Abstract
The general stress response of the bacterium Bacillus subtilis is controlled by the ςB transcription factor. Here we show that loss of ςB reduces stationary-phase viability 10-fold in either alkaline or acidic media and reduces cell yield in media containing ethanol. We further show that loss of the developmental transcription factor ςH also has a marked effect on stationary-phase viability under these conditions and that this effect is independent from the simple loss of sporulation ability.
The general stress response of the bacterium Bacillus subtilis is controlled by ςB, an alternative ς factor that binds RNA polymerase to direct the transcription of over 50 genes (7, 11, 18, 36). The stresses that activate ςB include heat, osmotic, and ethanol shocks to exponentially growing cells, as well as the energy limitations imposed on starving cells (2, 6, 11, 13, 35, 36). These stress signals are conveyed to ςB by a signal transduction network that functions by the newly discovered “partner switching” mechanism, in which key protein interactions are controlled by reversible phosphorylation events (2, 3, 39).
Based on the stresses which lead to ςB activation, genes expressed under ςB control are thought to enhance survival when cells persist in a nongrowing state (11, 19, 36). However, early studies found no clear phenotype for ςB null mutants (8, 16, 21, 23). Consequently, two complementary approaches have been used to determine the physiological role of the ςB regulon. In the first, two-dimensional gel analysis identified proteins whose synthesis was ςB dependent (7, 36). A significant discovery of this line of investigation was that ςB is required for oxidative stress resistance in starved cells (4, 5, 17, 32). In the second approach, a genetic screen identified genes controlled by ςB, or csb genes, with the view that their predicted products would provide important clues to ςB function (12, 14). Of the six csb transcription units identified, four encode products that are either known or suspected to interact with the cell envelope (1, 14, 27, 33). Furthermore, one of the six is under the dual control of both ςB and ςH, suggesting that in addition to controlling sporulation initiation, ςH might also regulate a subclass of genes involved in a general stress response (34).
From the results of the csb gene studies, we hypothesized that ςB might contribute to cell viability under those growth conditions which induce ςB activity while simultaneously taxing cell envelope function. In support of this notion, we report here that loss of ςB reduces the viability of stationary-phase cells grown in alkaline or acidic media and also decreases cell yield in Luria broth (LB) containing high concentrations of ethanol. Significantly, loss of ςH also affects the viability of stationary-phase cells under these same growth conditions.
Strains and growth conditions.
We assayed the growth rate and viability of B. subtilis strains carrying a null mutation(s) in sigB, sigH, or both (Table 1). These strains were inoculated into LB, grown at 37°C to early logarithmic stage, and then diluted 1:100 into one of the three stress media described in the figure legends: alkali, acid, or ethanol stress medium. Cells were grown with vigorous shaking in flasks containing 1/10 volume of medium.
TABLE 1.
B. subtilis strains used in this study
| Strain | Genotype | Reference or construction |
|---|---|---|
| PB2 | trpC2 | Wild-type Marburg strain |
| PB61 | spo0A12 trpC2 | 10 |
| PB153 | sigBΔ2::cat trpC2 | 14 |
| PB384 | sigHΔ::cat trpC2 | 34 |
| PB386 | sigBΔ3::spc sigHΔ::cat trpC2 | 34 |
| PB513 | spoIIA::spc trpC2 | SM69-1→PB2a |
| SM69-1 | spoIIA::spc | R. Losick (unpublished data) |
The arrow indicates transformation from donor to recipient.
Loss of ςB function results in diminished survival at alkaline pH.
Maintenance of proton motive force at alkaline pH is thought to be a major energy drain which becomes especially severe during prolonged starvation (38). Bacteria are believed to respond to this stress by partially shifting energy-consuming processes from H+- to Na+-based energetics or by adopting membrane modifications that may provide an alternative means of maintaining proton motive force (9, 26, 28).
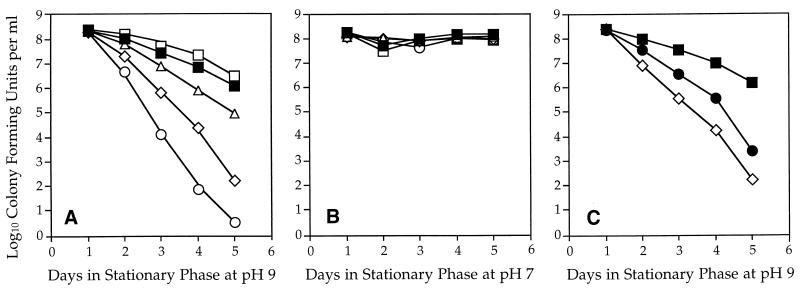
To probe the possible role of the ςB regulon under these conditions, we tested the growth and survival properties of wild-type and sigB null strains at alkaline pH. In LB medium buffered to pH 9, there was no difference in logarithmic growth rate between the wild type and the sigB mutant. Both strains had a generation time of 50 min in this medium, compared to 20 min in LB medium buffered to pH 7. However, after 5 days in stationary phase at pH 9, we saw a reproducible 10-fold loss of viability in the sigB null mutant (Fig. 1A). This difference was statistically significant by Student’s two-tailed t test, assuming unequal variance (P < 0.01). In contrast, there was no decrease in viability when the sigB null mutant was maintained in medium buffered to pH 7 (Fig. 1B). We conclude that loss of ςB function has a modest but significant effect on viability during prolonged incubation at alkaline pH.
FIG. 1.
Survival of B. subtilis strains under alkaline conditions. The data points shown in all three panels are averages of four independent experiments; differences noted are significant according to a one-factor analysis of variance (P < 0.001). The significance of key pairwise comparisons determined by Student’s t test (unequal variance) is discussed in the text. (A) Strains bearing a null mutation(s) in sigB (PB153; ▵), in sigH (PB384; ◊), or in both sigB and sigH (PB386; ○) were grown to stationary phase in LB medium lacking salt (11) buffered to pH 9.0 with 100 mM 3-(1,1-dimethyl-2-hydroxyethyl amino)-2-hydroxypropanesulfonic acid. The measured pH of the medium was 8.5 at the end of 5 days of incubation. Cell growth was monitored with a Klett-Summerson colorimeter (red filter), and cell viability was determined by serial dilution and plating on tryptose blood agar (Difco Laboratories). Controls included wild-type strain PB2 (▪) and spoIIA (sigF) null mutant PB513 (□), which lacks ςF activity. (B) The same experiment conducted with phosphate-buffered LB medium, pH 7.0. The symbols are the same as in panel A. (C) Comparison of the alkaline (pH 9.0) viability of strains bearing null mutations in sigH (PB384; ◊) and spo0A (PB61; •) to that of wild-type strain PB2 (▪).
Consistent with the notion that ςH is also involved in a stress response distinct from sporulation (34), we found that loss of ςH function was extremely deleterious to survival under prolonged alkaline incubation, with a loss of viability exceeding 106 after 5 days at pH 9 (Fig. 1A). In contrast, no such decrease was observed following prolonged incubation at neutral pH (Fig. 1B). To address the possibility that this loss of viability was primarily a consequence of the impaired sporulation ability of the sigH null mutant, we also tested the viability of a sigF null mutant. ςF is a forespore-specific transcription factor which acts immediately downstream from ςH in the developmental cascade (29). Notably, a sigF null mutant was indistinguishable from the wild type during prolonged alkaline incubation (Fig. 1A). We concluded that the diminished viability of the sigH null mutant was specifically due to the loss of ςH function and not due to a general loss of sporulation ability.
One function of ςH is to direct the stationary-phase transcription of spo0A, which encodes a response regulator with multiple roles in growing and nongrowing cells (15, 20, 31). Because loss of spo0A function is known to pleiotropically affect stationary-phase viability, we tested whether the decreased alkaline viability observed in a sigH null mutant could be entirely attributed to decreased spo0A function. We first determined that survival of a spo0A null mutant was indistinguishable from that of wild-type cells at pH 7 (data not shown). In contrast, the spo0A null mutation dramatically affected viability under prolonged incubation at pH 9 and the sigH null mutation reproducibly caused a further 15-fold loss of viability (Fig. 1C). This additional 15-fold loss was statistically significant (P < 0.05). Therefore, most, but not all, of the viability loss in the sigH null mutant was due to the loss of spo0A function. Moreover, the comparison shown in Fig. 1C likely overestimated the extent to which loss of spo0A function contributes to loss of alkaline viability in a sigH null mutant. In addition to its ςH-dependent stationary-phase promoter, spo0A also has a ςA-like vegetative promoter to provide maintenance levels of Spo0A (15, 20). Therefore, in comparison to the spo0A null mutant, the sigH null mutant would be expected to retain at least some spo0A function. We conclude that, in addition to its role in spo0A transcription, ςH must control at least one additional gene that significantly contributes to survival under alkaline conditions. And, because the effects of the sigB and sigH null mutations were roughly additive (Fig. 1A), we infer that these two transcription factors control largely independent stress response systems.
Loss of ςB function results in diminished survival at acidic pH.
We next assayed the growth and survival of wild-type and mutant strains during acid stress. In LB medium buffered to pH 5, there was no difference in logarithmic growth rate between the wild type and the sigB mutant. Both strains had a generation time of 60 to 65 min in this medium, compared to 20 min in LB buffered to pH 7. However, during prolonged incubation at pH 5, we saw a reproducible 10-fold loss of viability in the sigB and sigH null single mutants and a 100-fold loss in the sigB-sigH double mutant (Fig. 2). These differences were statistically significant (P < 0.03). Because the effects of the sigB and sigH mutations were cumulative, ςB and ςH contribute independently to stationary-phase survival at acidic pH. And, because a sigF null mutant (Fig. 2) and a spo0A null mutant (data not shown) were similar to the wild type in viability, the diminished acid survival of the sigH mutant was not simply due to an inability to sporulate or an inability to transcribe spo0A during stationary phase.
FIG. 2.
Survival of B. subtilis strains under acidic conditions. The data points shown are averages of four independent experiments; differences noted are significant according to a one-factor analysis of variance (P < 0.001). Strains bearing a null mutation(s) in sigB (PB153; ▵), in sigH (PB384; ◊), or in both sigB and sigH (PB386; ○) were grown to stationary phase in LB medium buffered to pH 5.0 with 200 mM 2-(N-morpholino)ethanesulfonic acid. The measured pH of the medium was 5.5 at the end of 5 days of incubation. Cell growth and viability were monitored as described in the Fig. 1 legend. Controls included wild-type strain PB2 (▪) and spoIIA (sigF) null mutant PB513 (□).
Loss of ςB function results in decreased cell yield in ethanol-containing media.
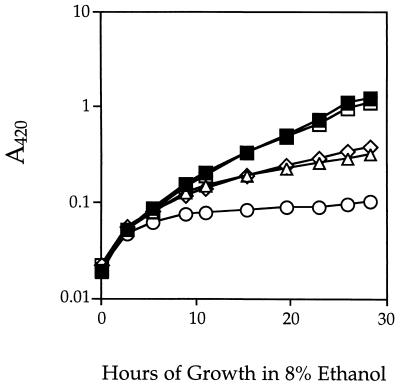
ςB activity is strongly induced by ethanol shock (11, 36), and ethanol is known to have multiple effects on membrane function (reviewed in reference 22). Consequently, we tested the influence of various concentrations of ethanol on the growth of sigB and sigH null mutants. Our initial experiments found that growth of B. subtilis cells in LB medium led to rapid alkalification whether or not ethanol was present (data not shown). To eliminate the influence of alkalification, we therefore performed experiments with phosphate-buffered LB medium. At ethanol concentrations of up to 6% (wt/vol), there was no difference in growth rate between the wild-type and mutant strains; in greater-than-9% ethanol, neither the wild type nor the mutants could grow (data not shown), but with ethanol concentrations of 7, 8, and 9%, there was a clear difference in cell yield between the wild-type and mutant strains. Figure 3 shows the results of a typical growth experiment with 8% ethanol, in which sigB and sigH single mutants showed a fourfold-lower cell yield than either the wild-type or sigF control strain and the sigB-sigH double mutant manifested a 10-fold-lower cell yield. Because the effects of the sigB and sigH null mutations were approximately additive, ςB and ςH contribute independently to the ability of B. subtilis to grow in ethanol-containing LB medium.
FIG. 3.
Growth and yield of B. subtilis strains in LB medium containing 8% ethanol. The A420 of strains bearing a null mutation(s) in sigB (PB153; ▵), in sigH (PB384; ◊), or in both sigB and sigH (PB386; ○) was monitored by placing samples into 0.9% (vol/vol) formaldehyde and measuring A420 in a Varian DMS100 spectrophotometer, essentially as described by Neidhardt et al. (30). Over the range shown, A420 was found to be directly correlated with viable cell counts, with 1 U of A420 equal to 108 CFU/ml. Growth medium was phosphate-buffered LB lacking salt (11) and supplemented with 8% (wt/vol) ethanol. Controls included wild-type strain PB2 (▪) and spoIIA (sigF) null mutant PB513 (□).
Conclusions.
Because a number of csb gene products appear to be associated with the cell envelope, we hypothesized that loss of ςB function would become evident under environmental conditions that challenge envelope function. The results presented here indicate that ςB significantly contributes to the survival of B. subtilis cells during prolonged starvation at extremes of pH and also contributes to the ability to grow in medium containing high ethanol concentrations. Although the molecular basis for these observations is not known, our findings do extend the phenotype of a sigB null mutant beyond the previously described sensitivity to oxidative stress (4, 5, 17, 32).
Other studies have suggested additional roles for the ςB regulon in preserving cell viability but have not associated these roles with a clear phenotype for a sigB null mutant. In one study, the ClpC operon was shown to be under the dual control of a ςA-like promoter and a ςB-dependent promoter (24). In addition to the ClpC protein, which is a pleiotropic regulator of stress responses, the ClpC operon also encodes products involved in DNA repair (25). In another study, expression of the OpuE transporter, which is required for proline-dependent osmoprotection, was also found to be under the control of dual ςA and ςB promoters, both of which are osmoregulated (37). These other studies, together with our results, implicate ςB in the control of genes which are important for resistance to oxidative stress, for repair of DNA damage, for osmoprotection, for growth in ethanol, and for viability at extreme pH.
One unexpected finding is that in addition to its well-established role in controlling the sporulation process, ςH also contributes to stress resistance in nonsporulating cells. From the cumulative effects of ςH and ςB under the three stress conditions tested, it is clear that these transcription factors independently contribute to stress response. Two different scenarios are consistent with this result. First, all alkali, acid, and ethanol stress genes in the ςH and ςB regulons could be under dual ςH and ςB control. In this view, the cumulative effect of these two transcription factors would primarily reflect their relative contribution to the expression of the same subset of stress genes. Alternatively, ςH and ςB could regulate largely independent sets of stress genes, only some of which are under the control of both transcription factors. In this latter case, the cumulative effect of ςH and ςB would then reflect the relative importance of these independent gene sets to survival of a given stress.
Acknowledgments
We thank R. Losick for providing strain SM69-1 bearing the sigF null allele and A. L. Sonenshein for helpful comments on the manuscript.
This research was supported by Public Health Service grant GM42077 from the National Institute of General Medical Sciences.
REFERENCES
- 1.Akbar S, Price C W. Isolation and characterization of csbB, a gene controlled by Bacillus subtilis general stress transcription factor ςB. Gene. 1996;177:123–128. doi: 10.1016/0378-1119(96)00287-9. [DOI] [PubMed] [Google Scholar]
- 2.Alper S, Duncan L, Losick R. An adenosine nucleotide switch controlling the activity of a cell type-specific transcription factor in B. subtilis. Cell. 1994;77:195–205. doi: 10.1016/0092-8674(94)90312-3. [DOI] [PubMed] [Google Scholar]
- 3.Alper S, Dufour A, Garsin D A, Duncan L, Losick R. Role of adenosine nucleotides in the regulation of a stress-response transcription factor in Bacillus subtilis. J Mol Biol. 1996;260:165–177. doi: 10.1006/jmbi.1996.0390. [DOI] [PubMed] [Google Scholar]
- 4.Antelmann H, Engelmann S, Schmid R, Hecker M. General and oxidative stress responses in Bacillus subtilis: cloning, expression, and mutation of the alkyl hydroperoxide reductase operon. J Bacteriol. 1996;178:6571–6578. doi: 10.1128/jb.178.22.6571-6578.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antelmann H, Engelmann S, Schmid R, Sorokin A, Lapidus A, Hecker M. Expression of a stress- and starvation-induced dps/pexB-homologous gene is controlled by the alternative sigma factor ςB in Bacillus subtilis. J Bacteriol. 1997;179:7251–7256. doi: 10.1128/jb.179.23.7251-7256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benson A K, Haldenwang W G. The ςB dependent promoter of the Bacillus subtilis sigB operon is induced by heat shock. J Bacteriol. 1993;175:1929–1935. doi: 10.1128/jb.175.7.1929-1935.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernhardt J, Völker U, Völker A, Antelmann H, Schmid R, Mach H, Hecker M. Specific and general stress proteins in Bacillus subtilis—a two-dimensional protein electrophoresis study. Microbiology. 1997;143:999–1017. doi: 10.1099/00221287-143-3-999. [DOI] [PubMed] [Google Scholar]
- 8.Binnie C, Lampe M, Losick R. Gene encoding the sigma-37 species of RNA polymerase sigma factor from Bacillus subtilis. Proc Natl Acad Sci USA. 1986;83:5943–5947. doi: 10.1073/pnas.83.16.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogachev A V, Murtazina R A, Shestopalov A I, Skulachev V P. Induction of the Escherichia coli cytochrome-d by low ΔμH+ and by sodium ions. Eur J Biochem. 1995;232:304–308. doi: 10.1111/j.1432-1033.1995.tb20812.x. [DOI] [PubMed] [Google Scholar]
- 10.Boylan S A, Chun K T, Edson B A, Price C W. Early-blocked sporulation mutations alter expression of enzymes under carbon control in Bacillus subtilis. Mol Gen Genet. 1988;212:271–280. doi: 10.1007/BF00334696. [DOI] [PubMed] [Google Scholar]
- 11.Boylan S A, Redfield A R, Brody M S, Price C W. Stress-induced activation of the ςB transcription factor of Bacillus subtilis. J Bacteriol. 1993;175:7931–7937. doi: 10.1128/jb.175.24.7931-7937.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boylan S A, Redfield A R, Price C W. Transcription factor ςB of Bacillus subtilis controls a large stationary-phase regulon. J Bacteriol. 1993;175:3957–3963. doi: 10.1128/jb.175.13.3957-3963.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boylan S A, Rutherford A, Thomas S M, Price C W. Activation of Bacillus subtilis transcription factor ςB by a regulatory pathway responsive to stationary-phase signals. J Bacteriol. 1992;174:3695–3706. doi: 10.1128/jb.174.11.3695-3706.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boylan S A, Thomas M D, Price C W. Genetic method to identify regulons controlled by nonessential elements: isolation of a gene dependent on alternate transcription factor ςB of Bacillus subtilis. J Bacteriol. 1991;173:7856–7866. doi: 10.1128/jb.173.24.7856-7866.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chibazakura T, Kawamura F, Takahashi H. Differential regulation of spo0A transcription in Bacillus subtilis: glucose represses promoter switching at the initiation of sporulation. J Bacteriol. 1991;176:1977–1984. doi: 10.1128/jb.173.8.2625-2632.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duncan M L, Kalman S S, Thomas S M, Price C W. Gene encoding the 37,000-dalton minor sigma factor of Bacillus subtilis RNA polymerase: isolation, nucleotide sequence, chromosomal locus, and cryptic function. J Bacteriol. 1987;169:771–778. doi: 10.1128/jb.169.2.771-778.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engelmann S, Hecker M. Impaired oxidative stress resistance of Bacillus subtilis sigB mutants and the role of katA and katE. FEMS Microbiol Lett. 1996;145:63–69. doi: 10.1111/j.1574-6968.1996.tb08557.x. [DOI] [PubMed] [Google Scholar]
- 18.Haldenwang W G, Losick R. A novel RNA polymerase sigma factor from Bacillus subtilis. Proc Natl Acad Sci USA. 1980;77:7000–7005. doi: 10.1073/pnas.77.12.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hecker M, Schumann W, Völker U. Heat-shock and general stress response in Bacillus subtilis. Mol Microbiol. 1996;19:417–428. doi: 10.1046/j.1365-2958.1996.396932.x. [DOI] [PubMed] [Google Scholar]
- 20.Hoch J A. Regulation of the phosphorelay and the initiation of sporulation in Bacillus subtilis. Annu Rev Microbiol. 1993;47:441–465. doi: 10.1146/annurev.mi.47.100193.002301. [DOI] [PubMed] [Google Scholar]
- 21.Igo M, Lampe M, Ray C, Schafer W, Moran C P, Losick R. Genetic studies of a secondary RNA polymerase sigma factor in Bacillus subtilis. J Bacteriol. 1987;169:3464–3469. doi: 10.1128/jb.169.8.3464-3469.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ingram L O, Buttke T M. Effects of alcohols on microorganisms. Adv Microb Physiol. 1984;25:253–300. doi: 10.1016/s0065-2911(08)60294-5. [DOI] [PubMed] [Google Scholar]
- 23.Kalman S, Duncan M L, Thomas S M, Price C W. Similar organization of the sigB and spoIIA operons encoding alternate sigma factors of Bacillus subtilis RNA polymerase. J Bacteriol. 1990;172:5575–5585. doi: 10.1128/jb.172.10.5575-5585.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krüger E, Msadek T, Hecker M. Alternative promoters direct stress-induced transcription of the Bacillus subtilis clpC operon. Mol Microbiol. 1996;20:713–723. doi: 10.1111/j.1365-2958.1996.tb02511.x. [DOI] [PubMed] [Google Scholar]
- 25.Krüger E, Msadek T, Ohlmeir S, Hecker M. The Bacillus subtilis clpC operon encodes DNA repair and competence proteins. Microbiology. 1996;143:1309–1316. doi: 10.1099/00221287-143-4-1309. [DOI] [PubMed] [Google Scholar]
- 26.Krulwich T A, Ito M, Gilmour R, Sturr M G, Guffanti A A, Hicks D B. Energetic problems of extremely alkaliphilic aerobes. Biochim Biophys Acta. 1996;1275:21–26. doi: 10.1016/0005-2728(96)00044-8. [DOI] [PubMed] [Google Scholar]
- 27.Lee S-Y. Ph.D. thesis. University of California, Davis; 1995. [Google Scholar]
- 28.Lewis K, Naroditskaya V, Ferrante A, Folkina I. Bacterial resistance to uncouplers. J Bioenerg Biomembr. 1994;20:639–646. doi: 10.1007/BF00831539. [DOI] [PubMed] [Google Scholar]
- 29.Losick R, Stragier P. Crisscross regulation of cell-type specific gene expression during development in B. subtilis. Nature (London) 1992;355:601–604. doi: 10.1038/355601a0. [DOI] [PubMed] [Google Scholar]
- 30.Neidhardt F C, Bloch P L, Smith D F. Culture medium for enterobacteria. J Bacteriol. 1974;119:736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Predich M, Nair G, Smith I. Bacillus subtilis early sporulation genes kinA, spo0F, and spo0A are transcribed by RNA polymerase containing ςH. J Bacteriol. 1992;174:2771–2778. doi: 10.1128/jb.174.9.2771-2778.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scharf C, Riethdorf S, Ernst H, Engelmann S, Völker U, Hecker M. Thioredoxin is an essential protein induced by multiple stresses in Bacillus subtilis. J Bacteriol. 1998;180:1869–1877. doi: 10.1128/jb.180.7.1869-1877.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varón D, Boylan S A, Okamoto K, Price C W. Bacillus subtilis gtaB encodes UDP-glucose pyrophosphorylase and is controlled by stationary-phase transcription factor ςB. J Bacteriol. 1993;175:3964–3971. doi: 10.1128/jb.175.13.3964-3971.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varón D, Brody M S, Price C W. Bacillus subtilis operon under the dual control of the general stress transcription factor ςB and the sporulation transcription factor ςH. Mol Microbiol. 1996;20:339–350. doi: 10.1111/j.1365-2958.1996.tb02621.x. [DOI] [PubMed] [Google Scholar]
- 35.Voelker U, Voelker A, Maul B, Hecker M, Dufour A, Haldenwang W G. Separate mechanisms activate ςB of Bacillus subtilis in response to environmental and metabolic stresses. J Bacteriol. 1995;177:3771–3780. doi: 10.1128/jb.177.13.3771-3780.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Völker U, Engelmann S, Maul B, Riethdorf S, Völker A, Schmid R, Mach H, Hecker M. Analysis of the induction of general stress proteins of Bacillus subtilis. Microbiology. 1994;140:741–752. doi: 10.1099/00221287-140-4-741. [DOI] [PubMed] [Google Scholar]
- 37.von Blohn C, Kempf B, Kappes R M, Bremer E. Osmostress response in Bacillus subtilis: characterization of a proline uptake system (OpuE) regulated by high osmolarity and the alternative transcription factor sigma-B. Mol Microbiol. 1997;25:175–187. doi: 10.1046/j.1365-2958.1997.4441809.x. [DOI] [PubMed] [Google Scholar]
- 38.Weiner L, Model P. Role of an Escherichia coli stress-induced operon in stationary phase survival. Proc Natl Acad Sci USA. 1994;91:2191–2195. doi: 10.1073/pnas.91.6.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang X, Kang C M, Brody M S, Price C W. Opposing pairs of serine protein kinases and phosphatases transmit signals of environmental stress to activate a bacterial transcription factor. Genes Dev. 1996;10:2265–2275. doi: 10.1101/gad.10.18.2265. [DOI] [PubMed] [Google Scholar]