Abstract
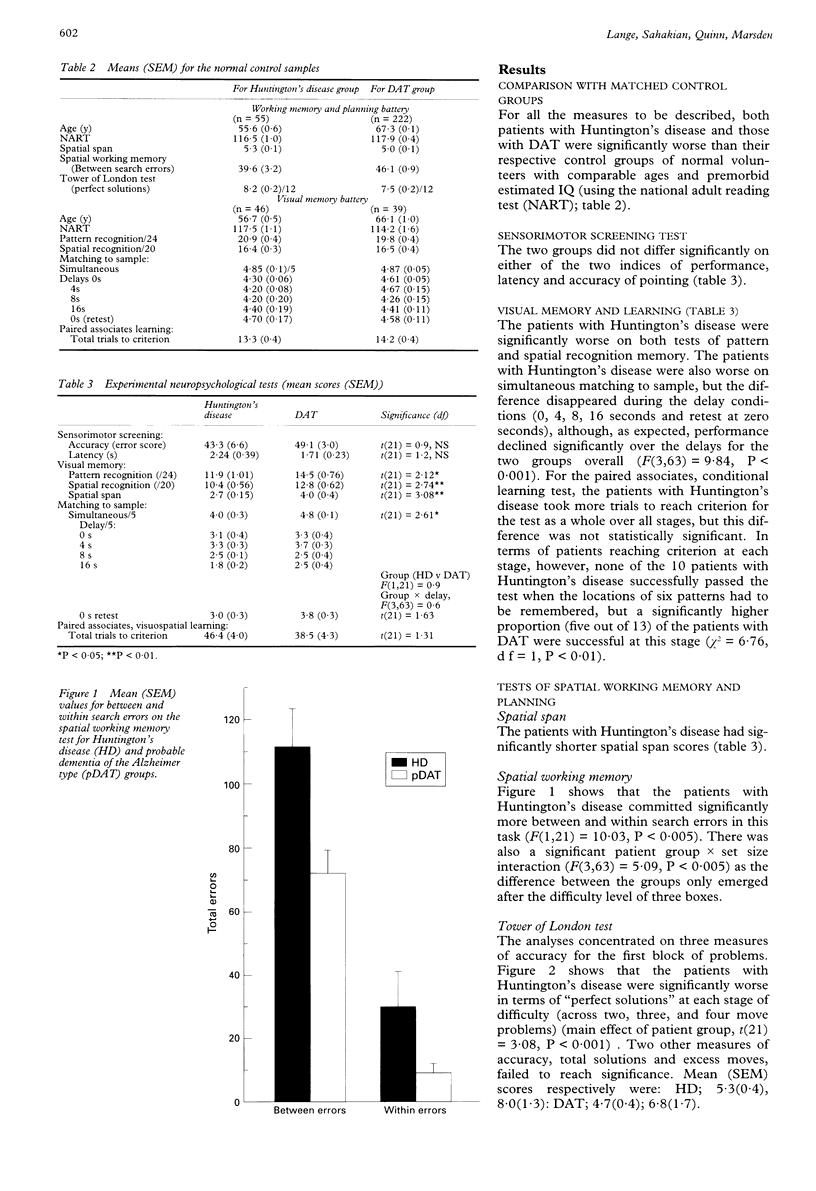
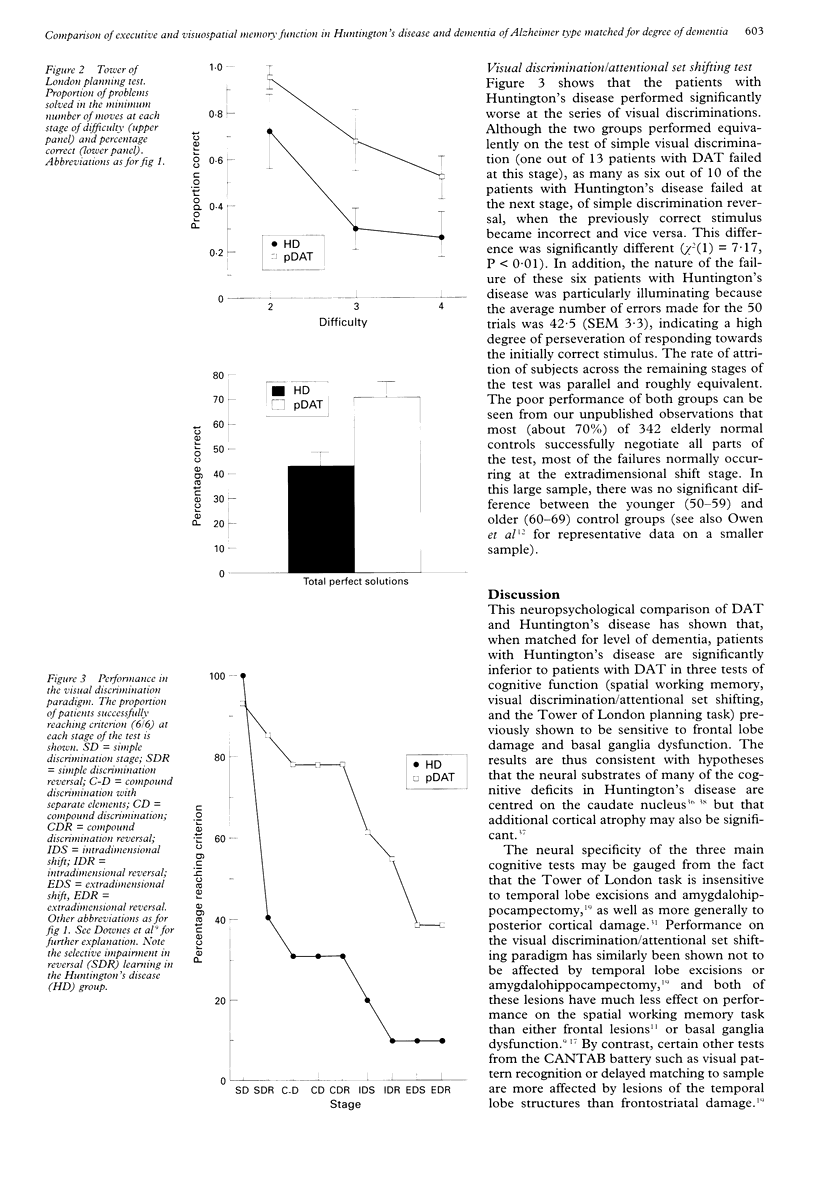
Groups of patients with Hungington's disease and probable dementia of Alzheimer type (DAT) matched for level of dementia on the basis of mini mental state examination scores were compared in several tests of visual memory and tests sensitive to frontal lobe dysfunction. Whereas recall of patients with DAT tended to be worse on the Kendrick object learning test, the two groups were equivalent on tests of sensorimotor ability and delayed matching to sample performance. By contrast, the patients with Huntington's disease were significantly worse on tests of pattern and spatial recognition, simultaneous matching to sample, visuospatial paired associates, and on three tests sensitive to frontal lobe dysfunction--namely, the Tower of London test of planning, spatial working memory, and a visual discrimination learning and reversal paradigm. The impairments in these tests, however, did not always qualitatively resemble those seen in patients with frontal lobe damage and may be more characteristic of primary neostriatal deficit. In the visual discrimination paradigm the patients with Hungtington's disease were significantly worse than the patients with DAT at the simple reversal stage, where they displayed significant preservation to the previously rewarded alternative. The results are consistent with the hypothesis that patients with Huntington's disease exhibit deficits in tests sensitive to frontostriatal dysfunction and that this form of intellectual deterioration is qualitatively distinct from that seen in Alzheimer's disease.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert M. L., Feldman R. G., Willis A. L. The 'subcortical dementia' of progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 1974 Feb;37(2):121–130. doi: 10.1136/jnnp.37.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander G. E., DeLong M. R., Strick P. L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Bamford K. A., Caine E. D., Kido D. K., Plassche W. M., Shoulson I. Clinical-pathologic correlation in Huntington's disease: a neuropsychological and computed tomography study. Neurology. 1989 Jun;39(6):796–801. doi: 10.1212/wnl.39.6.796. [DOI] [PubMed] [Google Scholar]
- Beatty W. W., Salmon D. P., Butters N., Heindel W. C., Granholm E. L. Retrograde amnesia in patients with Alzheimer's disease or Huntington's disease. Neurobiol Aging. 1988 Mar-Apr;9(2):181–186. doi: 10.1016/s0197-4580(88)80048-4. [DOI] [PubMed] [Google Scholar]
- Berent S., Giordani B., Lehtinen S., Markel D., Penney J. B., Buchtel H. A., Starosta-Rubinstein S., Hichwa R., Young A. B. Positron emission tomographic scan investigations of Huntington's disease: cerebral metabolic correlates of cognitive function. Ann Neurol. 1988 Jun;23(6):541–546. doi: 10.1002/ana.410230603. [DOI] [PubMed] [Google Scholar]
- Brouwers P., Cox C., Martin A., Chase T., Fedio P. Differential perceptual-spatial impairment in Huntington's and Alzheimer's dementias. Arch Neurol. 1984 Oct;41(10):1073–1076. doi: 10.1001/archneur.1984.04050210071017. [DOI] [PubMed] [Google Scholar]
- Brown R. G., Marsden C. D. 'Subcortical dementia': the neuropsychological evidence. Neuroscience. 1988 May;25(2):363–387. doi: 10.1016/0306-4522(88)90246-1. [DOI] [PubMed] [Google Scholar]
- Butters N., Wolfe J., Granholm E., Martone M. An assessment of verbal recall, recognition and fluency abilities in patients with Huntington's disease. Cortex. 1986 Mar;22(1):11–32. doi: 10.1016/s0010-9452(86)80030-2. [DOI] [PubMed] [Google Scholar]
- Bylsma F. W., Rebok G. W., Brandt J. Long-term retention of implicit learning in Huntington's disease. Neuropsychologia. 1991;29(12):1213–1221. doi: 10.1016/0028-3932(91)90035-7. [DOI] [PubMed] [Google Scholar]
- Cummings J. L., Benson D. F. Subcortical dementia. Review of an emerging concept. Arch Neurol. 1984 Aug;41(8):874–879. doi: 10.1001/archneur.1984.04050190080019. [DOI] [PubMed] [Google Scholar]
- David A. S., Jeste D. V., Folstein M. F., Folstein S. E. Voluntary movement dysfunction in Huntington's disease and tardive dyskinesia. Acta Neurol Scand. 1987 Feb;75(2):130–139. doi: 10.1111/j.1600-0404.1987.tb07907.x. [DOI] [PubMed] [Google Scholar]
- Divac I., Rosvold H. E., Szwarcbart M. K. Behavioral effects of selective ablation of the caudate nucleus. J Comp Physiol Psychol. 1967 Apr;63(2):184–190. doi: 10.1037/h0024348. [DOI] [PubMed] [Google Scholar]
- Downes J. J., Roberts A. C., Sahakian B. J., Evenden J. L., Morris R. G., Robbins T. W. Impaired extra-dimensional shift performance in medicated and unmedicated Parkinson's disease: evidence for a specific attentional dysfunction. Neuropsychologia. 1989;27(11-12):1329–1343. doi: 10.1016/0028-3932(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Eagger S. A., Levy R., Sahakian B. J. Tacrine in Alzheimer's disease. Lancet. 1991 Apr 27;337(8748):989–992. doi: 10.1016/0140-6736(91)92656-m. [DOI] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E., McHugh P. R. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Heindel W. C., Salmon D. P., Shults C. W., Walicke P. A., Butters N. Neuropsychological evidence for multiple implicit memory systems: a comparison of Alzheimer's, Huntington's, and Parkinson's disease patients. J Neurosci. 1989 Feb;9(2):582–587. doi: 10.1523/JNEUROSCI.09-02-00582.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges J. R., Salmon D. P., Butters N. Differential impairment of semantic and episodic memory in Alzheimer's and Huntington's diseases: a controlled prospective study. J Neurol Neurosurg Psychiatry. 1990 Dec;53(12):1089–1095. doi: 10.1136/jnnp.53.12.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges J. R., Salmon D. P., Butters N. The nature of the naming deficit in Alzheimer's and Huntington's disease. Brain. 1991 Aug;114(Pt 4):1547–1558. doi: 10.1093/brain/114.4.1547. [DOI] [PubMed] [Google Scholar]
- Hughes C. P., Berg L., Danziger W. L., Coben L. A., Martin R. L. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982 Jun;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Jones B., Mishkin M. Limbic lesions and the problem of stimulus--reinforcement associations. Exp Neurol. 1972 Aug;36(2):362–377. doi: 10.1016/0014-4886(72)90030-1. [DOI] [PubMed] [Google Scholar]
- Knopman D., Nissen M. J. Procedural learning is impaired in Huntington's disease: evidence from the serial reaction time task. Neuropsychologia. 1991;29(3):245–254. doi: 10.1016/0028-3932(91)90085-m. [DOI] [PubMed] [Google Scholar]
- McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E. M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984 Jul;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Milner B. Interhemispheric differences in the localization of psychological processes in man. Br Med Bull. 1971 Sep;27(3):272–277. doi: 10.1093/oxfordjournals.bmb.a070866. [DOI] [PubMed] [Google Scholar]
- Moss M. B., Albert M. S., Butters N., Payne M. Differential patterns of memory loss among patients with Alzheimer's disease, Huntington's disease, and alcoholic Korsakoff's syndrome. Arch Neurol. 1986 Mar;43(3):239–246. doi: 10.1001/archneur.1986.00520030031008. [DOI] [PubMed] [Google Scholar]
- Owen A. M., Beksinska M., James M., Leigh P. N., Summers B. A., Marsden C. D., Quinn N. P., Sahakian B. J., Robbins T. W. Visuospatial memory deficits at different stages of Parkinson's disease. Neuropsychologia. 1993 Jul;31(7):627–644. doi: 10.1016/0028-3932(93)90135-m. [DOI] [PubMed] [Google Scholar]
- Owen A. M., Downes J. J., Sahakian B. J., Polkey C. E., Robbins T. W. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28(10):1021–1034. doi: 10.1016/0028-3932(90)90137-d. [DOI] [PubMed] [Google Scholar]
- Owen A. M., James M., Leigh P. N., Summers B. A., Marsden C. D., Quinn N. P., Lange K. W., Robbins T. W. Fronto-striatal cognitive deficits at different stages of Parkinson's disease. Brain. 1992 Dec;115(Pt 6):1727–1751. doi: 10.1093/brain/115.6.1727. [DOI] [PubMed] [Google Scholar]
- Owen A. M., Roberts A. C., Polkey C. E., Sahakian B. J., Robbins T. W. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1991;29(10):993–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
- Owen A. M., Sahakian B. J., Semple J., Polkey C. E., Robbins T. W. Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1995 Jan;33(1):1–24. doi: 10.1016/0028-3932(94)00098-a. [DOI] [PubMed] [Google Scholar]
- Pillon B., Dubois B., Lhermitte F., Agid Y. Heterogeneity of cognitive impairment in progressive supranuclear palsy, Parkinson's disease, and Alzheimer's disease. Neurology. 1986 Sep;36(9):1179–1185. doi: 10.1212/wnl.36.9.1179. [DOI] [PubMed] [Google Scholar]
- Robbins T. W., James M., Lange K. W., Owen A. M., Quinn N. P., Marsden C. D. Cognitive performance in multiple system atrophy. Brain. 1992 Feb;115(Pt 1):271–291. doi: 10.1093/brain/115.1.271. [DOI] [PubMed] [Google Scholar]
- Robbins T. W., James M., Owen A. M., Lange K. W., Lees A. J., Leigh P. N., Marsden C. D., Quinn N. P., Summers B. A. Cognitive deficits in progressive supranuclear palsy, Parkinson's disease, and multiple system atrophy in tests sensitive to frontal lobe dysfunction. J Neurol Neurosurg Psychiatry. 1994 Jan;57(1):79–88. doi: 10.1136/jnnp.57.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins T. W., James M., Owen A. M., Sahakian B. J., McInnes L., Rabbitt P. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994 Sep-Oct;5(5):266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- Roberts A. C., De Salvia M. A., Wilkinson L. S., Collins P., Muir J. L., Everitt B. J., Robbins T. W. 6-Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analog of the Wisconsin Card Sort Test: possible interactions with subcortical dopamine. J Neurosci. 1994 May;14(5 Pt 1):2531–2544. doi: 10.1523/JNEUROSCI.14-05-02531.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. C., Robbins T. W., Everitt B. J. The effects of intradimensional and extradimensional shifts on visual discrimination learning in humans and non-human primates. Q J Exp Psychol B. 1988 Nov;40(4):321–341. [PubMed] [Google Scholar]
- Rothlind J. C., Brandt J. A brief assessment of frontal and subcortical functions in dementia. J Neuropsychiatry Clin Neurosci. 1993 Winter;5(1):73–77. doi: 10.1176/jnp.5.1.73. [DOI] [PubMed] [Google Scholar]
- Sahakian B. J., Downes J. J., Eagger S., Evenden J. L., Levy R., Philpot M. P., Roberts A. C., Robbins T. W. Sparing of attentional relative to mnemonic function in a subgroup of patients with dementia of the Alzheimer type. Neuropsychologia. 1990;28(11):1197–1213. doi: 10.1016/0028-3932(90)90055-s. [DOI] [PubMed] [Google Scholar]
- Sahakian B. J., Morris R. G., Evenden J. L., Heald A., Levy R., Philpot M., Robbins T. W. A comparative study of visuospatial memory and learning in Alzheimer-type dementia and Parkinson's disease. Brain. 1988 Jun;111(Pt 3):695–718. doi: 10.1093/brain/111.3.695. [DOI] [PubMed] [Google Scholar]
- Sahakian B. J., Owen A. M. Computerized assessment in neuropsychiatry using CANTAB: discussion paper. J R Soc Med. 1992 Jul;85(7):399–402. [PMC free article] [PubMed] [Google Scholar]
- Starkstein S. E., Brandt J., Bylsma F., Peyser C., Folstein M., Folstein S. E. Neuropsychological correlates of brain atrophy in Huntington's disease: a magnetic resonance imaging study. Neuroradiology. 1992;34(6):487–489. doi: 10.1007/BF00598956. [DOI] [PubMed] [Google Scholar]
- Tröster A. I., Salmon D. P., McCullough D., Butters N. A comparison of the category fluency deficits associated with Alzheimer's and Huntington's disease. Brain Lang. 1989 Oct;37(3):500–513. doi: 10.1016/0093-934x(89)90032-1. [DOI] [PubMed] [Google Scholar]
- Weinberger D. R., Berman K. F., Iadarola M., Driesen N., Zec R. F. Prefrontal cortical blood flow and cognitive function in Huntington's disease. J Neurol Neurosurg Psychiatry. 1988 Jan;51(1):94–104. doi: 10.1136/jnnp.51.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]


