Abstract
Background
We studied the pattern of herbal and dietary supplement (HDS) use in patients with chronic liver disease (CLD) during the first year of the COVID-19 pandemic.
Methods
A questionnaire/survey was sent to hepatology patients with CLD under the care of hepatologists at Johns Hopkins University School of Medicine.
Results
The 5 most taken dietary supplements during the pandemic included vitamin B12 (27.7%), vitamin C (32.4%), vitamin D (54.6%), zinc (25.4%) and green tea extract (20.8%). Most participants (82.3%) did not discuss their HDS use with their hepatology providers.
Conclusions
Healthcare providers should be mindful of potential HDS use in patients with CLD.
Introduction
Despite having the potential for hepatotoxicity, dietary supplement use in the United States and Europe is widespread. The National Center for Complementary and Integrative Health defines dietary supplements as herbs, vitamins and probiotics. Analyses of an extensive study of 55,388 individuals from the National Health and Nutrition Examination Surveys (NHANES) from 2001 to 2012 observed that 7.2% of patients with chronic liver disease (CLD) reported using dietary supplements.1 The US Food and Drug Administration (FDA) regulates dietary supplements as food but not as drugs under the Dietary Supplement Health and Education Act of 1994.2 Liver injury from herbal and dietary supplements (HDS) is not uncommon and is responsible for up to 20% of drug-induced liver injury, according to the Drug-Induced Liver Injury Network (DILIN).3
A systematic review and meta-analysis of 11 studies reported that the pooled prevalence of COVID-19-related liver injury was 22.1%.4 Although the mechanisms of liver injury during COVID-19 are complex and involve direct and indirect effects of the virus, drug or dietary supplement-induced liver injury may be an overlooked yet preventable cause. In response to the COVID-19 pandemic, many individuals, including healthcare professionals, consumed and recommended a variety of non-prescription dietary supplements with potential hepatotoxicity.5 To better understand the patterns of dietary supplements during the first year of the COVID-19 pandemic taken by patients with CLD, we surveyed our patients for HDS usage through a secure electronic medical portal.
Materials and Methods
Human Study Participants
The study and recruitment strategy were approved by the Johns Hopkins University School of Medicine Institutional Review Board. All adult patients (age ≥18 years) with CLD receiving hepatology care at the Johns Hopkins Hospital and USA affiliates were invited to participate. CLD was defined as a preexisting liver disease with a duration of >6 months. A query of the electronic medical records was completed on all hepatology department outpatients using the ICD-10 codes defining CLD.
Survey Distribution
A survey questionnaire geared towards understanding the pattern of dietary supplement use during the COVID-19 pandemic was developed under the guidance of a board-certified nutrition specialist (GEM) and set up in REDCap. The survey included standard questions on demographics, health conditions, liver diseases, medications, HDS use and COVID-19 pandemic-related questions. The study team pilot tested the survey, confirming the data entry, flow and logic of responses and content validity. A response to all questions was not required in order to encourage survey completion. A link to this questionnaire, along with a letter explaining the purpose of this study, was then sent to the patients via a patient messaging portal (Epic electronic medical record [Epic Systems, Verona, Wisconsin USA], MyChart patient portal). The survey link was not individualized for each patient to ensure anonymity and confidentiality. Patient responses were recorded in REDCap in a de-identified fashion. Patients received the message to fill out the survey between February 3, 2021, and February 9, 2021, and then again between March 9, 2021, and March 12, 2021. Each patient received 2 messages.
Data Analysis
All analyses were performed using Stata 15 (StataCorp, College Station, Texas USA) and SAS® v9.4 (SAS Institute, Inc., Cary, North Carolina USA), with P < .05 indicating statistical significance. Descriptive statistics, including means and standard deviations, medians and interquartile ranges and frequencies and proportions, were examined. Differences between groups of interest were assessed using Chi-squared or Fisher’s exact tests, as appropriate.
Results
Survey Completion Assessment
A total of 535 participants (24.6%) responded to the questionnaire survey; 2 respondents reported being pregnant and were excluded from further analysis, yielding a sample of 533 respondents. Of these 533, the majority (n = 403; 75.6%) reached the end of the survey, while some (n = 71; 13.3%) answered more than half of the survey questions and some (n = 59; 11.1%) answered less than half of the survey questions. Of the 533 respondents, 298 reached the end of the survey and acknowledged having CLD. This denominator (298) was used to calculate the study results. Furthermore, a sensitivity analysis was limited to participants who answered all the HDS use questions in order to validate the study results. The mean patient age in this cohort was 57.1 ± 13.4) years; 186 of the 298 participants (62.6%) were women. A total of 78% identified themselves as white, 8.7% as African American or black, 7.0% as Asian and 5.3% as multiracial or other. Of the respondents, 73% had either a bachelor’s (36.8%) or a master’s degree (36.4%).
Etiologies of Chronic Liver Disease
The etiology of CLD in the 267 non-liver transplantation participants is summarized in the Table. A total of 28 participants (9.4%) were taking tacrolimus, followed by prednisone (9.7%), mycophenolate (8.7%) and azathioprine (8.0%).
Table.
Liver Diseases in Patients Who Had Not Had a Liver Transplant (N=267)
| Liver Disease | n (%) |
|---|---|
| Hepatitis B | 13 (4.87) |
| Hepatitis C | 8(3) |
| Alcoholic liver disease | 13 (4.87) |
| Non-alcoholic liver disease (NASH/NAFLD) | 88 (32.96) |
| Autoimmune hepatitis | 46 (17.23) |
| Primary biliary cholangitis | 38 (14.23) |
| Primary sclerosing cholangitis | 26 (9.74) |
| Cirrhosis | 63 (23.6) |
| Liver cancer | 3 (1.12) |
| Other | 21 (7.87) |
Herbal and Dietary Supplement Use During the COVID Pandemic
Of the 298 participants, 68.2% reported using HDS during the first year of the COVID-19 pandemic. The most commonly taken HDS during the pandemic included fish oil (7.5%), garlic (9.8%), ginger (17.1%), green tea (20.8%), milk thistle (12.6%), probiotics (25.3%), turmeric/curcumin (14.9%), vitamin B12 (27.7%), vitamin C (32.4%), vitamin D (54.6%), zinc (25.4%) and other (22.1%) (Figure). In a sensitivity analysis limited to patients who answered all of the HDS use questions (n=118), results were similar to the primary analysis, including all respondents with varying sample sizes per question. There were very few statistically significant differences in HDS use by race observed. Participants self-describing as Asian (n = 15) were more likely to report Asian herbal supplements (9.5%), and participants self-describing as multiracial or other races were more likely to report chamomile. Asians are more susceptible to drug-induced liver injury (DILI) and Asian herbals are known to be potentially adulterated and hepatotoxic.6
Figure.
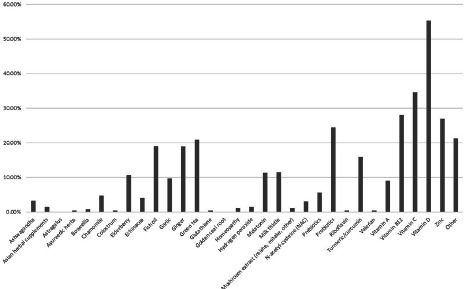
Dietary supplement use during the COVID-19 pandemic. The most common dietary supplements used during the COVID-19 pandemic. The most taken dietary supplement during the pandemic was vitamin D, likely due to its presumed antiviral and immune benefits. The other widely used dietary supplements with potential immune-modulating properties included (in descending order of frequency) vitamin C, zinc, probiotics, green tea, fish oil, ginger, turmeric/curcumin and garlic.
The most commonly used HDS among the 31 liver transplant patients was vitamin D (41.9%), followed by vitamin C (19.4%), probiotics (9.6%), melatonin (9.6%), zinc (9.6%), green tea extract (6.5%) and ginger (6.5%). In a similar fashion, 82 patients with autoimmune disease endorsed taking vitamin D (45.1%), vitamin C (21.9%), zinc (17.1%), vitamin B12 (20.7%), green tea (13.4%) and probiotics (13.4%). A total of 121 participants (36.7%) felt their use of HDS improved their overall health. Most participants (82.3%) did not discuss their HDS use with their hepatology provider. Of the participants, 9 reported that they added supplements to their regimens during the pandemic; the most common supplements added included vitamin D (n = 4), vitamin C (n = 3) and zinc (n = 5).
Discussion
Our study shows that the overall use of HDS among patients with CLD during the COVID-19 pandemic was 68.2%. Vitamin D was the most utilized supplement, with more than 50% reporting its usage. Of note, most participants (63.2%) did not feel that HDS use improved their health during the pandemic. An even greater percentage of participants (82.3%) did not contact their physicians to discuss ongoing dietary supplementation use.
Prior to COVID-19, NHANES demonstrated that dietary supplement use increased from 49.5% in the 2009-2010 cycle to 57.4% in the 2017-2018 cycle.7-8 Our study demonstrated a high utilization of dietary supplements (68.2%) in adults with CLD. Only 9 participants reported new HDS use during the study period, and we were unable to demonstrate an increase in use during the COVID-19 pandemic due to an absence of a baseline comparison group. Iron was not included in our questionnaire for HDS, which potentially underestimated reporting compared with NHANES.
Liver injury from HDS use is potentially harmful in patients with CLD. A prospective study by the DILIN reported an increase in HDS-induced liver injuries from 7% in 2004-2005 to 20% in 2013-2014 (P = .0007) due to their increased use.9 The major implicated agents include anabolic steroids, green tea extract and multi-ingredient nutritional supplements (MINS). A cross-sectional study using an NHANES database between 2001and 2011 reported that 41 out of 573 patients with CLD were taking HDS with potential hepatotoxicity; the most common were saw palmetto, green tea extract, Hydroxycut® and Herbalife.10 In our survey, 20.8% of study participants, 6.5% of high-risk post-transplant patients and 13.4% of the patients with autoimmune liver disease confirmed green tea extract use, a class A hepatotoxic agent per the liver toxicology database.11 In addition, 30% of our participants reported taking immunosuppressants, with tacrolimus (9.4%) being the most common. A recent review reported a significant interaction between tacrolimus and cytochrome inhibitor-modulating supplements such as ginger and turmeric, potentially enhancing drug nephrotoxicity.12-13 Our autoimmune liver disease and liver transplant participants used immune-enhancing supplements that could adversely affect their immunosuppression.
In our study, 82.3% of participants responded that they did not discuss using dietary supplements with their liver specialists. This finding is in line with a previous study by Gardiner, et al., who observed a non-reporting rate of 69%.14 Patients may not consider dietary supplements medically relevant to disclose or feel embarrassed to admit their use.15
Conclusions
We present the only study on HDS use in patients with CLD during the COVID-19 pandemic. Healthcare providers should be mindful that more than 50% of patients with CLD use HDS and more than 80% do not talk with their healthcare provider about this use. The potential benefits and risks of HDS use are largely unmonitored in patients with CLD. We remind all healthcare providers to be mindful of potential dietary supplementation use, especially in patients with CLD, and to educate all patients about the potential risks and benefits of using dietary supplements.
Acknowledgments
None.
Footnotes
Conflict of Interest
None.
Financial Support and Disclosures
Avleen Kaur: none, Ahyoung Kim: none; Lisa Yanek: none; Yisi Liu: none; Xueting Tao: none; Anna Peeler: none; Doug Mogul: Consults for Mirum Pharmaceuticals; James Hamilton: No financial disclosures; Grant Support: Copper Homeostasis and Liver Function, NIH/NIDDK, R01DK117396; Gerard E. Mullin: No financial disclosures, Grant Support: Biofilm Epidemiology and Mechanisms in Colon Cancer, National Cancer Institute. R01CA196845.
Financial support statement
This project was supported by the Johns Hopkins School of Medicine Biostatistics, Epidemiology and Data Management (BEAD) Core through the department of medicine at Johns Hopkins University School of Medicine to defray the cost of consultation and dissemination of the survey, data analysis and interpretation.
References
- 1.Zheng M, Yalamanchili S, Shah M, Reddy SS, Navarro V, Halegoua-De Marzio D. Herbal and Dietary Supplement Use among US Adults with Chronic Liver Disease. Gastroenterology. 2017;152(5):S1110-S1110. doi:10.1016/S0016-5085(17)33738-1 [Google Scholar]
- 2.Burnett BP, Mullin G. Therapeutic food claims: a global perspective. Curr Opin Clin Nutr Metab Care. 2017;20(6):522-528. doi:10.1097/MCO.0000000000000418 [DOI] [PubMed] [Google Scholar]
- 3.Navarro VJ, Khan I, Björnsson E, Seeff LB, Serrano J, Hoofnagle JH. Liver injury from herbal and dietary supplements. Hepatology. 2017;65(1):363-373. doi:10.1002/hep.28813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merola E, Pravadelli C, de Pretis G. Prevalence of liver injury in patients with coronavirus disease 2019 (COVID-19) : a systematic review and meta-analysis. Acta Gastroenterol Belg. 2020;83(3):454-460. [PubMed] [Google Scholar]
- 5.Bulatova N, Younes S, Arabiyat M, et al. Use of traditional and complementary medicine for COVID 19 prophylaxis among healthcare professionals and students in Jordan: A cross-sectional study. PLoS One. 2022;17(10):e0276015. doi:10.1371/journal.pone.0276015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kesar V, Channen L, Masood U, et al. Liver Transplantation for Acute Liver Injury in Asians Is More Likely Due to Herbal and Dietary Supplements. Liver Transpl. 2022;28(2):188-199. doi:10.1002/lt.26260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lam CS, Koon HK, Chung VC, Cheung YT. A public survey of traditional, complementary and integrative medicine use during the COVID-19 outbreak in Hong Kong. PLoS One. 2021;16(7):e0253890. doi:10.1371/journal.pone.0253890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao L, Tyson N, Liu J, Hébert J, Steck S. Trends in Dietary Supplement Use Among US Adults Between 2009 and 2018. Curr Dev Nutr. 2021;5(suppl 2):701-701. doi:10.1093/cdn/nzab045_083 [Google Scholar]
- 9.Navarro VJ, Barnhart H, Bonkovsky HL, et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology. 2014;60(4):1399-1408. doi:10.1002/hep.27317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navarro VJ, Khan I, Björnsson E, Seeff LB, Serrano J, Hoofnagle JH. Liver injury from herbal and dietary supplements. Hepatology. 2017;65(1):363-373. PMID:27677775 doi:10.1002/hep.28813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green Tea. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet] National Institute of Diabetes and Digestive and Kidney Diseases; 2020. [Google Scholar]
- 12.Parvez MK, Rishi V. Herb-Drug Interactions and Hepatotoxicity. Curr Drug Metab. 2019;20(4):275-282. doi:10.2174/1389200220666190325141422 [DOI] [PubMed] [Google Scholar]
- 13.Miedziaszczyk M, Bajon A, Jakielska E, et al. Controversial interactions of tacrolimus with dietary supplements, herbs and food. Pharmaceutics. 2022;14(10):2154. doi:10.3390/pharmaceutics14102154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardiner P, Graham RE, Legedza AT, Eisenberg DM, Phillips RS. Factors associated with dietary supplement use among prescription medication users. Arch Intern Med. 2006;166(18):1968-1974. doi:10.1001/archinte.166.18.1968 [DOI] [PubMed] [Google Scholar]
- 15.de Boer YS, Sherker AH. Herbal and Dietary Supplement-Induced Liver Injury. Clin Liver Dis. 2017;21(1):135-149. doi:10.1016/j.cld.2016.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]


