Abstract
Dietary management is a crucial component of non-pharmacological treatment for hyperuricemia, yet there is a paucity of research on the impact of dietary habits on the survival outcomes of individuals with hyperuricemia. The objective of this study is to examine the association between dietary inflammatory index (DII) and the all-cause and cardiovascular disease (CVD) mortality in individuals with hyperuricemia. This study included 3093 adult participants from National Health and Nutrition Examination Survey (NHANES) 2001 to 2010. Participants were categorized into 4 groups based on quartiles of DII to demonstrate data characteristics, with sample weights considered. The relationship between DII and the risk of hyperuricemia was examined using multivariable logistic regression models. Kaplan–Meier models and Cox proportional hazards models were employed to assess the relationship between DII levels and the all-cause mortality in individuals with hyperuricemia, with the non-linear relationship tested using restricted cubic splines (RCS). Competing risk models were employed to investigate the association between DII levels and the CVD mortality in individuals diagnosed with hyperuricemia. Subgroup and sensitivity analysis were performed to confirm the robustness and reliability of the findings. Among the participants, 47.95% were aged over 60 years. A positive association observed between the highest quartile of DII level and the incidence of hyperuricemia (OR: 1.34, CI [1.13, 1.57]). Elevated DII levels were correlated with increased all-cause mortality (P value < .001) and CVD mortality (P value < .001) in participants. In comparison to the lowest quartile, the highest quartile of DII exhibited a 31% rise in all-cause mortality (HR: 1.31, CI [1.01, 1.68]) and a 50% increase in CVD mortality (HR: 1.50, CI [1.00, 2.26]). No indication of a nonlinear association between DII levels and all-cause mortality (p-non-linear = .43). These findings indicate a positive correlation between the pro-inflammatory diet and the incidence of hyperuricemia. Additionally, a pro-inflammatory diet may elevate the all-cause and CVD mortality in individuals with hyperuricemia.
Keywords: cohort study, dietary inflammatory index, hyperuricemia, mortality, NHANES
1. Introduction
Hyperuricemia is a metabolic disorder resulting from prolonged dysfunction in purine metabolism, excessive purine intake, or impaired elimination of uric acid. In the nationally representative sample survey conducted in 2015 to 2016, the occurrence rate of hyperuricemia among adult individuals in the United States was recorded at 20%, and this prevalence remained stable over a period of 10 years.[1] In China, data from 2018 to 2019 indicated an overall prevalence of hyperuricemia among the adult population at 14%, with a significantly higher prevalence among males (24.4%) compared to females (3.6%). Furthermore, there has been an upward trend in the overall prevalence of hyperuricemia within a span of 5 years in China.[2] Chronic hyperuricemia leads to the deposition of urate crystals in joints and surrounding soft tissues, resulting in recurrent episodes of acute joint and soft tissue inflammation, commonly known as gout.[3] Recent epidemiological studies have also found that cumulative exposure to elevated blood uric acid levels may be associated with elevated morbidity of non-articular conditions such as stroke, cardiovascular events, chronic kidney disease, liver disease and metabolic syndrome.[4–8] Furthermore, it has been observed that there exists a positive correlation between hyperuricemia and elevated increased all-cause mortality in the general population, as well as in specific populations such as individuals with diabetes, chronic heart failure, chronic kidney disease, and metabolic dysfunction-associated fatty liver disease (MAFLD).[4,5,9–11] Therefore, the threat posed by hyperuricemia to public health is highly intricate and may potentially surpass people conventional estimations. In studies conducted, a positive correlation has been observed between white blood cell count and the prevalence of hyperuricemia in individuals without underlying diseases. Additionally, individuals with hyperuricemia have shown significant elevations in serum levels of C-reactive protein (CRP), tumor necrosis factor (TNF), interleukin-6 (IL-6), and other inflammatory mediators.[12] In recent years, mechanistic studies have revealed that the NF-κB pathway is a key pathway involved in the induction of inflammation by uric acid crystals.[13] This implies that inflammation could potentially exert a substantial influence on the pathological process of hyperuricemia.
Dietary management is a crucial component of non-pharmacological treatment for hyperuricemia, and a low-purine diet is highly recommended by clinicians as it is believed to improve hyperuricemia and prevent gout attacks.[14] Furthermore, research has found that anthocyanins, probiotics, and other bioactive compounds have potential in ameliorating hyperuricemia.[15,16] Although these dietary components are believed to have anti-inflammatory effects, the overall inflammatory potential of the diet and its relationship with the physical health of individuals with hyperuricemia remains unknown. The DII is a scoring tool that quantifies the overall inflammatory potential of an individual diet. It was first proposed by Shivappa et al in 2014.[17] By reviewing published literature, they identified the impact of overall dietary components on 6 inflammatory markers (IL-1β, IL-4, IL-6, IL-10, TNF-α, and CRP) and calculated pro-inflammatory and anti-inflammatory scores for each food parameter based on study characteristics, resulting in an overall inflammation effect score specific to each food parameter. Lower scores indicate a stronger anti-inflammatory capacity of the diet, while higher scores indicate a stronger pro-inflammatory capacity. In recent years, the DII has been extensively employed in clinical research to explore the association between dietary inflammatory potential and disease.[18–20] However, to date, no studies have revealed the relationship between the DII and all-cause and CVD mortality in individuals with hyperuricemia.
The present study employed data from the NHANES from 2001 to 2010. The primary objective was to examine the correlation between DII and all-cause and CVD mortality in individuals with hyperuricemia. The findings of this study aim to provide new insights and evidence for improving the survival outcomes of individuals with hyperuricemia through dietary management strategies.
2. Method
2.1. Study population and data source
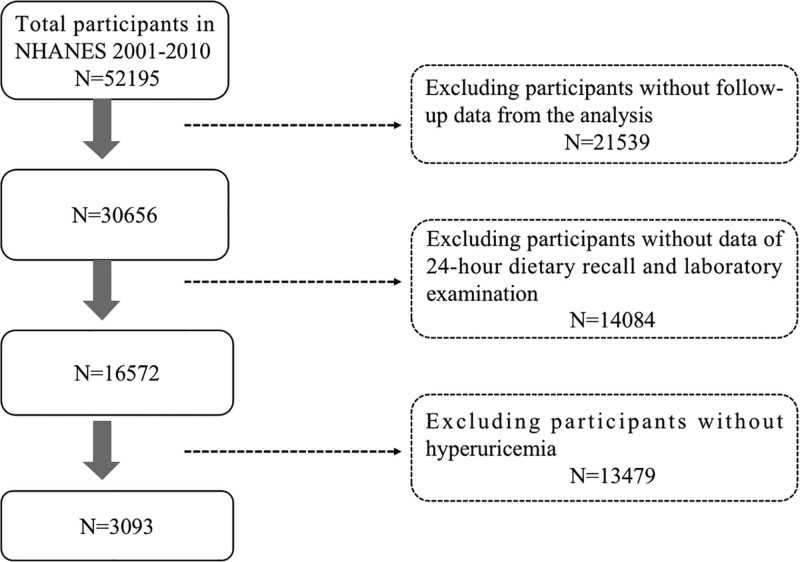
This cohort study included 3093 adult participants aged ≥ 20 years from the NHANES conducted between 2001 and 2010. NHANES is a national program designed to investigate the health and nutritional condition of adults and children residing in the United States. It collects health information from community residents across various regions in the United States through questionnaire surveys, physical examinations, and laboratory tests. Exclusion criteria for the study participants included missing survival data, missing blood uric acid results, missing 24-hour dietary recall records, and missing other covariates (serum creatinine, triglycerides, cholesterol, sociodemographic information, medical history). Approval for this study was granted by the Ethics Committee of the National Center for Health Statistics, and all participants provided written consent after being fully informed. The schematic representation of the study design can be observed in Figure 1.
Figure 1.
Flow chart of the screening process for the selection of eligible participants. NHANES = National Health and Nutrition Examination Survey.
2.2. Dietary inflammatory index
The DII serves as a scoring index that quantifies the overall inflammatory capacity of individual diet. Shivappa et al conducted a comprehensive review of published literature to ascertain the impact of whole foods and dietary constituents on 6 inflammatory markers (IL-1β, IL-4, IL-6, IL-10, TNF-α, CRP). To calculate the overall inflammatory potential of an individual diet, Shivappa et al assigned pro-inflammatory and anti-inflammatory scores to each food parameter based on the characteristics of the studies reviewed. These scores were then weighted to obtain an overall inflammatory effect score for each specific food parameter. A lower DII score indicates a diet with a stronger capacity to reduce inflammation, while a higher DII score indicates a diet with a stronger capacity to promote inflammation.[17] DII can evaluate the inflammatory effects of 45 food components, but previous studies have shown that using <30 food components does not affect the evaluation ability of DII.[18] The DII was computed in this study utilizing data from 24-hour dietary recall, and a total of 27 nutrients were employed in the computation of the DII score containing alcohol, vitamins B12/B6, β-carotene, caffeine, carbohydrates, cholesterol, total fat, fiber, folate, iron, magnesium, zinc, selenium, mono unsaturated fat acid (MUFA), niacin, n-3 fatty acids, n-6 fatty acids, protein, polyunsaturated fatty acid (PUFA), riboflavin, saturated fat, thiamin, and vitamins A/C/D/E. The food parameters used to calculate DII are shown in Supplementary Table 1, http://links.lww.com/MD/K876.
2.3. Hyperuricemia
The timed endpoint method was used to measure plasma uric acid concentration. Uric acid is oxidized by uricase, producing allatoin and hydrogen peroxide. Hydrogen peroxide reacts with 4-aminoantipyrine (4-AAP) and 3,5-dichloro-2-hydroxybenzene sulfonic acid (DCHBS) to generate a colored product in a reaction catalyzed by peroxidase. The system monitors the absorbance changes at 520 nanometers at fixed time intervals. The changes in absorbance are directly proportional to the concentration of uric acid in the sample. The Beckman Synchron LX20 instrument was used for measurements from 2001 to 2007, and it was replaced by the Beckman Coulter UniCel DxC800 instrument after 2008. The detailed information regarding the detection can be found at https://www.cdc.gov/nchs/nhanes/index.htm. Hyperuricemia is defined as serum uric acid (SUA) levels ≥ 420 μmol/L (7mg/dl) for males and ≥ 360 μmol/L (6 mg/dl) for females.[21]
2.4. Main outcome
The primary outcomes were all-cause and CVD mortality. The death dates and causes of death for study participants were validated by linking with the National Death Index (NDI) public dataset (https://www.cdc.gov/nchs/data-linkage/mortality-public.htm). The International Classification of Diseases, 10th Revision (ICD-10) codes were used to classify the causes of death. CVD deaths were defined by ICD-10 codes I00-I09, I11, I13, I20-I51, or I60-I69.
2.5. Covariates
Information regarding demographic characteristics, lifestyle factors, and health status was collected. Sociodemographic characteristics included age, gender, race/ethnicity (non-Hispanic White, Mexican American, non-Hispanic Black, other Hispanic, other race), educational attainment (<9th grade, 9–11th grade, high school graduate or equivalent, some college or associate degree, college graduate or above), and poverty income ratio (PIR). Lifestyle information included smoking status (never smoker, former smoker, current smoker) and alcohol consumption (nondrinker, drinker). Health information included body mass index (BMI), history of diabetes (hemoglobin A1C concentration ≥ 6.5%, fasting blood glucose ≥ 126 mg/dL,[22] or self-reported diabetes), history of hypertension (average of 3 consecutive measurements with systolic blood pressure ≥ 130 mm Hg or diastolic blood pressure ≥ 80 mm Hg,[23] self-reported history of hypertension, or use of antihypertensive medication), estimated glomerular filtration rate (eGFR), serum total cholesterol, and serum triglycerides.
2.6. Statistical analysis
To show the characteristics of participants with different dietary patterns, we divided all participants into 4 groups based on quartiles of DII. Continuous variables that followed a normal distribution were reported as means and standard deviations, whereas categorical variables were presented as frequencies and proportions. The significance of differences between means of continuous variables and proportions of categorical variables was determined using analysis of variance (ANOVA) and Pearson chi-square test, respectively. This analysis utilized sample weights to ensure the representativeness of the results for the U.S. community population. First, to investigate the potential link between DII and the risk of hyperuricemia, a multivariable logistic regression analysis was utilized. We assessed the differences in all-cause and CVD mortality among individuals with hyperuricemia at different levels of DII using the Kaplan–Meier model and the competing risk model respectively. We also employed the Cox proportional hazards model and the competing risk model to investigate the impact of different DII levels on the all-cause and CVD mortality in individuals with hyperuricemia respectively. Model 1 involved a univariate analysis of DII levels, while Model 2 adjusted for age, gender, race, education level, and PIR. Model 3 further included smoking, alcohol consumption, hypertension, diabetes, eGFR, serum cholesterol, and serum triglycerides as covariates on top of Model 2. To assess the potential non-linear association between DII and the all-cause mortality among individuals with hyperuricemia, RCS were utilized for the continuous DII values. Three knots were placed at the 5th, 50th, and 95th percentiles.
2.7. Subgroup analyses and sensitivity analyses
To test the stability of results, we conducted subgroup and sensitivity analyses. We performed subgroup analyses on the logistic regression model and the Cox proportional hazards model to determine the influence of demographic characteristics and comorbidities on the outcomes. To mitigate the possibility of reverse causality, Cox proportional hazards analysis was conducted, excluding participants who experienced mortality within a 24-month follow-up period. High uric acid participants who were initially excluded due to incomplete data were re-included, and the impact of DII on the all-cause mortality was analyzed using the Kaplan–Meier model. Additionally, we also conducted analyses in the non-hyperuricemia population. The statistical analyses for this study were performed using R version 4.2.2, and all statistical tests were conducted with a 2-sided approach. A significance level of 0.05 was employed to ascertain statistical significance.
3. Result
3.1. Baseline characteristics of the participants
Among the initial 52,195 participants, a total of 3093 participants met the inclusion criteria, as shown in Figure 1. Table 1 presents the characteristics of the participants. Participants aged over 60 accounted for 47.95%, and females accounted for 44.1%. 76.2% were classified as obese, and 54.35% were non-Hispanic white. Participants with higher DII levels had a higher proportion of low education levels and lower PIR. They also had lower alcohol consumption rates, higher incidence rates of diabetes and hypertension, lower levels of eGFR, and lower levels of serum triglycerides.
Table 1.
Characteristics of participants aged 20 years and older from the National Health and Nutrition Examination Survey (2001 to 2010).
| Characteristics | Dietary inflammatory index | P value | ||||
|---|---|---|---|---|---|---|
| Total (N = 3093) | Q1 (N = 672) | Q2 (N = 748) | Q3 (N = 794) | Q4 (N = 879) | ||
| Gender, n(%) | <.001 | |||||
| Female | 1364 (44.1) | 208 (15.89) | 280 (22.05) | 362 (26.32) | 514 (35.73) | |
| Male | 1729 (55.9) | 464 (30.21) | 468 (28.96) | 432 (23.48) | 365 (17.35) | |
| Age, n(%) | <.003 | |||||
| 20–40 years old | 728 (23.54) | 173 (26.75) | 180 (25.24) | 207 (27.37) | 168 (20.65) | |
| 40–60 years old | 882 (28.52) | 218 (26.21) | 221 (28.06) | 219 (22.84) | 224 (22.90) | |
| 60–80 years old | 1483 (47.95) | 281 (20.03) | 347 (24.60) | 368 (24.46) | 487 (30.91) | |
| Race, n(%) | <.002 | |||||
| Non-Hispanic White | 1681 (54.35) | 388 (24.97) | 435 (27.16) | 417 (23.71) | 441 (24.16) | |
| Mexican American | 397 (12.84) | 93 (29.21) | 98 (25.83) | 106 (26.79) | 100 (18.18) | |
| Non-Hispanic Black | 718 (23.21) | 115 (15.88) | 142 (19.12) | 204 (29.06) | 257 (35.95) | |
| Other Hispanic | 159 (5.14) | 33 (21.32) | 38 (29.08) | 36 (22.97) | 52 (26.63) | |
| Other Race | 138 (4.46) | 43 (27.86) | 35 (24.61) | 31 (27.03) | 29 (20.50) | |
| Education status, n(%) | <.001 | |||||
| <9th Grade | 360 (11.64) | 59 (17.06) | 73 (18.23) | 101 (30.25) | 127 (34.46) | |
| 9–11th Grade | 487 (15.75) | 65 (15.61) | 104 (20.70) | 134 (27.42) | 184 (36.27) | |
| High School Grad | 807 (26.09) | 175 (23.51) | 191 (25.61) | 201 (23.02) | 240 (27.86) | |
| College | 901 (29.13) | 199 (22.81) | 230 (26.97) | 243 (27.86) | 229 (22.36) | |
| College Graduate or above | 538 (17.39) | 174 (33.51) | 150 (30.25) | 115 (19.31) | 99 (16.93) | |
| PIR, mean (SD) | 2.98 (0.05) | 3.35 (0.07) | 3.15 (0.09) | 2.84 (0.09) | 2.58 (0.10) | <.001 |
| BMI, n(%) | <.01 | |||||
| Underweight (<18.5) | 14 (0.45) | 3 (10.02) | 2 (13.87) | 4 (36.43) | 5 (39.68) | |
| Normal (18.5–24) | 277 (8.96) | 62 (25.01) | 78 (31.05) | 64 (25.75) | 73 (18.19) | |
| Overweight (24–27) | 445 (14.39) | 116 (31.27) | 112 (26.99) | 110 (24.79) | 107 (16.95) | |
| Obesity (>27) | 2357 (76.2) | 491 (22.78) | 556 (25.35) | 616 (24.47) | 694 (27.40) | |
| Smoking, n(%) | =.05 | |||||
| Never Smoker | 1493 (48.27) | 328 (23.93) | 368 (26.46) | 366 (24.17) | 431 (25.45) | |
| Former Smoker | 1030 (33.3) | 241 (27.20) | 261 (27.68) | 256 (22.94) | 272 (22.17) | |
| Current Smoker | 570 (18.43) | 103 (19.76) | 119 (22.29) | 172 (28.92) | 176 (29.03) | |
| Drinking, n(%) | <.001 | |||||
| No | 928 (30) | 151 (17.69) | 198 (22.48) | 237 (24.42) | 342 (35.41) | |
| Yes | 2165 (70) | 521 (26.52) | 550 (27.34) | 557 (24.77) | 537 (21.37) | |
| Diabetes, n(%) | <.01 | |||||
| No | 2437 (78.79) | 559 (25.39) | 598 (26.60) | 617 (24.18) | 663 (23.82) | |
| Yes | 656 (21.21) | 113 (17.97) | 150 (23.21) | 177 (27.26) | 216 (31.56) | |
| Hypertension, n(%) | <.04 | |||||
| Non-hypertension | 919 (29.71) | 224 (25.88) | 236 (26.58) | 235 (26.49) | 224 (21.05) | |
| Hypertension | 2174 (70.29) | 448 (23.33) | 512 (25.79) | 559 (23.74) | 655 (27.13) | |
| DII, mean (SD) | 1.61 (0.06) | −0.91 (0.05) | 1.17 (0.02) | 2.45 (0.02) | 3.65 (0.02) | <.001 |
| eGFR, mean (SD) | 83.16 (0.67) | 85.79 (1.10) | 83.10 (1.13) | 82.97 (1.43) | 80.88 (1.13) | <.01 |
| Uricacid, mean (SD) | 444.73 (1.44) | 448.85 (2.20) | 445.07 (2.36) | 446.35 (3.25) | 438.78 (3.02) | =.08 |
| Cholesterol, mean (SD) | 5.34 (0.04) | 5.24 (0.07) | 5.35 (0.07) | 5.40 (0.06) | 5.35 (0.05) | =.29 |
| Triglyceride, mean (SD) | 2.16 (0.04) | 2.11 (0.07) | 2.25 (0.09) | 2.29 (0.10) | 1.98 (0.05) | <.01 |
Normally distributed continuous variables were displayed as means and standard deviations (SDs), categorical variables were displayed as proportions. Analysis of Variance (ANOVA) were used for comparison of Continuous variables and Pearson χ2 test were used for comparison of categorical variables. The mobile examination center sample weights were accounted and the results represented the US civilian resident population. Quartiles of DII showed as Q1 (−4.11 to 0.66), Q2 (0.66–2.08), Q3 (2.08–3.17), Q4 (3.17–5.24).
BMI = body mass index, DII = dietary inflammatory index, eGFR = estimated glomerular filtration rate, PIR = poverty index rate, SD = standard deviation.
3.2. DII and hyperuricemia
We developed 3 logistic regression models while considering weights (The population characteristics for the logistic regression analysis were presented in Supplementary Table 2, http://links.lww.com/MD/K877). Model 1 conducted a univariate analysis of DII and hyperuricemia. Model 2 included gender, age, race, education level, and PIR in addition to Model 1. Model 3 further incorporated BMI, smoking history, alcohol consumption history, hypertension, diabetes, eGFR, serum cholesterol, and serum triglycerides on top of Model 2. In Model 1, DII levels at Q2, Q3, and Q4 were all positively associated with the risk of hyperuricemia. After adjusting for covariates, this association persisted in Model 3 specifically for DII levels at Q4 (OR: 1.34, CI [1.13, 1.57], P value < .001) (Table 2).
Table 2.
The relationship between dietary inflammatory index and risk of hyperuricemia among participants aged 20 years and older from the National Health and Nutrition Examination Survey (2001 to 2010).
| DII | range | OR (95% CI), P value | |||||
|---|---|---|---|---|---|---|---|
| Model 1a | Model 2b | Model 3c | |||||
| Q1 | −5.28 to 0.37 | Ref | Ref | Ref | |||
| Q2 | 0.37 to 1.84 | 1.21 (1.04,1.42) | <.02 | 1.22 (1.02,1.46) | <.03 | 1.12 (0.92,1.35) | =.24 |
| Q3 | 1.84 to 3.00 | 1.24 (1.08,1.43) | <.002 | 1.31 (1.13,1.53) | <.001 | 1.19 (0.99,1.43) | <.07 |
| Q4 | 3.00 to 5.42 | 1.38 (1.19,1.61) | <.001 | 1.49 (1.27,1.74) | <.001 | 1.34 (1.13,1.57) | <.001 |
Sampling weights were considered in logistic regression analyses to obtain nationally representative estimates.
DII = dietary inflammatory index, OR = odd rate, Q = quartile; ref = reference.
Model 1: Unadjusted.
Model 2: Adjusted for gender, age, race, education level, and PIR.
Model 3: Model 2 + adjusted for smoking history, alcohol consumption history, hypertension, diabetes, eGFR, serum cholesterol, and serum triglycerides.
3.3. DII and mortality
Throughout the follow-up period, a total of 892 deaths were recorded, out of which 254 were attributed to CVD causes. The median duration of follow-up was 136 months. The
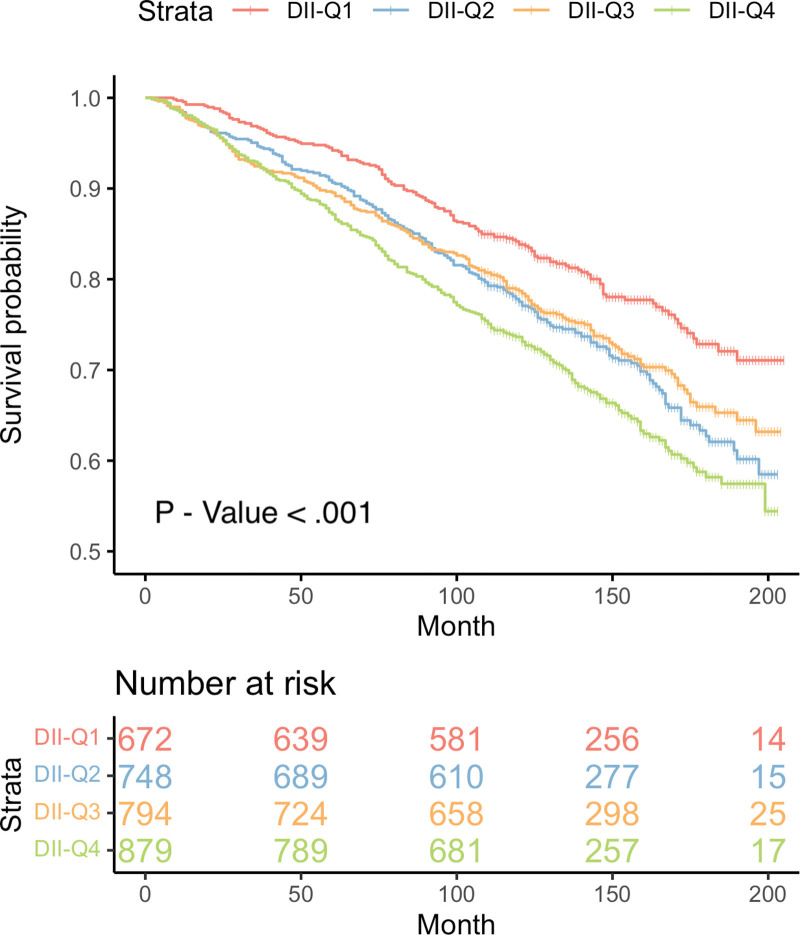
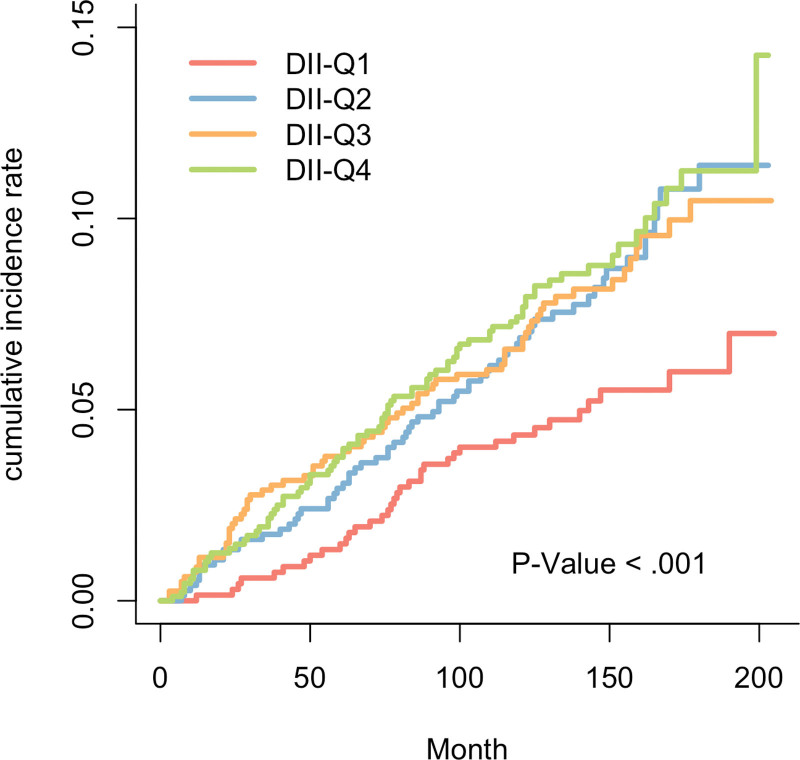
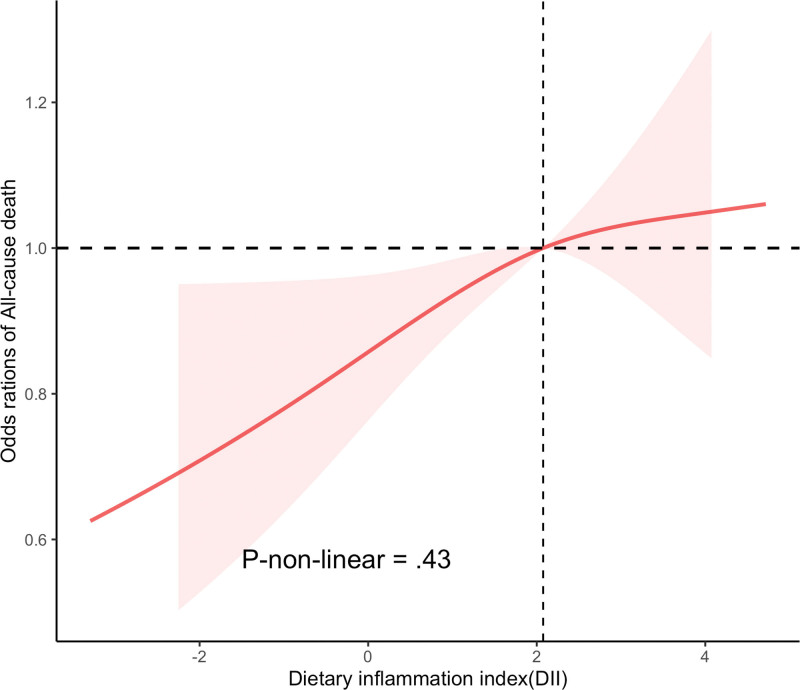
Kaplan–Meier curve demonstrated a significantly higher all-cause mortality in hyperuricemia participants with higher DII levels (P value < .001) (Fig. 2). Similar results were observed in the competing risk model for DII and CVD mortality (P value < .001) (Fig. 3). After further adjusting for covariates, the Cox proportional hazards model revealed that compared to the Q1 level, the Q4 level of DII increased the all-cause mortality by 31% in individuals with hyperuricemia (HR: 1.31, CI [1.01, 1.68], P value < .04) (Table 3). Similarly, in the adjusted competing risk model, the Q3 level increased the CVD mortality by 59% (HR: 1.59, CI [1.06, 2.40], P value < .026), and the Q4 level increased it by 50% (HR: 1.50, CI [1.00, 2.26], P value < .048). Additionally, in the Cox proportional hazards model where DII is a continuous variable, DII is positively correlated with all-cause mortality (Table 4). Finally, the RCS results showed no evidence of a non-linear relationship between DII levels and the all-cause mortality in individuals with hyperuricemia (p-non-linear = .43) (Fig. 4).
Figure 2.
The cumulative incidence of all-cause death in the 4 groups of DII during the follow-up period. DII = dietary inflammatory index.
Figure 3.
The cumulative incidence of cardiovascular-cause death in the 4 groups of DII during the follow-up period. DII = dietary inflammatory index.
Table 3.
All-cause mortality according to quartiles of dietary inflammatory index among participants with hyperuricemia aged 20 years and older from the National Health and Nutrition Examination Survey (2001 to 2010).
| DII | range | Event/total | HR (95% CI), P value | |||||
|---|---|---|---|---|---|---|---|---|
| Model 1a | Model 2b | Model 3c | ||||||
| Q1 | −4.11 to 0.66 | 146/672 | Ref | Ref | Ref | |||
| Q2 | 0.66 to 2.08 | 225/748 | 1.56 (1.17,2.07) | <.002 | 1.28 (0.95,1.73) | 0.11 | 1.32 (0.98,1.79) | 0.072 |
| Q3 | 2.08 to 3.17 | 223/794 | 1.53 (1.19,1.97) | <.001 | 1.21 (0.95,1.56) | 0.13 | 1.21 (0.94,1.56) | 0.15 |
| Q4 | 3.17 to 5.24 | 298/879 | 1.99 (1.55,2.55) | <.001 | 1.35 (1.05,1.73) | <.018 | 1.31 (1.01,1.68) | <.04 |
| Continuous | −5.28 to 5.42 | 892/3093 | 1.14 (1.09,1.19) | <.001 | 1.06 (1.01,1.11) | <.001 | 1.05 (1.00,1.11) | <.03 |
Sampling weights were considered in Cox proportional hazard model to obtain nationally representative estimates.
DII = dietary inflammatory index, HR = hazard rate, Q = quartile, ref = reference.
Model 1: Unadjusted.
Model 2: Adjusted for gender, age, race, education level, and PIR.
Model 3: Model 2 + adjusted for smoking history, alcohol consumption history, hypertension, diabetes, eGFR, serum cholesterol, and serum triglycerides.
Table 4.
Cardiovascular disease mortality according to quartiles of dietary inflammatory index among participants with hyperuricemia aged 20 years and older from the National Health and Nutrition Examination Survey (2001 to 2010).
| DII | range | Event/total | HR (95% CI), P value | |||||
|---|---|---|---|---|---|---|---|---|
| Modela | Model 2b | Model 3c | ||||||
| Q1 | −4.11 to 0.66 | 36/672 | Ref | Ref | Ref | |||
| Q2 | 0.66 to 2.08 | 67/748 | 1.69 (1.13,2.54) | <.011 | 1.50 (1.00,2.27) | <.049 | 1.50 (1.00,2.25) | =.05 |
| Q3 | 2.08 to 3.17 | 69/794 | 1.65 (1.10,2.47) | <.014 | 1.54 (1.02,2.32) | <.042 | 1.59 (1.06,2.40) | <.026 |
| Q4 | 3.17 to 5.24 | 82/879 | 1.81 (1.22,2.67) | <.003 | 1.44 (0.95,2.17) | =.082 | 1.50 (1.00,2.26) | <.048 |
Sampling weights were considered in competing risk model to obtain nationally representative estimates.
DII = dietary inflammatory index, HR = hazard rate, Q = quartile, ref = reference.
Model 1: Unadjusted.
Model 2: Adjusted for gender, age, race, education level, and PIR.
Model 3: Model 2 + adjusted for smoking history, alcohol consumption history, hypertension, diabetes, eGFR, serum cholesterol, and serum triglycerides.
Figure 4.
Restricted cubic spline (RCS) analyses between DII and all-cause mortality of participants with hyperuricemia. DII = dietary inflammatory index.
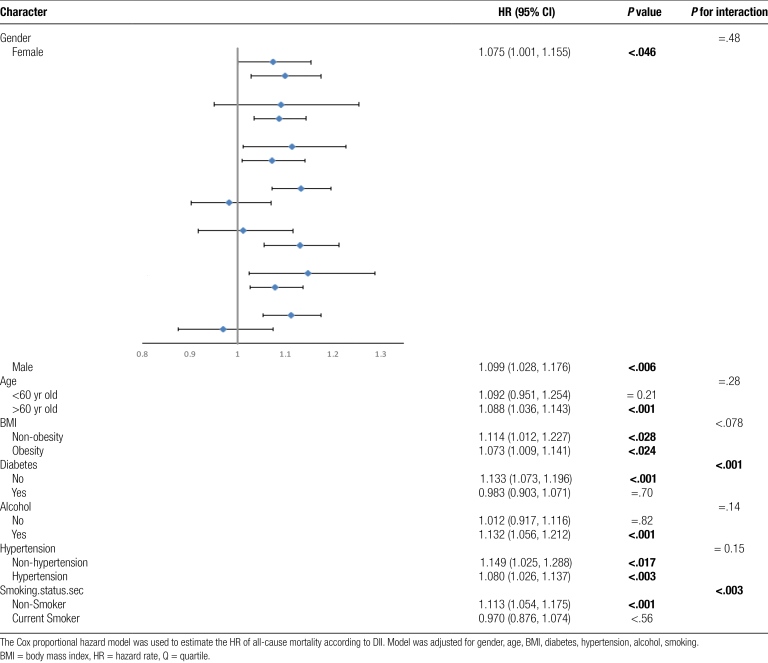
3.4. Subgroup analyses and sensitivity analyses
Subgroup analyses were conducted to determine whether demographic characteristics and comorbidities influenced the relationship between DII and the all-cause mortality in individuals with hyperuricemia. The results showed consistent findings when stratified by gender, age, BMI, alcohol consumption, and hypertension (p for interaction > .05). However, interactions were observed between DII and diabetes as well as smoking history (Table 5). Additionally, we performed stratified analyses on the logistic regression of DII and hyperuricemia, and consistent results were observed when stratified by gender, age, smoking history, alcohol consumption history, hypertension, and diabetes, with only an interaction observed with BMI (p for interaction < .008) (Supplementary table 3, http://links.lww.com/MD/K878). Sensitivity analyses excluding participants who died within 24 months of follow-up yielded similar results (Supplementary table 4, http://links.lww.com/MD/K879). The relationship between DII and the all-cause mortality persisted in the complete population of participants who were initially excluded due to data incompleteness (Supplementary figure 1, http://links.lww.com/MD/K880) and in the non-hyperuricemia population (Supplementary figure 2, http://links.lww.com/MD/K881).
Table 5.
Subgroup analyses of the associations between dietary inflammatory indexa and all-cause mortality among participants with hyperuricimia aged 20 years and older from the National Health and Nutrition Examination Survey (2001 to 2010).
4. Discussion
This study utilized the nationally representative samples from NHANES between 2001 and 2010 to explore the correlation between DII and the prevalence of hyperuricemia, as well as the all-cause and CVD mortality among individuals with hyperuricemia. The results revealed that, after adjusting for confounding factors, there existed a positive correlation between DII levels and the prevalence of hyperuricemia in the community population. Furthermore, among individuals diagnosed with hyperuricemia, a positive association was observed between DII and both all-cause and CVD mortality. Furthermore, higher levels of DII are associated with increased all-cause and CVD mortality in individuals with hyperuricemia.
Previous studies have identified a potential association between dietary inflammatory potential and the prevalence of hyperuricemia. Cross-sectional studies conducted in communities from East Asian countries consistently demonstrated that higher DII scores, indicative of a pro-inflammatory diet, were associated with an increased risk of hyperuricemia,[24,25] which is consistent with our findings. Individuals with a pro-inflammatory diet have been found to have higher levels of white blood cells and CRP in their blood,[26–28] but currently, there is no compelling evidence to suggest a causal relationship between higher levels of inflammation in the body and the occurrence of hyperuricemia. One potential underlying cause for the occurrence of hyperuricemia is the state of renal health, as the kidneys play a crucial role in maintaining the uric acid homeostasis in the human body, with approximately 70% of uric acid being excreted through the kidneys.[29] Chronic inflammation is recognized as one of the primary mechanisms of renal tubular damage, which can ultimately affect uric acid excretion and lead to the development of hyperuricemia.[30] The relationship between DII and the incidence of hyperuricemia is also likely to be explained by dietary components. The Western dietary pattern, characterized by a higher proportion of total fat, saturated fat, red meat, processed meat, and simple carbohydrates, not only promotes inflammation but also contains a higher content of purines, which are metabolized into uric acid in the body.[31,32] Flavonoids, folate, probiotics, anthocyanins, fibers and other anti-inflammatory dietary components have been shown to reduce blood uric acid levels and improve hyperuricemia through mechanisms such as downregulating inflammatory pathways and modulating intestinal homeostasis.[15,16,33–37] Further research is needed to elucidate the relationship between the inflammatory potential of food and hyperuricemia.
Diet is one of the important factors influencing levels of systemic inflammation. A dietary pattern characterized by a higher proportion of fruits, vegetables, whole grains, and fish (such as the Mediterranean diet) is associated with lower levels of systemic inflammation. Conversely, a dietary pattern characterized by a higher proportion of total fat, saturated fat, protein, and simple carbohydrates (such as the Western diet) is associated with higher levels of systemic inflammation.[26,31] Possible mechanisms include the influence of different dietary components on the stability of the gut microbiota, which in turn affects gut permeability and subsequently impacts systemic inflammation levels.[38,39] Additionally, food metabolites from different breakdown processes possess distinct pro-inflammatory characteristics, thereby shaping different pro-inflammatory environments.[40] In population with hyperuricemia, the progression of their health conditions is significantly influenced by the pivotal role of inflammation. A cross-sectional study conducted in the United States demonstrated that, even after adjusting for confounding factors, hyperuricemia independently increased the likelihood of elevated high-sensitivity CRP (hs-CRP) levels.[41] Similarly, a cross-sectional study conducted in southern China revealed that among suspected coronary heart disease patients, those with elevated uric acid levels upon admission exhibited significantly higher white blood cell count, neutrophil count, and CRP levels compared to non-hyperuricemic patients.[42] These findings suggest that higher levels of uric acid in the body may contribute to a sustained, asymptomatic state of inflammation. The mechanisms underlying uric acid-induced inflammation leading to tissue pathological changes and pathophysiological processes are gradually being elucidated. In vitro experiments have demonstrated that uric acid can activate human hepatoma HepG2 cells, inducing the expression of pro-inflammatory NF-κB signaling cascade and exhibiting a dose-dependent effect.[43] In animal models, elevated levels of uric acid activate vascular activity and inflammatory processes, leading to hypertension, which may serve as the initiating process for subclinical renal injury.[44] For vascular endothelial cells, uric acid activates adrenergic receptors, triggering endothelial inflammation and intensifying reactive oxygen species, thereby promoting the progression of atherosclerosis.[45,46] These mechanisms may potentially elucidate the underlying reasons why the pro-inflammatory capacity of diet can impact the all-cause and CVD mortality in individuals with hyperuricemia.
Although food is recognized as an important factor influencing the level of inflammation in the body, there is currently a lack of research exploring the potential pro-inflammatory effects of food and its association with mortality in individuals with hyperuricemia. As an assessment tool, DII is capable of effectively evaluating the overall dietary intake of populations and quantifying the potential impact of food on the inflammatory levels in the human body.[17] Previous cohort studies have consistently demonstrated a positive association between DII and all-cause mortality in general populations, cancer populations, populations with metabolic disorders, and elderly populations.[47–50] Furthermore, a meta-analysis incorporating 17 cohort studies confirmed that a pro-inflammatory diet increases the risk of all-cause, cancer, and CVD mortality.[51] Our study extends these findings for the first time to individuals with hyperuricemia. This suggests that the inflammatory status induced by dietary patterns can significantly impact the survival outcomes of individuals with hyperuricemia, highlighting the potential of anti-inflammatory diets as an important lifestyle intervention for improving the prognosis of individuals with hyperuricemia.
The necessity of pharmacological treatment for asymptomatic hyperuricemia remains a subject of significant debate, leading to an increasing focus on dietary management among individuals with hyperuricemia and clinical practitioners. Previous research has primarily concentrated on dietary patterns that can reduce serum uric acid levels and preventing episodes of gout,[52,53] with less emphasis on the impact of dietary patterns on the survival outcomes of individuals with hyperuricemia.[54] To the best of our knowledge, our study is the first to utilize the DII to assess the pro-inflammatory potential of daily diets in individuals with hyperuricemia and examine its association with all-cause and CVD mortality. It partly addresses the limitations of using single dietary components hard to represent the overall pro-inflammatory potential of diets, providing new insights and evidence for improving the prognosis of individuals with hyperuricemia through dietary management.
However, our study has limitations. Firstly, dietary information was self-reported by participants, which inevitably introduces recall bias and cannot represent participants’ fixed dietary patterns, even though we used data from 2 rounds of 24-hour dietary recall records. Secondly, although we adjusted for potential confounding factors that may influence the outcomes in our models, there may still be other unmeasured confounders. Thirdly, the observational study design cannot establish a causal relationship between pro-inflammatory diets and the mortality in individuals with hyperuricemia.
5. Conclusion
DII levels were positively correlated with the incidence of hyperuricemia, and a pro-inflammatory diet may elevate the all-cause and CVD mortality in individuals with hyperuricemia. These findings suggest that DII has independent prognostic value, and modifying pro-inflammatory dietary habits may be a potential factor in improving the prognosis of individuals with hyperuricemia.
Acknowledgments
We thank to the National Center for Health Statistics at the CDC for their excellent work in collecting the NHANES data and for making it accessible to the public.
Author contributions
Conceptualization: Jingda Huang, Yandong Zhang, Mindan Sun.
Data curation: Jingda Huang.
Formal analysis: Jingda Huang.
Investigation: Jingda Huang, Yandong Zhang.
Methodology: Jingda Huang, Yihui Wei.
Software: Jingda Huang.
Validation: Jingda Huang, Mindan Sun.
Visualization: Jingda Huang, Huimin Li.
Writing – original draft: Jingda Huang, Jiajie Li.
Writing – review & editing: Jingda Huang, Mindan Sun.
Supplementary Material
Abbreviations:
- AAP
- aminoantipyrine
- ANOVA
- analysis of variance
- BMI
- body mass index
- CRP
- C-reactive protein
- CVD
- cardiovascular disease
- DCHBS
- 3,5-dichloro-2-hydroxybenzene sulfonic acid
- DII
- dietary inflammatory index
- eGFR
- estimated glomerular filtration rate
- ICD
- international classification of diseases
- IL
- interleukin
- MAFLD
- metabolic dysfunction-associated fatty liver disease
- MUFA
- mono unsaturated fat acid
- NDI
- national death index
- NHANES
- national health and nutrition examination survey
- PIR
- poverty income ration
- PUFA
- polyunsaturated fatty acid
- RCS
- restricted cubic splines
- SUA
- serum uric acid
- TNF
- tumor necrosis factor
The CDC/NCHS Research Ethics Review Board granted approval for the detailed methods and protocols employed in the NHANES study. These procedures, including informed consent protocols for participants, can be accessed on the CDC.gov website. All methods adhered to the applicable guidelines and regulations. Since the data used in this study are publicly available, it was exempt from ethical review involving human subjects.
The datasets generated during and/or analyzed during the current study are publicly available.
Supplemental Digital Content is available for this article.
The authors have no conflicts of interest to disclose.
This study was supported by The Special Fund for Medical and Health Talents of Jilin Province, No.JLSWSRCZX2023-33.
How to cite this article: Huang J, Zhang Y, Li J, Li H, Wei Y, Sun M. Association of dietary inflammatory index with all-cause and cardiovascular disease mortality in hyperuricemia population: A cohort study from NHANES 2001 to 2010. Medicine 2023;102:51(e36300).
Contributor Information
Jingda Huang, Email: huangjingda_jilinu@163.com.
Yandong Zhang, Email: zyd@jlu.edu.cn.
Jiajie Li, Email: 1410963077@qq.com.
Huimin Li, Email: 1410963077@qq.com.
Yihui Wei, Email: weiyihui22021@163.com.
References
- [1].Chen-Xu M, Yokose C, Rai SK, et al. Contemporary prevalence of gout and hyperuricemia in the United States and Decadal Trends: the national health and nutrition examination Survey, 2007-2016. Arthritis Rheumatol. 2019;71:991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhang M, Zhu X, Wu J, et al. Prevalence of hyperuricemia among chinese adults: findings from two nationally representative cross-sectional surveys in 2015-16 and 2018-19. Front Immunol. 2021;12:791983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dalbeth N, Gosling AL, Gaffo A, et al. Gout. Lancet. 2021;397:1843–55. [DOI] [PubMed] [Google Scholar]
- [4].Liu Z, Huang H, Xie J, et al. Associations of serum uric acid levels with liver disease-related morbidity and mortality: a prospective cohort study of the UK Biobank. Liver Int. 2023;43:1046–55. [DOI] [PubMed] [Google Scholar]
- [5].Muiesan ML, Agabiti Rosei C, Paini A, et al. Serum uric acid and left ventricular mass index independently predict cardiovascular mortality: the uric acid right for heart health (URRAH) project. Eur J Intern Med. 2023. [DOI] [PubMed] [Google Scholar]
- [6].Tian X, Chen S, Xu Q, et al. Cumulative serum uric acid exposure and its time course with the risk of incident stroke. Stroke. 2023;54. [DOI] [PubMed] [Google Scholar]
- [7].Tsao HM, Lai TS, Chang YC, et al. Serum urate and risk of chronic kidney disease: a mendelian randomization study using Taiwan Biobank. Mayo Clin Proc. 2023;98:513–21. [DOI] [PubMed] [Google Scholar]
- [8].Tu YC, Liu YH, Chen SC, et al. Metabolic syndrome and its components are associated with new-onset hyperuricemia in a large Taiwanese population follow-up study. Nutrients. 2023;15:1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li B, Chen L, Hu X, et al. Association of serum uric acid with all-cause and cardiovascular mortality in diabetes. Diabetes Care. 2023;46:425–33. [DOI] [PubMed] [Google Scholar]
- [10].Wang C, Che H, Zhou Y, et al. Joint association of hyperuricemia and chronic kidney disease with mortality in patients with chronic heart failure. Front Endocrinol (Lausanne). 2023;14:1131566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang SX, Yu YL, Tang ST, et al. Association of serum uric acid levels with cardiovascular and all-cause mortality in hypertensive patients in China: a cohort study. Postgrad Med J. 2023;99:708–14. [DOI] [PubMed] [Google Scholar]
- [12].Liu J, Shen P, Ma X, et al. White blood cell count and the incidence of hyperuricemia: insights from a community-based study. Front Med. 2019;13:741–6. [DOI] [PubMed] [Google Scholar]
- [13].Isaka Y, Takabatake Y, Takahashi A, et al. Hyperuricemia-induced inflammasome and kidney diseases. Nephrol Dial Transplant. 2016;31:890–6. [DOI] [PubMed] [Google Scholar]
- [14].Nielsen SM, Zobbe K, Kristensen LE, et al. Nutritional recommendations for gout: an update from clinical epidemiology. Autoimmun Rev. 2018;17:1090–6. [DOI] [PubMed] [Google Scholar]
- [15].Yang Y, Zhang JL, Zhou Q. Targets and mechanisms of dietary anthocyanins to combat hyperglycemia and hyperuricemia: a comprehensive review. Crit Rev Food Sci Nutr. 2022;62:1119–43. [DOI] [PubMed] [Google Scholar]
- [16].Zhao H, Lu Z, Lu Y. The potential of probiotics in the amelioration of hyperuricemia. Food Funct. 2022;13:2394–414. [DOI] [PubMed] [Google Scholar]
- [17].Shivappa N, Steck SE, Hurley TG, et al. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang J, Jia J, Lai R, et al. Association between dietary inflammatory index and atherosclerosis cardiovascular disease in US adults. Front Nutr. 2022;9:1044329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhang X, Guo Y, Yao N, et al. Association between dietary inflammatory index and metabolic syndrome: analysis of the NHANES 2005-2016. Front Nutr. 2022;9:991907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Botelho J, Leira Y, Viana J, et al. The role of inflammatory diet and vitamin D on the link between periodontitis and cognitive function: a mediation analysis in older adults. Nutrients. 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Han Y, Cao Y, Han X, et al. Hyperuricemia and gout increased the risk of long-term mortality in patients with heart failure: insights from the National Health and Nutrition Examination Survey. J Transl Med. 2023;21:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Golabi P, Paik JM, Kumar A, et al. Nonalcoholic fatty liver disease (NAFLD) and associated mortality in individuals with type 2 diabetes, pre-diabetes, metabolically unhealthy, and metabolically healthy individuals in the United States. Metabolism. 2023;146:155642. [DOI] [PubMed] [Google Scholar]
- [23].Carey RM, Whelton PK, 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension Guideline. Ann Intern Med. 2018;168:351–8. [DOI] [PubMed] [Google Scholar]
- [24].Kim HS, Kwon M, Lee HY, et al. Higher pro-inflammatory dietary score is associated with higher hyperuricemia risk: results from the case-controlled Korean Genome and Epidemiology Study_Cardiovascular Disease Association Study. Nutrients. 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ye C, Huang X, Wang R, et al. Dietary inflammatory index and the risk of hyperuricemia: a cross-sectional study in Chinese Adult Residents. Nutrients. 2021;13:4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wirth MD, Sevoyan M, Hofseth L, et al. The Dietary Inflammatory Index is associated with elevated white blood cell counts in the National Health and Nutrition Examination Survey. Brain Behav Immun. 2018;69:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shin D, Hur J, Cho EH, et al. Pre-pregnancy body mass index is associated with dietary inflammatory index and C-reactive protein concentrations during pregnancy. Nutrients. 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shivappa N, Wirth MD, Hurley TG, et al. Association between the dietary inflammatory index (DII) and telomere length and C-reactive protein from the National Health and Nutrition Examination Survey-1999-2002. Mol Nutr Food Res. 2017;61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lipkowitz MS. Regulation of uric acid excretion by the kidney. Curr Rheumatol Rep. 2012;14:179–88. [DOI] [PubMed] [Google Scholar]
- [30].Halperin Kuhns VL, Woodward OM. Urate transport in health and disease. Best Pract Res Clin Rheumatol. 2021;35:101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Di Giosia P, Stamerra CA, Giorgini P, et al. The role of nutrition in inflammaging. Ageing Res Rev. 2022;77:101596. [DOI] [PubMed] [Google Scholar]
- [32].Rai SK, Fung TT, Lu N, et al. The Dietary Approaches to Stop Hypertension (DASH) diet, Western diet, and risk of gout in men: prospective cohort study. Bmj. 2017;357:j1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cao J, Wang T, Liu Y, et al. Lactobacillus fermentum F40-4 ameliorates hyperuricemia by modulating the gut microbiota and alleviating inflammation in mice. Food Funct. 2023;14:3259–68. [DOI] [PubMed] [Google Scholar]
- [34].Shi R, Ye J, Fan H, et al. Lactobacillus plantarum LLY-606 supplementation ameliorates hyperuricemia via modulating intestinal homeostasis and relieving inflammation. Food Funct. 2023;14:5663–77. [DOI] [PubMed] [Google Scholar]
- [35].Wang P, Zhang X, Zheng X, et al. Folic acid protects against hyperuricemia in C57BL/6J Mice via ameliorating gut-kidney axis dysfunction. J Agric Food Chem. 2022;70:15787–803. [DOI] [PubMed] [Google Scholar]
- [36].Wang X, Dong L, Dong Y, et al. Corn Silk Flavonoids Ameliorate Hyperuricemia via PI3K/AKT/NF-κB Pathway. J Agric Food Chem. 2023;71:9429–40. [DOI] [PubMed] [Google Scholar]
- [37].Zhu Q, Yu L, Li Y, et al. Association between Dietary Fiber Intake and Hyperuricemia among Chinese Adults: Analysis of the China Adult Chronic Disease and Nutrition Surveillance (2015). Nutrients. 2022;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Malesza IJ, Malesza M, Walkowiak J, et al. High-fat, western-style diet, systemic inflammation, and gut microbiota: a narrative review. Cells. 2021;10:3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kim KA, Gu W, Lee IA, et al. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One. 2012;7:e47713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zheng J, Hoffman KL, Chen JS, et al. Dietary inflammatory potential in relation to the gut microbiome: results from a cross-sectional study. Br J Nutr. 2020;124:931–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kaspar CDW, Lu J. Hyperuricemia, elevated body mass index, female sex, and albuminuria increase the probability of elevated high-sensitivity C-Reactive protein: results from the National Health and Nutrition Examination Survey 2015-2018. Front Public Health. 2021;9:689219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hu X, Liu J, Li W, et al. Elevated serum uric acid was associated with pre-inflammatory state and impacted the role of HDL-C on carotid atherosclerosis. Nutr Metab Cardiovasc Dis. 2022;32:1661–9. [DOI] [PubMed] [Google Scholar]
- [43].Spiga R, Marini MA, Mancuso E, et al. Uric acid is associated with inflammatory biomarkers and induces inflammation via activating the NF-κB Signaling Pathway in HepG2 Cells. Arterioscler Thromb Vasc Biol. 2017;37:1241–9. [DOI] [PubMed] [Google Scholar]
- [44].Yanai H, Adachi H, Hakoshima M, et al. Molecular biological and clinical understanding of the pathophysiology and treatments of hyperuricemia and its association with metabolic syndrome, cardiovascular diseases and chronic kidney disease. Int J Mol Sci . 2021;22:9221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yang X, Gu J, Lv H, et al. Uric acid induced inflammatory responses in endothelial cells via up-regulating(pro)renin receptor. Biomed Pharmacother. 2019;109:1163–70. [DOI] [PubMed] [Google Scholar]
- [46].Lu J, Sun M, Wu X, et al. Urate-lowering therapy alleviates atherosclerosis inflammatory response factors and neointimal lesions in a mouse model of induced carotid atherosclerosis. FEBS J. 2019;286:1346–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tomata Y, Shivappa N, Zhang S, et al. Dietary inflammatory index and disability-free survival in community-dwelling older adults. Nutrients. 2018;10:1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tan J, Liu N, Sun P, et al. A proinflammatory diet may increase mortality risk in patients with diabetes mellitus. Nutrients. 2022;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yuan S, Song C, Zhang R, et al. Dietary inflammation index and its association with long-term all-cause and cardiovascular mortality in the general US population by baseline glycemic status. Nutrients. 2022;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zheng J, Tabung FK, Zhang J, et al. Association between dietary inflammatory potential and mortality after cancer diagnosis in the Women’s Health Initiative. Br J Cancer. 2023;128:606–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhang J, Feng Y, Yang X, et al. Dose-response association of dietary inflammatory potential with all-cause and cause-specific mortality. Adv Nutr. 2022;13:1834–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Danve A, Sehra ST, Neogi T. Role of diet in hyperuricemia and gout. Best Pract Res Clin Rheumatol. 2021;35:101723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yokose C, McCormick N, Choi HK. Dietary and lifestyle-centered approach in gout care and prevention. Curr Rheumatol Rep. 2021;23:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Vedder D, Walrabenstein W, Heslinga M, et al. Dietary interventions for gout and effect on cardiovascular risk factors: a systematic review. Nutrients. 2019;11:2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.