilateral precuneus) and decreased mean number of syllables during the phonemic fluency task in cognitively healthy older adults. Dotted curves indicate 95% confidence interval around the mean. Notes: PF, phonemic fluency task; S1, seed 1; S2, seed 2.
Post-hoc Group × Cerebellum FC on Fluency Performance
To assess the group × cerebellum FC on fluency performance, the average z-scored connectivity within the Seed 1 and Seed 2 that displayed a significant correlation between ET-related changes in cerebellum FC and verbal fluency in HC, respectively, were also extracted from subject-level correlation maps of MCI participants. Our post-hoc interaction analyses results showed a significant interactive effect between group and ΔCerebellum-FC (Seed 1 and right IPL) on Δmean log frequency during the phonemic fluency task [F(1,29) = 5.181, p = 0.022, η2p = 0.167] (Figure 4; Panel A). There was also a significant group × ΔCerebellum-FC (Seed 2 and right precuneus) on the phonemic fluency Δmean number of syllables [F(1,29) = 4.500, p = 0.042, η2p = 0.134] (Figure 4; Panel B).
Fig 4.
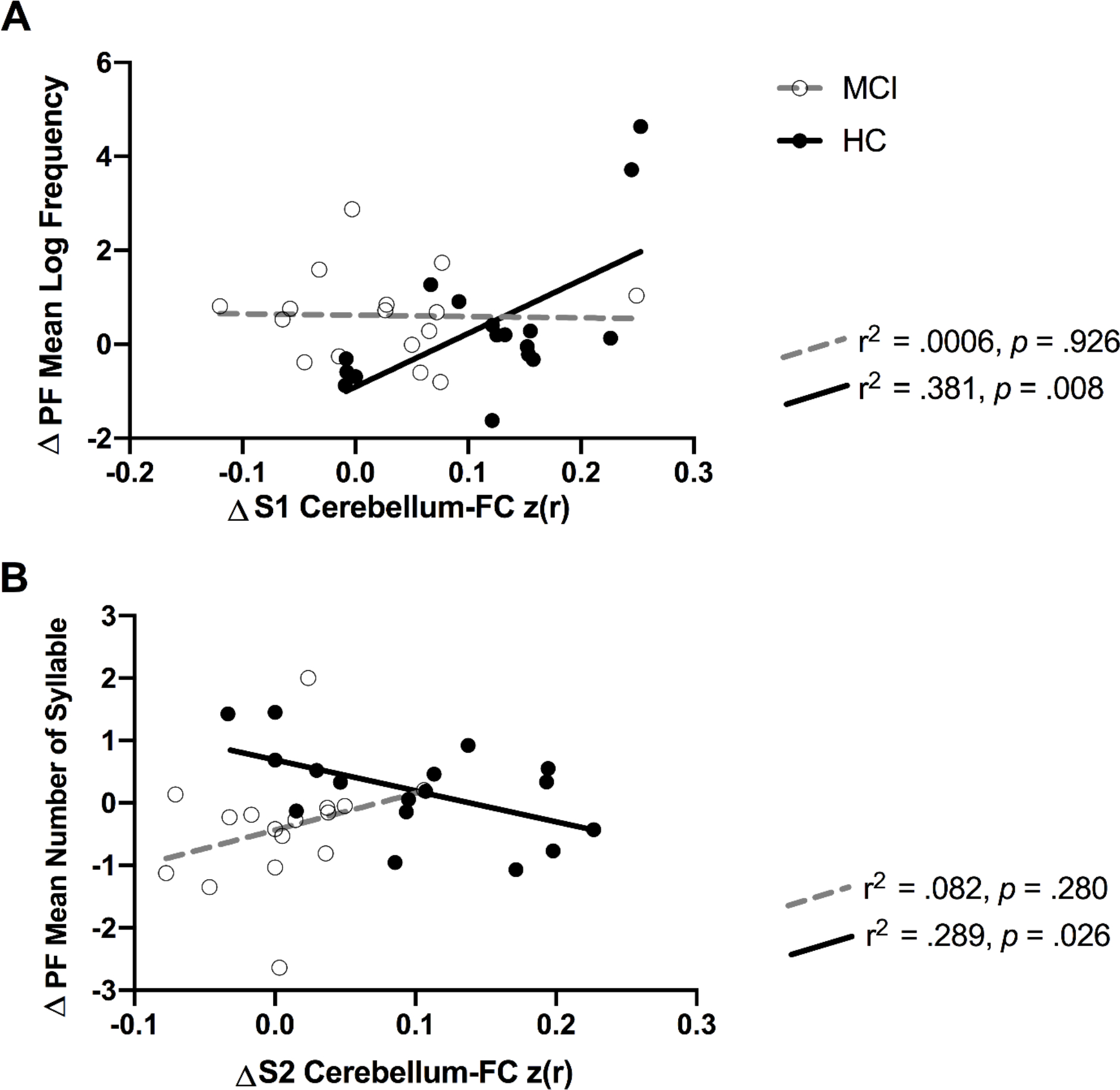
Significant interaction bewteen group (MCI vs HC) and cerebellum-FC on the phonemic flunecy task performance. The r2 and p-values reflect the correlation of cerebellum-FC and fluency performance for each group. Notes: PF, phonemic fluency task; S1, seed 1; S2, seed 2.
DISCUSSION
The present study investigated the effects of ET on fluency task response patterns and its association with cerebellar connectivity in older adults. There were two major findings from the current analyses. First, a 12-week walking intervention significantly enhanced cardiorespiratory fitness (approximately 9% increase in ) and frequency and switches of the words produced during the phonemic fluency task across groups. ET was also associated with significantly increased mean switches and cluster size during the semantic fluency task, which was specific to HC. Second, there were significant associations between ET-induced increased cerebellum FC and improvements in lexical characteristics during the phonemic fluency task in cognitively healthy older adults. In contrast, this relationship was not found in those with MCI.
In our past work using the same cohort, there was a significant ET-related improvements in the number of words produced during the phonemic fluency task [18]. Here, we extend the previous study by revealing the improved lexical characteristics of the phonemic fluency responses. According to Troyer et al., participants begin to access the mental lexicon and retrieve words that are frequently encountered. Typically, word searching and switching strategies tend to be smoothly executed during the early phase of the test (~15 seconds) [7]. When exemplars with high frequency are exhausted, participants search for more difficult and less-frequently-used words from the mental lexicon [53]. Hence, the improved mean switches and frequency after ET are indicatives of delayed exhaustion of the high-frequency words and quick shifts to search for and retrieve new words. Particularly, the greater switches reflect better cognitive flexibility, which is corroborated by the evidence of ET-elicited improvements in task-switching performance in older adults [54]. Taken together, the present results, in conjunction with our previous findings, propose that ET-induced enhancements in switches and frequency are key components contributing to increased total number of words produced during the phonemic fluency task in older adults.
Of note, there were significant associations between ET-related greater recruitment of cerebellar connectivity and enhanced lexical characteristics during the phonemic fluency task. A key ability required during fluency task is constantly comparing prior and ongoing stimuli (i.e., sequencing ability) to avoid repeated words [55]. To accomplish this, the last word is sequentially coupled with the new words throughout the fluency task. Cerebellum plays an important role in the sequencing procedure as well as in accelerating the phonemic cue retrieval [55,56]. It is hypothesized that the cerebellum’s contribution to cognitive function occurs through cerebello-cerebrocortical pathways which connect cerebellum to the cognitive networks such as the executive control network (e.g., prefrontal and parietal cortices) [57] and the default mode network (DMN; e.g., cingulate gyrus and precuneus) [58]. This pathway demonstrates topographically organized feedforward projections to the cerebral cortex [59] that enable cerebellum to engage in the higher-order cognitive behaviors including language [60]. Indeed, neuroimaging evidence delineates that cerebellum is functionally connected to the prefrontal and parietal regions [23,24] and the DMN [58]. Given the IPL is a key anchor of the executive control network [61] and both IPL and precuneus are highly connected hubs of the DMN [62], our findings of augmented cerebellar connectivity with IPL and precuneus offer evidence that the ET-elicited increased connectivity between cerebellum and key hub regions of the cognitive networks. Furthermore, both involvement of IPL [63] and precuneus [64] are implicated during verbal fluency task. Thus, the ET-related greater connectivity between cerebellum and the cognitive networks may be determinants of enhanced lexical characteristics (i.e., increased cluster size), cognitive flexibility (i.e., increased switches), and efficiency of word processing (i.e., increased frequency and decreased number of syllables in order to generate more words under time constraints) during the phonemic fluency task. Moreover, two indicators of efficiency during the phonemic fluency task (mean log frequency and number of syllables) were not statistically correlated (correlation between Δlog frequency and Δnumber of syllables p = 0.608, data not presented), further suggesting that functional cerebellar connection with IPL (associated with enhanced frequency; Figure 3, Panel A) and precuneus (associated with lower number of syllables; Figure 3, Panel D) may be distinctively contributing to the improved fluency task performance after ET.
Because of the well-documented role of IFG in verbal fluency task [40,50] and selective exercise-induced enhancement in the frontal regions of the brain [65,66], we conducted a priori analysis to compute the resting-state connectivity between cerebellum and IFG. However, none of the significant main effects of time and group by time interaction were found in resting-state connectivity between cerebellar seeds with IFG. This suggests that ET-related alterations in functional connectivity between cerebellar regions involved in processing verbal function and IFG may not be the underlying neural changes associated with ET-induced improvements in the fluency task performance in older adults.
Notably, the association between ET-related changes in cerebellar FC and improvements in fluency performance was specific to the phonemic fluency task. Although both phonemic and semantic fluency tests share many common attributes, there are key differences between the two tasks. Suppressing semantically relevant words and novel retrieval strategies under the given verbal cue are essential to successfully accomplish the phonemic fluency task [67]. Conversely, the ability to retrieve the linkage between members under a given category is important for semantic fluency task performance [67]. As such, our results indicate that the ET-related functional changes in the cerebellar network may be associated with the improvements in novel retrieval strategies, not with retrieval of the link between associative concepts.
Using the same sample analyzed in the current study, we previously reported that older individuals diagnosed with MCI demonstrated an augmented synchronization in the default-mode network (DMN) [68], greater neural efficiency during semantic memory task [35], and increased cortical thickness [69] after ET. Based on these neuroprotective effects of ET, we expected ET-related cerebellar hyperconnectivity in MCI, suggesting maintenance of neural reserve by elevating age-related compensatory responses [68], which was not supported in the present analyses. According to a prior evidence, there was an altered cerebero-cerebellar network in older adults with MCI compared to the age-matched cognitively healthy control [70]. In the present results, such differences were not found in the baseline cerebellar connectivity between HC and MCI. Given the enhanced phonemic fluency task performance in older adults with MCI following ET, the neural mechanism responsible for the ET-related verbal fluency improvements may differ between HC and MCI. However, this interpretation should be viewed with caution since our current results are limited due to small sample size.
Potential Mechanisms
Earlier exercise neuroimaging work observed a decreased fMRI BOLD activation in the IFG during the fluency task in those who completed the ET in comparison with the control group [71]. Similarly, during the semantic fluency task, physically active older adults exhibited reduced functional activations in the brain regions related to attention-language compared to their age-matched sedentary counterparts [72]. These fMRI findings consistently suggest the ET-induced neural efficiency improvement through attenuated BOLD activation during the successful cognitive task performance [73]. Chronic exercise may facilitate increased capacity within the neural networks, through repeated upregulation and expression of neurotransmitters and neurotrophic factors [74,75]. In rodent work, ET has shown to induce angiogenesis [76] and upregulation of neurotrophins (e.g., serum insulin like growth factor-1 (IGF-1)) [77] in cerebellum. These neural adaptations to ET promote survival and maintenance of neurons through the growth and development of dendritic connections and building of a stronger neural scaffolding [73], ultimately resulting in more efficient and resilient neural capacity to withstand the age-related decline in brain function [78,79]. Collectively, this underlying neural mechanism may elucidate the ET-induced stronger task-independent functional connectivity and reduced task-induced neural activation to efficiently perform cognitive tasks. However, this hypothesis is speculative and further mechanistic and translational evidence are warranted to robustly test this hypothesis.
Strengths and Limitations
We evaluated the effects of ET on lexical characteristics, contributing to the existing literature that was limited to the analysis of the number of words produced during fluency tasks. Additionally, our participants had a high adherence rate to the exercise program (96.1±5%) and their cardiorespriatory fitness was succesfully improved after the training (approximately 9%). We also tested older adults with MCI who have been understudied in the aging neuroscience literature. Lastly, we used a stringent voxel-wise probability threshold (e.g., p < 0.001) in accordance with the current fMRI thresholding recommendations to reduce the vulnerability to noise and possibility of cluster-level false positive rate [80].
The present study has several limitations. Due to relatively small (n = 35) and homogenous characteristic of the sample (women 71% and highly educated), our results may not be generalizable to the entire older population. Additionally, our exercise intervention was relatively short (12 weeks) that might have prevented us to from observing potential effects acquired after longer exercise intervention (e.g., 6 months). The present study is subject to the limitations of lacking non-exercise control group, warranting some caution in interpretation of the results. Although we cannot directly account for the effects possibly induced by the passage of time or other nonspecific intervention, pretest-posttest designs are commonly used to examine intervention effects over time and the present results are in line with our prior studies using the same sample demonstrating the beneficial effects of ET on brain function [18,68]. Next, because of using the same version of phonemic and semantic fluency tests at before and after ET, some improvement due to a practice effect might be expected. However, the familiarity effect in older individuals is typically observed as a stable performance over time. Thus, the cognitive improvement in our sample may exceed what could be expected with repeated test administration [81]. Despite lacking a non-exercise control condition, the improvement in phonemic fluency task performance after ET compared to before ET reflects significant effects induced by ET. Indeed, achieving cognitive stability in older adults is considered a marker of treatment success in ET interventions [82]. Moreover, the improvement in phonemic fluency performance among HC participants co-occurred with the changes in cerebellum FC, supporting the hypothesis that ET may impact on the neural networks related to phonemic fluency performance. In addition, due to potential bias in seed selection, the interpretation of the seed-based correlation analyses should be viewed cautiously [83]. However, we selected the seeds based on the prior meta-analysis that rigorously examined cerebellum functional network based on existing neuroimaging literature [48]. Lastly, transcription of the words in verbal fluency data was in writing rather typing which could have led to systematic bias. To reduce this bias, independent raters performed cross-checked the transcription.
CONCLUSIONS
In older adults with both intact and impaired cognition, 12 weeks of regular walking ET improved frequency and switches during the phonemic fluency task. Moreover, increased cerebellar functional network connectivity may be an important determinant of the ET-induced behavioral enhancements during the phonemic fluency performance among older adults with HC. The increased neural synchronization in cerebellar network may have been induced by the ET-related adaptations to the building of a stronger neural scaffolding. Conversely, the associations between ET-stimulated increased cerebellar connectivity and improved lexical characteristics were not found in those with MCI. This suggests that there may be different neural mechanisms in MCI for the phonemic fluency improvements induced by ET. However, interpretation of the results warrants some caution due to relatively small sample size, lack of control group, and using same form of fluency tasks. An important direction for future studies is to replicate the findings presented in this study using a larger sample size, non-exercise control group, and alternates form of fluency task at before and after ET intervention.
ACKNWOLEDGEMENTS
We thank the participants for their dedication while participating in this study, and Drs. Nathan Hantke and Alissa Butts for their assistance with participant assessment. This study was supported by the University of Wisconsin-Milwaukee Graduate School Research Growth Initiative; and the National Center for Advancing Translational Sciences, NIH grant numbers 8UL1TR000055, 8KL2TR000056. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
CONFLICTS OF INTEREST
The authors declared no conflicts of interest with respect to the research, authorship, and publication of this article.
REFERENCES
- 1.Clark LJ, Gatz M, Zheng L, Chen Y-L, McCleary C, Mack WJ. Longitudinal verbal fluency in normal aging, preclinical, and prevalent Alzheimer’s disease. Am J Alzheimers Dis Dementias ®. 2009;24(6):461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kempler D, Zelinski EM. Language in dementia and normal aging. Dement Norm Aging. 1994;331–365. [Google Scholar]
- 3.Burke DM, Shafto MA. Aging and language production. Curr Dir Psychol Sci. 2004;13(1):21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, Belleville S, Brodaty H, Bennett D, Chertkow H, Cummings JL. Mild cognitive impairment. The lancet. 2006;367(9518):1262–1270. [DOI] [PubMed] [Google Scholar]
- 5.Lezak MD, Howieson DB, Loring DW, Fischer JS. Neuropsychological assessment. Oxford University Press, USA; 2004. [Google Scholar]
- 6.Fisk JE, Sharp CA. Age-related impairment in executive functioning: Updating, inhibition, shifting, and access. J Clin Exp Neuropsychol. 2004;26(7):874–890. [DOI] [PubMed] [Google Scholar]
- 7.Troyer AK, Moscovitch M, Winocur G, Alexander MP, Stuss DON. Clustering and switching on verbal fluency: The effects of focal frontal-and temporal-lobe lesions. Neuropsychologia. 1998;36(6):499–504. [DOI] [PubMed] [Google Scholar]
- 8.Henry JD, Crawford JR, Phillips LH. Verbal fluency performance in dementia of the Alzheimer’s type: a meta-analysis. Neuropsychologia. 2004;42(9):1212–1222. [DOI] [PubMed] [Google Scholar]
- 9.Abwender DA, Swan JG, Bowerman JT, Connolly SW. Qualitative analysis of verbal fluency output: Review and comparison of several scoring methods. Assessment. 2001;8(3):323–338. [DOI] [PubMed] [Google Scholar]
- 10.Bélanger S, Belleville S. Semantic inhibition impairment in mild cognitive impairment: A distinctive feature of upcoming cognitive decline? Neuropsychology. 2009;23(5):592. [DOI] [PubMed] [Google Scholar]
- 11.Duong A, Whitehead V, Hanratty K, Chertkow H. The nature of lexico-semantic processing deficits in mild cognitive impairment. Neuropsychologia. 2006;44(10):1928–1935. [DOI] [PubMed] [Google Scholar]
- 12.Brickman AM, Paul RH, Cohen RA, Williams LM, MacGregor KL, Jefferson AL, Tate DF, Gunstad J, Gordon E. Category and letter verbal fluency across the adult lifespan: relationship to EEG theta power. Arch Clin Neuropsychol. 2005;20(5):561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodríguez-Aranda C, Martinussen M. Age-related differences in performance of phonemic verbal fluency measured by Controlled Oral Word Association Task (COWAT): a meta-analytic study. Dev Neuropsychol. 2006;30(2):697–717. [DOI] [PubMed] [Google Scholar]
- 14.Mirandez RM, Aprahamian I, Talib LL, Forlenza OV, Radanovic M. Multiple category verbal fluency in mild cognitive impairment and correlation with CSF biomarkers for Alzheimer’s disease. Int Psychogeriatr. 2017;29(6):949–958. [DOI] [PubMed] [Google Scholar]
- 15.Brown BM, Peiffer JJ, Sohrabi HR, Mondal A, Gupta VB, Rainey-Smith SR, Taddei K, Burnham S, Ellis KA, Szoeke C, Masters CL. Intense physical activity is associated with cognitive performance in the elderly. Transl Psychiatry. 2012;2(11):e191–e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nocera JR, McGregor KM, Hass CJ, Crosson B. Spin exercise improves semantic fluency in previously sedentary older adults. J Aging Phys Act. 2015;23(1):90–94. [DOI] [PubMed] [Google Scholar]
- 17.Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Plymate SR, Fishel MA, Watson GS, Cholerton BA, Duncan GE. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67(1):71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alfini AJ, Weiss LR, Nielson KA, Verber MD, Smith JC. Resting cerebral blood flow after exercise training in mild cognitive impairment. J Alzheimers Dis. 2019;67(2):671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci. 2006;103(37):13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voss MW, Erickson KI, Prakash RS, Chaddock L, Malkowski E, Alves H, Kim JS, Morris KS, White SM, Wójcicki TR, Hu L. Functional connectivity: a source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia. 2010;48(5):1394–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, Alves H, Heo S, Szabo A, White SM, Wójcicki TR. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci. 2010;2:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(5):2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex. 2009;19(10):2485–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoodley CJ. The cerebellum and cognition: evidence from functional imaging studies. The Cerebellum. 2012;11(2):352–365. [DOI] [PubMed] [Google Scholar]
- 26.Buckner RL. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 2013;80(3):807–815. [DOI] [PubMed] [Google Scholar]
- 27.Keren-Happuch E, Chen S-HA, Ho M-HR, Desmond JE. A meta-analysis of cerebellar contributions to higher cognition from PET and fMRI studies. Hum Brain Mapp. 2014;35(2):593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richter S, Gerwig M, Aslan B, Wilhelm H, Schoch B, Dimitrova A, Gizewski ER, Ziegler W, Karnath HO, Timmann D. Cognitive functions in patients with MR-defined chronic focal cerebellar lesions. J Neurol. 2007;254(9):1193–1203. [DOI] [PubMed] [Google Scholar]
- 29.Rodríguez-Aranda C, Johnsen SH, Eldevik P, Sparr S, Wikran GC, Herder M, Vangberg TR. Neuroanatomical correlates of verbal fluency in early Alzheimer’s disease and normal aging. Brain Lang. 2016;155:24–35. [DOI] [PubMed] [Google Scholar]
- 30.Weiss EM, Siedentopf C, Hofer A, Deisenhammer EA, Hoptman MJ, Kremser C, Golaszewski S, Felber S, Fleischhacker WW, Delazer M. Brain activation pattern during a verbal fluency test in healthy male and female volunteers: a functional magnetic resonance imaging study. Neurosci Lett. 2003;352(3):191–194. [DOI] [PubMed] [Google Scholar]
- 31.Miró-Padilla A, Bueichekú E, Ventura-Campos N, Palomar-García M-Á, Ávila C. Functional connectivity in resting state as a phonemic fluency ability measure. Neuropsychologia. 2017;97:98–103. [DOI] [PubMed] [Google Scholar]
- 32.Angelucci F, De Bartolo P, Gelfo F, Foti F, Cutuli D, Bossù P, Caltagirone C, Petrosini L. Increased concentrations of nerve growth factor and brain-derived neurotrophic factor in the rat cerebellum after exposure to environmental enrichment. The Cerebellum. 2009;8(4):499–506. [DOI] [PubMed] [Google Scholar]
- 33.Vazquez-Sanroman D, Sanchis-Segura C, Toledo R, Hernández ME, Manzo J, Miquel M. The effects of enriched environment on BDNF expression in the mouse cerebellum depending on the length of exposure. Behav Brain Res. 2013;243:118–128. [DOI] [PubMed] [Google Scholar]
- 34.Ben-Soussan TD, Berkovich-Ohana A, Piervincenzi C, Glicksohn J, Carducci F. Embodied cognitive flexibility and neuroplasticity following Quadrato Motor Training. Front Psychol. 2015;6:1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith JC, Nielson KA, Antuono P, Lyons JA, Hanson RJ, Butts AM, Hantke NC, Verber MD. Semantic memory functional MRI and cognitive function after exercise intervention in mild cognitive impairment. J Alzheimers Dis. 2013;37(1):197–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. [DOI] [PubMed] [Google Scholar]
- 37.Yesavage JA. Geriatric depression scale. Psychopharmacol Bull. 1988;24(4):709–711. [PubMed] [Google Scholar]
- 38.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. The gerontologist. 1969;9(3_Part_1):179–186. [PubMed] [Google Scholar]
- 39.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruff RM, Light RH, Parker SB, Levin HS. Benton controlled oral word association test: Reliability and updated norms. Arch Clin Neuropsychol. 1996;11(4):329–338. [PubMed] [Google Scholar]
- 41.Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol. 1999;14(2):167–177. [PubMed] [Google Scholar]
- 42.Brysbaert M, New B. Moving beyond Kučera and Francis: A critical evaluation of current word frequency norms and the introduction of a new and improved word frequency measure for American English. Behav Res Methods. 2009;41(4):977–990. [DOI] [PubMed] [Google Scholar]
- 43.Bird H, Franklin S, Howard D. Age of acquisition and imageability ratings for a large set of words, including verbs and function words. Behav Res Methods Instrum Comput. 2001;33(1):73–79. [DOI] [PubMed] [Google Scholar]
- 44.Borg G Borg’s perceived exertion and pain scales. Human kinetics; 1998. [Google Scholar]
- 45.Fischl B FreeSurfer. Neuroimage. 2012;62(2):774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cox RW. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Comput Biomed Res. 1996. Jun 1;29(3):162–73. [DOI] [PubMed] [Google Scholar]
- 47.Beall EB, Lowe MJ. SimPACE: generating simulated motion corrupted BOLD data with synthetic-navigated acquisition for the development and evaluation of SLOMOCO: a new, highly effective slicewise motion correction. Neuroimage. 2014;101:21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44(2):489–501. [DOI] [PubMed] [Google Scholar]
- 49.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8(8):665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sutter C, Zöllig J, Martin M. Plasticity of verbal fluency in older adults: A 90-minute telephone-based intervention. Gerontology. 2013;59(1):53–63. [DOI] [PubMed] [Google Scholar]
- 51.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. [DOI] [PubMed] [Google Scholar]
- 52.Alfini AJ, Weiss LR, Leitner BP, Smith TJ, Hagberg JM, Smith JC. Hippocampal and cerebral blood flow after exercise cessation in master athletes. Front Aging Neurosci. 2016;8:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Juhasz BJ, Chambers D, Shesler LW, Haber A, Kurtz MM. Evaluating lexical characteristics of verbal fluency output in schizophrenia. Psychiatry Res. 2012;200(2–3):177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A. Ageing, fitness and neurocognitive function. Nature. 1999;400(6743):418–419. [DOI] [PubMed] [Google Scholar]
- 55.Leggio MG, Silveri MC, Petrosini L, Molinari M. Phonological grouping is specifically affected in cerebellar patients: a verbal fluency study. J Neurol Neurosurg Psychiatry. 2000;69(1):102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Molinari M, Chiricozzi FR, Clausi S, Tedesco AM, De Lisa M, Leggio MG. Cerebellum and detection of sequences, from perception to cognition. The Cerebellum. 2008;7(4):611–615. [DOI] [PubMed] [Google Scholar]
- 57.Ramnani N Frontal lobe and posterior parietal contributions to the cortico-cerebellar system. The Cerebellum. 2012;11(2):366–383. [DOI] [PubMed] [Google Scholar]
- 58.Alalade E, Denny K, Potter G, Steffens D, Wang L. Altered cerebellar-cerebral functional connectivity in geriatric depression. PloS One. 2011;6(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmahmann JD. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp. 1996;4(3):174–198. [DOI] [PubMed] [Google Scholar]
- 60.Leiner HC, Leiner AL, Dow RS. The human cerebro-cerebellar system: its computing, cognitive, and language skills. Behav Brain Res. 1991;44(2):113–128. [DOI] [PubMed] [Google Scholar]
- 61.Menon V Large-scale brain networks in cognition: Emerging principles. Anal Funct Large-Scale Brain Netw. 2010;14:43–54. [DOI] [PubMed] [Google Scholar]
- 62.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. 2008; [DOI] [PubMed] [Google Scholar]
- 63.Gourovitch ML, Kirkby BS, Goldberg TE, Weinberger DR, Gold JM, Esposito G, Van Horn JD, Berman KF. A comparison of rCBF patterns during letter and semantic fluency. Neuropsychology. 2000;14(3):353. [DOI] [PubMed] [Google Scholar]
- 64.Yin S, Zhu X, He R, Li R, Li J. Spontaneous activity in the precuneus predicts individual differences in verbal fluency in cognitively normal elderly. Neuropsychology. 2015;29(6):961. [DOI] [PubMed] [Google Scholar]
- 65.Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61(11):1166–1170. [DOI] [PubMed] [Google Scholar]
- 66.Won J, Alfini AJ, Weiss LR, Callow DD, Smith JC. Brain activation during executive control after acute exercise in older adults. Int J Psychophysiol. 2019;146:240–248. [DOI] [PubMed] [Google Scholar]
- 67.Luo L, Luk G, Bialystok E. Effect of language proficiency and executive control on verbal fluency performance in bilinguals. Cognition. 2010;114(1):29–41. [DOI] [PubMed] [Google Scholar]
- 68.Chirles TJ, Reiter K, Weiss LR, Alfini AJ, Nielson KA, Smith JC. Exercise training and functional connectivity changes in mild cognitive impairment and healthy elders. J Alzheimers Dis. 2017;57(3):845–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reiter K, Nielson KA, Smith TJ, Weiss LR, Alfini AJ, Smith JC. Improved cardiorespiratory fitness is associated with increased cortical thickness in mild cognitive impairment. J Int Neuropsychol Soc. 2015;21(10):757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qi Z, An Y, Zhang M, Li H-J, Lu J. Altered cerebro-cerebellar limbic network in AD spectrum: a resting-state fMRI study. Front Neural Circuits. 2019;13:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nocera J, Crosson B, Mammino K, McGregor KM. Changes in cortical activation patterns in language areas following an aerobic exercise intervention in older adults. Neural Plast. 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zlatar ZZ, Towler S, McGregor KM, Dzierzewski JM, Bauer A, Phan S, Cohen M, Marsiske M, Manini TM, Crosson B. Functional language networks in sedentary and physically active older adults. J Int Neuropsychol Soc. 2013;19(6):625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reuter-Lorenz PA, Park DC. How does it STAC up? Revisiting the scaffolding theory of aging and cognition. Neuropsychol Rev. 2014;24(3):355–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hyodo K, Dan I, Suwabe K, Kyutoku Y, Yamada Y, Akahori M, Byun K, Kato M, Soya H. Acute moderate exercise enhances compensatory brain activation in older adults. Neurobiol Aging. 2012;33(11):2621–2632. [DOI] [PubMed] [Google Scholar]
- 75.Meeusen R, Smolders I, Sarre S, De Meirleir K, Keizer H, Serneels M, Ebinger G, Michotte Y. Endurance training effects on neurotransmitter release in rat striatum: an in vivo microdialysis study. Acta Physiol Scand. 1997;159(4):335–341. [DOI] [PubMed] [Google Scholar]
- 76.Isaacs KR, Anderson BJ, Alcantara AA, Black JE, Greenough WT. Exercise and the brain: angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. J Cereb Blood Flow Metab. 1992;12(1):110–119. [DOI] [PubMed] [Google Scholar]
- 77.Carro E, Nuñez A, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci. 2000;20(8):2926–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Won J, Alfini AJ, Weiss LR, Michelson CS, Callow DD, Ranadive SM, Gentili RJ, Smith JC. Semantic memory activation after acute exercise in healthy older adults. J Int Neuropsychol Soc. 2019;25(6):557–568. [DOI] [PubMed] [Google Scholar]
- 79.Won J, Alfini AJ, Weiss LR, Hagberg JM, Smith JC. Greater Semantic Memory Activation After Exercise Training Cessation in Older Endurance-Trained Athletes. J Aging Phys Act. 2020;1(aop):1–9. [DOI] [PubMed] [Google Scholar]
- 80.Woo C-W, Krishnan A, Wager TD. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage. 2014;91:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Devanand DP, Liu X, Brown PJ, Huey ED, Stern Y, Pelton GH. A two-study comparison of clinical and MRI markers of transition from mild cognitive impairment to Alzheimer’s disease. Int J Alzheimer’s Dis. 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci. 2011;108(7):3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci. 2010;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]


