Abstract
Candida auris recently emerged as an urgent public health threat, causing outbreaks of invasive infections in healthcare settings throughout the world. This fungal pathogen persists on the skin of patients and on abiotic surfaces despite antiseptic and decolonization attempts. The heightened capacity for skin colonization and environmental persistence promotes rapid nosocomial spread. Following skin colonization, C. auris can gain entrance to the bloodstream and deeper tissues, often through a wound or an inserted medical device, such as a catheter. C. auris possesses a variety of virulence traits, including the capacity for biofilm formation, production of adhesins and proteases, and evasion of innate immune responses. In this review, we highlight the interactions of C. auris with the host, emphasizing the intersection of laboratory studies and clinical observations.
Scope of the public health threat
Since the initial description of this new species in 2009, Candida auris has caused devasting outbreaks of difficult-to-treat infections in healthcare facilities spanning 6 continents [1–8]. C. auris appears to have emerged relatively independently in separate locations and isolates cluster into at least 4 distinct geographic clades [2,9,10]. C. auris effectively colonizes the skin of patients, particularly those with extended hospital stays and prior antibiotic or antifungal exposure [11–13]. A subset of these patients can develop invasive infection, often in the setting of indwelling medical devices, feeding tubes, and other surgical procedures [11–13]. C. auris isolates exhibit high rates of drug resistance to common classes of antifungals, often narrowing treatment options [14,15]. Mortality rates vary across studies, but reports as high as 60% have been documented [2]. Due to these factors, the Centers for Disease Control and Prevention classifies drug-resistant C. auris as an urgent threat, placing the organism in the most serious category [16].
Although C. auris infections tend to cluster as epidemic outbreaks, the overall cases have continued to rise in recent years. Reports can include cases of infection, where C. auris is isolated from a site of infection, or cases of colonization, where C. auris is identified on the skin or other site and not producing symptomatic infection. In the United States, cases of C. auris skin colonization nearly tripled from 1,310 cases in 2020 to 4,041 cases in 2021 [17]. Cases of invasive candidiasis and candidemia due to C. auris are also on the rise in many areas. For example, a report examining critically ill patients in India with candidemia found C. auris to be the most common species, responsible for approximately 40% of all cases [18]. A study from Kuwait also reported an increasing proportion of C. auris bloodstream isolates (13.7%) compared to years prior [19]. In the US, the number of invasive clinical infections of C. auris also rose sharply, nearly doubling from 756 cases in 2020 to 1,471 cases in 2021 [17]. Clinical investigations have additionally questioned the likelihood of developing an invasive infection after C. auris establishes skin colonization. For patients previously known to harbor C. auris on skin, the rate of progression to candidemia ranges from <25% to 74.5% [20,21]. These findings point to the need for greater understanding of skin colonization not only to combat C. auris growth on skin but also to prevent subsequent invasive infections.
Skin colonization by C. auris
Multiple clinical studies have analyzed the prevalence of C. auris skin colonization in outbreak settings and have reported colonization for 37.5% to 86% of participants [4,22–25]. C. auris skin colonization cases appear to be increasing in recent years, paralleling the rise of invasive disease. For example, reported rates of skin colonization in the US increased over 200% in 2021 compared to 2020 [17]. Common sites of skin colonization sampling include the axilla and groin, but a recent work pointed to the importance of expanding the sampling areas to other highly colonized areas including nares, fingertips/palms, toe web, and perianal area [25]. Of note, oral C. auris colonization has not been commonly noted for patients. This is consistent with reports of poor oral colonization in mice and C. auris susceptibility to the salivary antimicrobial peptide histatin 5 [26,27]. Single-site testing found that nares was the most sensitive testing site for determining colonized patients (53.1% sensitivity), and the combination of nares with palm and fingertips yielded the highest 2-site sensitivity (76.1% sensitivity) [25]. The colonization of the palms/fingertips of patients is particularly concerning for efficient spread person-to-person or via high touch surfaces [25]. C. auris may also spread via contaminated gloves. In laboratory studies, viable colonies of C. auris could be recovered from fingertips of both latex and nitrile gloves, as well as from a urinary catheter surface after encounter with wet or dried contaminated gloves [28]. It is likely that the ability of C. auris to remain viable under dry conditions on numerous abiotic surfaces contributes to potential contamination of catheters, other medical devices, and shared medical equipment [23,29–31]. Hand hygiene remains a critical component of controlling outbreaks.
As high-burden skin colonization poses significant risk for hospital transmission and development of invasive infection, there has been great interest in exploring methods to decolonize skin. Bathing patients with a 2% solution of chlorhexidine gluconate (CHG) is a common approach for the cleansing of patient skin in many clinical settings, including intensive care units. In vitro studies show that C. auris isolates are inhibited by CHG at <0.02% [32,33]. However, despite this in vitro activity, C. auris can persist on patient skin in healthcare facilities that implement routine CHG bathing [23,25,34]. The reasons for this appear multifactorial. Routine bathing may not adequately distribute CHG to all colonized sites. In studying CHG bathing for colonized patients in a skilled nursing facility in Illinois, Proctor and colleagues found that fewer than 10% of skin sites received concentrations sufficient to reduce the odds of C. auris colonization, with the minimal concentration associated with significantly reduced colonization calculated to be 625 μg/ml [25]. This calculated CHG concentration is equivalent to approximately 0.6%, 20 to 39 times higher than the CHG concentration noted for growth inhibition of C. auris in vitro, suggesting decreased effectiveness on skin compared to in vitro conditions [25,32,33].
Animal models of C. auris skin colonization recapitulate the limitations of CHG treatment for decolonization of skin. In a murine skin model for prevention of C. auris colonization, preexposure and ongoing treatment with 2% CHG wipes could prevent colonization following low inoculum (107 CFU) exposure. However, treatment reduced but did not fully prevent C. auris colonization upon exposure to higher burden (109 CFU) [35]. When CHG was used as a treatment for established C. auris colonization, the viable burden on the skin surface decreased but was not fully eradicated. Furthermore, CHG treatment minimally impacted the viable burdens of deeper skin samples [35]. Similar observations were found using an ex vivo porcine skin model where 2% CHG treatment of C. auris on skin resulted in a meager 0.5 log-reduction in viable yeast [36]. While CHG treatment reduces C. auris growth on skin, it does not appear to eliminate it completely. C. auris likely persists in deeper tissues and follicles, allowing it the opportunity to proliferate [35,36].
C. auris exhibits the capacity to form biofilms during skin colonization (Fig 1) [37]. Growth in this form may further limit the activity of CHG, as in vitro biofilms (when compared to planktonic cells) are approximately 10-fold more resistant to the activity of CHG, and are not fully eradicated by treatment with 2% CHG [36,38]. Ex vivo studies suggest that the addition of 70% isopropyl alcohol and commonly used topical essential oils, including tea tree (Melaleuca alternifolia) oil and lemongrass (Cymbopogon flexuosus) oil, can improve the activity of CHG [36]. Study using a guinea pig model of skin colonization also suggests that the addition of systemic antifungal therapy may also help to decrease burden of skin colonization [39]. Given the lack of clinical data to show successful decolonization, there are currently no CDC-recommended strategies to eradicate C. auris from skin (https://www.cdc.gov/fungal/candida-auris/c-auris-infection-control.html).
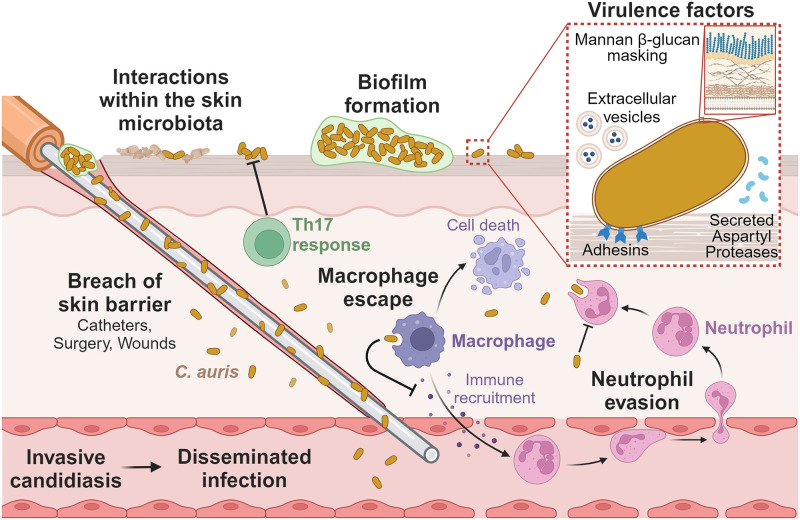
Fig 1. Candida auris mechanisms of pathogenesis.
C. auris colonizes and persists on the skin, and breaches of the skin barrier can lead to invasive candidiasis and hematogenous spread of infection. Interactions with other skin microbiota (e.g., Malassezia spp., Klebsiella pneumoniae, Staphylococcus hominis) influence the growth of C. auris on the skin. Biofilm formation of C. auris increases resistance to antimicrobial treatment on the skin surface. C. auris produces multiple virulence factors to release into the surrounding microenvironment including SAPs and EVs. Adhesins promote attachment to the skin surface, and mannans in the cell wall mask β-glucans from PRRs on immune cells. C. auris evades neutrophil recognition and phagocytosis. C. auris escapes macrophage killing, inhibits macrophage-mediated immune recruitment, and induces macrophage cell death. In contrast, the Th17 T-cell response inhibits C. auris skin colonization. The figure was designed using Biorender. EV, extracellular vesicle; PRR, pathogen recognition receptor; SAP, secreted aspartyl protease.
Acquisition of C. auris correlates with prior receipt of antibiotics and antifungals, suggesting a role for skin dysbiosis [11–13,25]. Proctor and colleagues examined the skin microbiome of patients with and without C. auris colonization. They found that patients with skin mycobiomes dominated by Malassezia species were at a lower risk of C. auris dominance on the skin [25]. Other mycobiomes were primarily dominated by a diverse group of Candida spp., which appeared to represent a transitional state of the skin mycobiome. Mycobiomes with shared dominance by a variety of Candida species switched to a singular dominance by C. auris 30% to 50% of the time. These data highlight the importance of studying Malassezia species during interactions within the skin microbiome (reviewed in [40]). The group also examined bacterial communities and identified organisms with higher abundance in C. auris-colonized patients (Proteus mirabilis, Klebsiella pneumoniae, Providencia stuartii, and Pseudomonas aeruginosa). In contrast, Staphylococcus hominis was more abundant in the patients without C. auris colonization. Further understanding of how the skin microbiome influences C. auris may help to identify strategies to prevent or eliminate colonization. For example, cleansing methods that promote healthy skin microbiota may help to decrease the rate of C. auris skin colonization.
Adhesion, biofilm formation, and environmental persistence
C. auris survives in harsh conditions on both nonliving and living surfaces in healthcare environments. C. auris has been isolated from multiple sources in hospitals including floors, bed rails, bed sheets, door handles, oxygen masks, and sinks [4,22]. The adaptability of C. auris for growth on a variety of abiotic surfaces has been described in laboratory studies, demonstrating survival of C. auris on plastic for multiple weeks in low-moisture environments [29,30,41]. A unique profile in withstanding salt stress and osmotic stress may play an addition role in long-term environmental persistence on surfaces in the healthcare settings [42,43]. Biofilm formation likely contributes to C. auris environmental persistence and tolerance of biocides. Clinical isolates form in vitro biofilms in tissue culture media (RPMI) on plastic with some heterogeneity in density noted among clades and isolates [27,44–46]. Even more dense biofilms form in synthetic skin/sweat media, suggesting that devices in contact with skin may be particularly prone to contamination by C. auris biofilms [37,47]. C. auris biofilms formed in this milieu can survive desiccation for weeks [37]. Kean and colleagues further showed that the biofilm phenotype promotes resistance to antiseptics, including H2O2, povidone iodine, and CHG, additionally contributing to persistence in healthcare settings [38]. Biofilms formed during skin colonization are expected to be highly tolerant to these therapies, as is observed clinically for CHG [23,25,34].
Surface adhesion is a critical first step for biofilm formation and skin colonization. For C. albicans and other Candida spp., roles for specific adhesins have been well described [48–50]. Members of the agglutin-like sequence (ALS) family of adhesins function in adherence and as virulence factors in Candida species and homologs to ALS family proteins have been identified in C. auris [51,52]. The roles of these adhesins appear to vary among isolates and across the C. auris life cycle. Using a 3D in vitro wound infection model, Brown and colleagues examined transcriptional patterns associated with biofilm formation [53]. They found ALS5 to be associated with biofilm formation for isolates with an aggregative/clustering growth phenotype, but not for those lacking this phenotype, suggesting a role for ALS5 during biofilm growth for these isolates. This study and others have noted heterogeneity in biofilm formation across clinical isolates [45,54], with one potential factor being the lack of cell wall and ALS-like genes in Clade II strains [55]. This finding may aid in explaining heterogeneity in biofilm formation, lack of outbreaks for Clade II isolates, and lower skin colonization of Clade II strains in a mouse model [35,55]. Bing and colleagues found that expression of ALS4 across C. auris isolates correlated with protein-dependent aggregation and heightened biofilm formation [56,57]. Whole genome analysis revealed genomic amplification for ALS4 with high copy number variations among clinical isolates. The replicative age of C. auris also influences expression of adhesins, where older C. auris cells show increased expression of ALS5 and exhibit thicker cell walls [58].
C. auris has also been shown to express at least one unique adhesin. Santana and colleagues identified the previously uncharacterized adhesin Scf1 with homologs only in C. auris and closely related strains of C. haemulonii [59]. Among C. auris isolates included in the study, transcriptional abundance of SCF1 positively correlated with adhesion of the isolates. Loss of SCF1 and IFF4109, a conserved member of the IPF Family F/Hyphally Regulated adhesin family, in C. auris strain resulted in decreased fungal loads in an immunocompromised mouse model of disseminated infection and decreased ability to colonize ex vivo human skin and proliferate on the luminal surface of a polyethylene rat central venous catheter. Scf1 interactions were linked to exposed cationic residues, in contrast to the hydrophobic interactions typically observed for fungal adhesions. This novel adhesin appears to contribute to the capacity of C. auris to colonize skin, form biofilm-associated infections, and persist on surfaces.
It is interesting to speculate how the skin microbiota may influence C. auris adhesion, biofilm formation, and skin colonization. Patients with skin mycobiomes dominated by Malassezia species appear to be at a lower risk of C. auris dominance on the skin [25]. Similarly, Staphylococcus hominis abundance negatively correlates with C. auris colonization. Mechanistic insight into these interactions is limited. However, fungi, including common probiotic strains Saccharomyces cerevisiae and Issatchenkia occidentalis, have been shown to reduce C. auris adhesion to plastic by up to 60% [60]. It is possible that skin microbiota modulate C. auris adherence properties.
Catheters, breach of skin, and other risk factors for C. auris-invasive disease
Patients with C. auris skin colonization become increasingly at risk for invasive disease when there is a breach of the skin barrier that can serve as a portal of entry to the bloodstream and deeper tissues. For example, medical device implantation, recent surgery, total parenteral nutrition (TPN) administration, and catheter placement are risk factors for development of invasive C. auris infection in adult populations [11,13,61]. Similarly, a study of pediatric patients with C. auris bloodstream infection found that 82% had undergone central venous catheter placement, and 56% had received TPN [5]. To better understand and potentially predict which skin-colonized patients may progress to develop invasive disease, Garcia-Bustos and colleagues used clinical and epidemiological factors from a single hospital outbreak to construct a scoring system [11]. This study provided a model to estimate the factors posing the greatest risk for C. auris candidemia. They found that TPN to be the greatest risk factor, with recent surgery, central venous catheter placement, and arterial catheter placement among the other independent predictors of candidemia. Catheters likely serve as both a substrate for biofilm formation and a bridge from the epidermal surface to bloodstream (Fig 1). Furthermore, the nutritional components of TPN may promote fungal growth. When compared to other Candida spp., C. auris may have a heightened capacity to cause catheter-associated candidemia, perhaps due to its propensity for skin colonization. Retrospective analysis examining patients with candidemia found that those with C. auris candidemia were more likely to carry the diagnosis of catheter-associated bloodstream infection (89%) than those with candidemia due to other species (47%) [61].
Animal models of catheter-associated infection mirror clinical observations of high-burden biofilm growth for C. auris in this setting (Fig 2 and Table 1). Using an indwelling rat jugular venous catheter model, Dominguez and colleagues found multiple C. auris clinical isolates to propagate as biofilms on luminal catheter surfaces [62]. Some isolates appeared to form even thicker biofilms with more extracellular matrix in vivo than in vitro. Vila and colleagues examined 2 C. auris clinical isolates in a murine model of subcutaneous catheter fragment implants [27]. The group included one isolate with high biofilm capacity under in vitro conditions and one with low capacity biofilm formation. Surprisingly, both isolates replicated to burdens beyond C. albicans on catheters in vivo. This suggests that some in vitro conditions may not be representative of biofilm formation in the context of infection and colonization. Although C. auris isolates exhibit heterogeneity in the capacity for biofilm formation, biofilms formed in standard laboratory media on plastic have generally been less dense in comparison to C. albicans [27,32,41,63]. However, biofilm formation within models mimicking the skin microenvironment and catheterization have yielded increased recovery for C. auris compared to C. albicans [27,41,47]. Catheter-associated biofilm formation by C. auris further complicates treatment due to increased tolerance of multiple antifungals that occurs during biofilm formation [32,62,64].
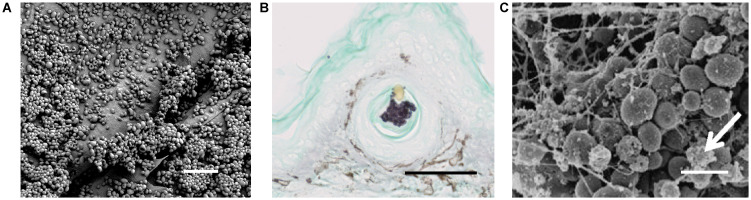
Fig 2. Skin colonization and catheter-associated infection models for C. auris.
(A) C. auris growing on the surface of porcine skin ex vivo, reproduced from Horton and colleagues [37]; measurement bar represents 10 μm. (B) C. auris replicating in the hair follicle of an immunosuppressive murine model of C. auris skin colonization, reproduced from Huang and colleagues [35]; measurement bar represents 50 μm. (C) C. auris growing as a biofilm on the luminal surface of a rat vascular catheter, reproduced from Dominguez and colleagues; measurement bar represents 5 μm [62].
Table 1. In vivo and ex vivo skin and catheter colonization models.
| Infection model | In vivo C. auris findings | Strain(s) | Clade(s), country of isolation | Comparisons and notes | Ref |
|---|---|---|---|---|---|
| Rat central venous catheter | Biofilm formation, drug sequestration | B11104, B11203 (AR-0387), B11211, B11219, B11220 (AR-0381), B11221 (AR-0383), B11785, B11799, B11801, B11804 | Pakistan, Colombia, India, Japan, South Africa, Colombia | Biofilm formation on in vivo catheters, deposition of extracellular matrix | [62] |
| Immunocompetent BALB/c mice, skin topical application | Higher fungal burdens in lung and brain tissue for filamentous cells | BJCA001 | Clade I, China | Morphologies include typical yeast (not induced to form filaments at low growing temperatures), filamentation-competent yeast, and filamentous cells | [69] |
| Ex vivo porcine skin | Biofilm formation and desiccation resistance in skin niche conditions | B11804, B11220 (AR-0381), B11221 (AR-0383), B11801, B11203 (AR-0389), B11219, B11211, B11104, B11799, B11785 | Clades I, II, III, IV, Columbia, Japan, South Africa, India, and Pakistan | C. auris had greater viability after desiccation than C. albicans; C. auris biofilms were denser on porcine skin model grown in sweat media than C. albicans grown with same conditions | [37] |
| BALB/c mice, subcutaneous catheter | Adhesion to catheter, high replication | AR-0382 and AR-0387 | Clade I | Increased recovery of low-biofilm and high-biofilm C. auris strains compared to C. albicans | [27] |
| In vivo guinea pig | MRL 35368 | Oral dosing of ibrexafungerp reduced the skin C. auris fungal burden compared with untreated controls | [39] | ||
| Wild-type and immunodeficient C57BL/6 and C57BL/10 mice, topical application on dorsal skin and pinna areas | Ability to reside in deep compartments of the skin for extended periods of time | AR-0387, AR-0381, AR-0383, AR-0385, NIH clinical center | Clades I, II, III, IV | IL-17R pathway limits C. auris skin colonization | [35] |
| Ex vivo porcine skin | Resistance to killing by chlorhexidine on the skin | B11203 (AR-0389) | Clade I, India | Addition of isopropanol, tea tree oil, or lemongrass oil could augment the activity of chlorhexidine | [36] |
| Ex vivo human and porcine skin | High biofilm burden on skin surface | B11203 (AR-0389), B11219, B11211, B11104, B11804, B11801, B11785, B11799, B11220 (AR-0381), B11221 (AR-0383) | Clades I, II, III, IV, Columbia, Japan, South Africa, India, and Pakistan | C. auris colonizes the skin to greater biofilm burdens than other Candida species, including C. haemulonii | [47] |
| Rat central venous catheter and ex vivo human skin | Increased biofilm colonization on skin and catheter surface with expression of adhesins | AR-0382, AR-0387, AR-0382 Δscf1/Δiff4109, AR-0382 pTEF1-SCF1 | Clade I | Overexpression of SCF1 in the AR-0387 was sufficient to induce skin and catheter colonization of this isolate | [59] |
Secreted aspartyl proteases (SAPs) and cellular morphology
Like other Candida spp., C. auris produces secreted aspartyl proteases (SAPs), which contribute to virulence through the cleavage of host proteins. SAPs can alter adhesive properties, promote tissue invasion, influence immune responses, and disrupt complement signaling [51,52,65,66]. Maybe not surprisingly, SAP production varies among C. auris isolates and environmental conditions [43,67]. For example, Fan and colleagues examined SAP activity for 2 isolates from China (Clade I and Clade III) [67]. The Clade III strain produced the most SAP activity at 37°C with lesser at lower temperatures (30°C and 25°C), while the Clade I strain produced similar amounts across the temperature range [67]. The Clade I strain was more virulent in Galleria and murine models of infection. However, a specific link to virulence is not entirely clear, as many differences were noted between the strains. In addition to the different clade designation, the Clade III isolate exhibited an aggregation phenotype and increased drug resistance, while the Clade I strain did not aggregate. The mechanisms of how SAP production influences C. auris–host interactions remains unclear. Considering the lower temperature of the skin surface, the authors note that decreased SAP production at these temperatures may be beneficial to dampen immune responses during long-term persistence on skin. In addition, C. auris has been noted to produce SAPs at high temperatures as high as 42°C [43]. This is consistent with its observed thermotolerance and suggests a role for SAPs in warm environmental conditions [68].
In addition to genetic factors, SAP production appears to be further influenced by cellular morphology and biofilm formation [46,69]. C. auris typically grows in yeast form, with more rare reports of filamentous structures [43]. However, Yue and colleagues described how passage of C. auris through a mouse via a tail vein bloodstream infection model yielded distinct morphologies [69]. These included typical yeast forms, filament competent forms, and filamentous morphologies. Interestingly, the yeast-filamentous transition was shown to be heritable, while the filament competent-filamentous transition was nonheritable and dependent on temperature. The finding that lower temperatures (20°C and 25°C) promoted filamentous growth for C. auris is distinct from C. albicans, where hyphal growth is triggered by higher temperatures. They examined the impact of morphology on virulence using a systemic infection model of BALB/c mice and found similar viable burdens in the kidney, spleen, and liver, but greater burdens from the brain and lungs of a mice infected with the filamentous form [69]. Upon examination of SAP production at 37°C, both the filament competent and filamentous phenotypes displayed more activity than the yeast forms, suggesting that SAPs may be contributing to the virulence. This was temperature dependent, as at low temperatures the yeast exhibited more activity than the other forms. Other work has shown the presence of elongated, aggregated, and mixed morphologies among C. auris clinical isolates independent of passage through a mammalian host [70]. Similar to the murine studies, the isolates with filamentous morphology appear more virulent in a Galleria mellonella infection model.
Extracellular vesicle formation by C. auris
Diverse fungal species, including C. albicans, secrete extracellular vesicles (EVs), structures of lipid bilayer-enclosed cargo that modulate morphologic changes, host interactions, and drug resistance [71,72]. Like C. albicans, C. auris also produces vesicles during planktonic and biofilm modes of growth; however, some of the cargo and properties differ [72,73]. Zamith-Miranda and colleagues examined C. auris vesicles produced during planktonic growth, comparing them to C. albicans vesicles. While vesicles from both species contained sterols, RNA, protein, and lipids, the specific contents analyzed by proteomics and lipidomics varied significantly, suggesting that their activities may diverge as well. In functional analysis, the group found that C. auris EVs could augment fungal adhesion to epithelial cells, while C. albicans EVs did not. For one (of two) of the C. auris strains tested, EV treatment enhanced replication and survival after phagocytosis by a murine-derived macrophage line. EVs from the 2 C. auris isolates were found to stimulate murine bone marrow–derived dendritic cells (BMDCs) by increasing expression of MHCII and costimulatory molecules in a pattern similar to C. albicans [73]. Comparison of an azole-resistant isolate to an azole-susceptible strain revealed differences in both content and functional activity, suggesting that EVs may be altered in the setting of drug resistance or that they may vary broadly across strains [73]. In other work, C. auris EVs were shown to augment C. auris survival in the presence of amphotericin B, while C. albicans EVs did not, further highlighting differences in EV activities between the species [74].
Zarnowski and colleagues analyzed C. auris EVs produced during biofilm growth, comparing their enclosed cargo to other Candida spp. [72]. The monosaccharide analysis revealed the presence of mannan and glucan in relatively similar ratios across EVs collected from C. albicans, C. parapsilosis, C. tropicalis, C. glabrata, and C. auris. The mannan:glucan ratio was also consistent with the ratio of these polysaccharides in the extracellular matrix of C. albicans biofilms, as EVs have been shown to deliver and deposit a mannan–glucan complex capable of antifungal sequestration [75]. On proteomic analysis, they found high variability across the EV proteomes but identified a set of cargo proteins common to all EVs. Genetic disruption of these proteins impacted biofilm-associated drug tolerance with the exogenous addition of EVs reversing the phenotype across species. This suggests that C. auris EVs may act cooperatively with other Candida spp. In subsequent work examining adhesion, competitive interactions for EVs across species were identified [76]. Little is known about the interaction of C. auris biofilm EVs with the host, but considering studies with planktonic EVs, they may influence adhesion to host and/or immune recognition [73].
Modeling of systemic C. auris infection
Many studies have analyzed C. auris virulence utilizing a variety of animal models, including mice, zebrafish, G. mellonella, and Caenorhabditis elegans (Tables 2 and 3). Murine models have primarily included pharmacologically immunosuppressed animals, but complement 5-deficient mice have been used as well [77]. C. auris exhibits increased mortality and higher fungal burden when compared to related and non-albicans species such as C. haemulonii, C. glabrata, and C. parapsilosis, in murine, zebrafish, and Galleria models [32,54,68,78–81]. However, there is some variability in mortality overserved for individual C. auris isolates in both murine and Galleria models [32,54,67,82]. Studies have been more mixed for comparison of C. auris and C. albicans. Murine models have generally observed lower mortality for C. auris, while Galleria and zebrafish studies have reported comparable or increased virulence compared to C. albicans [26,32,44,54,68,78–81,83,84]. In a Galleria model, higher mortality has been observed for isolates that do not aggregate compared to aggregating strains [32,54]. Studies using this model have also found variability in virulence based on the body site of isolate collection, with higher mortality for bloodstream isolates compared to urine or respiratory samples [44]. As patients with C. auris bloodstream infections are anticipated to have the same strains as colonizers of the skin, respiratory tract, and/or urine, this finding suggests a possible phenotypic alteration or switch during human bloodstream infection, as has been described in mice [44,69].
Table 2. Vertebrate models of invasive disease.
| Infection model | In vivo C. auris findings | Strain(s) | Clade(s), country of isolation | Comparisons and notes | Ref |
|---|---|---|---|---|---|
| Immunosuppressed BALB/c mice, disseminated candidiasis (cyclophosphamide-treated) | Burden in kidney, forming aggregates | TA005-14 | n/a, Israel | Increased fungal burden and mortality compared to C. hauemulonii, less than C. albicans | [68] |
| Immunocompetent BALB/c mice, disseminated candidiasis | Higher fungal burden in spleen compared to C. albicans | BJCA001 | Clade I, China | No lethality in mouse | [43] |
| Immunocompetent ICR mice, disseminated candidiasis | High burdens in kidney, also recovery from spleen, liver, and lungs | VPCI 479/P13 and VPCI 510/P14 | n/a, India | Comparable mortality and fungal burden to C. albicans | [78] |
| Zebrafish, larval hindbrain injection | Low neutrophil recruitment, impaired NETosis | B11203 (AR-0389) | Clade I, India | C. albicans triggers neutrophil responses in zebrafish to a greater extent than C. auris | [85] |
| Immunocompetent BALB/c mice, intraperitoneal injection, intravenous injection | Higher fungal burdens in lung and brain tissue for filamentous cells | BJCA001 | Clade I, China | Morphologies include typical yeast (not induced to form filaments at low growing temperatures), filamentation-competent yeast, and filamentous cells | [69] |
| ICR CD-1 immunosuppressed (cyclophosphamide and cortisone acetate treated), disseminated candidiasis and vaccination | Fungal burdens in kidney and heart; biofilm formation; resistance to killing | CAU-09 (AR-0389) | Clade I | NDV-3A vaccine reduced mortality in C. auris-infected mice | [93] |
| C5-deficient A/J mice, disseminated candidiasis (cyclophosphamide-treated) | Rapid proliferation in target organs and rapid fatal response | AR-0381 and AR-0386 | Clade II, Clade IV | NE−/− and C57BL/6J mice survived C. auris infection, even upon cyclophosphamide treatment | [95] |
| Immunosuppressed BALB/c mice | n/a | Ca432 and Ca446 | n/a, Colombia | Similar lethality to C. haemulonii species complex | [80] |
| Immunocompromised neutropenic BALB/c mice, disseminated candidiasis (cyclophosphamide-treated) | High burdens in heart and kidneys | C. auris 196, C. auris 164, NCPF 8984, CBS 12373, NCPF 13042, C. auris 204, C. auris I-24, C. auris 16565, | South Asian (Oman, England), East Asian (Japan, Korea), South African (England), South American (Israel, Chicago) | High lethality strains were similar to C. albicans in heart and kidney burden; intermediate and low lethality strains had lower burdens | [82] |
| Immunocompetent A/J mice and immunosuppressed (cyclophosphamide treated) C57BL/6 mice, disseminated candidiasis | Disseminated spread to tissues including kidney, brain, and heart | AR-0386 and AR-0389 | Clade I and IV | Monoclonal antibodies against β-1,2-mannotriose, hyphal wall protein 1, and phosphoglycerate kinase 1 enhanced survival and decreased target organ fungal burden | [77] |
| Zebrafish, larval hindbrain injection | Low neutrophil recruitment, high burden | B11203 (AR-0389) | Clade I, India | Disruption of PMR1 and VAN1 causes increased neutrophil recruitment and lower fungal burden compared to WT | [96] |
| Zebrafish, larval swim-bladder inoculation | Induce proinflammatory cytokine genes and down-regulate recruitment genes | C. auris SI-18-CAU-HEM | n/a, Thailand | Highest lethality and burden for C. auris compared to C. haemulonii and C. albicans | [79] |
| Immunocompetent BALB/c mice, intravenous injection | Greater production of SAPs at 25 and 30°C, greater copies of the active Zorro3 retrotransposon | BJCA001 and BJCA 002 | Clade I and III, China | [67] | |
| C57BL/6 immunocompetent mice, disseminated candidiasis | Low innate immune cell recruitment to kidneys and spleen, blunted proinflammatory cytokine response | BJCA001 | Clade I, China | Decreased immune cell recruitment compared to C. albicans | [87] |
| ICR CD-1 immunosuppressed (cyclophosphamide and cortisone acetate treated), disseminated candidiasis | Fungal burdens in kidney and heart | CAU-09 (AR-0389) | Clade I | HX01 mAb reduced mortality in mice infected with C. auris | [92] |
| ICR CD-1 immunosuppressed (cyclophosphamide and cortisone acetate treated), disseminated candidiasis | Increased mortality and higher fungal burdens in the kidneys and heart with expression of adhesins | AR-0382, AR-0387, AR-0382 Δscf1/Δiff4109, AR-0382 pTEF1-SCF1 | Clade I | Loss of adhesins SCF1 and IFF4109 increased survival and reduced dissemination to the kidneys and heart | [59] |
Table 3. Invertebrate models of invasive disease.
| Infection model | In vivo C. auris findings | Strain(s) | Clade(s), country of isolation | Comparisons and notes | Ref |
|---|---|---|---|---|---|
| Galleria mellonella | Psuedohyphae production, dissemination in vivo | Clinical isolates from National Health Service hospitals in the United Kingdom | n/a, United Kingdom | Aggregating isolates showed less lethality than non-aggregating | [32,54] |
| Galleria mellonella | Lysis of hemocytes, high fungal burden, tissue invasion | Ca432, Ca446, Ca386, Ca881, Ca885 | n/a, Colombia | Higher lethality than C. haemulonii species complex | [80] |
| Galleria mellonella | Melanization, decreased activity scores, cocoon formation | C. auris DSM 21092 Cau40, Cau63 | n/a, Colombia | Clinical strains were more virulent than reference strains, no difference in mortality between Agg vs. non-Agg strains | [84] |
| Galleria mellonella | Increased growth rate or secretion of virulence factors at 37°C compared with 30°C | BJCA001, RICU13 | Clade I, III | Typical yeast and aggregating forms less virulent than filamentous and nonaggregating forms | [70] |
| Galleria mellonella | Melanization, filament and pseudohyphal formation, and high tissue invasiveness | 2018-1-124819, Cj104, Cj98, 253107, 182482, 312755, Cj197, Cj198, Cj175, Cj173 | n/a, Spain | C. auris isolates less virulent than C. albicans but more virulent than C. parapsilosis; aggregative phenotypes less pathogenic than nonaggregative | [81] |
| Galleria mellonella and C. elegans | Clinical blood, oropharyngeal, and urine isolates from the Hospital Universitario y Politécnico La Fe of Valencia, Spain and the Institut für Hygiene und Mikrobiologie, Würzburg, Germany | n/a, Spain and Germany | Galleria model showed variability in virulence based on site of C. auris isolation with highest virulence in blood isolates | [44] | |
| Galleria mellonella | Greater production of SAPs at 25 and 30°C, greater copies of the active Zorro3 retrotransposon | BJCA001 and BJCA 002 | Clade I and III, China | [67] | |
| Galleria mellonella | Invades respiratory system, high immunogenic activity causing inflammatory nodules of aggregated plasmatocytes | CJ175, CJ101 | Clade III, n/a | Less pseudohyphae production than C. albicans and C. glabrata; unique tropism for C. auris | [97] |
Immune responses to C. auris ex vivo, during infection, and on skin
Innate immune responses to C. auris have been explored using a combination of murine models, zebrafish models, primary leukocytes, and cell lines. Examination of human neutrophil interactions with C. auris revealed impaired phagocytosis and killing of C. auris compared to C. albicans [85]. Poor neutrophil phagocytosis for C. auris was observed to be conserved across a variety of strains and clades [86]. Unlike the response to C. albicans, C. auris induced minimal reactive oxygen species (ROS) and did not trigger the formation of neutrophil extracellular traps (NETs). In other work, Wang and colleagues similarly examined ex vivo C. auris–neutrophil interactions [87]. Compared to C. albicans, they observed decreased phagocytosis of C. auris for both murine and human neutrophils. The impaired phagocytosis also correlated with a decreased ability of neutrophils to kill C. auris. Using an immunocompetent murine model of disseminated candidiasis, the group also showed diminished neutrophil recruitment to C. auris in the kidneys and spleen, which correlated with high fungal burdens in these organs when compared to C. albicans. To assess if the outer cell wall mannan layer may be involved in the poor phagocytosis of C. auris, Horton and colleagues constructed mutants with disruption of PMR1, a putative Golgi-associated ATP-ase pump required for N- and O-mannosylation, and VAN1, a putative N-linked mannosyltransferase. Both C. auris mutants exhibited broad disruption of the cell wall mannan layer. The mutants were more readily phagocytosed and killed by human neutrophils ex vivo and in a larval zebrafish hindbrain injection model [86]. The findings suggest that the outer cell wall layer protects C. auris from phagocytic responses.
Wang and colleagues further examined the role of mannosylation on innate immune responses, primarily focusing on murine bone marrow–derived macrophages (BMDMs) [87]. In addition to the observed poor neutrophil responses to C. auris when compared to C. albicans, they also found a blunted proinflammatory response broadly across innate immune cells in vivo and ex vivo. The C. auris cell wall contained more mannan than C. albicans, and the mannan fibrils of C. auris were twice as long when observed by electron microscopy. To analyze the role of C. auris mannans, they constructed mutants with disruption of genes with putative involvement in mannosylation, including PMR1, (N- and O-mannosylation), OCH1 (N-mannosylation), and PMT1 (O-mannosylation). Disruption of either N- or O-mannosylation resulted in proinflammatory responses by BMDMs, consistent with a critical role for mannosylation in C. auris immune evasion and protection from phagocytosis.
C. auris appears to utilize multiple mechanisms for immune evasion. A recent study examining macrophage–C. auris interactions found that C. auris could escape murine BMDMs after phagocytosis [88]. Following intracellular replication, C. auris was shown to essentially deplete macrophage glucose concentrations and provoke their killing without induction of inflammasome responses. Corresponding to decreased inflammasome activation, C. auris-infected BMDMs had decreased production of cytokine IL-1β in comparison to C. albicans-infected BMDMs.
While some studies have identified immune evasion phenotypes, other work has shown proinflammatory responses for C. auris [89]. Bruno and colleagues examined C. auris interactions with human peripheral blood mononuclear cells (PBMCs) and described a greater proinflammatory transcriptional response when compared to PBMCs exposed to C. albicans [89]. The authors hypothesized that altered mannosylation may play a role and identified a unique M-α-1-phosphate sidechain in the acid-labile portion of C. auris mannan. They found C. auris mannans to display lower binding affinity to recombinant human dectin-2 and mannose receptor pattern recognition receptors (PRRs) when compared to C. albicans mannans. The broad relevance of the mannan linkage is unclear. As other studies have not found this component, it may be dependent on strain or growth conditions [86]. In other work examining C. auris interactions with PBMCs, Navarro-Arias and colleagues found similar cytokine profiles and phagocytosis rates for PBMCs exposed to either C. auris or C. albicans [90]. It appears likely that immune responses vary among C. auris isolates, phagocytes, and model systems.
Understanding features of the C. auris cell wall may not only shed light on the pathogenesis of immune responses to C. auris but also provide insight into treatment strategies. For example, Yan and colleagues noted distinct structures in the mannan of C. auris that were not found in C. albicans, including high amounts of β-1,2-linkages in the terminal mannan chains [91]. The alteration was linked to differential IgG binding. In other work, Candida-specific antibodies targeting cell wall components (β-1,2-mannotriose, hyphal wall protein, or phosphoglycerate kinase 1) were protective in a disseminated murine model [77]. Monoclonal antibodies targeting the immunogenic cell wall protein of C. auris (hyphal-regulated protein Hyr1) were shown to prevent biofilm formation, promote opsonophagocytosis, and protect mice from disseminated infection [92]. In addition, the NDV-3A vaccine developed to target the major C. albicans adhesin Als3 also produces immunity against invasive C. auris infection in mice [93].
Immune responses to C. auris on skin have primarily been analyzed using in vivo murine models with skin colonization of the shaved back or ear pinna [35]. Examining sites of colonization in this model, Huang and colleagues showed accumulation of Th17 T cells, specifically in CD4+ IL-17A+ and CD4+ IL-17F+ cells [35]. In addition to this CD4+ T cell response, an abundance of CD8+ T cells producing IL-17A and IL-17F were observed. Upon disruption of the IL-17 receptor signaling pathway, they found increased recovery of C. auris from the skin, leading to the conclusion that the IL-17 axis participates in limiting C. auris skin colonization in mice. Previous work has reported the IL-17 response to be critical for controlling Candida fungal infections at mucosal surfaces, such as oropharyngeal candidiasis and across the broad spectrum of chronic mucocutaneous candidiasis (reviewed in [94]). It appears similar responses are involved in control of C. auris on skin.
Conclusions and future directions
C. auris is a newly emergent species that persists in the environment and on patient skin despite attempts at decolonization. Hospitalized patients undergoing catheterization and other surgical procedures are at particularly high risk for invasive infection. Cases of C. auris colonization and infection are on the rise, underscoring the need for effective mechanisms for decontamination and prevention of C. auris colonization on skin and abiotic surfaces. For invasive infection, development of new therapeutic options and enhancing immune response to C. auris will be crucial for combatting drug-resistant isolates. Current models for dissecting virulence and host factors involved in C. auris infection have gained traction for providing insight into C. auris pathogenesis. However, directly correlating laboratory and animal model findings with human clinical disease will remain a challenge.
A few outstanding questions remain:
How does Candida auris grow effectively in the skin microenvironment?
How does the human immune system recognize C. auris, and how can recognition be enhanced?
How does isolate-specific variation alter C. auris infection and colonization?
What are the associations among the biological factors of C. auris that contribute to virulence? How do these interface with antifungal resistance?
Funding Statement
This work was supported by the National Institutes of Health R01AI145939 and R21AI159583 to JEN. The funders had no role data analysis, decision to publish the manuscript, or manuscript preparation.
References
- 1.Magobo RE, Corcoran C, Seetharam S, Govender NP. Candida auris-associated candidemia, South Africa. Emerg Infect Dis. 2014;20(7):1250–1251. doi: 10.3201/eid2007.131765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin Infect Dis. 2017;64(2):134–140. Epub 2016/12/19. doi: 10.1093/cid/ciw691 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calvo B, Melo ASA, Perozo-Mena A, Hernandez M, Francisco EC, Hagen F, et al. First report of Candida auris in America: Clinical and microbiological aspects of 18 episodes of candidemia. J Infect. 2016;73(4):369–374. doi: 10.1016/j.jinf.2016.07.008 [DOI] [PubMed] [Google Scholar]
- 4.Escandón P, Chow NA, Caceres DH, Gade L, Berkow EL, Armstrong P, et al. Molecular Epidemiology of Candida auris in Colombia Reveals a Highly Related, Countrywide Colonization With Regional Patterns in Amphotericin B Resistance. Clin Infect Dis. 2018;68:15–21. doi: 10.1093/cid/ciy411 [DOI] [PubMed] [Google Scholar]
- 5.Berrio I, Caceres DH, Coronell RW, Salcedo S, Mora L, Marin A, et al. Bloodstream Infections With Candida auris Among Children in Colombia: Clinical Characteristics and Outcomes of 34 Cases. J Pediatric Infect Dis Soc. 2021;10(2):151–154. Epub 2020/05/07. doi: 10.1093/jpids/piaa038 . [DOI] [PubMed] [Google Scholar]
- 6.Lane CR, Seemann T, Worth LJ, Easton M, Pitchers W, Wong J, et al. Incursions of Candida auris into Australia, 2018. Emerg Infect Dis. 2020;26(6):1326–1328. Epub 2020/03/28. doi: 10.3201/eid2606.190936 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emara M, Ahmad S, Khan Z, Joseph L, Al-Obaid I, Purohit P, et al. Candida auris candidemia in Kuwait, 2014. Emerg Infect Dis. 2015;21(6):1091–1092. doi: 10.3201/eid2106.150270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satoh K, Makimura K, Hasumi Y, Nishiyama Y, Uchida K, Yamaguchi H. Candida auris sp nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol. 2009;53(1):41–44. doi: 10.1111/j.1348-0421.2008.00083.x [DOI] [PubMed] [Google Scholar]
- 9.Chow NA, de Groot T, Badali H, Abastabar M, Chiller TM, Meis JF. Potential Fifth Clade of Candida auris, Iran, 2018. Emerg Infect Dis. 2019;25(9):1780–1781. Epub 2019/07/17. doi: 10.3201/eid2509.190686 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spruijtenburg B, Badali H, Abastabar M, Mirhendi H, Khodavaisy S, Sharifisooraki J, et al. Confirmation of fifth Candida auris clade by whole genome sequencing. Emerg Microbes Infect. 2022;11(1):2405–2411. Epub 2022/09/27. doi: 10.1080/22221751.2022.2125349 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Bustos V, Salavert M, Ruiz-Gaitan AC, Cabanero-Navalon MD, Sigona-Giangreco IA, Peman J. A clinical predictive model of candidaemia by Candida auris in previously colonized critically ill patients. Clin Microbiol Infect. 2020. Epub 2020/02/18. doi: 10.1016/j.cmi.2020.02.001 . [DOI] [PubMed] [Google Scholar]
- 12.Sayeed MA, Farooqi J, Jabeen K, Awan S, Mahmood SF. Clinical spectrum and factors impacting outcome of Candida auris: a single center study from Pakistan. BMC Infect Dis. 2019;19(384). Epub 2019/05/08. doi: 10.1186/s12879-019-3999-y . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudramurthy SM, Chakrabarti A, Paul RA, Sood P, Kaur H, Capoor MR, et al. Candida auris candidaemia in Indian ICUs: analysis of risk factors. J Antimicrob Chemother. 2017;72(6):1794–1801. doi: 10.1093/jac/dkx034 [DOI] [PubMed] [Google Scholar]
- 14.Chowdhary A, Prakash A, Sharma C, Kordalewska M, Kumar A, Sarma S, et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009–17) in India: role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J Antimicrob Chemother. 2018;73:891–899. Epub 2018/01/13. doi: 10.1093/jac/dkx480 . [DOI] [PubMed] [Google Scholar]
- 15.Ostrowsky B, Greenko J, Adams E, Quinn M, O’Brien B, Chaturvedi V, et al. Candida auris isolates resistant to three classes of antifungal medications—New York, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(1):6–9. doi: 10.15585/mmwr.mm6901a2 Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Increasing Threat of Spread of Antimicrobial-resistant Fungus in Healthcare Facilities. 2023. https://www.cdc.gov/media/releases/2023/p0320-cauris.html#:~:text=CDC%20has%20deemed%20C.,infections%20with%20high%20death%20rates.
- 17.Lyman M, Forsberg K, Sexton DJ, Chow NA, Lockhart SR, Jackson BR, et al. Worsening Spread of Candida auris in the United States, 2019 to 2021. Ann Intern Med. 2023;176(4):489–495. Epub 2023/03/21. doi: 10.7326/M22-3469 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shastri PS, Shankarnarayan SA, Oberoi J, Rudramurthy SM, Wattal C, Chakrabarti A. Candida auris candidaemia in an intensive care unit—Prospective observational study to evaluate epidemiology, risk factors, and outcome. J Crit Care. 2020;57:42–48. Epub 2020/02/18. doi: 10.1016/j.jcrc.2020.01.004 . [DOI] [PubMed] [Google Scholar]
- 19.Ahmad S, Khan Z, Al-Sweih N, Alfouzan W, Joseph L. Candida auris in various hospitals across Kuwait and their susceptibility and molecular basis of resistance to antifungal drugs. Mycoses. 2020;63(1):104–112. Epub 2019/10/17. doi: 10.1111/myc.13022 . [DOI] [PubMed] [Google Scholar]
- 20.Mulet Bayona JV, Tormo Palop N, Salvador Garcia C, Herrero Rodriguez P, Abril Lopez de Medrano V, Ferrer Gomez C, et al. Characteristics and Management of Candidaemia Episodes in an Established Candida auris Outbreak. Antibiotics (Basel). 2020;9(9). Epub 2020/09/03. doi: 10.3390/antibiotics9090558 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Briano F, Magnasco L, Sepulcri C, Dettori S, Dentone C, Mikulska M, et al. Candida auris Candidemia in Critically Ill, Colonized Patients: Cumulative Incidence and Risk Factors. Infect Dis Ther. 2022;11(3):1149–1160. Epub 2022/04/12. doi: 10.1007/s40121-022-00625-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yadav A, Singh A, Wang Y, Haren MHV, Singh A, de Groot T, et al. Colonisation and Transmission Dynamics of Candida auris among Chronic Respiratory Diseases Patients Hospitalised in a Chest Hospital, Delhi, India: A Comparative Analysis of Whole Genome Sequencing and Microsatellite Typing. J Fungi (Basel). 2021;7(2). Epub 2021/02/04. doi: 10.3390/jof7020081 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schelenz S, Hagen F, Rhodes JL, Abdolrasouli A, Chowdhary A, Hall A, et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob Resist. Infect Control. 2016;5(35). doi: 10.1186/s13756-016-0132-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eyre DW, Sheppard AE, Madder H, Moir I, Moroney R, Quan TP, et al. A Candida auris outbreak and its control in an intensive care setting. N Engl J Med. 2018;379(14):1322–1331. doi: 10.1056/NEJMoa1714373 . [DOI] [PubMed] [Google Scholar]
- 25.Proctor DM, Dangana T, Sexton DJ, Fukuda C, Yelin RD, Stanley M, et al. Integrated genomic, epidemiologic investigation of Candida auris skin colonization in a skilled nursing facility. Nat Med. 2021;27(8):1401–1409. Epub 2021/06/23. doi: 10.1038/s41591-021-01383-w . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pathirana RU, Friedman J, Norris HL, Salvatori O, McCall AD, Kay J, et al. Fluconazole-resistant Candida auris is susceptible to salivary histatin 5 killing and to intrinsic host defenses. Antimicrob Agents Chemother. 2018;62(2). doi: 10.1128/AAC.01872-17 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vila T, Montelongo-Jauregui D, Ahmed H, Puthran T, Sultan AS, Jabra-Rizk MA. Comparative Evaluations of the Pathogenesis of Candida auris Phenotypes and Candida albicans Using Clinically Relevant Murine Models of Infections. mSphere. 2020;5(4). Epub 2020/08/08. doi: 10.1128/mSphere.00760-20 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jabeen K, Mal PB, Tharwani A, Hashmi M, Farooqi J. Persistence of Candida auris on latex and nitrile gloves with transmission to sterile urinary cathetersdouble dagger. Med Mycol. 2020;58(1):128–132. Epub 2019/04/30. doi: 10.1093/mmy/myz033 . [DOI] [PubMed] [Google Scholar]
- 29.Welsh RM, Bentz ML, Shams A, Houston H, Lyons A, Rose LJ, et al. Survival, persistence, and isolation of the emerging multidrug-resistant pathogenic yeast Candida auris on a plastic health care surface. J Clin Microbiol. 2017;55 (10):2996–3005. doi: 10.1128/JCM.00921-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furin WA, Tran LH, Chan MY, Lyons AK, Noble-Wang J, Rose LJ. Sampling efficiency of Candida auris from healthcare surfaces: culture and nonculture detection methods. Infect Control Hosp Epidemiol. 2022;43(10):1492–1494. Epub 2021/06/23. doi: 10.1017/ice.2021.220 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karmarkar EN, O’Donnell K, Prestel C, Forsberg K, Gade L, Jain S, et al. Rapid Assessment and Containment of Candida auris Transmission in Postacute Care Settings-Orange County, California, 2019. Ann Intern Med. 2021;174(11):1554–1562. Epub 2021/09/07. doi: 10.7326/M21-2013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sherry L, Ramage G, Kean R, Borman A, Johnson EM, Richardson MD, et al. Biofilm-forming capability of highly virulent, multidrug-resistant Candida auris. Emerg Infect Dis. 2017;23(2):328–331. doi: 10.3201/eid2302.161320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdolrasouli A, Armstrong-James D, Ryan L, Schelenz S. In vitro efficacy of disinfectants utilised for skin decolonisation and environmental decontamination during a hospital outbreak with Candida auris. Mycoses. 2017;60:758–763. Epub 2017/09/06. doi: 10.1111/myc.12699 . [DOI] [PubMed] [Google Scholar]
- 34.Biswal M, Rudramurthy SM, Jain N, Shamanth AS, Sharma D, Jain K, et al. Controlling a possible outbreak of Candida auris infection: lessons learnt from multiple interventions. J Hosp Infect. 2017;97:363–370. Epub 2017/09/25. doi: 10.1016/j.jhin.2017.09.009 . [DOI] [PubMed] [Google Scholar]
- 35.Huang X, Hurabielle C, Drummond RA, Bouladoux N, Desai JV, Sim CK, et al. Murine model of colonization with fungal pathogen Candida auris to explore skin tropism, host risk factors and therapeutic strategies. Cell Host Microbe. 2021;29(2):210–221.e6. doi: 10.1016/j.chom.2020.12.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson CJ, Eix EF, Lam BC, Wartman KM, Meudt JJ, Shanmuganayagam D, et al. Augmenting the Activity of Chlorhexidine for Decolonization of Candida auris from Porcine skin. J Fungi (Basel). 2021;7(10). doi: 10.3390/jof7100804 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horton MV, Johnson CJ, Kernien JF, Patel TD, Lam BC, Cheong JZA, et al. Candida auris forms high-burden biofilms in skin niche conditions and on porcine skin. mSphere. 2020;5(1):1–8. doi: 10.1128/mSphere.00910-19 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kean R, McKloud E, Townsend EM, Sherry L, Delaney C, Jones BL, et al. The comparative efficacy of antiseptics against Candida auris biofilms. Int J Antimicrob Agents. 2018;52(5):673–677. Epub 2018/05/19. doi: 10.1016/j.ijantimicag.2018.05.007 . [DOI] [PubMed] [Google Scholar]
- 39.Ghannoum M, Isham N, Angulo D, Borroto-Esoda K, Barat S, Long L. Efficacy of Ibrexafungerp (SCY-078) against Candida auris in an In Vivo Guinea Pig Cutaneous Infection Model. Antimicrob Agents Chemother. 2020;64(10). Epub 2020/07/29. doi: 10.1128/AAC.00854-20 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruchti F, LeibundGut-Landmann S. New insights into immunity to skin fungi shape our understanding of health and disease. Parasite Immunol. 2023;45(2):e12948. Epub 2022/09/02. doi: 10.1111/pim.12948 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horton MV, Johnson CJ, Kernien JF, Patel TD, Lam BC, Cheong JZA, et al. Candida auris Forms High-Burden Biofilms in Skin Niche Conditions and on Porcine Skin. mSphere. 2020;5(1). Epub 2020/01/24. doi: 10.1128/mSphere.00910-19 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Day AM, McNiff MM, da Silva DA, Gow NAR, Quinn J. Hog1 regulates stress tolerance and virulence in the emerging fungal pathogen Candida auris. mSphere. 2018;3(5). doi: 10.1128/mSphere.00506-18 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, Bing J, Zheng Q, Zhang F, Liu J, Yue H, et al. The first isolate of Candida auris in China: clinical and biological aspects. Emerg Microbes Infect. 2018;7(1):93. doi: 10.1038/s41426-018-0095-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hernando-Ortiz A, Mateo E, Perez-Rodriguez A, de Groot PWJ, Quindos G, Eraso E. Virulence of Candida auris from different clinical origins in Caenorhabditis elegans and Galleria mellonella host models. Virulence. 2021;12(1):1063–1075. Epub 2021/04/13. doi: 10.1080/21505594.2021.1908765 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh R, Kaur M, Chakrabarti A, Shankarnarayan SA, Rudramurthy SM. Biofilm formation by Candida auris isolated from colonising sites and candidemia cases. Mycoses. 2019;62(8):706–709. doi: 10.1111/myc.12947 . [DOI] [PubMed] [Google Scholar]
- 46.Brown JL, Delaney C, Short B, Butcher MC, McKloud E, Williams C, et al. Candida auris Phenotypic Heterogeneity Determines Pathogenicity In Vitro. mSphere. 2020;5(3). Epub 2020/06/26. doi: 10.1128/mSphere.00371-20 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eix EF, Johnson CJ, Wartman KM, Kernien JF, Meudt JJ, Shanmuganayagam D, et al. Ex Vivo Human and Porcine Skin Effectively Model Candida auris Colonization, Differentiating Robust and Poor Fungal Colonizers. J Infect Dis. 2022;225(10):1791–1795. doi: 10.1093/infdis/jiac094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Timmermans B, De Las Penas A, Castano I, Van Dijck P. Adhesins in Candida glabrata. J Fungi (Basel). 2018;4(2). Epub 2018/05/23. doi: 10.3390/jof4020060 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sundstrom P. Adhesion in Candida spp. Cell Microbiol 2002;4(8):461–469. Epub 2002/08/14. doi: 10.1046/j.1462-5822.2002.00206.x . [DOI] [PubMed] [Google Scholar]
- 50.Nobile CJ, Mitchell AP. Genetics and genomics of Candida albicans biofilm formation. Cell Microbiol. 2006;8(9):1382–1391. Epub 2006/07/20. doi: 10.1111/j.1462-5822.2006.00761.x . [DOI] [PubMed] [Google Scholar]
- 51.Chatterjee S, Alampalli SV, Nageshan RK, Chettiar ST, Joshi S, Tatu US. Draft genome of a commonly misdiagnosed multidrug resistant pathogen Candida auris. BMC Genomics. 2015;16(1):686. Epub 2015/09/09. doi: 10.1186/s12864-015-1863-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watkins RR, Gowen R, Lionakis MS, Ghannoum M. Update on the Pathogenesis, Virulence, and Treatment of Candida auris. Pathog Immun. 2022;7(2):46–65. Epub 2022/11/05. doi: 10.20411/pai.v7i2.535 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kean R, Delaney C, Sherry L, Borman A, Johnson EM, Richardson MD, et al. Transcriptome assembly and profiling of Candida auris reveals novel insights into biofilm-mediated resistance. mSphere. 2018;3(4). doi: 10.1128/mSphere.00334-18 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borman AM, Szekely A, Johnson EM. Comparative pathogenicity of United Kingdom isolates of the emerging pathogen Candida auris and other key pathogenic Candida species. mSphere. 2016;1(4). doi: 10.1128/mSphere.00189-16 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Munoz JF, Welsh RM, Shea T, Batra D, Gade L, Howard D, et al. Clade-specific chromosomal rearrangements and loss of subtelomeric adhesins in Candida auris. Genetics. 2021;218(1). Epub 2021/03/27. doi: 10.1093/genetics/iyab029 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bing J, Guan Z, Zheng T, Zhang Z, Fan S, Ennis CL, et al. Clinical isolates of Candida auris with enhanced adherence and biofilm formation due to genomic amplification of ALS4. PLoS Pathog. 2023;19(3):e1011239. Epub 2023/03/14. doi: 10.1371/journal.ppat.1011239 following competing interests: CJN is a cofounder of BioSynesis, Inc., a company developing therapeutics and diagnostics for biofilm infections. All other authors declare no conflict of interest. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Santana DJ, O’Meara TR. Forward and reverse genetic dissection of morphogenesis identifies filament-competent Candida auris strains. Nat Commun. 2021;12(1):7197. Epub 2021/12/12. doi: 10.1038/s41467-021-27545-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bhattacharya S, Holowka T, Orner EP, Fries BC. Gene Duplication Associated with Increased Fluconazole Tolerance in Candida auris cells of Advanced Generational Age. Sci Rep. 2019;9(1):5052. Epub 2019/03/27. doi: 10.1038/s41598-019-41513-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Santana DJ, Anku JAE, Zhao G, Zarnowski R, Johnson CJ, Hautau H, et al. A Candida auris-specific adhesin, Scf1, governs surface association, colonization, and virulence. Science. 2023;381(6665):1461–1467. Epub 2023/09/28. doi: 10.1126/science.adf8972 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kunyeit L, Kurrey NK, Anu-Appaiah KA, Rao RP. Probiotic Yeasts Inhibit Virulence of Non-albicans Candida Species. MBio. 2019;10(5). Epub 2019/10/17. doi: 10.1128/mBio.02307-19 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sayeed MA, Farooqi J, Jabeen K, Mahmood SF. Comparison of risk factors and outcomes of Candida auris candidemia with non-Candida auris candidemia: A retrospective study from Pakistan. Med Mycol. 2019. doi: 10.1093/mmy/myz112 [DOI] [PubMed] [Google Scholar]
- 62.Dominguez E. G. Z R, Choy H. L., Zhao M., Sanchez H., Nett Jeniel E., Andes D. R. Conservation and divergence in the Candida species biofilm matrix mannan-glucan complex structure, function, and genetic control. MSphere. 2019;4(1). doi: 10.1128/mSphereDirect.00680-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Larkin E, Hager C, Chandra J, Mukherjee PK, Retuerto M, Salem I, et al. The emerging pathogen Candida auris: Growth phenotype, virulence factors, activity of antifungals, and effect of SCY-078, a novel glucan synthesis inhibitor, on growth morphology and biofilm formation. Antimicrob Agents Chemother. 2017;61(5). doi: 10.1128/AAC.02396-16 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Romera D, Aguilera-Correa JJ, Gadea I, Vinuela-Sandoval L, Garcia-Rodriguez J, Esteban J. Candida auris: a comparison between planktonic and biofilm susceptibility to antifungal drugs. J Med Microbiol. 2019;68(9):1353–1358. doi: 10.1099/jmm.0.001036 . [DOI] [PubMed] [Google Scholar]
- 65.Rapala-Kozik M, Bochenska O, Zajac D, Karkowska-Kuleta J, Gogol M, Zawrotniak M, et al. Extracellular proteinases of Candida species pathogenic yeasts. Mol Oral Microbiol. 2018;33(2):113–124. Epub 2017/11/16. doi: 10.1111/omi.12206 . [DOI] [PubMed] [Google Scholar]
- 66.Silva NC, Nery JM, Dias AL. Aspartic proteinases of Candida spp.: role in pathogenicity and antifungal resistance. Mycoses. 2014;57(1):1–11. Epub 2013/06/06. doi: 10.1111/myc.12095 . [DOI] [PubMed] [Google Scholar]
- 67.Fan S, Zhan P, Bing J, Jiang N, Huang Y, Chen D, et al. A biological and genomic comparison of a drug-resistant and a drug-susceptible strain of Candida auris isolated from Beijing, China. Virulence 2021;12(1):1388–1399. Epub 2021/06/02. doi: 10.1080/21505594.2021.1928410 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ben-Ami R, Berman J, Novikov A, Bash E, Shachor-Meyouhas Y, Zakin S, et al. Emerg Infect Dis. 2017;23(1). doi: 10.3201/eid2302.161486 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yue H, Bing J, Zheng Q, Zhang Y, Hu T, Du H, et al. Filamentation in Candida auris, an emerging fungal pathogen of humans: passage through the mammalian body induces a heritable phenotypic switch. Emerg Microbes Infect. 2018;7(1):188. doi: 10.1038/s41426-018-0187-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fan S, Yue H, Zheng Q, Bing J, Tian S, Chen J, et al. Filamentous growth is a general feature of Candida auris clinical isolates. Med Mycol. 2021;59(7):734–740. Epub 2021/01/24. doi: 10.1093/mmy/myaa116 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Honorato L, de Araujo JFD, Ellis CC, Piffer AC, Pereira Y, Frases S, et al. Extracellular Vesicles Regulate Biofilm Formation and Yeast-to-Hypha Differentiation in Candida albicans. MBio. 2022;13(3):e0030122. Epub 2022/04/15. doi: 10.1128/mbio.00301-22 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zarnowski R, Sanchez H, Jaromin A, Zarnowska UJ, Nett JE, Mitchell AP, et al. A common vesicle proteome drives fungal biofilm development. Proc Natl Acad Sci U S A. 2022;119(38):e2211424119. Epub 2022/09/13. doi: 10.1073/pnas.2211424119 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zamith-Miranda D, Heyman HM, Couvillion SP, Cordero RJB, Rodrigues ML, Nimrichter L, et al. Comparative Molecular and Immunoregulatory Analysis of Extracellular Vesicles from Candida albicans and Candida auris. mSystems. 2021;6(4):e0082221. Epub 2021/08/25. doi: 10.1128/mSystems.00822-21 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chan W, Chow FW, Tsang CC, Liu X, Yao W, Chan TT, et al. Induction of amphotericin B resistance in susceptible Candida auris by extracellular vesicles. Emerg Microbes Infect. 2022;11(1):1900–1909. Epub 2022/07/06. doi: 10.1080/22221751.2022.2098058 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mitchell KF, Zarnowski R, Sanchez H, Edward JA, Reinicke EL, Nett JE, et al. Community participation in biofilm matrix assembly and function. Proc Natl Acad Sci U S A. 2015;112(13):4092–4097. doi: 10.1073/pnas.1421437112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zarnowski R, Massey J, Mitchell AP, Andes D. Extracellular Vesicles Contribute to Mixed-Fungal Species Competition during Biofilm Initiation. MBio. 2022;13(6):e0298822. doi: 10.1128/mbio.02988-22 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rosario-Colon J, Eberle K, Adams A, Courville E, Xin H. Candida Cell-Surface-Specific Monoclonal Antibodies Protect Mice against Candida auris Invasive Infection. Int J Mol Sci. 2021;22(11). Epub 2021/07/03. doi: 10.3390/ijms22116162 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fakhim H, Vaezi A, Dannaoui E, Chowdhary A, Nasiry D, Faeli L, et al. Comparative virulence of Candida auris with Candida haemulonii, Candida glabrata and Candida albicans in a murine model. Mycoses. 2018;61(6):377–382. doi: 10.1111/myc.12754 . [DOI] [PubMed] [Google Scholar]
- 79.Pharkjaksu S, Boonmee N, Mitrpant C, Ngamskulrungroj P. Immunopathogenesis of Emerging Candida auris and Candida haemulonii Strains. J Fungi (Basel). 2021;7(9). Epub 2021/09/29. doi: 10.3390/jof7090725 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Munoz JE, Ramirez LM, Dias LDS, Rivas LA, Ramos LS, Santos ALS, et al. Pathogenicity Levels of Colombian Strains of Candida auris and Brazilian Strains of Candida haemulonii Species Complex in Both Murine and Galleria mellonella Experimental Models. J Fungi (Basel). 2020;6(3). Epub 2020/07/16. doi: 10.3390/jof6030104 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garcia-Bustos V, Ruiz-Sauri A, Ruiz-Gaitan A, Sigona-Giangreco IA, Cabanero-Navalon MD, Sabalza-Baztan O, et al. Characterization of the Differential Pathogenicity of Candida auris in a Galleria mellonella Infection Model. Microbiol Spectr. 2021;9(1):e0001321. Epub 2021/06/10. doi: 10.1128/Spectrum.00013-21 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Forgacs L, Borman AM, Prepost E, Toth Z, Kardos G, Kovacs R, et al. Comparison of in vivo pathogenicity of four Candida auris clades in a neutropenic bloodstream infection murine model. Emerg Microbes Infect. 2020;9(1):1160–1169. doi: 10.1080/22221751.2020.1771218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carvajal SK, Alvarado M, Rodriguez YM, Parra-Giraldo CM, Varon C, Morales-Lopez SE, et al. Pathogenicity Assessment of Colombian Strains of Candida auris in the Galleria mellonella Invertebrate Model. J Fungi (Basel). 2021;7(6). Epub 2021/06/03. doi: 10.3390/jof7060401 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Romera D, Aguilera-Correa JJ, Garcia-Coca M, Mahillo-Fernandez I, Vinuela-Sandoval L, Garcia-Rodriguez J, et al. The Galleria mellonella infection model as a system to investigate the virulence of Candida auris strains. Pathog Dis. 2020;78(9). Epub 2020/10/25. doi: 10.1093/femspd/ftaa067 . [DOI] [PubMed] [Google Scholar]
- 85.Johnson CJ, Davis JM, Huttenlocher A, Kernien JF, Nett JE. Emerging Fungal Pathogen Candida auris Evades Neutrophil Attack. MBio. 2018;9(4):e01403–e01418. doi: 10.1128/mBio.01403-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Horton MV, Johnson CJ, Zarnowski R, Andes BD, Schoen TJ, Kernien JF, et al. Candida auris cell wall mannosylation contributes to neutrophil evasion through pathways civergent from Candida albicans and Candida glabrata. mSphere. 2021;6(3):e0040621. doi: 10.1128/mSphere.00406-21 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Y, Zou Y, Chen X, Li H, Yin Z, Zhang B, et al. Innate immune responses against the fungal pathogen Candida auris. Nat Commun. 2022;13(1):3553. doi: 10.1038/s41467-022-31201-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weerasinghe H, Simm C, Djajawi TM, Tedja I, Lo TL, Simpson DS, et al. Candida auris uses metabolic strategies to escape and kill macrophages while avoiding robust activation of the NLRP3 inflammasome response. Cell Rep. 2023;42(5):112522. Epub 2023/05/19. doi: 10.1016/j.celrep.2023.112522 . [DOI] [PubMed] [Google Scholar]
- 89.Bruno M, Kersten S, Bain JM, Jaeger M, Rosati D, Kruppa MD, et al. Transcriptional and functional insights into the host immune response against the emerging fungal pathogen Candida auris. Nat Microbiol. 2020;5(12):1516–1531. Epub 2020/08/26. doi: 10.1038/s41564-020-0780-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Navarro-Arias MJ, Hernandez-Chavez MJ, Garcia-Carnero LC, Amezcua-Hernandez DG, Lozoya-Perez NE, Estrada-Mata E, et al. Differential recognition of Candida tropicalis, Candida guilliermondii, Candida krusei, and Candida auris by human innate immune cells. Infect Drug Resist. 2019;12:783–794. Epub 2019/05/02. doi: 10.2147/IDR.S197531 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yan L, Xia K, Yu Y, Miliakos A, Chaturvedi S, Zhang F, et al. Unique Cell Surface Mannan of Yeast Pathogen Candida auris with Selective Binding to IgG. ACS Infect Dis. 2020;6(5):1018–1031. Epub 2020/04/03. doi: 10.1021/acsinfecdis.9b00450 . [DOI] [PubMed] [Google Scholar]
- 92.Singh S, Barbarino A, Youssef EG, Coleman D, Gebremariam T, Ibrahim AS. Protective Efficacy of Anti-Hyr1p Monoclonal Antibody against Systemic Candidiasis Due to Multi-Drug-Resistant Candida auris. J Fungi (Basel). 2023;9(1). Epub 2023/01/22. doi: 10.3390/jof9010103 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Singh S, Uppuluri P, Mamouei Z, Alqarihi A, Elhassan H, French S, et al. The NDV-3A vaccine protects mice from multidrug resistant Candida auris infection. PLoS Pathog. 2019;15(8):e1007460. doi: 10.1371/journal.ppat.1007460 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mengesha BG, Conti HR. The Role of IL-17 in Protection against Mucosal Candida Infections. J Fungi (Basel). 2017;3(4). Epub 2018/01/27. doi: 10.3390/jof3040052 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xin H, Mohiuddin F, Tran J, Adams A, Eberle K. Experimental Mouse Models of Disseminated Candida auris Infection. mSphere. 2019;4(5). Epub 2019/09/06. doi: 10.1128/mSphere.00339-19 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Horton MV, Johnson CJ, Zarnowski R, Andes BD, Schoen TJ, Kernien JF, et al. Candida auris Cell Wall Mannosylation Contributes to Neutrophil Evasion through Pathways Divergent from Candida albicans and Candida glabrata. mSphere. 2021;6(3):e0040621. Epub 2021/06/24. doi: 10.1128/mSphere.00406-21 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Garcia-Bustos V, Peman J, Ruiz-Gaitan A, Cabanero-Navalon MD, Cabanilles-Boronat A, Fernandez-Calduch M, et al. Host-pathogen interactions upon Candida auris infection: fungal behaviour and immune response in Galleria mellonella. Emerg Microbes Infect. 2022;11(1):136–146. Epub 2021/12/11. doi: 10.1080/22221751.2021.2017756 . [DOI] [PMC free article] [PubMed] [Google Scholar]