Abstract
PURPOSE
The Targeted Agent and Profiling Utilization Registry Study is a phase II basket study evaluating antitumor activity of commercially available targeted agents in patients with advanced cancers with genomic alterations known to be drug targets. The results in a cohort of patients with solid tumors with BRAF mutations treated with cobimetinib plus vemurafenib are reported.
METHODS
Eligible patients had measurable disease (RECIST v.1.1), Eastern Cooperative Oncology Group performance status 0-2, adequate organ function, and no standard treatment options. The primary end point was disease control (DC), defined as complete response (CR) or partial response (PR) or stable disease of at least 16-weeks duration (SD16+). Low-accruing histology-specific cohorts with BRAF mutations treated with cobimetinib plus vemurafenib were collapsed into a single histology-pooled cohort for this analysis. The results were evaluated on the basis of a one-sided exact binomial test with a null DC rate of 15% versus 35% (power, .82; α, .10). The secondary end points were objective response (OR), progression-free survival, overall survival, duration of response, duration of stable disease, and safety.
RESULTS
Thirty-one patients with solid tumors with BRAF mutations were enrolled. Twenty-eight patients were evaluable for efficacy. Patients had tumors with BRAF V600E (n = 26), K601E (n = 2), or other (n = 3) mutations. Two patients with CR (breast and ovarian cancers; V600E), 14 with PR (13 V600E, one N581I), and three with SD16+ (two V600E, one T599_V600insT) were observed with a DC rate of 68% (P < .0001; one-sided 90% CI, 54 to 100) and an OR rate of 57% (95% CI, 37 to 76). Nineteen patients experienced ≥one drug-related grade 3-5 adverse event or serious adverse event including one death attributed to treatment-related kidney injury.
CONCLUSION
Cobimetinib plus vemurafenib showed antitumor activity in patients with advanced solid tumors with BRAF V600E mutations; additional study is warranted to confirm the antitumor activity in tumors with non-V600E BRAF mutations.
INTRODUCTION
The BRAF gene encodes the BRAF protein, a serine/threonine kinase that regulates the mitogen-activated protein kinase (MAPK) pathway, which modulates cell growth and division. The most common BRAF mutation, BRAF V600E, constitutively activates the kinase. BRAF is mutated in 40% of melanomas, and the BRAF V600E mutation comprises more than 90% of BRAF mutations in melanoma.1,2 An analysis of the AACR GENIE database demonstrated that BRAF V600E mutations are also found frequently (>30%) in thyroid cancer and less commonly in colorectal cancer (CRC; 7.6%), cholangiocarcinoma (1.5%), and non–small-cell lung cancer (NSCLC; 1.3%).3
CONTEXT
Key Objective
The Targeted Agent and Profiling Utilization Registry Study aims to evaluate the antitumor activity of US Food and Drug Administration (FDA)–approved drugs used outside of their approved indication(s) in patients with advanced cancers with potentially actionable genomic variants. This cohort assessed whether the combination of vemurafenib, a BRAF inhibitor, plus cobimetinib, a MEK inhibitor, is efficacious in patients with solid tumors with BRAF mutations.
Knowledge Generated
The combination of cobimetinib plus vemurafenib demonstrated antitumor activity in heavily pretreated patients with BRAF-mutated solid tumors, primarily BRAF V600E.
Relevance
The FDA approval of dabrafenib plus trametinib for patients with advanced solid tumors with a BRAF V600E mutation with no satisfactory alternative treatment options demonstrates the benefit of the combination of BRAF and MEK inhibitors for this indication. The results of this study build upon these findings and demonstrate the efficacy of cobimetinib plus vemurafenib in patients with various solid tumors with BRAF mutations.
Vemurafenib, an ATP-competitive inhibitor of mutant BRAF V600E, improved overall survival (OS) and progression-free survival (PFS) compared with treatment with dacarbazine in patients with unresectable or metastatic melanoma bearing BRAF V600E mutations.4,5 This led to the approval of vemurafenib by the US Food and Drug Administration (FDA) in 2011. The limited efficacy observed with single-agent vemurafenib in BRAF V600E–mutant CRC raised concerns about the antitumor activity of BRAF inhibitors.6 However, a basket trial of vemurafenib in patients with solid tumors harboring a BRAF V600E mutation demonstrated a 33% overall response rate (ORR) and a 42% clinical benefit rate (CBR), suggesting that BRAF V600E is an actionable alteration across many tumor types.7
Preclinically, inhibition of BRAF induced MEK inhibitor–sensitive but RAF inhibitor–resistant ERK activation.8 This paradoxical ERK activation limited antitumor efficacy and contributed to toxicity. This established the rationale for clinical testing of BRAF/MEK inhibitor combinations, and the FDA has now approved three such combinations for treating advanced metastatic melanoma: vemurafenib and cobimetinib (2015), dabrafenib and trametinib (2022), and encorafenib and binimetinib (2018).
These combinations improved patient OS and PFS and lead to precision oncology trials targeting members of the MAPK pathway.9-11 For example, the combination of vemurafenib and cobimetinib, a highly selective small molecule inhibitor of MEK, exhibited an ORR of 68% in patients with melanoma.11
The Targeted Agent and Profiling Utilization Registry (TAPUR) Study is a phase II basket study evaluating the antitumor activity of commercially available targeted agents in patients with advanced cancers with genomic alterations known to be drug targets. We report the results in a cohort of patients with solid tumors with BRAF mutations treated with cobimetinib plus vemurafenib.
METHODS
The rationale, general design, and eligibility criteria of this trial were reported previously.12 The methods specific to the data collection and analysis of a cohort, defined in TAPUR as a group of patients with the same tumor genomic target, histology, and study treatment received, have been previously reported for other cohorts.13-17
Patients
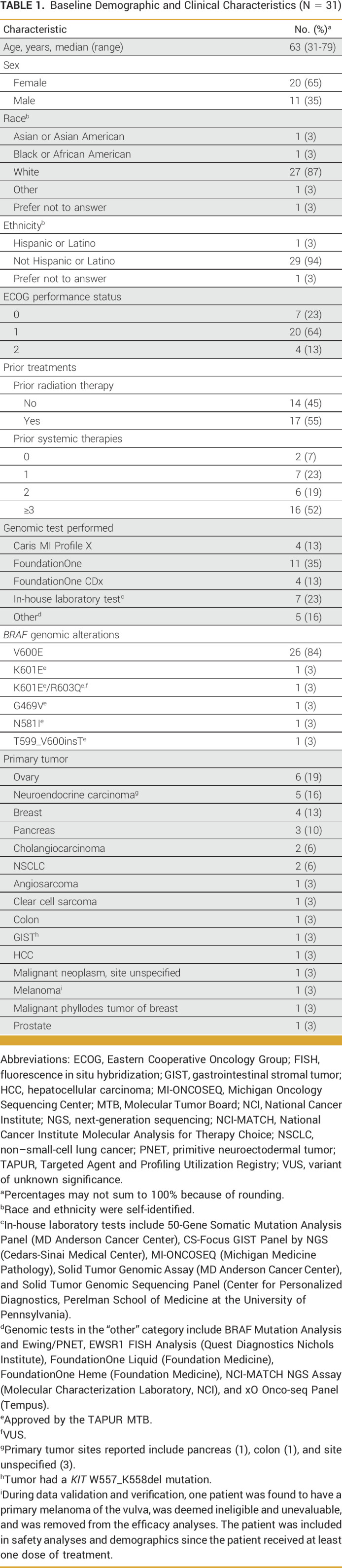
Eligible patients were required to meet both general and drug-specific eligibility criteria. General eligibility criteria included advanced or metastatic solid tumors measurable according to RECIST version 1.1.,18 Eastern Cooperative Oncology Group performance status of 0-2, and a protocol-specified genomic target identified by a Clinical Laboratory Improvement Amendments–certified and College of American Pathologists or NY State–accredited laboratory. Patients with BRAF V600D/E/K/R mutations without mutations in MAP2K1, MAP2K2, MEK1, MEK2, or NRAS were eligible for this study. Other BRAF mutations were acceptable if approved by the TAPUR Molecular Tumor Board (MTB). The TAPUR MTB is an ASCO-appointed group of experts in clinical oncology, pathology, genomics and cancer biology, and pharmacology, among others. Each case submitted to the MTB is thoroughly reviewed against relevant preclinical and clinical research, established gene mutation knowledge bases to assess the pathogenicity of the mutation, and the mechanism of action of various treatment options in or outside of the TAPUR study. Patients matched to cobimetinib plus vemurafenib must have been 18 years or older with no previous treatment with any BRAF or MEK inhibitor. Prior treatment with EGFR inhibitors was permitted. Patients with melanoma were excluded. Additional drug-specific exclusion criteria have been previously reported.19
Patients were treated with cobimetinib 60 mg orally once daily for 21 days followed by 7 days off and vemurafenib 960 mg orally twice daily until clinical and/or radiographic evidence of progressive disease or withdrawal because of unacceptable toxicity, patient preference, or physician recommendation.
Patients who were eligible but unevaluable were those for whom data on the primary end point were not available because of leaving the study before radiographic assessment; these patients were not included in the efficacy analyses but were included in safety analyses.
Study End Points
The primary end point was investigator assessment of disease control (DC), defined as objective response (OR) or stable disease of at least 16-weeks duration (SD16+) from the initiation of study treatment as determined by RECIST version 1.1.18 The assessment of complete response (CR) is based on radiographic assessment of measured target lesions and recorded nontarget lesions only. The secondary end points were OR, PFS, OS, duration of response, duration of SD, and safety. Duration of response was defined as the time from the participant's first documented OR until disease progression and was censored at the last time the patient was known to be progression free. Duration of SD was defined as the time from initiation of study treatment until disease progression. PFS was measured from the time of initiation of study treatment until clinical progression, radiographic progression, or death, whichever occurred first, and was censored at the last clinical evaluation at which the patient was still alive and progression free. OS was measured from time of initiation of study treatment until death from any cause or censored on the date of last follow-up of the surviving patients. Radiographic assessment and clinical evaluation for response were performed at 8 weeks and 16 weeks after treatment initiation and then every 12 weeks thereafter while the patient remained on treatment. DC was determined on the basis of the best response reported at 16 weeks after treatment initiation or later. An independent review of imaging studies was not performed. All serious adverse events (SAEs) and treatment-related adverse events (AEs) of grade 3-5 were reported according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Statistical Considerations
Low-accruing individual histology-specific cohorts of patients with BRAF-mutant solid tumors who were treated with cobimetinib plus vemurafenib were collapsed into a single cohort for this analysis. The primary objective was assessed on the basis of an exact binomial test with a null DC rate of .15 and one-sided α of .10. For this cohort of 28 evaluable patients, the null hypothesis was rejected if at least eight patients had DC. This design has a power of 82% if the true DC rate is .35. A one-sided 90% CI is also provided for the DC rate. Kaplan-Meier curves were used to estimate the PFS and OS distributions. Other efficacy end point estimates used 95% CIs. All patients receiving at least one dose of study treatment were included in the safety analysis.
Trial Oversight
The study protocol was approved by a central institutional review board and, in some cases, by a local institutional review board at participating sites. Patients provided written consent before any screening activities or data collection began. The study was designed by ASCO staff with input from ASCO volunteer members, patient advocates, and participating pharmaceutical companies. The TAPUR Data and Safety Monitoring Board (DSMB) is an ASCO-appointed independent board that meets biannually to monitor the study and review the safety and efficacy findings. In this cohort, multiple groups of patients with BRAF mutations treated with cobimetinib plus vemurafenib were collapsed into a single histology-pooled group. After review, the DSMB approved release of the outcome data reported herein.
RESULTS
Patient Characteristics
Thirty-one patients with solid tumors, representing 15 tumor types, were enrolled from December 2016 to January 2021 across 18 clinical sites (68% were enrolled from community-based sites). Twenty-eight patients were evaluable and were included in the efficacy analyses. Two patients left the study before the protocol-specified radiographic assessment at week 8 and were unevaluable while one patient was ineligible.
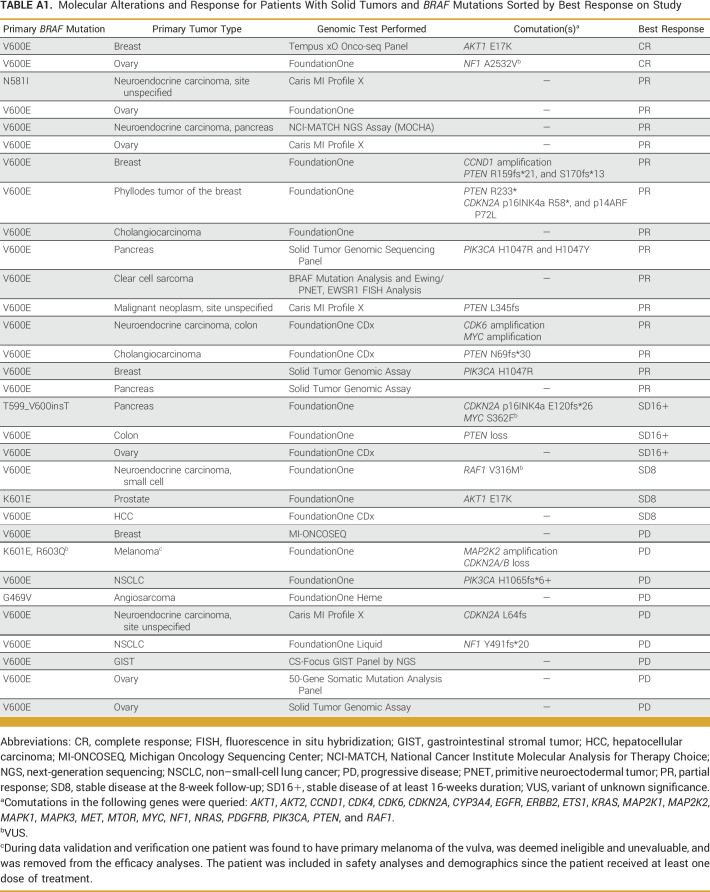
Patient characteristics are summarized in Table 1. Twenty-six of 31 patients (84%) had a tumor with a BRAF V600E mutation. Two patients had tumors with a BRAF K601E mutation, one of whom also had a BRAF R603Q mutation. The remaining three patients had tumors with unique BRAF mutations (G469V, N581I, and T599_V600insT). The two most common tumor types were ovary (19%) and neuroendocrine carcinoma of varying sites (16%). During data validation and verification, one patient was found to have primary melanoma of the vulva and was, therefore, deemed ineligible and removed from efficacy analyses. Genomic alterations, tumor types, and responses for all patients are shown in Appendix Table A1.
TABLE 1.
Baseline Demographic and Clinical Characteristics (N = 31)
Efficacy Results
Of 28 evaluable patients, two had CR, 14 had a partial response (PR), and three had SD16+. The DC rate was 68% (one-sided 90%, 54 to 100), and the OR rate was 57% (95% CI, 37 to 76). The null hypothesis of a 15% DC rate was rejected (P < .0001).
Seventeen of 19 patients with DC had tumors with a BRAF V600E mutation while the other two had tumors with an N581I (PR, neuroendocrine carcinoma, site unspecified) and a T599_V600insT (SD16+, pancreas) mutation. The two patients with CR had ER+/HER2– invasive ductal carcinoma and ovarian cancer with V600E mutations.
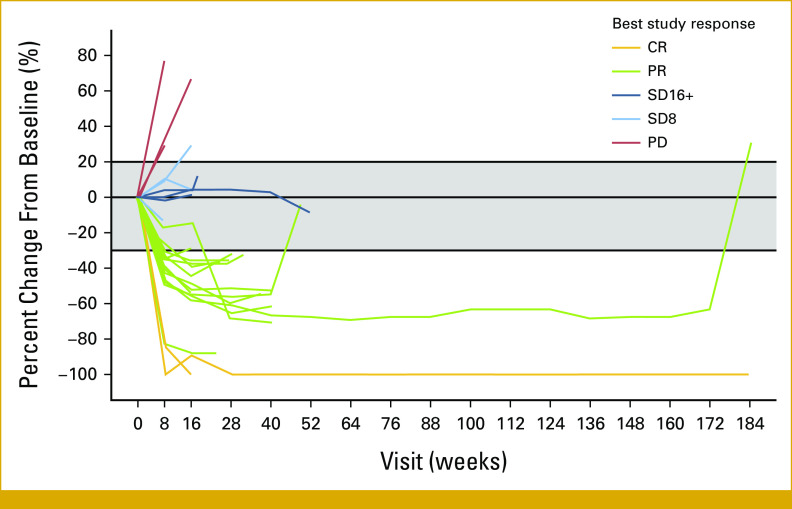
Maximum percent change in target lesion size from baseline is shown in Figure 1. Time on treatment for patients with a best response of OR or SD16+ is shown in Figure 2, and percent change in tumor burden over time for 28 patients is shown in Figure 3. The duration of response on study for the two patients with CR was 5 (ovary, left study because of rising creatinine levels) and 170 (invasive ductal carcinoma in the breast) weeks. The median duration of response for the patients with PR was 21 weeks (range, 8-176). Among the three patients with a best response of SD16+, the duration of SD was 20, 25, and 53 weeks, respectively.
FIG 1.
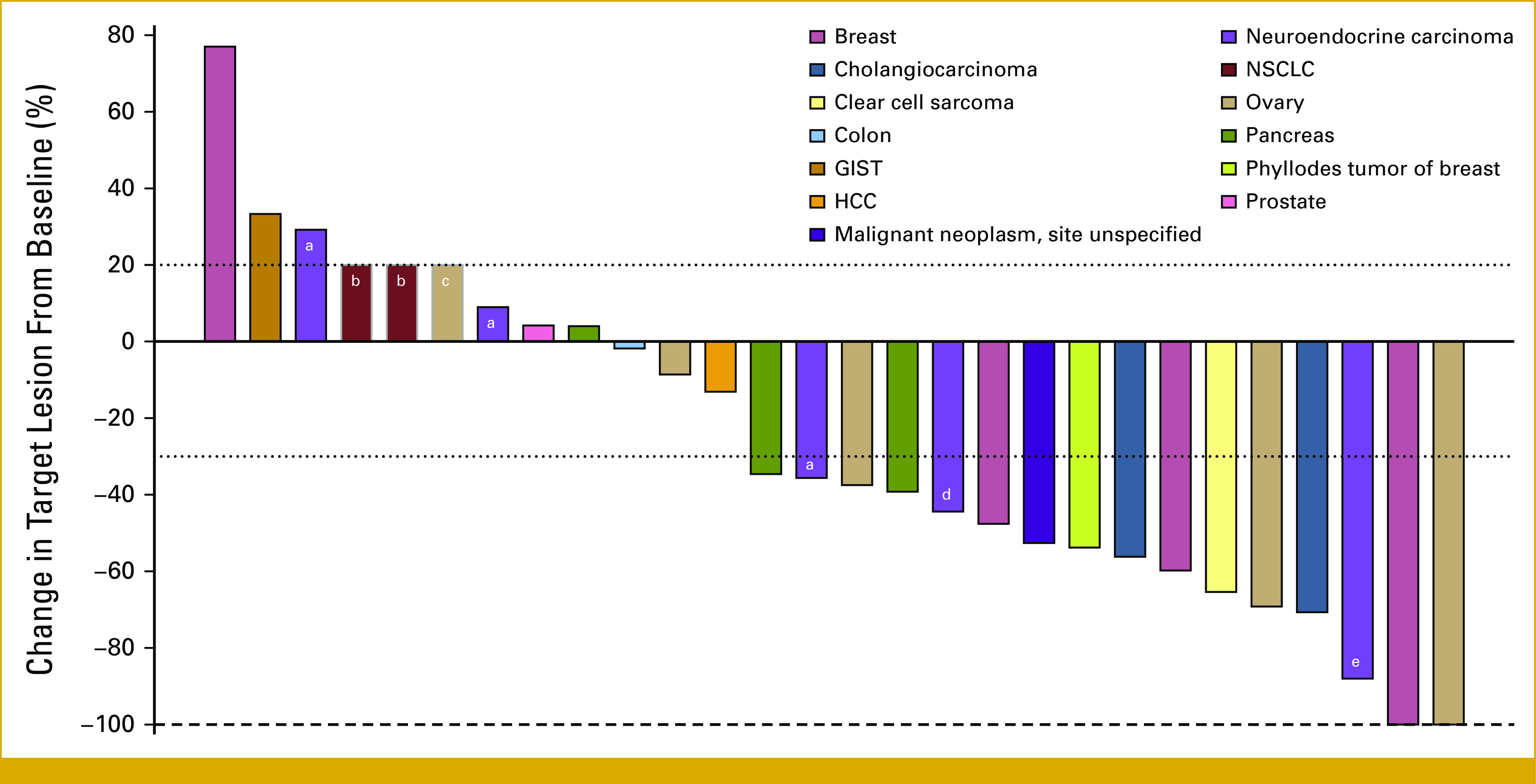
Maximum percent change from baseline in target lesions (n = 28). Color coding indicates primary tumor origin. All evaluable patients are included in this figure, including one patient who died due to acute kidney injury, a known side effect of vemurafenib treatment. aNeuroendocrine carcinoma, site unspecified. bFor two patients with clinical progression but no post-treatment tumor measurements, a 20% increase was assigned. cFor one patient who ended treatment due to death related to study treatment but no post-treatment tumor measurements, a 20% increase was assigned. dNeuroendocrine carcinoma of colon. eNeuroendocrine carcinoma of pancreas. GIST, gastrointestinal stromal tumor; HCC, hepatocellular carcinoma; NSCLC, non–small-cell lung cancer.
FIG 2.
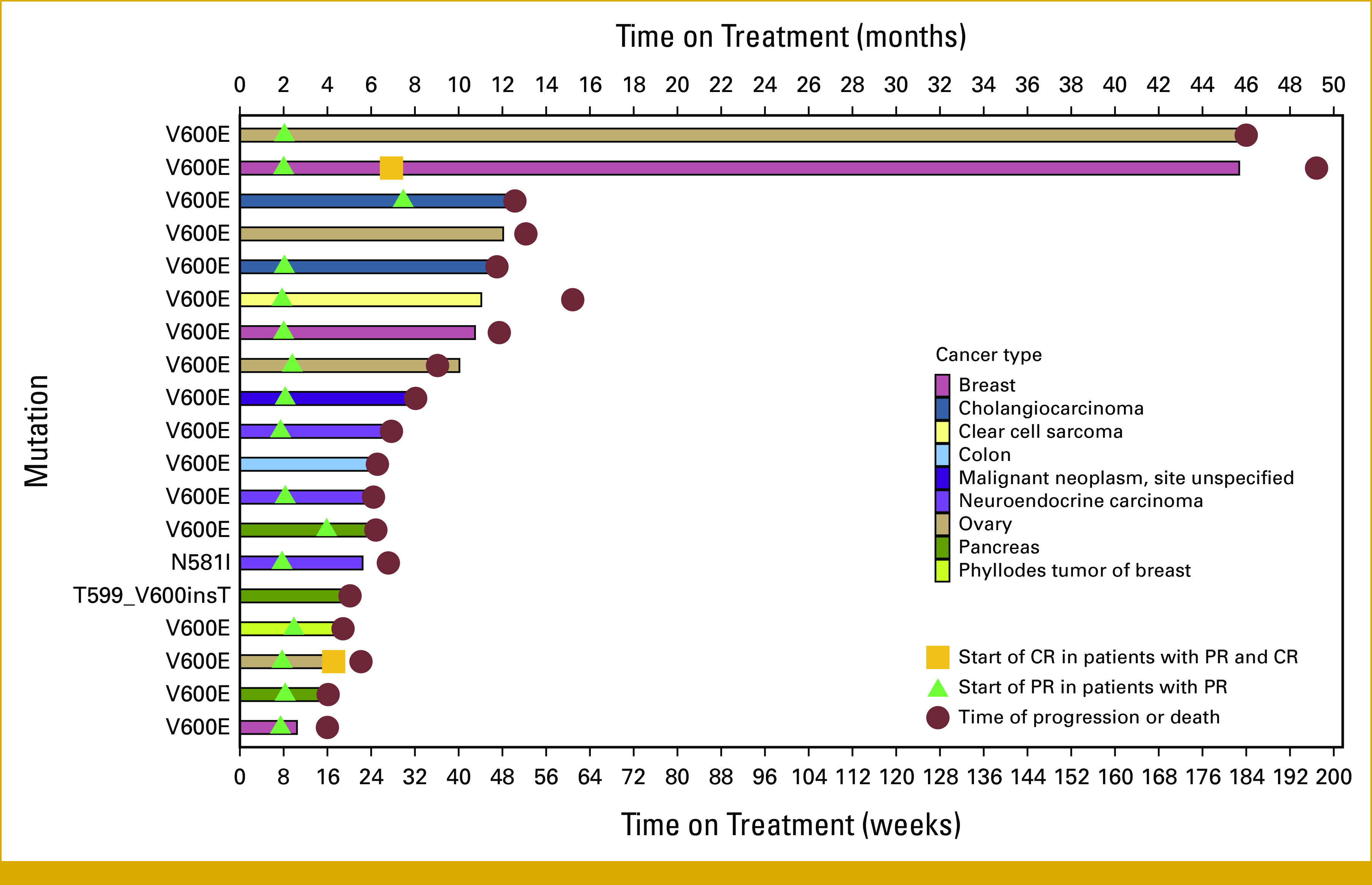
Time on treatment of 19 patients with OR or SD16+. Color coding indicates primary tumor origin. Sites for neuroendocrine carcinoma include pancreas (1), colon (1), and site unspecified (3). CR, complete response; OR, objective response; PR, partial response; SD16+, stable disease of at least 16-weeks duration.
FIG 3.
Spider plot of percent change from baseline in tumor burden during cobimetinib plus vemurafenib treatment in 28 patients with solid tumors with BRAF mutations. Three patients were not evaluable or had only a baseline visit because of leaving the study early and are not included in the spider plot: one enrolled on study, received treatment, and was later found to be ineligible, one had an unreportable AE, and one chose to discontinue participation in the study. Color coding in the plot showing CR, PR, SD16+, SD8, and PD indicates best response observed during the period of observation. AE, adverse event; CR, complete response; PD, progressive disease; PR, partial response; SD8, stable disease at the 8-week follow-up visit; SD16+, stable disease of at least 16-weeks duration.
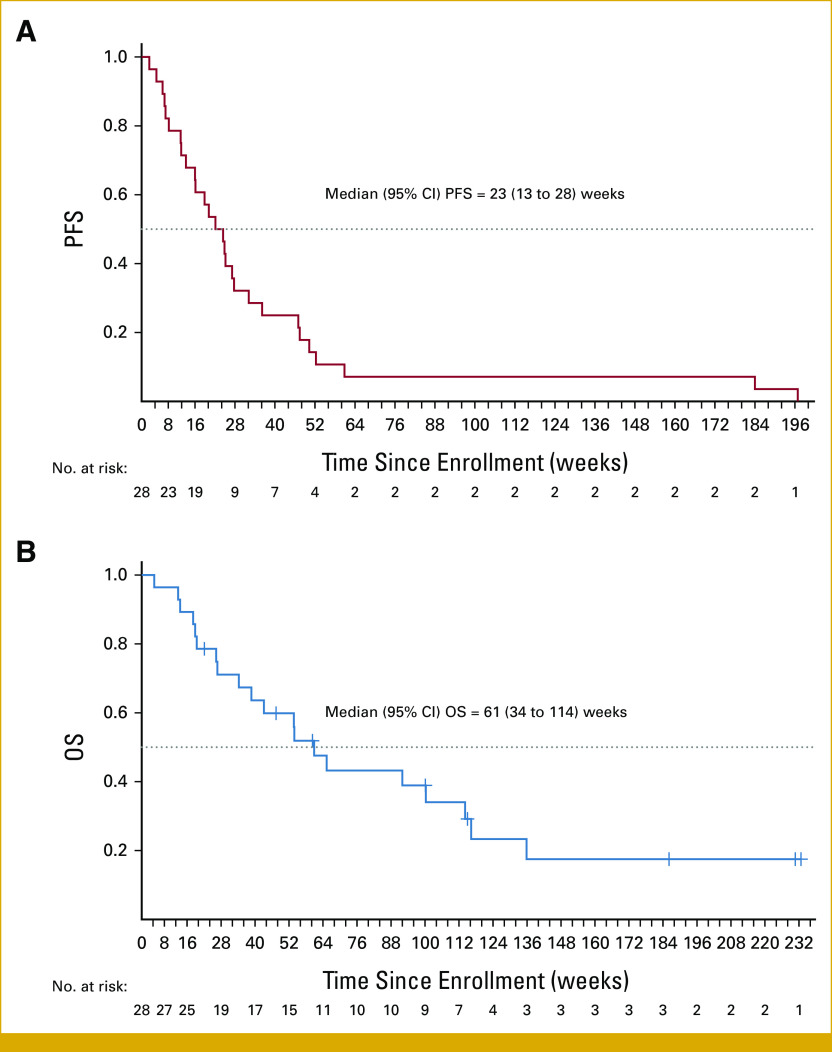
The median PFS for all evaluable patients was 23 weeks (95% CI, 13 to 28), and the median OS was 61 weeks (95% CI, 34 to 114), as shown in Figures 4A and 4B.
FIG 4.
(A) PFS and (B) OS in 28 patients with solid tumors with BRAF mutations treated with cobimetinib plus vemurafenib. OS, overall survival; PFS, progression-free survival.
Safety Results
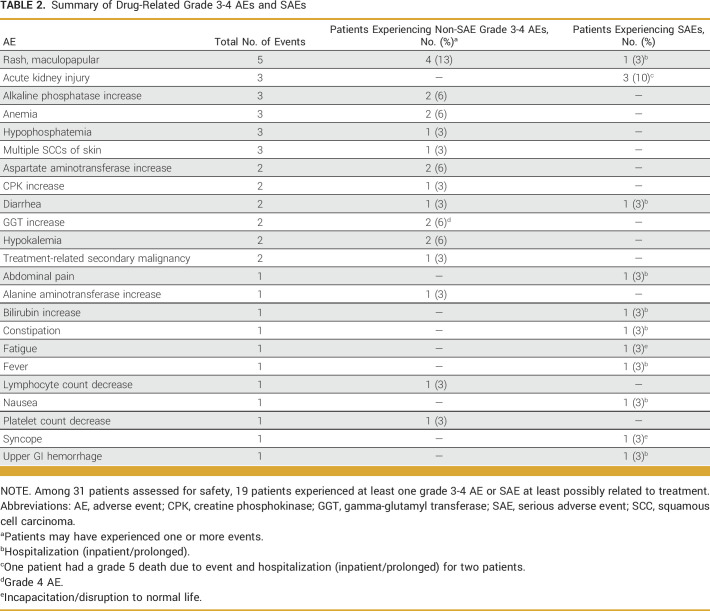
Drug-related grade 3-5 AEs and SAEs were reported in 19 of 31 patients (61%) included in the safety analysis (Table 2); drug-related SAEs were reported in 11 patients (35%). Two patients discontinued treatment because of AEs and were not evaluable: one experienced a grade 2 AE of rash, fatigue, dysgeusia, pain, and anorexia, and one experienced an unrelated grade 4 SAE of sepsis. One patient died from treatment-related acute kidney injury which is a known side effect of vemurafenib.
TABLE 2.
Summary of Drug-Related Grade 3-4 AEs and SAEs
Of 19 patients with drug-related AEs, six had dose adjustments and 11 had dose discontinuations. Those with dose discontinuations may have also experienced dose adjustments before discontinuation. Among 31 patients assessed for safety, 18 had dose adjustments or discontinuation regardless of the AE's relatedness to treatment, 10 of whom experienced DC. Dose adjustment was defined as an instance in which the treating physician changed treatment dosing for a patient because of an AE, and dose discontinuation was defined as an instance in which the treating physician temporarily or permanently stopped treatment for a patient because of an AE.
DISCUSSION
Genomic testing is increasingly used to inform the care of patients with advanced cancer. Multiple genotype-matched therapies are now FDA-approved for several disease types and tumor-agnostic biomarker-matched therapies, including agents targeted to NTRK and RET fusions, BRAF V600E mutations, and tumors with microsatellite instability-high status, and high tumor mutational burden are available for patients with any solid tumor. Recently, the ASCO Guideline Committee released a provisional clinical opinion on the clinical utility of genomic testing recommending that multigene panel testing be performed in patients with advanced solid tumors whenever more than one genomic biomarker has been linked to an approved therapy.3 They concluded that tumor agnostic approvals provide a rationale for genomic testing in all solid tumors and may identify additional targets in diseases without disease-specific drug approvals. Our study confirms that routine genomic testing can identify patients with actionable BRAF mutations in various cancers.
In the past decade, BRAF inhibitors have been approved for multiple BRAF V600-mutant tumors, as either monotherapy (vemurafenib for Erdheim-Chester disease) or in combination with MEK inhibitors (dabrafenib plus trametinib for anaplastic thyroid cancer, NSCLC, and unresectable or metastatic solid tumors) and in combination with EGFR inhibitors (encorafenib and cetuximab for CRC).20-22 In our study, cobimetinib plus vemurafenib demonstrated an OR rate of 57%, with responses and prolonged SD in multiple tumor types where vemurafenib, alone or in combination, has not yet been approved, notably breast cancer, ovarian cancer, and cholangiocarcinoma. AEs were consistent with known side effects of the drug combination. Our findings provide further evidence that BRAF V600 is an actionable driver mutation across multiple tumor types.
The VE-BASKET study enrolled 208 patients, 172 of whom were included in the efficacy analysis to investigate the efficacy of vemurafenib in BRAF V600E-mutant tumors.7,23 The study demonstrated an ORR of 33% and a CBR of 42%. The cohort presented here demonstrated higher antitumor activity, with an OR rate of 57% and a DC rate of 68%. Our observed median PFS of 23 weeks (5.75 months) comports with the VE BASKET trial, which reported a median PFS of 5.8 months.7 However, the patients included in this study were more heavily pretreated, with 52% having had three or more lines of systemic therapy, compared with 26% in the VE–BASKET trial. In addition, this study had few patients with lung cancer or cholangiocarcinoma, diseases where BRAF V600E mutations are more common and for which BRAF/MEK combination therapy (dabrafenib/trametinib) has already demonstrated efficacy.24,25
The phase II, open-label Rare Oncology Agnostic Research (ROAR) basket trial, which enrolled patients with BRAF V600E–mutated rare cancers (ClinicalTrials.gov identifier: NCT02034110), demonstrated activity of dabrafenib in combination with trametinib that led to initial FDA approvals in NSCLC and anaplastic thyroid cancer.24 This trial also demonstrated antitumor activity in rare cancers including adenocarcinoma of the small intestine (ORR, 67%), low-grade glioma (ORR, 54%), high-grade glioma (ORR, 33%), hairy cell leukemia (ORR, 89%), and multiple myeloma (ORR, 50%).26 The NCI-MATCH (ClinicalTrials.gov identifier: NCT02465060) study included 29 patients with various tumor types harboring BRAF V600E mutations and reported an ORR of 38% (90% CI, 22.9 to 54.9).27 Although, we report a higher OR rate, the similar CIs (57% [95% CI, 37 to 76]) in these studies suggest that either dabrafenib plus trametinib or cobimetinib plus vemurafenib are reasonable treatment options for patients with tumors with BRAF V600E mutations.
The collective data from the ROAR basket trial, NCI-MATCH trial, and the 36 pediatric patients from the CTMT212X2101 study (ClinicalTrials.gov identifier: NCT02124772),28 in which a 25% ORR was observed in response to combination dabrafenib and trametinib, supported the tumor-agnostic FDA approval for dabrafenib plus trametinib.29 For the 131 adult patients, 54 (41%; 95% CI, 33 to 50) experienced OR. The studies enrolled patients with 24 tumor types. Among the highest representative tumor types, ORR was 46% for biliary tract cancer, 33% for high-grade glioma, and 50% for low-grade glioma. TAPUR patients with various tumor types, including ER+ breast cancer, cholangiocarcinoma, CRC, neuroendocrine tumors (various sites), ovarian cancer, pancreatic cancer, and clear cell sarcoma, had ORs and/or clinical benefit, in some cases of several years' duration, providing further support for the efficacy of this treatment strategy across multiple tumor types.
Over the past few years, our understanding of BRAF signaling has significantly improved. BRAF mutations are subdivided into three classes on the basis of the activation mechanism of ERK signaling. Class I mutations (V600) signal as active monomers while class II mutations signal as active dimers. Class III mutants are often kinase impaired or dead and promote MAPK signaling in cooperation with RAS.30,31 Importantly, vemurafenib is ineffective at inhibiting MAPK signaling in cancer cells when the pathway is activated by class II and III BRAF mutations. In our study, patients were eligible if they had a BRAF V600E mutation; however, patients with other BRAF alterations were enrolled following review by the TAPUR MTB leading to inclusion of two patients with tumors harboring class II mutations (K601E and G469V) and one with a tumor with a class III mutation (N581I).32,33
Interestingly, the patient in this study with a neuroendocrine tumor (site unspecified) harboring a BRAF N581I mutation had a PR. BRAF N581I is an inactivating mutation insensitive to vemurafenib/BRAF inhibition but sensitive to MEK inhibition.31 Thus, the antitumor activity observed in this patient was likely attributable to cobimetinib, further highlighting the potential benefit of this combinatorial approach. Our study enrolled five patients with neuroendocrine tumors harboring BRAF mutations, three of whom obtained PRs. Another patient with a pancreatic tumor with a BRAF p.T599_V600insT mutation had a duration of SD of 20 weeks. Previous reports have documented sensitivity to BRAF/MEK inhibitors in patients with tumors harboring this mutation.34-36
There are limitations to this study. Patients were enrolled on the basis of local testing using a variety of genomic testing platforms, although this was intended in the design of TAPUR to try to replicate real-world clinical practice. However, not all genomic tests had the same coverage or depth of sequencing, resulting in variation in the mutational profiles reported for individual patients. As a result, we were unable to systematically evaluate the contribution of coalterations to treatment efficacy. In addition, during the study period, dabrafenib plus trametinib was FDA-approved for anaplastic thyroid and NSCLC, limiting the accrual of these sensitive tumor types to our study. Finally, our study did not have a control arm, and we were unable to assess the individual contributions of vemurafenib and cobimetinib. However, we are encouraged that patients with multiple different BRAF-mutant tumor types exhibited clinical benefit at a time when few other treatment options were available for them.
In summary, the TAPUR Study demonstrated that BRAF mutations (most commonly V600E) can be identified in many tumor types in patients undergoing routine genomic testing. Cobimetinib plus vemurafenib combination therapy had a toxicity profile consistent with the drug labels and showed antitumor activity in patients with multiple tumor types with advanced solid tumors with BRAF V600E mutations. Responses seen in patients with non-V600E mutations warrant further study to confirm antitumor activity.
ACKNOWLEDGMENT
The authors thank the patients who participated, the clinical centers, staff, and the TAPUR Study team for study conduct and manuscript support. Study activities are supported as noted in the Funding Support statement.
APPENDIX
TABLE A1.
Molecular Alterations and Response for Patients With Solid Tumors and BRAF Mutations Sorted by Best Response on Study
Funda Meric-Bernstam
Employment: MD Anderson Cancer Center
Consulting or Advisory Role: Roche, Zymeworks, Infinity Pharmaceuticals, AbbVie, Black Diamond Therapeutics, Eisai, OnCusp Therapeutics, Lengo Therapeutics, Tallac Therapeutics, Karyopharm Therapeutics, Biovica, AstraZeneca, Seagen, Loxo, PACT Pharmaceuticals, Apeiron Biologics, EcoR1 Capital, Menarini Group, Theratechnologies, Calibr, LegoChem Biosciences, Protai
Research Funding: Novartis (Inst), AstraZeneca (Inst), Taiho Pharmaceutical (Inst), Genentech (Inst), Calithera Biosciences (Inst), Debiopharm Group (Inst), Bayer (Inst), Aileron Therapeutics (Inst), Puma Biotechnology (Inst), CytomX Therapeutics (Inst), Jounce Therapeutics (Inst), Zymeworks (Inst), Curis (Inst), Pfizer (Inst), eFFECTOR Therapeutics (Inst), AbbVie (Inst), Boehringer Ingelheim (Inst), Guardant Health (Inst), Daiichi Sankyo (Inst), GlaxoSmithKline (Inst), Seagen (Inst), KLUS Pharma (Inst), Takeda (Inst)
Travel, Accommodations, Expenses: European Organisation for Research and Treatment of Cancer (EORTC), ESMO, Cholangiocarcinoma Foundation
Timothy L. Cannon
Honoraria: Deciphera, AstraZeneca, Bayer
Consulting or Advisory Role: Intermountain Healthcare
Other Relationship: Navican/Intermountain Healthcare
Steven Powell
This author is a member of the JCO Precision Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Bristol Myers Squibb (Inst)
Speakers' Bureau: Alkermes (Inst)
Research Funding: Merck Sharp & Dohme (Inst), Novartis (Inst), Genentech/Roche (Inst), Incyte (Inst), Bristol Myers Squibb (Inst), Pfizer (Inst), Vyriad (Inst), Actuate Therapeutics (Inst), AstraZeneca/MedImmune (Inst), Seagen (Inst), Molecular Templates (Inst), Sorrento Therapeutics (Inst)
John C. Krauss
Research Funding: Ignyta (Inst), ACCRU (Inst), NSABP Foundation (Inst), Amgen (Inst), Isofol Medical (Inst), Novartis (Inst), Hutchison MediPharma (Inst), Cardiff Oncology, AstraZeneca/MedImmune (Inst), Tempest Therapeutics (Inst), Pfizer (Inst), Alliance for Clinical Trials in Oncology (Inst), Daiichi Sankyo/Arqule (Inst), Bristol Myers Squibb/Medarex (Inst), Bristol Myers Squibb (Inst), Exelixis (Inst), Janssen Oncology (Inst)
Margaret von Mehren
Honoraria: Molleculin, Boehringer Ingelheim, Deciphera
Consulting or Advisory Role: deciphera
Research Funding: Novartis (Inst), Deciphera (Inst), ASCO (Inst), Solaris Health (Inst), Theseus (Inst), Sumitomo Pharma Oncology (Inst)
Patents, Royalties, Other Intellectual Property: Cell line
Travel, Accommodations, Expenses: Boehringer Ingelheim
Other Relationship: NCCN
Deepti Behl
Consulting or Advisory Role: AstraZeneca, Bristol Myers Squibb Foundation, Lilly, Novartis, Janssen Oncology, Novocure, Taiho Pharmaceutical
Speakers' Bureau: AstraZeneca, Janssen Oncology
Herbert L. Duvivier
Speakers' Bureau: Guardant Health, AstraZeneca, Regeneron
Henry G. Kaplan
Employment: Syapse, Medpace, Criterium, Inc
Stock and Other Ownership Interests: Abbott, Becton Dickinson, CVS Health, UnitedHealthcare, Humana, illumina
Consulting or Advisory Role: Syapse, Criterium
Research Funding: illumina, Seagen
Michael B. Livingston
Expert Testimony: Vander
Manish R. Sharma
Stock and Other Ownership Interests: Abbott Laboratories, AbbVie, Biogen, Bristol Myers Squibb, Lilly, Merck, Pfizer, Regeneron, Amgen, Gilead Sciences, Johnson & Johnson/Janssen, Moderna Therapeutics, Vertex, West Pharmaceutical
Consulting or Advisory Role: Pliant
Research Funding: Ascentage Pharma (Inst), AstraZeneca (Inst), Bolt Biotherapeutics (Inst), Bristol Myers Squibb (Inst), Compugen (Inst), Constellation Pharmaceuticals (Inst), CytomX Therapeutics (Inst), Exelixis (Inst), Ikena Oncology (Inst), InhibRx (Inst), Jounce Therapeutics (Inst), KLUS Pharma (Inst), Loxo (Inst), Macrogenics (Inst), Merck (Inst), Pfizer (Inst), Regeneron (Inst), Symphogen (Inst), Syros Pharmaceuticals (Inst), Astellas Pharma (Inst), Celgene (Inst), Treadwell Therapeutics (Inst), eFFECTOR Therapeutics (Inst), Genmab (Inst), Arrys Therapeutics (Inst), PureTech (Inst), GlaxoSmithKline/Tesaro (Inst), Seagen (Inst), Shattuck Labs (Inst), Sapience Therapeutics (Inst), Epizyme (Inst), Odonate Therapeutics (Inst), Tempest Therapeutics (Inst), Mersana (Inst), NGM Biopharmaceuticals (Inst), Samumed (Inst), Onconova Therapeutics (Inst), Gilead Sciences (Inst), AbbVie (Inst), Agenus (Inst), Alkermes (Inst), Alpine Immune Sciences (Inst), Alexo Therapeutics (Inst), Johnson & Johnson/Janssen (Inst), Seven and Eight Biopharmaceuticals (Inst), Cullinan Oncology (Inst), Debiopharm Group (Inst), Palleon Pharmaceuticals (Inst), Helsinn Therapeutics (Inst), Kinnate Biopharma (Inst), KSQ Therapeutics (Inst), Repare Therapeutics (Inst), SK Life Sciences (Inst), Theratechnologies (Inst), Tizona Therapeutics, Inc (Inst)
Walter J. Urba
Consulting or Advisory Role: Bristol Myers Squibb (Inst), AstraZeneca/MedImmune, AstraZeneca/MedImmune (Inst)
Research Funding: Bristol Myers Squibb (Inst), MedImmune (Inst), Merck (Inst), Galectin Therapeutics (Inst), AstraZeneca (Inst)
Patents, Royalties, Other Intellectual Property: MedImmune (Inst), Galectin Therapeutics (Inst)
Abigail Gregory
Employment: Pfizer, Biohaven Pharmaceuticals (I)
Stock and Other Ownership Interests: Biohaven Pharmaceuticals (I)
Susan Halabi
This author is a member of the JCO Precision Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Employment: ASCO
Honoraria: Sanofi, AVEO, Bristol Myers Squibb, Janssen
Richard L. Schilsky
Leadership: Clarified Precision Medicine, Leap Therapeutics
Stock and Other Ownership Interests: EQRx, Leap Therapeutics
Honoraria: Toray Industries
Consulting or Advisory Role: Cellworks, Scandion Oncology, Bryologyx, Illumina, EQRx, Syapse, Zephyr AI, AADi, Flatiron Health
Research Funding: AstraZeneca (Inst), Bayer (Inst), Bristol Myers Squibb (Inst), Genentech/Roche (Inst), Lilly (Inst), Merck (Inst), Pfizer (Inst), Boehringer Ingelheim (Inst), Seagen (Inst), Taiho Oncology (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1138818/summary
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in abstract and oral presentation format at the ASCO Annual Meeting on June 7, 2022, in Chicago, IL.
SUPPORT
Cobimetinib (Cotellic) plus vemurafenib (Zelboraf) and funding for the Targeted Agent and Profiling Utilization Registry (TAPUR) Study were provided by Genentech. Additional funding for TAPUR was provided by AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly and Company, Merck, Pfizer, Seagen and Taiho Oncology.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Pam K. Mangat, Elizabeth Garrett-Mayer, Susan Halabi, Richard L. Schilsky
Financial support: Funda Meric-Bernstam
Administrative support: Funda Meric-Bernstam, Gina N. Grantham, Dominique C. Hinshaw, Abigail Gregory
Provision of study materials or patients: Funda Meric-Bernstam, Pam K. Mangat, John C. Krauss, Christopher M. Reynolds, Margaret von Mehren, Deepti Behl, Herbert L. Duvivier, Michael B. Livingston, Manish R. Sharma, Walter J. Urba, Rodolfo Gutierrez
Collection and assembly of data: Funda Meric-Bernstam, Pam K. Mangat, Rodolfo Gutierrez, Eugene R. Ahn, Steven Powell, Christopher M. Reynolds, Margaret von Mehren, Deepti Behl, Manish R. Sharma, Gina N. Grantham, Dominique C. Hinshaw, Abigail Gregory
Data analysis and interpretation: Funda Meric-Bernstam, Michael Rothe, Pam K. Mangat, Elizabeth Garrett-Mayer, Eugene R. Ahn, Timothy L. Cannon, John C. Krauss, Christopher M. Reynolds, Deepti Behl, Carmen J. Calfa, Herbert L. Duvivier, Henry G. Kaplan, Michael B. Livingston, Manish R. Sharma, Walter J. Urba, Gina N. Grantham, Dominique C. Hinshaw, Abigail Gregory, Susan Halabi, Richard L. Schilsky
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Funda Meric-Bernstam
Employment: MD Anderson Cancer Center
Consulting or Advisory Role: Roche, Zymeworks, Infinity Pharmaceuticals, AbbVie, Black Diamond Therapeutics, Eisai, OnCusp Therapeutics, Lengo Therapeutics, Tallac Therapeutics, Karyopharm Therapeutics, Biovica, AstraZeneca, Seagen, Loxo, PACT Pharmaceuticals, Apeiron Biologics, EcoR1 Capital, Menarini Group, Theratechnologies, Calibr, LegoChem Biosciences, Protai
Research Funding: Novartis (Inst), AstraZeneca (Inst), Taiho Pharmaceutical (Inst), Genentech (Inst), Calithera Biosciences (Inst), Debiopharm Group (Inst), Bayer (Inst), Aileron Therapeutics (Inst), Puma Biotechnology (Inst), CytomX Therapeutics (Inst), Jounce Therapeutics (Inst), Zymeworks (Inst), Curis (Inst), Pfizer (Inst), eFFECTOR Therapeutics (Inst), AbbVie (Inst), Boehringer Ingelheim (Inst), Guardant Health (Inst), Daiichi Sankyo (Inst), GlaxoSmithKline (Inst), Seagen (Inst), KLUS Pharma (Inst), Takeda (Inst)
Travel, Accommodations, Expenses: European Organisation for Research and Treatment of Cancer (EORTC), ESMO, Cholangiocarcinoma Foundation
Timothy L. Cannon
Honoraria: Deciphera, AstraZeneca, Bayer
Consulting or Advisory Role: Intermountain Healthcare
Other Relationship: Navican/Intermountain Healthcare
Steven Powell
This author is a member of the JCO Precision Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Bristol Myers Squibb (Inst)
Speakers' Bureau: Alkermes (Inst)
Research Funding: Merck Sharp & Dohme (Inst), Novartis (Inst), Genentech/Roche (Inst), Incyte (Inst), Bristol Myers Squibb (Inst), Pfizer (Inst), Vyriad (Inst), Actuate Therapeutics (Inst), AstraZeneca/MedImmune (Inst), Seagen (Inst), Molecular Templates (Inst), Sorrento Therapeutics (Inst)
John C. Krauss
Research Funding: Ignyta (Inst), ACCRU (Inst), NSABP Foundation (Inst), Amgen (Inst), Isofol Medical (Inst), Novartis (Inst), Hutchison MediPharma (Inst), Cardiff Oncology, AstraZeneca/MedImmune (Inst), Tempest Therapeutics (Inst), Pfizer (Inst), Alliance for Clinical Trials in Oncology (Inst), Daiichi Sankyo/Arqule (Inst), Bristol Myers Squibb/Medarex (Inst), Bristol Myers Squibb (Inst), Exelixis (Inst), Janssen Oncology (Inst)
Margaret von Mehren
Honoraria: Molleculin, Boehringer Ingelheim, Deciphera
Consulting or Advisory Role: deciphera
Research Funding: Novartis (Inst), Deciphera (Inst), ASCO (Inst), Solaris Health (Inst), Theseus (Inst), Sumitomo Pharma Oncology (Inst)
Patents, Royalties, Other Intellectual Property: Cell line
Travel, Accommodations, Expenses: Boehringer Ingelheim
Other Relationship: NCCN
Deepti Behl
Consulting or Advisory Role: AstraZeneca, Bristol Myers Squibb Foundation, Lilly, Novartis, Janssen Oncology, Novocure, Taiho Pharmaceutical
Speakers' Bureau: AstraZeneca, Janssen Oncology
Herbert L. Duvivier
Speakers' Bureau: Guardant Health, AstraZeneca, Regeneron
Henry G. Kaplan
Employment: Syapse, Medpace, Criterium, Inc
Stock and Other Ownership Interests: Abbott, Becton Dickinson, CVS Health, UnitedHealthcare, Humana, illumina
Consulting or Advisory Role: Syapse, Criterium
Research Funding: illumina, Seagen
Michael B. Livingston
Expert Testimony: Vander
Manish R. Sharma
Stock and Other Ownership Interests: Abbott Laboratories, AbbVie, Biogen, Bristol Myers Squibb, Lilly, Merck, Pfizer, Regeneron, Amgen, Gilead Sciences, Johnson & Johnson/Janssen, Moderna Therapeutics, Vertex, West Pharmaceutical
Consulting or Advisory Role: Pliant
Research Funding: Ascentage Pharma (Inst), AstraZeneca (Inst), Bolt Biotherapeutics (Inst), Bristol Myers Squibb (Inst), Compugen (Inst), Constellation Pharmaceuticals (Inst), CytomX Therapeutics (Inst), Exelixis (Inst), Ikena Oncology (Inst), InhibRx (Inst), Jounce Therapeutics (Inst), KLUS Pharma (Inst), Loxo (Inst), Macrogenics (Inst), Merck (Inst), Pfizer (Inst), Regeneron (Inst), Symphogen (Inst), Syros Pharmaceuticals (Inst), Astellas Pharma (Inst), Celgene (Inst), Treadwell Therapeutics (Inst), eFFECTOR Therapeutics (Inst), Genmab (Inst), Arrys Therapeutics (Inst), PureTech (Inst), GlaxoSmithKline/Tesaro (Inst), Seagen (Inst), Shattuck Labs (Inst), Sapience Therapeutics (Inst), Epizyme (Inst), Odonate Therapeutics (Inst), Tempest Therapeutics (Inst), Mersana (Inst), NGM Biopharmaceuticals (Inst), Samumed (Inst), Onconova Therapeutics (Inst), Gilead Sciences (Inst), AbbVie (Inst), Agenus (Inst), Alkermes (Inst), Alpine Immune Sciences (Inst), Alexo Therapeutics (Inst), Johnson & Johnson/Janssen (Inst), Seven and Eight Biopharmaceuticals (Inst), Cullinan Oncology (Inst), Debiopharm Group (Inst), Palleon Pharmaceuticals (Inst), Helsinn Therapeutics (Inst), Kinnate Biopharma (Inst), KSQ Therapeutics (Inst), Repare Therapeutics (Inst), SK Life Sciences (Inst), Theratechnologies (Inst), Tizona Therapeutics, Inc (Inst)
Walter J. Urba
Consulting or Advisory Role: Bristol Myers Squibb (Inst), AstraZeneca/MedImmune, AstraZeneca/MedImmune (Inst)
Research Funding: Bristol Myers Squibb (Inst), MedImmune (Inst), Merck (Inst), Galectin Therapeutics (Inst), AstraZeneca (Inst)
Patents, Royalties, Other Intellectual Property: MedImmune (Inst), Galectin Therapeutics (Inst)
Abigail Gregory
Employment: Pfizer, Biohaven Pharmaceuticals (I)
Stock and Other Ownership Interests: Biohaven Pharmaceuticals (I)
Susan Halabi
This author is a member of the JCO Precision Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Employment: ASCO
Honoraria: Sanofi, AVEO, Bristol Myers Squibb, Janssen
Richard L. Schilsky
Leadership: Clarified Precision Medicine, Leap Therapeutics
Stock and Other Ownership Interests: EQRx, Leap Therapeutics
Honoraria: Toray Industries
Consulting or Advisory Role: Cellworks, Scandion Oncology, Bryologyx, Illumina, EQRx, Syapse, Zephyr AI, AADi, Flatiron Health
Research Funding: AstraZeneca (Inst), Bayer (Inst), Bristol Myers Squibb (Inst), Genentech/Roche (Inst), Lilly (Inst), Merck (Inst), Pfizer (Inst), Boehringer Ingelheim (Inst), Seagen (Inst), Taiho Oncology (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1138818/summary
No other potential conflicts of interest were reported.
REFERENCES
- 1.Hélias-Rodzewicz Z, Funck-Brentano E, Baudoux L, et al. : Variations of BRAF mutant allele percentage in melanomas. BMC Cancer 15:1-10, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ascierto PA, Kirkwood JM, Grob JJ, et al. : The role of BRAF V600 mutation in melanoma. J Transl Med 10:85, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakravarty D, Johnson A, Sklar J, et al. : Somatic genomic testing in patients with metastatic or advanced cancer: ASCO provisional clinical opinion. J Clin Oncol 40:1231-1258, 2022 [DOI] [PubMed] [Google Scholar]
- 4.Chapman PB, Hauschild A, Robert C, et al. : Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364:2507-2516, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lito P, Pratilas CA, Joseph EW, et al. : Relief of profound feedback inhibition of mitogenic signaling by RAF inhibitors attenuates their activity in BRAFV600E melanomas. Cancer Cell 22:668-682, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopetz S, Desai J, Chan E, et al. : Phase II pilot study of vemurafenib in patients with metastatic BRAF-mutated colorectal cancer. J Clin Oncol 33:4032-4038, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Subbiah V, Puzanov I, Blay JY, et al. : Pan-cancer efficacy of vemurafenib in BRAFV600-mutant non-melanoma cancers. Cancer Discov 10:657-663, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatzivassiliou G, Liu B, O’Brien C, et al. : ERK inhibition overcomes acquired resistance to MEK inhibitors. Mol Cancer Ther 11:1143-1154, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Robert C, Grob JJ, Stroyakovskiy D, et al. : Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med 381:626-636, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Dummer R, Flaherty KT, Robert C, et al. : COLUMBUS 5-year update: A randomized, open-label, phase III trial of encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF V600-mutant melanoma. J Clin Oncol 40:4178-4188, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larkin J, Ascierto PA, Dréno B, et al. : Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 371:1867-1876, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Mangat PK, Halabi S, Bruinooge SS, et al. : Rationale and design of the Targeted Agent and Profiling Utilization Registry Study. JCO Precis Oncol 2:1-14, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al Baghdadi T, Garrett-Mayer E, Halabi S, et al. : Sunitinib in patients with metastatic colorectal cancer (mCRC) with FLT-3 amplification: Results from the Targeted Agent and Profiling Utilization Registry (TAPUR) Study. Target Oncol 15:743-750, 2020 [DOI] [PubMed] [Google Scholar]
- 14.Ahn ER, Mangat PK, Garrett-Mayer E, et al. : Palbociclib in patients with non–small-cell lung cancer with CDKN2A alterations: Results from the Targeted Agent and Profiling Utilization Registry Study. JCO Precis Oncol 4:757-766, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Al Baghdadi T, Halabi S, Garrett-Mayer E, et al. : Palbociclib in patients with pancreatic and biliary cancer with CDKN2A alterations: Results from the Targeted Agent and Profiling Utilization Registry Study. JCO Precis Oncol 3:1-8, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Fisher JG, Tait D, Garrett-Mayer E, et al. : Cetuximab in patients with breast cancer, non-small cell lung cancer, and ovarian cancer without KRAS, NRAS, or BRAF mutations: Results from the Targeted Agent and Profiling Utilization Registry (TAPUR) Study. Target Oncol 15:733-741, 2020 [DOI] [PubMed] [Google Scholar]
- 17.Alva AS, Mangat PK, Garrett-Mayer E, et al. : Pembrolizumab in patients with metastatic breast cancer with high tumor mutational burden: Results from the Targeted Agent and Profiling Utilization Registry (TAPUR) Study. J Clin Oncol 39:2443-2451, 2021 [DOI] [PubMed] [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Klute KA, Rothe M, Garrett-Mayer E, et al. : Cobimetinib plus vemurafenib in patients with colorectal cancer with BRAF mutations: Results from the Targeted Agent and Profiling Utilization Registry (TAPUR) Study. JCO Precis Oncol 6:e2200191, 2022 [DOI] [PubMed] [Google Scholar]
- 20.FDA approves encorafenib in combination with cetuximab for metastatic colorectal cancer with a BRAF V600E mutation. FDA. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves- encorafenib-combination-cetuximab-metastatic-colorectal-cancer-braf-v600e-mutation
- 21.FDA granted approval to vemurafenib for Erdheim-Chester Disease. FDA. https://www.fda.gov/drugs/resources- information-approved-drugs/fda-granted-approval-vemurafenib-erdheim-chester- disease
- 22.Smith-Cohn M, Davidson C, Colman H, et al. : Challenges of targeting BRAF V600E mutations in adult primary brain tumor patients: A report of two cases. CNS Oncol 8:CNS48, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyman DM, Puzanov I, Subbiah V, et al. : Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med 373:726-736, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subbiah V, Lassen U, Gasal E, et al. : Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer—Authors' reply. Lancet Oncol 21:e516, 2020 [DOI] [PubMed] [Google Scholar]
- 25.Planchard D, Smit EF, Groen HJM, et al. : Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: An open-label, phase 2 trial. Lancet Oncol 18:1307-1316, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Subbiah V, Kreitman RJ, Wainberg ZA, et al. : Dabrafenib plus trametinib in BRAFV600E-mutated rare cancers: The phase 2 ROAR trial. Nat Med 2023:1-10, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salama AKS, Li S, Macrae ER, et al. : Dabrafenib and trametinib in patients with tumors with BRAFV600E mutations: Results of the NCI-MATCH trial subprotocol H. J Clin Oncol 38:3895-3904, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouffet E, Geoerger B, Moertel C, et al. : Efficacy and safety of trametinib monotherapy or in combination with dabrafenib in pediatric BRAF V600-mutant low-grade glioma. J Clin Oncol 41:664-674, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.FDA grants accelerated approval to dabrafenib in combination with trametinib for unresectable or metastatic solid tumors with BRAF V600E mutation. FDA. https://www.fda.gov/drugs/resources- information-approved-drugs/fda-grants-accelerated-approval-dabrafenib-combination- trametinib-unresectable-or-metastatic-solid
- 30.Yao Z, Torres NM, Tao A, et al. : BRAF mutants evade ERK-dependent feedback by different mechanisms that determine their sensitivity to pharmacologic inhibition. Cancer Cell 28:370-383, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao Z, Yaeger R, Rodrik-Outmezguine VS, et al. : Tumours with class 3 BRAF mutants are sensitive to the inhibition of activated RAS. Nature 548:234-238, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazieres J, Cropet C, Montané L, et al. : Vemurafenib in non-small-cell lung cancer patients with BRAFV600 and BRAFnonV600 mutations. Ann Oncol 31:289-294, 2020 [DOI] [PubMed] [Google Scholar]
- 33.Moiseyenko FV, Egorenkov VV, Kramchaninov MM, et al. : Lack of response to vemurafenib in melanoma carrying BRAF K601E mutation. Case Rep Oncol 12:339-343, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turshudzhyan A, Vredenburgh J: A rare p.T599dup BRAF mutant NSCLC in a non-smoker. Curr Oncol 28:196-202, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller KE, Schieffer KM, Grischow O, et al. : Clinical response to dabrafenib plus trametinib in a pediatric ganglioglioma with BRAF p.T599dup mutation. Cold Spring Harb Mol Case Stud 7:a006023, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marchand A, Tallet A, Collin C, et al. : A rare BRAF T599dup mutation conferring sensitivity to BRAF inhibitor in a patient with metastatic melanoma. Br J Dermatol 179:528-529, 2018 [DOI] [PubMed] [Google Scholar]