Abstract
Objective:
This study aimed to analyze risk factors and develop a predictive model for early allograft loss due to early graft dysfunction (EGD) in adult live-donor liver transplantation (LDLT).
Methods:
Data of patients who underwent LDLT from 2011 to 2019 were reviewed for EGD, associated factors, and outcomes. A homogeneous group of 387 patients was analyzed: random cohort A (n = 274) for primary analysis and random cohort B (n = 113) for validation.
Results:
Of 274 recipients, 92 (33.6%) developed EGD. The risk of graft loss within 90 days was 29.3% and 7.1% in those with and without EGD, respectively (P < 0.001). Multivariate logistic regression analysis determined donor age (P = 0.045), estimated (e) graft weight (P = 0.001), and the model for end-stage liver disease (MELD) score (0.001) as independent predictors of early graft loss due to EGD. Regression coefficients of these factors were employed to formulate the risk model: Predicted (P) early graft loss risk (e-GLR) score = 10 × [(donor age × 0.052) + (e-Graft weight × 1.681) + (MELD × 0.145)] − 8.606 (e-Graft weight = 0, if e-Graft weight ≥640 g and e-Graft weight = 1, and if e-Graft weight < 640 g). Internal cross-validation revealed a high predictive value (C-statistic = 0.858).
Conclusions:
Our novel risk score can efficiently predict early allograft loss following graft dysfunction, which enables donor-recipient matching, evaluation, and prognostication simply and reliably in adult LDLT.
Keywords: donor age, graft loss, graft weight, live-donor liver transplantation, MELD
Mini Abstract
Early graft dysfunction after live-donor liver transplantation can have a grave impact on graft survival and short-term outcomes. The early graft loss risk score includes donor age, estimated (e) graft weight, and pre-op model for end-stage liver disease score of the recipient. Preoperatively, it can effectively predict the early graft loss due to graft dysfunction. This score empowers donor-recipient matching and prognostication simply and reliably.
INTRODUCTION
Early graft dysfunction (EGD) is defined as graft dysfunction in the absence of vascular, biliary, or immunological issues. In live-donor liver transplantation (LDLT), EGD leading to graft loss has traditionally been examined through the lens of “small for size syndrome” (SFSS).1–3 However, EGD in LDLT is a multifactorial outcome of graft-to-recipient weight ratio (GRWR), graft inflow and outflow, recipient metabolic demand, graft volume, and graft quality. Expanding donor acceptance criteria and the inclusion of sicker recipients have added to this sea of possible factors affecting early graft loss. Despite striving for excellence, the lack of objectivity in the ‘best functional graft size and quality,’ the surgeon’s experience and discretion guide the course of graft harvesting, and recipient factors remain unmodifiable in most situations. The current study aimed to analyze the factors associated with early graft loss due to graft dysfunction and develop a preoperative predictive model for better selection in patients undergoing LDLT.
MATERIALS AND METHODS
A total of 645 primary liver transplant (LT) procedures were performed between January 2011 and December 2019. Recipients under 18 years of age (n = 66), those who had undergone transplantation for acute liver failure (ALF) (n = 42), deceased donor LTs (n = 51), and those who received right posterior sectoral grafts (n = 4) were excluded. In addition, to reduce the effect of confounding variables, recipients who had vascular (n = 20) and biliary (n = 40) complications and biopsy-proven acute rejections (n = 13) were excluded along with recipients who died due to causes other than EGD (n = 22). The final analysis included 387 recipients, randomly divided into cohorts A and B, consisting of 274 (70%) patients for primary analysis and 113 (30%) patients for validation (Fig. 1). Donor-, recipient-, and transplant-related factors were recorded in a prospectively maintained database. Patients were legally authorized by an independent committee, and written informed consent was obtained before surgery. This study was reported in line with the strengthening the reporting of cohort, cross-sectional and case-control studies in surgery criteria.4 The study was approved by the Institutional Review Board (no. IEC/2020/79/MA05).
FIGURE 1.
Composition of the study population.
Surgical Technique and Perioperative Management
The accepted donors were healthy adults between 18 and 50 years of age with no known comorbidity having a compatible blood group and body mass index (BMI) <28 kg/m2, a remnant liver volume of >30%, and liver attenuation index (LAI) ≥+5 Hounsfield unit (HU) on computed tomography (CT). They were either related to or emotionally attached to the patients and voluntarily opted for liver donation. Donors with BMI >28, dyslipidemia, steatosis on ultrasonography, and/or abnormal LAI (ie, <5 or >15 HU) were optimized by lifestyle changes, weight reduction, and dietary modifications. Liver biopsy was advised in donors with deranged liver function tests, persistently abnormal LAI scores on repeat evaluation, or those with BMI >28 who were on an optimization program intended for donation. Donors with up to 10% steatosis on liver biopsy were accepted for donation, provided that there was an adequate liver remnant of ≥35% on CT volumetry. We also performed liver biopsy selectively in potential donors aged >45 years, genetic relationship to a person with autoimmune or genetic liver disease, and hepatitis B virus core-positive serology. Volumetric assessment of the donor’s liver was performed using automated software (GE Discovery 750HD Single-source Dual Energy CT scanner, Myrian XP Liver 3D software). The examination parameters were a slice thickness of 5 mm and a 5-mm reconstruction interval. The images were reconstructed with 0.625-mm reconstruction intervals for detailed interpretation. For right and left hemiliver volume assessments, a plane was marked along the middle hepatic vein (MHV) on the venous phase of contrast CT of the abdomen. Volumetric calculations (considering liver density equivalent to 1 gm/mL) of the liver parenchyma were retrieved after excluding the major extrahepatic vessels (portal vein and inferior vena cava). The recipient condition and donor liver anatomy (vascular and biliary) dictated graft-type selection. In our center, a predicted GRWR ≥0.8% is the conventional acceptance criterion; however, with the escalation of expertise and experience, we have been utilizing liver grafts with predicted GRWR ≥0.6% to 0.8% since 2015 in selected recipients. Such donors were considered only in the absence of an alternative suitable donor with an informed higher than the normal risk in recipients with a low model for end-stage liver disease (MELD) score (≤20). We measured the definite graft weight after donor hepatectomy (after draining out the blood from the graft) just before flushing with histidine-tryptophan-ketoglutarate solution at 4°C. The pretransplant CT-estimated graft weight showed a good correlation (assessed by the coefficient of determination) with the actual graft weight (R2 = 0.781), as shown in Supplemental Figure S1, http://links.lww.com/AOSO/A256. The difference between the estimated (e) and actual (a) graft weights was within a ±6.5% margin (calculated using an equivalence test, P = 0.036). The MHV was routinely preserved in the donor, and the Neo-MHV was recreated on the bench. The inferior vena cava was preserved during the recipient’s hepatectomy. If a large (≥8 mm) splenorenal shunt and dilated coronary vein were present, the left renal vein and coronary vein were ligated to augment portal flow. We did not perform portal inflow modulation on our patients. The grafts were transplanted with a side-biting clamp into the recipient cava. All significant inferior veins (>5 mm) were anastomosed to the inferior vena cava. Intraoperative Doppler ultrasound (using a Toshiba Xario USG machine with a Doppler facility using curvilinear probes of 7–11 MHz frequency) was performed routinely to assess graft inflow and outflow. A Doppler examination was performed after the completion of the arterial anastomosis. The flow velocity and cross-sectional area of the portal vein were measured in Doppler mode. Portal flow was calculated using the flow velocity and portal vein diameter just beyond the portal vein anastomosis. The mean of 3 consecutive readings was used for the analysis. To avoid congestion of the liver graft, the central venous pressure was kept low (preferably <10 mm Hg).
In brief, our immunosuppression consisted of intravenous corticosteroids (100 mg methylprednisolone during the anhepatic phase, followed by a 5-day dose of an attenuating regimen), mycophenolate mofetil, and calcineurin inhibitors (dose adjusted according to blood levels). Dose modifications were performed on a case-to-case basis, depending on the clinical course. Perioperative antimicrobial prophylaxis comprised intravenous meropenem (2 g/d) twice daily and teicoplanin (400 mg/d) once daily for 72 hours. Doppler ultrasonography and routine hematological tests, in the form of serum biochemistry, hemogram, and coagulation studies, were performed twice a day for 7 days and then once daily until discharge.
Definitions
LAI was defined as the average attenuation value of the hepatic parenchyma minus the average attenuation value of the splenic parenchyma, and it was calculated on a non-contrast CT scan to assess hepatic steatosis. EGD was defined by the modified Olthoff et al5–7 criteria as bilirubin ≥10 mg/dL on day 7 and/or international normalized ratio ≥1.6 on day 7, excluding vascular, biliary, infectious, and immunological causes. Small-for-size syndrome was defined by Soejima et al8 criteria as total bilirubin >10 mg/dL at postoperative day 14 (without any other definitive causes for cholestasis) and intractable ascites (daily production of ascites of >1l at postoperative day 14 or >500 mL at postoperative day 28).8 The Clavein-Dindo classification was utilized to grade the postoperative complications.9 Bacterial sepsis was defined as a positive blood/fluid culture (excluding common skin commensals) within 90 days of surgery that is, transplantation, with clinical symptoms, including fever, chills and rigor, tachycardia, breathing difficulty, mental obtundation, hypotension, or decreased urine output of <0.5 mL/kg/hr for >2 hours despite fluid resuscitation. Early graft loss was defined as mortality due to graft loss occurring within 90 days. Recipient comorbidity was defined using the Modified Charlson Comorbidity Index with a score >1.10
Statistical methods
The chi-square test was used to compare categorical variables, which are presented as numbers (percentages). Mean (standard deviation, SD) represented the continuous variables, and an independent sample Student’s t-test was used for comparison. Demography, donor factors, recipient factors, and transplant-related variables were compared between the 2 groups (EGD and non-EGD groups) in the derivation cohort. Univariate analysis was performed to identify the variables associated with early allograft loss. Variables that had a value of P ≤ 0.1 (approaching significance) in the univariate analysis were considered for a multivariable logistic regression model to identify independent predictors of early graft loss. For the multivariate analysis, P values under 0.05 were taken as statistically significant. SPSS software version 22 (IBM Corp. Armonk, NY, USA) was used for the statistical analyses and the random division of the final study population into derivation (70%) and validation (30%) cohorts (using the following commands: (1) from the menus choose: data >select cases, (2) select random sample of cases, (3) click sample, and (4) enter the percentage or number of case).
RESULTS
Demographics
The study population comprised 343 men and 44 women with a mean (±SD) age of 46.7 ± 9.4 years. The mean (±SD) MELD score at transplantation was 23.5 ± 6.1. Right liver grafts were used in 330 (85.2%) patients, whereas 57 (14.8%) received left liver grafts. The mean GRWR was 0.98 ± 0.23 with 78 (20.2%) subjects having a GRWR <0.8%. The incidences of SFSS in recipients with GRWR ≥0.8 and <0.8% were 3.5% (11/309) and 6.4 % (5/78), respectively (P = 0.24). In recipients with GRWR ≥0.8 and <0.8%, the rates of EGD were 31.1% (96/309) and 39.7% (31/78), respectively (P = 0.14). Further, early graft loss following EGD was comparable between the 2 groups, 7.8% (24/309) vs. 14% (11/78), respectively (P = 0.08). The donor pool included 160 men and 227 women, with a mean (±SD) age of 30.6 ± 9.2 years (Table 1). There was no donor mortality, and the grade 3 and above complication rate was 4.6%. A total of 274 subjects formed cohort A and were considered for primary analysis, whereas 113 subjects were included in cohort B for validation. The donor and recipient data between these 2 cohorts did not show any significant differences (Table 1).
TABLE 1.
Characteristics of Patients in the Study Population, Derivation Cohort A and Validation Cohort B
| Characteristics | Study Population (n = 387) |
Derivation Cohort A (n = 274) | Validation Cohort B (n = 113) | P |
|---|---|---|---|---|
| Recipient age (years) Gender (male, %) BMI |
46.7 ± 9.4 344 (88.8 %) 25.78 ± 6.2 |
47.2 ± 9.5 245 (89.4) 25.62 ± 5.4 |
45.6 ± 9.1 99 (87.6) 24.54 ± 6.45 |
0.14 0.60 0.09 |
| Donor age (years) Gender (male, %) Liver attenuation index Steatotic graft |
30.6 ± 9.2 160 (41.3%) 10.78 ± 6.5 42 (10.8%) |
31.1 ± 9.4 115 (42) 10.56 ± 6.4 27 (9.8%) |
29.5 ± 8.5 45 (60.2) 9.68 ± 5.5 15 (13.3%) |
0.12 0.69 0.20 0.32 |
| MELD score at transplant | 23.8 ± 6.1 | 22.9 ± 7.8 | 24.4 ± 6.6 | 0.08 |
| Child-Pugh status A/B (%) | 48 (12.4) | 37 (13.5) | 11 (9.7) | 0.30 |
| C (%) | 339 (86.4%) | 237 (86.5) | 102 (90.3) | |
| Comorbidity (%) | 179 (46.2) | 131 (47.8) | 48 (42.4) | 0.34 |
| Presence of PVT (%) | 44 (11.4%) | 36 (13.1) | 8 (7.1) | 0.08 |
| Anhepatic phase (min) | 136 ± 44.7 | 138 ± 44.3 | 134.4 ± 45.8 | 0.47 |
| Cold ischemia time (min) | 99.8 ± 39.6 | 98.2 ± 31.1 | 103.8 ± 55.1 | 0.20 |
| Warm ischemia time (min) | 30.7 ± 10.8 | 31.3 ± 11 | 29.4 ± 10.1 | 0.11 |
| Graft type (%) | ||||
| Right Left |
330 (85.2%) 57 (14.8%) |
232 (84.7) 42 (15.3) |
98 (86.7) 15 (13.3) |
0.60 |
| Estimated graft weight (grams) | 682.13 ± 129.80 | 679.53 ± 122.29 | 688.45 ± 146.82 | 0.54 |
| GRWR% | 0.98 ± 0.23 | 0.98 ± 0.23 | 0.99 ± 0.24 | 0.77 |
| GRWR<0.8% | 78 (20.2%) | 56 (20.4%) | 22 (19.5%) | 0.83 |
| Portal venous flow (L/min) | 2.7 ± 1.1 | 2.77 ± 1.10 | 2.8 ± 1.16 | 0.72 |
| Blood loss (mL) | 2676 ± 1638 | 2644.8 ± 1551 | 2752.4 ± 1837.3 | 0.55 |
BMI = body mass index; GRWR = graft-to-recipient weight ratio (calculated by estimated graft weight); MELD = model for end-stage liver disease; PVT = portal vein thrombosis.
Graft and Patient Outcomes
In the derivation cohort, 92 of 274 (33.6%) recipients satisfied the EGD criteria, whereas 182 of 274 (66.4%) did not. International normalized ratio ≥1.6 on postoperative day 7 was the most frequently observed EGD-defining parameter (61 out of 92 patients). Both criteria were present in only 14 of the 92 patients. Recipients with EGD had higher mean preoperative MELD score (24.9 vs. 21.8; P < 0.001), higher mean donor age (35.6 vs. 29.9 years; P = 0.02), lower e-Graft weight (653.3 vs. 692.79 grams; P = 0.011), lower GRWR (0.86 vs. 0.92%; P = 0.013), and higher intraoperative blood loss (2961 vs. 2484 mL; P = 0.01). The EGD group had a higher portal flow but did not reach the significance (2.9 vs. 2.6L/min; P = 0.06). EGD was significantly associated with all analyzed postoperative outcomes, including the 30-day postoperative mortality rate (Table 2). Similarly, recipients meeting the definition of EGD had 29.3% (n = 27) graft loss at 90 days compared with 7.1% (n = 13) for those who did not fulfill the criteria (P < 0.001). Forty (14.6%) recipients died within 90 days after LDLT. The causes of early mortality were graft failure following EGD in 27 patients and infection in the remaining 13 patients (bacterial sepsis in 10 and fungal sepsis in 3). Graft loss was averted in 70.7% (65/92) of cases of graft dysfunction with supportive medical treatment (ie, enteral nutrition, intravenous broad-spectrum antibiotics based on cultures, avoidance of hepatotoxic medications, maintenance of fluid and electrolyte balance, TEG-based correction of coagulopathy, mechanical ventilatory and ionotropic support, and continuous renal replacement therapy whenever needed). Although we performed plasma exchange in 2 recipients with graft dysfunction, the graft could not be salvaged in either patient. Re-transplantation was not feasible in any of the 27 cases because of the lack of a suitable second live donor, nonavailability of deceased donor grafts, or financial constraints. Many times, the patient became too sick for re-transplantation.
TABLE 2.
Characteristics of Patients with EGD vs. no EGD in Derivation Cohort A
| Early Graft Dysfunction | |||
|---|---|---|---|
| Preoperative Characteristics | Yes (n = 92) | No (n = 182) | P |
| Recipient age (years) Gender (male, %) BMI |
46 ± 9.6 85 (92.4) 26.57 ± 5.2 |
47.7 ± 9.4 160 (87.9) 25.32 ± 4.6 |
0.15 0.25 0.10 |
| Donor age (years) Gender (male, %) Liver attenuation index Steatotic graft |
35.6 ± 9.5 40 (43.5) 9.84 ± 5.67 12 (13) |
29.9 ± 8.9 75 (41.2) 10.67 ± 5.05 15 (8.2) |
0.02 0.71 0.34 0.21 |
| MELD score at transplant | 24.9 ± 5.4 | 21.8 ± 5.7 | <0.001 |
| Child-Pugh status | |||
| A (%) | 0 (0.0) | 2 (1.1) | 0.36 |
| B (%) C (%) |
10 (10.9) 82 (89.1) |
25 (13.7) 155 (85.2) |
|
| Co-morbidities (%) | 41 (44.5) | 90 (49.4) | 0.52 |
| Chronic liver disease (%) ACLF (%) |
78 (84.7) 14 (15.3) |
163 (89.6) 19 (10.4) |
0.19 |
| Etiology (%) | |||
| Alcohol | 45 (48.9) | 85 (46.7) | 0.44 |
| Cryptogenic HBV HCV NASH Others |
14 (15.2) 7 (7.6) 5 (5.4) 12 (13) 9 (9.7) |
22 (12.1) 17 (9.3) 12 (6.5) 30 (16.5) 16 (8.9) |
|
| Estimated graft weight(grams) | 653.3 ± 124.71 | 692.79 ± 119.21 | 0.011 |
| GRWR (%) | 0.86 ± 0.24 | 0.92 ± 0.22 | 0.013 |
| Intraoperative parameters | |||
| Anhepatic phase (min) | 142 ± 46.2 | 136 ± 43.2 | 0.29 |
| Cold ischemia time (min) | 99 ± 33.1 | 97 ± 30 | 0.74 |
| Warm ischemia time (min) | 31.9 ± 9.1 | 31 ± 11.8 | 0.56 |
| Graft type (%) | 0.08 | ||
| Right Left |
73 (79.3) 19 (20.7) |
159 (87.4) 23 (12.6) |
|
| Presence of PVT (%) | 12 (13) | 24 (13.2) | 0.97 |
| Portal venous flow (L/min) | 2.9 ± 1.1 | 2.6 ± 1.1 | 0.06 |
| Blood loss (mL) | 2961 ± 1794 | 2484 ± 1391 | 0.01 |
| Postoperative parameters | |||
| Peak INR | 4.6 ± 1.4 | 3.8 ± 1.4 | <0.001 |
| Peak serum creatinine(mg/dL) | 1.5 ± 0.6 | 1.1 ± 0.6 | <0.001 |
| Peak T. bilirubin (mg/dL) | 13.7 ± 9.2 | 8.3 ± 4.7 | < 0.001 |
| Peak AST (IU/L) | 154.9 ± 111 | 59.3 ± 38.9 | <0.001 |
| Peak ALT (IU/L) | 116.2 ± 59.2 | 95.9 ± 83.2 | <0.001 |
| Ascites >1 L on day 14(%) | 41 (44.5) | 44 (24.1) | <0.001 |
| Bacterial sepsis (%) | 48 (52.1) | 53 (29.1) | <0.001 |
| Hospital stay (days) | 30.3 ± 23.8 | 24.4 ± 15.3 | 0.014 |
| ICU/HDU stay (days) | 19 ± 37.6 | 11 ± 11.3 | <0.001 |
| Retransfer to ICU (%) | 17 (21.7) | 18 (9.8) | 0.01 |
| Morbidity (CDC ≥IIIa) (%) | 44 (47.8) | 68 (37.3) | 0.009 |
| 30-day mortality (%) | 16 (17.3) | 7 (3.8) | <0.001 |
ACLF = acute on chronic liver failure; ALT = alanine transaminase; AST = aspirate transaminase; BMI = steatotic graft; CDC = Clavein-Dindo classification; GRWR = graft-to-recipient weight ratio; HBV = hepatitis B virus; HCV = hepatitis C virus; HDU = high dependency unit; ICU = intensive care unit; INR = international normalized ratio; MELD = model for end-stage liver disease; NASH = nonalcoholic steatohepatitis; PVT = portal vein thrombosis.
Risk Factors for Early Graft Loss
In the derivation cohort (n = 274), 27 patients experienced graft loss 90 days after graft dysfunction. Univariate analysis was performed using donor and recipient demographics and surgery-related factors. The variables associated with early graft loss due to EGD were estimated (e) graft weight (P = 0.001), GRWR (P = 0.026), and MELD score (P = 0.004). Multivariate logistic regression analysis identified only donor age (P = 0.045; OR, 1.053; 95% confidence interval [CI], 1.001–1.108), e-Graft weight (P = 0.001; OR, 5.359; 95% CI, 1.961–14.7037) and MELD score (P = 0.001; OR, 1.156; 95% CI, 1.062–1.258) as independent predictors of early graft loss (Table 3). Interestingly, on multivariate analysis, there were no significant correlations between graft loss and GRWR, type of graft, and portal blood flow, suggesting that these factors did not alter recovery from graft dysfunction. In addition, steatotic grafts did not affect the outcome because of our stringent donor selection criteria and the preoperative optimization of steatotic donors. We did not accept donors with >10% steatosis.
TABLE 3.
Univariate and Multivariate Analysis of Potential Donor and Recipient Factors for the Risk of Early Graft Loss (n = 27) Due to EGD in Derivation Cohort (n = 274)
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI | P | OR (95% CI | P | |
| Recipient age | 1.001 (0.954–1.050) | 0.965 | ||
| Recipient gender (male) | 0.402 (0.052–3.110) | 0.367 | ||
| Recipient BMI | 1.012 (0.923–1.091) | 0.852 | ||
| Donor age | 1.038 (0.991–1.087) | 0.060 | 1.053 (1.001–1.108) | 0.045 |
| Donor gender (male) | 0.780 (0.320–1.904) | 0.585 | ||
| Donor LAI | 1.024 (0.525–3.241) | 0.560 | ||
| Steatotic grafts | 1.153 (0.452–2.901) | 0.763 | ||
| Child C status | 1.074 (0.300–3.840) | 0.913 | ||
| MELD score | 1.122 (1.038–1.212) | 0.004 | 1.156 (1.062–1.258) | 0.001 |
| Presence of PVT | 2.323 (0.794–6.800) | 0.115 | ||
| Estimated graft weight | 4.358 (1.885–10.075) | 0.001 | 5.359 (1.961–14.703) | 0.001 |
| Graft type (left) | 1.331 (0.425–4.173) | 0.622 | ||
| GRWR | 0.054 (0.004–0.708) | 0.026 | 0.379 (0.024–7.086) | 0.569 |
| Blood loss | 1.000 (1.000–1.000) | 0.261 | ||
| Anhepatic phase | 0.999 (0.989–1.000) | 0.887 | ||
| Cold ischemia time | 1.004 (0.990–1.018) | 0.574 | ||
| Warm ischemia time | 1.002 (0.963–1.043) | 0.911 | ||
| Portal venous flow | 0.909 (0.596–1.385) | 0.656 | ||
BMI = steatotic graft; GRWR = graft-to-recipient weight ratio (calculated by estimated graft weight); LAI = liver attenuation index; MELD = model for end-stage liver disease; PVT = portal vein thrombosis.
As the use of a larger graft volume did not provide a survival benefit in LDLT, we determined a suitable cutoff value of e-Graft weight for discriminating early graft loss due to graft dysfunction. A statistical cutoff value for e-Graft weight was defined by receiver operating characteristic (ROC) curve analysis and showed a threshold of 640 g (sensitivity, 73%; specificity, 70.2%; c-statistic, 0.746) (Supplemental Figure S2, http://links.lww.com/AOSO/A256).
Predictive Score for Early Graft Loss
A formula was devised based on the multivariate logistic regression analysis results of significant donor and recipient factors and their regression coefficients (ie, ß coefficients). The following risk score was deduced for graft loss at 90 days.
Predicted (P) early graft loss risk (e-GLR) score:
10× ([donor age × 0.052] + [e-Graft weight × 1.681] + [MELD × 0.145]) − 8.606.
(Where e-Graft weight = 0, if graft volume ≥640 g and e-Graft weight = 1, if graft volume < 640 g.) The histogram (Supplemental Figure S3, http://links.lww.com/AOSO/A256) depicts the distribution of the (P) e-GLR scores in the derivation and validation cohorts.
This model conveniently predicts the risk of early graft loss secondary to graft dysfunction. The study cohort had a C-static value of 0.805. This formula was cross-validated in cohort B of randomly selected 113 subjects from the same study population, achieving a C-statistic of 0.858. There was no significant difference (P = 0.62) between the areas under the 2 independent ROC curves using the Hanley and McNeil test.11 The (P) e-GLR score correctly stratified the survival and mortality in both the derivation and validation cohorts by 93.8% and 92.3%, respectively. Further, the entire validation cohort (n = 113) was divided into 10 groups by percentile of the (P) e-GLR model, and model-predicted versus actual graft losses at 90 days were plotted according to each subgroup. The model was well calibrated, and the Hosmer–Lemeshow test12 showed no significance (P = 0.86) (Fig. 2).
FIGURE 2.
Calibration plot of observed to estimated 90 days graft losses following adult living-donor liver transplantation in validation dataset (n = 113).
Logistic regression analysis of the (P) e-GLR score was plotted against 90-day graft loss in the validation dataset. The cumulative logistic probability plot demonstrated a strong association between the e-GLR score and the risk of graft loss due to EGD, and there was a progressively increasing risk of graft loss at 90 days as the (P) e-GLR score increased (Fig. 3). Based on the ROC analysis, we derived a cutoff value of 54.2 (area under the curve = 0.791) to best differentiate early graft loss after graft dysfunction in adult LDLT (Supplemental Figure S4, http://links.lww.com/AOSO/A256). Up to this cutoff, the risk of graft loss was within 10.6%. Our model is dynamic, and we have derived another cutoff to reflect a lower graft failure rate of around 5%. As per Fig. 3, at the cutoff of 45.6, the risk of early graft loss following EGD was around 5%.
FIGURE 3.
The Cumulative logistic probability plot between predicated (P) e-GLR score and graft loss at 90 days.
Similarly, we derived the actual (A) e-GLR score based on the actual (a) graft weight (Supplemental Table 1, http://links.lww.com/AOSO/A256; the formula was derived based on the results of significant variables on multivariate analysis and their regression coefficients):
Actual (a) e-GLR score:
10 × ([Donor Age × 0.054] + [a-Graft Weight × 1.851] + [MELD × 0.128]) − 8.128.
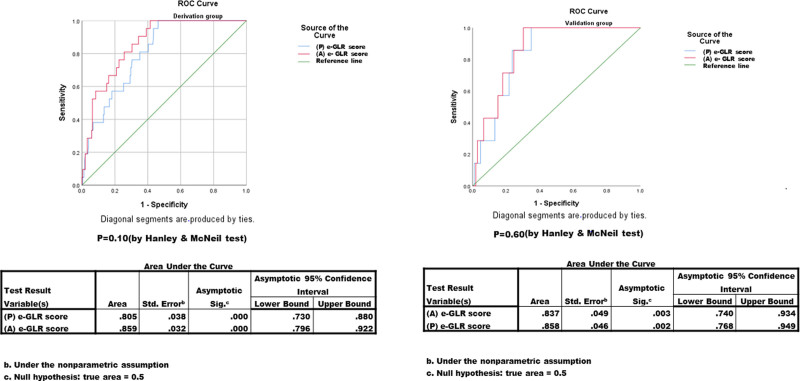
(Where a-Graft weight = 0, if a-Graft weight ≥610 g and a-Graft weight = 1, if a-Graft weight <610 g; statistical cutoff of 610 g was defined by ROC analysis with a sensitivity of 70% and specificity of 72%; c-statistic, 0.76.) The discriminative powers of both the (P) e-GLR and (A) e-GLR scores in the derivation and validation cohorts were comparable (Fig. 4). Based on the above results, we have derived a calculator (https://www.ilbs.in/download/eGLR_Calculator.xlsx) for the e-GLR score (Fig. 5).
FIGURE 4.
Receiver operating characteristic (ROC) curves of predicted (P) e-GLR and actual (A) e-GLR scores in relation to graft loss at 90 days following adult living-donor liver transplantation in the validation cohort (A) and derivation cohort (B).
FIGURE 5.
Snapshot of early graft loss risk (e-GLR) calculator
DISCUSSION
This is the first study to propose a simple continuous prognostic scoring system that indicates the risk of early graft loss following graft dysfunction using objective donor and recipient parameters in LDLT. In the context of LDLT, donor safety is paramount, and there should be good justification to offer LDLT to sick recipients. Many centers are skeptical about offering LDLT in patients with high MELD (>25).13 But, in countries like ours where organ donation is still in its infancy, even LDLT for ALF is being routinely performed, and more sick candidates with end-stage liver disease are being offered LDLT. In sick patients with high MELD scores who cannot wait long, LDLT is the sole option. Often, it is difficult to decide whether such sick patients should be offered LDLT, taking into consideration the donor risk at the cost of an uncertain recipient outcome. However, the donor is a close family member and is emotionally driven to save the life of a loved one. Denying LT with an available organ as a dedicated gift from a motivated family member is also an ethical issue. A simple predictive model such as this can help the transplant team to objectively define and quantify the risk of early graft loss preoperatively to prognosticate the patients and, most importantly, in the context of LDLT, justify donor hepatectomy in a healthy individual for the recipients’ credit. We present our results in a homogenous study cohort and enumerate this as a merit of our study. The incidence of graft dysfunction in our study group was 33%, which falls in the higher strata of the reported incidence range in the literature.14 A comparison with other landmark LDLT studies6,7,15–19 showing that our results with high EGD are not unexpected ones has been illustrated in Table 4. The overall mortality rate was 14.6%. Perioperative mortality rates reflect the patients’ clinical status at the time of transplantation. In comparison with published literature, the mean MELD of our recipients was 24 (range, 14–36), with a significant proportion (86.4%) of Child-Pugh class C patients. Owing to poor organ donation rates, LDLT is the predominant mode of transplantation at our center. As a result, most patients decide late for LT because of fear of surgery, limited fit donors in the family, live liver donor risk, financial issues, local referral practices, and so on. By the time they make up their mind, most of the patients have sarcopenia, refractory ascites, subacute bacterial peritonitis, hepatic encephalopathy, prior sepsis, and acute kidney injury leading to multiple hospital admissions before LT. Many of these are not reflected in MELD scores. The timing of the transplant is the key to improving the results. Skepticism in chronic liver disease patients regarding transplant results and apprehension in donors can be averted by timely counseling. This can avoid a sicker stage with high MELD at the time of transplant and improve the results by ameliorating post-transplant EGD and sepsis-related complications and mortality.
TABLE 4.
Major Published Series on EGD After LDLT
| Author/Year/Journal | Study Type | Country | N | MELD Score | EGD Criteria | EGD Incidence | Graft Loss |
|---|---|---|---|---|---|---|---|
| Pomposelli et al.6 2016 (A2ALL study) Transplantation |
Prospective | US and Canada | 631 | A2ALL-1 15.2 ± 5.4 A2ALL-2 15.8 ± 6.0 |
Bilirubin >10 mg/dL or INR >1.6 On POD 7 |
A2ALL-1:16% A2ALL-2:19% |
24% |
| Chae et al.15 2016 Ann Transplant |
Retrospective | Korea | 104 | EGD group 22.0 (12.0–30.0) Non-EGD group 13.5 (8.0–24.5) |
Olthoff’s Criteria |
29.8% | NA |
| Yadav et al.16 2017 Clinical Transplantation |
Retrospective | India | 151 849 |
≥25 <25 |
Olthoff’s Criteria |
25.2% 15.2% |
NA NA |
| Yang et al.17 2017 Hepatobiliary Pancreat Dis Int |
Retrospective | China | 231 | 20 (12–28) | Olthoff’s Criteria |
38.6% | 28.1% |
| Okamura et al.7 2018 Transplantation |
Retrospective | Japan | 260 | 18 (6–46) | TB of 10 mg/dL or greater and/or PT-INR of 1.6 or greater on POD 7 |
32.3% | 59% |
| Tasai et al.18 2021 Am J Transl Res |
Prospective | Taiwan | 74 | EGD group (21.22 ± 11.19) Non-EGD group (16.41 ± 8.37) |
Olthoff’s criteria |
29.7% | 22.7% |
| Singh et al.19 2022 Transplantation |
Prospective | India | 135 | 16 (6–45) | A2ALL study | 29.6% | 4.4% |
| Present study 2023 |
Retrospective | India | 387 | 24.9 ± 5.4 | Bilirubin ≥10 mg/dL and/or INR ≥1.6 on POD7, excluding vascular, biliary, infectious, and immunological causes | 33% | 9% |
EGD = early graft dysfunction; INR = international normalized ratio; MELD = model for end-stage liver disease; POD = postoperative day.
A predictive model was developed by considering the significant donor and recipient factors and their regression coefficients. Its internal cross-validation reproduced a good discrimination ability with a c-statistic of 0.858. Notably, all 3 continuous parameters of the model were available preoperatively. The optimal cutoff for differentiating graft loss due to EGD was a (P) e-GLR score of 54.2, beyond which the risk of graft loss increased steeply (Fig. 3), suggesting that LDLT should be performed after a comprehensive discussion with the donor and recipient. If we were to predict EGD-related graft loss using our score in the recipients, a total of 24/27 could be forecasted with poorer outcomes. With its high predictive value, the e-GLR score could not only help in the decision to transplant but also match the best donor-recipient pair if there are multiple donors available, help in paired donor exchange, prognosticate the patients, and give the transplant team the opportunity to guard against dire situations. Apart from optimizing the recipient and timing of the transplant, its clinical applicability also lies in avoiding futile transplants and live-donor risks. We have provided the Excel sheet for the e-GLR score calculator in Supplemental File B, http://links.lww.com/AOSO/A258 or https://www.ilbs.in/download/eGLR_Calculator.xlsx.
Previous attempts have been made to develop these models. The Kyushu group from Japan validated the D-MELD score (product of donor age and MELD), originally proposed for deceased donor liver transplantation, to predict early graft survival in LDLT.20 In their study a MELD score ≥20 was significantly associated with poor graft outcomes. However, only 22.8% (81/355) of subjects had a MELD score ≥20, and only 50/355 (14%) patients accounted for D-MELD class C (score ≥900) with a mean MELD score of 24.3. In addition, graft volume did not have a significant influence on graft survival. They defined primary graft dysfunction as delayed functional hyperbilirubinemia of ≥20 mg/dL after postoperative day 7 and persisting for ≥7 consecutive days. Although they did not include patients with ALF in their study, patients with technical (vascular and biliary) complications and acute rejections were not excluded. In the current study, the MELD score was predictive of early graft loss in a homogeneous cohort (Fig. 1) of recipients. However, the mean MELD in our study population was high (24), with 45% of the subjects having MELD ≥25. Apart from the MELD score and donor age, our study also revealed the critical role of the quantity of partial graft in deciding the outcomes of LDLT. Yoshizumi et al21 devised another model based on donor age, graft weight (% of the standard liver weight of a recipient), preoperative MELD score, and shunt (if present) to predict early graft function in LDLT. However, they assessed graft function by considering the SFSS. The study also analyzed a small cohort of low MELD recipients (n = 110), with only 6 patients having graft loss due to SFSS. In contrast, the current study assessed graft dysfunction in a large number of sick recipients with a higher MELD score and revealed the complex interaction of recipient and donor factors, concentrating on early graft loss. Graft dysfunction in adult LDLT has always been examined from the SFSS perspective, with an emphasis on GRWR and portal flow pressure. More recently, it has been shown that the outcome of LDLT is multifactorial. In the current study, the 3 main factors predictive of graft loss were donor age, graft weight, and MELD score in multivariate analysis. It is well recognized that the severity of underlying liver disease is of prime importance when considering patient outcomes. Multiple studies have shown that a high MELD score is predictive of graft loss and poor outcomes in LDLT.22,23 Moreover, we included patients with poor clinical conditions (excluding patients with ALF), which reflects the rate of graft dysfunction. This is in contrast to the existing studies on graft dysfunction in LDLT-dominant centers, where hepatocellular carcinoma was the prime transplant indication3 and the median MELD score was 15–18.6,7
In addition to MELD, the quality and quantity of the graft also play a pivotal role in determining the outcome of LDLT. One of the main determinants of graft quality is donor age. It is well known that advanced donor age is associated with poor outcomes due to poor regeneration and higher vascular resistance (lower compliance) of the hepatic parenchyma in older livers.24 As a result, these grafts are more vulnerable to ischemia-reperfusion injury and shear stress injury caused by portal hyperperfusion, leading to an increased probability of graft dysfunction and graft loss in the early post-transplant period. In our study, donor age had a significant impact on early graft function and outcome. The mean donor age was 30.89 years in the graft survival group. The optimal donor age is between 20 and 30 years. Recently, Kubota et al25 also showed that post-transplant survival was significantly higher in patients receiving livers from donors in their 20s. Because a closed relative is often the donor (spouse, parent, or sibling) in LDLT, the age limit cannot be capped at 30 years. The acceptable upper age limit of the donor can be extended (up to 50 years), depending on the recipient’s disease status on an individual basis. A combination of a young donor with a high MELD score recipient may work equivalently to a low MELD score recipient with an older donor. In this context, the timing of the LT is important. Waiting for long periods with a progressive increase in MELD and LDLT with older donors may have a direct impact on the outcome. If one has the choice of donor, scoring can help predict the outcome. Whether the late presentation or late referral is to be blamed for the high MELD score at the time of transplantation, the use of a smaller partial graft (such as the left lobe) even from a young donor becomes perilous in these patients. Thus, the quantity of partial grafts is also crucial for sicker recipients.
Another important factor that affects early graft function and recipient survival is the ‘total effectively functioning’ liver mass.26 As opposed to the traditional concept of GRWR, our study favors the importance of a minimum absolute graft volume. As the recipient’s weight is affected by ascites, volume overload due to third spacing, sarcopenia, and so on, the metabolic demand of the recipient and portal hypertension are not determined by the recipient’s weight. Hence, the GRWR may not be a true reflector of adequate graft size. Therefore, graft volume might have a greater impact on graft survival than GRWR, particularly in sick recipients. Although the optimal graft size in recipients is obscure, we deduced from the current analysis that the minimum graft volume of 610–640 g can assimilate the afferent portal blood flow without graft injury and sustain the high metabolic demand of sick recipients irrespective of their body weight. Of course, we believe that the outflow of the graft should be optimally reconstructed, ensuring the functionality of every segment of a partial graft and hence the maximal function of the graft volume. Furthermore, unimpeded hepatic venous outflow augments portal flow hemodynamics and equilibrates portal flow and pressure. Moon et al27 also highlighted the significance of adequate anterior sector drainage of the right lobe graft with donor age. Graft outcome and early survival were also not dependent on the GRWR in their study. Caution was advised when using small-sized grafts from elderly donors. Thus, a sufficient ‘functional liver mass’ of good quality (which in turn is dependent on donor age) is key to early graft function and recipient survival.
Few previous studies have signified the higher portal flow/pressure with poorer graft survival.28 However, the necessity of portal inflow modulation cannot be ascertained, as concord on the optimal portal venous flow that does not cause sheer injury to the graft is lacking. In addition, after implantation, the positive correlation between portal pressure and portal inflow to the graft cannot be maintained, as it is demonstrable before recipient hepatectomy.29 Despite these facts, in the current study, we did not measure portal pressure intraoperatively, as we believe portal flow is a more important factor than pressure. Portal pressure directly correlates with portal flow and liver parenchymal resistance. A cirrhotic or fibrotic liver offers more resistance to the flow, but portosystemic shunting may normalize the pressure despite the high resistance. In contrast, in LDLT, a transplanted partial graft with normal parenchyma usually has high portal pressure despite normal resistance due to high portal inflow (relative to a perfused smaller liver mass). Thus, using a larger graft or empirical decompression of portal hypertension by inflow modulation will not dilute or nullify the adverse impact of advanced donor age or the severity of the recipient disease. Though we have not performed portal flow modulation in any of the patients in the current study, the univariate and multivariate analyses (Table 3) have not revealed portal venous flow as an independent risk factor for EGD-related graft loss, implying no correlation between the portal inflow and the graft recovery from graft dysfunction. We believe that a good outflow is essential to evade SFSS, and this plays a key role in circumventing graft failure, even in grafts from elderly donors. In Asan’s experience, the graft could sustain a portal inflow of up to 450 mL/min/100 g GW with a perfect outflow.30 Further, it has been laid out that the high portal inflow may actually contribute to graft regeneration and hypertrophy.31 We also ligated large portosystemic shunts to avoid portal flow steal. Currently, there is no definitive index along with a cutoff value, if any, to assist in decision-making for portal inflow modulation.32 Even if some definite portal hemodynamic parameter is assigned along with a limit to dictate the decision to decompress the portal inflow, it may not be feasible to generalize it as every LT center has its own donor selection criteria, recipient indications, and surgical expertise. A recent study from Hong Kong with 587 LDLTs demonstrated that with precise graft weight estimation, appropriate recipient and donor selection, meticulous venous outflow construction, and portal inflow modification, whenever required, LDLTs were feasible even with GRWRs as low as 0.6%.33 They had a very low (4.8%) incidence of SFSS, despite the use of small-for-size grafts (GRWR<0.8) in one-third of the recipients. Merely 5% of patients needed modulation of portal inflow. Collectively, it may be deduced that portal inflow modulation is not imperative in most of the cases. Improvised donor and recipient matching, good graft design (balancing the donor risk and recipient benefit), and impeccable surgical technique are the true armaments to minimize EGD and EGD-related early graft loss.
The retrospective design and single-center experience are limitations of the current study. However, we analyzed our data using 2 random cohorts to dilute the disadvantages of the retrospective design and the impact of unknown factors. Importantly, the results of the validation cohort mirrored those of the derivation cohort, empowering the prognostic value of our model in estimating early graft loss due to graft dysfunction. Chronological bias might have seeped into this study because of the long period over which the study subjects were accrued. However, the randomization of the 2 study cohorts nullified this bias. Experiences from other tertiary care centers are needed to rationalize and strengthen our findings.
In conclusion, early graft dysfunction is a common and serious condition following LDLT and can have a significant impact on graft survival and short-term outcomes. A prognostic score is proposed based on the donor age, graft weight, and the preoperative MELD score of the recipient, which can effectively predict early graft loss preoperatively. This score empowers donor-recipient matching, evaluation, and prognostication simply and reliably in adult LDLT.
Supplementary Material
Footnotes
Published online 9 October 2023
Viniyendra Pamecha and Nilesh Sadashiv Patil contributed equally to this work.
The study was carried out in accordance with the Declaration of Helsinki principles (2000) as well as the Declaration of Istanbul (2008) for medical research involving human subjects. The study was approved from the Institute ethics committee (no: IEC/2020/79/MA05).
Disclosure: The authors declare that they have nothing to disclose.
All relevant data are within the paper and its Supporting Information files.
All authors have read the final version of the article and have provided consent for the article to be published in “Annals of Surgery”.
No animal research was done.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Dahm F, Georgiev P, Clavien PA. Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant. 2005;5:2605–2610. [DOI] [PubMed] [Google Scholar]
- 2.Kiuchi T, Tanaka K, Ito T, et al. Small-for-size graft in living donor liver transplantation: how far should we go? Liver Transpl. 2003;9:S29–S35. [DOI] [PubMed] [Google Scholar]
- 3.Ikegami T, Shirabe K, Yoshizumi T, et al. Primary graft dysfunction after living donor liver transplantation is characterized by delayed functional hyperbilirubinemia. Am J Transplant. 2012;12:1886–1897. [DOI] [PubMed] [Google Scholar]
- 4.Mathew G, Agha R, Albrecht J, et al. STROCSS 2021: strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg. 2021;37:100430. [DOI] [PubMed] [Google Scholar]
- 5.Olthoff KM, Emond JC, Shearon TH, et al. Liver regeneration after living donor transplantation: adult-to-adult living donor liver transplantation cohort study. Liver Transpl. 2015;21:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pomposelli JJ, Goodrich NP, Emond JC, et al. Patterns of Early Allograft Dysfunction (EAD) in adult live donor liver transplantation: the A2ALL experience. Transplantation. 2016;100:1490–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okamura Y, Yagi S, Sato T, et al. Coexistence of bilirubin ≥ 10 mg/dL and prothrombin time-international normalized ratio≥ 1.6 on day 7: a strong predictor of early graft loss after living donor liver transplantation. Transplantation. 2018;102:440–447. [DOI] [PubMed] [Google Scholar]
- 8.Soejima Y, Taketomi A, Yoshizumi T, et al. Feasibility of left lobe living donor liver transplantation between adults: an 8-year, single-center experience of 107 cases. Am J Transplant. 2006;6:1004–1011. [DOI] [PubMed] [Google Scholar]
- 9.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volk ML, Hernandez JC, Lok AS, et al. Modified Charlson Comorbidity Index for predicting survival after liver transplantation. Liver Transpl. 2007;13:1515–1520. [DOI] [PubMed] [Google Scholar]
- 11.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. [DOI] [PubMed] [Google Scholar]
- 12.Lemeshow S, Hosmer DW. A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol. 1982;115:92–106. [DOI] [PubMed] [Google Scholar]
- 13.Feng S. Living donor liver transplantation in high model for end-stage liver disease score patients. Liver Transpl. 2017;23:S9–S21. [DOI] [PubMed] [Google Scholar]
- 14.Hao C, Junjie X, Baiyong S, et al. Initial poor graft dysfunction and primary graft non-function after orthotopic liver transplantation. Liver biopsy in mod med. 2011;12:183−207. [Google Scholar]
- 15.Chae MS, Koo JM, Park CS. Predictive role of intraoperative serum brain natriuretic peptide for early allograft dysfunction in living donor liver transplantation. Ann Transplant. 2016;21:538–549. [DOI] [PubMed] [Google Scholar]
- 16.Yadav SK, Saraf N, Saigal S, et al. High MELD score does not adversely affect outcome of living donor liver transplantation: experience in 1000 recipients. Clin Transplant. 2017;31:e13006. [DOI] [PubMed] [Google Scholar]
- 17.Yang J, Wang HQ, Yang JY, et al. Role of the postoperative cholesterol in early allograft dysfunction and survival after living donor liver transplantation. Hepatobiliary Pancreat Dis Int. 2017;16:610–616. [DOI] [PubMed] [Google Scholar]
- 18.Tsai HI, Lo CJ, Lee CW, et al. A panel of biomarkers in the prediction for early allograft dysfunction and mortality after living donor liver transplantation. Am J Transl Res. 2021;13:372–382. [PMC free article] [PubMed] [Google Scholar]
- 19.Singh A, Singhal S, Venuthurimilli A, et al. HPi: a novel parameter to predict graft-related outcome in adult living donor liver transplant. Transplantation. 2022;106:767–780. [DOI] [PubMed] [Google Scholar]
- 20.Ikegami T, Imai D, Wang H, et al. D-MELD as a predictor of early graft mortality in adult-to-adult living-donor liver transplantation. Transplantation. 2014;97:457–462. [DOI] [PubMed] [Google Scholar]
- 21.Yoshizumi T, Taketomi A, Uchiyama H, et al. Graft size, donor age, and patient status are the indicators of early graft function after living donor liver transplantation. Liver Transpl. 2008;14:1007–1013. [DOI] [PubMed] [Google Scholar]
- 22.Morioka D, Egawa H, Kasahara M, et al. Outcomes of adult-to-adult living donor liver transplantation: a single institution’s experience with 335 consecutive cases. Ann Surg. 2007;245:315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaido T, Egawa H, Tsuji H, et al. In-hospital mortality in adult recipients of living donor liver transplantation: experience of 576 consecutive cases at a single center. Liver Transpl. 2009;15:1420–1425. [DOI] [PubMed] [Google Scholar]
- 24.Lué A, Solanas E, Baptista P, et al. How important is donor age in liver transplantation? World J Gastroenterol. 2016;22:4966–4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kubota T, Hata K, Sozu T, et al. Impact of donor age on recipient survival in adult-to-adult living-donor liver transplantation. Ann Surg. 2018;267:1126–1133. [DOI] [PubMed] [Google Scholar]
- 26.Soejima Y, Shirabe K, Taketomi A, et al. Left lobe living donor liver transplantation in adults. Am J Transplant. 2012;12:1877–1885. [DOI] [PubMed] [Google Scholar]
- 27.Moon JI, Kwon CH, Joh JW, et al. Safety of small-for-size grafts in adult-to-adult living donor liver transplantation using the right lobe. Liver Transpl. 2010;16:864–869. [DOI] [PubMed] [Google Scholar]
- 28.Ito T, Kiuchi T, Yamamoto H, et al. Changes in portal venous pressure in the early phase after living donor liver transplantation: pathogenesis and clinical implications. Transplantation. 2003;75:1313–1317. [DOI] [PubMed] [Google Scholar]
- 29.Chan SC, Lo CM, Ng KK, et al. Portal inflow and pressure changes in right liver living donor liver transplantation including the middle hepatic vein. Liver Transpl. 2011;17:115–121. [DOI] [PubMed] [Google Scholar]
- 30.Lee SG. A complete treatment of adult living donor liver transplantation: a review of surgical technique and current challenges to expand indication of patients. Am J Transplant. 2015;15:17–38. [DOI] [PubMed] [Google Scholar]
- 31.Byun SH, Yang HS, Kim JH. Liver graft hyperperfusion in the early postoperative period promotes hepatic regeneration 2 weeks after living donor liver transplantation: a prospective observational cohort study. Medicine (Baltimore). 2016;95:e5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rammohan A, Rela M, Kim DS, et al. ; ERAS4OLT.org Working Group. Does modification of portal pressure and flow enhance recovery of the recipient after living donor liver transplantation? A systematic review of literature and expert panel recommendations. Clin Transplant. 2022;36:e14657. [DOI] [PubMed] [Google Scholar]
- 33.Wong TC, Fung JY, Cui T, et al. The risk of going small: lowering GRWR and overcoming small for-size syndrome in adult living donor liver transplantation. Ann Surg. 2021;274:e1260–e1268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.