Abstract
Rationale:
The increasing use of immune checkpoint inhibitors (ICIs) for treating malignant tumors result in the concomitant rise of immune-related adverse events (irAEs). This case report may provide useful insight to understanding the etiology of ICI-induced hypophysitis, a severe irAE leading to potentially fatal secondary adrenal insufficiency.
Patient concerns:
An 81-year-old Japanese man was hospitalized for diabetic ketoacidosis following 4 courses of ICI combination therapy with nivolumab and ipilimumab for metastatic renal cell carcinoma.
Diagnosis:
Insulin secretion was depleted, leading to diagnosis of fulminant type 1 diabetes. Adrenocorticotropic hormone (ACTH) and cortisol levels were very high (60.8 pmol/L and 1575 nmol/L, respectively) upon admission. ACTH and cortisol returned to normal ranges on the 2nd day. On the 8th day, an ACTH loading test showed intact cortisol response (peak value 519 nmol/L). However, on the 14th day, there was a sharp decrease in ACTH and cortisol levels (10.5 pmol/L and 47 nmol/L, respectively) accompanied by fatigue and a drop in blood pressure to 97/63 mm Hg. As secondary adrenal insufficiency was suspected, hydrocortisone replacement was initiated. An ACTH loading test on the 17th day revealed low cortisol peak (peak value 232 nmol/L), indicating sudden disruption of adrenal function. Magnetic resonance imaging showed no abnormal findings and there was no other pituitary hormone deficiency. These findings, along with the patient clinical course, suggest that secondary adrenal insufficiency was caused by acute ACTH producing cell destruction as an irAE associated with ICI therapy.
Interventions:
The patient hyperglycemia and ketoacidosis were treated using extracellular fluid and insulin therapy. After development of adrenal insufficiency, hydrocortisone 20 mg was started, and the patient symptoms improved.
Outcomes:
He was continued on insulin therapy, hydrocortisone, and reinitiated nivolumab.
Lessons:
This case provides a detailed course of the fulminant onset of ACTH deficiency during ICI administration, emphasizing the importance of close monitoring.
Keywords: adrenocorticotropic hormone (ACTH), fulminant type 1 diabetes, hypophysitis, immune checkpoint inhibitor, secondary adrenal insufficiency
1. Introduction
The use of immune checkpoint inhibitors (ICIs) is increasing because of their broad indication for treatment of malignant tumors, resulting in the concomitant rise of immune-related adverse events (irAEs).[1] Several cases with plural irAEs have been previously reported, although the clinical course and underlying mechanisms are still unclear.[2–4] Hypophysitis is one of the critical irAEs, which develops acute secondary adrenal insufficiency, triggering to a fatal outcome. We previously reported nivolumab-induced hypophysitis leading to secondary adrenal insufficiency after transient adrenocorticotropic hormone (ACTH) elevation. This case report suggested that increased ACTH is an early indicator of the hypophysitis onset.[5] We here present the case of a patient with renal cell cancer who received nivolumab and ipilimumab combination therapy and developed thyroid dysfunction initially and was then hospitalized due to fulminant hypophysitis following diabetic ketoacidosis caused by the onset of fulminant type 1 diabetes (T1D). While hospitalized, the patient also developed adrenal insufficiency caused by the hypophysitis. Since we intensively followed the course of the hypophysitis onset by monitoring ACTH, cortisol, and ACTH loading at pre- and post-onset, this case report would give useful insight to understanding the etiology of fulminant ICI-induced hypophysitis.
2. Case presentation
An 81-year-old Japanese man was found to have a 21 mm left renal tumor after having an abdominal computed tomography scan. The patient was introduced to a nearby hospital, where a robot-assisted laparoscopic partial nephrectomy was performed, and the tumor was diagnosed as clear cell renal cell carcinoma. 7 months after, the patient complained of left back pain and a computed tomography scan showed bone metastasis in the 12th thoracic and 5th lumbar vertebrae. Denosumab was given, followed by initiation of ICI combination therapy with nivolumab and ipilimumab 3 months after starting denosumab treatment.
At a regular visit after 2 courses of ICI combination therapy, thyrotoxicosis (thyroid-stimulating hormone [TSH] 0.02 mIU/L, free triiodothyronine 8.66 pmol/L, free thyroxine 35.9 pmol/L) without any symptoms was discovered. Based on the finding of negative TSH receptor antibody but positive anti-thyroglobulin antibody without increased vascularity on color Doppler ultrasound, the patient was thought to have developed destructive thyroiditis due to ICI combination therapy with nivolumab and ipilimumab. The thyrotoxicosis had improved in natural course without medication.
Following 4 courses of ICI combination therapy, the patient presented at the hospital with nausea, loss of appetite, and thirst lasting for 3 days. Blood tests showed hyperglycemia (plasma glucose 34.6 mmol/L, hemoglobin A1c 47 mmol/mol) and ketoacidosis, and the patient was emergently hospitalized immediately (Table 1). Serum C-peptide immunoreactivity (CPR) levels were undetectable in the fasting state and after loading with 1 mg glucagon, exhibiting insulin depletion. Anti-GAD antibody was negative. The patient developed ketoacidosis within 1 week of symptom onset, with plasma glucose > 16.0 mmol/L (288 mg/dL), hemoglobin A1c (IFCC) < 72 mmol/mol (NGSP, <8.7%), and serum CPR < 0.17 nmol/L (<0.5 ng/mL) after glucagon load. Therefore, the patient was diagnosed with fulminant T1D mellitus.[6] The patient was identified as having the human leukocyte antigen (HLA) class II alleles DRB1*09:01 and DQB1*03:03, which are related to susceptibility to T1D.[7] The patient hyperglycemia and ketoacidosis were managed with extracellular fluid monitoring, continuous venous insulin infusion, and multiple daily insulin injections. ICI combination therapy was stopped.
Table 1.
Laboratory findings on admission.
| Hematology | Biochemistry | ||||
|---|---|---|---|---|---|
| White blood cell | 12.8 × 109 | /L | Total protein | 75 | g/L |
| Neutrophil | 86.0 | % | Albumin | 43 | g/L |
| Lymphocyte | 7.0 | % | Creatinine | 99.9 | µmol/L |
| Monocyte | 7.0 | % | Blood urea nitrogen | 15.7 | mmol/L |
| Hemoglobin | 129 | g/L | Sodium | 133 | mmol/L |
| Red blood cell | 4.90 × 1012 | /L | Potassium | 5.4 | mmol/L |
| Hematocrit | 0.404 | /L | Chloride | 93 | mmol/L |
| Platelet | 201 | /L | Aspartate aminotransferase | 35 | IU/L |
| Alanine aminotransferase | 59 | IU/L | |||
| Total cholesterol | 3.1 | mmol/L | |||
| Triglyceride | 0.3 | mmol/L | |||
| Postprandial plasma glucose | 34.6 | mmol/L | |||
| HbA1c | 47 | mmol/mol | |||
| Venous blood gas (room air) | |||||
| pH | 7.215 | ||||
| pCO2 | 30.1 | mm Hg | |||
| HCO3- | 11.7 | mmol/L | |||
| Base excess | −14.7 | mmol/L | |||
| Anion gap | 21.3 | mmol/L | |||
| Total ketone body | 13.468 | mmol/L | |||
| 3-hydroxybutyric acid | 10.496 | mmol/L | |||
| Acetoacetic acid | 2.972 | mmol/L | |||
| Human leukocyte antigen | DRB1*09:01-DQB1*03:03 | ||||
ACTH and cortisol levels were strikingly high (60.8 pmol/L and 1575 nmol/L, respectively) on the admission day (day 1) (Table 2), which may be explained by physical stress and diabetic ketoacidosis; however, having learned from a previously reported a case of nivolumab-induced hypophysitis leading to secondary adrenal insufficiency after transient ACTH elevation [5], we frequently monitored the patient ACTH and cortisol levels. ACTH and cortisol levels returned to normal ranges (8.2 pmol/L and 303 nmol/L) on day 2, and ACTH increased again to 24.2 pmol/L on day 6, whereas cortisol level remained normal (301 nmol/L). An ACTH loading test showed intact cortisol response (peak value: 519 nmol/L) on day 8. On day 14, ACTH and cortisol levels decreased sharply to 10.5 pmol/L and 47 nmol/L in the early morning (Fig. 1), and the patient complained of fatigue and the patient blood pressure had decreased to 97/63 mm Hg. Secondary adrenal insufficiency was suspected, and hydrocortisone replacement (15 and 5 mg, in the morning and evening, respectively) was started. An ACTH loading test was performed again, and basal cortisol value was < 28 nmol/L with peak value 232 nmol/L, suggesting adrenal insufficiency. Magnetic resonance imaging (MRI) showed no abnormal findings in the patient pituitary gland on day 20 (Fig. 2). A corticotropin-releasing hormone (CRH)-thyrotropin-releasing hormone-luteinizing hormone-releasing hormone loading test on day 21 showed normal responses for prolactin, luteinizing hormone, and follicle-stimulating hormone, however, ACTH showed low response to CRH, and cortisol did not respond to CRH increased ACTH (Fig. 3). Thyrotropin response to thyrotropin-releasing hormone was reduced because of levothyroxine administration. A growth hormone releasing peptide-2 loading test showed normal response of growth hormone.
Table 2.
Basal endocrinological data on admission.
| Endocrinology | ||
|---|---|---|
| Value | Unit (reference value) | |
| Adrenocorticotropic hormone | 60.8 | pmol/L (1.5–12.3) |
| Cortisol | 1575 | nmol/L (176–579) |
| Thyroid stimulating hormone | 0.01 | mU/L (0.38–4.31) |
| Free triiodothyronine | 2.65 | pmol/L (3.23–5.83) |
| Free thyroxine | 18.0 | pmol/L (10.6–21.0) |
| Anti-thyroglobulin antibody | 679.74 | IU/mL (0–0.2) |
| Anti-thyroid peroxidase antibody | <0.05 | IU/mL (0–0.2) |
| Growth hormone | 1.02 | µg/L (0.11–3.90) |
| Insulin-like growth factor-1 | 7.87 | nmol/L (6.4–23.6) |
| Prolactin | 12.6 | µg/L (4.1–28.9) |
| Luteinizing hormone | 5.1 | IU/L (1.8–5.2) |
| Follicle stimulating hormone | 20.2 | IU/L (2.9–8.2) |
| Testosterone (ECLIA) | 3.16 | nmol/L (6.93–26.35) |
Figure 1.
Changes in blood ACTH and cortisol levels. ACTH = adrenocorticotropic hormone.
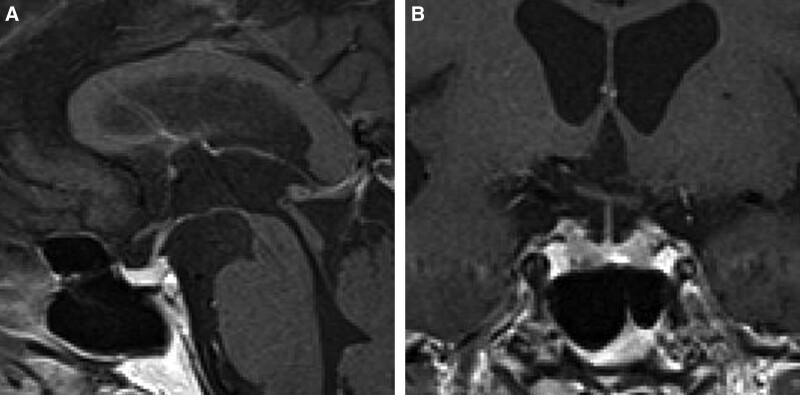
Figure 2.
Brain magnetic resonance imaging (MRI) on d 20. (A) Sagittal gadolinium-enhanced T1-weighted MRI. (B) Coronal gadolinium-enhanced T1-weighted MRI.
Figure 3.
Results of hormone stimulation tests. (A) Changes in cortisol after intravenous ACTH (250 µg) injection. (B) Changes in ACTH and cortisol after intravenous CRH (0.1 mg) injection on d 21. ACTH = adrenocorticotropic hormone, CRH = corticotropin-releasing hormone.
The above findings and the patient clinical course suggested secondary adrenal insufficiency arising from ICI-induced hypophysitis. Administration of 20 mg hydrocortisone was continued. During the course of hospitalization, a notable decline in thyroid hormones was observed on day 8, and as a therapeutic intervention, an initial dosage of 25 µg of levothyroxine was administered on day 9. Subsequently, the dosages were gradually escalated to 50 µg on day 15 and further increased to 75 µg on day 21, resulting in normalization of thyroid hormone levels. The patient was discharged on day 31. The monotherapy of nivolumab was started after discharge with hormone replacement. The patient hormone status was unchanged 4 months post discharge.
3. Discussion and conclusions
Here we report a case of ICI combination therapy-induced fulminant ACTH deficiency following thyroiditis and fulminant T1D, and we present, for the first time, the hormonal status pre- and post-onset of ACTH deficiency including ACTH loading test results. There was no other anterior pituitary hormone or arginine vasopressin deficiency observed.
Table 3 illustrates the knowledge of hypophysitis induced by ICIs. Several meta-analyses reported that the incidence rate of hypophysitis with ICI combination therapy is 7.7% to 10.5%.[8–12] Barroso-Sousa[8] reported that the patients who received ICI combination therapy were more likely to develop hypophysitis (odds ratio 2.2; 95%CI 1.39–3.60) than those who received ipilimumab monotherapy. Da[12] reported that ICI combination therapy significantly increased the risks of hypophysitis (relative risk 3.5; 95%CI 2.07–6.07) when compared with ICI monotherapy. Furthermore, other endocrine irAEs, such as hypothyroidism (risk ratio [RR], 2.17) and hyperthyroidism (RR, 3.13), and non-endocrine irAEs, including pneumonitis (RR, 2.31), and hepatitis (RR, 2.54), occurred more frequently with ICI combination therapy than with ICI monotherapy.[12] Therefore, the prevalence of developing multiple irAEs, such as in this case, is assumed to increase. The clinical features of hypopituitarism are contingent upon the specific type of ICI administered. For instance, nivolumab-induced hypophysitis frequently demonstrates no noticeable change in the hypopituitary gland on MRI,[13] with isolated ACTH deficiency (IAD) being a prevalent occurrence.[14] In contrast, enlarged pituitary gland appeared on MRI in 60 cases out of 61 patients with ipilimumab-induced hypophysitis,[13] with multiple anterior pituitary hormones being implicated. A review of the hormone disruption patterns revealed TSH 92%, luteinizing hormone/follicle-stimulating hormone 85.7%, ACTH 74%, prolactin 64.7%[15] in cases of ipilimumab-related hypophysitis. The incidence of GH deficiency was 41.9%, a comparatively low rate. Reports of diabetes insipidus remain scarce, with only 1 out of 75 cases,[16] whereas Atkins[17] documented a 36% rate (4 cases out of 11) of diabetes insipidus development.
Table 3.
Hypophysitis due to combination therapy.
| Nivolumab (Nivo) | Ipilimumab (Ipi) | Combination therapy* | |
|---|---|---|---|
| Incidence rate | 0.3%–1.1%[8–11] | 3.8%–5.6%[8–11] | 7.7%–10.5%[8–12] OR 2.2%–3.5%[8,12] (vs monotherapy) |
| MRI | No change[13] | Enlargement of pituitary[13] | Variable |
| Hormone deficiency | Isolated ACTH deficiency[14] | Multiple anterior pituitary hormone deficiency[15–17] | Variable |
| mechanism | Autoimmune response against corticotrophs by ectopic ACTH expression in tumors[16] | Binding to CTLA-4 and activated classical pathway[19] | Unknown |
ACTH = adrenocorticotropic hormone; CTLA-4 = cytotoxic T-lymphocyte associated antigen-4, MRI = magnetic resonance imaging, OR = odds ratio.
Combination of two immune checkpoint inhibitors, e.g. PD-1/PD-L1 plus CTLA-4 inhibitors.
Immune cells possess the capability to recognize and prepare for the obliteration of cancer cells. However, their activity is hindered by the presence of inhibitory receptors and ligands including cytotoxic T-lymphocyte associated antigen-4 (CTLA-4), programmed cell death protein 1 (PD-1), and programmed death-ligand 1 (PD-L1). Under physiological conditions, these immune checkpoint pathways uphold self-tolerance and restrict incidental tissue damage during antimicrobial immune responses. In the context of oncology, these pathways can be manipulated to evade immune-mediated tumor destruction. Therapeutic agents such as anti-CTLA-4, anti-PD-1, anti-PD-L1 inhibit these receptors and ligands, which promote sustained regression of neoplasms by mitigating the suppression of antitumor immune responses.[18] However, CTLA-4 antigens were expressed by pituitary endocrine cells of all subjects, including 6 patients and 11 unaffected controls who had not been received anti-CTLA-4 antibodies. However, expression levels varied. The binding of anti-CTLA-4 to CTLA-4 expressed in the pituitary gland stimulates macrophage phagocytosis and triggers the classical pathway, known as Type II hypersensitivity reactions.[16] In addition, case of autoimmune reactions against corticotrophs triggered by ectopic ACTH expression in tumors among patients receiving PD-1 have been reported.[19] T cell mediated immune destruction has been suggested.[20] In this case, there was no signs of pituitary enlargement on MRI, and only ACTH was disordered, a characteristic feature observed in nivolumab-induced hypophysitis.
Three irAEs, such as thyroid disease, diabetes mellitus, and hypophysitis, developed in this case. In 7 previous reports of triple endocrine irAEs due to ICI therapy (Table 4),[21–27] thyroid disease and/or T1D mellitus developed before onset of hypophysitis. In 3 cases, ICI therapies were continued or reinitiated after resolution of irAEs.[22,24,26] In 1 case, treatment was switched to sunitinib, but serum creatinine increased and nivolumab was resumed.[25] In these 7 cases, the symptoms of secondary adrenal insufficiency due to hypophysitis comprised refractory hypotension, nausea, fatigue, memory loss, malaise, loss of appetite, and hypoglycemia, which lack specificity. Hyponatremia and hyperkalemia were also signs of adrenal insufficiency. There was also no detailed investigation of hormones, such as pre- and post-hypophysitis onset ACTH loading tests. This case study represents the first documentation of ACTH deficiency manifesting within an extraordinarily condensed time frame, specifically within a few days, reflecting the rapid progression characteristic of fulminant T1D. We previously reported a case of hypophysitis leading to secondary adrenal insufficiency after transient ACTH elevation by nivolumab,[5] and the present report showed a similar course due to ICI combination therapy, suggesting that the observed ACTH increase may be a clue to the subsequent rapid decline in ACTH.
Table 4.
Cases that have 3 irAEs including secondary adrenal insufficiency due to ICI therapy.
| Case | Age | Sex | Primary disease | Therapy | Endocrine irAEs* | Signs of secondary adrenal insufficiency | D to hypophysitis diagnosis from first administration |
|---|---|---|---|---|---|---|---|
| Sum M (2018)[21] | 75 | M | Melanoma | Nivo + Ipi | T1D → AD = hypothyroidism | Hypoglycemia, malaise | NA |
| Khalid M (2019)[22] | 53 | M | Malignant melanoma | Nivo + Ipi | primary hypothyroidism → IAD → T1D | Refractory hypotension | 6 mo |
| Lanzolla G (2019)[23] | 60 | M | Lung adeno-carcinoma | Atezo | T1D → Addison disease = hypophysitis | Hyperkalemia and hyponatremia | 12 wk |
| Takata M (2022)[24] | 59 | M | Malignant melanoma | Nivo | Thyroiditis → IAD → acute T1D | Nausea | 14 wk |
| Hino C (2022)[25] | 45 | M | Sarcomatoid renal cell carcinoma | Nivo + Ipi | Thyroiditis → T1D → AD | Fatigue and memory loss | 244 d |
| Ishiguro A (2022)[26] | 67 | M | Malignant melanoma | Nivo | T1D = thyroiditis → IAD | Malaise and loss of appetite | 57 d |
| Luo J (2022)[27] | 76 | M | Non-small cell lung cancer | Pem | Hypothyroidism → T1D → AD | Hyponatremia | 14 mo |
| This case | 81 | M | Renal cell carcinoma | Nivo + Ipi | Thyroiditis → fulminant T1D → IAD | Hypotension and fatigue | 89 d |
Atezo = Atezolizumab, ICI = immune checkpoint inhibitor, Ipi = ipilimumab, Nivo = nivolumab, Pem = Pembrolizumab, T1D = type 1 diabetes.
Endocrine irAEs are listed in order of onset.
Table 5 shows cases reporting hypophysitis and T1D.[3,21–32] ICIs are anti-PD-1 monotherapy, anti-PD-L1 monotherapy, and combination therapy of anti-PD-1/anti-PD-L1 and anti-CTLA-4. No cases with hypophysitis and T1D due to ipilimumab monotherapy were reported. Three previous cases reported the presence of anti-glutamic acid decarboxylase antibody. T1D susceptibility-related HLA haplotypes, DRB1*08:02-DQB*03:02 and DRB1*04-DQB1*03 were reported in 2 separate cases, but no other reports of HLA alleles related to T1D susceptibility were found. Kobayashi et al[33] showed that some HLA alleles are often positive in hypophysitis due to ICIs. Risk-related HLA alleles are distinct, and they overlap between IAD and panhypopituitarism. The prevalence of HLA-Cw12, HLA-DR15, HLA-DQ7, and HLA-DPw9 was significantly higher in patients with IAD, whereas that of HLA-Cw12 and HLA-DR15 was significantly higher in patients with panhypopituitarism compared to controls. However, none of the reported HLA alleles overlapped with T1D. In the present case, the patient was negative for all autoimmune islet antibodies and the patient had a T1D risk-related HLA allele. The involvement of activated T cells in irAEs such as T1D[34] and hypophysitis[16] has been suggested as a mechanism, and HLA polymorphisms would play an important role in the development.[30,33]
Table 5.
Cases that have T1D and hypophysitis due to ICI therapy.
| Case | Age | Sex | Primary disease | Therapy | A term to hypophysitis diagnosis from T1D* | Hormone disorder | Enlarged pituitary by MRI | HbA1c (mmol/mol) | CPR (nmol/L) | Autoantibody | HLA† |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Okahata S (2019)[3] | 52 | F | Breast cancer | Nivo | −1 mo | IAD | no | 62 | 0.10 | GAD-/IA-2-/IAA- | DRB1*14:05,14:06 DQB1*03:01,03:03 |
| Sum M (2018)[21] | 75 | M | Melanoma | Nivo +Ipi |
NA (T1D → hypophysitis) |
AD | enlarged | 60 | 0.10 | GAD+/islet- | NA |
| Khalid M (2019)[22] | 53 | M | Malignant melanoma | Nivo +Ipi |
−18 mo | IAD | no | NA | un- detectable |
GAD+ | NA |
| Lanzolla G (2019)[23] | 60 | M | Lung adeno-carcinoma | Atezo | 1 wk | AD | no | NA | un- detectable |
pituitary+ /21-hydroxylase+ /GAD-/IA-2- |
DRB1*04- DQB1*03 |
| Takata M (2022)[24] | 59 | M | Malignant melanoma | Nivo | −22 wk | IAD | NA | 58 | 0.18 | NA | NA |
| Hino C (2022)[25] | 45 | M | Sarcomatoid renal cell carcinoma | Nivo +Ipi |
73 d | AD | NA | 60 | 0.17 | GAD-/IAA- | NA |
| Ishiguro A (2022)[26] | 67 | M | Malignant melanoma | Nivo | 168 d | IAD | no | 54 | 0.86 | GAD+ | DRB1*08:02-DQB*03:02 |
| Luo J (2022)[27] | 76 | M | Non-small cell lung cancer | Pem | 4 mo | AD | NA | 27 | 0.01 | GAD-/IA-2- /islet- |
HLA-DQA1/DQB1 |
| Marchand L (2017)[28] | 55 | M | Pulmonary pleomorphic carcinoma | Nivo | 1 mo | IAD | no | 66 | un-detectable | GAD-/IA-2-/IAA-/islet- /Zinc 8- |
NA |
| Porntharukchareon T (2020)[29] | 70 | M | Non-small cell lung cancer | Nivo +Ipi |
simultaneous | IAD | no | 48 | un-detectable | GAD-/IA-2- | NA |
| Kikuchi F (2021)[30] | 62 | M | Renal cell carcinoma | Nivo | −3 mo | IAD | no | 65 | 0.07 | GAD- | NA |
| Boswell L (2021)[31] | 51 | M | Malignant melanoma | Nivo +Ipi |
−75 wk | IAD | no | 63 | 0.32 | GAD-/IA-2-/IAA- | DRB*01:01, DRB1*12:01 |
| Min Li (2022)[32] | 45 | M | Non-small-cell lung cancer | KN046‡ | −2 mo | AD | no | 77 | <0.03 | GAD-/IA-2- /islet- |
NA |
| This case | 81 | M | Renal cell carcinoma | Nivo +Ipi |
14 d | IAD | no | 46 | 0.01 | GAD-/IA-2-/IAA-/islet- /Zinc 8- |
DRB1*09:01- DQB1*03:03 |
CPR = serum C-peptide, GAD = anti-glutamic acid decarboxylase antibody, HbA1c = hemoglobin A1c, IA-2 = anti-insulinoma-associated antigen-2 antibody, IAA = insulin auto-antibody, ICI = immune checkpoint inhibitor, islet = islet cell antibody, NA = not assessed, Zinc 8 = zinc transporter 8 antibody.
Minus (−) means hypophysitis precedes T1D.
Bold letters are HLAs susceptible to T1D.
‡Recombinant humanized PD-L1/CTLA-4 bispecific single-domain antibody Fc fusion protein injection.
Because adrenal insufficiency due to ICI therapy could be fatal, this condition should be more widely recognized by physicians with appropriate ACTH/cortisol monitoring as well as associated clinical manifestations. The present report provides a detailed course of hypophysitis, which will contribute to better care for patients receiving ICI therapy.
Author contributions
Conceptualization: Hiraku Kameda.
Investigation: Hiroshi Iesaka, Aika Miya, Hiroshi Nomoto, Kyu Yong Cho, Akinobu Nakamura, Takashige Abe.
Supervision: Nobuo Shinohara, Tatsuya Atsumi.
Writing – original draft: Hiroshi Iesaka.
Writing – review & editing: Hiraku Kameda.
Abbreviations:
- ACTH
- adrenocorticotropic hormone
- CRH
- corticotropin-releasing hormone
- CTLA-4
- cytotoxic T-lymphocyte associated antigen-4
- HLA
- human leukocyte antigen
- IAD
- isolated ACTH deficiency
- ICIs
- immune checkpoint inhibitors
- irAEs
- immune-related adverse events
- MRI
- magnetic resonance imaging
- PD-1
- programmed cell death protein 1
- PD-L1
- programmed death-ligand 1
- RR
- risk ratio
- T1D
- type 1 diabetes
- TSH
- thyroid-stimulating hormone
Our case report was waived from the ethical approval or institutional review board of Hokkaido University Hospital, based on their policy to review all interventional and observational studies except for a case report.
The patient provided written informed consent for the purpose of publication of his clinical data. The presented data are anonymized, and the risk of identification is minimal.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors have no funding and conflicts of interest to disclose.
How to cite this article: Iesaka H, Kameda H, Miya A, Nomoto H, Cho KY, Nakamura A, Abe T, Shinohara N, Atsumi T. Fulminant ACTH decrease following diabetic ketoacidosis induced by immune checkpoint inhibitor combination therapy with nivolumab and ipilimumab: A case report. Medicine 2023;102:51(e36664).
Contributor Information
Hiroshi Iesaka, Email: hnomoto@med.hokudai.ac.jp.
Aika Miya, Email: miyaxgag@gmail.com.
Hiroshi Nomoto, Email: hnomoto@med.hokudai.ac.jp.
Kyu Yong Cho, Email: ky9494@gmail.com.
Akinobu Nakamura, Email: akinbo@tim.hi-ho.ne.jp.
Takashige Abe, Email: takataka@rf6.so-net.ne.jp.
Nobuo Shinohara, Email: nozomis@mbj.nifty.com.
Tatsuya Atsumi, Email: at3tat@med.hokudai.ac.jp.
References
- [1].Lowe JR, Perry DJ, Salama AK, et al. Genetic risk analysis of a patient with fulminant autoimmune type 1 diabetes mellitus secondary to combination ipilimumab and nivolumab immunotherapy. J ImmunoTher Cancer. 2016;4:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sakurai K, Niitsuma S, Sato R, et al. Painless thyroiditis and fulminant type 1 diabetes mellitus in a patient treated with an immune checkpoint inhibitor, Nivolumab. Tohoku J Exp Med. 2018;244:33–40. [DOI] [PubMed] [Google Scholar]
- [3].Okahata S, Sakamoto K, Mitsumatsu T, et al. Fulminant type 1 diabetes associated with isolated ACTH deficiency induced by anti-programmed cell death 1 antibody-insight into the pathogenesis of autoimmune endocrinopathy. Endocr J. 2019;66:295–300. [DOI] [PubMed] [Google Scholar]
- [4].Zeng MF, Chen LL, Ye HY, et al. Primary hypothyroidism and isolated ACTH deficiency induced by nivolumab therapy: case report and review. Medicine (Baltim). 2017;96:e8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sekizaki T, Kameda H, Oba C, et al. Nivolumab-induced hypophysitis causing secondary adrenal insufficiency after transient ACTH elevation. Endocr J. 2019;66:937–41. [DOI] [PubMed] [Google Scholar]
- [6].Imagawa A, Hanafusa T, Awata T, et al. Report of the Committee of the Japan Diabetes Society on the Research of fulminant and acute-onset type 1 diabetes mellitus: new diagnostic criteria of fulminant type 1 diabetes mellitus. J Diabetes Investig. 2012;3:536–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kawabata Y, Ikegami H, Kawaguchi Y, et al. Asian-specific HLA haplotypes reveal heterogeneity of the contribution of HLA-DR and -DQ haplotypes to susceptibility to type 1 diabetes. Diabetes. 2002;51:545–51. [DOI] [PubMed] [Google Scholar]
- [8].Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. 2018;4:173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].de Filette J, Andreescu CE, Cools F, et al. A systematic review and meta-analysis of endocrine-related adverse events associated with immune checkpoint inhibitors. Horm Metab Res. 2019;51:145–56. [DOI] [PubMed] [Google Scholar]
- [10].Lu J, Li L, Lan Y, et al. Immune checkpoint inhibitor-associated pituitary-adrenal dysfunction: a systematic review and meta-analysis. Cancer Med. 2019;8:7503–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Almutairi AR, McBride A, Slack M, et al. Potential immune-related adverse events associated with monotherapy and combination therapy of ipilimumab, nivolumab, and pembrolizumab for advanced melanoma: a systematic review and meta-analysis. Front Oncol. 2020;10:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Da L, Teng Y, Wang N, et al. Organ-specific immune-related adverse events associated with immune checkpoint inhibitor monotherapy versus combination therapy in cancer: a meta-analysis of randomized controlled trials. Front Pharmacol. 2020;10:1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Faje A, Reynolds K, Zubiri L, et al. Hypophysitis secondary to nivolumab and pembrolizumab is a clinical entity distinct from ipilimumab-associated hypophysitis. Eur J Endocrinol. 2019;181:211–9. [DOI] [PubMed] [Google Scholar]
- [14].Ohara N, Ohashi K, Fujisaki T, et al. Isolated adrenocorticotropin deficiency due to nivolumab-induced hypophysitis in a patient with advanced lung adenocarcinoma: a case report and literature review. Intern Med. 2018;57:527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Faje A. Immunotherapy and hypophysitis: clinical presentation, treatment, and biologic insights. Pituitary. 2016;19:82–92. [DOI] [PubMed] [Google Scholar]
- [16].Caturegli P, Di Dalmazi G, Lombardi M, et al. Hypophysitis secondary to cytotoxic T-Lymphocyte-associated protein 4 blockade: insights into pathogenesis from an autopsy series. Am J Pathol. 2016;186:3225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Atkins P, Ur E. Primary and ipilimumab-induced hypophysitis: a single-center case series. Endocr Res. 2020;45:246–53. [DOI] [PubMed] [Google Scholar]
- [18].Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kanie K, Iguchi G, Bando H, et al. Mechanistic insights into immune checkpoint inhibitor-related hypophysitis: a form of paraneoplastic syndrome. Cancer Immunol Immunother. 2021;70:3669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Torino F, Barnabei A, Paragliola RM, et al. Endocrine side-effects of anti-cancer drugs: mAbs and pituitary dysfunction: clinical evidence and pathogenic hypotheses. Eur J Endocrinol. 2013;169:R153–64. [DOI] [PubMed] [Google Scholar]
- [21].Sum M, Garcia FV. Immunotherapy-induced autoimmune diabetes and concomitant hypophysitis. Pituitary. 2018;21:556–7. [DOI] [PubMed] [Google Scholar]
- [22].Khalid M, Aziz SW. Endocrinopathies related to immune checkpoint inhibitors. Am J Ther. 2019;26:e608–9. [DOI] [PubMed] [Google Scholar]
- [23].Lanzolla G, Coppelli A, Cosottini M, et al. Immune checkpoint blockade Anti-PD-L1 as a trigger for autoimmune polyendocrine syndrome. J Endocr Soc. 2019;3:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Takata M, Nomura M, Yamamura K, et al. Autoimmune polyendocrine syndrome type 3, characterized by autoimmune thyroid disease, type 1 diabetes mellitus, and isolated ACTH deficiency, developed during adjuvant nivolumab treatment. Asia Pac J Clin Oncol. 2022;18:481–2. [DOI] [PubMed] [Google Scholar]
- [25].Hino C, Nishino K, Pham B, et al. Nivolumab plus ipilimumab induced endocrinopathy and acute interstitial nephritis in metastatic sarcomatoid renal-cell carcinoma: a case report and review of literature. Front Immunol. 2022;13:993622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ishiguro A, Ogata D, Ohashi K, et al. Type 1 diabetes associated with immune checkpoint inhibitors for malignant melanoma: a case report and review of 8 cases. Medicine (Baltim). 2022;101:993622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Luo J, Feng J, Liu C, et al. Type 1 diabetes mellitus induced by PD-1 inhibitors in China: a report of two cases. J Int Med Res. 2022;50:3000605221121940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Marchand L, Paulus V, Fabien N, et al. Nivolumab-induced acute diabetes mellitus and hypophysitis in a patient with advanced pulmonary pleomorphic carcinoma with a prolonged tumor response. J Thorac Oncol. 2017;12:e182–4. [DOI] [PubMed] [Google Scholar]
- [29].Porntharukchareon T, Tontivuthikul B, Sintawichai N, et al. Pembrolizumab- and ipilimumab-induced diabetic ketoacidosis and isolated adrenocorticotropic hormone deficiency: a case report. J Med Case Rep. 2020;14:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kikuchi F, Saheki T, Imachi H, et al. Nivolumab-induced hypophysitis followed by acute-onset type 1 diabetes with renal cell carcinoma: a case report. J Med Case Rep. 2021;15:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Boswell L, Casals G, Blanco J, et al. Onset of fulminant type 1 diabetes mellitus following hypophysitis after discontinuation of combined immunotherapy. A case report. J Diabetes Investig. 2021;12:2263–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Li M, Wu C, Liu Y, et al. Autoimmune polyendocrinopathy induced by an antibody (KN046) that simultaneously inhibits PD-L1 and CTLA-4: a case report and literature review. Diabetes Metab Syndr Obes. 2022;15:1253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kobayashi T, Iwama S, Sugiyama D, et al. Anti-pituitary antibodies and susceptible human leukocyte antigen alleles as predictive biomarkers for pituitary dysfunction induced by immune checkpoint inhibitors. J ImmunoTher Cancer. 2021;9:e002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sznol M, Postow MA, Davies MJ, et al. Endocrine-related adverse events associated with immune checkpoint blockade and expert insights on their management. Cancer Treat Rev. 2017;58:70–6. [DOI] [PubMed] [Google Scholar]