Abstract
Advanced HIV disease (AHD) remains a significant burden, despite the widespread use of antiretroviral therapy (ART) programs. Individuals with AHD are at a high risk of death even after starting ART. We characterized treatment naïve and treatment experienced clients presenting with AHD in western Kenya to inform service delivery and program improvement. We conducted a retrospective study using routinely collected program data from October 2016 to September 2019 for AHD clients in eight facilities in Homa Bay County, Kenya. Demographic and clinical data were abstracted from the medical records of AHD clients, defined as HIV-positive clients aged ≥ 5 years with documented CD4 count < 200 cells/mm3 and/or WHO clinical stage II/IV. Associations were assessed using Pearson’s chi-square and Mann–Whitney Rank-Sum tests at 5% level of significance. Of the 19,427 HIV clients at the eight facilities, 6649 (34%) had a CD4 count < 200 cells/mm3 or a WHO III/IV stage. Of these, 1845 were randomly selected for analysis. Over half (991) of participants were aged 45 + years and 1040 (56%) were female. The median age was 46.0 years (interquartile range: 39.2–54.5); 1553 (84%) were in care at county and sub-county hospitals; and 1460 (79%) were WHO stage III/IV at enrollment. At ART initiation, 241 (13%) had tuberculosis, 192 (10%) had chronic diarrhea, and 94 (5%) had Pneumocystis jiroveci pneumonia. At the time of data collection, 89 (5%) participants had died and 140 (8%) were lost to follow-up. Eighteen percent (330) of participants were ART-experienced (on ART for ≥ 3 months). The proportions of ART-experienced and -naïve clients regarding age, sex and marital status were similar. However, a higher proportion of ART-experienced clients received care at primary care facilities, (93(28%) vs. 199 (13%); P < .001); were WHO stage 3/4 at AHD diagnosis, 273 (84%) vs. 1187 (79%) (P = .041); and had died or been LTFU, (124 (38%) vs. 105 (7%); P < .001). With increasing prevalence of patients on ART, the proportion of AHD treatment-experienced clients may increase without effective interventions to ensure that these patients remain in care.
Keywords: advanced HIV disease, antiretroviral therapy, ART-experienced, ART-naive, morbidity, viral load
1. Introduction
Recent estimates suggest that about 30% to 40% of people living with HIV (PLHIV) starting antiretroviral therapy (ART) in low- and middle-income settings have a CD4 cell count of less than 200 cells/mm3, and 20% have a CD4 cell count of less than 100 cells/mm3.[1,2] In some settings, up to half of people present to care with advanced HIV disease (AHD), defined as clients 5 years of age and older with a CD4 cell count < 200 cells/mm3 and/or WHO stage III or IV condition. All children younger than 5 years old and living with HIV are considered as having AHD.[3] Clients presenting with AHD have an increased risk of morbidity and mortality, compared to those without AHD.[4,5] Leading causes of mortality among adults with AHD globally include tuberculosis (TB), severe bacterial infections, cryptococcal meningitis, toxoplasmosis and P jirovecii pneumonia.[3,6,7] In children with AHD, TB, severe bacterial infections, P jirovecii pneumonia, diarrheal diseases, malnutrition and wasting are the leading causes of death.[5,6]
In Kenya, 96% of PLHIV know their status, and 86% of PLHIV were estimated to be receiving ART in 2020.[8] Despite these achievements, studies in South Africa, and in Cameroon, Mozambique, Uganda and Zimbabwe, have found that about 30% of newly-diagnosed PLHIV have AHD at the time of diagnosis and an increasing proportion of PLHIV with AHD are likely to have had a history of prior HIV diagnosis and ART receipt, but subsequently disengaged from care.[9,10] With the increasing proportion of PLHIV accessing care and treatment, we sought to assess the extent to which those presenting with AHD in Homa Bay, Western Kenya were treatment experienced as compared with treatment naïve.
The objectives of this study were: to determine the proportion of newly initiating treatment (treatment naïve) and treatment-experienced clients among clients with AHD; to describe the outcomes for the treatment-naïve and treatment experienced AHD PLHIV at 3, 6 and 12 months following the diagnosis of AHD; to describe VL suppression rates (<1000 copies/ml3) among treatment naïve and treatment experience PLHIV with AHD with documented viral loads (VLs) 3 and 6 months after diagnosis of AHD, and to describe the factors associated with advanced HIV disease among the treatment naïve and treatment experienced clients. Data were collected at facilities supported by the Elizabeth Glaser Pediatric AIDS Foundation (EGPAF) in Homa Bay County. Situated in western Kenya, Homa Bay County has the highest HIV prevalence in the country at 19.6%, which is four times the national prevalence.[11]
2. Methods
2.1. Study design and setting
We conducted a retrospective study of routinely collected data in eight out of the 20 EGPAF-supported health facilities in Homa Bay County that were implementing the Electronic Medical Records (EMR) system at the time of study implementation. The 20 health facilities implementing EMR had over 80% concordance between paper-based and EMR data. In addition, the 20 health facilities had both paper and EMR records to verify data quality. We selected the eight sites with the highest numbers of recorded AHD clients, accounting for 78% of all AHD cases at the 20 facilities. The eight selected health facilities included two dispensaries, two health centers, three sub-county hospitals, and the Homa Bay County Teaching Referral Hospital.
2.2. Study population and eligibility criteria
The study population included children > 5 years old, adolescents, and adults who were on ART between October 2016 and September 2019 at the selected facilities. Clients were eligible if they had a documented HIV diagnosis in hospital records at the time of enrollment in care and CD4 count < 200 cells/mm3 and/or WHO clinical stage III or IV, during follow up, thus meeting the diagnosis of AHD. Clients without AHD were excluded.
We defined, newly initiated on treatment as treatment-naïve PLHIV who, at enrollment into care, had not been on ART before or those who had been on ART for up to 3 months, treatment-experienced clients as PLHIV who had been on ART for more than 3 months. This included clients who had defaulted or been lost to follow-up (LTFU) but then returned to care or those who had died, LTFU -as PLHIV who had been on ART, but had missed their last appointment by 30 days or more with no documentation of death or transfer out of the study site, and PLHIV with a diagnosis of AHD at enrollment to care as a client who had documented in hospital records, a CD4 count < 200 cells/mm3 and or WHO clinical stage III or IV. Clients who had missing CD4 or WHO disease staging were included in the analysis under missing. Clients for whom there was no documentation of opportunistic infections (OI) were assumed not to have any OIs.
2.3. Sampling and sample size
Using program data for HIV clients routinely collected between October 2016 and September 2019, we randomly selected AHD records (those meeting the criteria above), using probability proportional to the number of clients presenting at the eight facilities. We also proportionated the sample by year; October 2016 to September 2017, October 2017 to September 2018, October 2018 to September 2019, to ensure representation by time. October to September is the programmatic reporting cycle.
Our sample size was based on logistic considerations. Over a two-month data collection period, we estimated that the four trained research assistants (RAs) would collect data for about 1600 records, which would provide sufficient numbers for the planned analyses.
2.4. Data collection
The Research assistants abstracted demographic and clinical data from both electronic and paper based records of PLHIV aged 5 years and above, with documented CD4 count < 200 cells/mm3 and/or WHO clinical stage III/IV, attending HIV care at the selected facilities between October 2016 and September 2019. At the time of data collection, the EMR system was not real-time; thus, we used the paper-based records to supplement information that was not yet in the EMR system. For example, viral load data was sometimes missing from the EMR as these data had not yet been keyed in. We abstracted data for each enrolled participant from AHD diagnosis to 12 months following the AHD diagnosis. Abstracted demographic data included age (years), sex, and marital status.
For clinical data, we captured any OIs and/or hospitalizations documented at HIV diagnosis (as recorded in the patient records), ART initiation and at the time of data collection (current), as well as CD4 count and WHO stage at ART initiation. We also documented dates of clinic attendance, transfers out and recorded deaths. We collected viral load at different time points and the 12-month viral load results were used for this analysis. Data were collected from January to May 2020. Data were entered into an Open Data Kit database on Wi-Fi enabled study tablets, with built-in checks to reduce errors. Each patient was assigned a unique study identification (ID) number and database records were linked to the patient using this unique ID only. The data abstraction team maintained a master document that allowed linking of files of participants to the unique ID number. Access to this document was limited to only trained members of the study team. All research assistants completed an online ethics course certification and signed a data handling and confidentiality agreement.
Data were summarized using frequencies, means, medians and proportions. Statistical associations were assessed using Pearson’s chi-square and Wilcoxon Rank-Sum tests at 5% level of significance. Analysis was conducted in STATA version 16.0.
3. Ethics approval and consent to participate
The study was conducted under the patient and program outcome protocol approved by Kenyatta National Hospital-University of Nairobi Ethics Research Committee and Advarra Institutional Review Board in the US. The protocol was also reviewed in accordance with the U.S. Center for Disease Control and Prevention human research protection procedures and was determined to be research, but the Center for Disease Control and Prevention investigators did not interact with human subjects or have access to identifiable data or specimens for research purposes. We requested for waiver of informed consent from the ethics committee. Since the evaluation was retrospective in nature, involved no more than minimal risk to human subjects, and analyzed both aggregate and individual level data; the evaluation would not adversely affect the rights and welfare of the subjects; the evaluation could not practicably be carried out without the waiver because it was not possible to track down and obtain consent from clinic patients; and, if tracing of patients to their homesteads was attempted, this could reasonably constitute a violation of privacy and confidentiality. We received a waiver of informed consent for the use of all retrospective data including data for children below 18 years. All data were kept confidential and only the study team had access to patient data.
4. Results
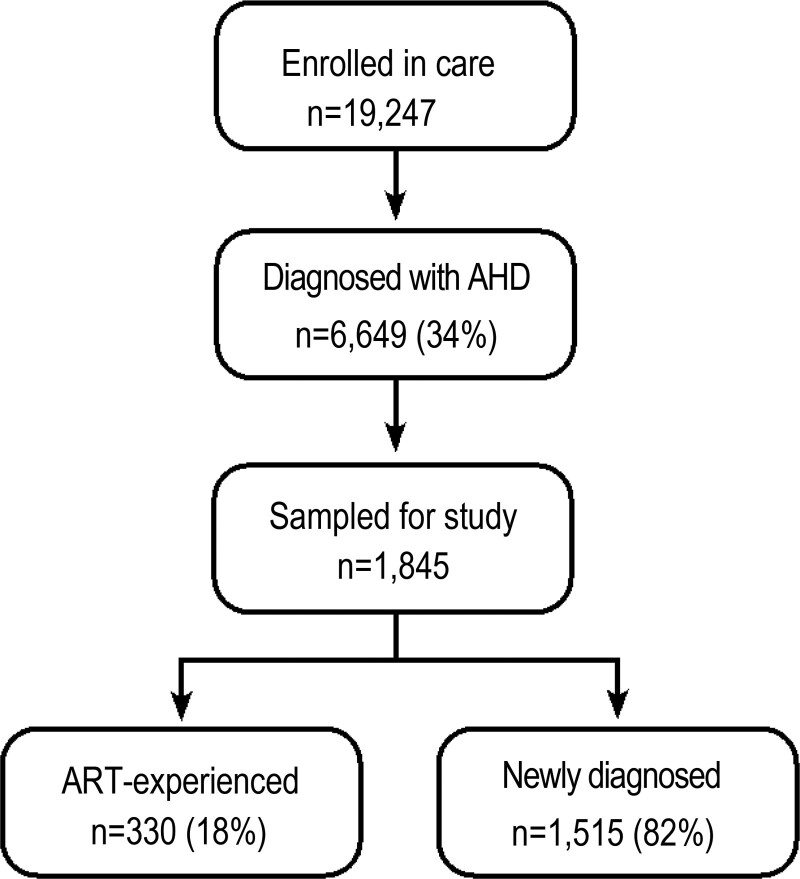
Of the 19,427 PLHIV enrolled in care at the eight study sites during the study period, 6649 (34%) had a CD4 count < 200 cells/mm3 and/or WHO Stage III/IV, of which 1845 were randomly selected for this analysis. Of those in this analysis, the majority were diagnosed with AHD at the time of HIV diagnosis, 1515 (82%), Figure 1.
Figure 1.
Study Flowchart. Study flow chart of sampled AHD clients in selected sites in Homa Bay County. The flow chart shows the sampled AHD clients in selected sites in Homa Bay county, disaggregated by ART-experienced and newly diagnosed. The sample was drawn from patients who were enrolled in care from October 2016 to September 2019 in the selected study facilities.
Table 1 presents the demographic and clinical characteristics of AHD clients at AHD diagnosis stratified by whether they were ART-experienced or newly diagnosed. The median age was 46 years, [interquartile range (IQR):39.2–54.5], and 1040 (56.4%) were women. Only 9 (0.5%) participants were children < 15 years. A total of 1275 (70%), were married/living with a partner, including polygamous marriages, and 330 (18.6%) were widowed.
Table 1.
Demographic and clinical characteristics of AHD clients at AHD diagnosis, stratified by ART- experience or new diagnosis, in Homa Bay County, Kenya from October 2016 to September 2019.
| ART-Experienced (n = 330) N (%) |
Newly diagnosed with HIV (n = 1515) N (%) |
Total (n = 1845) N (%) |
P value | |
|---|---|---|---|---|
| Age (yr)* | ||||
| Median (IQR) | 44.7 (36.8–53.2) | 46.4 (39.6–54.6) | 46.0 (39.2–54.5) | .006 |
| <15 | 3 (0.9) | 6 (0.4) | 9 (0.5) | |
| 15–24 | 8 (2.4) | 29 (1.9) | 37 (2.0) | |
| 25–45 | 158 (47.9) | 650 (42.9) | 808 (43.8) | |
| 45+ | 161 (48.8) | 830 (54.8) | 991 (53.7) | |
| Sex* | ||||
| Male | 145 (43.9) | 659 (43.5) | 804 (43.6) | .890 |
| Female | 185 (56.1) | 855 (56.4) | 1040 (56.4) | |
| Missing | 1 | 1 | ||
| Marital status* | ||||
| Married/living with partner | 168 (52.3) | 776 (53.5) | 944 (53.3) | <.001 |
| Polygamous marriage | 57 (17.8) | 274 (18.9) | 331 (18.7) | |
| Divorced/separated | 17 (5.3) | 34 (2.3) | 51 (2.8) | |
| Widowed | 44 (13.7) | 286 (19.7) | 330 (18.6) | |
| Single, minor | 35 (10.9) | 80 (5.5) | 114 (6.5) | |
| Facility type* | ||||
| County referral/Sub-County Hospital | 237 (71.82) | 1316 (86.86) | 1553 (84.17) | <.001 |
| Dispensaries/Health Centers | 93 (28.18) | 199 (13.14) | 292 (15.83) | |
| CD4 count at ART initiation | ||||
| Median (IQR) | 169.0 (90.0–296.0) | 159.0 (83.0–273.0) | 161.0 (84.0–277.0) | .090 |
| <100 | 72 (27.1) | 410 (29.3) | 482 (28.9) | .600 |
| 100–200 | 89 (33.4) | 480 (34.3) | 569 (34.2) | |
| >200 | 105 (39.5) | 509 (36.4) | 614 (36.9) | |
| Missing | 64 | 116 | 180 | |
| WHO stage at ART initiation | ||||
| 1/II | 54 (16.5) | 326 (21.5) | 380 (20.7) | .041 |
| III/IV | 273 (83.5) | 1187 (78.5) | 1460 (79.3) | |
| Missing | 3 | 2 | 5 | |
| ART Regimen (current)# | ||||
| NNRTI Based | 216 (65.6) | 374 (24.8) | 590 (32.1) | <.001 |
| DTG Based | 68 (20.7) | 940 (62.2) | 1008 (54.8) | |
| PI Based | 45 (13.7) | 196 (12.9) | 241 (13.1) | |
| DRV Based | 1 (0.1) | 1 (0.1) | ||
| Missing | 1 | 4 | 5 | |
| ART Regimen (at ART initiation) | ||||
| NNRTI Based | 321 (97.6) | 1492 (98.6) | 1813 (98.4) | .390 |
| DTG Based | 5 (1.5) | 13 (0.9) | 18 (1.0) | |
| PI Based | 3 (0.9) | 8 (0.5) | 11 (0.6) | |
| Missing | 1 | 2 | 3 |
ART = antiretroviral therapy, DRV = darunavir, DTG = dolutegravir, IQR = interquartile range, NNRTI = non-nucleoside reverse transcriptase inhibitors, PI = protease inhibitor.
At AHD diagnosis
#At time of data collection.
Overall 1051(62%) of the AHD clients had a CD4 count < 200 cells/mm3 at ART initiation, with similar proportions among the ART-experienced and newly-diagnosed clients, 161 (60%) and 890 (64%), respectively. At ART initiation, 614 (37%) of clients who had CD4 count > 200 cells/mm3 were diagnosed with AHD using WHO clinical staging. At ART initiation, 1460 (79%) were assessed to be WHO stage III/IV, with a significantly higher proportion among the ART-experienced compared to newly-diagnosed clients, 273 (84%) vs 1187 (79%), respectively, P = .041. Overall, 590 (32%) were currently on NNRTI-based regimens; 216 (66%) of the ART-experienced clients were still on these regimens compared to 25% of newly-diagnosed clients. Almost all participants (98.4%) had been initiated on a NNRTI-based regimen. Over half (55%) had been optimized to a dolutegravir (DTG)-based regimen at the time of data collection, but this was lower among ART-experienced clients 68 (21%) compared to newly-diagnosed clients 940 (62%).
At AHD diagnosis, 645 (35%) of AHD clients had documented OIs. Of these, 587 (39%) of newly-diagnosed clients had OIs, which was significantly more than ART-experienced clients, 18% (n = 58), P < .001. Chronic diarrhea seemed to be more commonly diagnosed among newly-diagnosed clients, as compared to ART-experienced (32% vs 7%), P < .001, while cryptococcal meningitis was significantly more common among ART-experienced clients as compared to newly-diagnosed clients (5.2% vs 1.4% respectively), P = .035. Overall, only 4 clients (0.2%) were hospitalized at the time of AHD diagnosis (Table 2).
Table 2.
Opportunistic infections at AHD diagnosis and hospitalization experience, stratified by ART-experienced and newly-diagnosed clients.
| ART-Experienced (n = 330) N (%) |
Newly diagnosed with HIV (n = 1515) N (%) |
Total (n = 1845) N (%) |
P value | |
|---|---|---|---|---|
| Opportunistic infections at diagnosis of AHD | 58 (17.6) | 587 (38.8) | 645 (35.0) | <.001 |
| TB | 19 (32.8) | 222 (37.8) | 241 (37.4) | .447 |
| Chronic diarrhea | 4 (6.9) | 188 (32.0) | 192 (29.8) | <.001 |
| Pneumocystis jirovecii | 5 (8.6) | 89 (15.2) | 94 (14.9) | .175 |
| Kaposi Sarcoma | 2 (3.5) | 14 (2.4) | 16 (2.5) | .625 |
| Cryptococcal Meningitis | 3 (5.2) | 8 (1.4) | 11 (1.7) | .035 |
| Other* | 27 (46.6) | 291 (49.6) | 318 (49.3) | .663 |
| Hospitalization at time AHD diagnosis | 3 (1.0) | 1 (0.1) | 4 (0.2) |
This table shows different opportunistic infections at AHD diagnosis and hospitalization experience, stratified by ART-experienced and newly-diagnosed clients. Participants may have had more than 1 opportunistic infection, ranging from 1 to 6, accounting for the percentage being above 100.
AHD = Advanced HIV disease, OI = opportunistic infections, TB = Tuberculosis.
Other (includes non-WHO stage III/IV): oral thrush (197), herpes simplex (77), vaginitis (38), toxoplasmosis (3), mycobacterium avian complex (2), Non-Hodgkin’s Lymphoma (1).
Overall, 1524 (83%) of AHD clients remained active in care through 12 months of follow-up. ART-experienced clients, however, experienced significantly more attrition (death/LTFU/transfer out) at all time periods of follow-up, when compared to newly-diagnosed clients, (P < .001), particularly LTFU. The mean time of follow up was 11.9 months(SD = 0.8). Although 12-month viral load results were only available for 76(20%) of the ART-experienced and 1050 (60%) of the newly-diagnosed clients, the proportion of clients virally suppressed was above 80% in both groups; 65 (85%) and 905 (86%) amongst ART-experienced and newly-diagnosed clients respectively (Table 3).
Table 3.
Outcomes among AHD clients, at 3, 6 and 12 mo following AHD diagnosis, stratified by ART-experienced and newly-diagnosed clients in Homa Bay County, Kenya, October 2016 to September 2019.
| ART-Experienced (n = 330) N (%) |
Newly diagnosed with HIV (n = 1515) N (%) |
Total (n = 1845) N (%) |
P value | |
|---|---|---|---|---|
| Treatment outcome at 3 mo | ||||
| Active in care | 286 (86.7) | 1356 (89.5) | 1642 (89.0) | .135 |
| Dead | 2 (0.6) | 5 (0.3) | 7 (0.4) | .460 |
| LTFU | 22 (6.7) | 21 (1.4) | 43 (2.3) | <.001 |
| Transfer out | 16 (4.9) | 4 (0.3) | 20 (1.1) | <.001 |
| Unknown | 4 | 129 | 133 | <.001 |
| Treatment outcome at 6 mo | ||||
| Active in care | 230 (69.7) | 1340 (88.4) | 1570 (85.1) | <.001 |
| Dead | 10 (3.0) | 6 (0.4) | 16 (0.9) | <.001 |
| LTFU | 46 (13.9) | 35 (2.3) | 81 (4.4) | <.001 |
| Transfer out | 35 (10.6) | 11 (0.7) | 46 (2.5) | <.001 |
| Unknown | 7 | 118 | 125 | <.001 |
| Treatment outcome at 12 mo | ||||
| Active in care | 175 (53.0) | 1349 (89.0) | 1524 (82.6) | <.001 |
| Dead | 10 (3.0) | 6 (0.4) | 16 (0.9) | <.001 |
| LTFU | 61 (18.5) | 28 (1.8) | 89 (4.8) | <.001 |
| Transfer out | 65 (19.7) | 17 (1.1) | 82 (4.4) | <.001 |
| Unknown | 7 | 104 | 111 | .001 |
| Viral load outcome (12 mo post AHD diagnosis) | n = 76 | n = 1050 | n = 1126 | |
| Suppressed (VL < 1000) | 65 (85.5) | 905 (86.2) | 970 (86.1) | .870 |
| Unsuppressed (VL > 1000) | 11 (14.5) | 145 (13.8) | 156 (13.9) | |
| Missing | 254 | 465 | 719 |
This table presents the outcomes among AHD clients, at 3, 6, and 12 mo following AHD diagnosis, stratified by ART-experienced and newly-diagnosed clients.
LTFU = loss to follow up.
At the time of data collection, 1345 (73%) of AHD clients were active in care, while only 71 (21.5%) of ART-experienced clients were still active in care at that time, which is significantly less than those newly diagnosed, P < .001. A total of 124 (37.6%) ART-experienced clients were dead or LTFU at the time of data collection (Table 4).
Table 4.
Patient outcomes at time of data collection, stratified by ART-experienced or newly diagnosed in Homa Bay County, Kenya October 2016 to September 2019.
| ART-Experienced (n = 330) N (%) |
Newly diagnosed with HIV (n = 1515) N (%) |
Total (n = 1845) N (%) |
P value | |
|---|---|---|---|---|
| Active in care | 71 (21.5) | 1274 (84.4) | 1345 (73.1) | <.001 |
| Dead | 48 (14.6) | 41 (2.7) | 89 (4.8) | <.001 |
| LTFU | 76 (23.0) | 64 (4.2) | 140 (7.6) | <.001 |
| Transfer out | 135 (40.9) | 130 (8.6) | 265 (14.4) | <.001 |
| Missing | 0 | 6 | 6 | N/A |
This table presents patient outcomes at time of data collection, stratified by ART-experienced or newly diagnosed.
LTFU = loss to follow up.
5. Discussion
In summary, we found that about 34% of HIV clients had AHD at enrollment in care in the eight study facilities, similar to findings in South Africa (both 33%), and Nairobi, Kenya (33%).[1,12,13] Nearly 20% of AHD clients were ART-experienced. A two-country study of hospitalized AHD clients found that 47% were ART-experienced (ART > 3 months).[14] A population-based survey of AHD in three countries, (Kenya, Malawi and South Africa), found that about 40% of AHD clients had ever been on ART; and in the Kenya (Ndhiwa) site this was 33%.[15] This estimate is nearly double what we found In our setting in Kenya, there may be more unmet need for HIV services, due to poor health seeking behavior, and available testing strategies may not be optimized to reach all those who do not know their status, resulting in a higher proportion of newly diagnosed HIV clients having AHD.
Clients who were ART-experienced were similar in age, sex and marital status to newly-diagnosed clients but were significantly more likely to be from dispensaries or health centers (primary care facilities), 28% vs 13%, P < .001. ART-experienced clients are more likely to have been previously accessing care through the nearest health facility and have returned there on reentry into care. An alternative explanation is that HIV care at these lower-level facilities may not have been optimal, resulting in clients being temporarily LTFU. The causes for disengagement from HIV care include structural (transport, time, cost, work conflicts), psychosocial (stigma, fear of disclosure, feeling healthy), and facility-related quality of care (wait-time, disrespectful facility staff).[16–18] A study has also suggested lack of health services responsiveness to life changes and mobility as reason for disengagement.[19] ART-experienced clients were also more likely to have been in WHO stage III/IV at enrollment (P = .041), indicating that they may have been sicker than the newly-diagnosed clients. A higher proportion of the ART-experienced clients were currently on NNRTI regimen, (66% vs 25%); this was most likely because they had not yet been switched to the more current and optimal DTG regimen.
Significantly fewer ART-experienced clients compared to those newly diagnosed were active in care at the time of study data collection (22% vs 84%; P < .001) with 41% having transferred out, and 23% LTFU and 15% died. While studies have shown that the highest mortality among AHD occurred within 4 weeks of engagement or reengagement with care, we found that attrition from care (dead and LTFU) for the ART-experienced clients occurred at all observational time points, but particularly at 6 months, where 15% were LTFU compared to 7% at 3 months, and there were 10 deaths (3%) compared to 2 deaths (0.6%) at 3 months.[20] Many ART-experienced clients may have been quite ill, and data show that immune reconstitution is lower among those whose immune system was already impaired.[21,22] Additionally, LTFU could be an indicator of death.[23,24] It is possible that some of the ART-experienced clients previously initiated and reengaged in care may have been failing on treatment but continued to receive suboptimal non-suppressive treatment and hence had increased risk of mortality. A systematic review and meta-analysis from sub-Saharan Africa reported a mean delay between confirmation of treatment failure and switch of therapy of 530 days.[25] In Kenya, a review of program data reported the average time taken to ART regimen switch in clients with treatment failure is 411 days.
Our study has some limitations, including missing data for some variables such as CD4 data and viral load outcome. The significant amount of missing CD4 data and unknown treatment outcomes could influence the percentages particularly and the overall results. As this was a retrospective analysis of routinely collected data, it may be possible that some clients designated as LTFU may have self-transferred to other facilities. Similarly, data on OIs may have included both suspected and confirmed diagnoses. Finally, the facilities in this study may not be representative of those in Kenya as a whole, but the sampling method of probability proportional-to-size sampling minimized bias, as clients in the study sites had an equal chance of being selected. However, strengths of the study include the relatively large sample size and that it was undertaken in an area of high HIV prevalence in Kenya. Also, our study was conducted under programmatic conditions (real-world conditions) using routine medical records at public health facilities, demonstrating the burden of AHD in real-world conditions, therefore, maximizing generalizability.
In conclusion, the finding that 20% of clients with AHD were treatment-experienced, highlights the critical importance of maintaining clients in HIV-care, and reducing loss to follow-up. With increasing prevalence of patients on ART, the proportion of AHD treatment-experienced clients may increase without effective interventions to ensure that these patients remain in care.
Acknowledgments
The authors would like to thank the investigators, EGPAF program staff and the team of research assistants for their essential role in this study and the study participants, without whom this research would not be possible. We also acknowledge the collaboration of the Homa Bay County Ministry of Health and CDC for their technical and funding support throughout this study. Finally, we greatly thank Shannon Viana of EGPAF for editing and formatting this paper.
Author contributions
Conceptualization: Rose Otieno Masaba, Allan Mayi, Judith Kose, Boniface Ochanda, Eliud Mwangi, Gordon Okomo, Godfrey Woelk.
Data curation: Nicole Herrera, Stephen Siamba.
Formal analysis: Nicole Herrera.
Funding acquisition: Eliud Mwangi.
Investigation: Rose Otieno Masaba, Godfrey Woelk.
Methodology: Rose Otieno Masaba, Corneleous Okal, Judith Kose, James Ndimbii, Boniface Ochanda, Gordon Okomo, Godfrey Woelk.
Project administration: Corneleous Okal, James Ndimbii.
Resources: Eliud Mwangi.
Software: Stephen Siamba.
Supervision: Millicent Ouma, Corneleous Okal, James Ndimbii, Gordon Okomo.
Validation: Rose Otieno Masaba, Nicole Herrera, Stephen Siamba, Allan Mayi, Boniface Ochanda, Godfrey Woelk.
Visualization: Rose Otieno Masaba, Nicole Herrera, Stephen Siamba, Godfrey Woelk.
Writing – original draft: Rose Otieno Masaba.
Writing – review & editing: Rose Otieno Masaba, Nicole Herrera, Stephen Siamba, Millicent Ouma, Corneleous Okal, Allan Mayi, Judith Kose, James Ndimbii, Boniface Ochanda, Eliud Mwangi, Gordon Okomo, Godfrey Woelk.
Abbreviations:
- ART
- AHD-Advanced HIV disease, antiretroviral therapy
- EGPAF
- Elizabeth Glaser Pediatric AIDS Foundation
- EMR
- electronic medical records
- ID
- identification document
- IQR
- interquartile range
- LTFU
- loss to follow up
- OI
- opportunistic infections
- PLHIV
- people living with HIV
- RA
- research assistant
- VL
- viral load
- WHO
- World Health Organization
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors have no conflicts of interest to disclose.
This study was supported by the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) through the U.S. Centers for Disease Control and Prevention (CDC) under the terms of Cooperative Agreement No. NU2GGH001948-01-00.
The findings and conclusions in this manuscript are those of the author(s) and do not necessarily represent the official position of the funding agencies.
How to cite this article: Masaba RO, Herrera N, Siamba S, Ouma M, Okal C, Mayi A, Kose J, Ndimbii J, Ochanda B, Mwangi E, Okomo G, Woelk G. Advanced HIV disease in Homa Bay County, Kenya: Characteristics of newly-diagnosed and antiretroviral therapy-experienced clients. Medicine 2023;102:51(e36716).
Contributor Information
Nicole Herrera, Email: nherrera@pedaids.org.
Stephen Siamba, Email: stephen.siamba@gmail.com.
Millicent Ouma, Email: oumamillicent500@gmail.com.
Corneleous Okal, Email: okalcorneleous@gmail.com.
Allan Mayi, Email: amayi@pedaids.org.
Judith Kose, Email: otienoj@africa-union.org.
James Ndimbii, Email: jndimbii@pedaids.org.
Boniface Ochanda, Email: ivq2@cdc.gov.
Eliud Mwangi, Email: emwangi@pedaids.org.
Gordon Okomo, Email: okomogordon@homabayhealth.or.ke.
Godfrey Woelk, Email: gwoelk@pedaids.org.
References
- [1].Carmona S, Bor J, Nattey C, et al. Persistent high burden of advanced HIV disease among patients seeking care in South Africa’s national HIV program: data from a nationwide laboratory cohort. Clin Infect Dis. 2018;66(suppl_2):S111–7. Available at: https://pubmed.ncbi.nlm.nih.gov/29514238/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Osler M, Hilderbrand K, Goemaere E, et al. The continuing burden of advanced HIV disease over 10 years of increasing antiretroviral therapy coverage in South Africa. Clin Infect Dis. 2018;66(suppl_2):S118–25. Available at: https://pubmed.ncbi.nlm.nih.gov/29514233/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].World Health Organization. Guidelines for Managing Advanced HIV Disease and Rapid Initiation of Antiretroviral Therapy. Geneva, Switzerland: World Health Organization. 2017. Available at: https://www.who.int/publications/i/item/9789241550062. [Access date December 20, 2022]. [PubMed] [Google Scholar]
- [4].Egger M, May M, Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Available at: https://www.thelancet.com/journals/lancet/article/PIIS0140673602094114/fulltext [access date December 10, 2022]. [DOI] [PubMed]
- [5].Walker AS, Prendergast AJ, Mugyenyi P, et al. Mortality in the year following antiretroviral therapy initiation in HIV-infected adults and children in Uganda and Zimbabwe. Clin Infect Dis. 2012;55:1707–18 Available at: https://www.thelancet.com/journals/lanhiv/article/PIIS2352-3018(15)00137-X/fulltext. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ford N, Shubber Z, Meintjes G, et al. Causes of hospital admission among people living with HIV worldwide: a systematic review and meta-analysis. Lancet HIV. 2015;2:e438–44. Available at: 10.1016/S2352-3018(15)00137-X. [DOI] [PubMed] [Google Scholar]
- [7].Ford N, Doherty M. The enduring challenge of advanced HIV infection. N Engl J Med. 2017;377:283–4. Available at: https://www.nejm.org/doi/full/10.1056/NEJMe1707598. [DOI] [PubMed] [Google Scholar]
- [8].UNAIDS; Kenya Country Factsheet 2020 Available at: https://www.unaids.org/en/regionscountries/countries/kenya. [Access date December 27, 2021].
- [9].Calmy A, Ford N, Meintjes G. The persistent challenge of advanced HIV disease and AIDS in the Era of antiretroviral therapy. Clin Infect Dis. 2018;66(suppl 2):S103–SS105. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5850411/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lamp K, McGovern S, Fong Y, et al. Proportions of CD4 test results indicating advanced HIV disease remain consistently high at primary health care facilities across four high HIV burden countries. PLoS One. 2020;15:e0226987. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6946176/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].National AIDS and STI Control Programme (NASCOP), Preliminary Kenya Population-based HIV Impact Assessment (KENPHIA) 2018 Report. NASCOP. 2020. Available at: https://phia.icap.columbia.edu/wp-content/uploads/2020/04/KENPHIA-2018_Preliminary-Report_final-web.pdf. [Google Scholar]
- [12].Fomundam HN, Tesfay AR, Mushipe SA, et al. Prevalence and predictors of late presentation for HIV care in South Africa. S Afr Med J. 2017;107:1058–64. Available at: https://pubmed.ncbi.nlm.nih.gov/29262956/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].van der Kop ML, Thabane L, Awiti PO, et al. Advanced HIV disease at presentation to care in Nairobi, Kenya: late diagnosis or delayed linkage to care? - - a cross-sectional study. BMC Infect Dis. 2016;16:169. Available at: https://pubmed.ncbi.nlm.nih.gov/27091128/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ousley J, Niyibizi AA, Wanjala S, et al. High proportions of patients with advanced HIV are antiretroviral therapy experienced: hospitalization outcomes from 2 Sub-Saharan African Sites. Clin Infect Dis. 2018;66(suppl_2):S126–31. Available at: https://pubmed.ncbi.nlm.nih.gov/29514239/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chihana ML, Huerga H, Van Cutsem G, et al. Distribution of advanced HIV disease from three high HIV prevalence settings in Sub-Saharan Africa: a secondary analysis data from three population-based cross-sectional surveys in Eshowe (South Africa), Ndhiwa (Kenya) and Chiradzulu (Malawi). Glob Health Action. 2019;12:1679472. Available at: https://pubmed.ncbi.nlm.nih.gov/31679482/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sikazwe I, Eshun-Wilson I, Sikombe K, et al. Patient-reported reasons for stopping care or switching clinics in Zambia: a multisite, regionally representative estimate using a multistage sampling-based approach in Zambia. Clin Infect Dis. 2021;73:e2294–302. Available at: https://pubmed.ncbi.nlm.nih.gov/33011803/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mody A, Sikombe K, Beres LK, et al. Profiles of HIV care disruptions among adult patients lost to follow-up in Zambia: a latent class analysis. J Acquir Immune Defic Syndr. 2021;86:62–72. Available at: https://pubmed.ncbi.nlm.nih.gov/33105396/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Camlin CS, Neilands TB, Odeny TA, et al. Patient-reported factors associated with reengagement among HIV-infected patients disengaged from care in East Africa. AIDS. 2016;30:495–502. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4996364/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bisnauth MA, Davies N, Monareng S, et al. Why do patients interrupt and return to antiretroviral therapy? Retention in HIV care from the patient’s perspective in Johannesburg, South Africa. PLoS One. 2021;16:e0256540. Available at: https://pubmed.ncbi.nlm.nih.gov/34473742/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Post FA, Szubert AJ, Prendergast AJ, et al. Causes and timing of mortality and morbidity among late presenters starting antiretroviral therapy in the REALITY Trial. Clin Infect Dis. 2018;66(suppl_2):S132–9. Available at: https://pubmed.ncbi.nlm.nih.gov/29514234/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sereti I, Sheikh V, Shaffer D, et al. Prospective international study of incidence and predictors of immune reconstitution inflammatory syndrome and death in people living with human immunodeficiency virus and severe lymphopenia. Clin Infect Dis. 2020;71:652–60. Available at: https://pubmed.ncbi.nlm.nih.gov/31504347/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Vinhaes CL, Araujo-Pereira M, Tibúrcio R, et al. Systemic inflammation associated with immune reconstitution inflammatory syndrome in persons living with HIV. Life (Basel). 2021;11:65. Available at: https://pubmed.ncbi.nlm.nih.gov/33477581/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wekesa P, McLigeyo A, Owuor K, et al. Factors associated with 36-month loss to follow-up and mortality outcomes among HIV-infected adults on antiretroviral therapy in Central Kenya. BMC Public Health. 2020;20:328. Available at: https://pubmed.ncbi.nlm.nih.gov/32171279/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jiamsakul A, Gani Y, Avihingsanon A, et al. Brief report: mortality after loss to follow-up-a linkage study of people living with HIV in Thailand and Malaysia. J Acquir Immune Defic Syndr. 2022;91:290–5. Available at: https://pubmed.ncbi.nlm.nih.gov/35969472/#:~:text=Results%3A%20Data%20linkages%20were%20performed,4.89%20per%20100%20person%2Dyears. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bernabé KJ, Siedner M, Tsai AC, et al. Detection of HIV virologic failure and switch to second line therapy: a systematic review and meta-analysis of data from Sub-Saharan Africa. Open Forum Infect Dis. 2022;9:ofac121. Available at: https://pubmed.ncbi.nlm.nih.gov/35434173/. [DOI] [PMC free article] [PubMed] [Google Scholar]