Abstract
Objective:
The aim of this study was to systematically review the literature for each surgical step of the minimally invasive right hemicolectomy (MIRH) for non-locally advanced colon cancer, to define the most optimal procedure with the highest level of evidence.
Background:
High variability exists in the way MIRH is performed between surgeons and hospitals, which could affect patients’ postoperative and oncological outcomes.
Methods:
A systematic search using PubMed was performed to first identify systematic reviews and meta-analyses, and if there were none then landmark papers and consensus statements were systematically searched for each key step of MIRH. Systematic reviews were assessed using the AMSTAR-2 tool, and selection was based on highest quality followed by year of publication.
Results:
Low (less than 12 mmHg) intra-abdominal pressure (IAP) gives higher mean quality of recovery compared to standard IAP. Complete mesocolic excision (CME) is associated with lowest recurrence and highest 5-year overall survival rates, without worsening short-term outcomes. Routine D3 versus D2 lymphadenectomy showed higher LN yield, but more vascular injuries, and no difference in overall and disease-free survival. Intracorporeal anastomosis is associated with better intra- and postoperative outcomes. The Pfannenstiel incision gives the lowest chance of incisional hernias compared to all other extraction sites.
Conclusion:
According to the best available evidence, the most optimal MIRH for colon cancer without clinically involved D3 nodes entails at least low IAP, CME with D2 lymphadenectomy, an intracorporeal anastomosis and specimen extraction through a Pfannenstiel incision.
Keywords: colon cancer, laparoscopic, minimally invasive, right hemicolectomy, right-sided colon cancer, robot-assisted, surgical steps
This systematic review provides a comprehensive overview of the best evidence of all surgical steps of the minimally invasive right hemicolectomy (MIRH) for colon cancer. The ideal MIRH includes at least low intra-abdominal pressure, complete mesocolic excision with D2 lymphadenectomy, an intracorporeal anastomosis and specimen extraction through a Pfannenstiel incision.
INTRODUCTION
Colon cancer (CC) significantly contributes to cancer-related mortality worldwide, with a 5-year overall survival (OS) of about 65%.1 CC has long been considered a single disease entity besides rectal cancer, but existing heterogeneity with implications for treatment and prognosis is increasingly being acknowledged. Based on its anatomical location, CC is categorized as right-sided or left-sided with the splenic flexure as demarcation point.
The corner stone of CC treatment in a curative setting is surgery, except for the early cancers that can be treated by polypectomy alone. Curative surgery is based on general oncological principles, comprising radical resection of the tumor with a complete and intact colonic mesentery containing all tumor-draining lymph nodes (LN), thereby aiming to achieve the best long-term disease-free survival (DFS). Furthermore, the aim is to preserve abdominal wall integrity, restore bowel continuity, and minimize associated morbidity. The introduction of minimally invasive (laparoscopic) surgery has significantly improved short-term clinical outcomes and provides similar long-term outcomes.2–4 Among colorectal surgeons, the minimally invasive right hemicolectomy (MIRH) is considered to be one of the most commonly performed, straightforward colorectal procedures.5 Nevertheless, the procedure is still associated with substantial short-term morbidity and mortality.6
Current clinical practice for MIRH shows high variability in different steps and anatomical surgical dissection planes, based on previous surgical training and local habits. Although variance in surgery is inevitable, recent research suggests that quality of surgery substantially influences clinical outcomes for colorectal cancer.7–9 The OS of right-sided CC (RCC) is inferior to that of left-sided CC but the mechanisms responsible for this difference have yet to be elucidated.10–13 There are differences in biological characteristics, with increased MSI-high and BRAF-mutant cancers in RCC, but this does probably not entirely explain the observed OS difference.14 Population-based data show that especially LN positive RCC is associated with worse OS, with a potential role for optimized mesocolic excision.12
Continuous surgical research on various procedural steps is providing new evidence that has the potential to improve clinical outcomes. However, these improvements are only variably adapted in clinical practice.15–17
Attempts have been made to review and standardize elements of the MIRH to improve the proficiency in surgical training programs.18–20 In the last decades, many innovations have been shown to improve outcomes for patients who underwent MIRH. Not only the complete mesocolic excision (CME) for RCC has been introduced, but also other elements such as low-pressure pneumoperitoneum, new techniques of performing the anastomosis, and the location of the extraction site.21–24 Consequently, no up-to-date comprehensive report on the preferred methods for all steps of a MIRH has been published in recent years. Therefore, the aim of the present systematic review was to comprehensively evaluate all recent literature for every surgical step of MIRH to determine the optimal evidence-based procedure.
METHODS
Surgical key steps of MIRH were defined based on previous literature that describes the different steps of the procedure, literature addressing MIRH-related research topics, and discussion amongst involved surgeons (B.R.T., P.J.T., and J.B.T.). A review of the literature regarding each surgical key step was performed, primarily based on identification of systematic reviews. If not available for certain steps, a narrative approach was used with description of relevant guidelines, consensus statements, and individual landmark studies.
Definitions of Oncological Terms
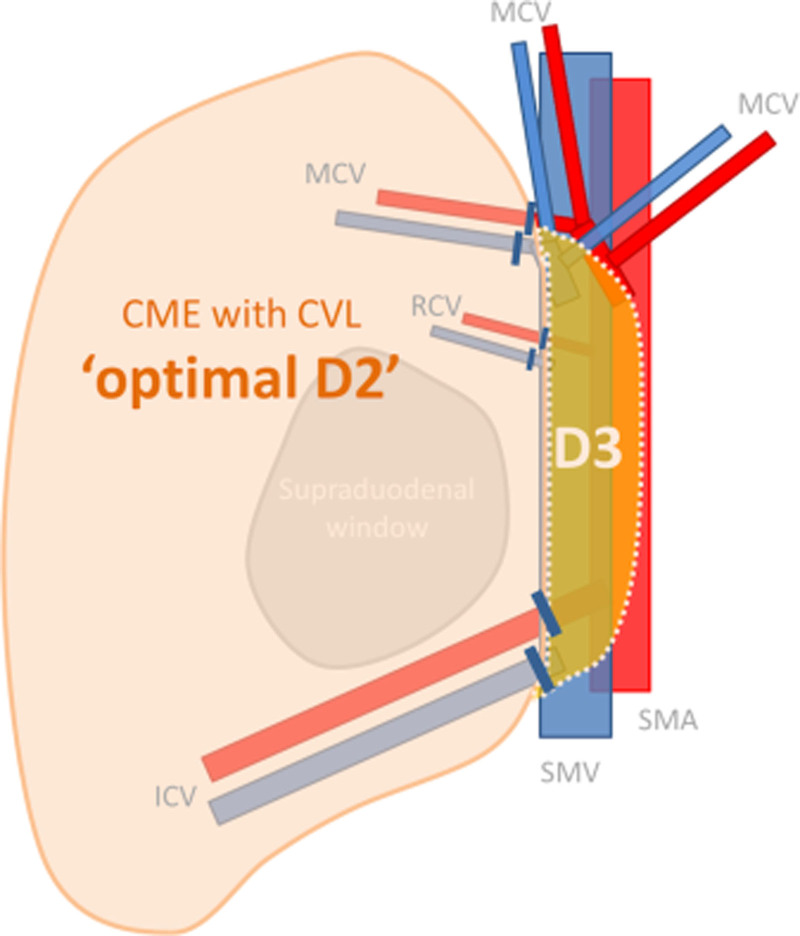
Because of still existing controversy on the terms used in literature to describe the extent of the resection in MIRH, we defined these terms for clarity. D2 lymphadenectomy is defined as resection of the mesentery up to the right lateral border of the superior mesenteric vein (SMV), while D3 lymphadenectomy comprises an additional resection of the mesentery up to the left lateral border of the superior mesenteric artery (SMA). CME is defined as a dissection along the mesofascial interface and thereby preserving the integrity of the mesentery. The CME principle per definition includes central vascular ligation (CVL), to achieve an undamaged specimen with an intact duodenal window and the surgical trunk on its medial side, which constitutes an optimal D2 lymphadenectomy25 (Figure 1).
FIGURE 1.
The schematic overview showing the difference of complete mesocolic excision (CME), central vascular ligation (CVL), ‘optimal D2’, and D3 dissection.
Search Strategy
For each surgical key step, specific outcomes were defined before setting up the literature search (Supplementary Table 1, http://links.lww.com/AOSO/A260). PubMed was separately searched by 2 investigators (A.G. and J.S.) regarding all (sub)steps. Search strategies were formulated with the support of a medical information specialist on each subject. The searches were specified for MIRH but were broadened to colorectal or general laparoscopic surgery if deemed applicable. No language restrictions or limits on publication date were applied. References of included studies were checked for other eligible studies. Details of the systematic searches are provided in the supplementary file.
Study Selection
The same 2 investigators independently performed the study selection by title and abstract screening. In case of disagreement, a third investigator was consulted. Potentially eligible articles were assessed in full text to identify systematic reviews that were in accordance with the guidance from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist.26 Systematic reviews prevailed over other types of evidence. If appropriate papers meeting the selection criteria were published after selected systematic review, these were also incorporated. If more than one systematic review was available regarding a certain subject with overlapping outcomes, the AMSTAR-2 tool was used to identify highest quality reviews.27 If several systematic reviews were of the same quality, the most recent one was included. When no systematic reviews on the specific topic were published, consensus/guideline papers and landmark studies with highest level of evidence were selected.
Quality Assessment
The Level of Evidence scale by the Oxford Centre for Evidence-Based Medicine was used to rate the evidence of all outcomes.28 The AMSTAR-2 checklist (Assessing the Methodological Quality of Systematic Reviews) was used to assess the quality of the identified systematic reviews. The AMSTAR-2 checklist is a critical assessment tool that allows detailed assessment of systematic reviews that include randomized and/or non-randomized studies of clinical interventions.27
RESULTS
Literature Search
Supplementary Table 2, http://links.lww.com/AOSO/A261 provides an overview of the search result, with number of systematic reviews, the number and reason for unselected systematic reviews, the number of selected systematic reviews, the number of articles selected in addition to or in the absence of systematic reviews within the search and the total number of selected papers for best evidence per surgical key step (including other relevant articles found by cross-linking and by other searches). For comprehensive details of the systematic searches on PubMed of the various sections, see Supplementary File 1, http://links.lww.com/AOSO/A259.
Quality Assessment and Level of Evidence
The quality assessment of the identified systematic reviews is shown in Supplementary Table 3, http://links.lww.com/AOSO/A262. In 2 systematic reviews, the score was high, in 5 low and 9 were scored with a critically low score. The available level of evidence of the included studies for each topic ranged from 1A to 5 (Supplementary Table 4, http://links.lww.com/AOSO/A263).
Best Evidence for Each Surgical Step of Minimally Invasive Right Hemicolectomy
Preoperative Assessment of Vascular Anatomy
The vascular anatomy of the right-sided colon is complex and many variations exist.29 To facilitate planning of MIRH and anticipating anatomical variance, preoperative assessment of the central vascular anatomy may be of additional value. A review by Sun et al (2020) describes intraoperative incidence rates of the middle colic artery (MCA) at 100%, the ileocolic artery at 96.7% to 100% and the right colic artery of 12.2% to 55.0%. One consistent finding was the anatomy of the MCA, which always crosses the SMV in an anterior fashion.30 The right colic artery crosses the SMV anteriorly in 83.6%, but the ileocolic artery crosses the SMV frequently in both an anterior (52.5%) as well as posterior fashion (47.5%). The ileocolic vein drains into the SMV in the majority of cases and sporadically into the gastrocolic trunk of Henle (1.9%).29 The middle colic vein drains into the SMV in most patients, whereas the right colic vein usually drains into the gastrocolic trunk of Henle, which afterward converges into the SMV. Preoperative assessment to assess this variance of vascular anatomy is recommended by experts although no prospective clinical trials have evaluated the assessment on clinical outcomes.31 This is level 4 evidence for preoperative assessment of vascular anatomy (Supplementary Table 4, http://links.lww.com/AOSO/A263).
Patient Positioning
There are 3 commonly used options for patient positioning in abdominal surgery: French (supine, split-leg), lithotomy (supine, split-leg, flexed hips and knees), and American/supine. Definitions of these positions vary in the literature. For example, French and lithotomy positions are often combined. Existing heterogeneity makes it difficult to interpret papers. No comparative studies have been published on positioning for MIRH. In the Delphi procedure of the procedural key steps for laparoscopic right hemicolectomy by Dijkstra et al (2015), consensus about the positioning of the patient was reached: patients should be placed on a vacuum mattress, with leg holders and the left arm alongside their body.18 Kramp et al (2013) performed an ergonomic assessment of surgeons for lithotomy versus American/supine positioning in laparoscopic cholecystectomy.32 No statistical difference was observed in body posture of the neck and trunk and the orientation of the head. The lithotomy position can be associated with lower extremity neuropathy.33 The French position (supine, split-leg) allows for more standing positions for the operating team.34 According to the consensus article by Dijkstra et al, patients undergoing MIRH should be placed in right lateral tilt and in (anti)Trendelenburg position depending on the phase of the procedure.18 2a is the highest level of evidence for patient positioning (Supplementary Table 4, http://links.lww.com/AOSO/A263).
Number and Size of Trocars
The systematic review by Ahmad et al (2019) did not show a difference in rates of vascular injury between an open or Veress needle introduction.35 The Delphi procedure by Dijkstra et al (2015) concluded that a 12 mm trocar should be introduced paraumbilical using a Veress needle or an open technique.18
Regarding number of trocars, short-term outcomes of a 3-port procedure versus a 5-port procedure were similar in the retrospective study by Shi et al (2020).36 After propensity score matching, the complication rate was 25.6% versus 17.9% (P = 0.584), but the hospital stay did not differ (11.02 ± 4.69 vs 10.36 ± 2.69 days, P = 0.443). The OS and 5-year DFS of 3-port versus 5-port procedures were also similar in a retrospective study by Zhang et al (2021).37 Internationally, 4 laparoscopic ports were most commonly used by surgeons compared to 3 or 5.17 No difference in the number of trocars was seen with regard to the amount of performed cases and years of experience. Chiung Ta Lu et al (2021) compared the total oral morphine equivalent daily dose required for different trocar amounts and sizes in laparoscopic cholecystectomy (group A: 12/5/5 trocars [total number of trocars 3], group B: 12/5/5/5 trocars [4 trocars], groups C: 12/10/5/5 [4 trocars]).38 Compared to group A, the total oral morphine equivalent daily dose was 21 mg higher in group B and 30.7 mg in group C (P < 0.001). The highest level of evidence for number of trocars is 2b (Supplementary Table 4, http://links.lww.com/AOSO/A263).
The systematic review by Swank et al (2012) observed a higher rate of trocar site hernia (TSH) for 12 mm than for 10 mm trocars (odds ratio [OR] 13, 1.5–110; P < 0.01).39 No difference was seen between 5 mm and 10 mm trocars. The prospective cohort study by Wang et al (2017) concluded that the size of the port sites is the main determinant of port-specific pain in gynecological laparoscopy.40 The highest level of evidence for size of trocars is 2a (Supplementary Table 4, http://links.lww.com/AOSO/A263).
Pneumoperitoneum
Warm Humidified CO2
Intraoperative hypothermia may be associated with postoperative adverse events.41,42 To reduce hypothermia, warm humidified CO2 during laparoscopy was introduced in 1996.43 Although a meta-analysis by Dean et al (2017) concluded that the use of warmed humidified CO2 is associated with an increased intraoperative core temperature,42 the systematic review of Birch et al (2016) showed no clinical effect of this warm humidified CO2 on patient outcomes such as postoperative pain scores and major adverse events.44 The highest level of evidence for this subject is 1a (Supplementary Table 4, http://links.lww.com/AOSO/A263).
Intra-Abdominal Pressure
The systematic review by Gurusamy et al (2014) compared low (<12 mmHg) and standard (12–14 mmHg) intra-abdominal pressure (IAP) in laparoscopic cholecystectomy.45 There was no difference between the 2 groups concerning serious adverse events (0.6% vs 0%, RR 3.00, 95% CI 0.14–65.90) and operating time (MD 1.51 minutes, 95% CI 0.07–2.94). Raval et al (2020) evaluated the effect of low (<12 mmHg), standard (12–14 mmHg), and high (≥15 mmHg) IAP in a systematic review and meta-analysis.46 Significant lower pain scores at 24h postoperative (MD: −0.70; 95% CI −1.26 to −0.13) and less shoulder pain (OR 0.24; 95% CI 0.12–0.48) were seen in the low IAP group compared to standard IAP. Low IAP was not compared with high IAP in the included studies. No significant difference was seen between the 3 IAP groups with respect to conversion rate. A high heterogeneity was described in the included studies. In the RECOVER trial by Albers et al (2022), the mean quality of recovery (QoR-40) was higher for patients operated with low IAP (8 mmHg) compared to standard IAP of 12 mmHg (MD 8.3, 95% CI 2.5–14.1, P = 0.005).22 The highest level of evidence for this subject is 1a (Supplementary Table 4, http://links.lww.com/AOSO/A263).
Surgical Approach
Several surgical dissection approaches can be applied in MIRH. The most commonly used approaches are medial-to-lateral (MtL), lateral-to-medial (LtM), caudal-to-cranial, and cranial-to-caudal (CrtCa). A meta-analysis of Li et al (2017) investigated 3 of them (MtL, LtM, and CrtCa) and concluded that all those methods are safe and acceptable for MIRH.15 Length of hospital stay (weighted median difference [WMD] = 0.29, 95% CI 0.08–0.50, P < 0.05) and postoperative flatus recovery time (WMD = 1.4, 95% CI 0.13–2.67, P < 0.05) of the LtM approach were shorter compared with MtL approach. The CrtCa approach might have slightly less overall postoperative complications compared with MtL approach (OR 3.37, 95% CI 1.06–10.70, P < 0.05) according to this paper. No reports on important long-term oncological outcome for patients undergoing MIRH, such as DFS and local recurrence, were made when comparing those different surgical approaches. The meta-analysis of Li et al analyzed only the number of harvested LN between those 3 approaches, and no differences were found. The highest level of evidence for this subject is 2a (Supplementary Table 4, http://links.lww.com/AOSO/A263).
Complete Mesocolic Excision
Systematic reviews on CME versus conventional resections are hampered by the heterogeneity of the definitions of experimental and control interventions amongst the individual studies. In 2021, the meta-analysis of Anania et al compared CME with the traditional approach, and found a higher LN yield (MD 0.75, 95% CI 4.06–10.04), and an increased 3-year OS (RR 0.42, 95% CI 0.27–0.66) and 5-year DFS rate (RR 0.36, 95% CI 0.17–0.56).47 In only one of the included studies, local recurrence and systemic recurrence rates were compared between the 2 groups. Both were lower in the CME group, namely 2.97% versus 7.84% local recurrence and 5.94% versus 15.6% systemic recurrence. In terms of safety, this meta-analysis showed that CME is not inferior in blood loss (MD −32.48, 95 CI −98.54 to −33.58), anastomotic leakage (RR 0.82, 95% CI 0.38–1.79), overall postoperative complications (RR 1.36, 95% CI 0.82–2.28) and reoperation rate (RR 0.65, 95% CI 0.26–1.75). However, comparing the intraoperative data, the traditional group was associated with lower conversion rate (RR 1.72, 95% CI 1.00–2.96) and shorter operating time (MD 16.43, 95% CI 4.27–28.60). The highest level of evidence for this subject is 2a (Supplementary Table 4, http://links.lww.com/AOSO/A263).
D2 or D3 Lymphadenectomy
There were no systematic reviews selectively comparing D2 and D3 lymphadenectomy, with the same approach and quality of the retroperitoneal dissection and both using CVL. One large Chinese randomized controlled trial (RCT) was designed to answer this research question. Survival data are not available yet, but short-term outcomes of 995 patients have been published.48 No difference in postoperative surgical complications (20% in D3 group vs 22% in D2 group, 95% CI −7.2 to 2.8), no difference in death (0% vs 0%), no statistical difference in chyle leak (5% in D3 group vs 3% in D2 group, 95% CI −4.4 to 0.3) and a higher number of median harvested LNs in favor of the D3 group (26 [19–35] vs 23 [17.5–29]) were found. However, vascular injury was significantly more common in the D3 group (3% in D3 group vs 1% in D2 group, 95% CI 0.04–3.6) and the median duration of the operation was significantly higher in the D3 group (163 [135–195] min in D3 group vs 150.5 [125–180] min in D2 group). Based on a comparative cohort study, Sammour et al (2020) concluded that CME with D2 dissection should be the minimal standard, and that CME with D3 dissection could be considered in patients with suspicious central (D3) LN.49 The results showed that the D3 group had a significantly higher median LN retrieval compared to the D2 group (31 vs 27, P = 0.011), but there was no difference in OS (P = 0.538) and DFS (P = 0.780) after a median follow up of 25.2 months. The highest level of evidence for this subject is 1b (Supplementary Table 4, http://links.lww.com/AOSO/A263).
Vessel Ligation
The 2 main options available for vessel ligation during MIRH are energy devices (electrothermal bipolar or ultrasonic) and clips. One RCT by Adamina et al compared clips with energy devices in colorectal procedures.50 Their results showed a significant time reduction (mean 6.9 [0.5–13.2] min, P = 0.031) and significantly reduced costs of US $80.7 per patient (P = 0.043) when energy devices were used for vascular control. In 2007, a comparative study between electrothermal bipolar vessel sealing (EBVS) and ultrasonic coagulating shears (UCS) in laparoscopic colectomies was performed. A statistically significant difference was found in mean blood loss in favor of the EBVS group (115 [30–160] ml vs 370 [150–680] mL, P < 0.001), but no significant differences in mean postoperative hospital stay (5.2 [4–6] days in EBVS group vs 6.1 [5–7] days in UCS group) and mean operative time (111 [70–195] min in EBVS group vs 133 [95–190] min in UCS group) were found.51
A prospective randomized study by Marcello et al (2006) comparing EBVS with disposable clip appliers showed a higher cost-effectiveness of the EBVS during right, left, and total laparoscopic colectomy.52 The mean cost per case for vessel ligation was significantly lower in the EBVS group ($317 ± 0$ vs $400 ± $112, P < 0.001), with a decreased failure rate in the LIG group (3.0% vs 9.2%, P = 0.02), but no significant difference in median blood loss (50 [20–50] ml in S/C group vs 100 [25–800] ml in LIG group, P = 0.054). The highest level of evidence for this subject is 1b (Supplementary Table 4, http://links.lww.com/AOSO/A263).
Anastomosis
The ileocolonic anastomosis can be created inside or outside the abdomen (intracorporeal [ICA] vs extracorporeal [ECA]) and in an iso- or antiperistaltic fashion. An ECA may be handsewn or stapled, whereas an ICA is almost exclusively stapled. The remaining enterotomy after intracorporeal stapling may be closed using a single- or double-layer (DL) technique.
Intracorporeal vs Extracorporeal
Aiolfi et al (2020) published a meta-analysis including 3755 patients, of which 1720 underwent ICA and 2035 underwent ECA. They concluded that ICA during MIRH is a safe, reliable and convenient procedure that offers benefits in terms of less postoperative infectious complications (RR 0.51, 95% CI: 0.31–0.84, P = 0.009), and less overall complications (RR 0.78, 95% CI: 0.62–0.97, P = 0.028). An ICA was superior in terms of time to first flatus (MD −16.68, P < 0.001), first defecation (MD −25.94, P < 0.001), first oral diet (MD −16.35, P < 0.001) and length of hospital stay (MD −0.72, P < 0.001).53 The highest level of evidence for this subject is 1a (Supplementary Table 4, http://links.lww.com/AOSO/A263).
Isoperistaltic Versus Antiperistaltic
In 2019, an RCT was published comparing iso- and antiperistaltic anastomosis during laparoscopic right hemicolectomy.54 The authors concluded that both techniques present similar results. In the long-term, there seemed to be a shorter intestinal transit time in the antiperistaltic group and a statistically non-significant tendency to a higher chronic diarrhea rate, which did not result in a decreased reported quality of life. The results showed no significant differences in surgical time (P = 0.481), anastomotic time (P = 0.207), postoperative complications (P = 0.693), postoperative ileus (P = 0.112), anastomotic leakage (P = 1.00), hospital stay (P = 0.236) or in chronic diarrhea rates measured with the GIQLI score (P = 0.541). Differences were found in time (days) to first flatus and time to first defecation in favor of the antiperistaltic group (P = 0.004 and P = 0.017). The highest level of evidence for this subject is 1b (Supplementary Table 4, http://links.lww.com/AOSO/A263).
Single-Layer Versus Double-Layer Enterotomy Closure
No systematic reviews or randomized trials on this topic for MIRH were identified. Reggio et al (2015) compared a DL closure technique with the single-layer (SL) technique in a single-center comparative cohort study, when performing an intracorporeal ileocolic anastomosis.55 In both techniques, an absorbable running suture was placed. They reported that DL was associated with a significantly lower incidence of anastomotic leakage rate (1.2% in DL vs 7.8% in SL, P = −0.044) and a significantly shorter median hospital stay (8 [range: 4–34] days in SL and 6 [range: 4–26] days in DL, P = 0.011). Moreover, the overall median operative time (118 [range: 75–226] min in SL and 128 [range: 98–228] min in DL) and the median time to perform the anastomosis (17 [range: 12–26] min in SL and 20 [range: 14–33] min in DL) was similar between the 2 techniques. Another multicenter case-controlled study found a significant reduction in terms of ALs in the DL group (4.5% in DL vs 7.1% in SL, P = 0.02) and there was no stenosis in either the SL or DL group. In this study, both techniques were performed using running sutures.56 The highest level of evidence for this subject is 2b (Supplementary Table 4, http://links.lww.com/AOSO/A263).
Longitudinal Resection Margin
The impact of the longitudinal resection margin (LRM) on oncological outcomes was retrospectively evaluated by Lee et al (2017).57 An overall median LRM length of 5.0 cm (range: 0.5–26.0) was reported, in accordance to several guidelines. When comparing 3 groups with different LRM (<3, ≥3 <5, and ≥5 cm), the LRM did not influence the 3-year DFS (89.2%, 89.0%, and 87.0%; P = 0.629). There was also no significant difference in 5-year OS between the 3 groups (89.0%, 92.1%, and 91.8%, P = 0.679), even though patients with a longer LRM had larger tumor sizes and more advanced T stages. The number of LN retrieved increased with a higher LRM. The highest level of evidence for this subject is 2b (Supplementary Table 4, http://links.lww.com/AOSO/A263).
Indocyanine Green for Vascular Assessment
For this topic, no systematic review was found specifically for MIRH. Systematic reviews on colorectal procedures were excluded due to the heterogeneity. Morales-Conde et al (2020) performed a prospective study and found that the use of ICG to assess colonic perfusion in laparoscopic colorectal procedures led to an increased number of changes in the chosen resection margin.58 However, in the laparoscopic right hemicolectomy group alone, the use of ICG resulted in a changed resection margin in only 6% (4/67) of the cases when compared to laparoscopic left hemicolectomy (21/81, 25.9%, P = 0.0016) and laparoscopic anterior resection of the rectum (9/35, 25.7%, P = 0.0095). Moreover, in another study of the use of ICG used in MIRH, none of the 40 evaluated cases underwent a change in the resection line after assessing the perfusion with ICG.59 Mangano et al showed that ICG assessment of colonic perfusion in robotic right hemicolectomy led to a change of the resection line in 1 of 3 cases, but there was no control group and it is unclear if this enhanced clinical outcomes.60 The highest level of evidence for this subject is 2b (Supplementary Table 4 http://links.lww.com/AOSO/A263).
Extraction Site
Different sites of the abdominal wall can be used for specimen extraction. A meta-analysis by Lee et al (2017) concluded that midline incisions for specimen extraction in laparoscopic colorectal surgery are at significantly higher risk of incisional hernia (IH) when compared to the transverse and Pfannenstiel incisions.24 The pooled incidence of IH was 0.9% in Pfannenstiel, 3.7% in transverse, and 10.6% in midline incisions. Midline incisions were associated with a significantly higher risk of IH when compared with all off-midline incisions (OR 4.1, 95% CI 2.0–8.3). Midline incisions remained at a higher incidence of IH than both transverse (OR 3.0, 95% CI 1.4–6.7) and Pfannenstiel (OR 8.6, 95% CI 3.0–24.6) incisions when the off-midline comparison group was subcategorized into a transverse or Pfannenstiel incision. In 2021, Greemland et al published a retrospective study specifically on laparoscopic right hemicolectomy patients that confirmed the findings of the meta-analysis.61 In their study, midline extractions, compared with off-midline extractions resulted in a significantly higher risk of IH (58.3% vs 18.8%, P < 0.0001). Off-midline incisions included incisions in the para-midline, transverse left abdomen, transverse right abdomen, and transverse mid-abdomen. The highest level of evidence for this subject is 2a (Supplementary Table 4, http://links.lww.com/AOSO/A263).
Wound Management
An important risk factor for TSH after laparoscopic surgery is the size of the trocar. A systematic review by Swank et al (2012) observed a slightly higher prevalence of TSH if at the end of the procedure, the fascia of the 12 mm port sites are left open (OR 3.9, range 0.6–24).39 The closure of the extraction site and port sites of 10 mm or greater is labeled as a key step in laparoscopic right hemicolectomy by Dijkstra et al (2015).18 The highest level of evidence for this subject is 2a (Supplementary Table 4, http://links.lww.com/AOSO/A263).
Several retrospective cohort studies suggest that the use of a wound protector is associated with a reduced incidence of a surgical site infection (SSI).62–64 No systematic reviews on this topic were identified. In a retrospective case-cohort study by Capolupo et al (2019), the wound protector was inserted immediately after opening the peritoneum, maintained during the extraction of the surgical specimen, and removed after ICA was completed and final closure started.64 Overall SSI rate was 10.12% in the group with wound protector and 19% without. The Delphi consensus article by Dijkstra et al (2015) also advises the use of a wound protector.18 Lauricella et al (2021) assessed the cost-effectiveness of an O-ring wound protector in elective laparoscopic colorectal surgery in a retrospective cohort study.65 They saw an SSI rate of 5.7% with wound protectors and 15.6% without. The total costs of both groups were similar. There is no clear evidence regarding the influence of wound protectors on the risk of abdominal wall metastases by tumor implants. The highest level of evidence for this subject is 2b (Supplementary Table 4, http://links.lww.com/AOSO/A263).
The systematic review of Norman et al (2020) concluded that the prophylactic use of negative pressure wound therapy probably reduces the rate of SSI compared to standard wound dressing in primary closed wounds overall.66 There was no clear evidence of wound dehiscence and the influence on secondary outcomes was uncertain. In a prospective cohort study by Abadía et al (2021) in 200 elective colorectal surgery patients, the use of negative pressure wound therapy was associated with a reduced risk of SSIs compared to closed dressing (incidence 9% vs 19%, OR: 0.3; 95% CI 0.11–0.83, P = 0.02).67 The highest level of evidence for this subject is 1a (Supplementary Table 4, http://links.lww.com/AOSO/A263).
No clear evidence on the use of wound irrigation to reduce the risk of an SSI or wound dehiscence is found in the systematic review of Norman et al (2017).68 The highest level of evidence for this subject is 1a (Supplementary Table 4, http://links.lww.com/AOSO/A263).
DISCUSSION
This systematic review provides a comprehensive overview of the best evidence for every surgical step in MIRH. According to the highest level of evidence (level 1a/b), the MIRH should be performed with low IAP (less than 12 mmHg) pneumoperitoneum and an ICA. The MIRH should ideally include (level evidence 2a/b) CME with CVL and D2 lymphadenectomy, closure of the fascia of 12 mm ports, DL technique when closing the enterotomy during creation of the anastomosis and extraction of the specimen by a Pfannenstiel incision with the use of a wound protector. In addition, it could include (expert opinion) preoperative vascular anatomy assessment and French position with trocar positioning that seems to facilitate CME with dissection along the SMV. No supporting evidence was found for the routine use of a D3 lymphadenectomy, the standard use of ICG in creation of the anastomosis, the best surgical approach for the retroperitoneal dissection, the use of warm humidified CO2, a minimum LRM, the superiority of an anti- or isoperistaltic anastomosis or wound irrigation in MIRH. This review could be the basis for a most optimal and standardized MIRH. By adapting all the elements instead of a few, the most significant contribution towards better clinical outcomes can be achieved.
Implementation of a procedure based on the best evidence of all separate surgical steps of an MIRH has never been investigated. One may hypothesize that several interactions with overlap in measured effects exists on one hand, but it may also be true that the whole is more than the sum of its parts. From an oncological point of view, the discussion on CME and extent of vertical lymphadenectomy seems to be the most important topic, but postoperative complications that are influenced by several other steps of MIRH are also associated with survival.69 This illustrates that we should probably strive for an integrated approach to optimize surgery for RCC. The present systematic review focused on the surgical procedure, but one should realize that differences in perioperative care can also affect (short-term) outcomes.70
With regards to the CME and D3 lymphadenectomy, it is important to emphasize that throughout literature, terms are often used interchangeably and are sometimes incorrectly referred to as synonyms.71 Similar to total mesorectal excision, applying the CME principle should be separated from the discussion about the additive value of D3 lymphadenectomy. One article included “D3 Lymphadenectomy” in the search.72 In the selected systematic review by Xu et al (2021), the short-term outcomes of the RELARC study were included, and D3 dissection was considered an integral part of CME in that study.48 Although there is still some remaining controversy about the details of this procedure and exact definition, following, the CME principle results in optimized long-term oncological outcomes and has been demonstrated to be safe regarding postoperative complications, as nicely shown in the study by Bertelsen et al.73 Given the increased morbidity for D3 lymphadenectomy, and even mortality in some reports as well as the still unproven additive oncological benefit compared to CME D2, this should only be applied in highly selected cases with clinically involved D3 nodes, and not on a routine basis.48,49,74–77 One of those studies reported that D3 nodes of 8 mm or larger or with internal heterogeneity or irregular margins are visible on preoperative imaging in less than 2%.49
A significant amount of literature on MIRH is about the debate regarding ICA versus ECA, with an increasing body of evidence in favor of ICA. The known advantages are the non-necessity to lift enterotomies outside the abdomen, less application of traction on the future anastomosis, and an optimal vision during the creation of the anastomosis in its future natural position.23,53,78–88 Additionally, ICA allows for specimen extraction through a Pfannenstiel incision, thereby minimizing the risk of incisional hernias.24,61
The optimal level of IAP is still under debate, although there is evidence that shows less postoperative pain for low IAP without negative effects on the conversion or adverse event rates. In addition, the observation that low pressure is associated with less infectious complications is interesting as found in the recent RECOVER trial.22 Nevertheless surgical safety is a primary objective and the concern of low IAP is that the vision of the surgical field might be impaired. Optimizing the surgical space conditions could then be facilitated with a deep neuromuscular block.89 But otherwise, the pressure might be increased if necessary for proper exposure.
With the introduction of CME and CVL, the SMV has become an important landmark in MIRH for RCC, with implications for patient and trocar positioning, despite the absence of high level of evidence. Regarding patient positioning, lithotomy should be avoided due to the risk of lower extremity neuropathies, while French (supine split-leg) position provides the surgeon performing conventional laparoscopy the option to stand between the legs, which is helpful during central dissection of the SMV. Another non-evidence-based topic related to CME is the importance of having knowledge of the anatomy of the SMV and SMA and its segmental branches, and preoperative imaging assessment is therefore advised.30
Innovations in surgery have only been marginally adopted in community hospitals.90 The strength of standardization and subsequent implementation of all best evidence steps of an operation in surgical education and surgical practice will potentially have a much bigger effect on outcomes than implementation of one of the steps of an operation. In surgical training, standardization of a procedure allows a safer and quicker learning curve. In general practice, it allows proper auditing and interventions, and it enables accurate comparison in research. Trying to define an optimal standardized MIRH based on the highest level of evidence for each surgical step using a systematic approach likely contributes to this goal. It is just not yet clear whether combining multiple evidence-based recommendations and thus an entire standardized technique will lead to much better clinical outcomes. This needs to be further explored in the future with proper scientific clinical research.
To implement all innovations within MIRH, it is important to keep the influence of the learning curve on patient outcomes as low as possible. For example, in the included meta-analysis comparing CME to the traditional approach, some intraoperative complications did occur more often in the CME group during their learning curve.47 Surgical learning curves should be considered, due to their association with a negative influence on patient outcomes.91 Training before implementation and the help of proctoring during the first period of implementing new techniques seem both essential to reduce the influence of the learning curve on patient outcomes.92 Within this process, surgical quality assessment (SQA) is an important process to facilitate learning. For example, many surgeons are often convinced that they are performing a D2 lymphadenectomy, but in reality, it is a so-called D1.5 or D1+ lymphadenectomy. Video-based SQA tools can be used by surgeons to anonymously judge whether an optimal D2 lymphadenectomy with CVL has been performed. The same applies to several other aspects of MIRH, such as the quality of CME dissection and ICA. In future studies, video-based SQA might become a crucial component to avoid bias related to heterogeneity in surgical quality.
CONCLUSIONS
This systematic review aimed to give a comprehensive overview of the best evidence regarding all surgical steps of the MIRH. Based on best available evidence, the CME principle including CVL resulting in an optimal D2 lymphadenectomy should be followed using low IAP pneumoperitoneum, after which an ICA is made, with preferably DL closure of the enterotomy, closure of the fascia of the ports ≥12 mm, and extraction of the specimen by a Pfannenstiel incision covered with a wound protector. D3 lymphadenectomy should be reserved for rare cases with clearly suspicious nodes in that area. This stepwise procedure can potentially improve clinical and oncological outcomes of patients with RCC if implemented on a large scale.
Supplementary Material
Footnotes
Published online 5 October 2023
Alexander A.J. Grüter and Julie M.L. Sijmons contributed equally to this work.
Disclosure: The authors declare that they have nothing to disclose.
None of the authors have a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Aguiar Junior S, Oliveira MM, Silva DRME, et al. Survival of patients with colorectal cancer in a cancer center. Arq Gastroenterol. 2020;57:172–177. [DOI] [PubMed] [Google Scholar]
- 2.van der Pas MH, Haglind E, Cuesta MA, et al. ; COlorectal cancer Laparoscopic or Open Resection II (COLOR II) Study Group. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210–218. [DOI] [PubMed] [Google Scholar]
- 3.Bonjer HJ, Deijen CL, Abis GA, et al. ; COLOR II Study Group. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015;372:1324–1332. [DOI] [PubMed] [Google Scholar]
- 4.Lei X, Wang Y, Shan F, et al. Short-and long-term outcomes of laparoscopic versus open gastrectomy in patients with gastric cancer: a systematic review and meta-analysis of randomized controlled trials. World J Surg Oncol. 2022;20:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jamali FR, Soweid AM, Dimassi H, et al. Evaluating the degree of difficulty of laparoscopic colorectal surgery. Arch Surg. 2008;143:762–7; discussion 768. [DOI] [PubMed] [Google Scholar]
- 6.Bosker RJI, Van't Riet E, de Noo M, et al. Minimally invasive versus open approach for right-sided colectomy: a study in 12,006 patients from the Dutch surgical colorectal audit. Dig Surg. 2019;36:27–32. [DOI] [PubMed] [Google Scholar]
- 7.Curtis NJ, Foster JD, Miskovic D, et al. Association of surgical skill assessment with clinical outcomes in cancer surgery. JAMA Surg. 2020;155:590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackenzie H, Ni M, Miskovic D, et al. Clinical validity of consultant technical skills assessment in the English national training programme for laparoscopic colorectal surgery. Br J Surg. 2015;102:991–997. [DOI] [PubMed] [Google Scholar]
- 9.Stulberg JJ, Huang R, Kreutzer L, et al. Association between surgeon technical skills and patient outcomes. JAMA Surg. 2020;155:960–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dekker E, Tanis PJ, Vleugels JLA, et al. Colorectal cancer. Lancet. 2019;394:1467–1480. [DOI] [PubMed] [Google Scholar]
- 11.Hamfjord J, Myklebust TA, Larsen IK, et al. Survival trends of right- and left-sided colon cancer across four decades: a Norwegian population-based study. Cancer Epidemiol Biomarkers Prev. 2022;31:342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodges N, Mackenzie H, D'Souza N, et al. Survival outcomes for right-versus left-sided colon cancer and rectal cancer in England: a propensity-score matched population-based cohort study. Eur J Surg Oncol. 2022;48:841–849. [DOI] [PubMed] [Google Scholar]
- 13.Tom CM, Mankarious MM, Jeganathan NA, et al. Characteristics and outcomes of right- versus left-sided early onset colorectal cancer. Dis Colon Rectum. 2022;66:498–510. [DOI] [PubMed] [Google Scholar]
- 14.Lee MS, Menter DG, Kopetz S. Right versus left colon cancer biology: integrating the consensus molecular subtypes. J Natl Compr Canc Netw. 2017;15:411–419. [DOI] [PubMed] [Google Scholar]
- 15.Li F, Zhou X, Wang B, et al. Comparison between different approaches applied in laparoscopic right hemi-colectomy: a systematic review and network meta-analysis. Int J Surg. 2017;48:74–82. [DOI] [PubMed] [Google Scholar]
- 16.Matsuda T, Yamashita K, Hasegawa H, et al. Current status and trend of laparoscopic right hemicolectomy for colon cancer. Ann Gastroenterol Surg. 2020;4:521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Taher M, Okamoto N, Mutter D, et al. International survey among surgeons on laparoscopic right hemicolectomy: the gap between guidelines and reality. Surg Endosc. 2022;36:5840–5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dijkstra FA, Bosker RJI, Veeger NJGM, et al. Procedural key steps in laparoscopic colorectal surgery, consensus through Delphi methodology. Surg Endosc. 2015;29:2620–2627. [DOI] [PubMed] [Google Scholar]
- 19.Palter VN, MacRae HM, Grantcharov TP. Development of an objective evaluation tool to assess technical skill in laparoscopic colorectal surgery: a Delphi methodology. Am J Surg. 2011;201:251–259. [DOI] [PubMed] [Google Scholar]
- 20.Haug TR, Miskovic D, Ørntoft MW, et al. Development of a procedure-specific tool for skill assessment in left- and right-sided laparoscopic complete mesocolic excision. Colorectal Dis. 2022;25:31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hohenberger W, Weber K, Matzel K, et al. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis. 2009;11:354–64; discussion 364. [DOI] [PubMed] [Google Scholar]
- 22.Albers KI, Polat F, Helder L, et al. ; RECOVER Study Collaborators. Quality of recovery and innate immune homeostasis in patients undergoing low-pressure versus standard-pressure pneumoperitoneum during laparoscopic colorectal surgery (RECOVER): a randomized controlled trial. Ann Surg. 2022;276:e664–e673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Oostendorp S, Elfrink A, Borstlap W, et al. Intracorporeal versus extracorporeal anastomosis in right hemicolectomy: a systematic review and meta-analysis. Surg Endosc. 2017;31:64–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee L, Abou-Khalil M, Liberman S, et al. Incidence of incisional hernia in the specimen extraction site for laparoscopic colorectal surgery: systematic review and meta-analysis. Surg Endosc. 2017;31:5083–5093. [DOI] [PubMed] [Google Scholar]
- 25.Benz S, Tannapfel A, Tam Y, et al. Proposal of a new classification system for complete mesocolic excison in right-sided colon cancer. Tech Coloproctol. 2019;23:251–257. [DOI] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. [DOI] [PubMed] [Google Scholar]
- 27.Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oxford Centre for Evidence-Based Medicine: Levels of Evidence. 2009. [Google Scholar]
- 29.Negoi I, Beuran M, Hostiuc S, et al. Surgical anatomy of the superior mesenteric vessels related to colon and pancreatic surgery: a systematic review and meta-analysis. Sci Rep. 2018;8:4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun KK, Zhao H. Vascular anatomical variation in laparoscopic right hemicolectomy. Asian J Surg. 2020;43:9–12. [DOI] [PubMed] [Google Scholar]
- 31.Ogino T, Takemasa I, Horitsugi G, et al. Preoperative evaluation of venous anatomy in laparoscopic complete mesocolic excision for right colon cancer. Ann Surg Oncol. 2014;21(Suppl 3):S429–S435. [DOI] [PubMed] [Google Scholar]
- 32.Kramp KH, van Det MJ, Totte ER, et al. Ergonomic assessment of the French and American position for laparoscopic cholecystectomy in the MIS Suite. Surg Endosc. 2014;28:1571–1578. [DOI] [PubMed] [Google Scholar]
- 33.Warner MA, Warner DO, Harper CM, et al. Lower extremity neuropathies associated with lithotomy positions. Anesthesiology. 2000;93:938–942. [DOI] [PubMed] [Google Scholar]
- 34.Thiruchelvam N, Lee SY, Chiow AK. Patient and port positioning in laparoscopic liver resections. Hepatoma Res. 2021;7:22. [Google Scholar]
- 35.Ahmad G, Baker J, Finnerty J, et al. Laparoscopic entry techniques. Cochrane Database Syst Rev. 2019;1:CD006583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi Y, Song Z, Gu Y, et al. Short-term outcomes of three-port laparoscopic right hemicolectomy versus five-port laparoscopic right hemicolectomy: with a propensity score matching analysis. J Invest Surg. 2020;33:822–827. [DOI] [PubMed] [Google Scholar]
- 37.Zhang T, Zhang Y, Shen X, et al. Longterm outcomes of three-port laparoscopic right hemicolectomy versus five-port laparoscopic right hemicolectomy: a retrospective study. Front Oncol. 2021;11:762716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiung Ta Lu T, Gan P, Versace V. Fewer ports cut opioid use and length of stay in elective laparoscopic cholecystectomy. JSLS. 2021;25:e2020.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swank HA, Mulder IM, la Chapelle CF, et al. Systematic review of trocar-site hernia. Br J Surg. 2012;99:315–323. [DOI] [PubMed] [Google Scholar]
- 40.Wang Q, Huang L, Zeng W, et al. Assessment of port-specific pain after gynecological laparoscopy: a prospective cohort clinical trial. J Laparoendosc Adv Surg Tech A. 2017;27:597–604. [DOI] [PubMed] [Google Scholar]
- 41.Slotman GJ, Jed EH, Burchard KW. Adverse effects of hypothermia in postoperative patients. Am J Surg. 1985;149:495–501. [DOI] [PubMed] [Google Scholar]
- 42.Dean M, Ramsay R, Heriot A, et al. Warmed, humidified CO2 insufflation benefits intraoperative core temperature during laparoscopic surgery: a meta-analysis. Asian J Endosc Surg. 2017;10:128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korell M, Schmaus F, Strowitzki T, et al. Pain intensity following laparoscopy. Surg Laparosc Endosc. 1996;6:375–379. [PubMed] [Google Scholar]
- 44.Birch DW, Dang JT, Switzer NJ, et al. Heated insufflation with or without humidification for laparoscopic abdominal surgery. Cochrane Database Syst Rev. 2016;10:CD007821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gurusamy KS, Vaughan J, Davidson BR. Low pressure versus standard pressure pneumoperitoneum in laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2014:CD006930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raval AD, Deshpande S, Koufopoulou M, et al. The impact of intra-abdominal pressure on perioperative outcomes in laparoscopic cholecystectomy: a systematic review and network meta-analysis of randomized controlled trials. Surg Endosc. 2020;34:2878–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anania G, Davies RJ, Bagolini F, et al. Right hemicolectomy with complete mesocolic excision is safe, leads to an increased lymph node yield and to increased survival: results of a systematic review and meta-analysis. Tech Coloproctol. 2021;25:1099–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu L, Su X, He Z, et al. ; RELARC Study Group. Short-term outcomes of complete mesocolic excision versus D2 dissection in patients undergoing laparoscopic colectomy for right colon cancer (RELARC): a randomised, controlled, phase 3, superiority trial. Lancet Oncol. 2021;22:391–401. [DOI] [PubMed] [Google Scholar]
- 49.Sammour T, Malakorn S, Thampy R, et al. Selective central vascular ligation (D3 lymphadenectomy) in patients undergoing minimally invasive complete mesocolic excision for colon cancer: optimizing the risk-benefit equation. Colorectal Dis. 2020;22:53–61. [DOI] [PubMed] [Google Scholar]
- 50.Adamina M, Champagne BJ, Hoffman L, et al. Randomized clinical trial comparing the cost and effectiveness of bipolar vessel sealers versus clips and vascular staplers for laparoscopic colorectal resection. Br J Surg. 2011;98:1703–1712. [DOI] [PubMed] [Google Scholar]
- 51.Campagnacci R, de Sanctis A, Baldarelli M, et al. Electrothermal bipolar vessel sealing device vs. ultrasonic coagulating shears in laparoscopic colectomies: a comparative study. Surg Endosc. 2007;21:1526–1531. [DOI] [PubMed] [Google Scholar]
- 52.Marcello PW, Roberts PL, Rusin LC, et al. Vascular pedicle ligation techniques during laparoscopic colectomy. A prospective randomized trial. Surg Endosc. 2006;20:263–269. [DOI] [PubMed] [Google Scholar]
- 53.Aiolfi A, Bona D, Guerrazzi G, et al. Intracorporeal versus extracorporeal anastomosis in laparoscopic right colectomy: an updated systematic review and cumulative meta-analysis. J Laparoendosc Adv Surg Tech A. 2020;30:402–412. [DOI] [PubMed] [Google Scholar]
- 54.Ibanez N, Abrisqueta J, Luján J, et al. Isoperistaltic versus antiperistaltic ileocolic anastomosis. Does it really matter? Results from a randomised clinical trial (ISOVANTI). Surg Endosc. 2019;33:2850–2857. [DOI] [PubMed] [Google Scholar]
- 55.Reggio S, Sciuto A, Cuccurullo D, et al. Single-layer versus double-layer closure of the enterotomy in laparoscopic right hemicolectomy with intracorporeal anastomosis: a single-center study. Tech Coloproctol. 2015;19:745–750. [DOI] [PubMed] [Google Scholar]
- 56.Milone M, Elmore U, Allaix ME, et al. Fashioning enterotomy closure after totally laparoscopic ileocolic anastomosis for right colon cancer: a multicenter experience. Surg Endosc. 2020;34:557–563. [DOI] [PubMed] [Google Scholar]
- 57.Lee SY, Kim CH, Kim YJ, et al. Prognostic impact of the length of the longitudinal resection margin in colon cancer. Colorectal Dis. 2017;19:634–640. [DOI] [PubMed] [Google Scholar]
- 58.Morales-Conde S, Alarcón I, Yang T, et al. Fluorescence angiography with indocyanine green (ICG) to evaluate anastomosis in colorectal surgery: where does it have more value? Surg Endosc. 2020;34:3897–3907. [DOI] [PubMed] [Google Scholar]
- 59.Boni L, David G, Dionigi G, et al. Indocyanine green-enhanced fluorescence to assess bowel perfusion during laparoscopic colorectal resection. Surg Endosc. 2016;30:2736–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mangano A, Fernandes E, Gheza F, et al. Near-infrared indocyanine green-enhanced fluorescence and evaluation of the bowel microperfusion during robotic colorectal surgery: a retrospective original paper. Surg Technol Int. 2019;34:93–100. [PubMed] [Google Scholar]
- 61.Greemland I, Raveh G, Gavrielli S, et al. High rates of incisional hernia after laparoscopic right colectomy with midline extraction site. Surg Laparosc Endosc Percutan Tech. 2021;31:722–728. [DOI] [PubMed] [Google Scholar]
- 62.Luo Y, Qiu Y-E, Mu Y-F, et al. Plastic wound protectors decreased surgical site infections following laparoscopic-assisted colectomy for colorectal cancer: a retrospective cohort study. Medicine (Baltim). 2017;96:e7752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arenal JJ, Martínez A, Maderuelo MV, et al. Reduced wound infection in colorectal resection by using a wound auto-retractor. Infez Med. 2016;24:310–317. [PubMed] [Google Scholar]
- 64.Capolupo GT, Lauricella S, Mascianà G, et al. O-ring protector in prevention of SSIs in laparoscopic colorectal surgery. JSLS. 2019;23:e2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lauricella S, Caricato M, Mascianà G, et al. Cost-effectiveness analysis of O-ring wound retractor in elective laparoscopic colorectal surgery. Ann Ital Chir. 2021;92:460–464. [PubMed] [Google Scholar]
- 66.Norman G, Goh EL, Dumville JC, et al. Negative pressure wound therapy for surgical wounds healing by primary closure. Cochrane Database Syst Rev. 2020;5:CD009261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abadia P, Ocaña J, Ramos D, et al. Prophylactic use of negative pressure wound therapy reduces surgical site infections in elective colorectal surgery: a prospective cohort study. Surg Infect (Larchmt). 2021;22:234–239. [DOI] [PubMed] [Google Scholar]
- 68.Norman G, Atkinson RA, Smith TA, et al. Intracavity lavage and wound irrigation for prevention of surgical site infection. Cochrane Database Syst Rev. 2017;10:CD012234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Warps AK, Tollenaar RAEM, Tanis PJ, et al. ; Dutch ColoRectal Audit. Postoperative complications after colorectal cancer surgery and the association with long-term survival. Eur J Surg Oncol. 2022;48:873–882. [DOI] [PubMed] [Google Scholar]
- 70.Sun SD, Wu P-P, Zhou J-F, et al. Failure of enhanced recovery after surgery in laparoscopic colorectal surgery: a systematic review. Int J Colorectal Dis. 2020;35:1007–1014. [DOI] [PubMed] [Google Scholar]
- 71.Sica GS, Vinci D, Siragusa L, et al. Definition and reporting of lymphadenectomy and complete mesocolic excision for radical right colectomy: a systematic review. Surg Endosc. 2022;37:846–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ferri V, Vicente E, Quijano Y, et al. Right-side colectomy with complete mesocolic excision vs conventional right-side colectomy in the treatment of colon cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2021;36:1885–1904. [DOI] [PubMed] [Google Scholar]
- 73.Bertelsen CA, Neuenschwander AU, Jansen JE, et al. 5-year outcome after complete mesocolic excision for right-sided colon cancer: a population-based cohort study. Lancet Oncol. 2019;20:1556–1565. [DOI] [PubMed] [Google Scholar]
- 74.Feng X, Li H, Lu X, et al. Regional lymph nodes distribution pattern in central area of right-sided colon cancer: in-vivo detection and the update on the clinical exploration. Am J Cancer Res. 2021;11:2095–2105. [PMC free article] [PubMed] [Google Scholar]
- 75.Yamaoka Y, Kinugasa Y, Shiomi A, et al. The distribution of lymph node metastases and their size in colon cancer. Langenbecks Arch Surg. 2017;402:1213–1221. [DOI] [PubMed] [Google Scholar]
- 76.Prevost GA, Odermatt M, Furrer M, et al. Postoperative morbidity of complete mesocolic excision and central vascular ligation in right colectomy: a retrospective comparative cohort study. World J Surg Oncol. 2018;16:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bertelsen CA, Neuenschwander AU, Jansen JE, et al. ; Copenhagen Complete Mesocolic Excision Study (COMES). Short-term outcomes after complete mesocolic excision compared with ‘conventional’ colonic cancer surgery. Br J Surg. 2016;103:581–589. [DOI] [PubMed] [Google Scholar]
- 78.Brown RF, Cleary RK. Intracorporeal anastomosis versus extracorporeal anastomosis for minimally invasive colectomy. J Gastrointest Oncol. 2020;11:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carnuccio P, Jimeno J, Pares D. Laparoscopic right colectomy: a systematic review and meta-analysis of observational studies comparing two types of anastomosis. Tech Coloproctol. 2014;18:5–12. [DOI] [PubMed] [Google Scholar]
- 80.Cirocchi R, Trastulli S, Farinella E, et al. Intracorporeal versus extracorporeal anastomosis during laparoscopic right hemicolectomy - systematic review and meta-analysis. Surg Oncol. 2013;22:1–13. [DOI] [PubMed] [Google Scholar]
- 81.Creavin B, Balasubramanian I, Common M, et al. Intracorporeal vs extracorporeal anastomosis following neoplastic right hemicolectomy resection: a systematic review and meta-analysis of randomized control trials. Int J Colorectal Dis. 2021;36:645–656. [DOI] [PubMed] [Google Scholar]
- 82.Emile SH, Elfeki H, Shalaby M, et al. Intracorporeal versus extracorporeal anastomosis in minimally invasive right colectomy: an updated systematic review and meta-analysis. Tech Coloproctol. 2019;23:1023–1035. [DOI] [PubMed] [Google Scholar]
- 83.Feroci F, Lenzi E, Garzi A, et al. Intracorporeal versus extracorporeal anastomosis after laparoscopic right hemicolectomy for cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2013;28:1177–1186. [DOI] [PubMed] [Google Scholar]
- 84.Milone M, Elmore U, Vignali A, et al. Recovery after intracorporeal anastomosis in laparoscopic right hemicolectomy: a systematic review and meta-analysis. Langenbecks Arch Surg. 2018;403:1–10. [DOI] [PubMed] [Google Scholar]
- 85.Ricci C, Casadei R, Alagna V, et al. A critical and comprehensive systematic review and meta-analysis of studies comparing intracorporeal and extracorporeal anastomosis in laparoscopic right hemicolectomy. Langenbecks Arch Surg. 2017;402:417–427. [DOI] [PubMed] [Google Scholar]
- 86.Selvy M, Mattevi C, Slim K, et al. Intra-versus extracorporeal anastomosis in laparoscopic right colectomy: a meta-analysis of 3699 patients. Int J Colorectal Dis. 2020;35:1673–1680. [DOI] [PubMed] [Google Scholar]
- 87.Wu Q, Jin C, Hu T, et al. Intracorporeal versus extracorporeal anastomosis in laparoscopic right colectomy: a systematic review and meta-analysis. J Laparoendosc Adv Surg Tech A. 2017;27:348–357. [DOI] [PubMed] [Google Scholar]
- 88.Zheng JC, Zhao S, Chen W, et al. Comparison of intracorporeal and extracorporeal anastomosis and resection in right colectomy: a systematic review and meta-analysis. Langenbecks Arch Surg. 2021;406:1789–1801. [DOI] [PubMed] [Google Scholar]
- 89.Bruintjes MH, van Helden EV, Braat AE, et al. Deep neuromuscular block to optimize surgical space conditions during laparoscopic surgery: a systematic review and meta-analysis. Br J Anaesth. 2017;118:834–842. [DOI] [PubMed] [Google Scholar]
- 90.Roberts DJ, Zygun DA, Ball CG, et al. Challenges and potential solutions to the evaluation, monitoring, and regulation of surgical innovations. BMC Surg. 2019;19:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mackenzie H, Markar SR, Askari A, et al. National proficiency-gain curves for minimally invasive gastrointestinal cancer surgery. Br J Surg. 2016;103:88–96. [DOI] [PubMed] [Google Scholar]
- 92.Bosker R, Groen H, Hoff C, et al. Effect of proctoring on implementation and results of elective laparoscopic colon surgery. Int J Colorectal Dis. 2011;26:941–947. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.