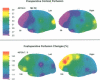
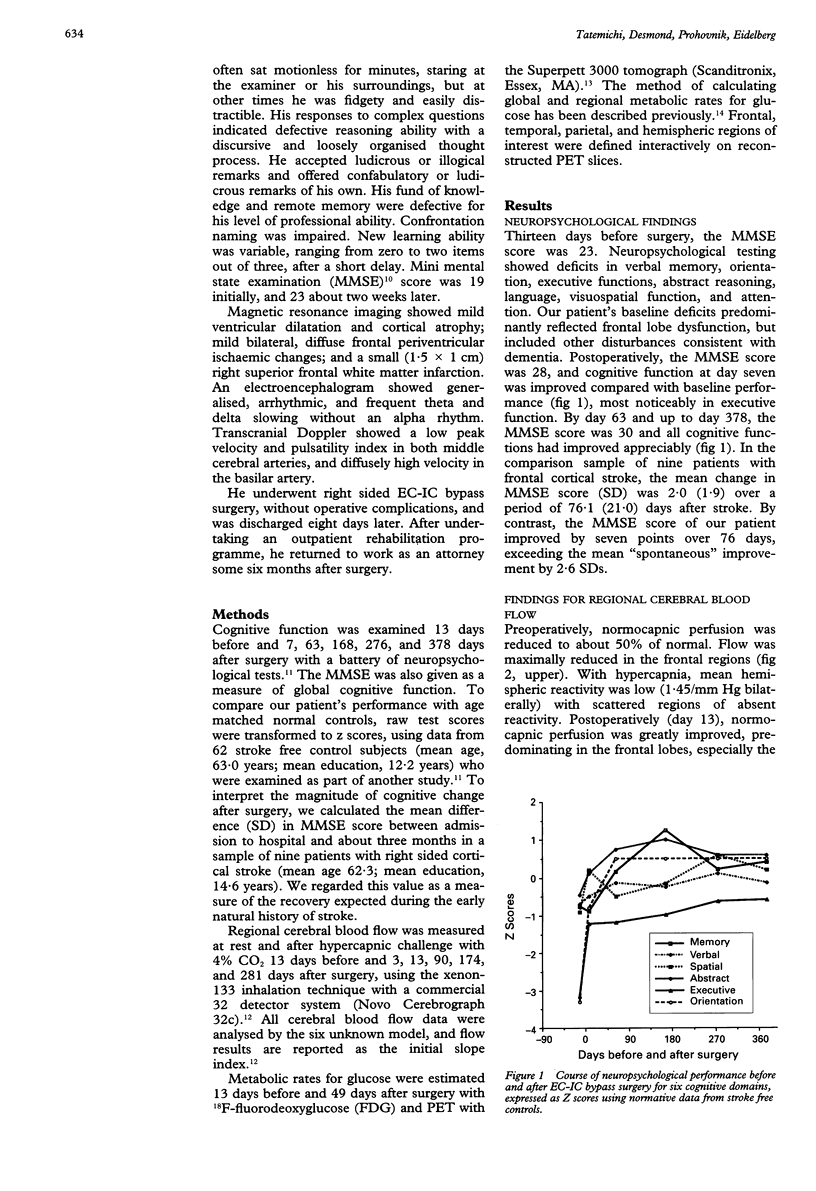
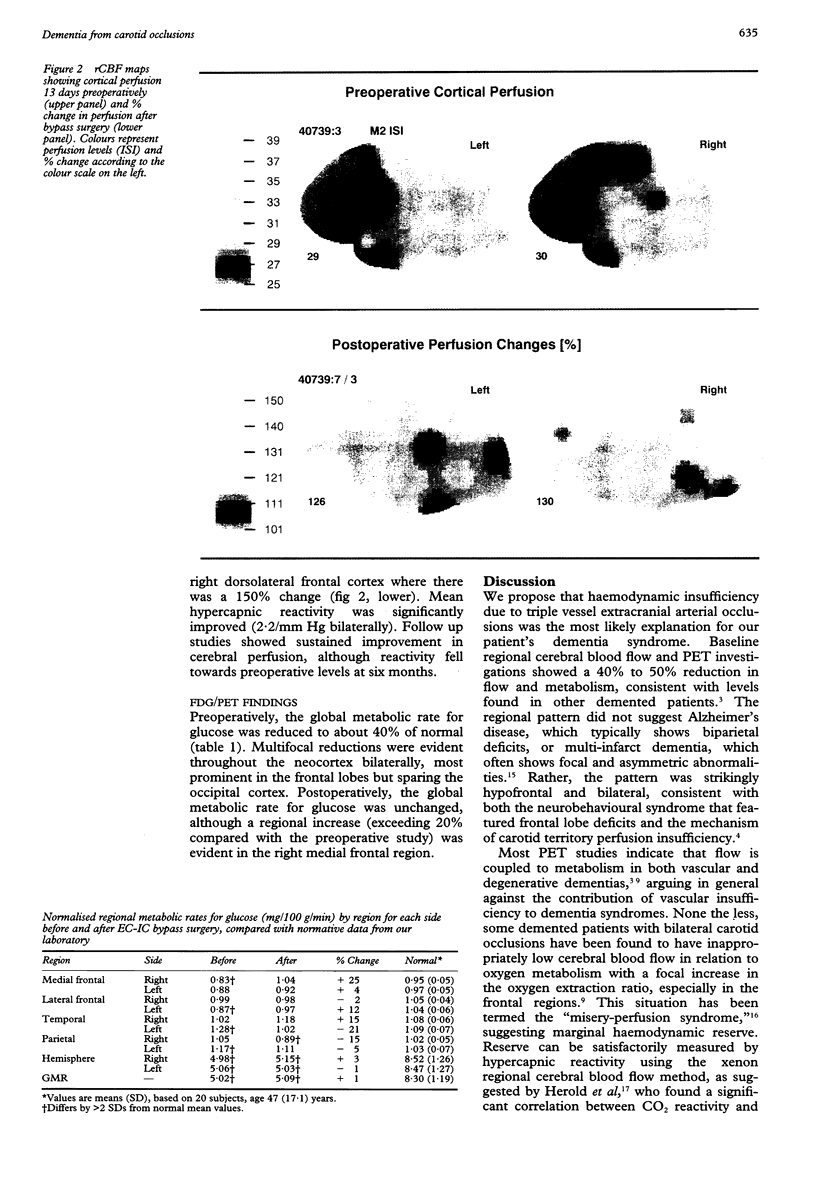
Abstract
A 55 year old man with bilateral internal carotid and unilateral vertebral artery occlusions presented subacutely with profound behavioural and cognitive changes featuring frontal lobe deficits. Neuropsychological testing showed severe cognitive impairment compatible with dementia. Anatomical imaging showed only a small right superior frontal infarction. Cerebral blood flow was severely reduced, with profound hypofrontality and limited hypercapnic reactivity, and cerebral metabolism was reduced primarily in the medial frontal lobes. After right sided extracranial to intracranial cerebral bypass surgery, both flow and metabolism improved, as did behavioural and neuropsychological deficits. Perfusion insufficiency from bilateral carotid occlusions, with secondarily reduced metabolism in the frontal zones bilaterally, may be an unusual cause of a reversible frontal dementia syndrome.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abiko S., Matsunaga T., Yamashita T., Fujii M., Aoki H. [Anastomosis of the superficial temporal artery to the middle cerebral artery for occlusive disease of the bilateral internal carotid arteries with dementia]. No Shinkei Geka. 1990 Nov;18(11):1047–1052. [PubMed] [Google Scholar]
- Baron J. C., Bousser M. G., Rey A., Guillard A., Comar D., Castaigne P. Reversal of focal "misery-perfusion syndrome" by extra-intracranial arterial bypass in hemodynamic cerebral ischemia. A case study with 15O positron emission tomography. Stroke. 1981 Jul-Aug;12(4):454–459. doi: 10.1161/01.str.12.4.454. [DOI] [PubMed] [Google Scholar]
- Benson D. F., Kuhl D. E., Hawkins R. A., Phelps M. E., Cummings J. L., Tsai S. Y. The fluorodeoxyglucose 18F scan in Alzheimer's disease and multi-infarct dementia. Arch Neurol. 1983 Nov;40(12):711–714. doi: 10.1001/archneur.1983.04050110029003. [DOI] [PubMed] [Google Scholar]
- Eidelberg D., Moeller J. R., Dhawan V., Spetsieris P., Takikawa S., Ishikawa T., Chaly T., Robeson W., Margouleff D., Przedborski S. The metabolic topography of parkinsonism. J Cereb Blood Flow Metab. 1994 Sep;14(5):783–801. doi: 10.1038/jcbfm.1994.99. [DOI] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E., McHugh P. R. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frackowiak R. S., Pozzilli C., Legg N. J., Du Boulay G. H., Marshall J., Lenzi G. L., Jones T. Regional cerebral oxygen supply and utilization in dementia. A clinical and physiological study with oxygen-15 and positron tomography. Brain. 1981 Dec;104(Pt 4):753–778. doi: 10.1093/brain/104.4.753. [DOI] [PubMed] [Google Scholar]
- Gibbs J. M., Frackowiak R. S., Legg N. J. Regional cerebral blood flow and oxygen metabolism in dementia due to vascular disease. Gerontology. 1986;32 (Suppl 1):84–88. doi: 10.1159/000212835. [DOI] [PubMed] [Google Scholar]
- Herold S., Brown M. M., Frackowiak R. S., Mansfield A. O., Thomas D. J., Marshall J. Assessment of cerebral haemodynamic reserve: correlation between PET parameters and CO2 reactivity measured by the intravenous 133 xenon injection technique. J Neurol Neurosurg Psychiatry. 1988 Aug;51(8):1045–1050. doi: 10.1136/jnnp.51.8.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc R., Tyler J. L., Mohr G., Meyer E., Diksic M., Yamamoto L., Taylor L., Gauthier S., Hakim A. Hemodynamic and metabolic effects of cerebral revascularization. J Neurosurg. 1987 Apr;66(4):529–535. doi: 10.3171/jns.1987.66.4.0529. [DOI] [PubMed] [Google Scholar]
- Tatemichi T. K., Desmond D. W., Mayeux R., Paik M., Stern Y., Sano M., Remien R. H., Williams J. B., Mohr J. P., Hauser W. A. Dementia after stroke: baseline frequency, risks, and clinical features in a hospitalized cohort. Neurology. 1992 Jun;42(6):1185–1193. doi: 10.1212/wnl.42.6.1185. [DOI] [PubMed] [Google Scholar]
- Tatemichi T. K., Young W. L., Prohovnik I., Gitelman D. R., Correll J. W., Mohr J. P. Perfusion insufficiency in limb-shaking transient ischemic attacks. Stroke. 1990 Feb;21(2):341–347. doi: 10.1161/01.str.21.2.341. [DOI] [PubMed] [Google Scholar]
- Wiebers D. O., Adams H. P., Jr, Whisnant J. P. Animal models of stroke: are they relevant to human disease? Stroke. 1990 Jan;21(1):1–3. doi: 10.1161/01.str.21.1.1. [DOI] [PubMed] [Google Scholar]