Abstract
BACKGROUND:
Despite chronic therapies, atrial fibrillation (AF) leads to rapid ventricular rates (RVR) often requiring intravenous treatments. Etripamil is a fast-acting, calcium-channel blocker administered intranasally affecting the atrioventricular node within minutes.
METHODS:
Reduction of Ventricular Rate in Patients with Atrial Fibrillation evaluated the efficacy and safety of etripamil for the reduction of ventricular rate (VR) in patients presenting urgently with AF-RVR (VR ≥110 beats per minute [bpm]), was randomized, double-blind, placebo-controlled, and conducted in Canada and the Netherlands. Patients presenting urgently with AF-RVR were randomized (1:1, etripamil nasal spray 70 mg: placebo nasal spray). The primary objective was to demonstrate the effectiveness of etripamil in reducing VR in AF-RVR within 60 minutes of treatment. Secondary objectives assessed achievement of VR <100 bpm, reduction by ≥10% and ≥20%, relief of symptoms and treatment effectiveness; adverse events; and additional measures to 360 minutes.
RESULTS:
Sixty-nine patients were randomized, 56 dosed with etripamil (n=27) or placebo (n=29). The median age was 65 years; 39% were female patients; proportions of AF types were similar between groups. The difference of mean maximum reductions in VR over 60 minutes, etripamil versus placebo, adjusting for baseline VR, was −29.91 bpm (95% CI, −40.31 to −19.52; P<0.0001). VR reductions persisted up to 150 minutes. Significantly greater proportions of patients receiving etripamil achieved VR reductions <100 bpm (with longer median duration <100 bpm), or VR reduction by ≥10% or ≥20%, versus placebo. VR reduction ≥20% occurred in 66.7% of patients in the etripamil arm and no patients in placebo. Using the Treatment Satisfaction Questionnaire for Medication-9, there was significant improvement in satisfaction on symptom relief and treatment effectiveness with etripamil versus placebo. Serious adverse events were rare; 1 patient in the etripamil arm experienced transient severe bradycardia and syncope, assessed as due to hypervagotonia.
CONCLUSIONS:
Intranasal etripamil 70 mg reduced VR and improved symptom relief and treatment satisfaction. These data support further development of self-administered etripamil for the treatment of AF-RVR.
REGISTRATION:
URL: https://www.clinicaltrials.gov; Unique Identifier: NCT04467905
Keywords: atrial fibrillation, ECG, emergency department, etripamil, heart rate, nasal sprays
WHAT IS KNOWN?
Patients with atrial fibrillation and rapid ventricular rate currently have few options available for immediate treatment and frequently present to an emergency department where treatment consists of intravenous β-blocker, intravenous calcium channel blocker, or electrical cardioversion.
Quickly controlling rapid ventricular rate could have utility to alleviate symptoms early and as a precursor to other treatments.
WHAT THE STUDY ADDS
ReVeRA ([Reduction of Ventricular Rate in Patients With Atrial Fibrillation] multicenter, randomized, double-blind, placebo-controlled study) evaluated the efficacy and safety of etripamil nasal spray 70 mg in patients presenting to an emergency department with atrial fibrillation and rapid ventricular rate to assess for acute reduction in ventricular rate and measures of symptoms.
Etripamil, a novel, fast-acting calcium channel blocker, demonstrated significant reductions in ventricular rate versus placebo, persisting for at least 60 minutes and up to 150 minutes, in patients with symptomatic atrial fibrillation and rapid ventricular rate.
Patients treated with etripamil showed significant improvement in satisfaction on relief of symptoms and satisfaction of effectiveness of treatment, versus placebo, with a low occurrence of adverse events.
Atrial fibrillation (AF) is the most common arrhythmia that affects over 6 million patients in the United States1 and over 37 million worldwide.2 More than 12 million in the United States may be affected by 2030.3
Patients with AF frequently experience episodes of rapid ventricular rate (RVR) associated with burdensome symptoms, including palpitations, shortness of breath, chest pain, fatigue, and anxiety, which often require medical intervention.4 Patients with AF-RVR currently have few options available for immediate treatment and frequently present to an emergency department where treatment consists of intravenous β-blocker, IV calcium channel blocker (CCB), or electrical cardioversion.5,6 There is a need for a self-administered medication that can rapidly reduce symptomatically elevated heart rates, both to quickly alleviate problematic symptoms and to precede longer–term treatment options.
Guidelines from the American College of Cardiology/American Heart Association/Heart Rhythm Society and the European Society of Cardiology recommend, for the acute management of AF, reducing the heart rate using IV medications, thereby emphasizing the importance of rapid treatment.5,7 Chronic therapy for prevention can be associated with ineffective rate control with breakthrough RVRs. When acutely administered for episodes of RVR, oral therapies (ie, pill-in-pocket β-blocker or L-type CCB) do not provide immediate rate control due to a delayed onset of action and have associated adverse events (AEs).8–10
Etripamil nasal spray (NS) is a fast-acting, self-administered CCB and new chemical entity that prolongs refractoriness and conduction velocity through the atrioventricular node.11 The drug is rapidly absorbed by the nasal mucosa, with a maximum concentration reached within 7 minutes after 70 mg dose. The efficacy, safety, and tolerability of the self-administered drug, have been studied in patients with paroxysmal supraventricular tachycardia (PSVT), demonstrating significant and rapid termination of PSVT with preceding slowing of the tachycardia rate and reduced emergency department care.12–14 Preclinical and clinical data show that etripamil results in slower rates of tachycardias conducted utilizing the atrioventricular node soon after intranasal administration, illustrating the drug’s action.12 Furthermore, self-administered etripamil has been observed to slow the ventricular rate (VR) in a small cohort of patients with symptomatic AF-RVR.15 Clinical data show general tolerability and limited AEs in >1600 patients with PSVT, supporting the likely safety of investigating etripamil in patients with AF-RVR.12–14
The ReVeRA (Reduction of Ventricular Rate in Patients with Atrial Fibrillation) phase 2 trial was designed to assess the efficacy and safety of etripamil NS administered by medical staff to patients presenting to an emergency department with AF-RVR.
Methods
Due to the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to datasharing@milestonepharma.com.
Study Overview and Eligibility
ReVeRA was a randomized, double-blind, placebo-controlled study conducted in 23 sites in Canada and the Netherlands from November 19, 2020 (first enrollment) to September 12, 2023 (database lock). Institutional review boards at participating sites approved the protocol, and all participants provided written informed consent before participation.
Patients ≥18 years of age were screened after urgently presenting with paroxysmal, persistent, or permanent AF and a VR of ≥110 beats per minute (bpm). Key exclusion criteria were evident atrial flutter; history of stroke, transient ischemic attack, or peripheral embolism within 3 months; receipt of IV flecainide, procainamide, digoxin, β-blocker, or CCB, within 1 hour of administering the study drug; signs of severe heart failure or hypotension (systolic blood pressure <90 mm Hg or diastolic blood pressure <60 mm Hg); or history of second- or third-degree atrioventricular block. Complete eligibility criteria are in Supplemental Methods SI.
Study Design
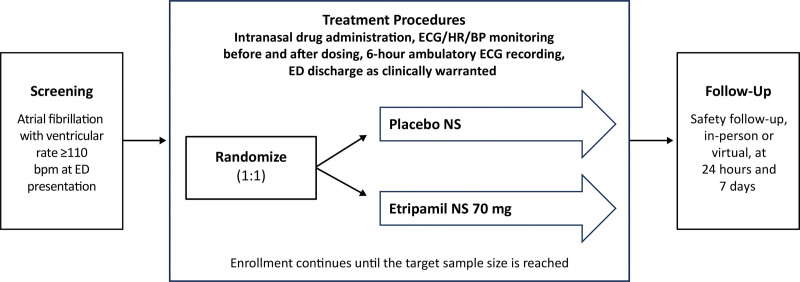
AF with a VR ≥110 bpm (assessed by continuous and 12-lead ECG) and blood pressure criteria were confirmed before study drug treatment (etripamil 70 mg NS: placebo NS, 1:1 allocation; Figure 1). The study drug was administered by medical staff, 1 spray in each nostril, each having half of the relevant dose, with the patient in a seated position (NS Bidose system). Placebo NS study drug contained carrier matched to that of etripamil NS and was administered intranasally. Monitoring in the emergency department occurred for at least 1 hour following the study drug, and an ECG cardiac monitoring system (M12A Ambulatory Holter by Global Instrumentation; 3 leads) was placed for a total of 6 hours of ECG acquisition post-drug, allowing for measurement of cardiac rhythm and rate, duration of any treatment effects, and safety findings. Patients completed the Treatment Satisfaction Questionnaire for Medication-9 (TSQM-9).16 The TSQM-9 is a psychometrically robust and validated patient-reported outcome tool used to assess patient satisfaction in domains of effectiveness, global satisfaction, and convenience. Each domain contains 3 questions, each answered on a 7-point anchored scale. Domain score is expressed on 0 to 100 point scale.16–18 Further TSQM-9 details are in Supplemental Methods S2. Additional rate-control treatment could be given if needed, but not until 1 hour after the study drug. AEs were recorded, and the patient could be discharged with the ECG cardiac monitoring system, if clinically appropriate, after 1 hour. Safety follow-up was performed at 24 hours (in person) and 1 week (in person or virtual) post-dose and included recording of any AEs or medications taken since study drug.
Figure 1.
Study design. ReVeRA (Reduction of Ventricular Rate in Patients with Atrial Fibrillation) phase 2, multicenter, randomized, double-blind, placebo-controlled study for evaluating the efficacy and safety of etripamil nasal spray (NS) administered intranasally to patients presenting to an emergency department (ED) with atrial fibrillation-rapid ventricular rate (AF-RVR). BP indicates blood pressure; bpm, beats per minute; HR, heart rate.
Assessment of Outcomes
The primary end point, the maximum reduction in VR from baseline over the 60 minutes after drug administration, utilized ECG cardiac monitoring system measurements in the efficacy population (patients confirmed to be in AF at the time of study drug administration and for 60 minutes post-drug). Baseline VR was defined as the average heart rate over the 5 minutes immediately before drug administration, and nadir was defined as the lowest heart rate (5-minute moving average) recorded during the 60-minute evaluation period. Secondary end points of the study, measured within 60 minutes of dosing, were elapsed time from drug administration to nadir heart rate (lowest 5-minute moving); percentage of patients achieving VR of <100 bpm; duration of reduction of VR to <100 bpm; percentage of patients with ≥10% and ≥20% reduction in VR; and patient satisfaction using the TSQM-9. Sensitivity analyses were performed on the primary and secondary end points, including using differing time windows of moving average of VR. Additional end points are described in the Supplemental Methods SIII. Safety end points included AEs, vital signs, and potential arrhythmia findings from ECG data.
Statistical Methods
It was estimated that 25 patients per group would provide 93% power at a 0.05 two-sided significance level to detect 20 bpm absolute difference in maximum reduction in VR from baseline between active drug and placebo arms, assuming an SD of 20 bpm. As prespecified in the protocol and statistical analysis plan, primary, and secondary analyses were performed on the efficacy population (defined as all randomized patients receiving the study drug and remaining in AF with adequate diagnostic ECG data for at least 60 minutes). An intention-to-treat population was not in the analysis plans for this study; it was anticipated that during the period of time immediately following randomization but before dosing, some patients would no longer remain eligible to receive study drug (eg, if no longer meeting specified criteria of being in AF or having a VR ≥110 bpm) and thus were excluded. The modified intention-to-treat (mITT) population (defined as randomized patients receiving study drug and with postbaseline ECG data) was analyzed, in prespecified sensitivity analyses, for efficacy end points, using statistical approaches identical to those used for primary and secondary analyses on the efficacy population. Safety assessments were performed on the safety population (defined as all patients receiving study drug).
The primary analysis was an ANCOVA model on the maximum reduction in VR from baseline, adjusting for the VR at baseline, performed using the efficacy population. This model allows for the comparison of adjusted means that are corrected for any between-group differences in baseline VR. These adjusted means are presented with 95% CI. Group comparisons for the secondary end points, elapsed time from drug administration to nadir, duration of VR <100 bpm over the 60 minutes following drug administration, and duration of ≥10% and ≥20% reduction in VR from baseline during that 60-minute period, were also performed using an ANCOVA. Percentages of patients achieving VR <100 bpm and percentages of patients achieving ≥10% and ≥20% reductions in VR from baseline were compared across groups using a χ2 test. The Kaplan-Meier method and the Wilcoxon test for censored data were used to compare the 2 groups for duration of time of VR <100 bpm or of VR reduced by ≥10% and ≥20%. Patient satisfaction with treatment measured using the TSQM-9 was analyzed using a t test. Interim analysis was performed to inform planning of next studies in the program, as stated in the Statistical Analysis Plan, with no alteration or termination of the study based on the interim review and no communication of results beyond the principal authors. All statistical tests were 2 sided and performed at a significance level of 0.05. Statistical analyses were conducted using SAS (version 9.4).
Results
Patients
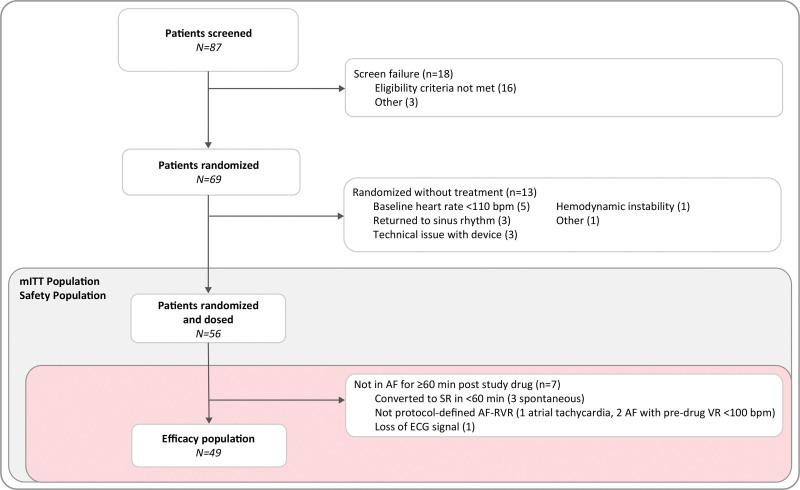
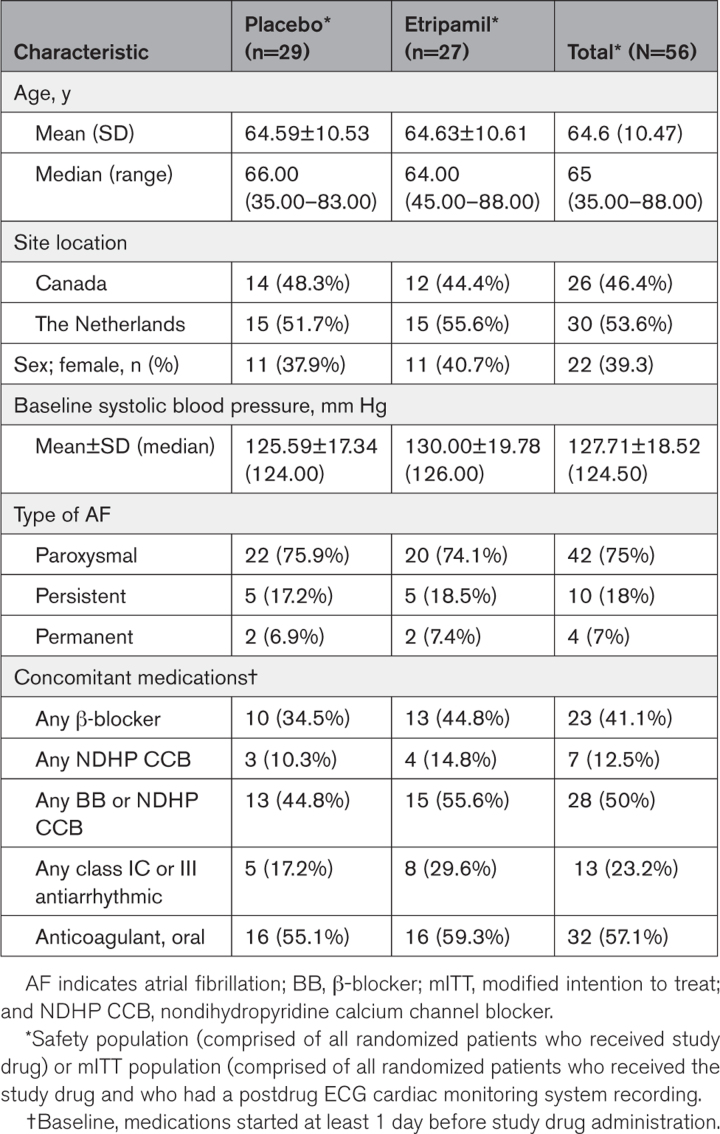
A total of 87 patients were screened, 69 were randomized, and 56 received study drug. No patients were lost to follow-up. Thirteen randomized patients were not included in the mITT population as they were no longer eligible to receive study drug (per protocol) because baseline heart rate was <110 bpm (n=5); conversion to sinus rhythm (n=3); hemodynamic instability based on specified definition (n=1); technical issues with the ECG cardiac monitoring device (n=3); and other (site misinterpretation of the protocol, n=1; Figure 2). The reasons to exclude these patients from the mITT population were independent of the treatment assigned, which was blinded at the time of the decision. Of the 56 patients comprising the mITT population, 27 received etripamil and 29 received placebo. The efficacy (n=49), mITT (n=56), and safety (n=56) populations are shown in Figure 2. Baseline characteristics were generally balanced between the treatment groups. The median age was 64 years (range, 45–88) in the etripamil and 66 years (range, 35–83) in the placebo arms 39% of participants were female patients and 75%, 18%, and 7% of participants had a history of paroxysmal, persistent, and permanent AF, respectively. There was minimal imbalance in the preenrollment use of oral β-blockers and antiarrhythmic drugs between etripamil and placebo arms (Table 1).
Figure 2.
Patient disposition. The safety population is comprised of all randomized patients receiving study drug. The modified intention-to-treat (mITT) population is comprised of all randomized patients who received the study drug and who had a postdrug ECG cardiac monitoring system recording. The efficacy population is comprised of all mITT patients (randomized patients receiving study drug) who remained in atrial fibrillation with adequately diagnostic ECG recordings for at least 60-minute postdrug. One patient had 2 reasons for screen failure. AF indicates atrial fibrillation; AF-RVR, atrial fibrillation-rapid ventricular rate; SR, sinus rhythm; and VR, ventricular rate.
Table 1.
Demographics and Baseline Characteristics
Efficacy Outcomes
Primary End Point
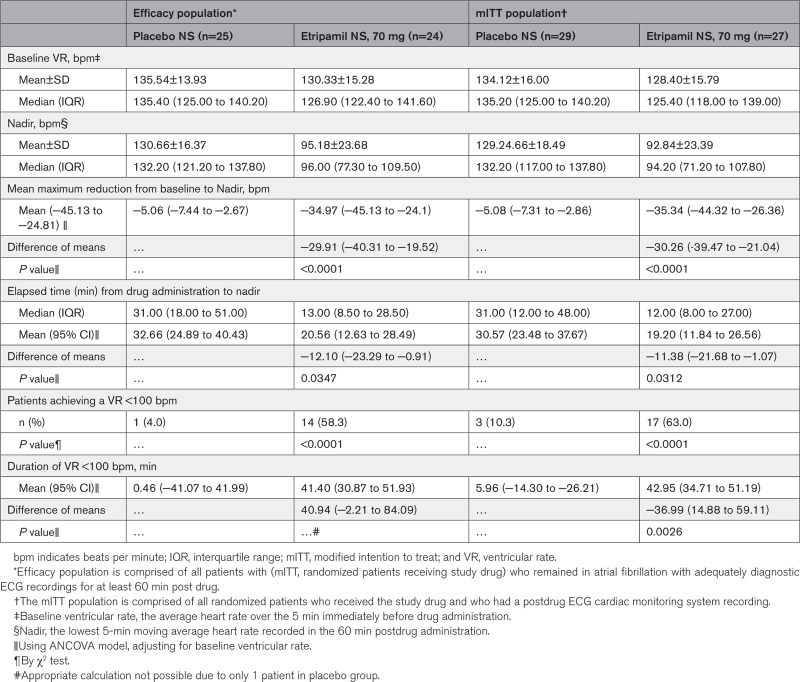
The primary efficacy analysis showed that the adjusted means (95% CI) of maximum reduction from baseline in VR were −34.97 (−45.13 to −24.81) bpm in the etripamil arm and −5.06 (−7.44 to −2.67) bpm in the placebo arm, for a difference of −29.91 (−40.31 to −19.52) bpm (P<0.0001; Table 2). A prespecified sensitivity analysis of the primary end point, performed in the mITT population, demonstrated consistency with a difference in adjusted means of maximum reduction in VR of −30.26 (−39.47 to −21.04; P<0.0001; Table 2).
Table 2.
Primary and Key Secondary Analyses Performed on the Efficacy Population, Sensitivity Analyses Performed on the mITT Population
Secondary End Points
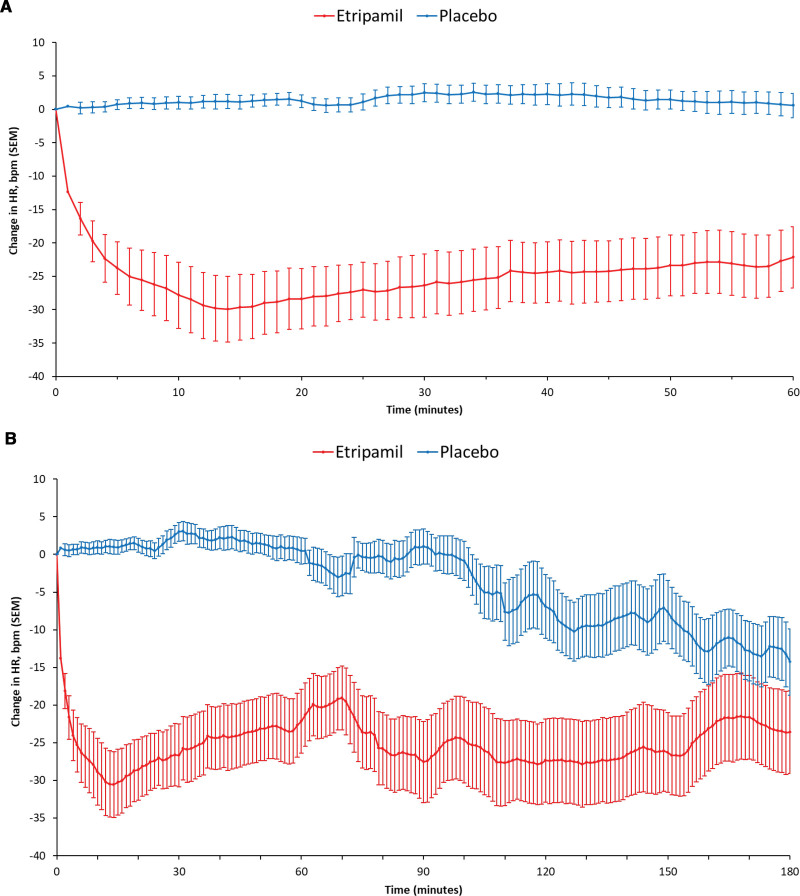
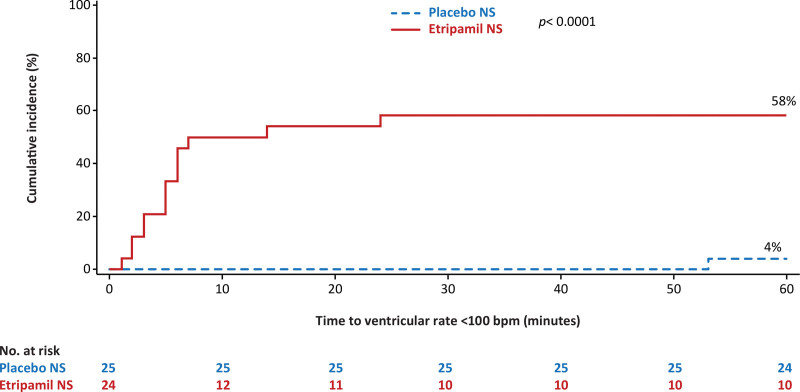
The median (interquartile range [IQR]) elapsed time from drug dosing to the nadir in VR was 13 (8.50–28.50) minutes for the etripamil arm and 31 (18.00–51.00) minutes for placebo. The adjusted mean (95% CI) for elapsed time from etripamil dosing to nadir in VR was 20.6 (12.6–28.5) minutes for the etripamil arm compared with 32.7 (24.9–40.4) minutes for placebo (P=0.0347; Table 2). The greater reduction in VR from baseline in the etripamil versus placebo arms, shown in the 6-hour collection of ECG data, persists for up to 150 minutes (Figure 3B). More patients in the etripamil versus placebo arm achieved a VR reduction of >20 bpm by 60 (62.5% versus 0%; P<0.0001) minutes by 90 (54.2% versus 8.0%; P=0.0005) minutes, and by 180 (50.0% versus 32.0%; P=0.20) minutes; ECG artifact precluded analysis at 360 minutes (Table S1). The proportion of patients achieving a VR of <100 bpm during the first 60-minute postdrug administration was higher for patients receiving etripamil (58.3% [14/24]) than for those receiving placebo (4.0% [1/25]); P<0.0001 by the χ2 test; and persisted for at least 60 minutes. Elapsed time from study drug administration to VR <100 bpm, illustrated in Figure 4, shows the majority of the patients in the etripamil arm achieving a VR <100 bpm did so within 10 minutes. The median duration of maintaining a VR <100 bpm, among patients achieving a VR <100 bpm during the first 60 minutes post-drug, was 45.50 (IQR, 24.00–56.00) minutes in the etripamil arm versus 7.00 minutes (IQR not applicable) in the placebo arm (Figure 4; Table S2). A reduction in VR by ≥20% from baseline VR occurred in 66.7% of patients in the etripamil arm by 60 minutes compared with no patients in the placebo arm; the median duration of achieving a ≥20% reduction in VR by 60 minutes was 48.00 (14.50–57.50) minutes in the etripamil arm (placebo arm, not applicable). A reduction in VR by ≥10% from baseline VR occurred in 95.8% of patients in the etripamil arm by 60 minutes compared with 20% of those in the placebo arm; the median duration (IQR) of achieving a ≥10% reduction in VR by 60 minutes was 49.00 (30.00–57.00) minutes in the etripamil arm and 5.00 (2.00–6.00) minutes in the placebo arm. Sensitivity analyses of proportions of patients achieving specified levels of VR reduction performed on the mITT population demonstrated results consistent with those shown in the efficacy population (Tables S2 and S3). Sensitivity analyses of the primary end point, performed with differing time windows of VR moving average demonstrated results consistent with those obtained from a 5-minute moving average (Table S4).
Figure 3.
Mean Change (± SEM) from Baseline in Ventricular Rate (bpm) over 60 minutes and 180 minutes. A, Mean change (±SEM) from baseline in ventricular rate (beats per minute [bpm]) over 60 minutes. B, Mean change (±SEM) from baseline in ventricular rate (bpm) over 180 minutes. Primary end point was mean maximum reduction in ventricular rate from adjusted baseline by ANCOVA; yielding P<0.0001 for difference in mean maximum reductions, placebo vs etripamil. Analysis performed separately from plot in A. *Separation of the placebo vs etripamil groups over the 180 minutes after drug administration was assessed based on a t test of difference between the areas under the curves (AUC0→180) of plots of absolute mean heart rate, yielding P<0.00001.
Figure 4.
Time from drug administration to achieving ventricular rate (VR) <100 beats per minute (bpm). The cumulative incidence of achieving VR <100 bpm represents the proportions of patients achieving a VR <100 bpm by 60 minutes; 58.3% in the etripamil arm vs 4.0% in the placebo arm (P<0.0001). *By χ2 test. NS indicates nasal spray.
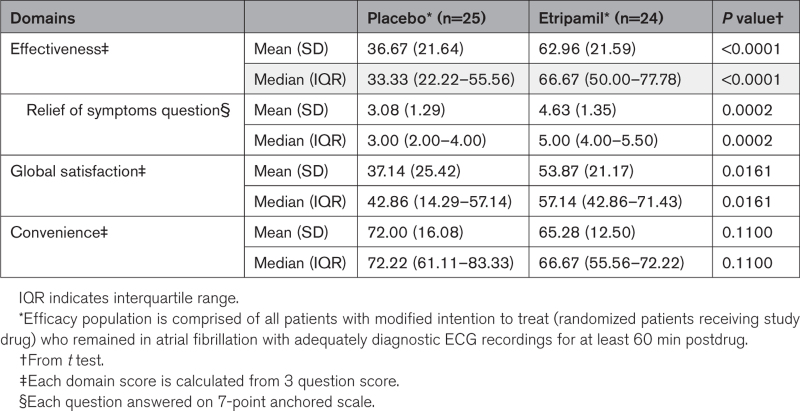
Patients in the etripamil arm showed significant improvement in satisfaction of effectiveness of treatment and satisfaction on relief of symptoms versus those on placebo, as measured by the TSQM-9 Effectiveness Domain and its Relief of Symptoms Question, respectively. Details on TSQM-9 calculations including verbatim questions are in Supplemental Methods S2. Measurements of satisfaction of effectiveness of treatment were, on the Effectiveness Domain 0-to-100 scale score, mean±SD of 62.96±21.59 for patients in the etripamil arm, 36.67±21.64 for those in the placebo arm, with a difference in means (95% CI) of 26.30 (13.87–38.72; P<0.0001, by t test); median (IQR) of 66.67 (50.0–77.78) in patients receiving etripamil and 33.33 (22.22–55.56) in those receiving placebo. Measurements of the Relief of Symptoms Question were, on its 7-point anchored scale, a mean±SD of4.63±1.35 for patients in the etripamil arm, 3.08±1.29 for those in the placebo arm, with difference in means (95% CI) of 1.55 (0.79–2.30; P=0.0002, by t test); and with a median (IQR) of 5.0 (4.0–5.5) in the etripamil arm and 3.0 (2.0–4.0) in the placebo arm (Table 3). All sensitivity analyses performed for end points of symptomatic relief on the mITT population demonstrated results consistent with those shown in the efficacy population (Table S5).
Table 3.
Summary of Patient Satisfaction With Treatment Measured by Treatment Satisfaction Questionnaire for Medication-9
Analyses of areas under the curve (AUC) were performed for plots of VR reduction from baseline (and absolute VR) versus time, for 0 to 60, 180, and 360 minutes. Significant differences between the AUCs of the placebo- and etripamil plots were shown for 0 to 60 minutes, P<0.0001 (by ANCOVA model for AUCs of VR reduction from baseline); 0 to 180 minutes, P<0.00001 (by t test of AUCs of mean VR); and 0 to 360 minutes, P=0.0015 (by the ANCOVA model). Sensitivity analyses performed on the mITT population demonstrated AUC differences consistent with those shown in the efficacy population (Table S3; Figure 3).
Additional treatment with rate control and antiarrhythmic drugs was allowed to be administered 60 minutes after study drug (and only within 60 minutes if deemed necessary). No patients received additional medication within the first 60 minutes. Within 24 hours of study drug (but >60 minutes), fewer patients in the etripamil arm (22.2%) were given treatment with a nondihydropyridine CCB or β-blocker than patients in the placebo arm (51.7%), either by the IV or oral route. Digoxin was given to fewer patients in the etripamil arm (11.1%) than in the placebo arm (20.7%) >60 minutes and ≤24 hours after study drug. Use of class IC or class III agents was similar between the groups during >60 minutes and ≤24 hours after study drug (29.6% of etripamil arm, 27.6% of placebo arm; Table S6).
Safety Outcomes
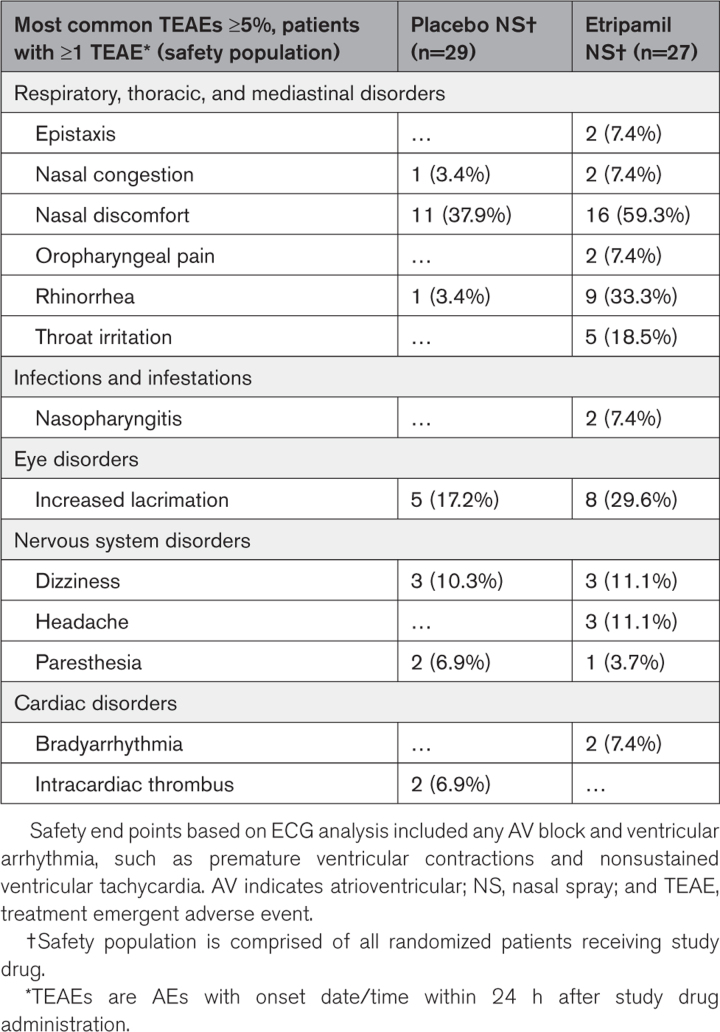
In the safety population, the most common (≥5%) AEs were nasal discomfort, nasal burning, rhinorrhea, and dizziness and were mild or moderate in intensity (Table 4). There was no increase in epistaxis in patients taking oral anticoagulation. Treatment-emergent serious AEs were rare, occurring in 1 patient (3.7%) in the etripamil arm and 2 patients (6.9%) in the placebo arm. The treatment-emergent serious AEs in the etripamil arm (transient severe bradycardia and syncope, assessed as due to hypervagotonia) occurred in a patient with a history of vagal events and fully resolved when placing the patient supine and was without sequelae. These treatment-emergent serious AEs, and those in the placebo arm, are described in Tables S7 and S8. There were no other arrhythmic (bradycardic or tachycardic) AEs or findings on ECG cardiac monitoring system, including an absence of pauses or atrioventricular block in the 4 patients converting from AF to sinus rhythm (3 spontaneously, 1 by electrical cardioversion).
Table 4.
Treatment Emergent Adverse Events
Discussion
The ReVeRA trial evaluated etripamil NS for the treatment of AF-RVR, demonstrating that the drug was effective at substantially reducing VR as shown by the difference between etripamil and placebo in maximum VR reduction (−29.91 bpm; P<0.0001), from visual inspection of time courses of VR reduction (Figure 3), and the significantly different AUCs of these plots. Responder analyses also showed substantial treatment effect: significantly greater proportions of patients achieved VR reductions of ≥10% or ≥20% from baseline with etripamil compared with placebo by 60 minutes; a VR reduction of ≥20% was not observed in the placebo arm. Acutely attaining the levels of VR responses demonstrated in ReVeRA is aligned with clinical practice goals for treatment of AF-RVR5,7 and occurred concurrently with significant relief of symptoms (the potential meaningfulness of which is discussed below).
Etripamil’s action to reduce VR in patients with AF-RVR occurred with a rapid timing, aligned with drug’s pharmacokinetics,19 a critical attribute given the burdensome symptoms of AF-RVR, the limitations of current options for prompt treatment, and the need to achieve rate control of AF before instituting longer term or rhythm-control strategies. Patients treated with etripamil in ReVeRA showed VR reduction as early as 2 minutes, with a median time to maximal reduction of 13 minutes, and with the majority of etripamil-treated patients achieving a defined responder level (eg, VR <100 bpm) within 10 minutes.
Regarding duration of effect, findings of reduction in VR, from either baseline or relative to placebo treatment, persisted for up to 150 minutes in patients with AF-RVR treated with etripamil. For patients achieving a VR <100 bpm over the first 60 minutes post drug, the median duration of maintaining a VR <100 bpm was 45.5 minutes in the etripamil arm. The duration of pharmacodynamic effects of etripamil exceed the duration of peak plasma levels of drug. This persistence of pharmacodynamic effect, or the drug having atrioventricular-nodal electrophysiological impact longer than predicted solely by pharmacokinetics, may allow for the ≈150-minute duration of VR reduction relative to placebo observed in ReVeRA. As well, early VR reduction and symptom relief may attenuate the perpetuation of AF-RVR. The observed duration of drug effect could be an important attribute to allow for rate control before orally administered therapies becoming effective.
In ReVeRA, etripamil treatment was associated with significant improvements in satisfaction of effectiveness of treatment (TSQM-9 mean domain score of 63 in the etripamil arm and a difference in means compared with the placebo arm of 26) and in relief of symptoms (difference in means of 1.55 on a 7-point scale). These values are interpreted as meaningful because: a domain score ≥50 is considered favorable (50 corresponds to a response of somewhat satisfactory); a delta between domain scores of at least 4.95 to 7.60 indicates a minimally important difference,16–18 and a difference in scores of ≥1 on a 7-point anchored scale is also considered meaningful.18,20 Although improvement in relief of symptoms was seen frequently and with significance, the absolute scores should be noted; this improvement is based on mean values (SD) in the treated group of 4.68 (1.35, corresponds between a score of 4 [somewhat satisfied] and 5 [satisfied]) versus in the placebo group of 3.08 (1.29, 3 reflecting dissatisfied). Overall, the substantial and rapid VR reductions shown in ReVeRA were associated with levels of symptom relief and satisfaction that suggest meaningful impact.
Additional medications could be administered after 60 minutes following study drug, and use was captured for up to at least 24 hours. Administration of rate-control agents (CCBs, β-blockers, and digoxin, IV or oral) during this period was lower in the etripamil arm than in the placebo arm, showing a lower need for further acute rate control in patients having received etripamil. Class IC and Class III antiarrhythmic treatment (IV or oral) was similar between the 2 groups, post-study drug, in this trial.
These efficacy results are consistent with earlier data, from a small cohort of patients with AF-RVR, showing a rapid and sustained reduction in VR following etripamil administration (NODE-303, Safety Study of Etripamil Nasal Spray for Patients With Paroxysmal Supraventricular Tachycardia https://www.clinicaltrials.gov; unique identifier: NCT04072835).15 Moreover, the currently reported findings can be viewed in the context of the efficacy demonstrated in phase 3 double-blind randomized, and open-label trials of self-administered intranasal etripamil 70 mg to rapidly terminate PSVT and to reduce tachycardia rate before termination.12,13 These efficacy data in patients with PSVT and the currently reported data in patients with AF-RVR reflect the impact of etripamil on atrioventricular-nodal conduction and properties during tachycardic rates.
The majority of common AEs in ReVeRA were localized to the drug’s administration site and mild-moderate, as has been observed with etripamil administration in patients with PSVT,12,13 and serious treatment emergent AEs were rare (occurring in 1 and 2 patients in the etripamil and placebo arms, respectively). These safety findings, taken together with the extensive data set from the etripamil program in PSVT,12,13 including intranasal administration to >1600 PSVT patients, indicate that the drug is well tolerated.
AF is associated with multiple morbidities3 and leads to >785 000 emergency department visits and hospitalizations and 158 000 deaths in the United States annually.3,21,22 Regardless of type of AF, exacerbations of the arrhythmia often require acute care for RVR and may necessitate IV medication to reduce RVR to improve cardiac output.23 AF-RVR, left untreated, increases risk of complications (eg, heart failure, tachycardia-induced cardiomyopathy). In a recent case-crossover analysis, AF-RVR that persisted for >6 hours weekly was associated with increased odds of death.24 Promptly treating the rapid VR in patients with AF-RVR is customary practice,5,7 is recommended before rhythm control strategies, and, importantly, would rapidly address AF-RVR symptoms that many patients experience.
Chronic, oral medication strategies include rate control with CCBs or β-blockers and rhythm control with class IC or class III antiarrhythmic drugs. These treatments can have limited effectiveness and AEs and when administered acutely, are associated with a delayed response. Moreover, before AF treatment with an antiarrhythmic drug (pill in the pocket) or a nonurgent cardioversion, control of RVR is critical. Thus, a drug administered to patients with AF-RVR to quickly control rapid rates would have utility to precede other treatments. Etripamil has not been associated with drug-drug interactions through the drug’s development; furthermore, concomitant treatment with cardiac medications was allowed in both the PSVT program and in the ReVeRA study. Data on etripamil NS, reported here, imply the potential for this drug, rapidly deployed, to have role in conjunction with or before other treatments, and should be an area of further investigation.
Promptly alleviating symptoms of AF-RVR is also important as many symptoms tend to perpetuate rapid rates. Furthermore, quickly implemented rate control and symptom attenuation could potentially lower the need for emergency room visits. Indeed, significantly reduced rates of emergency department care have been observed with rapid, at-home treatment of symptomatic PSVT episodes with etripamil NS.13,25 Therefore, the management of AF-RVR is important to improve marked symptoms, to reduce emergency department visits, as a potential first step before other treatments, and likely to prevent further deleterious effects. The rapidity of etripamil to reduce VR in AF-RVR shown in ReVeRA may be impactful for the intranasal drug to serve as a precursor treatment while longer–term oral therapies become effective.
Limitations
ReVeRA, performed in patients presenting to an emergency department, enrolled patients with characteristics and heart rates of AF-RVR that may be different in patients before such a presentation. In ReVeRA, intranasal drug was administered by medical staff, not patient administered. These are important considerations in generalizing this study’s findings and in designing studies of at-home administration of etripamil. In many trials, the ITT population consists of all randomized patients and is preferred for analyses. However, in ReVeRA, as described in the Methods section, due to the rapid course of the study and the need to reconfirm eligibility criteria immediately predrug administration, not all randomized patients were given study drug; this was performed per protocol, tracked, and blinded to treatment allocation so was unlikely to lead to bias. Of note, prespecified sensitivity efficacy analyses, performed in the mITT population, demonstrate results highly consistent with those from the efficacy population. Based on the study’s sizing, conclusions regarding the incidence of potential safety events may be limited; however, the extensive safety data sets from etripamil administration during PSVT attenuate this limitation.12–14
The majority of patients in this study had paroxysmal AF though those with persistent and permanent AF were enrolled; the future studies should include all 3 types. Additionally, only a single dose of etripamil NS was administered in this study. Achievement of a VR <100 bpm or a ≥20% or ≥10% reduction from baseline was observed in 58%, 67%, and 96%, respectively, of etripamil-treated patients, percentages that are substantial; however, even greater responder rates may be possible in some patients. Further investigation can assess whether increased responder rates would be observed with a repeat-dose etripamil regimen, such as has been shown in the conversion of PSVT by etripamil treatment.13 Additional end points evaluating the impact of etripamil on reducing emergency department visits and hospitalizations will be important to assess in the future investigation. Although the use of additional medication for rate and rhythm control was captured, this study did not directly examine the role of oral atrioventricular nodal blocking or antiarrhythmic agents (pill in the pocket) and thus cannot compare effectiveness.
The efficacy and safety of a repeat-dose regimen of etripamil, self-administered outside the health care setting, should be evaluated in the future studies to optimize effectiveness, as has been done in patients with PSVT.13
Conclusions
In this phase 2 randomized trial, etripamil NS 70 mg significantly reduced RVR from baseline by −29.91 bpm (placebo adjusted), with a median time to maximum reduction of 13 minutes, a median time to achieving a VR <100 bpm of 7 minutes, and with a duration of effect of up to 150 minutes. Etripamil-treated patients experienced a significant improvement in symptoms and treatment satisfaction, the drug was well-tolerated, and there was a lower use of additional acute rate-control treatment in those having received the drug. These data indicate a potential role of etripamil NS 70 mg to reduce the RVR in patients with symptomatic AF-RVR and support the future investigation of etripamil 70 mg as a self-administered treatment outside the health care setting.
ARTICLE INFORMATION
Acknowledgments
Medical writing support was provided by Two Labs (United States), which was in accordance with Good Publication Practice guidelines. Two Labs received funds from Milestone Pharmaceuticals (Montréal, Québec, Canada) to support medical writing.
Source of Funding
This study was funded by Milestone Pharmaceuticals (Montréal, Québec, Canada).
Disclosures
Dr Camm has received grants and personal fees from Boehringer Ingelheim, Bayer, Pfizer, Bristol-Myers Squibb, and Daiichi Sankyo; personal fees from Medtronic, Boston Scientific, Menarini, and Biotronik; and support from Anthos, Sanofi, Abbott, GlaxoSmithKline, and Johnson & Johnson. Dr Piccini has received grants for clinical research from Abbott, the American Heart Association, the Association for the Advancement of Medical Instrumentation, Bayer, Boston Scientific, iRhythm, and Philips and serves as a consultant to Abbott, AbbVie, ARCA biopharma, Bayer, Boston Scientific, Bristol-Myers Squibb (Myokardia), Element Science, Itamar Medical, LivaNova, Medtronic, ElectroPhysiology Frontiers, ReCor, Sanofi, Philips, and UpToDate. Dr Alings has no disclosures to report. Drs Dorian, Ip, and Stambler serve on the steering committee for Milestone Pharmaceuticals. Dr Gosselin has no disclosures to report. Drs Kowey and Mondésert are consultants for Milestone Pharmaceuticals. Drs Prins, Roux, van Eck, and Al Windy have no disclosures to report. Drs Thermil, Shardonofsky, and Bharucha are employees of Milestone Pharmaceuticals. Dr Roy has no disclosures to report.
Supplemental Material
List of Investigators
Supplemental Methods S1–S3
Tables S1–S8
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AE
- adverse event
- AF
- atrial fibrillation
- bpm
- beats per minute
- CCB
- calcium channel blocker
- mITT
- modified intention to treat
- NS
- nasal spray
- PSVT
- paroxysmal supraventricular tachycardia
- RVR
- rapid ventricular rate
- ReVeRA
- Reduction of Ventricular Rate in Patients With Atrial Fibrillation
- TSQM-9
- Treatment Satisfaction Questionnaire for Medication-9
- VR
- ventricular rate
For Sources of Funding and Disclosures, see page 649.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCEP.123.012567.
Contributor Information
Jonathan P. Piccini, Email: jonathan.piccini@duke.edu.
Marco Alings, Email: marco@alings.org.
Paul Dorian, Email: paul.dorian@unityhealth.to.
Gilbert Gosselin, Email: g.gosselin@videotron.ca.
Marie-Claude Guertin, Email: marie-claude.guertin@mhicc.org.
James E. Ip, Email: jei9008@med.cornell.edu.
Peter R. Kowey, Email: koweyp@mlhs.org.
Blandine Mondésert, Email: blandine.mondesert@icm-mhi.org.
Fransisco J. Prins, Email: fj.prins@elkerliek.nl.
Jean-Francois Roux, Email: jean-francois.roux@usherbrooke.ca.
Bruce S. Stambler, Email: bstambler1@gmail.com.
JWM van Eck, Email: m.v.eck@jbz.nl.
Nadea Al Windy, Email: n.al.windy@gelre.nl.
Nathalie Thermil, Email: nthermil@gmail.com.
Silvia Shardonofsky, Email: sshardonofsky@milestonepharma.com.
David B. Bharucha, Email: dbharucha@milestonepharma.com.
References
- 1.Turakhia MP, Guo JD, Keshishian A, Delinger R, Sun X, Ferri M, Russ C, Cato M, Yuce H, Hlavacek P. Contemporary prevalence estimates of undiagnosed and diagnosed atrial fibrillation in the United States. Clin Cardiol. 2023;46:484–493. doi: 10.1002/clc.23983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dai H, Zhang Q, Much AA, Maor E, Segev A, Beinart R, Adawi S, Lu Y, Bragazzi NL, Wi J. Global, regional, and national prevalence, incidence, mortality, and risk factors for atrial fibrillation, 1990-2017: results from the global burden of disease study 2017. Eur Heart J Qual Care Clin Outcomes. 2021;7:574–582. doi: 10.1093/ehjqcco/qcaa061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prevention CDC. Atrial fibrillation. Accessed 28 July 2023. https://www.cdc.gov/heartdisease/atrial_fibrillation.htm
- 4.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 5.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74:104–132. doi: 10.1016/j.jacc.2019.01.011 [DOI] [PubMed] [Google Scholar]
- 6.Siu CW, Lau CP, Lee WL, Lam KF, Tse HF. Intravenous diltiazem is superior to intravenous amiodarone or digoxin for achieving ventricular rate control in patients with acute uncomplicated atrial fibrillation. Crit Care Med. 2009;37:2174–2179; quiz 2180. doi: 10.1097/CCM.0b013e3181a02f56 [DOI] [PubMed] [Google Scholar]
- 7.Kirchof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Catella M, Diener HC, Heidbuchel H, Hendriks J, et al. 2016 ESC guidelines for or the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- 8.Reiffel JA, Blomström-Lundqvist C, Boriani G, Goette A, Kowey PR, Merino JL, Piccini JP, Saksena S, Camm AJ. Real-world utilization of the pill-in-the-pocket method for terminating episodes of atrial fibrillation: data from the multinational Antiarrhythmic Interventions for Managing Atrial Fibrillation (AIM-AF) survey. Europace. 2023;25:euad162. doi: 10.1093/europace/euad162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alboni P, Botto G, Baldi N, Luzi M, Russo V, Gianfranchi L, Marchi P, Calzolari M, Solano A, Baroffio R. Outpatient treatment of recent-onset atrial fibrillation with the “pill-in-the-pocket” approach. N Eng J Med. 2004;351:2384–2391. doi: 10.1056/NEJMoa041233 [DOI] [PubMed] [Google Scholar]
- 10.Saborido CM, Hockenhull J, Bagust A, Boland A, Dickson R, Todd D. Systematic review and cost-effectiveness evaluation of “pill-in-the-pocket” strategy for paroxysmal atrial fibrillation compared to episodic in-hospital treatment or continuous antiarrhythmic drug therapy. Health Technol Assess. 2010;14:1–75. doi: 10.3310/hta14310 [DOI] [PubMed] [Google Scholar]
- 11.Plat F, Broughton A, Douville P, Sanger P, Soh B, Wight D. The electrocardiographic effects of intranasal formulations of a new calcium channel blocker, MSP-2017, are consistent with a potential treatment of paroxysmal supraventricular tachycardia: results of a phase I dose escalation study. Circulation. 2015;132:A19713. doi: 10.1161/circ.132.suppl_3.19713 [Google Scholar]
- 12.Stambler BS, Plat F, Sager PT, Shardonofsky S, Wight D, Potvin D, Pandey AS, Ip JE, Coutu B, Mondésert B, et al. First randomized, multicenter, placebo-controlled study of self-administered intranasal etripamil for acute conversion of spontaneous paroxysmal supraventricular tachycardia (NODE-301). Circ Arrhythm Electrophysiol. 2022;15:e010915. doi: 10.1161/CIRCEP.122.010915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stambler BS, Camm AJ, Alings M, Dorian P, Heidbuchel H, Houtgraaf J, Kowey PR, Merino JL, Mondésert B, Piccini JP, et al. ; RAPID Investigators. Self-administered intranasal etripamil using a symptom-prompted, Repeat-Dose Regimen for Atrioventricular-Nodal-Dependent Supraventricular Tachycardia (RAPID): a multicentre, randomised trial. Lancet. 2023;402:118–128. doi: 10.1016/S0140-6736(23)00776-6 [DOI] [PubMed] [Google Scholar]
- 14.Stambler BS, Dorian P, Sager PT, Wight D, Douville P, Potvin D, Shamszad P, Haberman RJ, Kuk RS, Lakkireddy DR, et al. Etripamil nasal spray for rapid conversion of supraventricular tachycardia to sinus rhythm. J Am Coll Cardiol. 2018;72:489–497. doi: 10.1016/j.jacc.2018.04.082 [DOI] [PubMed] [Google Scholar]
- 15.Dorian P, Coutu B, Ip JE, Martinez FA, Piccini JP, Stambler BS, Thermil N, Omodele S, Shardonofsky S, Plat F, et al. Effect of etripamil nasal spray on ventricular rate in patients experiencing symptomatic atrial fibrillation: NODE-303 atrial fibrillation heart rate analysis [abstract MP-453091-1]. HRS. 2023;20:S109. doi: 10.1016/j.hrthm.2023.03.424 [Google Scholar]
- 16.Vermersch P, Hobart J, Dive-Pouletty C, Bozzi S, Hass S, Coyle PK. Measuring treatment satisfaction in MS: is the Treatment Satisfaction Questionnaire for Medication fit for purpose? Mult Scler. 2017;23:604–613. doi: 10.1177/1352458516657441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bharmal M, Payne K, Atkinson MJ, Desrosiers MP, Morisky DE, Gemmen E. Validation of an abbreviated Treatment Satisfaction Questionnaire for Medication (TSQM-9) among patients on antihypertensive medications. Health Qual Life Outcomes. 2009;7:36. doi: 10.1186/1477-7525-7-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atkinson MJ, Sinha A, Hass SL, Colman SS, Kumar RN, Brod M, Rowland CR. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:12. doi: 10.1186/1477-7525-2-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wight D, Yue CS, Nguyen D, Plat F. Safety, tolerability, pharmacokinetics, and pharmacodynamics of intranasal etripamil in healthy Japanese and non-Japanese adults. J Am Coll Cardiol. 2022;79(9_Supplement):43. [Google Scholar]
- 20.US Department of Health and Human Services Food and Drug Administration. Guidance for industry patient-reported outcome measures: use in medical product development to support labeling claims, clinical/medical. 2009. Accessed September 12, 2023. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims
- 21.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 22.Healthcare Cost and Utilization Project (HCUP). Content last reviewed December 2022. agency for healthcare research and quality, Rockville, MD. Accessed November 2023. https://www.ahrq.gov/data/hcup/index.html [PubMed]
- 23.Camm AJ, Savelieva I, Lip GYH; Guideline Development Group for the NICE Clinical Guideline for the Management of Atrial Fibrillation. Rate control in the medical management of atrial fibrillation. Heart. 2007;93:35–38. doi: 10.1136/hrt.2006.099903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piccini JP, Passman R, Turakhia M, Connolly AT, Nabutovsky Y, Varma N. Atrial fibrillation burden, progression, and the risk of death: a case-crossover analysis in patients with cardiac implantable electronic devices. Europace. 2019;21:404–413. doi: 10.1093/europace/euy222 [DOI] [PubMed] [Google Scholar]
- 25.Pokorney SD, Camm AJ, Dorian P, Ip JE, Kowey PR, Stambler BS, Bharucha DB, Piccini JP. Impact of investigational, at-home, self-administered, intranasal etripamil on the need for additional medical intervention in patients with supraventricular tachycardia [abstract C)143]. Value in Health. 2023;26:S2. doi: 10.1016/j.jval.2023.03.216 [Google Scholar]